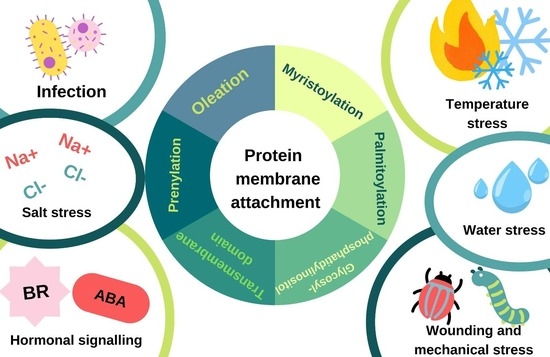
The Re-Localization of Proteins to or Away from Membranes as an Effective Strategy for Regulating Stress Tolerance in Plants
Abstract
:1. Introduction
2. Protein Re-Localization upon Water-Related Stress
2.1. Enhanced Localization of Annexin 1 at the Cell Membrane upon Plasmolysis
2.2. De-S-Palmitoylation of MfNACsa to Activate Its Transcriptional Regulatory Function
2.3. Redistribution of Aquaporins under Water-Related Stresses
3. Protein Re-Localization upon Salt Stress
3.1. Reduction of Aquaporins in the Plasma Membrane upon Salt Stress
3.2. Recruitment of SOS2 to the Plasma Membrane upon Salt Stress
3.3. Stabilization of MdCBL1 at the Plasma Membrane by Palmitoylation upon Salt Stress
4. Protein Re-Localization upon Heat/Cold Stress
4.1. Promotion of DnaJ Lipidation by Heat Shock
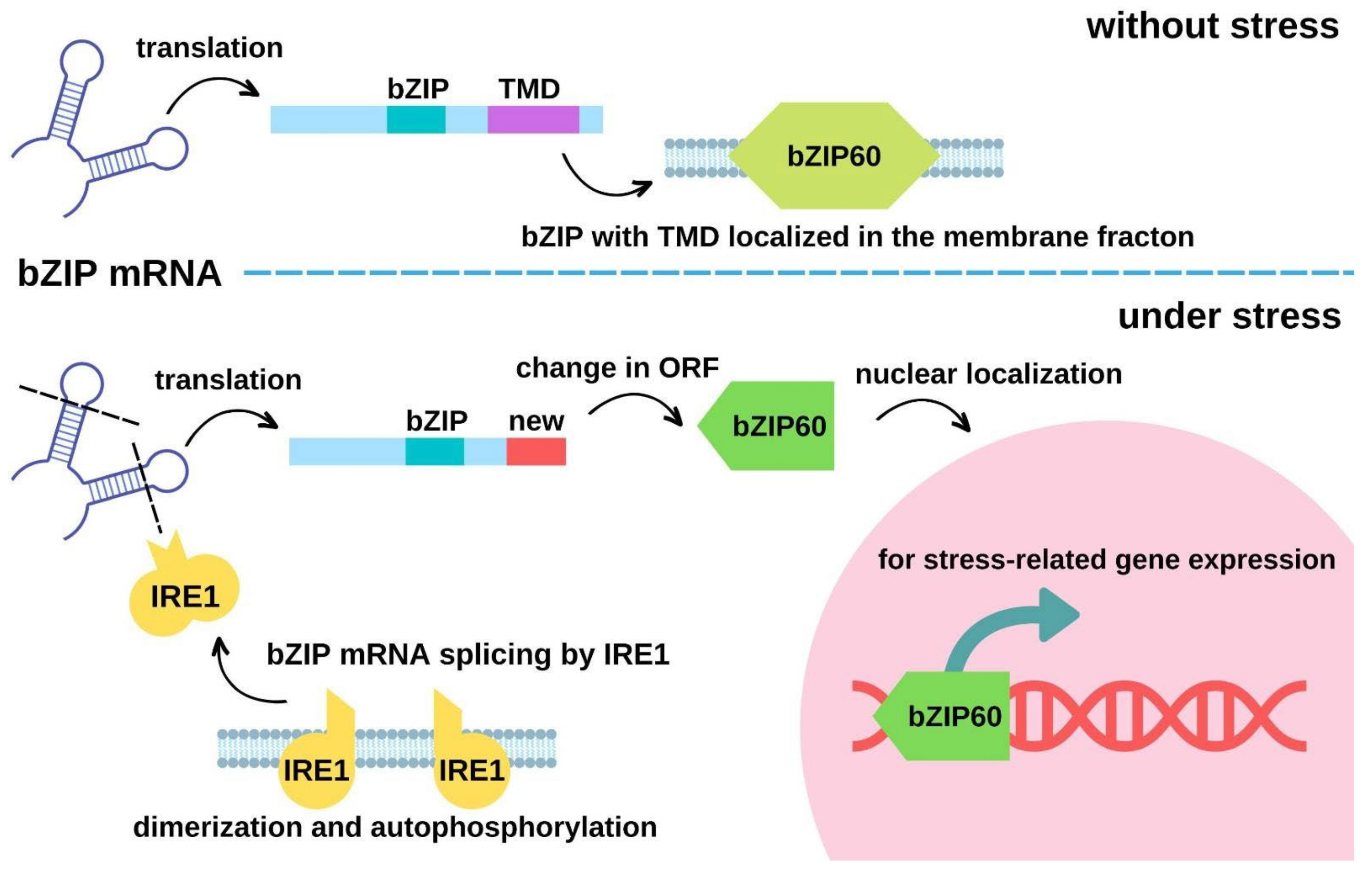
4.2. Protein Re-Localization by Transmembrane Domain Cleavage to Regulate Gene Expressions upon Heat Stress
4.3. Alteration of Protein Localization by mRNA cleavage upon Heat Stress
4.4. Translocation of Proteins to the Nucleus to Regulate Gene Expressions upon Cold Stress
4.5. Changes in Protein Subcellular Localization in Response to Heat/Cold Stress-Induced Ca2+ Signaling
5. Protein Re-Localization upon Mechanical Stress for Protein Activation
6. Protein Re-Localization upon Biotic Stress
7. Protein Re-Localization upon Oxidative Stress
8. Protein Re-Localization in Response to Stress Hormones
8.1. Regulation of NMT1 Expression by ABA
8.2. Mediation of Brassinosteroid Signaling by Myristoylated BSK1
9. Protein Re-Localization in Response to Other Signaling Events
9.1. Regulation of SnRK Signaling by N-Myristoylation
9.2. Light/Sugar Sensing
10. Discussion and Conclusions
| Type of Stress | Species | Protein | Description of the Relocation | Mechanism of the Relocation | Functional Significance of the Relocation | References |
|---|---|---|---|---|---|---|
| Water- related stress | Arabidopsis thaliana | ANN1 | Accumulation of ANN1 upon osmotic stress (plasmolysis) | Unknown | Association with Hechtian strands and reticulum at the plasma membrane for protection against osmotic stress | [24] |
| Medicago falcata | MfNACsa | Relocated from the plasma membrane to the nucleus upon drought stress | De-S-palmitoylation of MfNACsa | MfNACsa activates the expression of MtGly1 in the model plant Medicago truncatula for maintaining the glutathione pool in the reduced state to achieve drought tolerance | [29] | |
| Oryza sativa | OsPIP1;1, OsPIP2;4, and OsPIP2;5 | Relocated away from the plasma membrane | Endocytosis of OsPIP2;5 is enhanced by salt stress | Regulation of water transport | [33] | |
| Populus tomentosa | PtoPIP1;1 | Exhibited polar-like localization at the plasma membrane compared to the relatively even distribution at the plasma membrane under normal conditions | Not mentioned | Regulation of water transport | [35] | |
| Mesembryanthemum crystallinum | McTIP1;2 | Exhibited promoted localization in the tonoplast | Not mentioned | Promotion of osmotic adjustment in the cell | [37] | |
| Salt stress | Arabidopsis thaliana | SOS2 | Enhanced plasma membrane localization upon salt stress | The plasma membrane localization is enhanced by VPS23A | For the activation of SOS1, a membrane-bound Na+/H+ exchanger | [52] |
| Malus domestica | MdCBL1 | Stabilized plasma membrane localization upon salt stress | The expression of MdPAT16 is induced upon salt stress. MdPAT16 mediates the plasma membrane localization of MsCBL1 by palmitoylation | Regulation of sugar accumulation | [53] | |
| Arabidopsis thaliana | AtPIP2;1 | Reduced plasma membrane localization upon osmotic stress and salt stress | Enhanced internalization of AtPIP2;1 upon water-deficit stress through endocytic pathways | Regulation of the water permeability of plasma membrane to protect cells from water-deficit stress | [41] | |
| Arabidopsis thaliana | TIP1;1 | From the tonoplast to intracellular spherical structures | Not mentioned | Regulation of water transport inside the cell | [34] | |
| Heat stress | Arabidopsis thaliana | AtJ3 | From cytosol to membrane-less heat stress granules | Not mentioned | For the formation of HSP70/HSP40-based chaperones. Mutants failed to undergo AtJ3 farnesylation, leading to heat stress intolerance. AtJ3 farnesylation is responsible for directing HSP70 to the misfolded protein | [52,54,56] |
| Arabidopsis thaliana | AGO1; AtJ2/AtJ3 | From cytosol to membrane | Proposed model of farnesylation promotes the AtJ3-membrane interaction and AGO1-membrane interaction via J3, which further alters the loading of AGO1-miRNA to the polysome | Farnesyl transferase-deficient and farnesylation-deficient j2/j3 mutants had increased levels of the miRNA-associated membrane-bound polysomes | [65] | |
