Critical Flux and Fouling Analysis of PVDF-Mixed Matrix Membranes for Reclamation of Refinery-Produced Wastewater: Effect of Mixed Liquor Suspended Solids Concentration and Aeration
Abstract
1. Introduction
2. Experimental
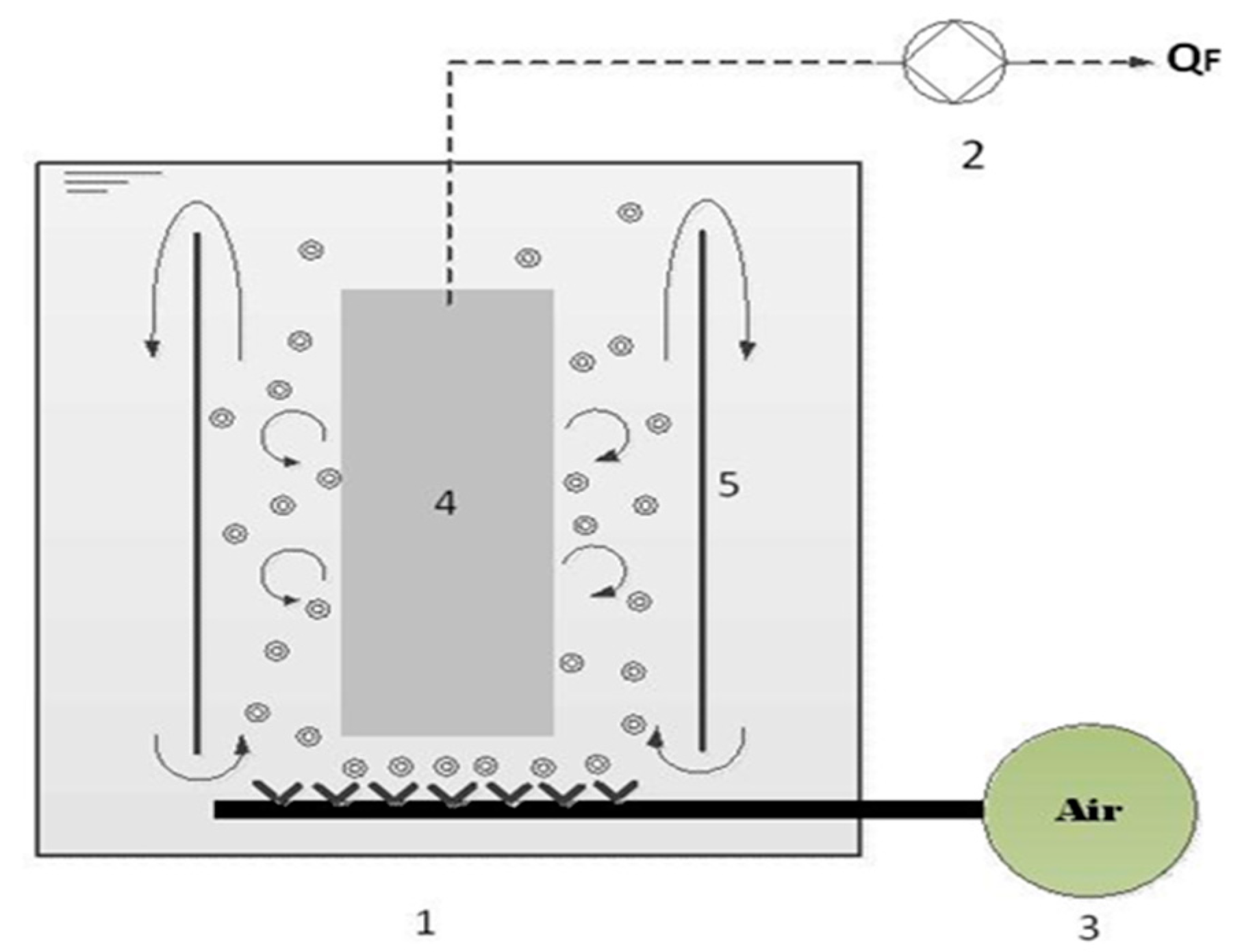
2.1. Preparation of Ultrafiltration System
2.2. Membrane Characterization
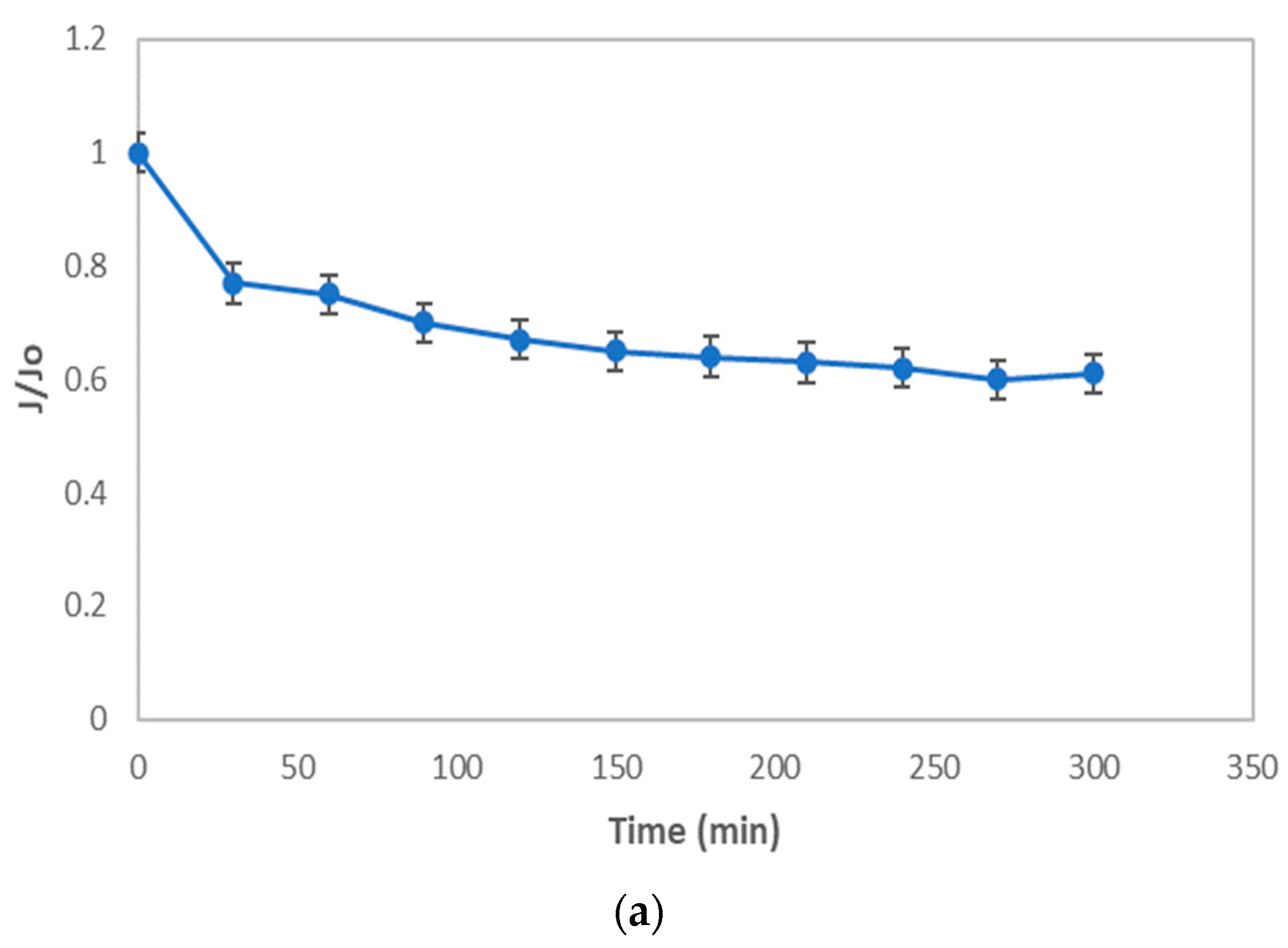
2.3. Critical Flux Evaluation
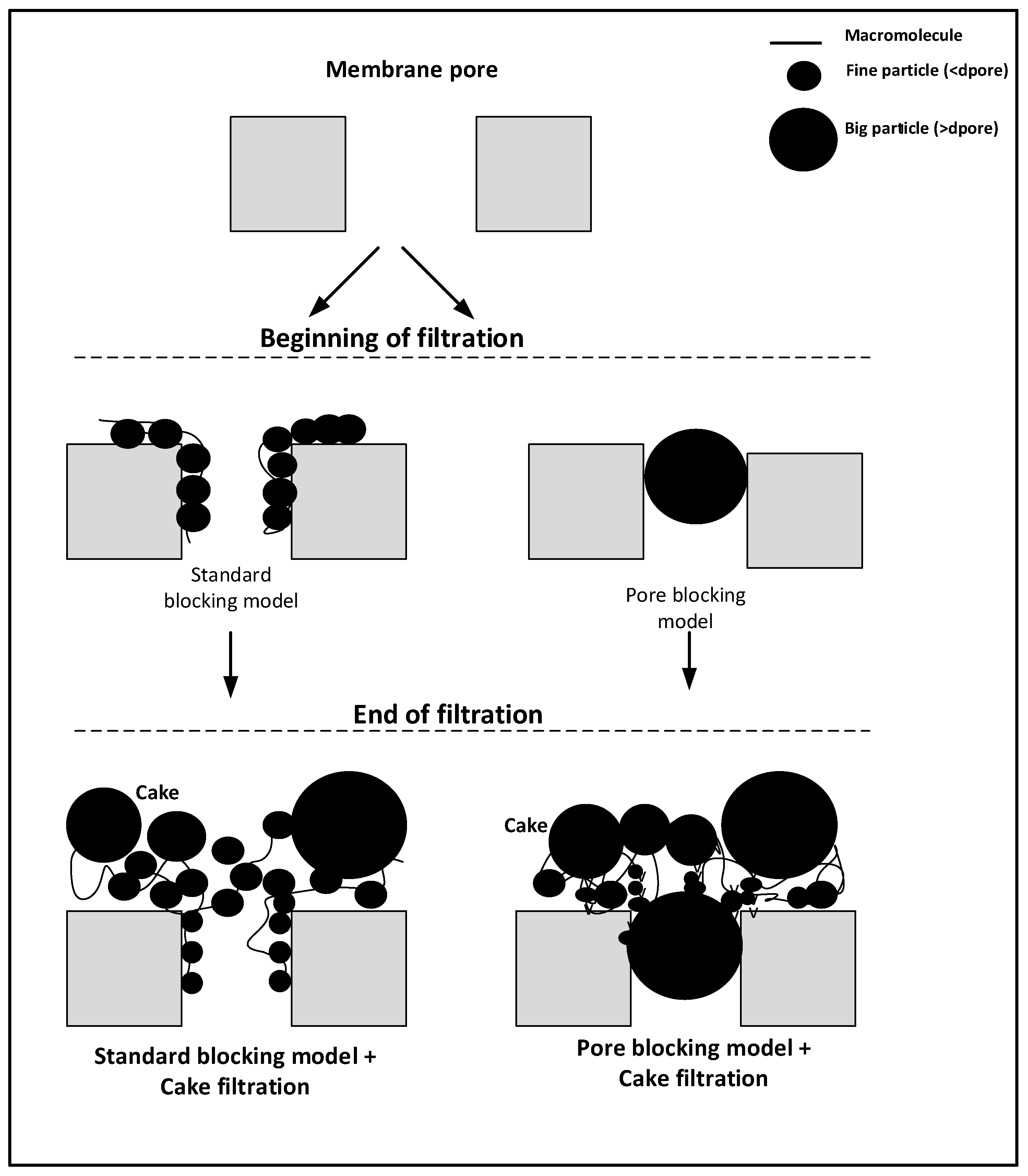
2.4. Fouling Mechanism
