Advances in Purification, Modification, and Application of Extracellular Vesicles for Novel Clinical Treatments
Abstract
1. Introduction
2. Constitution and Characterization of Exosomes
3. Molecular Regulation and Biomarkers with Exosome in Diseases
4. Applications of Exosome as Novel Drug Delivery System
5. Purification Methods and Drawbacks of Exosome
6. Application of Exosome in Plant and Food as DDS
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bazzan, E.; Tinè, M.; Casara, A.; Biondini, D.; Semenzato, U.; Cocconcelli, E.; Balestro, E.; Damin, M.; Radu, C.M.; Turato, G.; et al. Critical Review of the Evolution of Extracellular Vesicles’ Knowledge: From 1946 to Today. Int. J. Mol. Sci. 2021, 22, 6417. [Google Scholar] [CrossRef]
- Osaki, M.; Okada, F. Exosomes and Their Role in Cancer Progression. Yonago Acta. Med. 2019, 62, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Soleymaninejadian, E. Exosome a Story from Waste to Become a Gold Mine. Jenta. J. Cell. Mol. Biol. 2020, 11, e107622. [Google Scholar] [CrossRef]
- Negrete-García, M.C.; de Jesús Ramos-Abundis, J.; Alvarado-Vasquez, N.; Montes-Martínez, E.; Montaño, M.; Ramos, C.; Sommer, B. Exosomal Micro-RNAs as Intercellular Communicators in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2022, 23, 11047. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Sun, R.; Song, X.; Gu, W.; Shao, Y. Mechanism and clinical value of exosomes and exosomal contents in regulating solid tumor radiosensitivity. J. Transl. Med. 2022, 20, 189. [Google Scholar] [CrossRef]
- Xu, Y.X.; Pu, S.D.; Li, X.; Yu, Z.W.; Zhang, Y.T.; Tong, X.W.; Shan, Y.Y.; Gao, X.Y. Exosomal ncRNAs: Novel therapeutic target and biomarker for diabetic complications. Pharmacol. Res. 2022; in press. [Google Scholar] [CrossRef]
- Ipinmoroti, A.O.; Pandit, R.; Matthews, Q.L. Regenerative mesenchymal stem cell-derived extracellular vesicles: A potential alternative to cell-based therapy in viral infection and disease damage control. WIREs Mech. Dis. 2022, 14, e1574. [Google Scholar] [CrossRef]
- Meng, F.; Xue, X.; Yin, Z.; Gao, F.; Wang, X.; Geng, Z. Research Progress of Exosomes in Bone Diseases: Mechanism, Diagnosis and Therapy. Front. Bioeng. Biotechnol. 2022, 10, 866627. [Google Scholar] [CrossRef]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef]
- Pan, Z.; Zhao, R.; Li, B.; Qi, Y.; Qiu, W.; Guo, Q.; Zhang, S.; Zhao, S.; Xu, H.; Li, M.; et al. EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3. Mol. Cancer 2022, 21, 16. [Google Scholar] [CrossRef]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Liaw, W.S.; Chen, C.A.; Zhou, Q.A. Exosomes─Nature’s Lipid Nanoparticles, a Rising Star in Drug Delivery and Diagnostics. ACS Nano. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Schmidt, K.F.; Farhoud, M.; Zi, T.; Jang, S.C.; Dooley, K.; Kentala, D.; Dobson, H.; Economides, K.; Williams, D.E. In vivo tracking of [89Zr]Zr-labeled engineered extracellular vesicles by PET reveals organ-specific biodistribution based upon the route of administration. Nucl. Med. Biol. 2022, 112–113, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Ogami, K.; Suzuki, H.I. Nuclear RNA Exosome and Pervasive Transcription: Dual Sculptors of Genome Function. Int. J. Mol. Sci. 2021, 22, 13401. [Google Scholar] [CrossRef]
- Jiang, X.; You, L.; Zhang, Z.; Cui, X.; Zhong, H.; Sun, X.; Ji, C.; Chi, X. Biological Properties of Milk-Derived Extracellular Vesicles and Their Physiological Functions in Infant. Front. Cell Dev. Biol. 2021, 9, 693534. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.H.; Shao, S.L.; Guo, D.; Ge, L.N.; Wang, Z.; Liu, P.; Tao, Y.Y. Roles of microRNAs and exosomes in Helicobacter pylori associated gastric cancer. Mol. Biol. Rep. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, N.; Hu, X.; Wang, H. Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol. Cancer 2021, 20, 117. [Google Scholar] [CrossRef]