| Arabidopsis thaliana | SKD1 | From cytosol to messenger ribonucleoproteins | Not mentioned | Possibly involved in the selection of proteins to be localized to mRNP under stress conditions | [120] | |
| Arabidopsis thaliana | bZIP28 | From membrane to endoplasmic reticulum and cytosol | Heat-induced cleavage at the membrane- tethering C-terminus | The re-localized bZIP28 up-regulates heat stress-coping genes such as the ER-localized chaperone BiP2 in Arabidopsis for coping with heat stress | [66,67] | |
| Arabidopsis thaliana | AtbZIP60 | From membrane to nucleus | The ER-localized inositol-requiring enzyme 1 (AtIRE1) mediates the unconventional mRNA splicing of AtbZIP60 by open reading frame shift | Membrane-localized active AtbZIP6 promotes the expression of stress-related genes | [69] | |
| Atriplex nummularia | ANJ1 | Not mentioned | Not mentioned | Heat shock enhances the amount of the prenylated DnaJ protein in the membrane fraction; potentially functions in heat tolerance | [58] | |
| Oryza sativa | OsNTL3 | From membrane to nucleus | Not mentioned | OsNTL3 binds to the promoter region of OsbZIP74 for stress response activation | [68] | |
| Oryza sativa | OsbZIP74 | From membrane to nucleus | The ER-localized inositol-requiring enzyme 1 (OsIRE1) cleaves off the C-terminal transmembrane domain of OsbZIP74 | Membrane-localized active OsbZIP74 promotes the expression of stress-related genes | [70] | |
| Zea mays | ZmCDPK7 | From membrane to cytosol | Not mentioned | ZmCDPK7 activates sHSP17.4 via phosphorylation in the cytoplasm for maintaining protein stability | [90,91] | |
| Cold stress | Arabidopsis thaliana | Trx-h2 | From membrane to nucleus | De-myristoylation of Trx-h2 | Trx-h2 reduces the disulfide-bonded inactive CBF oligomers to form the active monomers that bind the promoter regions of cold-responsive (COR) genes | [71,72] |
| Arabidopsis thaliana | EGR2 | Not mentioned | Not mentioned | Low temperature attenuates the formation of the NMT1-EGR2 protein complex, leading to the suppression of the myristoylation of EGR2, and releasing its inhibition on OST1 for the proper activation of the CBF pathway and freezing tolerance | [73] | |
| Arabidopsis thaliana | STRP | Decrease in the membrane fraction of STRP | Not mentioned | Potentially affects the expressions of cold-activated genes, protects the chromatin structure, and stabilizes the membrane structure | [74] | |
| Hordeum vulgare | HvFP1 | Not mentioned | Not mentioned | Farnesylation of HvFP1 is important for its precise nuclear localization | [121] | |
| Arabidopsis thaliana | HIPP26 | Not mentioned | Not mentioned | Isoprenylation of HIPP26 is important for its precise nuclear localization | [122] | |
| Mechanical stress | Oryza sativa | OsYchF1 | From cytosol to membrane | Interact with the membrane-anchored interacting partner, OsGAP1 | Proposed re-localization of the negative regulator of stress to alleviate stress susceptibility | [97,98] |
| Biotic stress | Arabidopsis thaliana | CPK16 | From plasma membrane to chloroplast | Removal of N-myristoylation of RGLG1 | Allows CPK16 to work in chloroplast and enhances the resistance towards Pseudomonas syringae pv. tomato DC3000 and tomato yellow leaf curl virus | [101] |
| Oryza sativa | OsERG1 | From cytosol to plasma membrane | Elevation of cellular calcium level | OsERG1 is induced by elicitor from the fungal blast Magnaporthe grisea and is believed to play roles in fungal disease defense responses. The OsERG1 protein is relocated from cytosol to plasma membrane upon fungal elicitor treatment and calcium signals | [99] | |
| Arabidopsis thaliana | BSK1 | From plasma membrane to cytoplasm and endoplasmic reticulum | Loss of N-myristoylation | BSK1 is plasma membrane-bound and interacts with BRI1 and FLS2 for triggering BR signaling or defense response. When flagellin is perceived, BSK1 would relocate to the non-membrane raft for functioning. If BSK1 fails to be modified by N-myristoylation, it would no longer associate with the plasma membrane and would be degraded through the autophagic pathway | [115,116] | |
| Biotic stress and hormone | Triticum aestivum | TaERG3 | From nucleus to plasma membrane | Increase in cellular calcium level | TaERG3 plays roles in ABA signaling and acts as a positive regulator of responses to high salt and low temperature. It also enhances resistance towards Puccinia striiformis f. sp. tritici (the pathogen causing stripe rust). It is predominantly localized in plasmalemma and nucleus | [100] |
| Stress hormone | Arabidopsis thaliana | RGLG1 | From plasma membrane to nucleus | Reduction of N-myristoylation of RGLG1 | Allows the binding of RGLG1 to PP2CA in the nucleus which is a negative regulator of ABA signaling | [113] |
