Overview of Membrane Science and Technology in Portugal
Abstract
1. Introduction
2. Historical Perspective on Membrane Science and Technology
2.1. Instituto Superior Técnico—Universidade de Lisboa (IST)
2.2. NOVA School of Science and Technology—Universidade Nova de Lisboa (FCT NOVA)
2.3. Faculdade de Engenharia—Universidade do Porto (FEUP)
3. Activities on Membrane Science and Technology
3.1. Water
3.1.1. Drinking Water
3.1.2. Wastewater
Industrial Effluents
Sewage (Domestic Wastewater)
3.2. Membrane Applications in Food and Health
3.2.1. Membrane Processes in Biorefinery
3.2.2. Valorisation of Food Industry Wastewaters and Microalgae
3.2.3. Applications of Membranes in Health
3.3. Membranes for Energy Applications
3.3.1. Membranes for Fuel Cells
3.3.2. Membranes for Batteries
3.3.3. Reverse Electrodialysis for “Blue Energy” Generation
3.3.4. Membranes for Electrolytic Hydrogen Production
3.4. Membranes for Gas Separation and Pervaporation
3.4.1. Membranes for Gas Separation
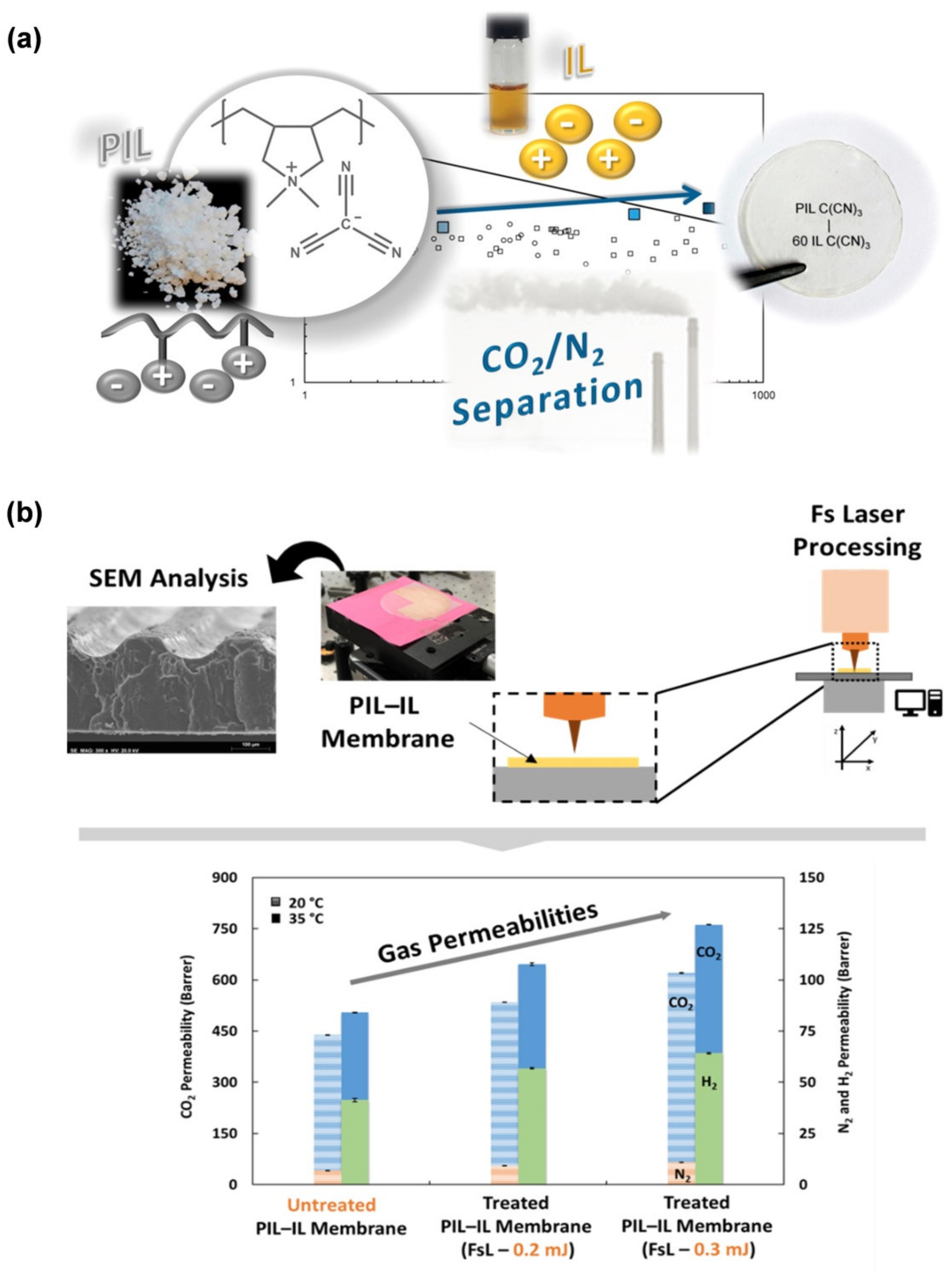
3.4.2. Membranes for Pervaporation
4. Future Perspectives
- -
- The development of membranes from natural, renewable resources, namely plant and bacterial cellulose and other natural (bio)polymers, which can be modified and functionalised for a diversity of applications;
- -
- The recovery of chemical elements that are becoming rare and are required for many applications associated with a sustainable lifestyle: (bio)refining of wastewaters aiming the recovery of essential elements such as phosphorus; sea mining, seeking the recovery of a large diversity of elements, including lithium and rare metals;
- -
- The use of renewable sources of energy, namely the integration of solar and membrane technology, but also the exploitation of salinity gradients for the harvesting of energy, supported on membrane-assisted processes.
- -
- Membranes with new functionalities, including research for membranes with controlled pore size (i.e., isoporous) and pore size distribution, with extremely high hydrophilicity or extremely high hydrophobicity namely for membrane contactors applications, highly resistant to organic solvents, namely using natural polymers as starting raw materials, and responsive to external stimuli (photo and magnetic stimuli are among approaches under study) aiming different applications, notably in the biomedical area;
- -
- Development and fabrication of membranes using less toxic, sustainable solvents, such as supercritical fluids, room temperature ionic liquids and deep eutectic solvents, among others;
- -
- Recycling/reusing membranes after use, namely by modifying their properties extending their service time.
- -
- Intelligent use of energy, avoiding processes that involve phase transition and applying energy where required: at the interface where mass and energy transfer takes place. For this purpose, membrane research aiming the design of membranes with a designed topography that promotes turbulence near their surface, assuring improved mass and heat transfer conditions, are currently under development;
- -
- Module design, as well as their components, using computational fluid dynamics is also a hot subject, aiming higher throughput by assuring better mass and heat transfer conditions, and minimising fouling with a minimum expenditure of energy. Spacer design and new (revisited) fouling control strategies are under consideration, such as the use of rotating and vibrating membranes.
- -
- Efficient monitoring of membrane processes, seeking on-line, real-time monitoring, making possible the establishment of advanced automation and better process control strategies. This approach may require the use of different sources of information/monitoring signals, some of them rather complex that, using data-driven modelling and machine learning approaches, may lead to advanced control with an impact on process efficiency;
- -
- Process integration in close interaction with corporate partners aiming the implementation and validation of membrane processes in all relevant domains with impact in social development: safe food and water production, valorisation and management of waste (regarded as by-products), environmental protection, energy production and storage, production of goods and health protection.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| AEM | anion-exchange membrane |
| ASI | Advanced Study Institute |
| BioPEM | biopolymer electrolyte membrane |
| BNC | bacterial nanocellulose |
| BPM | bipolar membrane |
| CA | cellulose acetate |
| CAn | carbonic anhydrase |
| CFD | computational fluid dynamics |
| CMS | carbon molecular sieve |
| CNC | cellulose nanocrystal |
| CNF | cellulose nanofibril |
| DBFC | direct borohydride fuel cell |
| DCMD | direct contact membrane distillation |
| DD | Donnan dialysis |
| DES | deep eutectic solvent |
| DF | dia-ultrafiltration |
| DLFC | direct liquid fuel cell |
| DMFC | direct methanol fuel cell |
| EEM | excitation-emission matrix |
| EIS | electrochemical impedance spectroscopy |
| EPS | exopolysaccharide |
| FCT NOVA | Nova School of Science and Technology |
| FEUP | Faculdade de Engenharia da Universidade do Porto |
| HER | hydrogen evolution reaction |
| IEMB | ion-exchange membrane bioreactor |
| IL | ionic liquid |
| IST | Instituto Superior Técnico |
| LIB | lithium-ion battery |
| LNEC | Laboratório Nacional de Engenharia Civil |
| MBO | membrane blood oxygenator |
| MEA | membrane-electrode assembly |
| MF | microfiltration |
| MFC | microbial fuel cell |
| MOF | metal-organic framework |
| NAqRFB | non-aqueous redox flow battery |
| NF | nanofiltration |
| NOM | natural organic matter |
| OER | oxygen evolution reaction |
| OMW | olive mill wastewater |
| OTC | oxytetracycline |
| PAA | poly(acrylic) acid |
| PAC | powdered activated carbon |
| PCA | principal components analysis |
| PCL | polycaprolactone |
| PDMS | polydimethylsiloxane |
| PEG | poly(ethylene glycol) |
| PEI | polyetherimide |
| PEM | proton exchange membrane |
| PES | polyethersulfone |
| PIL | poly (ionic liquid) |
| POMS/PEI | polyoctylmethylsiloxane/polyetherimide |
| PU | polyurethane |
| PVA | poly(vinyl alcohol) |
| PVDF-TrFE | poly(vinylidene fluoride-co-trifluoroethylene) |
| RED | reverse electrodialysis |
| RO | reverse osmosis |
| SILM | supported ionic liquid membrane |
| SGO | sulphonated graphene oxide |
| sPEEK | sulfonated poly (ether ether ketone) |
| SS | silica spheres |
| UF | ultrafiltration |
References
- Bungay, P.M.; Londsdale, H.K.; de Pinho, M.N. (Eds.) Synthetic Membranes: Science, Engineering and Applications; Springer: Dordrecht, The Netherlands, 1986. [Google Scholar] [CrossRef]
- Zhao, C.-T.; Norberta de Pinho, M. Design of polypropylene oxide/polybutadiene bi-soft segment urethane/urea polymer for pervaporation membranes. Polymer 1999, 40, 6089–6097. [Google Scholar] [CrossRef]
- Queiroz, D.P.; Pinto, I.M.; Besteiro, M.C.F.; Silva, A.F.M.; Gil, M.H.; Guiomar, A.J.; De Pinho, M.N. Surface and Hemocompatibility Studies of Bi-Soft Segment Polyurethane Membranes. Int. J. Artif. Organs 2006, 29, 866–872. [Google Scholar] [CrossRef] [PubMed]
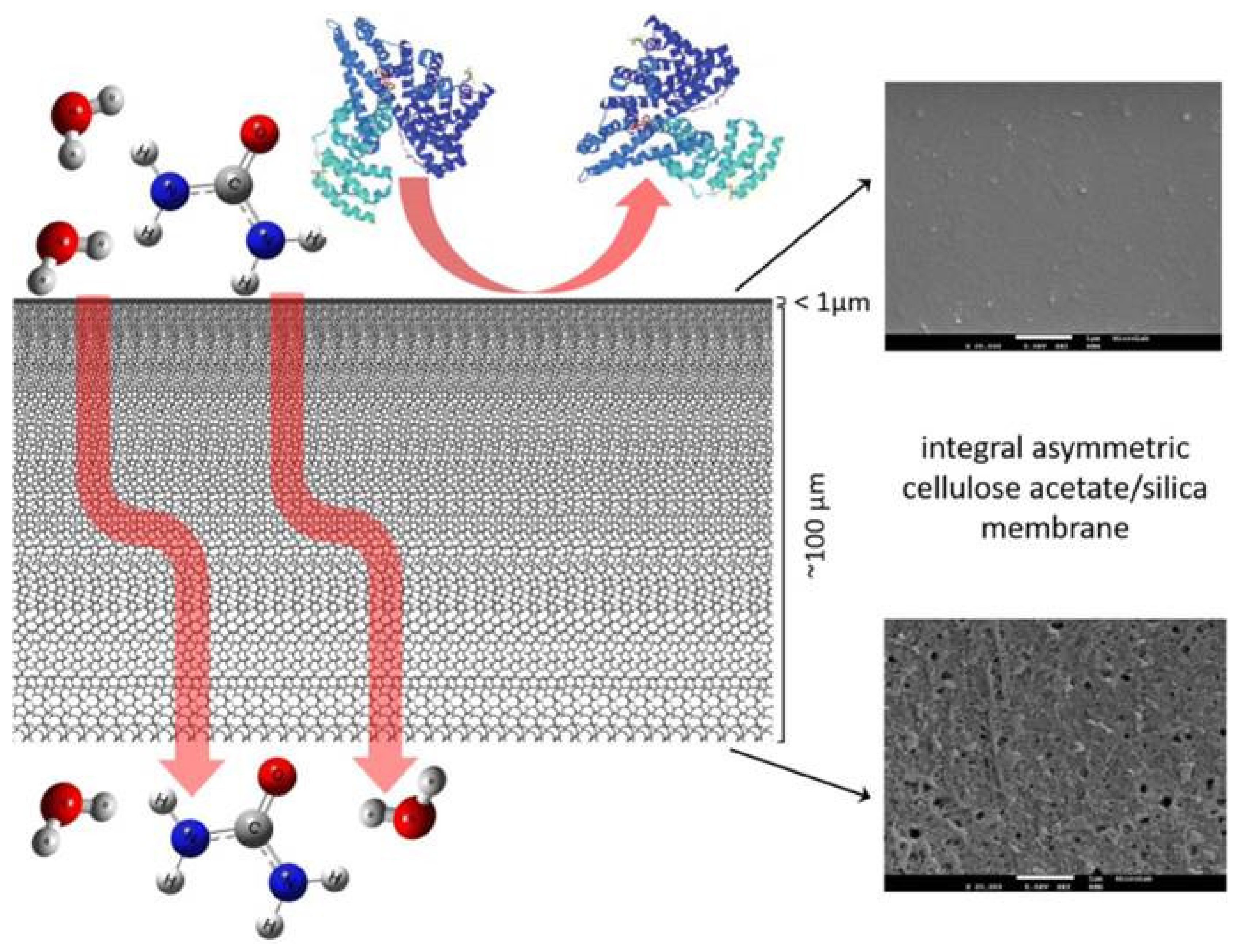
- Mendes, G.; Faria, M.; Carvalho, A.; Gonçalves, M.C.; de Pinho, M.N. Structure of water in hybrid cellulose acetate-silica ultrafiltration membranes and permeation properties. Carbohydr. Polym. 2018, 189, 342–351. [Google Scholar] [CrossRef]
- Beisl, S.; Monteiro, S.; Santos, R.; Figueiredo, A.S.; Sánchez-Loredo, M.G.; Lemos, M.A.; Lemos, F.; Minhalma, M.; de Pinho, M.N. Synthesis and bactericide activity of nanofiltration composite membranes—Cellulose acetate/silver nanoparticles and cellulose acetate/silver ion exchanged zeolites. Water Res. 2019, 149, 225–231. [Google Scholar] [CrossRef]
- De Pinho, M.N.; Geraldes, V.M.; Rosa, M.J.; Afonso, M.D.; Figueira, H.; Taborda, F.; Almeida, G.; Ganho, R.; Creusen, R.; Hanemaaijer, J.; et al. Water recovery from bleached pulp effluents. Tappi J. 1996, 79, 117–124. [Google Scholar]