- Aimaletdinov, A.M.; Gomzikova, M.O. Tracking of Extracellular Vesicles’ Biodistribution: New Methods and Approaches. Int. J. Mol. Sci. 2022, 23, 11312. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Kee, L.T.; Al-Masawa, M.E.; Lee, Q.H.; Subramaniam, T.; Kok, D.; Ng, M.H.; Law, J.X. Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review. Int. J. Mol. Sci. 2022, 23, 7986. [Google Scholar] [CrossRef] [PubMed]
- Arifin, D.R.; Witwer, K.W.; Bulte, J.W.M. Non-Invasive imaging of extracellular vesicles: Quo vaditis in vivo? J. Extracell. Vesicles 2022, 11, e12241. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Therapeutic Strategy of Mesenchymal-Stem-Cell-Derived Extracellular Vesicles as Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 6480. [Google Scholar] [CrossRef] [PubMed]
- Vinaiphat, A.; Sze, S.K. Proteomics for comprehensive characterization of extracellular vesicles in neurodegenerative disease. Exp. Neurol. 2022, 355, 114149. [Google Scholar] [CrossRef] [PubMed]
- Avalos, P.N.; Forsthoefel, D.J. An Emerging Frontier in Intercellular Communication: Extracellular Vesicles in Regeneration. Front. Cell Dev. Biol. 2022, 10, 849905. [Google Scholar] [CrossRef]
- Trisko, J.; Fleck, J.; Kau, S.; Oesterreicher, J.; Holnthoner, W. Lymphatic and Blood Endothelial Extracellular Vesicles: A Story Yet to Be Written. Life 2022, 12, 654. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Chang, X.; Fang, L.; Wang, Z. Subgroups of Extracellular Vesicles: Can They Be Defined by “Labels?”. DNA Cell Biol. 2022, 41, 249–256. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Extracellular Vesicles and Thrombogenicity in Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 1774. [Google Scholar] [CrossRef]
- Soler-Botija, C.; Monguió-Tortajada, M.; Munizaga-Larroudé, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S. Mechanisms governing the therapeutic effect of mesenchymal stromal cell-derived extracellular vesicles: A scoping review of preclinical evidence. Biomed. Pharmacother. 2022, 147, 112683. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, Y.; Han, G. Advances of Mesenchymal Stem Cells Released Extracellular Vesicles in Periodontal Bone Remodeling. DNA Cell Biol. 2022, 41, 935–950. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.J.; Tang, Y.C.; Hao, X.Y.; Xu, W.J.; Xiang, D.X.; Wu, J.Y. Apoptotic bodies for advanced drug delivery and therapy. J. Control. Release 2022, 351, 394–406. [Google Scholar] [CrossRef]
- Collado, A.; Gan, L.; Tengbom, J.; Kontidou, E.; Pernow, J.; Zhou, Z. Extracellular vesicles and their non-coding RNA cargos: Emerging players in cardiovascular disease. J. Physiol. 2022; in press. [Google Scholar] [CrossRef]
- Ding, X.; Wang, X.; Du, J.; Han, Q.; Zhang, D.; Zhu, H. A systematic review and Meta-analysis of urinary extracellular vesicles proteome in diabetic nephropathy. Front. Endocrinol. 2022, 13, 866252. [Google Scholar] [CrossRef] [PubMed]
- Al-Koussa, H.; AlZaim, I.; El-Sabban, M.E. Pathophysiology of Coagulation and Emerging Roles for Extracellular Vesicles in Coagulation Cascades and Disorders. J. Clin. Med. 2022, 11, 4932. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yin, X.M. The Role of Extracellular Vesicles in Liver Pathogenesis. Am. J. Pathol. 2022, 192, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Pan, Y.; Yang, X.; Chen, Z.; Heng, Y.; Yang, B.; Pu, M.; Zuo, J.; Lai, Z.; Tang, Y.; et al. The Emerging Role of the Interaction of Extracellular Vesicle and Autophagy-Novel Insights into Neurological Disorders. J. Inflamm. Res. 2022, 15, 3395–3407. [Google Scholar] [CrossRef]
- Ginini, L.; Billan, S.; Fridman, E.; Gil, Z. Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate. Cells 2022, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Gul, B.; Syed, F.; Khan, S.; Iqbal, A.; Ahmad, I. Characterization of extracellular vesicles by flow cytometry: Challenges and promises. Micron 2022, 161, 103341. [Google Scholar] [CrossRef] [PubMed]