| Nutritional starvation, biotic and abiotic stresses | Arabidopsis thaliana | SnRK1 | From plasma membrane to nucleus | Removal of N-myristoylation of SnRK1 β1 subunit | Allows the binding of SnRK1 to its transcription factor targets including bZIP63, FUS3, IDD8, EIN3, WRI1, MYC2 in the nucleus. Upon phosphorylation by SnRK1, these transcription factors would either have reduced activities or be degraded. In turn, sugar and amino acid metabolism, oil synthesis, seed maturation and germination, flowering, jasmonic acid, ethylene, and abscisic acid signaling controlled by these transcription factors would be affected | [118] |
| Sugar sensing and signal trans- duction | Petunia hybrida | CaM53 | From plasma membrane to nucleus | Loss of geranylation by geranylgeranyl transferases (GGTase-I) | In darkness or at low sugar levels, CaM53 is not geranylated and is localized in the nucleus. With light and sugar accumulation, CaM53 is geranylated by GGTase-I and becomes plasma membrane-bound | [119] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Novaković, L.; Guo, T.; Bacic, A.; Sampathkumar, A.; Johnson, K.L. Hitting the wall—Sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants 2018, 7, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giglione, C.; Fieulaine, S.; Meinnel, T. N-terminal protein modifications: Bringing back into play the ribosome. Biochimie 2015, 114, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.I.; Duronio, R.J.; Rudnick, D.A.; Adams, S.P.; Gokel, G.W. Protein N-myristoylation. J. Biol. Chem. 1991, 266, 8647–8650. [Google Scholar] [CrossRef]
- Bhatnagar, R.S.; Ashrafi, K.; Fütterer, K.; Waksman, G.; Gordon, J.I. Biology and enzymology of protein N-myristoylation. Enzymes 2001, 21, 241–290. [Google Scholar]
- Boisson, B.; Giglione, C.; Meinnel, T. Unexpected protein families including cell defense components feature in the N-myristoylome of a higher eukaryote. J. Biol. Chem. 2003, 278, 43418–43429. [Google Scholar] [CrossRef] [Green Version]
- Marmagne, A.; Ferro, M.; Meinnel, T.; Bruley, C.; Kuhn, L.; Garin, J.; Barbier-Brygoo, H.; Ephritikhine, G. A high content in lipid-modified peripheral proteins and integral receptor kinases features in the Arabidopsis plasma membrane proteome. Mol. Cell. Proteom. 2007, 6, 1980–1996. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.L.; Casey, P.J. Protein prenylation: Molecular mechanisms and functional consequences. Annu. Rev. Biochem. 1996, 65, 241–469. [Google Scholar] [CrossRef]
- Casey, P.J.; Seabra, M.C. Protein prenyltransferases. J. Biol. Chem. 1996, 271, 5289–5292. [Google Scholar] [CrossRef] [Green Version]
- Kinsella, B.T.; Maltese, W.A. Rab GTP-binding proteins implicated in vesicular transport are isoprenylated in vitro at cysteines within a novel carboxyl-terminal motif. J. Biol. Chem. 1991, 266, 8540–8544. [Google Scholar] [CrossRef]
- Hemsley, P.A.; Weimar, T.; Lilley, K.; Dupree, P.; Grierson, C. Palmitoylation in plants. Plant Signal. Behav. 2013, 8, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Akimzhanov, A.M.; Boehning, D.; Snyder, S.H. Rapid and transient palmitoylation of the tyrosine kinase Lck mediates Fas signaling. Proc. Natl. Acad. Sci. USA 2015, 112, 11876–11880. [Google Scholar] [CrossRef]
- Roth, A.F.; Feng, Y.; Chen, L.; Davis, N.G. The yeast DHHC cysteine-rich domain protein Akr1p is a palmitoyl transferase. J. Cell Biol. 2002, 159, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Hemsley, P.A.; Weimar, T.; Lilley, K.S.; Dupree, P.; Grierson, C.S. A proteomic approach identifies many novel palmitoylated proteins in Arabidopsis. New Phytol. 2013, 197, 805–814. [Google Scholar] [CrossRef] [Green Version]
- Harwood, J. Fatty acid metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1988, 39, 101–138. [Google Scholar] [CrossRef]
- Shanklin, J.; Cahoon, E.B. Desaturation and related modifications of fatty acids. Annu. Rev. Plant Biol. 1998, 49, 611–641. [Google Scholar] [CrossRef] [Green Version]
- Vidugiriene, J.; Menon, A.K. Biosynthesis of glycosylphosphatidylinositol anchors. Methods Enzymol. 1995, 250, 513–535. [Google Scholar]
- Oxley, D.; Bacic, A. Structure of the glycosylphosphatidylinositol anchor of an arabinogalactan protein from Pyrus communis suspension-cultured cells. Proc. Natl. Acad. Sci. USA 1999, 96, 14246–14251. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, T.; Fujita, M. Biosynthesis of GPI-anchored proteins: Special emphasis on GPI lipid remodeling. J. Lipid Res. 2016, 57, 6–24. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K. Glycosylphosphatidylinositol-anchored proteins in Arabidopsis and one of their common roles in signaling transduction. Front. Plant Sci. 2019, 10, 1022. [Google Scholar] [CrossRef] [Green Version]
- Takeda, J.; Kinoshita, T. GPI-anchor biosynthesis. Trends Biochem. Sci. 1995, 20, 367–371. [Google Scholar] [CrossRef]
- Eisenhaber, B.; Maurer-Stroh, S.; Novatchkova, M.; Schneider, G.; Eisenhaber, F. Enzymes and auxiliary factors for GPI lipid anchor biosynthesis and post-translational transfer to proteins. Bioessays 2003, 25, 367–385. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 2008, 9, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Cozier, G.E.; Carlton, J.; Bouyoucef, D.; Cullen, P.J. Membrane targeting by pleckstrin homology domains. Curr. Top. Microbiol. Immunol. 2003, 282, 49–88. [Google Scholar]