- Geraldes, V.; de Pinho, M.N. Process water recovery from pulp bleaching effluents by an NF/ED hybrid process. J. Membr. Sci. 1995, 102, 209–221. [Google Scholar] [CrossRef]
- Minhalma, M.; de Pinho, M.N. Development of nanofiltration/steam stripping sequence for coke plant wastewater treatment. Desalination 2002, 149, 95–100. [Google Scholar] [CrossRef]
- Korzenowski, C.; Minhalma, M.; Bernardes, A.M.; Ferreira, J.Z.; de Pinho, M.N. Nanofiltration for the treatment of coke plant ammoniacal wastewaters. Sep. Purif. Technol. 2011, 76, 303–307. [Google Scholar] [CrossRef]
- Minhalma, M.; de Pinho, M.N. Flocculation/Flotation/Ultrafiltration Integrated Process for the Treatment of Cork Processing Wastewaters. Environ. Sci. Technol. 2001, 35, 4916–4921. [Google Scholar] [CrossRef] [PubMed]
- Rosa Santos, F.; Catarino, I.; Geraldes, V.; Pinho, M.N. Concentration and Rectification of Grape Must by Nanofiltration. Am. J. Enol. Vitic. 2008, 59, 446–450. [Google Scholar]
- Minhalma, M.; de Pinho, M.N. Tannic-membrane interactions on ultrafiltration of cork processing wastewaters. Sep. Purif. Technol. 2001, 22–23, 479–488. [Google Scholar] [CrossRef]
- Minhalma, M.; Domínguez, J.R.; de Pinho, M.N. Cork processing wastewaters treatment by an ozonization/ultrafiltration integrated process. Desalination 2006, 191, 148–152. [Google Scholar] [CrossRef]
- Geraldes, V.; Minhalma, M.; Pinho, M.N.; Anil, A.; Ozgunay, H.; Bitlisli, B.O.; Sari, O. Nanofiltration of Cork Wastewaters and Their Possible Use in Leather Industry as Tanning Agents. Pol. J. Environ. Stud. 2009, 18, 353–357. [Google Scholar]
- Streit, K.F.; Ferreira, J.Z.; Bernardes, A.M.; Norberta De Pinho, M. Ultrafiltration/Nanofiltration for the Tertiary Treatment of Leather Industry Effluents. Environ. Sci. Technol. 2009, 43, 9130–9135. [Google Scholar] [CrossRef]
- Catarino, J.; Mendonça, E.; Picado, A.; Lança, A.; Silva, L.; Pinho, M.D.P.N.D. Membrane-based treatment for tanning wastewatersA paper submitted to the Journal of Environmental Engineering and Science. Can. J. Civ. Eng. 2009, 36, 356–362. [Google Scholar] [CrossRef]
- Brites Alves, A.M.; Norberta de Pinho, M. Ultrafiltration for colour removal of tannery dyeing wastewaters. Desalination 2000, 130, 147–154. [Google Scholar] [CrossRef]
- Afonso, M.D.; De Pinho, M.N. Nanofiltration of Bleaching Pulp and Paper Effluents in Tubular Polymeric Membranes. Sep. Sci. Technol. 1997, 32, 2641–2658. [Google Scholar] [CrossRef]
- Rosa, M.J.; de Pinho, M.N. The role of ultrafiltration and nanofiltration on the minimisation of the environmental impact of bleached pulp effluents. J. Membr. Sci. 1995, 102, 155–161. [Google Scholar] [CrossRef]
- Afonso, M.D.; Geraldes, V.; Rosa, M.J.; de Pinho, M.N. Nanofiltration removal of chlorinated organic compounds from alkaline bleaching effluents in a pulp and paper plant. Water Res. 1992, 26, 1639–1643. [Google Scholar] [CrossRef]
- Afonso, M.D.; Pinho, M.N. Membrane separation processes in pulp and paper production. Filtr. Sep. 1991, 28, 45–48. [Google Scholar] [CrossRef]
- Afonso, M.D.; De Pinho, M.N. Ultrafiltration of bleach effluents in cellulose production. Desalination 1990, 79, 115–124. [Google Scholar] [CrossRef]
- Magueijo, V.; de Pinho, M.N.; Geraldes, V. Numerical and experimental study of mass transfer in lysozyme ultrafiltration. Desalination 2002, 145, 193–199. [Google Scholar] [CrossRef]
- De Pinho, M.N.; Rautenbach, R.; Herion, C. Mass transfer in radiation-grafted pervaporation membranes. J. Membr. Sci. 1990, 54, 131–143. [Google Scholar] [CrossRef]
- Neto, J.M.; Pinho, M.N. Mass transfer modelling for solvent dehydration by pervaporation. Sep. Purif. Technol. 2000, 18, 151–161. [Google Scholar] [CrossRef]
- Geraldes, V.; Semiao, V.; Norberta de Pinho, M. The effect on mass transfer of momentum and concentration boundary layers at the entrance region of a slit with a nanofiltration membrane wall. Chem. Eng. Sci. 2002, 57, 735–748. [Google Scholar] [CrossRef]
- Murphy, D.; de Pinho, M.N. An ATR-FTIR study of water in cellulose acetate membranes prepared by phase inversion. J. Membr. Sci. 1995, 106, 245–257. [Google Scholar] [CrossRef]
- Stamatialis, D.F.; Dias, C.R.; Norberta de Pinho, M. Atomic force microscopy of dense and asymmetric cellulose-based membranes. J. Membr. Sci. 1999, 160, 235–242. [Google Scholar] [CrossRef]
- Crespo, J.; Moura, M.; Carrondo, M. Some engineering parameters for propionic acid fermentation coupled with ultrafiltration. Appl. Biochem. Biotechnol. 1990, 24, 613–625. [Google Scholar] [CrossRef]
- Barreiros, A.; Rodrigues, C.; Crespo, J.P.; Reis, M. Membrane bioreactor for drinking water denitrification. Bioprocess. Eng. 1998, 18, 297–302. [Google Scholar] [CrossRef]
- Velizarov, S.; Rodrigues, C.M.; Reis, M.A.; Crespo, J.G. Mechanism of charged pollutants removal in an ion exchange membrane bioreactor: Drinking water denitrification. Biotechnol. Bioeng. 2000, 71, 245–254. [Google Scholar] [CrossRef]
- Velizarov, S.; Crespo, J.G.; Reis, M.A. Ion exchange membrane bioreactor for selective removal of nitrate from drinking water: Control of ion fluxes and process performance. Biotechnol. Progr. 2002, 18, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Velizarov, S.; Reis, M.A.; Crespo, J.G. Removal of trace mono-valent inorganic pollutants in an ion exchange membrane bioreactor: Analysis of transport rate in a denitrification process. J. Membr. Sci. 2003, 217, 269–284. [Google Scholar] [CrossRef]
- Sousa, H.A.; Crespo, J.G.; Afonso, C.A. Asymmetric hydrolysis of a meso-diester using pig liver esterase immobilised in hollow fibre ultrafiltration membrane. Tetrahedro Asymmetry 2000, 11, 929–934. [Google Scholar] [CrossRef]
- Sousa, H.A.; Rodrigues, C.; Klein, E.; Afonso, C.A.M.; Crespo, J.G. Immobilisation of pig liver esterase in hollow fibre membranes. Enzym. Microb. Technol. 2001, 29, 625–634. [Google Scholar] [CrossRef]
- Schäffer, T.; Crespo, J.G. Recovery of aroma compounds from fermentation by pervaporation. Environ. Prot. Eng. 1999, 25, 73–85. [Google Scholar]
- Schäfer, T.; Bengtson, G.; Pingel, H.; Böddeker, K.; Crespo, J. Recovery of aroma compounds from a wine-must fermentation by organophilic pervaporation. Biotechnol. Bioeng. 1999, 62, 412–421. [Google Scholar] [CrossRef]
- Crespo, J.; Trotin, M.; Hough, D.; Howell, J. Use of fluorescence labelling to monitor protein fractionation by ultrafiltration under controlled permeate flux. J. Membr. Sci. 1999, 155, 209–230. [Google Scholar] [CrossRef]
- Coelhoso, I.; Moura, T.; Crespo, J.; Carrondo, M. Transport mechanisms in liquid membranes with ion exchange carriers. J. Membr. Sci. 1995, 108, 231–244. [Google Scholar] [CrossRef]
- Coelhoso, I.; Crespo, J.; Carrondo, M. Modeling of ion-pairing extraction with quaternary amines. Sep. Sci. Technol. 1996, 31, 491–511. [Google Scholar] [CrossRef]
- Coelhoso, I.; Silvestre, P.; Viegas, R.; Crespo, J.; Carrondo, M. Membrane-based solvent extraction and stripping of lactate in hollow-fibre contactors. J. Membr. Sci. 1997, 1, 19–32. [Google Scholar] [CrossRef]
- Fortunato, R.; Afonso, C.A.; Reis, M.; Crespo, J.G. Supported liquid membranes using ionic liquids: Study of stability and transport mechanisms. J. Membr. Sci. 2004, 242, 197–209. [Google Scholar] [CrossRef]
- Fortunato, R.; González-Muñoz, M.J.; Kubasiewicz, M.; Luque, S.; Alvarez, J.; Afonso, C.A.; Coelhoso, I.M.; Crespo, J.G. Liquid membranes using ionic liquids: The influence of water on solute transport. J. Membr. Sci. 2005, 249, 153–162. [Google Scholar] [CrossRef]
- Neves, L.A.; Crespo, J.G.; Coelhoso, I.M. Gas permeation studies in supported ionic liquid membranes. J. Membr. Sci. 2010, 357, 160–170. [Google Scholar] [CrossRef]
- Neves, L.A.; Afonso, C.; Coelhoso, I.M.; Crespo, J.G. Integrated CO2 capture and enzymatic bioconversion in supported ionic liquid membranes. Sep. Purif. Technol. 2012, 97, 34–41. [Google Scholar] [CrossRef]
- Polino, M.; Portugal, C.A.M.; Le The, H.; Tiggelaar, R.; Eijkel, J.; Crespo, J.G.; Coelhoso, I.M.; Pina, M.P.; Mallada, R. Enhanced Protein Crystallization on Nafion Membranes Modified by Low-Cost Surface Patterning Techniques. Cryst. Growth Des. 2020, 20, 2174–2186. [Google Scholar] [CrossRef]