- Awoyemi, T.; Iaccarino, D.A.; Motta-Mejia, C.; Raiss, S.; Kandzija, N.; Zhang, W.; Vatish, M. Neuropilin-1 is uniquely expressed on small syncytiotrophoblast extracellular vesicles but not on medium/large vesicles from preeclampsia and normal placentae. Biochem. Biophys. Res. Commun. 2022, 619, 151–158. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borràs, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcón-Pérez, J.M.; et al. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles 2021, 10, e12093. [Google Scholar] [CrossRef]
- Börger, V.; Weiss, D.J.; Anderson, J.D.; Borràs, F.E.; Bussolati, B.; Carter, D.R.F.; Dominici, M.; Falcón-Pérez, J.M.; Gimona, M.; Hill, A.F.; et al. International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: Considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy 2020, 22, 482–485. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 17, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Higginbotham, J.N.; Jeppesen, D.K.; Yang, Y.P.; Li, W.; McKinley, E.T.; Graves-Deal, R.; Ping, J.; Britain, C.M.; Dorsett, K.A.; et al. Transfer of Functional Cargo in Exomeres. Cell Rep. 2019, 27, 940–954.e6. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Kim, H.S.; Bojmar, L.; Gyan, K.E.; Cioffi, M.; Hernandez, J.; Zambirinis, C.P.; Rodrigues, G.; Molina, H.; Heissel, S.; et al. Extracellular Vesicle and Particle Biomarkers Define Multiple Human Cancers. Cell 2020, 182, 1044–1061.e18. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Franklin, J.L.; Crowe, J.E., Jr.; Coffey, R.J. Angiotensin-converting Enzyme 2-containing Small Extracellular Vesicles and Exomeres Bind the Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein. Gastroenterology 2021, 160, 958–961.e3. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.Y.; Sung, Y.C.; Chen, Y.J.; Chou, S.T.; Guo, V.; Chien, J.C.; Ko, J.J.; Yang, A.L.; Huang, H.C.; Chuang, J.C.; et al. Multiresolution Imaging Using Bioluminescence Resonance Energy Transfer Identifies Distinct Biodistribution Profiles of Extracellular Vesicles and Exomeres with Redirected Tropism. Adv. Sci. 2020, 7, 2001467. [Google Scholar] [CrossRef]
- Anand, S.; Samuel, M.; Mathivanan, S. Exomeres: A New Member of Extracellular Vesicles Family. Subcell. Biochem. 2021, 97, 89–97. [Google Scholar] [CrossRef]
- Bojmar, L.; Kim, H.S.; Tobias, G.C.; Pelissier Vatter, F.A.; Lucotti, S.; Gyan, K.E.; Kenific, C.M.; Wan, Z.; Kim, K.A.; Kim, D.; et al. Extracellular vesicle and particle isolation from human and murine cell lines, tissues, and bodily fluids. STAR Protoc. 2020, 2, 100225. [Google Scholar] [CrossRef]
- Liangsupree, T.; Multia, E.; Forssén, P.; Fornstedt, T.; Riekkola, M.L. Kinetics and interaction studies of anti-tetraspanin antibodies and ICAM-1 with extracellular vesicle subpopulations using continuous flow quartz crystal microbalance biosensor. Biosens. Bioelectron. 2022, 206, 114151. [Google Scholar] [CrossRef]
- An, H.J.; Cho, H.K.; Song, D.H.; Kee, C. Quantitative analysis of exosomes in the aqueous humor of Korean patients with pseudoexfoliation glaucoma. Sci. Rep. 2022, 12, 12875. [Google Scholar] [CrossRef]
- Liu, H.; Tian, Y.; Xue, C.; Niu, Q.; Chen, C.; Yan, X. Analysis of extracellular vesicle DNA at the single-vesicle level by nano-flow cytometry. J. Extracell. Vesicles 2022, 11, e12206. [Google Scholar] [CrossRef]