2.5. Water Analysis Methods
3. Results and Discussions
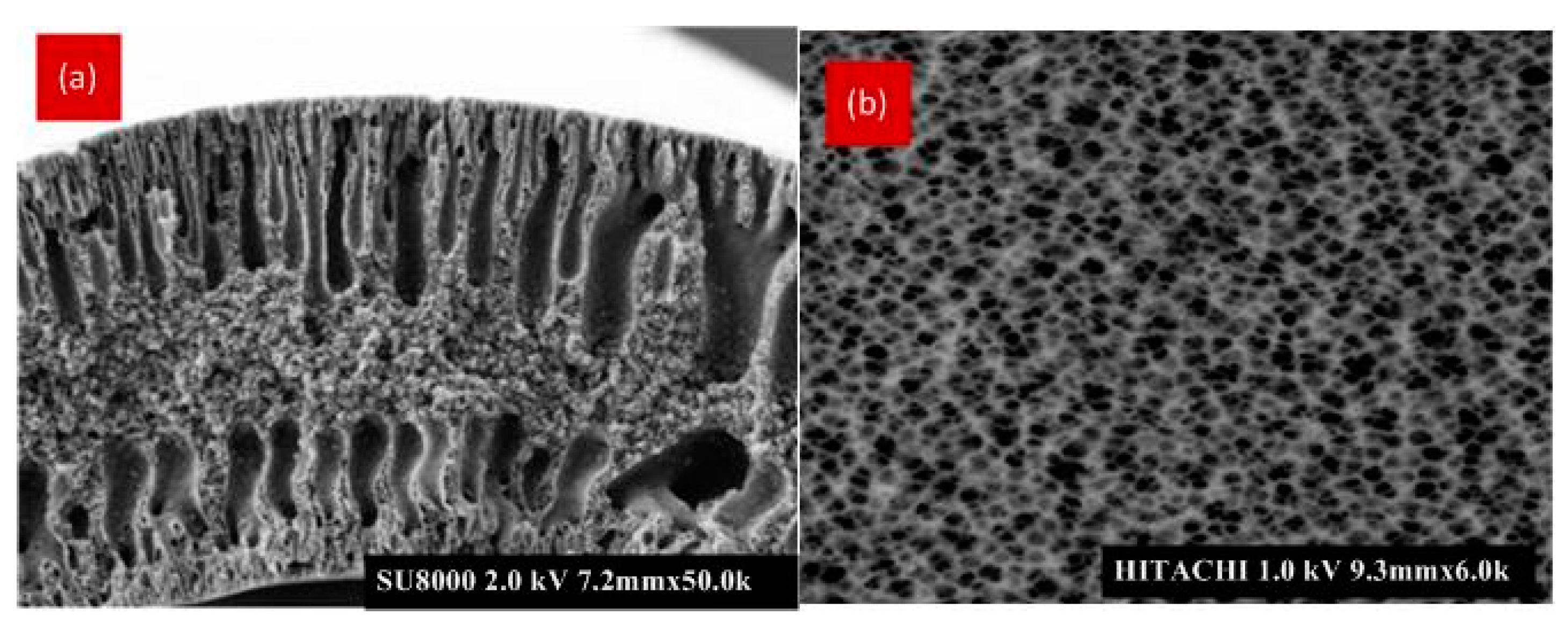
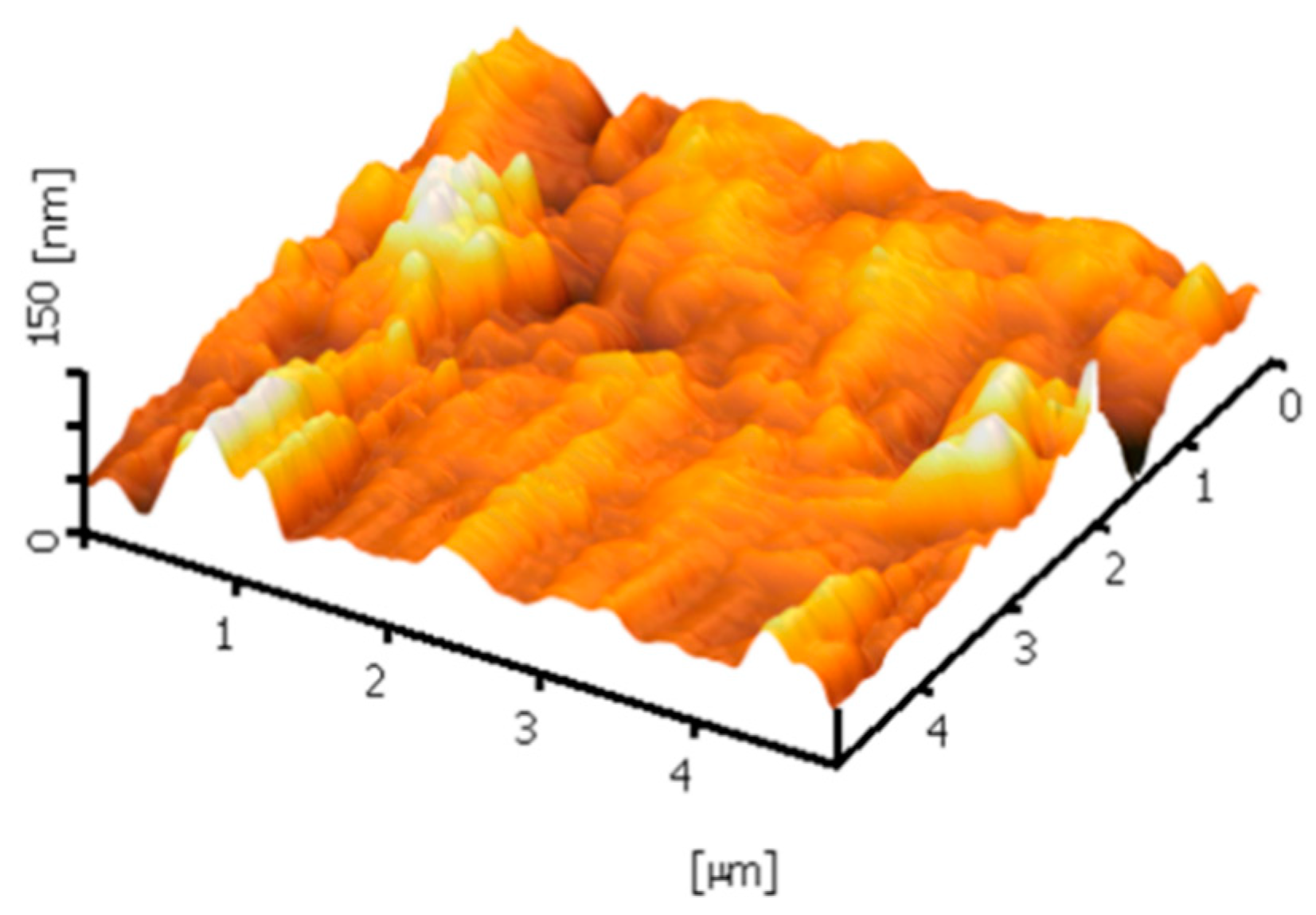
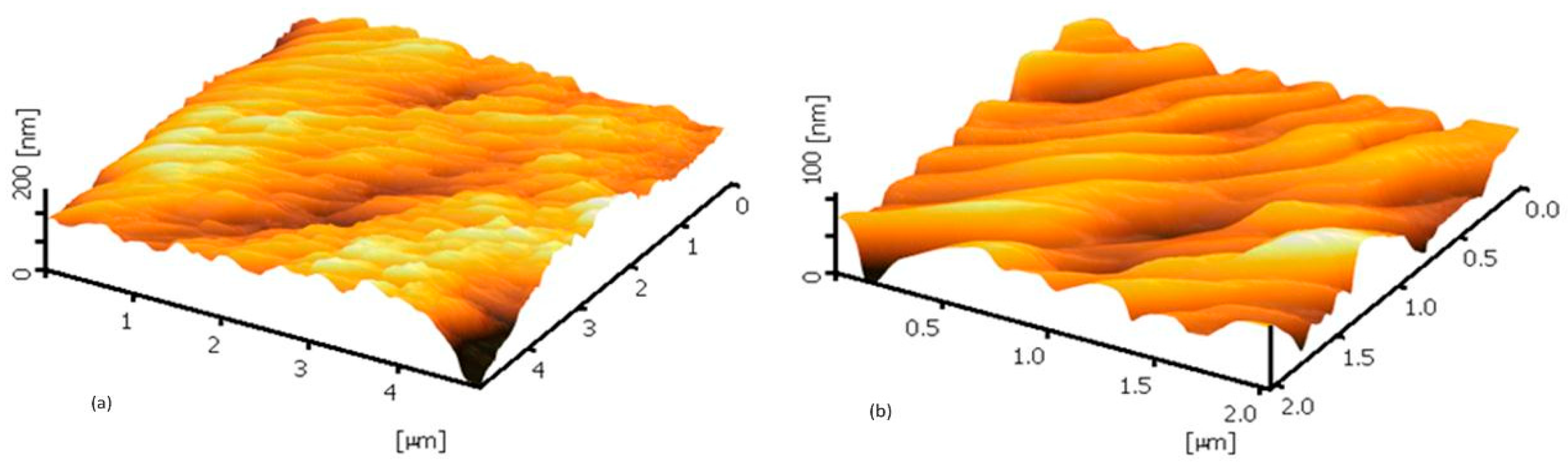
3.1. Microscopic Analysis Using FESEM and AFM