- Tichá, M.; Richter, H.; Ovečka, M.; Maghelli, N.; Hrbáčková, M.; Dvořák, P.; Šamaj, J.; Šamajová, O. Advanced microscopy reveals complex developmental and subcellular localization patterns of ANNEXIN 1 in Arabidopsis. Front. Plant Sci. 2020, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Ben Saad, R.; Ben Romdhane, W.; Ben Hsouna, A.; Mihoubi, W.; Harbaoui, M.; Brini, F. Insights into plant annexins function in abiotic and biotic stress tolerance. Plant Signal. Behav. 2020, 15, e1699264. [Google Scholar] [CrossRef] [PubMed]
- Pont-Lezica, R.F.; McNally, J.G.; Pickard, B.G. Wall-to-membrane linkers in onion epidermis: Some hypotheses. Plant. Cell Environ. 1993, 16, 111–123. [Google Scholar] [CrossRef]
- Rui, Y.; Dinneny, J.R. A wall with integrity: Surveillance and maintenance of the plant cell wall under stress. New Phytol. 2020, 225, 1428–1439. [Google Scholar] [CrossRef] [Green Version]
- Hoang, X.L.T.; Nguyen, Y.-N.H.; Thao, N.P.; Tran, L.-S.P. NAC transcription factors in drought and salinity tolerance. In Salt and Drought Stress Tolerance in Plants, Signaling and Communication in Plants; Hasanuzzaman, M., Tanveer, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 351–366. ISBN 9783030402778. [Google Scholar]
- Duan, M.; Zhang, R.; Zhu, F.; Zhang, Z.; Gou, L.; Wen, J.; Dong, J.; Wang, T. A lipid-anchored NAC transcription factor is translocated into the nucleus and activates Glyoxalase I expression during drought stress. Plant Cell 2017, 29, 1748–1772. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Deshmukh, R.; Muthamilarasan, M.; Rani, R.; Prasad, M. Versatile roles of aquaporin in physiological processes and stress tolerance in plants. Plant Physiol. Biochem. 2020, 149, 178–189. [Google Scholar] [CrossRef]
- Kapilan, R.; Vaziri, M.; Zwiazek, J.J. Regulation of aquaporins in plants under stress. Biol. Res. 2018, 51, 4. [Google Scholar] [CrossRef]
- Lopez, D.; Venisse, J.S.; Fumanal, B.; Chaumont, F.; Guillot, E.; Daniels, M.J.; Cochard, H.; Julien, J.L.; Gousset-Dupont, A. Aquaporins and leaf hydraulics: Poplar sheds new light. Plant Cell Physiol. 2013, 54, 1963–1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, T.T.H.; Hoang, T.G.; Trinh, D.C.; Bureau, C.; Meynard, D.; Vernet, A.; Ingouff, M.; Do, N.V.; Périn, C.; Guiderdoni, E.; et al. Sub-cellular markers highlight intracellular dynamics of membrane proteins in response to abiotic treatments in rice. Rice 2018, 11, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boursiac, Y.; Chen, S.; Luu, D.T.; Sorieul, M.; Van Den Dries, N.; Maurel, C. Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef] [PubMed]
- Leng, H.; Jiang, C.; Song, X.; Lu, M.; Wan, X. Poplar aquaporin PIP1;1 promotes Arabidopsis growth and development. BMC Plant Biol. 2021, 21, 253. [Google Scholar] [CrossRef] [PubMed]
- Bohnert, H.J.; Cushman, J.C. The ice plant cometh: Lessons in abiotic stress tolerance. J. Plant Growth Regul. 2000, 19, 334–346. [Google Scholar] [CrossRef]
- Vera-Estrella, R.; Barkla, B.J.; Bohnert, H.J.; Pantoja, O. Novel regulation of aquaporins during osmotic stress. Plant Physiol. 2004, 135, 2318–2329. [Google Scholar] [CrossRef] [Green Version]
- Ariani, A.; Barozzi, F.; Sebastiani, L.; di Toppi, L.S.; di Sansebastiano, G.P.; Andreucci, A. AQUA1 is a mercury sensitive poplar aquaporin regulated at transcriptional and post-translational levels by Zn stress. Plant Physiol. Biochem. 2019, 135, 588–600. [Google Scholar] [CrossRef]
- Leshem, Y.; Seri, L.; Levine, A. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance. Plant J. 2007, 51, 185–197. [Google Scholar] [CrossRef]
- Luu, D.-T.; Martinière, A.; Sorieul, M.; Runions, J.; Maurel, C. Fluorescence recovery after photobleaching reveals high cycling dynamics of plasma membrane aquaporins in Arabidopsis roots under salt stress. Plant J. 2012, 69, 894–905. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Yang, Y.; Li, R.; He, Q.; Fang, X.; Luu, D.-T.; Maurel, C.; Lin, J. Single-molecule analysis of PIP2;1 dynamics and partitioning reveals multiple modes of Arabidopsis plasma membrane aquaporin regulation. Plant Cell 2011, 23, 3780–3797. [Google Scholar] [CrossRef] [Green Version]
- Boursiac, Y.; Boudet, J.; Postaire, O.; Luu, D.-T.; Tournaire-Roux, C.; Maurel, C. Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J. 2008, 56, 207–218. [Google Scholar] [CrossRef]
- Dhonukshe, P.; Aniento, F.; Hwang, I.; Robinson, D.G.; Mravec, J.; Stierhof, Y.-D.; Frimi, J. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol. 2007, 17, 520–527. [Google Scholar] [CrossRef]