- Droste, M.; Tertel, T.; Jeruschke, S.; Dittrich, R.; Kontopoulou, E.; Walkenfort, B.; Börger, V.; Hoyer, P.F.; Büscher, A.K.; Thakur, B.K.; et al. Single Extracellular Vesicle Analysis Performed by Imaging Flow Cytometry and Nanoparticle Tracking Analysis Evaluate the Accuracy of Urinary Extracellular Vesicle Preparation Techniques Differently. Int. J. Mol. Sci. 2021, 22, 12436. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hanayama, R. TIM4-Affinity Methods Targeting Phosphatidylserine for Isolation or Detection of Extracellular Vesicles. Methods Mol. Biol. 2022, 2466, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Liu, X.; Yin, J.; Hao, L.; Diao, Y.; Zhong, J. Exosomes and autophagy in ocular surface and retinal diseases: New insights into pathophysiology and treatment. Stem Cell Res. Ther. 2022, 13, 174. [Google Scholar] [CrossRef] [PubMed]
- Choezom, D.; Gross, J.C. Neutral sphingomyelinase 2 controls exosome secretion by counteracting V-ATPase-mediated endosome acidification. J. Cell Sci. 2022, 135, jcs259324. [Google Scholar] [CrossRef]
- Bischoff, J.P.; Schulz, A.; Morrison, H. The role of exosomes in intercellular and inter-organ communication of the peripheral nervous system. FEBS Lett. 2022, 596, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Hsu, W.F.; Wo, A.M. Exosomes-Potential for Blood-Based Marker in Alzheimer’s Disease. Acta. Neurol. Taiwan 2022, 31, 1–6. [Google Scholar]
- Han, C.; Yang, J.; Sun, J.; Qin, G. Extracellular vesicles in cardiovascular disease: Biological functions and therapeutic implications. Pharmacol. Ther. 2022, 233, 108025. [Google Scholar] [CrossRef] [PubMed]
- Stotz, H.U.; Brotherton, D.; Inal, J. Communication is key: Extracellular vesicles as mediators of infection and defence during host-microbe interactions in animals and plants. EMS Microbiol. Rev. 2022, 46, fuab044. [Google Scholar] [CrossRef]
- Alptekin, A.; Parvin, M.; Chowdhury, H.I.; Rashid, M.H.; Arbab, A.S. Engineered exosomes for studies in tumor immunology. Immunol. Rev. 2022, 312, 76–102. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh, M.; Sokullu, E.; Saghati, S.; Karimipour, M.; Rahbarghazi, R. Insights into the Critical Role of Exosomes in the Brain; from Neuronal Activity to Therapeutic Effects. Mol. Neurobiol. 2022, 59, 4453–4465. [Google Scholar] [CrossRef]
- Liu, Q.W.; He, Y.; Xu, W.W. Molecular functions and therapeutic applications of exosomal noncoding RNAs in cancer. Exp. Mol. Med. 2022, 54, 216–225. [Google Scholar] [CrossRef]
- Safari, B.; Aghazadeh, M.; Davaran, S.; Roshangar, L. Exosome-loaded hydrogels: A new cell-free therapeutic approach for skin regeneration. Eur. J. Pharm. Biopharm. 2022, 171, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.S.; Gandley, R.E.; Lackner, E.; Dolinish, A.; Ouyang, Y.; Powers, R.W.; Morelli, A.E.; Hubel, C.A.; Sadovsky, Y. Small extracellular vesicles from plasma of women with preeclampsia increase myogenic tone and decrease endothelium-dependent relaxation of mouse mesenteric arteries. Pregnancy Hypertens. 2022, 28, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.F.; Li, W.J.; Hu, K.S.; Gao, J.; Zhai, W.L.; Yang, J.H.; Zhang, S.J. Exosome biogenesis: Machinery, regulation, and therapeutic implications in cancer. Mol. Cancer 2022, 21, 207. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.J.; Diaz, A.; Jafari, M.; Khawaja, R.R.; Agullo-Pascual, E.; Santiago-Fernández, O.; Richards, A.L.; Chen, K.H.; Dmitriev, P.; Sun, Y.; et al. Reduced endosomal microautophagy activity in aging associates with enhanced exocyst-mediated protein secretion. Aging Cell 2022, 21, e13713. [Google Scholar] [CrossRef] [PubMed]
- Neves, K.B.; Rios, F.J.; Sevilla-Montero, J.; Montezano, A.C.; Touyz, R.M. Exosomes and the cardiovascular system: Role in cardiovascular health and disease. J. Physiol. 2022; in press. [Google Scholar] [CrossRef]
- Rashidi, M.; Bijari, S.; Khazaei, A.H.; Shojaei-Ghahrizjani, F.; Rezakhani, L. The role of milk-derived exosomes in the treatment of diseases. Front. Genet. 2022, 13, 1009338. [Google Scholar] [CrossRef] [PubMed]
- Carnino, J.M.; Lee, H. Extracellular vesicles in respiratory disease. Adv. Clin. Chem. 2022, 108, 105–127. [Google Scholar] [CrossRef]