- Syed, U.T.; Leonardo, I.; Lahoz, R.; Gaspar, F.B.; Huertas, R.; Crespo, M.T.B.; Arruebo, M.; Crespo, J.G.; Sebastian, V.; Brazinha, C. Microengineered Membranes for Sustainable Production of Hydrophobic Deep Eutectic Solvent-Based Nanoemulsions by Membrane Emulsification for Enhanced Antimicrobial Activity. ACS Sustain. Chem. Eng. 2020, 8, 16526–16536. [Google Scholar] [CrossRef]
- Schäfer, T.; Vital, J.; Crespo, J.G. Coupled pervaporation/mass spectrometry for investigating membrane mass transport phenomena. J. Membr. Sci. 2004, 241, 197–205. [Google Scholar] [CrossRef]
- Brazinha, C.; Fonseca, A.P.; Teodoro, O.M.N.D.; Crespo, J.G. On-line and real-time monitoring of organophilic pervaporation by mass spectrometry. J. Membr. Sci. 2010, 347, 83–92. [Google Scholar] [CrossRef]
- Fraga, S.C.; Trabucho, L.; Brazinha, C.; Crespo, J.G. Characterisation and modelling of transient transport through dense membranes using on-line mass spectrometry. J. Membr. Sci. 2015, 479, 213–222. [Google Scholar] [CrossRef]
- Wolf, G.; Crespo, J.G.; Reis, M.A. Optical and spectroscopic methods for biofilm examination and monitoring. Rev. Environ. Sci. Biotechnol. 2002, 1, 227–251. [Google Scholar] [CrossRef]
- Wolf, G.; Almeida, J.S.; Pinheiro, C.; Correia, V.; Rodrigues, C.; Reis, M.A.; Crespo, J.G. Two-dimensional fluorometry coupled with artificial neural networks: A novel method for on-line monitoring of complex biological processes. Biotechnol. Bioeng. 2001, 72, 297–306. [Google Scholar] [CrossRef]
- Portugal, C.A.; Lima, J.; Crespo, J.G. Probing the change of enzymatic activity of horseradish peroxidase induced by membrane permeation using tryptophan fluorescence. J. Membr. Sci. 2006, 284, 180–192. [Google Scholar] [CrossRef]
- Portugal, C.A.M.; Lima, J.C.; Crespo, J.G. Fluorescence probing of structural and functional changes of proteins induced by ultrafiltration. Desalination 2006, 199, 547–549. [Google Scholar] [CrossRef]
- Galinha, C.F.; Carvalho, G.; Portugal, C.A.; Guglielmi, G.; Reis, M.A.; Crespo, J.G. Two-dimensional fluorescence as a fingerprinting tool for monitoring wastewater treatment systems. J. Chem. Technol. Biotechnol. 2011, 86, 985–992. [Google Scholar] [CrossRef]
- Galinha, C.; Carvalho, G.; Portugal, C.; Guglielmi, G.; Oliveira, R.; Crespo, J.; Reis, M. Real-time monitoring of membrane bioreactors with 2D-fluorescence data and statistically based models. Water Sci. Technol. 2011, 63, 1381–1388. [Google Scholar] [CrossRef]
- Galinha, C.F.; Carvalho, G.; Portugal, C.A.M.; Guglielmi, G.; Reis, M.A.M.; Crespo, J.G. Multivariate statistically-based modelling of a membrane bioreactor for wastewater treatment using 2D fluorescence monitoring data. Water Res. 2012, 46, 3623–3636. [Google Scholar] [CrossRef]
- Izák, P.; Godinho, M.; Brogueira, P.; Figueirinhas, J.; Crespo, J. 3D topography design of membranes for enhanced mass transport. J. Membr. Sci. 2008, 321, 337–343. [Google Scholar] [CrossRef]
- Pawlowski, S.; Geraldes, V.; Crespo, J.G.; Velizarov, S. Computational fluid dynamics (CFD) assisted analysis of profiled membranes performance in reverse electrodialysis. J. Membr. Sci. 2016, 502, 179–190. [Google Scholar] [CrossRef]
- Pereira, V.J.; Galinha, J.; Barreto Crespo, M.T.; Matos, C.T.; Crespo, J.G. Integration of nanofiltration, UV photolysis, and advanced oxidation processes for the removal of hormones from surface water sources. Sep. Purif. Technol. 2012, 95, 89–96. [Google Scholar] [CrossRef]
- Sanches, S.; Penetra, A.; Rodrigues, A.; Cardoso, V.; Ferreira, E.; Benoliel, M.; Crespo, M.B.; Crespo, J.; Pereira, V. Removal of pesticides from water combining low pressure UV photolysis with nanofiltration. Sep. Purif. Technol. 2013, 115, 73–82. [Google Scholar] [CrossRef]
- Lagorsse, S.; Magalhães, F.D.; Mendes, A. Carbon molecular sieve membranes: Sorption, kinetic and structural characterization. J. Membr. Sci. 2004, 241, 275–287. [Google Scholar] [CrossRef]
- Lagorsse, S.; Magalhães, F.D.; Mendes, A. Aging study of carbon molecular sieve membranes. J. Membr. Sci. 2008, 310, 494–502. [Google Scholar] [CrossRef]
- Campo, M.C.; Magalhães, F.D.; Mendes, A. Carbon molecular sieve membranes from cellophane paper. J. Membr. Sci. 2010, 350, 180–188. [Google Scholar] [CrossRef]
- Teixeira, M.; Campo, M.C.; Tanaka, D.A.P.; Tanco, M.A.L.; Magen, C.; Mendes, A. Composite phenolic resin-based carbon molecular sieve membranes for gas separation. Carbon 2011, 49, 4348–4358. [Google Scholar] [CrossRef]
- Teixeira, M.; Campo, M.; Tanaka, D.A.; Tanco, M.A.; Magen, C.; Mendes, A. Carbon–Al2O3–Ag composite molecular sieve membranes for gas separation. Chem. Eng. Res. Des. 2012, 90, 2338–2345. [Google Scholar] [CrossRef]
- Araújo, T.; Andrade, M.; Bernardo, G.; Mendes, A. Stable cellulose-based carbon molecular sieve membranes with very high selectivities. J. Membr. Sci. 2022, 641, 119852. [Google Scholar] [CrossRef]
- Pereira, A.I.; Pérez, P.; Rodrigues, S.C.; Mendes, A.; Madeira, L.M.; Tavares, C.J. Deposition of Pd–Ag thin film membranes on ceramic supports for hydrogen purification/separation. Mater. Res. Bull. 2015, 61, 528–533. [Google Scholar] [CrossRef]
- Brandão, L.; Madeira, L.M.; Mendes, A.M. Propyne hydrogenation in a continuous polymeric catalytic membrane reactor. Chem. Eng. Sci. 2007, 62, 6768–6776. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Abdollahzadeh, M.; Boaventura, M.; Mendes, A. H2 production with low carbon content via MSR in packed bed membrane reactors for high-temperature polymeric electrolyte membrane fuel cell. Appl. Energy 2017, 188, 409–419. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Sousa, J.M.; Mendes, A. Hydrogen production by methanol steam reforming in a membrane reactor: Palladium vs. carbon molecular sieve membranes. J. Membr. Sci. 2009, 339, 160–170. [Google Scholar] [CrossRef]
- Sá, S.; Sousa, J.M.; Mendes, A. Methanol steam reforming in a dual-bed membrane reactor for producing PEMFC grade hydrogen. Catal. Today 2010, 156, 254–260. [Google Scholar] [CrossRef]
- Silva, V.S.; Ruffmann, B.; Vetter, S.; Mendes, A.; Madeira, L.M.; Nunes, S.P. Characterization and application of composite membranes in DMFC. Catal. Today 2005, 104, 205–212. [Google Scholar] [CrossRef]
- Silva, V.; Schirmer, J.; Reissner, R.; Ruffmann, B.; Silva, H.; Mendes, A.; Madeira, L.; Nunes, S. Proton electrolyte membrane properties and direct methanol fuel cell performance: II. Fuel cell performance and membrane properties effects. J. Power Sources 2005, 140, 41–49. [Google Scholar] [CrossRef]
- Boaventura, M.; Ponce, M.; Brandao, L.; Mendes, A.; Nunes, S. Proton conductive membranes based on doped sulfonated polytriazole. Int. J. Hydrogen Energy 2010, 35, 12054–12064. [Google Scholar] [CrossRef]
- Campo, M.C.; Magalhães, F.D.; Mendes, A. Separation of nitrogen from air by carbon molecular sieve membranes. J. Membr. Sci. 2010, 350, 139–147. [Google Scholar] [CrossRef]
- Cruz, P.; Santos, J.C.; Magalhães, F.D.; Mendes, A. Simulation of separation processes using finite volume method. Comput. Chem. Eng. 2005, 30, 83–98. [Google Scholar] [CrossRef]
- Sousa, J.M.; Cruz, P.; Magalhães, F.D.; Mendes, A. Modeling catalytic membrane reactors using an adaptive wavelet-based collocation method. J. Membr. Sci. 2002, 208, 57–68. [Google Scholar] [CrossRef]
- Mendes, D.; Sá, S.; Tosti, S.; Sousa, J.M.; Madeira, L.M.; Mendes, A. Experimental and modeling studies on the low-temperature water-gas shift reaction in a dense Pd–Ag packed-bed membrane reactor. Chem. Eng. Sci. 2011, 66, 2356–2367. [Google Scholar] [CrossRef]
- Sousa, J.M.; Cruz, P.; Mendes, A. Modeling a catalytic polymeric non-porous membrane reactor. J. Membr. Sci. 2001, 181, 241–252. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Abdollahzadeh, M.; Pereira, A.; Relvas, F.; Boaventura, M.; Mendes, A. High temperature PEM fuel cell integrated with a cellular membrane methanol steam reformer: Experimental and modelling. Appl. Energy 2018, 215, 659–669. [Google Scholar] [CrossRef]