- Ueda, M.; Tsutsumi, N.; Fujimoto, M. Salt stress induces internalization of plasma membrane aquaporin into the vacuole in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2016, 474, 742–746. [Google Scholar] [CrossRef]
- Ji, H.; Pardo, J.M.; Batelli, G.; Van Oosten, M.J.; Bressan, R.A.; Li, X. The salt overly sensitive (SOS) pathway: Established and emerging roles. Mol. Plant 2013, 6, 275–286. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Q.-S.; Guo, Y.; Dietrich, M.A.; Schumaker, K.S.; Zhu, J.-K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 2002, 99, 8436–8441. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ishitani, M.; Halfter, U.; Kim, C.-S.; Zhu, J.-K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 2000, 97, 3730–3734. [Google Scholar] [CrossRef]
- Halfter, U.; Ishitani, M.; Zhu, J.-K. The Arabidopsis SOS2 protein kinase physically interacts with and is activated by the calcium-binding protein SOS3. Proc. Natl. Acad. Sci. USA 2000, 97, 3735–3740. [Google Scholar] [CrossRef]
- Ishitani, M.; Liu, J.; Halfter, U.; Kim, C.-S.; Shi, W.; Zhu, J.-K. SOS3 function in plant salt tolerance requires N-myristoylation and calcium binding. Plant Cell 2000, 12, 1667–1677. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Halfter, U.; Ishitani, M.; Zhu, J.-K. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 2001, 13, 1383–1399. [Google Scholar] [CrossRef] [Green Version]
- Quan, R.; Lin, H.; Mendoza, I.; Zhang, Y.; Cao, W.; Yang, Y.; Shang, M.; Chen, S.; Pardo, J.M.; Guo, Y. SCABP8/CBL10, a putative calcium Sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 2007, 19, 1415–1431. [Google Scholar] [CrossRef] [Green Version]
- Lou, L.; Yu, F.; Tian, M.; Liu, G.; Wu, Y.; Wu, Y.; Xia, R.; Pardo, J.M.; Guo, Y.; Xie, Q. ESCRT-I component VPS23A sustains salt tolerance by strengthening the SOS module in Arabidopsis. Mol. Plant 2020, 13, 1134–1148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Qi-Jun, M.; Zhong, M.-S.; Gao, H.-N.; Li, Y.-Y.; Hao, Y.-J. The apple palmitoyltransferase MdPAT16 influences sugar content and salt tolerance via an MdCBL1–MdCIPK13–MdSUT2.2 pathway. Plant J. 2021, 106, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Suzuki, S. Environmental stresses induce misfolded protein aggregation in plant cells in a microtubule-dependent manner. Int. J. Mol. Sci. 2013, 14, 7771–7783. [Google Scholar] [CrossRef] [PubMed]
- Baniwal, S.K.; Bharti, K.; Chan, K.Y.; Fauth, M.; Arnab, G.; Kotak, S.; Mishra, S.K.; Nover, L.; Port, M.; Scharf, K.-D.; et al. Heat stress response in plants: A complex game with chaperones and more than twenty heat stress transcription factors. J. Biosci. 2004, 29, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, J.; Ranjan, K.; Kumar, P.; Khanna, S.; Gupta, M.; Kumar, V.; Wani, S.H.; Sirohi, A. Heat shock proteins: Master players for heat-stress tolerance in plants during climate change. In Heat Stress Tolerance in Plants: Physiological, Molecular and Genetic Perspectives; Wani, S.H., Kumar, V., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 9781119432364. [Google Scholar]
- Zhu, J.K.; Bressan, R.A.; Hasegawa, P.M. Isoprenylation of the plant molecular chaperone ANJ1 facilitates membrane association and function at high temperature. Proc. Natl. Acad. Sci. USA 1993, 90, 8557–8561. [Google Scholar] [CrossRef] [Green Version]
- Preisig-Müller, R.; Muster, G.; Kindl, H. Heat shock enhances the amount of prenylated Dnaj protein at membranes of glyoxysomes. Eur. J. Biochem. 1994, 219, 57–63. [Google Scholar] [CrossRef]
- Wu, J.R.; Wang, T.Y.; Weng, C.P.; Duong, N.K.T.; Wu, S.J. AtJ3, a specific HSP40 protein, mediates protein farnesylation-dependent response to heat stress in Arabidopsis. Planta 2019, 250, 1449–1460. [Google Scholar] [CrossRef]
- Barghetti, A.; Sjögren, L.; Floris, M.; Paredes, E.B.; Wenkel, S.; Brodersen, P. Heat-shock protein 40 is the key farnesylation target in meristem size control, abscisic acid signaling, and drought resistance. Genes Dev. 2017, 31, 2282–2295. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.Y.; Wu, J.R.; Duong, N.K.T.; Lu, C.A.; Yeh, C.H.; Wu, S.J. HSP70-4 and farnesylated AtJ3 constitute a specific HSP70/HSP40-based chaperone machinery essential for prolonged heat stress tolerance in Arabidopsis. J. Plant Physiol. 2021, 261, 153430. [Google Scholar] [CrossRef]
- Wu, J.R.; Wang, L.C.; Lin, Y.R.; Weng, C.P.; Yeh, C.H.; Wu, S.J. The Arabidopsis heat-intolerant 5 (hit5)/enhanced response to aba 1 (era1) mutant reveals the crucial role of protein farnesylation in plant responses to heat stress. New Phytol. 2017, 213, 1181–1193. [Google Scholar] [CrossRef]
- Li, G.L.; Chang, H.; Li, B.; Zhou, W.; Sun, D.Y.; Zhou, R.G. The roles of the atDjA2 and atDjA3 molecular chaperone proteins in improving thermotolerance of Arabidopsis thaliana seedlings. Plant Sci. 2007, 173, 408–416. [Google Scholar] [CrossRef]