- Li, S.; Chen, L. Exosomes in Pathogenesis, Diagnosis, and Treatment of Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 793432. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, K.; Ranjbar, M.; Pirpour Tazehkand, A.; Asgharian, P.; Montazersaheb, S.; Tarhriz, V.; Ghasemnejad, T. Evaluation of exosomal non-coding RNAs in cancer using high-throughput sequencing. J. Transl. Med. 2022, 20, 30. [Google Scholar] [CrossRef]
- Paramanantham, A.; Asfiya, R.; Das, S.; McCully, G.; Srivastava, A. Extracellular Vesicle (EVs) Associated Non-Coding RNAs in Lung Cancer and Therapeutics. Int. J. Mol. Sci. 2022, 23, 13637. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, N.; Faghihkhorasani, F.; Fakhr, S.S.; Moghaddam, P.R.; Yazdani, E.; Kheradmand, Z.; Rezaei-Tazangi, F.; Adelian, S.; Mobarak, H.; Hamblin, M.R.; et al. Tumor-derived exosomal non-coding RNAs as diagnostic biomarkers in cancer. Cell Mol. Life Sci. 2022, 79, 572. [Google Scholar] [CrossRef]
- Zhou, Y.; Xiao, Z.; Zhu, W. The roles of small extracellular vesicles as prognostic biomarkers and treatment approaches in triple-negative breast cancer. Front. Oncol. 2022, 12, 998964. [Google Scholar] [CrossRef] [PubMed]
- Sataer, X.; Qifeng, Z.; Yingying, Z.; Chunhua, H.; Bingzhenga, F.; Zhiran, X.; Wanli, L.; Yuwei, Y.; Shuangfeng, C.; Lingling, W.; et al. Exosomal microRNAs as diagnostic biomarkers and therapeutic applications in neurodegenerative diseases. Neurol. Res. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Dhar, R.; Mukherjee, S.; Mukerjee, N.; Mukherjee, D.; Devi, A.; Ashraf, G.M.; Alserihi, R.F.; Tayeb, H.H.; Hashem, A.M.; Alexiou, A.; et al. Interrelation between extracellular vesicles miRNAs with chronic lung diseases. J. Cell Physiol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Ozkocak, D.C.; Phan, T.K.; Poon, I.K.H. Translating extracellular vesicle packaging into therapeutic applications. Front. Immunol. 2022, 13, 946422. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, J.; Akbari, A.; Rahbarghazi, R. Inhibition of extracellular vesicle biogenesis in tumor cells: A possible way to reduce tumorigenesis. Cell Biochem. Funct. 2022, 40, 248–262. [Google Scholar] [CrossRef]
- Moon, B.; Chang, S. Exosome as a Delivery Vehicle for Cancer Therapy. Cells 2022, 11, 316. [Google Scholar] [CrossRef]
- Yang, L.; Patel, K.D.; Rathnam, C.; Thangam, R.; Hou, Y.; Kang, H.; Lee, K.B. Harnessing the Therapeutic Potential of Extracellular Vesicles for Biomedical Applications Using Multifunctional Magnetic Nanomaterials. Small 2022, 18, e2104783. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, U. Exosomes in cardiovascular diseases: A blessing or a sin for the mankind. Mol. Cell Biochem. 2022, 477, 833–847. [Google Scholar] [CrossRef]
- Sun, K.; Zheng, X.; Jin, H.; Yu, F.; Zhao, W. Exosomes as CNS Drug Delivery Tools and Their Applications. Pharmaceutics 2022, 14, 2252. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, R.; Hou, Y.; Wang, H.; Li, Y.; Zhu, J.; Fu, C. Application of mesenchymal stem cell-derived exosomes from different sources in intervertebral disc degeneration. Front. Bioeng. Biotechnol. 2022, 10, 1019437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, R.; Wang, J.; Hao, Y.; Xie, Q.; Wang, L.; Wang, X. Melatonin pretreatment on exosomes: Heterogeneity, therapeutic effects, and usage. Front. Immunol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Song, B.; Yuan, L.; Yang, G. Multiplexed strategies toward clinical translation of extracellular vesicles. Theranostics 2022, 12, 6740–6761. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, D.; Kim, W.; Lee, S.; Gwon, Y.; Park, S.; Kim, J. Therapeutic Strategies and Enhanced Production of Stem Cell-Derived Exosomes for Tissue Regeneration. Tissue Eng. Part. B Rev. 2022; in press. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Meng, H.; Liao, S.; Zhang, J.; Guan, Y.; Tian, H.; Peng, J. Research Progress on Exosomes in Osteonecrosis of the Femoral Head. Orthop. Surg. 2022, 14, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, T.; Zhou, M. Immune-Cell-Derived Exosomes for Cancer Therapy. Mol. Pharm. 2022, 19, 3042–3056. [Google Scholar] [CrossRef]