3.2. Membrane Fouling Mechanism
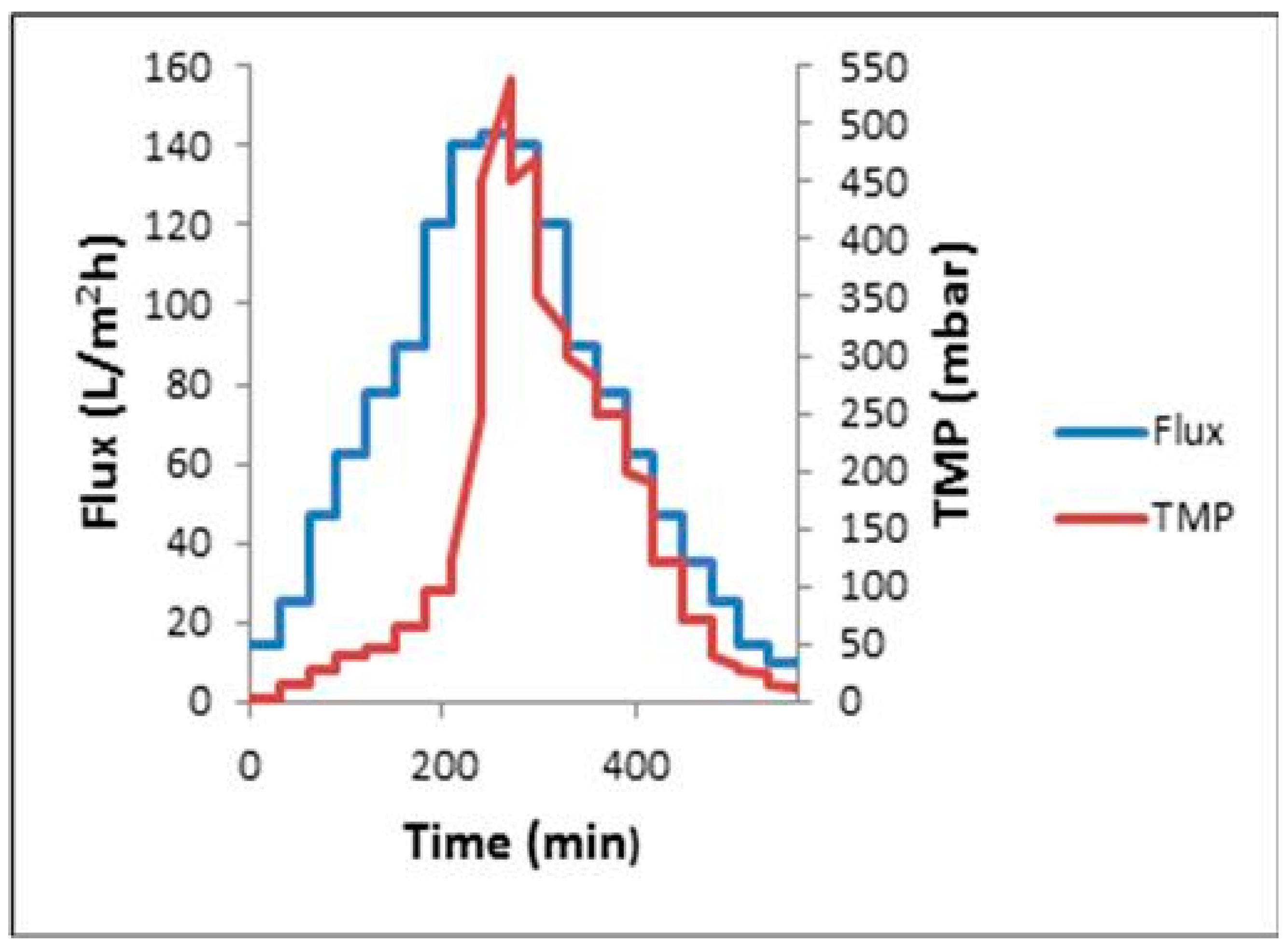
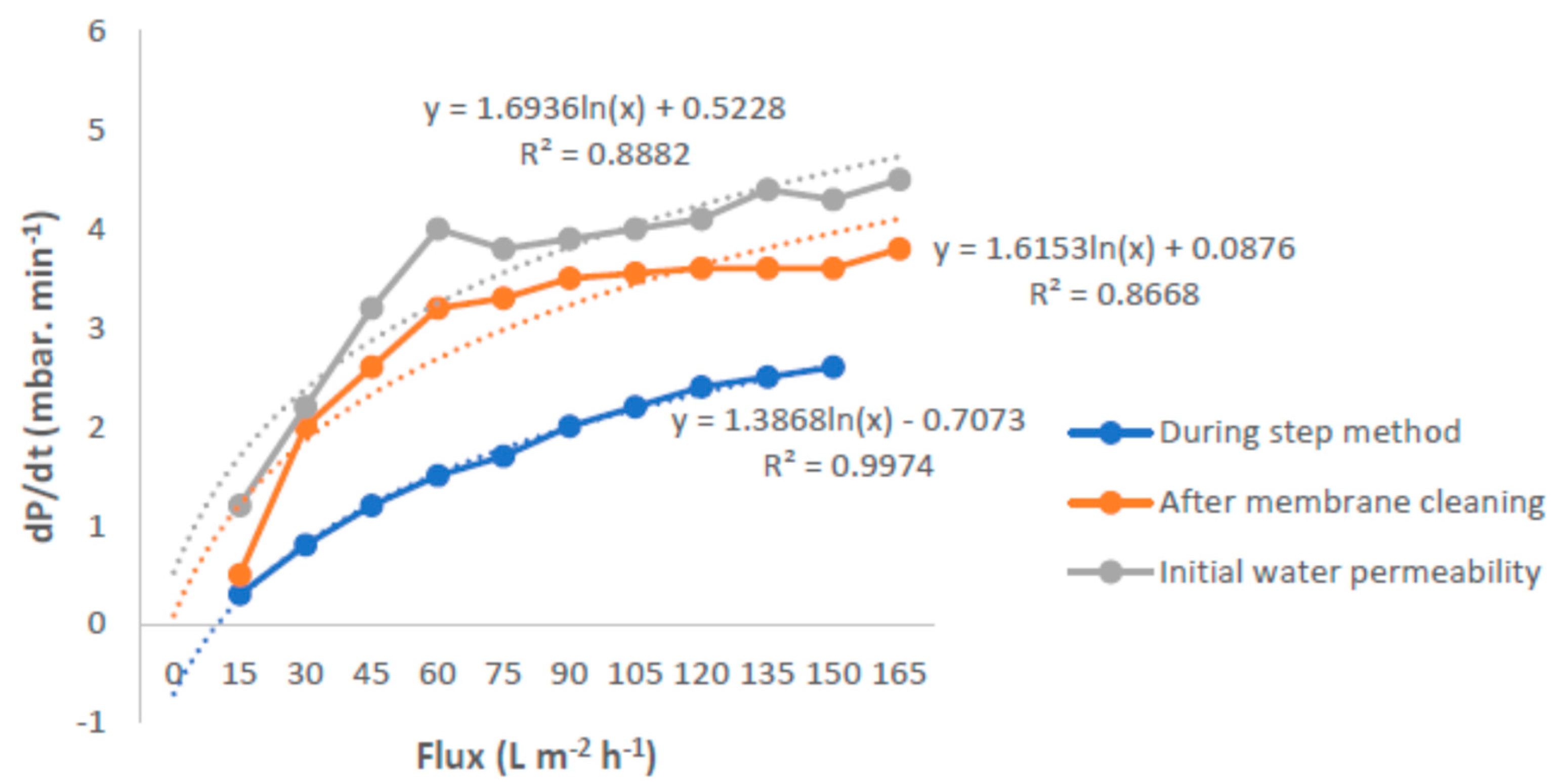
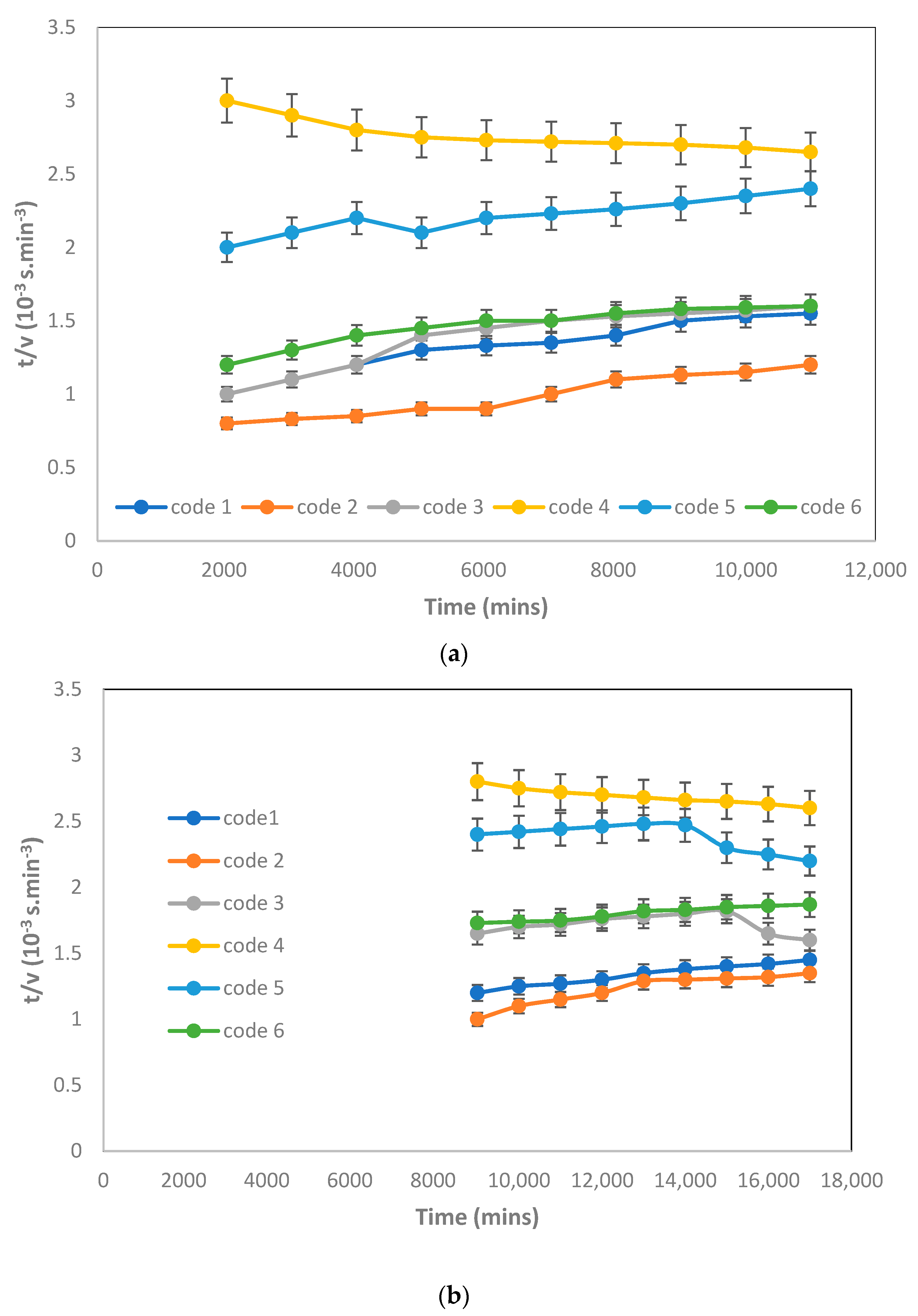
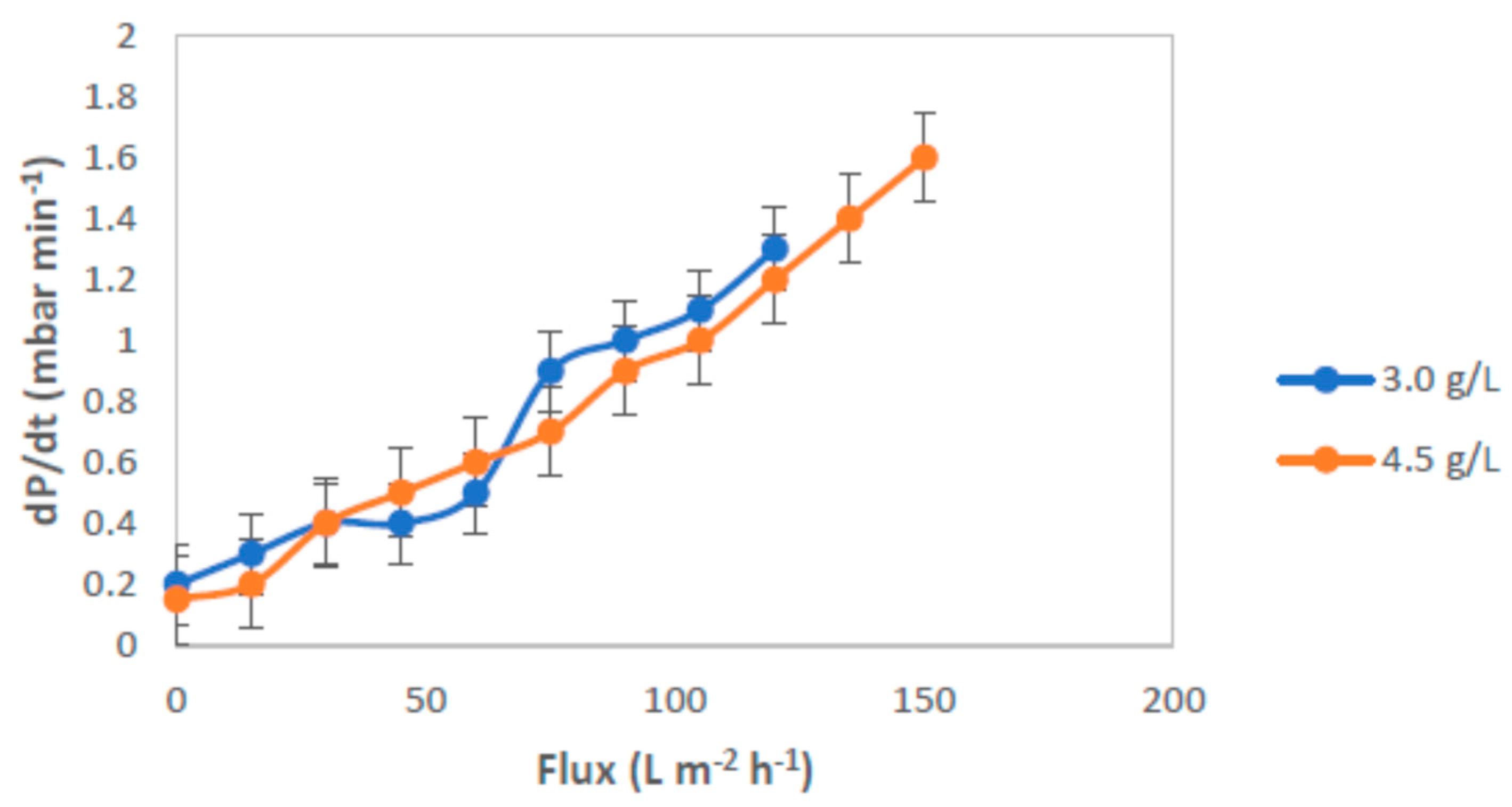
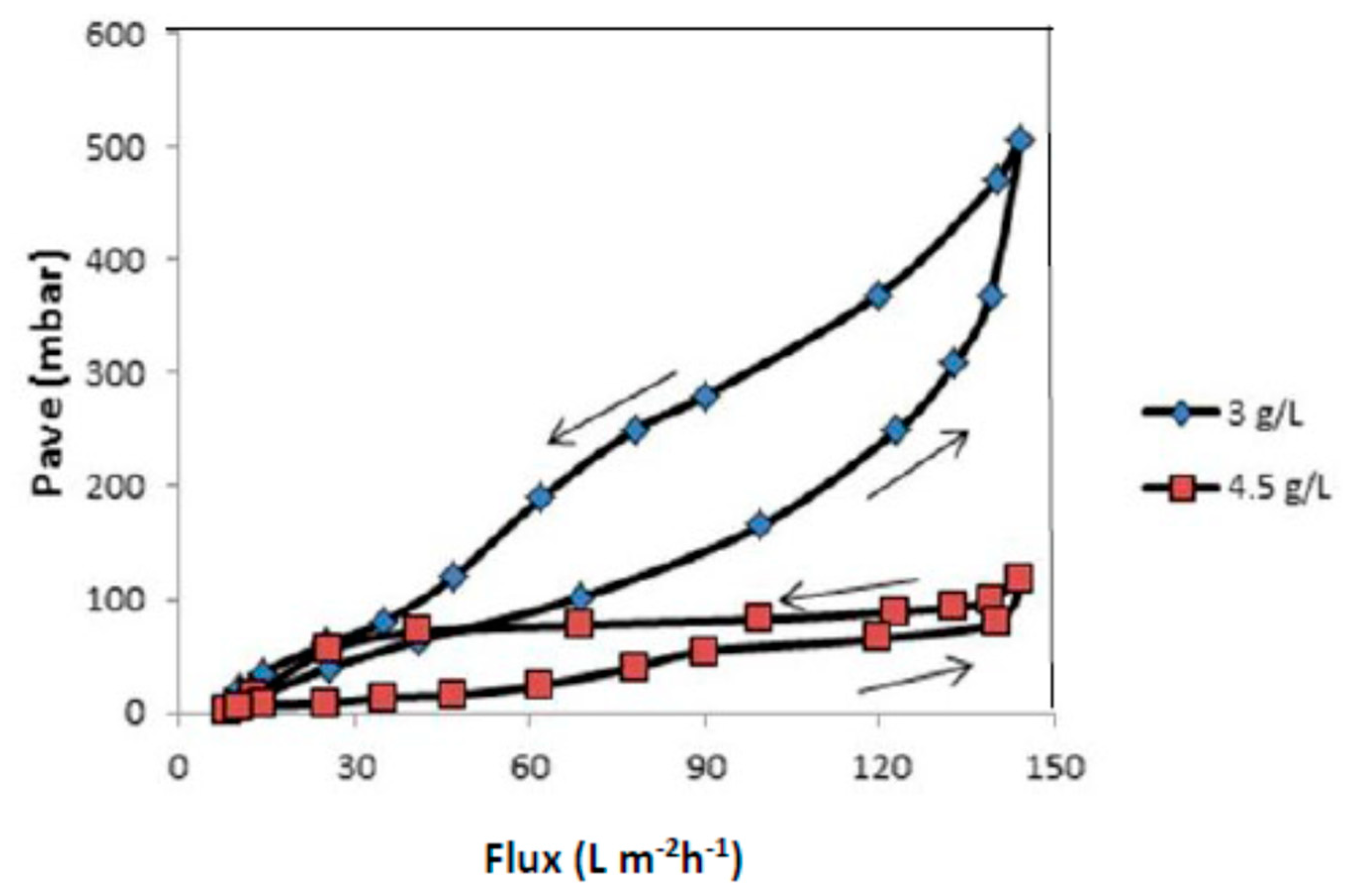