- Sjögren, L.; Floris, M.; Barghetti, A.; Völlmy, F.; Linding, R.; Brodersen, P. Farnesylated heat shock protein 40 is a component of membrane-bound RISC in Arabidopsis. J. Biol. Chem. 2019, 293, 16608–16622. [Google Scholar] [CrossRef]
- Gao, H.; Brandizzi, F.; Benning, C.; Larkin, R.M. A membrane-tethered transcription factor defines a branch of the heat stress response in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 16397–16403. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhang, S.S.; Lu, S.J.; Liu, J.X. Site-1 protease cleavage site is important for the ER stress-induced activation of membrane-associated transcription factor bZIP28 in Arabidopsis. Sci. China Life Sci. 2015, 58, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.H.; Lyu, Y.S.; Yang, W.; Yang, Z.T.; Lu, S.J.; Liu, J.X. A membrane-associated NAC transcription factor OsNTL3 is involved in thermotolerance in rice. Plant Biotechnol. J. 2020, 18, 1317–1329. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Humbert, S.; Liu, J.X.; Srivastava, R.; Rothstein, S.J.; Howell, S.H. Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 7247–7252. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.J.; Yang, Z.T.; Sun, L.; Sun, L.; Song, Z.T.; Liu, J.X. Conservation of IRE1-regulated bZIP74 mRNA unconventional splicing in rice (Oryza sativa L.) involved in ER stress responses. Mol. Plant 2012, 5, 504–514. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.S.; Park, J.H.; Wi, S.D.; Chae, H.B.; Paeng, S.K.; Bae, S.B.; Phan, K.A.T.; Kim, M.G.; Kwak, S.S.; Kim, W.Y.; et al. Demyristoylation of the cytoplasmic redox protein trx-h2 is critical for inducing a rapid cold stress response in plants. Antioxidants 2021, 10, 1287. [Google Scholar] [CrossRef]
- Lee, E.S.; Park, J.H.; Wi, S.D.; Kang, C.H.; Chi, Y.H.; Chae, H.B.; Paeng, S.K.; Ji, M.G.; Kim, W.Y.; Kim, M.G.; et al. Redox-dependent structural switch and CBF activation confer freezing tolerance in plants. Nat. Plants 2021, 7, 914–922. [Google Scholar] [CrossRef]
- Ding, Y.; Lv, J.; Shi, Y.; Gao, J.; Hua, J.; Song, C.; Gong, Z.; Yang, S. EGR2 phosphatase regulates OST1 kinase activity and freezing tolerance in Arabidopsis. EMBO J. 2019, 38, e99819. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, A.; Mattei, M.; Aducci, P.; Visconti, S.; Camoni, L. The salt tolerance related protein (STRP) mediates cold stress responses and abscisic acid signalling in Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Saidi, Y.; Finka, A.; Muriset, M.; Bromberg, Z.; Weiss, Y.G.; Maathuis, F.J.M.; Goloubinoff, P. The heat shock response in moss plants is regulated by specific calcium-permeable channels in the plasma membrane. Plant Cell 2009, 21, 2829–2843. [Google Scholar] [CrossRef] [PubMed]
- Monroy, A.F.; Sarhan, F.; Dhindsa, R.S. Cold-induced changes in freezing tolerance, protein phosphorylation, and gene expression: Evidence for a role of calcium. Plant Physiol. 1993, 102, 1227–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knight, H. Calcium signaling during abiotic stress in plants. Int. Rev. Cytol. 1999, 195, 269–324. [Google Scholar]
- Liu, Y.; Xu, C.; Zhu, Y.; Zhang, L.; Chen, T.; Zhou, F.; Chen, H.; Lin, Y. The calcium-dependent kinase OsCPK24 functions in cold stress responses in rice. J. Integr. Plant Biol. 2018, 60, 173–188. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Feng, X.; Yao, L.; Ding, C.; Lei, L.; Hao, X.; Li, N.; Zeng, J.; Yang, Y.; Wang, X. Characterization of CBL–CIPK signaling complexes and their involvement in cold response in tea plant. Plant Physiol. Biochem. 2020, 154, 195–203. [Google Scholar] [CrossRef]
- Ludwig, A.A.; Romeis, T.; Jones, J.D.G. CDPK-mediated signalling pathways: Specificity and cross-talk. J. Exp. Bot. 2004, 55, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Wu, C.; Luo, C.; Wei, M.; Qu, S.; Wang, S. Overexpression of MdCPK1a gene, a calcium dependent protein kinase in apple, increase tobacco cold tolerance via scavenging ROS accumulation. PLoS ONE 2020, 15, e0242139. [Google Scholar] [CrossRef]
- Tao, W.; Lijuan, L.; Zeyu, L.; Lianguang, S.; Quan, W. Cloning and characterization of protein prenyltransferase alpha subunit in rice. Rice Sci. 2021, 28, 557–566. [Google Scholar] [CrossRef]
- Tsai, T.M.; Chen, Y.R.; Kao, T.W.; Tsay, W.S.; Wu, C.P.; Huang, D.D.; Chen, W.H.; Chang, C.C.; Huang, H.J. PaCDPK1, a gene encoding calcium-dependent protein kinase from orchid, Phalaenopsis amabilis, is induced by cold, wounding, and pathogen challenge. Plant Cell Rep. 2007, 26, 1899–1908. [Google Scholar] [CrossRef]