- Ribeirinha, P.; Abdollahzadeh, M.; Sousa, J.; Boaventura, M.; Mendes, A. Modelling of a high-temperature polymer electrolyte membrane fuel cell integrated with a methanol steam reformer cell. Appl. Energy 2017, 202, 6–19. [Google Scholar] [CrossRef]
- Delgado, N.M.; Monteiro, R.; Abdollahzadeh, M.; Ribeirinha, P.; Bentien, A.; Mendes, A. 2D-dynamic phenomenological modelling of vanadium redox flow batteries—Analysis of the mass transport related overpotentials. J. Power Sources 2020, 480, 229142. [Google Scholar] [CrossRef]
- Delgado, N.M.; Monteiro, R.; Mendes, A. The first approach to dynamic modeling of a solar vanadium redox flow cell. Nano Energy 2021, 89, 106372. [Google Scholar] [CrossRef]
- Janknecht, P.; Lopes, A.D.; Mendes, A.M. Removal of Industrial Cutting Oil from Oil Emulsions by Polymeric Ultra- and Microfiltration Membranes. Environ. Sci. Technol. 2004, 38, 4878–4883. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.; Mendes, A.; Madeira, L.M.; Ferreira, A. Alcohol Removal From Beer by Reverse Osmosis. Sep. Sci. Technol. 2007, 42, 3011–3027. [Google Scholar] [CrossRef]
- Catarino, M.; Mendes, A. Dealcoholizing wine by membrane separation processes. Innov. Food Sci. Emerg. Technol. 2011, 12, 330–337. [Google Scholar] [CrossRef]
- Catarino, M.; Mendes, A. Non-alcoholic beer—A new industrial process. Sep. Purif. Technol. 2011, 79, 342–351. [Google Scholar] [CrossRef]
- Gales, L.; Mendes, A.; Costa, C. Removal of acetone, ethyl acetate and ethanol vapors from air using a hollow fiber PDMS membrane module. J. Membr. Sci. 2002, 197, 211–222. [Google Scholar] [CrossRef]
- Brandão, L.; Madeira, L.M.; Mendes, A.M. Mass transport on composite dense PDMS membranes with palladium nanoclusters. J. Membr. Sci. 2007, 288, 112–122. [Google Scholar] [CrossRef]
- Pantaleão, I.; Portugal, A.F.; Mendes, A.; Gabriel, J. Carbon Dioxide Absorption in a Membrane Contactor with Color Change. J. Chem. Educ. 2010, 87, 1377–1379. [Google Scholar] [CrossRef]
- Portugal, A.F.; Magalhães, F.D.; Mendes, A. Carbon dioxide removal from anaesthetic gas circuits using hollow fiber membrane contactors with amino acid salt solutions. J. Membr. Sci. 2009, 339, 275–286. [Google Scholar] [CrossRef]
- Cabral, J.; Pantateão, I.; Rodrigues, S.; Catarino, M.; Magalhães, F.; Mendes, A. Kinetics of the Carbon Dioxide Absorption and Desorption with Amino Acid Salt Solutions using Hollow Fiber Membrane Contactors. Procedia Eng. 2012, 44, 1223–1224. [Google Scholar] [CrossRef][Green Version]
- Silva, V.S.; Mendes, A.; Madeira, L.M.; Nunes, S.P. Proton exchange membranes for direct methanol fuel cells: Properties critical study concerning methanol crossover and proton conductivity. J. Membr. Sci. 2006, 276, 126–134. [Google Scholar] [CrossRef]
- Silva, V.S.; Ruffmann, B.; Silva, H.; Silva, V.B.; Mendes, A.; Madeira, L.M.; Nunes, S. Zirconium oxide hybrid membranes for direct methanol fuel cells—Evaluation of transport properties. J. Membr. Sci. 2006, 284, 137–144. [Google Scholar] [CrossRef]
- Brandão, L.; Rodrigues, J.; Madeira, L.M.; Mendes, A. Methanol crossover reduction by Nafion modification with palladium composite nanoparticles: Application to direct methanol fuel cells. Int. J. Hydrogen Energy 2010, 35, 11561–11567. [Google Scholar] [CrossRef]
- Boaventura, M.; Mendes, A. Activation procedures characterization of MEA based on phosphoric acid doped PBI membranes. Int. J. Hydrogen Energy 2010, 35, 11649–11660. [Google Scholar] [CrossRef]
- Boaventura, M.; Sander, H.; Friedrich, K.A.; Mendes, A. The influence of CO on the current density distribution of high temperature polymer electrolyte membrane fuel cells. Electrochim. Acta 2011, 56, 9467–9475. [Google Scholar] [CrossRef]
- Boaventura, M.; Alves, I.; Ribeirinha, P.; Mendes, A. The influence of impurities in high temperature polymer electrolyte membrane fuel cells performance. Int. J. Hydrogen Energy 2016, 41, 19771–19780. [Google Scholar] [CrossRef]
- Ponce, M.; Boaventura, M.; Gomes, D.; Mendes, A.; Madeira, L.; Nunes, S. Proton conducting membranes based on benzimidazole sulfonic acid doped sulfonated poly (oxadiazole–triazole) copolymer for low humidity operation. Fuel Cells 2008, 8, 209–216. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Ribeiorinha, P.; Li, H.; Kong, X.; Boaventura, M. A proton conductor electrolyte based on molten CsH5(PO4)2 for intermediate-temperature fuel cells. RSC Adv. 2018, 8, 5225–5232. [Google Scholar] [CrossRef]
- Delgado, S.; Lagarteira, T.; Mendes, A. Air bleeding strategies to increase the efficiency of proton exchange membrane fuel cell stationary applications fuelled with CO ppm-levels. Int. J. Electrochem. Sci. 2020, 15, 613–627. [Google Scholar] [CrossRef]
- Pais, J.A.G.C.R.; Ferreira, L.M.G.A. Performance study of an industrial RO plant for seawater desalination. Desalination 2007, 208, 269–276. [Google Scholar] [CrossRef]
- Geraldes, V.; Pereira, N.E.; Norberta de Pinho, M. Simulation and Optimization of Medium-Sized Seawater Reverse Osmosis Processes with Spiral-Wound Modules. Ind. Eng. Chem. Res. 2005, 44, 1897–1905. [Google Scholar] [CrossRef]
- Afonso, M.D.; Jaber, J.O.; Mohsen, M.S. Brackish groundwater treatment by reverse osmosis in Jordan. Desalination 2004, 164, 157–171. [Google Scholar] [CrossRef]
- Sanches, S.; Penetra, A.; Rodrigues, A.; Ferreira, E.; Cardoso, V.V.; Benoliel, M.J.; Barreto Crespo, M.T.; Pereira, V.J.; Crespo, J.G. Nanofiltration of hormones and pesticides in different real drinking water sources. Sep. Purif. Technol. 2012, 94, 44–53. [Google Scholar] [CrossRef]
- Velizarov, S.; Crespo, J.G.; Reis, M.A. Removal of inorganic anions from drinking water supplies by membrane bio/processes. Rev. Environ. Sci. Bio/Technol. 2004, 3, 361–380. [Google Scholar] [CrossRef]
- Matos, C.T.; Sequeira, A.M.; Velizarov, S.; Crespo, J.G.; Reis, M.A.M. Nitrate removal in a closed marine system through the ion exchange membrane bioreactor. J. Hazard. Mater. 2009, 166, 428–434. [Google Scholar] [CrossRef]
- Gando-Ferreira, L.M.; Gaspar, C.S.S.; Monteiro, M.; Moreira, M.J.A. Studies on integration of ion exchange and nanofiltration for water desalination. Sep. Sci. Technol. 2017, 52, 2600–2610. [Google Scholar] [CrossRef]
- Costa, A.R.; de Pinho, M.N. Comparison of the performance of ultrafiltration and nanofiltration in surface water treatment. Desalination 2006, 199, 73–75. [Google Scholar] [CrossRef]
- Costa, A.R.; de Pinho, M.N. Performance and cost estimation of nanofiltration for surface water treatment in drinking water production. Desalination 2006, 196, 55–65. [Google Scholar] [CrossRef]
- Costa, A.R.; de Pinho, M.N. Coagulation/flocculation/ultrafiltration for natural organic matter removal in drinking water production. Water Supply 2004, 4, 215–222. [Google Scholar] [CrossRef]
- Campinas, M.; Viegas, R.M.C.; Coelho, R.; Lucas, H.; Rosa, M.J. Adsorption/Coagulation/Ceramic Microfiltration for Treating Challenging Waters for Drinking Water Production. Membranes 2021, 11, 91. [Google Scholar] [CrossRef]
- Velizarov, S.; Reis, M.A.; Crespo, J.G. The Ion Exchange Membrane Bioreactor Developments and Perspectoves in Drinking Water Treatment Dordrecht. In Water Purification and Management; Springer: Dordrecht, The Netherlands, 2011; pp. 119–145. [Google Scholar]
- Pessoa Lopes, M.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. Optimisation of arsenate removal from water by an integrated ion-exchange membrane process coupled with Fe co-precipitation. Sep. Purif. Technol. 2020, 246, 116894. [Google Scholar] [CrossRef]
- Seabra Pinto, A.; Cabral Rolo, J. Chapter 22. Trade, logistics and agro-food strategies in Portugal. In MediTERRA 2014 (English); Presses de Sciences Po: Paris, France, 2014; pp. 377–386. [Google Scholar]
- Costa, J.M.; Oliveira, M.; Egipto, R.J.; Cid, J.F.; Fragoso, R.A.; Lopes, C.M.; Duarte, E.N. Water and wastewater management for sustainable viticulture and oenology in South Portugal—A review. Ciênc. Téc. Vitiv. 2020, 35, 1–15. [Google Scholar] [CrossRef]