3.3. Critical Flux, Resistance, and Filtrate Analysis of Refinery-Produced Wastewater
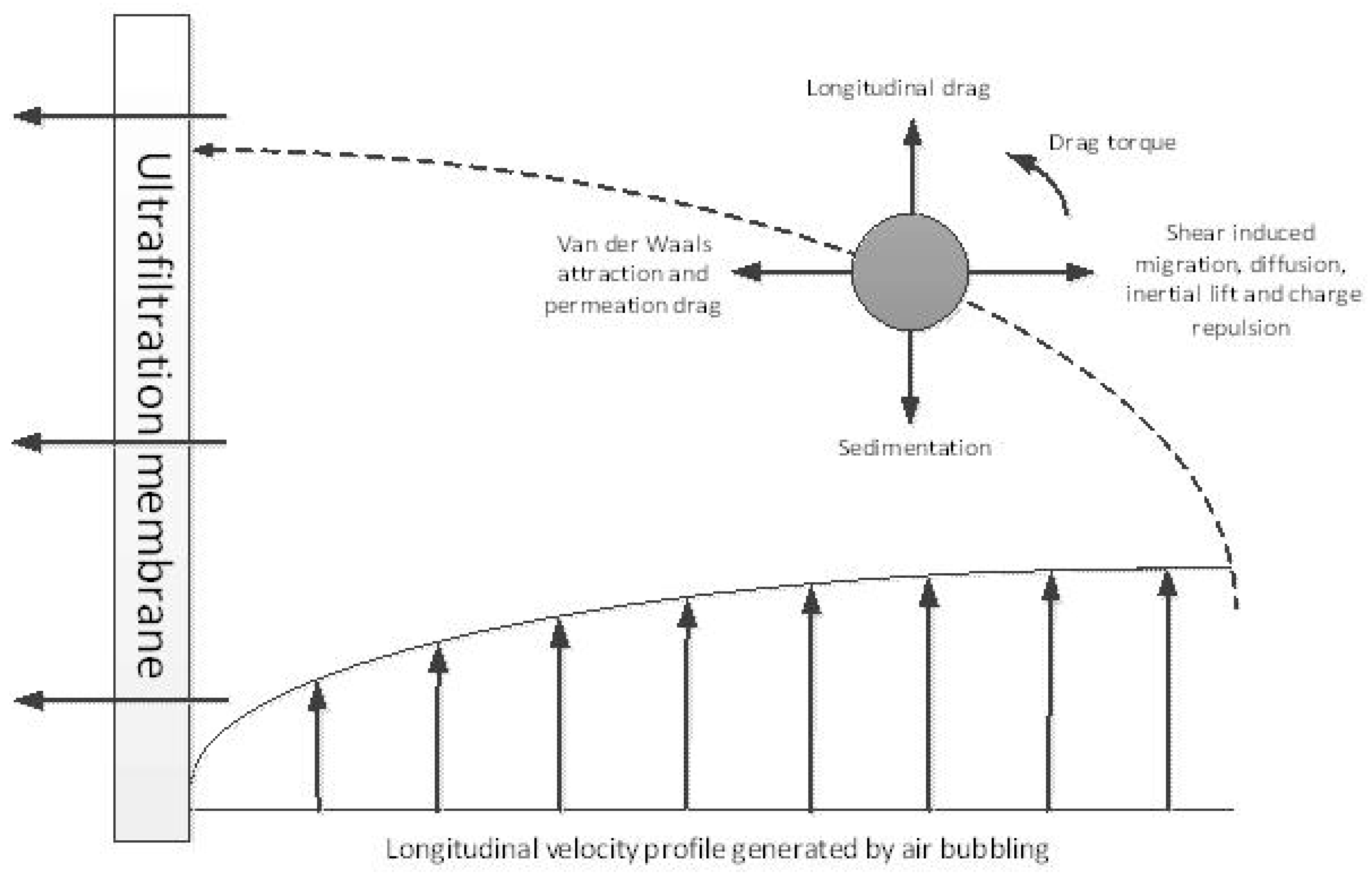
3.4. Effect of Air Bubbles Flow Rate on the Performance of Membrane Ultrafiltration
3.5. Effect of MLSS Concentration on the Critical Flux and Fouling Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ministry of Environmental of Republic Indonesia, Parameter Limits of Effluent. 2019. Available online: www.menlhk.go.id (accessed on 23 May 2020).