- Boyd-Gibbins, N.; Karagiannis, P.; Hwang, D.W.; Kim, S.I. iPSCs in NK Cell Manufacturing and NKEV Development. Front. Immunol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.; Al-Massri, K. New Approaches for Enhancement of the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cardiovascular Diseases. Tissue Eng. Regen. Med. 2022; in press. [Google Scholar] [CrossRef]
- Rezaie, J.; Nejati, V.; Mahmoodi, M.; Ahmadi, M. Mesenchymal stem cells derived extracellular vesicles: A promising nanomedicine for drug delivery system. Biochem. Pharmacol. 2022, 203, 115167. [Google Scholar] [CrossRef]
- Song, Q.; Yu, H.; Han, J.; Lv, J.; Lv, Q.; Yang, H. Exosomes in urological diseases—Biological functions and clinical applications. Cancer Lett. 2022, 544, 215809. [Google Scholar] [CrossRef] [PubMed]
- Hade, M.D.; Suire, C.N.; Mossell, J.; Suo, Z. Extracellular vesicles: Emerging frontiers in wound healing. Med. Res. Rev. 2022, 42, 2102–2125. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.; Wei, Z.; Gan, L.; Yang, X. Extracellular-Vesicle-Based Drug Delivery Systems for Enhanced Antitumor Therapies through Modulating the Cancer-Immunity Cycle. Adv. Mater. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, H.; Ghorbani, F.; Abbaspour-Aghdam, S.; Kamrani, A.; Valizadeh, H.; Nadiri, M.; Sadeghi, A.; Shamsasenjan, K.; Jadidi-Niaragh, F.; Roshangar, L.; et al. Chronic obstructive pulmonary disease and asthma: Mesenchymal stem cells and their extracellular vesicles as potential therapeutic tools. Stem Cell Res. Ther. 2022, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, X.; Zhong, Y.; Shen, J.; An, W. Biomimetic Exosomes: A New Generation of Drug Delivery System. Front. Bioeng. Biotechnol. 2022, 10, 865682. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, J.; Wang, S.; Zhou, A.; Zhao, G.; Li, P. Roles of small extracellular vesicles in the development, diagnosis and possible treatment strategies for hepatocellular carcinoma (Review). Int. J. Oncol. 2022, 61, 91. [Google Scholar] [CrossRef]
- Moayedfard, Z.; Sani, F.; Alizadeh, A.; Bagheri Lankarani, K.; Zarei, M.; Azarpira, N. The role of the immune system in the pathogenesis of NAFLD and potential therapeutic impacts of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2022, 13, 242. [Google Scholar] [CrossRef]
- Zhao, J.; An, Q.; Zhu, X.; Yang, B.; Gao, X.; Niu, Y.; Zhang, L.; Xu, K.; Ma, D. Research status and future prospects of extracellular vesicles in primary Sjögren’s syndrome. Stem Cell Res. Ther. 2022, 13, 230. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, H.; Guo, J.; Yuan, Y.; Ni, G. Effects of BMSC-Derived EVs on Bone Metabolism. Pharmaceutics 2022, 14, 1012. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, Q.; Jiang, L. Current Knowledge on Exosome Biogenesis, Cargo-Sorting Mechanism and Therapeutic Implications. Membranes 2022, 12, 498. [Google Scholar] [CrossRef]
- Xiong, Y.; Song, J.; Huang, X.; Pan, Z.; Goldbrunner, R.; Stavrinou, L.; Lin, S.; Hu, W.; Zheng, F.; Stavrinou, P. Exosomes Derived From Mesenchymal Stem Cells: Novel Effects in the Treatment of Ischemic Stroke. Front. Neurosci. 2022, 16, 899887. [Google Scholar] [CrossRef] [PubMed]
- Malekian, F.; Shamsian, A.; Kodam, S.P.; Ullah, M. Exosome engineering for efficient and targeted drug delivery: Current status and future perspective. J. Physiol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Goutas, D.; Pergaris, A.; Goutas, N.; Theocharis, S. Utilizing Exosomal-EPHs/Ephrins as Biomarkers and as a Potential Platform for Targeted Delivery of Therapeutic Exosomes. Int. J. Mol. Sci. 2022, 23, 3551. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dong, B.; Huang, L.; Zhou, J.; Huang, H. Research progress on the role and mechanism of action of exosomes in autoimmune thyroid disease. Int. Rev. Immunol. 2022; in press. [Google Scholar] [CrossRef]