- Velizarov, S.; Crespo, J.G. Membrane Processing for the Recovery of Bioactive Compounds in Agro-Industries. In Innovation in Food Engineering: New Techniques and Products, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 137–160. [Google Scholar] [CrossRef]
- Fraga, M.C.; Sanches, S.; Crespo, J.G.; Pereira, V.J. Assessment of a New Silicon Carbide Tubular Honeycomb Membrane for Treatment of Olive Mill Wastewaters. Membranes 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.C.; Huertas, R.M.; Crespo, J.G.; Pereira, V.J. Novel Submerged Photocatalytic Membrane Reactor for Treatment of Olive Mill Wastewaters. Catalysts 2019, 9, 769. [Google Scholar] [CrossRef]
- Kamali, M.; Suhas, D.P.; Costa, M.E.; Capela, I.; Aminabhavi, T.M. Sustainability considerations in membrane-based technologies for industrial effluents treatment. Chem. Eng. J. 2019, 368, 474–494. [Google Scholar] [CrossRef]
- Salazar, H.; Martins, P.M.; Santos, B.; Fernandes, M.M.; Reizabal, A.; Sebastián, V.; Botelho, G.; Tavares, C.J.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Photocatalytic and antimicrobial multifunctional nanocomposite membranes for emerging pollutants water treatment applications. Chemosphere 2020, 250, 126299. [Google Scholar] [CrossRef]
- De Almeida, M.S.; Martins, R.C.; Quinta-Ferreira, R.M.; Gando-Ferreira, L.M. Optimization of operating conditions for the valorization of olive mill wastewater using membrane processes. Environ. Sci. Pollut. Res. 2018, 25, 21968–21981. [Google Scholar] [CrossRef]
- Ribeiro, B.; Torrado, I.; Di Berardino, S.; Paixão, S.M.; Rusan, M.J.; Bani Amer, A.; Zuraiqi, S.; Eusébio, A. Jet-loop reactor with cross-flow ultrafiltration membrane system for treatment of olive mill wastewater. Water Pract. Technol. 2018, 13, 247–256. [Google Scholar] [CrossRef]
- Antónia Nunes, M.; Pawlowski, S.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P.; Velizarov, S. Valorization of olive pomace by a green integrated approach applying sustainable extraction and membrane-assisted concentration. Sci. Total Environ. 2019, 652, 40–47. [Google Scholar] [CrossRef]
- Gomes, L.; Borges, C.; Serralheiro, M.L.M.; Minhalma, M.; Pacheco, R. Cork processing wastewaters components fractioned by ultrafiltration membranes—Studies of antioxidant and antitumoral activity. J. Chem. Technol. Biotechnol. 2018, 93, 861–870. [Google Scholar] [CrossRef]
- Giacobbo, A.; Moura Bernardes, A.; Filipe Rosa, M.J.; De Pinho, M.N. Concentration Polarization in Ultrafiltration/Nanofiltration for the Recovery of Polyphenols from Winery Wastewaters. Membranes 2018, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Leandro, M.J.; Marques, S.; Ribeiro, B.; Santos, H.; Fonseca, C. Integrated Process for Bioenergy Production and Water Recycling in the Dairy Industry: Selection of Kluyveromyces Strains for Direct Conversion of Concentrated Lactose-Rich Streams into Bioethanol. Microorganisms 2019, 7, 545. [Google Scholar] [CrossRef]
- Bastos, P.D.A.; Santos, M.A.; Carvalho, P.J.; Velizarov, S.; Crespo, J.G. Pilot scale reverse osmosis refinery wastewater treatment—A techno-economical and sustainability assessment. Environ. Sci. Water Res. Technol. 2021, 7, 549–561. [Google Scholar] [CrossRef]
- Zakmout, A.; Sadi, F.; Portugal, C.A.M.; Crespo, J.G.; Velizarov, S. Tannery Effluent Treatment by Nanofiltration, Reverse Osmosis and Chitosan Modified Membranes. Membranes 2020, 10, 378. [Google Scholar] [CrossRef] [PubMed]
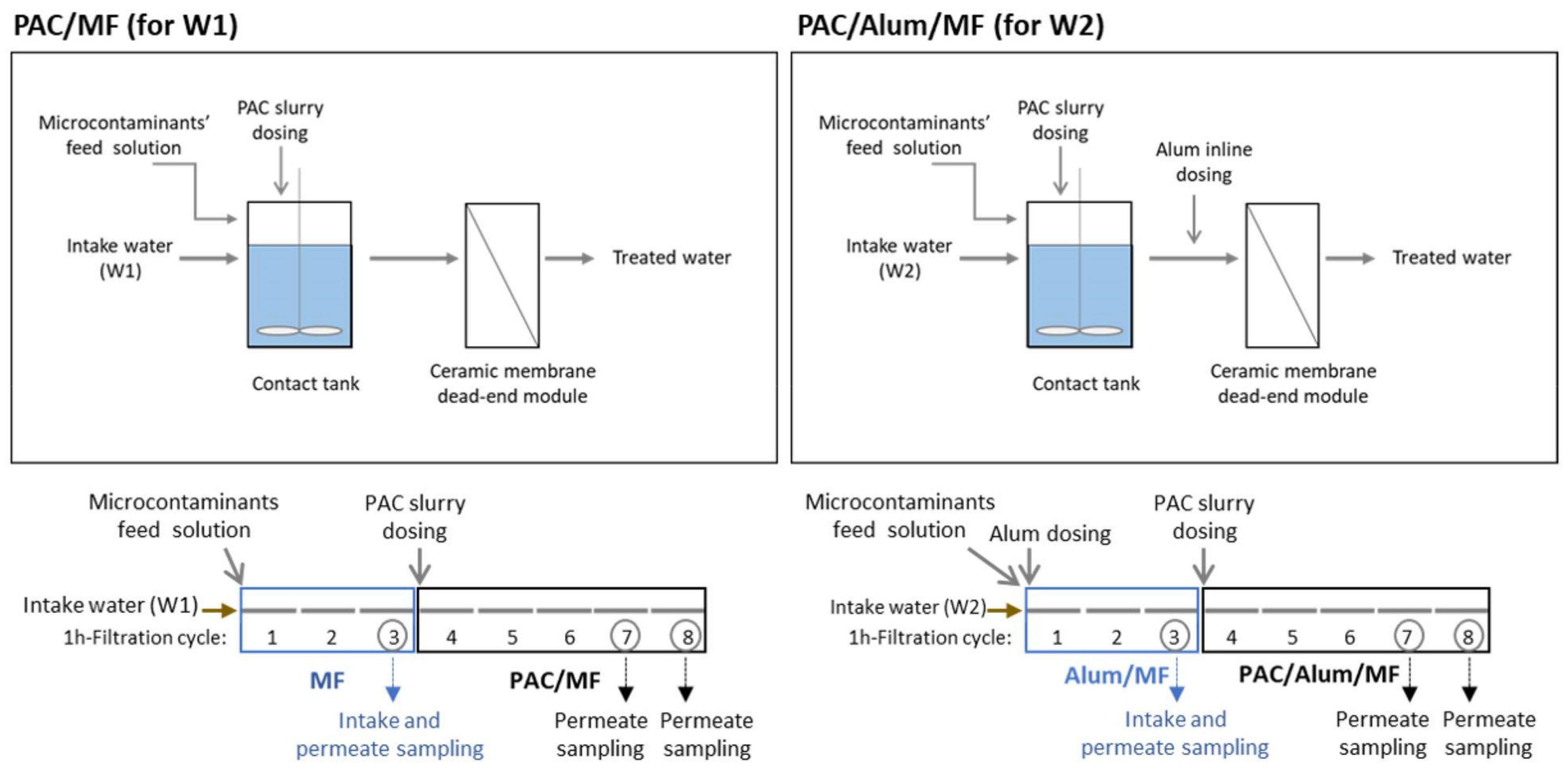
- Viegas, R.M.C.; Mesquita, E.; Campinas, M.; Rosa, M.J. Pilot Studies and Cost Analysis of Hybrid Powdered Activated Carbon/Ceramic Microfiltration for Controlling Pharmaceutical Compounds and Organic Matter in Water Reclamation. Water 2020, 12, 33. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Cristóvão, R.O.; Mendes, A.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic membrane reactor performance towards oxytetracycline removal from synthetic and real matrices: Suspended vs. immobilized TiO2-P25. Chem. Eng. J. 2019, 378, 122114. [Google Scholar] [CrossRef]
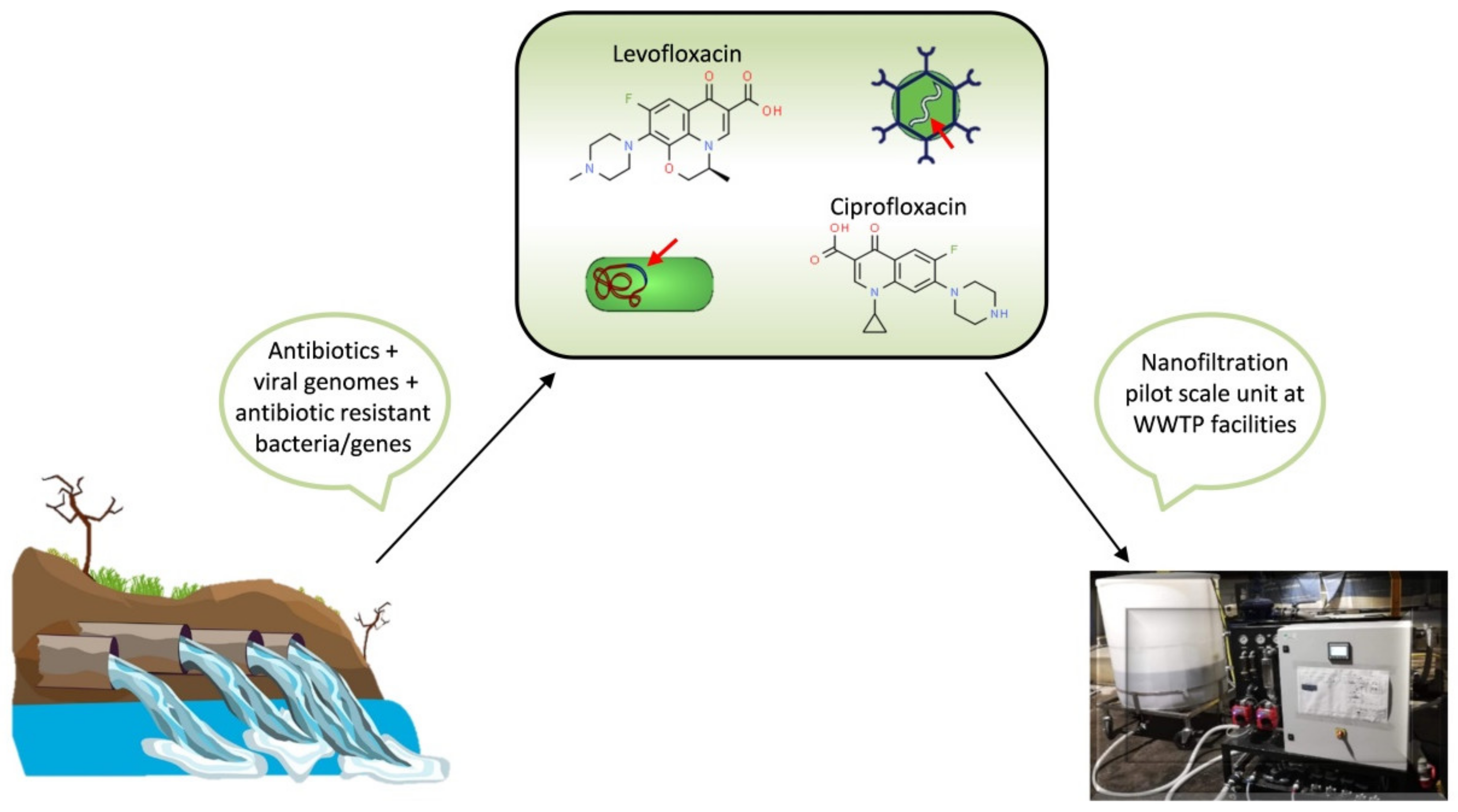
- Cristóvão, M.B.; Tela, S.; Silva, A.F.; Oliveira, M.; Bento-Silva, A.; Bronze, M.R.; Crespo, M.T.B.; Crespo, J.G.; Nunes, M.; Pereira, V.J. Occurrence of Antibiotics, Antibiotic Resistance Genes and Viral Genomes in Wastewater Effluents and Their Treatment by a Pilot Scale Nanofiltration Unit. Membranes 2021, 11, 9. [Google Scholar] [CrossRef]
- Cristóvão, M.B.; Torrejais, J.; Janssens, R.; Luis, P.; Van der Bruggen, B.; Dubey, K.K.; Mandal, M.K.; Bronze, M.R.; Crespo, J.G.; Pereira, V.J. Treatment of anticancer drugs in hospital and wastewater effluents using nanofiltration. Sep. Purif. Technol. 2019, 224, 273–280. [Google Scholar] [CrossRef]
- Vinoth Kumar, R.; Barbosa, M.O.; Ribeiro, A.R.; Morales-Torres, S.; Pereira, M.F.R.; Silva, A.M.T. Advanced oxidation technologies combined with direct contact membrane distillation for treatment of secondary municipal wastewater. Process. Saf. Environ. Prot. 2020, 140, 111–123. [Google Scholar] [CrossRef]
- Arboleda Mejia, J.A.; Ricci, A.; Figueiredo, A.S.; Versari, A.; Cassano, A.; Parpinello, G.P.; De Pinho, M.N. Recovery of Phenolic Compounds from Red Grape Pomace Extract through Nanofiltration Membranes. Foods 2020, 9, 1649. [Google Scholar] [CrossRef]
- Syed, U.T.; Brazinha, C.; Crespo, J.G.; Ricardo-da-Silva, J.M. Valorisation of grape pomace: Fractionation of bioactive flavan-3-ols by membrane processing. Sep. Purif. Technol. 2017, 172, 404–414. [Google Scholar] [CrossRef]
- Valério, R.; Crespo, J.G.; Galinha, C.F.; Brazinha, C. Effect of Ultrafiltration Operating Conditions for Separation of Ferulic Acid from Arabinoxylans in Corn Fibre Alkaline Extract. Sustainability 2021, 13, 4682. [Google Scholar] [CrossRef]
- Pinto, P.R.; Mota, I.F.; Pereira, C.M.; Ribeiro, A.M.; Loureiro, J.M.; Rodrigues, A.E. Separation and recovery of polyphenols and carbohydrates from Eucalyptus bark extract by ultrafiltration/diafiltration and adsorption processes. Sep. Purif. Technol. 2017, 183, 96–105. [Google Scholar] [CrossRef]
- Giacobbo, A.; Dias, B.B.; Onorevoli, B.; Bernardes, A.M.; de Pinho, M.N.; Caramão, E.B.; Rodrigues, E.; Jacques, R.A. Wine lees from the 1st and 2nd rackings: Valuable by-products. J. Food Sci. Technol. 2019, 56, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Giacobbo, A.; Bernardes, A.M.; de Pinho, M.N. Sequential pressure-driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine lees. Sep. Purif. Technol. 2017, 173, 49–54. [Google Scholar] [CrossRef]
- Restolho, J.A.; Prates, A.; de Pinho, M.N.; Afonso, M.D. Sugars and lignosulphonates recovery from eucalyptus spent sulphite liquor by membrane processes. Biomass Bioenergy 2009, 33, 1558–1566. [Google Scholar] [CrossRef]
- Ghalamara, S.; Silva, S.; Brazinha, C.; Pintado, M. Valorization of Fish by-Products: Purification of Bioactive Peptides from Codfish Blood and Sardine Cooking Wastewaters by Membrane Processing. Membranes 2020, 10, 44. [Google Scholar] [CrossRef]
- Monte, J.; Ribeiro, C.; Parreira, C.; Costa, L.; Brive, L.; Casal, S.; Brazinha, C.; Crespo, J.G. Biorefinery of Dunaliella salina: Sustainable recovery of carotenoids, polar lipids and glycerol. Bioresour. Technol. 2020, 297, 122509. [Google Scholar] [CrossRef] [PubMed]
- Polino, M.; Portugal, C.A.M.; Di Profio, G.; Coelhoso, I.M.; Crespo, J.G. Protein Crystallization by Membrane-Assisted Technology. Cryst. Growth Des. 2019, 19, 4871–4883. [Google Scholar] [CrossRef]
- Polino, M.; Rho, H.S.; Pina, M.P.; Mallada, R.; Carvalho, A.L.; Romão, M.J.; Coelhoso, I.; Gardeniers, J.G.E.; Crespo, J.G.; Portugal, C.A.M. Protein Crystallization in a Microfluidic Contactor with Nafion®117 Membranes. Membranes 2021, 11, 549. [Google Scholar] [CrossRef] [PubMed]
- Polino, M.; Carvalho, A.L.s.; Juknaitė, L.; Portugal, C.A.M.; Coelhoso, I.M.; Romão, M.J.; Crespo, J.G. Ion-Exchange Membranes for Stable Derivatization of Protein Crystals. Cryst. Growth Des. 2017, 17, 4563–4572. [Google Scholar] [CrossRef]