- Abdulsalam, H.M.; Che Man, H.; Idris, A.I.; Yunos, K.F.; Abidin, Z.Z. Treatment of palm oil mill effluent using membrane bioreactor: Novel Processes and their major drawbacks. Water 2018, 10, 1165. [Google Scholar] [CrossRef]
- Paramitadevi, Y.V. Technical problems of wastewater treatment in crude palm oil industry: A case study in PT Soefin Indonesia-Kebun Sungai Liput Naggroe aceh darussalam province. J. Earth Environ. 2017, 65, 012048. [Google Scholar]
- Judd, S. The MBR Book, Principles and Applications of Membrane Bioreactors in Water and Wastewaters Treatment; Elsevier: Oxford, UK, 2006. [Google Scholar]
- Jyothi, M.S.; Nayak, V.; Padaki, M.; Balakrishna, R.G.; Soontarapa, K. Aminated polysulfone/TiO2 composite membranes for an effective removal of Cr(VI). Chem. Eng. J. 2016, 283, 1494–1505. [Google Scholar] [CrossRef]
- Buscio, V.; Crespi, M.; Gutiérrez-Bouzan, C. Application of PVDF ultrafiltration membranes to treat and reuse textile wastewater. Desalination Water Treat. 2016, 57, 8090–8096. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, S.; Chen, L.; Fang, H. Controlling interlayer spacings of graphene oxide membranes with cationic for precise sieving of mono-/multi-valent ions. Sep. Purif. Technol. 2020, 241, 116738. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Z.; Lu, Y.; Liu, S.; Li, H.; Ye, G.; Chen, J. Graphene Oxide Membranes for Tunable Ion Sieving in Acidic Radioactive Waste. Adv. Sci. 2021, 8, 2002717. [Google Scholar] [CrossRef]
- Zheng, S.; Tu, Q.; Urban, J.J.; Li, S.; Mi, B. Swelling of Graphene Oxide Membranes in Aqueous Solution: Characterization of Interlayer Spacing and Insight into Water Transport Mechanisms. ACS Nano 2017, 11, 6440–6450. [Google Scholar] [CrossRef]
- Yeh, C.-N.; Raidongia, K.; Shao, J.; Yang, Q.-H.; Huang, J. On the origin of the stability of graphene oxide membranes in Water. Nat. Chem. 2015, 7, 166–170. [Google Scholar] [CrossRef]
- Kang, Y.; Xia, Y.; Wang, H.; Zhang, X. 2D Laminar Membranes for Selective Water and Ion Transport. Adv. Funct. Mater. 2019, 29, 1902014. [Google Scholar] [CrossRef]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Yuliwati, E.; Porawati, H.; Elfidiah, E.; Melani, A. Performance of Composite Membrane for Palm oil Wastewater Treatment. J. Appl. Membr. Sci. Technol. 2019, 23, 1–10. [Google Scholar] [CrossRef][Green Version]
- Tana, Y.H.; Goh, P.S.; Lai, G.S.; Lau, W.J.; Ismail, A.F. Treatment of aerobic treated palm oil mill effluent (AT-POME) by using TiO2 photocatalytic Process. J. Teknol. 2014, 70, 61–63. [Google Scholar] [CrossRef]
- Ulatowski, K.; Sobieszuk, P. Influence of liquid flowrate on size of nanobubbles generated by porous-membrane modules. Chem. Process Eng. 2018, 39, 335–345. [Google Scholar]
- Mansourpanah, Y.; Momeni-Habili, E. Investigation the separation properties of TiO2-TFC nanocomposite membranes by comparing the presence or absence of UV irradiation; membrane preparation and characterization. J. Membr. Sci. Res. 2015, 1, 26–33. [Google Scholar]
- Li, W.; Wu, W.; Li, Z. Controlling Interlayer Spacing of Graphene Oxide Membranes by External Pressure Regulation. ACS Nano 2018, 12, 9309–9317. [Google Scholar] [CrossRef]
- Liang, S.; Zhu, L.; Wang, S.; Chen, L.; Fang, H. Fast Reduced Graphene-Based Membranes with High desalination performance. Membranes 2021, 11, 846. [Google Scholar] [CrossRef]
- Wang, Y.J.; Kim, D.J. Crystallinity, morphology, mechanical properties and conductivity study of in situ formed PVDF/LiClO4/TiO2 nanocomposite polymer electrolytes. Electrochim. Acta 2007, 52, 3181–3189. [Google Scholar] [CrossRef]
- Wu, T.T.; Jahim, J.M.; Muhammad, A.W.; Anuar, N. Palm oil mill effluent (POME) treatment and bio resources recovery using ultrafiltration membrane: Effect of pressure on membrane fouling. Biochem. Eng. J. 2007, 35, 309–317. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F. Effect of additives concentration on the surface properties and performance of PVDF ultrafiltration membranes for refinery produced wastewater treatment. Desalination 2010, 273, 226–234. [Google Scholar] [CrossRef]
- Jarma, Y.A.; Parlar, I.; Pek, T.O.; Kayral, K.; Kabay, K.; Yigit, N.O.; Kitis, M.; Yuksel, M. Study on operational conditions to minimize membrane fouling in membrane bioreactor (MBR) system for wastewater treatment-preliminary pilot tests. J. Membr. Sci. Res. 2018, 4, 212–217. [Google Scholar]
- Lukina, A.O.; Boutin, C.; Rowland, O.; Carpenter, D.J. Evaluating trivalent chromium toxicity on wild terrestrial and wetland plants. Chemosphere 2016, 162, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Bokhary, A.; Tikka, A.; Leitch, M.; Liao, B. Membrane fouling prevention and control strategies in pulp and paper industry appalication: A review. J. Membr. Sci. Res. 2018, 4, 181–197. [Google Scholar]