- Chen, L.; Wang, L.; Zhu, L.; Xu, Z.; Liu, Y.; Li, Z.; Zhou, J.; Luo, F. Exosomes as Drug Carriers in Anti-Cancer Therapy. Front. Cell Dev. Biol. 2022, 10, 728616. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.C.; Li, C.; Zhang, W.; Pi, W.; Han, N. Potential Effects of Exosomes and their MicroRNA Carrier on Osteoporosis. Curr. Pharm. Des. 2022, 28, 899–909. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, W.; Ye, J.; Wang, Y. Potential Role of Exosomes in Ischemic Stroke Treatment. Biomolecules 2022, 12, 115. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L. The roles and therapeutic approaches of MSC-derived exosomes in colorectal cancer. Clin. Transl. Oncol. 2022, 24, 959–967. [Google Scholar] [CrossRef]
- He, J.; Ren, W.; Wang, W.; Han, W.; Jiang, L.; Zhang, D.; Guo, M. Exosomal targeting and its potential clinical application. Drug Deliv. Transl. Res. 2022, 12, 2385–2402. [Google Scholar] [CrossRef]
- Ren, J.; He, W.; Zheng, L.; Duan, H. From structures to functions: Insights into exosomes as promising drug delivery vehicles. Biomater. Sci. 2016, 4, 910–921. [Google Scholar] [CrossRef]
- Bie, N.; Yong, T.; Wei, Z.; Gan, L.; Yang, X. Extracellular vesicles for improved tumor accumulation and penetration. Adv. Drug Deliv. Rev. 2022, 188, 114450. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, Y.; Yashiro, R. Molecular Docking and Intracellular Translocation of Extracellular Vesicles for Efficient Drug Delivery. Int. J. Mol. Sci. 2022, 23, 12971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Gao, W.; Xie, N. Exosomes as Anticancer Drug Delivery Vehicles: Prospects and Challenges. Front. Biosci. (Landmark Ed) 2022, 27, 293. [Google Scholar] [CrossRef]
- Yang, F.; Wang, M.; Guan, X. Exosomes and mimics as novel delivery platform for cancer therapy. Front. Pharmacol. 2022, 13, 1001417. [Google Scholar] [CrossRef]
- Dalmizrak, A.; Dalmizrak, O. Mesenchymal stem cell-derived exosomes as new tools for delivery of miRNAs in the treatment of cancer. Front. Bioeng. Biotechnol. 2022, 10, 956563. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.A.; Mohammed, J.S.; Rabiu, S. Exosomes as Delivery Systems for Targeted Tumour Therapy: A Systematic Review and Meta-analysis of In vitro Studies. Pharm. Nanotechnol. 2022; in press. [Google Scholar] [CrossRef]
- Rezaie, J.; Etemadi, T.; Feghhi, M. The distinct roles of exosomes in innate immune responses and therapeutic applications in cancer. Eur. J. Pharmacol. 2022, 933, 175292. [Google Scholar] [CrossRef]
- Rezaie, J.; Feghhi, M.; Etemadi, T. A review on exosomes application in clinical trials: Perspective, questions, and challenges. Cell Commun. Signal. 2022, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Mansourabadi, A.H.; Aghamajidi, A.; Faraji, F.; Taghizadeh, S.; Mohamed Khosroshahi, L.; Bahramkiya, M.; Azimi, M. Mesenchymal stem cells- derived exosomes inhibit the expression of Aquaporin-5 and EGFR in HCT-116 human colorectal carcinoma cell line. BMC Mol. Cell Biol. 2022, 23, 40. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, L.; Li, B.; Li, Y.; Shen, T.; Zhao, B. The Exosome Journey: From Biogenesis to Regulation and Function in Cancers. J. Oncol. 2022; in press. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Y.; Huang, M.; Huang, Z.; Wang, Q.; Qing, L.; Li, L.; Xu, S.; Jia, B. Current Status, Opportunities, and Challenges of Exosomes in Oral Cancer Diagnosis and Treatment. Int. J. Nanomed. 2022, 17, 2679–2705. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhang, L.; Zhang, Y.; Lu, R. Plant-Derived Exosomes as a Drug-Delivery Approach for the Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Pharmaceutics 2022, 14, 822. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Thiruvengadam, M.; Venkidasamy, B.; Alomary, M.N.; Salawi, A.; Chung, I.M.; Shariati, M.A.; Rebezov, M. Exosome-based nanomedicine for cancer treatment by targeting inflammatory pathways: Current status and future perspectives. Semin. Cancer Biol. 2022, 86, 678–696. [Google Scholar] [CrossRef]