- Almadanim, M.C.; Alexandre, B.M.; Rosa, M.T.G.; Sapeta, H.; Leitão, A.E.; Ramalho, J.C.; Lam, T.K.T.; Negrão, S.; Abreu, I.A.; Oliveira, M.M. Rice calcium-dependent protein kinase OsCPK17 targets plasma membrane intrinsic protein and sucrose-phosphate synthase and is required for a proper cold stress response. Plant Cell Environ. 2017, 40, 1197–1213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawełek, A.; Duszyn, M.; Świeżawska, B.; Szmidt-Jaworska, A.; Jaworski, K. Transcriptional response of a novel HpCDPK1 kinase gene from Hippeastrum x hybr. to wounding and fungal infection. J. Plant Physiol. 2017, 216, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Hettenhausen, C.; Sun, G.; He, Y.; Zhuang, H.; Sun, T.; Qi, J.; Wu, J. Genome-wide identification of calcium-dependent protein kinases in soybean and analyses of their transcriptional responses to insect herbivory and drought stress. Sci. Rep. 2016, 6, 18973. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, F.; Zhu, S.; Li, X. The maize NBS-LRR gene ZmNBS25 enhances disease resistance in rice and Arabidopsis. Front. Plant Sci. 2018, 9, 1033. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, T.; Poovaiah, B.W. Calcium signaling-mediated plant response to cold stress. Int. J. Mol. Sci. 2018, 19, 3896. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Du, H.; Wang, Y.; Wang, H.; Yang, S.; Li, C.; Chen, N.; Yang, H.; Zhang, Y.; Zhu, Y.; et al. The calcium-dependent protein kinase ZmCDPK7 functions in heat-stress tolerance in maize. J. Integr. Plant Biol. 2021, 63, 510–527. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, S.; Qin, J.; Sun, C.; Liu, F. The lipid transfer protein OsLTPL159 is involved in cold tolerance at the early seedling stage in rice. Plant Biotechnol. J. 2020, 18, 756–769. [Google Scholar] [CrossRef]
- Hu, X.; Wu, L.; Zhao, F.; Zhang, D.; Li, N.; Zhu, G.; Li, C.; Wang, W. Phosphoproteomic analysis of the response of maize leaves to drought, heat and their combination stress. Front. Plant Sci. 2015, 6, 298. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Che, Z.; Zeng, X.; Zhou, X.; Sitoe, H.M.; Wang, H.; Yu, D. Genome-wide analysis of calcium-dependent protein kinases and their expression patterns in response to herbivore and wounding stresses in soybean. Funct. Integr. Genom. 2016, 16, 481–493. [Google Scholar] [CrossRef]
- Kanchiswamy, C.N.; Takahashi, H.; Quadro, S.; Maffei, M.E.; Bossi, S.; Bertea, C.; Zebelo, S.A.; Muroi, A.; Ishihama, N.; Yoshioka, H.; et al. Regulation of Arabidopsis defense responses against Spodoptera littoralis by CPK-mediated calcium signaling. BMC Plant Biol. 2010, 10, 97. [Google Scholar] [CrossRef] [Green Version]
- Chico, J.M.; Raíces, M.; Téllez-Iñón, M.T.; Ulloa, R.M. A calcium-dependent protein kinase is systemically induced upon wounding in tomato plants. Plant Physiol. 2002, 128, 256–270. [Google Scholar] [CrossRef]
- Szczegielniak, J.; Klimecka, M.; Liwosz, A.; Ciesielski, A.; Kaczanowski, S.; Dobrowolska, G.; Harmon, A.C.; Muszyńska, G. A wound-responsive and phospholipid-regulated maize calcium-dependent protein kinase. Plant Physiol. 2005, 139, 1970–1983. [Google Scholar] [CrossRef] [Green Version]
- Romeis, T.; Piedras, P.; Jones, J.D.G. Resistance gene-dependent activation of a calcium-dependent protein kinase in the plant defense response. Plant Cell 2000, 12, 803–815. [Google Scholar] [CrossRef]
- Cheung, M.Y.; Zeng, N.Y.; Tong, S.W.; Li, W.Y.F.; Xue, Y.; Zhao, K.J.; Wang, C.; Zhang, Q.; Fu, Y.; Sun, Z.; et al. Constitutive expression of a rice GTPase-activating protein induces defense responses. New Phytol. 2008, 179, 530–545. [Google Scholar] [CrossRef]
- Cheung, M.Y.; Xue, Y.; Zhou, L.; Li, M.W.; Sun, S.S.M.; Lam, H.M. An ancient P-loop GTPase in rice is regulated by a higher plant-specific regulatory protein. J. Biol. Chem. 2010, 285, 37359–37369. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.Y.; Koo, Y.D.; Jin, J.B.; Moon, B.C.; Kang, C.H.; Kim, S.T.; Park, B.O.; Lee, S.Y.; Kim, M.L.; Hwang, I.; et al. Rice C2-domain proteins are induced and translocated to the plasma membrane in response to a fungal elicitor. Biochemistry 2003, 42, 11625–11633. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, Y.F.; Li, Y.M.; Dong, Y.L.; Huang, X.L.; Yu, Y.T.; Wang, J.M.; Wang, X.M.; Wang, X.J.; Kang, Z.S. Characterization of a wheat C2 domain protein encoding gene regulated by stripe rust and abiotic stresses. Biol. Plant. 2013, 57, 701–710. [Google Scholar] [CrossRef]
- Medina-Puche, L.; Tan, H.; Dogra, V.; Wu, M.; Rosas-Diaz, T.; Wang, L.; Ding, X.; Zhang, D.; Fu, X.; Kim, C.; et al. A defense pathway linking plasma membrane and chloroplasts and co-opted by pathogens. Cell 2020, 182, 1109–1124.e25. [Google Scholar] [CrossRef]
- Nomura, H.; Komori, T.; Uemura, S.; Kanda, Y.; Shimotani, K.; Nakai, K.; Furuichi, T.; Takebayashi, K.; Sugimoto, T.; Sano, S.; et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 2012, 3, 926. [Google Scholar] [CrossRef] [Green Version]
- Arimura, G.I.; Maffei, M.E. Calcium and secondary CPK signaling in plants in response to herbivore attack. Biochem. Biophys. Res. Commun. 2010, 400, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Chiappetta, A.; Bruno, L.; Bitonti, M.B. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3544. [Google Scholar]