- Martins, C.F.; Neves, L.A.; Chagas, R.; Ferreira, L.M.; Coelhoso, I.M.; Crespo, J.G. Removing CO2 from Xenon anaesthesia circuits using an amino-acid ionic liquid solution in a membrane contactor. Sep. Purif. Technol. 2021, 275, 119190. [Google Scholar] [CrossRef]
- Martins, C.F.; Neves, L.A.; Chagas, R.; Ferreira, L.M.; Afonso, C.A.M.; Crespo, J.G.; Coelhoso, I.M. CO2 removal from anaesthesia circuits using gas-ionic liquid membrane contactors. Sep. Purif. Technol. 2020, 250, 116983. [Google Scholar] [CrossRef]
- Malankowska, M.; Martins, C.F.; Rho, H.S.; Neves, L.A.; Tiggelaar, R.M.; Crespo, J.G.; Pina, M.P.; Mallada, R.; Gardeniers, H.; Coelhoso, I.M. Microfluidic devices as gas—Ionic liquid membrane contactors for CO2 removal from anaesthesia gases. J. Membr. Sci. 2018, 545, 107–115. [Google Scholar] [CrossRef]
- Eusébio, T.M.; Martins, A.R.; Pon, G.; Faria, M.; Morgado, P.; Pinto, M.L.; Filipe, E.J.M.; de Pinho, M.N. Sorption/Diffusion Contributions to the Gas Permeation Properties of Bi-Soft Segment Polyurethane/Polycaprolactone Membranes for Membrane Blood Oxygenators. Membranes 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Moreira, C.; Mendonça Eusébio, T.; de Pinho, M.N.; Brogueira, P.; Semião, V. Oxygen mass transfer in a gas/membrane/liquid system surrogate of membrane blood oxygenators. AlChE J. 2018, 64, 3756–3763. [Google Scholar] [CrossRef]
- Faria, M.; Moreira, C.; Eusébio, T.; Brogueira, P.; de Pinho, M.N. Hybrid flat sheet cellulose acetate/silicon dioxide ultrafiltration membranes for uremic blood purification. Cellulose 2020, 27, 3847–3869. [Google Scholar] [CrossRef]
- De Pascale, M.; Faria, M.; Boi, C.; Semiao, V.; de Pinho, M.N. The effect of ultrafiltration transmembrane permeation on the flow field in a surrogate system of an artificial kidney. Exp. Results 2021, 2, e16. [Google Scholar] [CrossRef]
- Teixeira, F.C.; de Sá, A.I.; Teixeira, A.P.S.; Rangel, C.M. Nafion phosphonic acid composite membranes for proton exchange membranes fuel cells. Appl. Surf. Sci. 2019, 487, 889–897. [Google Scholar] [CrossRef]
- Teixeira, F.C.; de Sá, A.I.; Teixeira, A.P.S.; Rangel, C.M. Enhanced proton conductivity of Nafion-azolebisphosphonate membranes for PEM fuel cells. New J. Chem. 2019, 43, 15249–15257. [Google Scholar] [CrossRef]
- Teixeira, F.C.; de Sá, A.I.; Teixeira, A.P.S.; Ortiz-Martínez, V.M.; Ortiz, A.; Ortiz, I.; Rangel, C.M. New modified Nafion-bisphosphonic acid composite membranes for enhanced proton conductivity and PEMFC performance. Int. J. Hydrogen Energy 2021, 46, 17562–17571. [Google Scholar] [CrossRef]
- Vilela, C.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Nanocellulose-based materials as components of polymer electrolyte fuel cells. J. Mater. Chem. A 2019, 7, 20045–20074. [Google Scholar] [CrossRef]
- Vilela, C.; Martins, A.P.C.; Sousa, N.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Poly(bis[2-(methacryloyloxy)ethyl] phosphate)/Bacterial Cellulose Nanocomposites: Preparation, Characterization and Application as Polymer Electrolyte Membranes. Appl. Sci. 2018, 8, 1145. [Google Scholar] [CrossRef]
- Vilela, C.; Gadim, T.D.O.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Nanocellulose/poly(methacryloyloxyethyl phosphate) composites as proton separator materials. Cellulose 2016, 23, 3677–3689. [Google Scholar] [CrossRef]
- Vilela, C.; Morais, J.D.; Silva, A.C.Q.; Muñoz-Gil, D.; Figueiredo, F.M.L.; Silvestre, A.J.D.; Freire, C.S.R. Flexible Nanocellulose/Lignosulfonates Ion-Conducting Separators for Polymer Electrolyte Fuel Cells. Nanomaterials 2020, 10, 1713. [Google Scholar] [CrossRef]
- Vilela, C.; Silva, A.C.Q.; Domingues, E.M.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.L.; Santos, S.A.O.; Freire, C.S.R. Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: The case of bacterial cellulose and fucoidan. Carbohydr. Polym. 2020, 230, 115604. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Cordeiro, D.M.; Boas, J.V.; Barbosa, P.; Nolasco, M.; Vaz, P.D.; Rudić, S.; Ribeiro-Claro, P.; Silvestre, A.J.D.; Oliveira, V.B.; et al. Poly(4-styrene sulfonic acid)/bacterial cellulose membranes: Electrochemical performance in a single-chamber microbial fuel cell. Bioresour. Technol. Rep. 2020, 9, 100376. [Google Scholar] [CrossRef]
- Vilela, C.; Sousa, N.; Pinto, R.J.B.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Exploiting poly(ionic liquids) and nanocellulose for the development of bio-based anion-exchange membranes. Biomass Bioenergy 2017, 100, 116–125. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Falcão, D.S.; Pinto, A.M.F.R. Simulation of membrane chemical degradation in a proton exchange membrane fuel cell by computational fluid dynamics. Int. J. Hydrogen Energy 2021, 46, 1106–1120. [Google Scholar] [CrossRef]
- Rambabu, G.; Bhat, S.D.; Figueiredo, F.M.L. Carbon Nanocomposite Membrane Electrolytes for Direct Methanol Fuel Cells—A Concise Review. Nanomaterials 2019, 9, 1292. [Google Scholar] [CrossRef]
- Ben Jadi, S.; El Guerraf, A.; Bazzaoui, E.A.; Wang, R.; Martins, J.I.; Bazzaoui, M. Synthesis, characterization, and transport properties of Nafion-polypyrrole membrane for direct methanol fuel cell (DMFC) application. J. Solid State Electrochem. 2019, 23, 2423–2433. [Google Scholar] [CrossRef]
- Ben Jadi, S.; El Guerraf, A.; Kiss, A.; El Azrak, A.; Bazzaoui, E.A.; Wang, R.; Martins, J.I.; Bazzaoui, M. Analyses of scanning electrochemical microscopy and electrochemical impedance spectroscopy in direct methanol fuel cells: Permeability resistance and proton conductivity of polyaniline modified membrane. J. Solid State Electrochem. 2020, 24, 1551–1565. [Google Scholar] [CrossRef]
- De Oliveira, P.N.; Mendes, A.M.M. Preparation and characterization of an eco-friendly polymer electrolyte membrane (PEM) Based in a Blend of Sulphonated Poly(Vinyl Alcohol)/Chitosan Mechanically Stabilised by Nylon 6,6. Mater. Res. Bull. 2016, 19, 954–962. [Google Scholar] [CrossRef]
- Šljukić, B.; Santos, D.M.F. Chapter 10—Direct Borohydride Fuel Cells (DBFCs). In Direct Liquid Fuel Cells; Akay, R.G., Yurtcan, A.B., Eds.; Academic Press: New York, NY, USA, 2021; pp. 203–232. [Google Scholar] [CrossRef]
- Gouda, M.H.; Gouveia, W.; Afonso, M.L.; Šljukić, B.; El Essawy, N.A.; Nassr, A.A.A.; Santos, D.M.F. Poly(vinyl alcohol)-based crosslinked ternary polymer blend doped with sulfonated graphene oxide as a sustainable composite membrane for direct borohydride fuel cells. J. Power Sources 2019, 432, 92–101. [Google Scholar] [CrossRef]
- Gouda, M.H.; Gouveia, W.; Elessawy, N.A.; Šljukić, B.; Nassr, A.A.A.; Santos, D.M.F. Simple design of PVA-based blend doped with SO4(PO4)-functionalised TiO2 as an effective membrane for direct borohydride fuel cells. Int. J. Hydrogen Energy 2020, 45, 15226–15238. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Kundu, M.; Gören, A.; Silva, M.M.; Costa, C.M.; Liu, L.; Lanceros-Méndez, S. Optimization of filler type within poly(vinylidene fluoride-co-trifluoroethylene) composite separator membranes for improved lithium-ion battery performance. Compos. Part B Eng. 2016, 96, 94–102. [Google Scholar] [CrossRef]
- Costa, C.M.; Kundu, M.; Cardoso, V.F.; Machado, A.V.; Silva, M.M.; Lanceros-Méndez, S. Silica/poly(vinylidene fluoride) porous composite membranes for lithium-ion battery separators. J. Membr. Sci. 2018, 564, 842–851. [Google Scholar] [CrossRef]
- Gonçalves, R.; Lizundia, E.; Silva, M.M.; Costa, C.M.; Lanceros-Méndez, S. Mesoporous Cellulose Nanocrystal Membranes as Battery Separators for Environmentally Safer Lithium-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 3749–3761. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Correia, D.M.; Gonçalves, R.; de Zea Bermudez, V.; Silva, M.M.; Lanceros-Mendez, S.; Costa, C.M. Enhanced ionic conductivity in poly(vinylidene fluoride) electrospun separator membranes blended with different ionic liquids for lithium ion batteries. J. Colloid Interface Sci. 2021, 582, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, C.; Tufa, R.A.; Aili, D.; Fischer, P.; Velizarov, S. Membranes for zinc-air batteries: Recent progress, challenges and perspectives. J. Power Sources 2020, 475, 228689. [Google Scholar] [CrossRef]