- Eternadi, H.; Qazvini, H. Investigation of alumina nanoparticles role on the critical flux and performance of polyvinyl chloride membrane in a submerged membrane system for the removal of humic acid. Polym. Bull. 2021, 78, 2645–2662. [Google Scholar]
- Moustakes, N.G.; Katsaros, F.K.; Kontos, A.G.; Ramanos, G.E.; Dionysiou, D.D.; Falaras, P. Visible light active TiO2 photocatalytic filtration membranes with improved permeability and low energy consumption. Catal. Today 2014, 224, 56–69. [Google Scholar] [CrossRef]
- Dzinun, H.; Ichikawa, Y.; Honda, M.; Zhang, O. Efficient immobilised TiO2 in polyvinylidene fluoride (PVDF) membrane for photocatalytic degradation of methylene blue. J. Membr. Sci. Res. 2020, 6, 188–195. [Google Scholar]
- Aryanti, N.; Saraswati, A.; Putra, R.P.; Nafiunisa, A.; Wardhani, D.H. Fouling Mechanism of Micelle Enhanced Ultrafiltration with SDS Surfactant for Indigozol Dye Removal. J. Teknol. 2018, 80, 31–39. [Google Scholar] [CrossRef][Green Version]
- Huang, J.; Peng, L.; Zeng, G.; Li, X.; Zhao, Y.; Liu, L.; Li, F.; Chai, Q. Evaluation of micellar enhanced ultrafiltration for removing methylene blue and cadmium ion simultaneously with mixed surfactants. Sep. Purif. Technol. 2014, 125, 83–89. [Google Scholar] [CrossRef]
- Schwarze, M. Micellar-enhanced ultrafiltration (MEUF)—State of the art. Environ. Sci. Water Res. Technol. 2017, 3, 598–624. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]





| Parameter | Unit | Value | |
|---|---|---|---|
| Real | Synthetic | ||
| Oil and grease | mg/L | 170 | 200 |
| Chemical oxygen demand (COD) | mg/L | 555 | 398 |
| Total suspended solids (TSS) | mg/L | 213 | 543 |
| Ammonia nitrogen (NH3-N) | mg/L | 29.10 | 27.60 |
| pH | - | 6.70 | 6.70 |
| Temperature | °C | 27–28 | 26–28 |
| Code | Variables | |
|---|---|---|
| MLSS (mg/L) | Air Bubbles Flow Rate (mL/min) | |
| 1 | 3 | 1.2 |
| 2 | 3 | 2.4 |
| 3 | 3 | 3.0 |
| 4 | 4.5 | 1.2 |
| 5 | 4.5 | 2.4 |
| 6 | 4.5 | 3.0 |
| Parameter | Membrane |
|---|---|
| Membrane configuration | Hollow fiber |
| Membrane material Hydrophilic additive added | PVDF LiCl, TiO2 |
| Outer diameter (mm) | 1.10 |
| Inner diameter (mm) | 0.55 |
| Pore size (nm) | 34.05 |
| Porosity | 88.42% |
| Contact angle (o) | 47 |
| Zeta potential (mV at pH 6.9) | 62.00 |
| Tensile strength (MPa) | 3.37 ± 0.13 |
| Young’s modulus (GPa) | 3.81 ± 0.21 |
| Pure water flux (L m−2 h−1) | 82.95 at 250 mmHg |
| pH feed solution (pH) | 6.70 |
| Air bubbles flow rate (mL/min) | 1.20, 2.40, 3.00 |
| Mixed liquor suspended solids concentration (g/L) | 3.00, 4.50 |
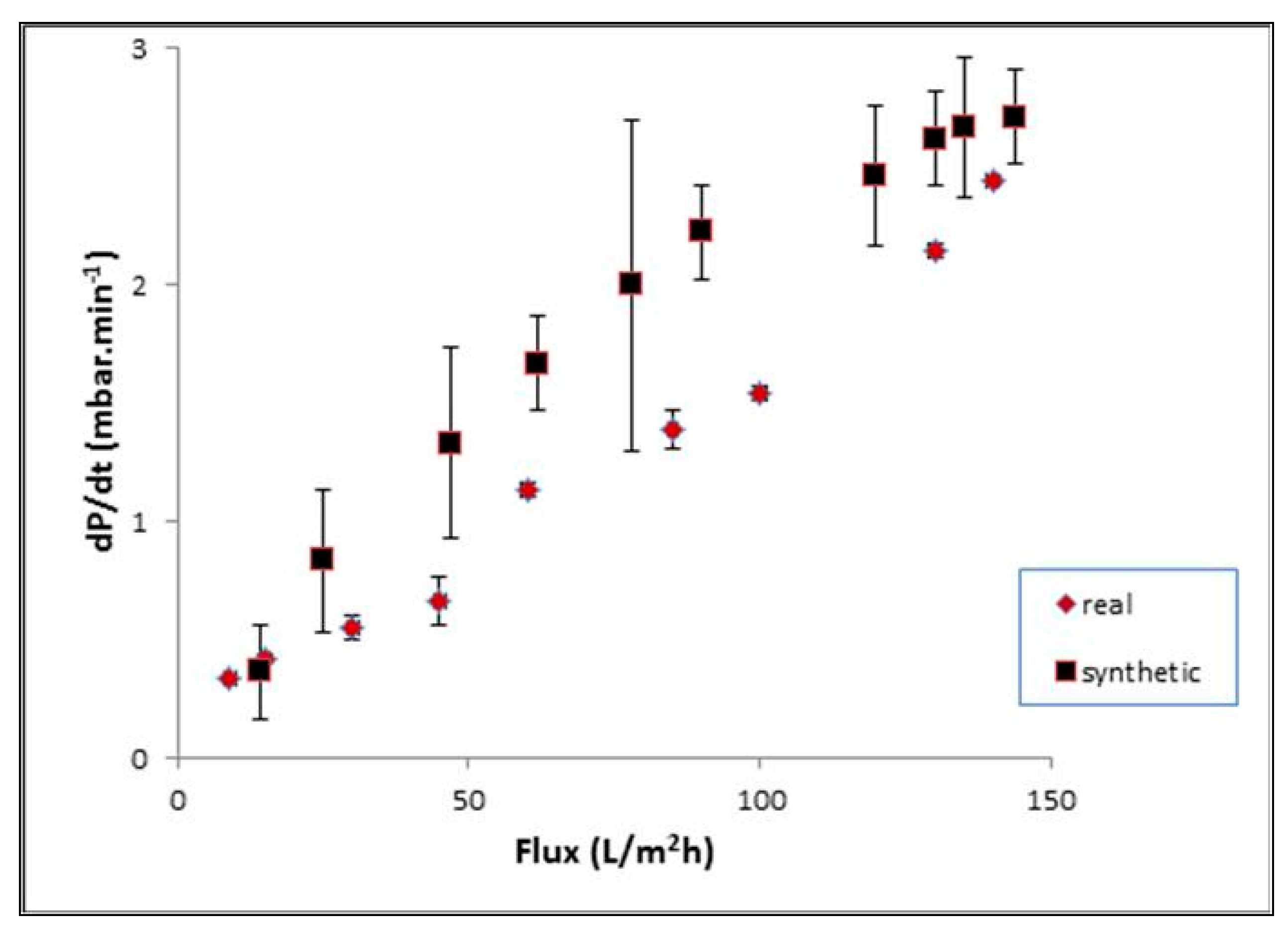
| Flux (L/m2h) | Pave (mbar) | Lp (L/m2h bar) | ΔP0 (mbar) | dP/dt (bar/min) | Rf/Rt (%) |
|---|---|---|---|---|---|
| 0 | 0 | 0 | |||
| 8.5 | 3 | 2.83 | 3 | 0.1 | 23.89 |
| 12.9 | 15 | 0.86 | 12 | 0.2 | 26.21 |
| 25.4 | 39 | 0.65 | 24 | 0.3 | 28.02 |
| 41.2 | 64 | 0.64 | 25 | 0.21 | 28.01 |
| 68.8 | 102 | 0.67 | 38 | 0.25 | 27.98 |
| 99.5 | 165 | 0.60 | 63 | 0.35 | 32.96 |
| 123.1 | 250 | 0.49 | 85 | 0.40 | 35.20 |
| 133.1 | 310 | 0.43 | 60 | 0.25 | 30.11 |
| 139.1 | 370 | 0.38 | 60 | 0.22 | 31.12 |
| 144.4 | 507 | 0.28 | 137 | 0.46 | 39.30 |
| Parameter of Permeate Removal (%) | Refinery Produced Wastewater | |
|---|---|---|
| Synthetic | Real | |
| Oil and grease | 97.35 | 97.82 |
| COD | 88.05 | 90.30 |
| TSS | 99.60 | 99.81 |
| NH3-N | 90.65 | 91.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuliwati, E.; Ismail, A.F.; Othman, M.H.D.; Shirazi, M.M.A. Critical Flux and Fouling Analysis of PVDF-Mixed Matrix Membranes for Reclamation of Refinery-Produced Wastewater: Effect of Mixed Liquor Suspended Solids Concentration and Aeration. Membranes 2022, 12, 161. https://doi.org/10.3390/membranes12020161
Yuliwati E, Ismail AF, Othman MHD, Shirazi MMA. Critical Flux and Fouling Analysis of PVDF-Mixed Matrix Membranes for Reclamation of Refinery-Produced Wastewater: Effect of Mixed Liquor Suspended Solids Concentration and Aeration. Membranes. 2022; 12(2):161. https://doi.org/10.3390/membranes12020161
Chicago/Turabian StyleYuliwati, Erna, Ahmad Fauzi Ismail, Mohd Hafiz Dzarfan Othman, and Mohammad Mahdi A. Shirazi. 2022. "Critical Flux and Fouling Analysis of PVDF-Mixed Matrix Membranes for Reclamation of Refinery-Produced Wastewater: Effect of Mixed Liquor Suspended Solids Concentration and Aeration" Membranes 12, no. 2: 161. https://doi.org/10.3390/membranes12020161
APA StyleYuliwati, E., Ismail, A. F., Othman, M. H. D., & Shirazi, M. M. A. (2022). Critical Flux and Fouling Analysis of PVDF-Mixed Matrix Membranes for Reclamation of Refinery-Produced Wastewater: Effect of Mixed Liquor Suspended Solids Concentration and Aeration. Membranes, 12(2), 161. https://doi.org/10.3390/membranes12020161