- Mansoor, S.; Wani, O.A.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Tao, Y.; Wang, Z.; Shen, D.; Dong, H. Transmembrane helices 2 and 3 determine the localization of plasma membrane intrinsic proteins in eukaryotic cells. Front. Plant Sci. 2020, 10, 1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wudick, M.M.; Li, X.; Valentini, V.; Geldner, N.; Chory, J.; Lin, J.; Maurel, C.; Luu, D.-T. Subcellular redistribution of root aquaporins induced by hydrogen peroxide. Mol. Plant 2015, 8, 1103–1114. [Google Scholar] [CrossRef]
- Del Rodríguez-Gacio, M.C.; Matilla-Vázquez, M.A.; Matilla, A.J. Seed dormancy and ABA signaling: The breakthrough goes on. Plant Signal. Behav. 2009, 4, 1035–1048. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.S.P.; Urao, T.; Qin, F.; Maruyama, K.; Kakimoto, T.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 20623–20628. [Google Scholar] [CrossRef] [Green Version]
- Máthé, C.; Garda, T.; Freytag, C.; M-Hamvas, M. The role of serine-threonine protein phosphatase PP2A in plant oxidative stress signaling-facts and hypotheses. Int. J. Mol. Sci. 2019, 20, 3028. [Google Scholar] [CrossRef] [Green Version]
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-Y.; Fung, P.; Nishimura, N.; Jensen, D.R.; Fujii, H.; Zhao, Y.; Lumba, S.; Santiago, J.; Rodrigues, A.; Chow, T.-F.F.; et al. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 2009, 324, 1068–1071. [Google Scholar] [CrossRef] [Green Version]
- Belda-Palazon, B.; Julian, J.; Coego, A.; Wu, Q.; Zhang, X.; Batistic, O.; Alquraishi, S.A.; Kudla, J.; An, C.; Rodriguez, P.L. ABA inhibits myristoylation and induces shuttling of the RGLG1 E3 ligase to promote nuclear degradation of PP2CA. Plant J. 2019, 98, 813–825. [Google Scholar] [CrossRef]
- Clause, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Biol. 1998, 49, 427–451. [Google Scholar] [CrossRef] [Green Version]
- Su, B.; Zhang, X.; Li, L.; Abbas, S.; Yu, M.; Cui, Y.; Baluška, F.; Hwang, I.; Shan, X.; Lin, J. Dynamic spatial reorganization of BSK1 complexes in the plasma membrane underpins signal-specific activation for growth and immunity. Mol. Plant 2021, 14, 588–603. [Google Scholar] [CrossRef]
- Su, B.; Wang, A.; Shan, X. The role of N-myristoylation in homeostasis of brassinosteroid signaling kinase 1. Planta 2022, 255, 73. [Google Scholar] [CrossRef]
- Wurzinger, B.; Nukarinen, E.; Nägele, T.; Weckwerth, W.; Teige, M. The SnRK1 kinase as central mediator of energy signaling between different organelles. Plant Physiol. 2018, 176, 1085–1094. [Google Scholar] [CrossRef]
- Pierre, M.; Traverso, J.A.; Boisson, B.; Domenichini, S.; Bouchez, D.; Giglione, C.; Meinnel, T. N-myristoylation regulates the SnRK1 pathway in arabidopsis. Plant Cell 2007, 19, 2804–2821. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Concepción, M.; Yalovsky, S.; Zik, M.; Fromm, H.; Gruissem, W. The prenylation status of a novel plant calmodulin directs plasma membrane or nuclear localization of the protein. EMBO J. 1999, 18, 1996–2007. [Google Scholar] [CrossRef] [Green Version]
- Wolff, H.; Jakoby, M.; Stephan, L.; Koebke, E.; Hülskamp, M. Heat stress-dependent association of membrane trafficking proteins with mRNPs is selective. Front. Plant Sci. 2021, 12, 670499. [Google Scholar] [CrossRef]
- Barth, O.; Zschiesche, W.; Siersleben, S.; Humbeck, K. Isolation of a novel barley cDNA encoding a nuclear protein involved in stress response and leaf senescence. Physiol. Plant. 2004, 121, 282–293. [Google Scholar] [CrossRef]
- Barth, O.; Vogt, S.; Uhlemann, R.; Zschiesche, W.; Humbeck, K. Stress induced and nuclear localized HIPP26 from Arabidopsis thaliana interacts via its heavy metal associated domain with the drought stress related zinc finger transcription factor ATHB29. Plant Mol. Biol. 2009, 69, 213–226. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ku, Y.-S.; Cheng, S.-S.; Cheung, M.-Y.; Law, C.-H.; Lam, H.-M. The Re-Localization of Proteins to or Away from Membranes as an Effective Strategy for Regulating Stress Tolerance in Plants. Membranes 2022, 12, 1261. https://doi.org/10.3390/membranes12121261
Ku Y-S, Cheng S-S, Cheung M-Y, Law C-H, Lam H-M. The Re-Localization of Proteins to or Away from Membranes as an Effective Strategy for Regulating Stress Tolerance in Plants. Membranes. 2022; 12(12):1261. https://doi.org/10.3390/membranes12121261
Chicago/Turabian StyleKu, Yee-Shan, Sau-Shan Cheng, Ming-Yan Cheung, Cheuk-Hin Law, and Hon-Ming Lam. 2022. "The Re-Localization of Proteins to or Away from Membranes as an Effective Strategy for Regulating Stress Tolerance in Plants" Membranes 12, no. 12: 1261. https://doi.org/10.3390/membranes12121261
APA StyleKu, Y.-S., Cheng, S.-S., Cheung, M.-Y., Law, C.-H., & Lam, H.-M. (2022). The Re-Localization of Proteins to or Away from Membranes as an Effective Strategy for Regulating Stress Tolerance in Plants. Membranes, 12(12), 1261. https://doi.org/10.3390/membranes12121261