- Alday, P.P.; Barros, S.C.; Alves, R.; Esperança, J.M.S.S.; Navarro-Segarra, M.; Sabaté, N.; Silva, M.M.; Esquivel, J.P. Biopolymer Electrolyte Membranes (BioPEMs) for Sustainable Primary Redox Batteries. Adv. Sustain. Syst. 2020, 4, 1900110. [Google Scholar] [CrossRef]
- Mushtaq, K.; Lagarteira, T.; Zaidi, A.A.; Mendes, A. In-situ crossover diagnostics to assess membrane efficacy for non-aqueous redox flow battery. J. Energy Storage 2021, 40, 102713. [Google Scholar] [CrossRef]
- Pawlowski, S.; Galinha, C.F.; Crespo, J.G.; Velizarov, S. 2D fluorescence spectroscopy for monitoring ion-exchange membrane based technologies—Reverse electrodialysis (RED). Water Res. 2016, 88, 184–198. [Google Scholar] [CrossRef] [PubMed]
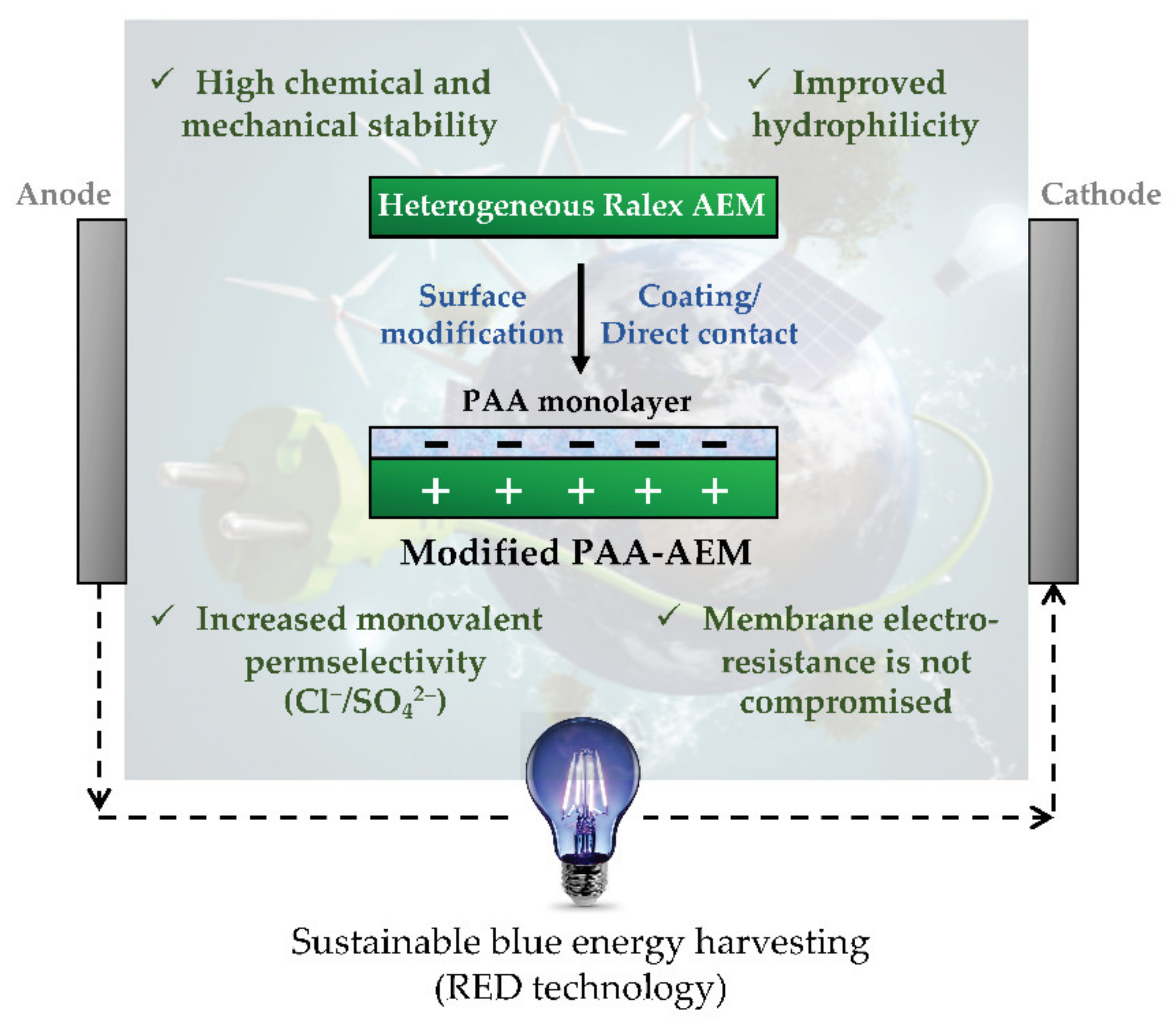
- Merino-Garcia, I.; Kotoka, F.; Portugal, C.A.M.; Crespo, J.G.; Velizarov, S. Characterization of Poly(Acrylic) Acid-Modified Heterogenous Anion Exchange Membranes with Improved Monovalent Permselectivity for RED. Membranes 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Amorim, I.; Li, Y.; Li, J.; Yu, Z.; Zhang, B.; Araujo, A.; Zhang, N.; Liu, L. Stable overall water splitting in an asymmetric acid/alkaline electrolyzer comprising a bipolar membrane sandwiched by bifunctional cobalt-nickel phosphide nanowire electrodes. Carbon Energy 2020, 2, 646–655. [Google Scholar] [CrossRef]
- Dias, A.C. Chlor-Alkali Membrane Cell Process: Study and Characterization. Ph.D. Thesis, University of Porto, Porto, Portugal, 2010. [Google Scholar]
- Dias, A.C.; Pereira, M.J.; Brandão, L.; Araújo, P.; Mendes, A. Characterization of the Chlor-Alkali Membrane Process by EIS. J. Electrochem. Soc. 2010, 157, E75. [Google Scholar] [CrossRef]
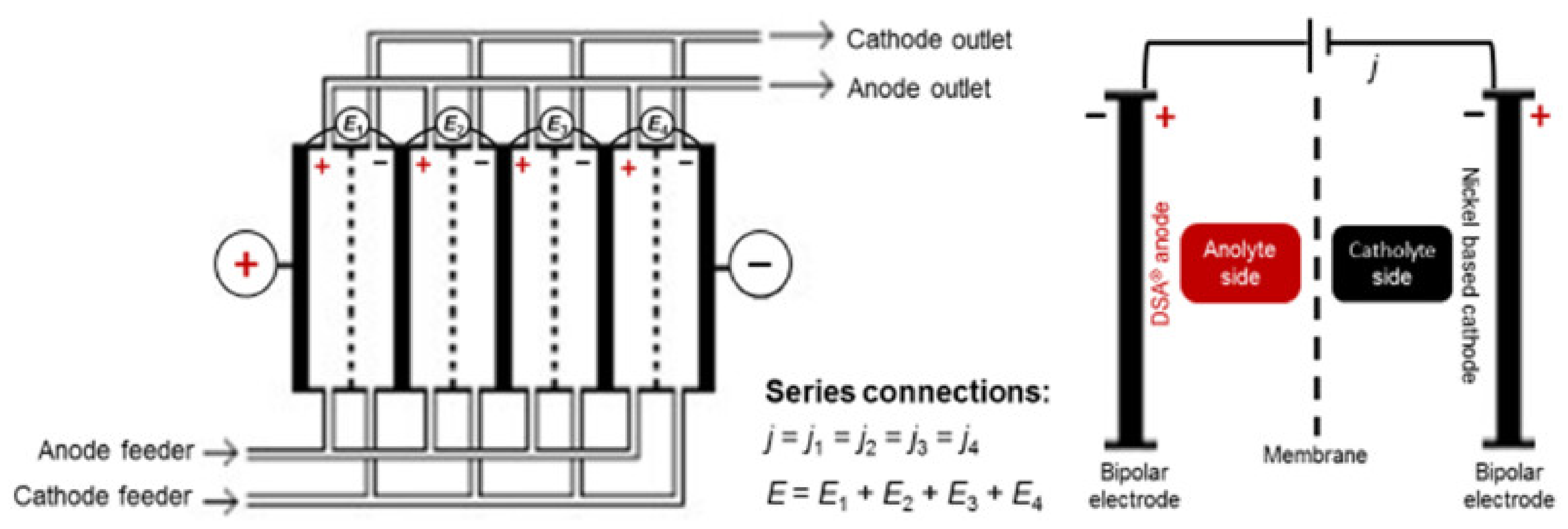
- Franco, F.; Prior, J.; Velizarov, S.; Mendes, A. A Systematic Performance History Analysis of a Chlor-Alkali Membrane Electrolyser under Industrial Operating Conditions. Appl. Sci. 2019, 9, 284. [Google Scholar] [CrossRef]
- Tomé, L.C.; Marrucho, I.M. Ionic liquid-based materials: A platform to design engineered CO2 separation membranes. Chem. Soc. Rev. 2016, 45, 2785–2824. [Google Scholar] [CrossRef]
- Albo, J.; Santos, E.; Neves, L.A.; Simeonov, S.P.; Afonso, C.A.M.; Crespo, J.G.; Irabien, A. Separation performance of CO2 through Supported Magnetic Ionic Liquid Membranes (SMILMs). Sep. Purif. Technol. 2012, 97, 26–33. [Google Scholar] [CrossRef]
- Santos, E.; Albo, J.; Daniel, C.I.; Portugal, C.A.M.; Crespo, J.G.; Irabien, A. Permeability modulation of Supported Magnetic Ionic Liquid Membranes (SMILMs) by an external magnetic field. J. Membr. Sci. 2013, 430, 56–61. [Google Scholar] [CrossRef]
- Tomé, L.C.; Patinha, D.J.S.; Freire, C.S.R.; Rebelo, L.P.N.; Marrucho, I.M. CO2 separation applying ionic liquid mixtures: The effect of mixing different anions on gas permeation through supported ionic liquid membranes. RSC Adv. 2013, 3, 12220–12229. [Google Scholar] [CrossRef]
- Tomé, L.C.; Patinha, D.J.S.; Ferreira, R.; Garcia, H.; Silva Pereira, C.; Freire, C.S.R.; Rebelo, L.P.N.; Marrucho, I.M. Cholinium-based Supported Ionic Liquid Membranes: A Sustainable Route for Carbon Dioxide Separation. ChemSusChem 2014, 7, 110–113. [Google Scholar] [CrossRef]
- Tomé, L.C.; Florindo, C.; Freire, C.S.R.; Rebelo, L.P.N.; Marrucho, I.M. Playing with ionic liquid mixtures to design engineered CO2 separation membranes. Phys. Chem. Chem. Phys. 2014, 16, 17172–17182. [Google Scholar] [CrossRef]
- Couto, R.; Neves, L.; Simões, P.; Coelhoso, I. Supported Ionic Liquid Membranes and Ion-Jelly® Membranes with [BMIM][DCA]: Comparison of Its Performance for CO2 Separation. Membranes 2015, 5, 13–21. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Tomé, L.C.; Marrucho, I.M. Towards the potential of cyano and amino acid-based ionic liquid mixtures for facilitated CO2 transport membranes. J. Membr. Sci. 2016, 510, 174–181. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Tomé, L.C.; Lozinskaya, E.I.; Shaplov, A.S.; Vygodskii, Y.S.; Marrucho, I.M. Exploring the effect of fluorinated anions on the CO2/N2 separation of supported ionic liquid membranes. Phys. Chem. Chem. Phys. 2017, 19, 28876–28884. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.Y.M.; Martins, C.F.; Neves, L.A.; Capasso, C.; Supuran, C.T.; Coelhoso, I.M.; Crespo, J.G.; Barboiu, M. Supported ionic liquid membranes immobilized with carbonic anhydrases for CO2 transport at high temperatures. J. Membr. Sci. 2017, 528, 225–230. [Google Scholar] [CrossRef]
- Tomé, L.C.; Gouveia, A.S.L.; Ab Ranii, M.A.; Lickiss, P.D.; Welton, T.; Marrucho, I.M. Study on Gas Permeation and CO2 Separation through Ionic Liquid-Based Membranes with Siloxane-Functionalized Cations. Ind. Eng. Chem. Res. 2017, 56, 2229–2239. [Google Scholar] [CrossRef]
- Craveiro, R.; Neves, L.A.; Duarte, A.R.C.; Paiva, A. Supported liquid membranes based on deep eutectic solvents for gas separation processes. Sep. Purif. Technol. 2021, 254, 117593. [Google Scholar] [CrossRef]
- Castro, A.M.d.; Prasavath, D.; Bevilaqua, J.V.; Portugal, C.A.M.; Neves, L.A.; Crespo, J.G. Role of water on deep eutectic solvents (DES) properties and gas transport performance in biocatalytic supported DES membranes. Sep. Purif. Technol. 2021, 255, 117763. [Google Scholar] [CrossRef]
- Tomé, L.C.; Gouveia, A.S.L.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Polymeric ionic liquid-based membranes: Influence of polycation variation on gas transport and CO2 selectivity properties. J. Membr. Sci. 2015, 486, 40–48. [Google Scholar] [CrossRef]
- Tomé, L.C.; Mecerreyes, D.; Freire, C.S.R.; Rebelo, L.P.N.; Marrucho, I.M. Pyrrolidinium-based polymeric ionic liquid materials: New perspectives for CO2 separation membranes. J. Membr. Sci. 2013, 428, 260–266. [Google Scholar] [CrossRef]
- Tomé, L.C.; Aboudzadeh, M.A.; Rebelo, L.P.N.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Polymeric ionic liquids with mixtures of counter-anions: A new straightforward strategy for designing pyrrolidinium-based CO2 separation membranes. J. Mater. Chem. A 2013, 1, 10403–10411. [Google Scholar] [CrossRef]
- Teodoro, R.M.; Tomé, L.C.; Mantione, D.; Mecerreyes, D.; Marrucho, I.M. Mixing poly(ionic liquid)s and ionic liquids with different cyano anions: Membrane forming ability and CO2/N2 separation properties. J. Membr. Sci. 2018, 552, 341–348. [Google Scholar] [CrossRef]
- Tomé, L.C.; Guerreiro, D.C.; Teodoro, R.M.; Alves, V.D.; Marrucho, I.M. Effect of polymer molecular weight on the physical properties and CO2/N2 separation of pyrrolidinium-based poly(ionic liquid) membranes. J. Membr. Sci. 2018, 549, 267–274. [Google Scholar] [CrossRef]
- Tomé, L.C.; Isik, M.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Novel pyrrolidinium-based polymeric ionic liquids with cyano counter-anions: High performance membrane materials for post-combustion CO2 separation. J. Membr. Sci. 2015, 483, 155–165. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Ventaja, L.; Tomé, L.C.; Marrucho, I.M. Towards Biohydrogen Separation Using Poly(Ionic Liquid)/Ionic Liquid Composite Membranes. Membranes 2018, 8, 124. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Malcaitè, E.; Lozinskaya, E.I.; Shaplov, A.S.; Tomé, L.C.; Marrucho, I.M. Poly(ionic liquid)–Ionic Liquid Membranes with Fluorosulfonyl-Derived Anions: Characterization and Biohydrogen Separation. ACS Sustain. Chem. Eng. 2020, 8, 7087–7096. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Yáñez, M.; Alves, V.D.; Palomar, J.; Moya, C.; Gorri, D.; Tomé, L.C.; Marrucho, I.M. CO2/H2 separation through poly(ionic liquid)–ionic liquid membranes: The effect of multicomponent gas mixtures, temperature and gas feed pressure. Sep. Purif. Technol. 2021, 259, 118113. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Oliveira, V.; Ferraria, A.M.; Do Rego, A.M.B.; Ferreira, M.J.; Tomé, L.C.; Almeida, A.; Marrucho, I.M. Processing of poly(ionic liquid)–ionic liquid membranes using femtosecond (fs) laser radiation: Effect on CO2 separation performance. J. Membr. Sci. 2022, 642, 119903. [Google Scholar] [CrossRef]
- Nabais, A.R.; Martins, A.P.S.; Alves, V.D.; Crespo, J.G.; Marrucho, I.M.; Tomé, L.C.; Neves, L.A. Poly(ionic liquid)-based engineered mixed matrix membranes for CO2/H2 separation. Sep. Purif. Technol. 2019, 222, 168–176. [Google Scholar] [CrossRef]
- Sampaio, A.M.; Nabais, A.R.; Tomé, L.C.; Neves, L.A. Impact of MOF-5 on Pyrrolidinium-Based Poly(ionic liquid)/Ionic Liquid Membranes for Biogas Upgrading. Ind. Eng. Chem. Res. 2020, 59, 308–317. [Google Scholar] [CrossRef]
- Monteiro, B.; Nabais, A.R.; Casimiro, M.H.; Martins, A.P.S.; Francisco, R.O.; Neves, L.A.; Pereira, C.C.L. Impact on CO2/N2 and CO2/CH4 Separation Performance Using Cu-BTC with Supported Ionic Liquids-Based Mixed Matrix Membranes. Membranes 2018, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.; Ferreira, T.J.; Barbosa, A.D.S.; de Castro, B.; Ribeiro, R.P.P.L.; Mota, J.P.B.; Alves, V.D.; Cunha-Silva, L.; Esteves, I.A.A.C.; Neves, L.A. Cr-based MOF/IL composites as fillers in mixed matrix membranes for CO2 separation. Sep. Purif. Technol. 2021, 276, 119303. [Google Scholar] [CrossRef]
- Pardo, F.; Gutiérrez-Hernández, S.V.; Hermida-Merino, C.; Araújo, J.M.M.; Piñeiro, M.M.; Pereiro, A.B.; Zarca, G.; Urtiaga, A. Integration of Stable Ionic Liquid-Based Nanofluids into Polymer Membranes. Part II: Gas Separation Properties toward Fluorinated Greenhouse Gases. Nanomaterials 2021, 11, 582. [Google Scholar] [CrossRef]
- Hermida-Merino, C.; Pardo, F.; Zarca, G.; Araújo, J.M.M.; Urtiaga, A.; Piñeiro, M.M.; Pereiro, A.B. Integration of Stable Ionic Liquid-Based Nanofluids into Polymer Membranes. Part I: Membrane Synthesis and Characterization. Nanomaterials 2021, 11, 607. [Google Scholar] [CrossRef]
- Lito, P.F.; Cardoso, S.P.; Rodrigues, A.E.; Silva, C.M. Kinetic Modeling of Pure and Multicomponent Gas Permeation Through Microporous Membranes: Diffusion Mechanisms and Influence of Isotherm Type. Sep. Purif. Rev. 2015, 44, 283–307. [Google Scholar] [CrossRef]
- Cardoso, S.P.; Azenha, I.S.; Lin, Z.; Portugal, I.; Rodrigues, A.E.; Silva, C.M. Inorganic Membranes for Hydrogen Separation. Sep. Purif. Rev. 2018, 47, 229–266. [Google Scholar] [CrossRef]
- Bernardo, G.; Araújo, T.; da Silva Lopes, T.; Sousa, J.; Mendes, A. Recent advances in membrane technologies for hydrogen purification. Int. J. Hydrogen Energy 2020, 45, 7313–7338. [Google Scholar] [CrossRef]
- Cardoso, S.P.; Azenha, I.S.; Portugal, I.; Lin, Z.; Rodrigues, A.E.; Silva, C.M. Single and binary surface diffusion permeation through zeolite membranes using new Maxwell-Stefan factors for Dubinin-type isotherms and occupancy-dependent kinetics. Sep. Purif. Technol. 2017, 182, 207–218. [Google Scholar] [CrossRef]
- Cardoso, S.P.; Lin, Z.; Portugal, I.; Rodrigues, A.E.; Silva, C.M. Synthesis, dynamic characterization, and modeling studies of an AM-3 membrane for light gases separation. Microporous Mesoporous Mater. 2018, 261, 170–180. [Google Scholar] [CrossRef]
- Araújo, T.; Bernardo, G.; Mendes, A. Cellulose-Based Carbon Molecular Sieve Membranes for Gas Separation: A Review. Molecules 2020, 25, 3532. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.C.; Andrade, M.; Moffat, J.; Magalhães, F.D.; Mendes, A. Carbon membranes with extremely high separation factors and stability. Energy Technol. 2019, 7, 1801089. [Google Scholar] [CrossRef]
- Rodrigues, S.C.; Andrade, M.; Moffat, J.; Magalhães, F.D.; Mendes, A. Preparation of carbon molecular sieve membranes from an optimized ionic liquid-regenerated cellulose precursor. J. Membr. Sci. 2019, 572, 390–400. [Google Scholar] [CrossRef]
- Crespo, J.G.; Brazinha, C. 1—Fundamentals of pervaporation. In Pervaporation, Vapour Permeation and Membrane Distillation; Basile, A., Figoli, A., Khayet, M., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 3–17. [Google Scholar] [CrossRef]
- Meireles, I.T.; Brazinha, C.; Crespo, J.G.; Coelhoso, I.M. A new microbial polysaccharide membrane for ethanol dehydration by pervaporation. J. Membr. Sci. 2013, 425–426, 227–234. [Google Scholar] [CrossRef]
- Meireles, I.T.; Portugal, C.; Alves, V.D.; Crespo, J.G.; Coelhoso, I.M. Impact of biopolymer purification on the structural characteristics and transport performance of composite polysaccharide membranes for pervaporation. J. Membr. Sci. 2015, 493, 179–187. [Google Scholar] [CrossRef]
- Meireles, I.T.; Brazinha, C.; Coelhoso, I.M.; Crespo, J.G. 10—Membranes for ethanol dehydration. In Membrane Technologies for Biorefining; Figoli, A., Cassano, A., Basile, A., Eds.; Woodhead Publishing: Oxford, UK, 2016; pp. 241–262. [Google Scholar] [CrossRef]
- Meireles, I.T.; Huertas, R.M.; Torres, C.A.V.; Coelhoso, I.M.; Crespo, J.G. Development and characterisation of hybrid polysaccharide membranes for dehydration processes. Carbohydr. Polym. 2018, 191, 216–224. [Google Scholar] [CrossRef]
- Boutikos, P.; Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Performance evaluation of silica membrane for water–n-butanol binary mixture. Sep. Purif. Technol. 2014, 127, 18–28. [Google Scholar] [CrossRef]
- Brazinha, C.; Crespo, J.G. Aroma recovery from hydro alcoholic solutions by organophilic pervaporation: Modelling of fractionation by condensation. J. Membr. Sci. 2009, 341, 109–121. [Google Scholar] [CrossRef]
- Brazinha, C.; Alves, V.D.; Viegas, R.M.C.; Crespo, J.G. Aroma recovery by integration of sweeping gas pervaporation and liquid absorption in membrane contactors. Sep. Purif. Technol. 2009, 70, 103–111. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Brazinha, C.; Afonso, C.A.M.; Crespo, J.G. Selective extraction of natural products with benign solvents and recovery by organophilic pervaporation: Fractionation of d-limonene from orange peels. Green Chem. 2010, 12, 1990–1994. [Google Scholar] [CrossRef]
- Brazinha, C.; Barbosa, D.S.; Crespo, J.G. Sustainable recovery of pure natural vanillin from fermentation media in a single pervaporation step. Green Chem. 2011, 13, 2197–2203. [Google Scholar] [CrossRef]
- Fraga, S.C.; Kujawska, A.; Kujawski, W.; Brazinha, C.; Crespo, J.G. Transport of dilute organics through dense membranes: Assessing impact on membrane-solute interactions. J. Membr. Sci. 2017, 523, 346–354. [Google Scholar] [CrossRef]
- Catarino, M.; Ferreira, A.; Mendes, A. Study and optimization of aroma recovery from beer by pervaporation. J. Membr. Sci. 2009, 341, 51–59. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Pinho, S.P.; Rodrigues, A.E. Batch and continuous studies for ethyl lactate synthesis in a pervaporation membrane reactor. J. Membr. Sci. 2010, 361, 43–55. [Google Scholar] [CrossRef]
- Constantino, D.S.M.; Faria, R.P.V.; Ribeiro, A.M.; Loureiro, J.M.; Rodrigues, A.E. Performance Evaluation of Pervaporation Technology for Process Intensification of Butyl Acrylate Synthesis. Ind. Eng. Chem. Res. 2017, 56, 13064–13074. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomé, L.C.; Santos, D.M.F.; Velizarov, S.; Coelhoso, I.M.; Mendes, A.; Crespo, J.G.; de Pinho, M.N. Overview of Membrane Science and Technology in Portugal. Membranes 2022, 12, 197. https://doi.org/10.3390/membranes12020197
Tomé LC, Santos DMF, Velizarov S, Coelhoso IM, Mendes A, Crespo JG, de Pinho MN. Overview of Membrane Science and Technology in Portugal. Membranes. 2022; 12(2):197. https://doi.org/10.3390/membranes12020197
Chicago/Turabian StyleTomé, Liliana C., Diogo M. F. Santos, Svetlozar Velizarov, Isabel M. Coelhoso, Adélio Mendes, João G. Crespo, and Maria Norberta de Pinho. 2022. "Overview of Membrane Science and Technology in Portugal" Membranes 12, no. 2: 197. https://doi.org/10.3390/membranes12020197
APA StyleTomé, L. C., Santos, D. M. F., Velizarov, S., Coelhoso, I. M., Mendes, A., Crespo, J. G., & de Pinho, M. N. (2022). Overview of Membrane Science and Technology in Portugal. Membranes, 12(2), 197. https://doi.org/10.3390/membranes12020197