- Vahabi, A.; Rezaie, J.; Hassanpour, M.; Panahi, Y.; Nemati, M.; Rasmi, Y.; Nemati, M. Tumor Cells-derived exosomal CircRNAs: Novel cancer drivers, molecular mechanisms, and clinical opportunities. Biochem. Pharmacol. 2022, 200, 115038. [Google Scholar] [CrossRef]
- Jiang, J.; Li, J.; Zhou, X.; Zhao, X.; Huang, B.; Qin, Y. Exosomes Regulate the Epithelial-Mesenchymal Transition in Cancer. Front. Oncol. 2022, 12, 864980. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, K.; Zhao, H.; Di, L.; Wang, L.; Wang, R. Tumor-derived extracellular vesicles as messengers of natural products in cancer treatment. Theranostics 2022, 12, 1683–1714. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Rathore, S.; Munshi, A.; Ramesh, R. Organically derived exosomes as carriers of anticancer drugs and imaging agents for cancer treatment. Semin. Cancer Biol. 2022, 86, 80–100. [Google Scholar] [CrossRef]
- Ferreira, D.; Moreira, J.N.; Rodrigues, L.R. New advances in exosome-based targeted drug delivery systems. Crit. Rev. Oncol. Hematol. 2022, 172, 103628. [Google Scholar] [CrossRef]
- St-Denis-Bissonnette, F.; Khoury, R.; Mediratta, K.; El-Sahli, S.; Wang, L.; Lavoie, J.R. Applications of Extracellular Vesicles in Triple-Negative Breast Cancer. Cancers 2022, 14, 451. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, H.; Liu, S.; Hou, Y.; Chi, G. The Dual Role of Astrocyte-Derived Exosomes and Their Contents in the Process of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Delcorte, O.; Degosserie, J.; Pierreux, C.E. Role of Extracellular Vesicles in Thyroid Physiology and Diseases: Implications for Diagnosis and Treatment. Biomedicines 2022, 10, 2585. [Google Scholar] [CrossRef] [PubMed]
- Nila, I.S.; Sumsuzzman, D.M.; Khan, Z.A.; Jung, J.H.; Kazema, A.S.; Kim, S.J.; Hong, Y. Identification of exosomal biomarkers and its optimal isolation and detection method for the diagnosis of Parkinson’s disease: A systematic review and meta-analysis. Ageing Res. Rev. 2022, 82, 101764. [Google Scholar] [CrossRef]
- Zhou, H.; Wan, H.; Feng, Y.; Zhu, L.; Mi, Y. The diagnostic role and mechanistic functions of exosomal lncRNAs in prostate cancer. Clin. Transl. Oncol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Li, Y. The emerging roles of exosome-derived noncoding RNAs in the tumor immune microenvironment and their future applications. Biomed. Pharmacother. 2022, 156, 113863. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cai, H.; Deng, R.; Cheng, J.; Shi, Y. Effects of exosomes on tumor immunomodulation and their potential clinical applications (Review). Int. J. Oncol. 2022, 61, 147. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, Y.; Chen, D.; Zheng, Y.; Jiang, J. Exosomes and Exosomal Cargos: A Promising World for Ventricular Remodeling Following Myocardial Infarction. Int. J. Nanomedicine 2022, 17, 4699–4719. [Google Scholar] [CrossRef]
- Hullin-Matsuda, F.; Colosetti, P.; Rabia, M.; Luquain-Costaz, C.; Delton, I. Exosomal lipids from membrane organization to biomarkers: Focus on an endolysosomal-specific lipid. Biochimie, 2022; in press. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, C.; Xiong, J. Emerging role of extracellular vesicles in kidney diseases. Front. Pharmacol. 2022, 13, 985030. [Google Scholar] [CrossRef]
- Qian, K.; Fu, W.; Li, T.; Zhao, J.; Lei, C.; Hu, S. The roles of small extracellular vesicles in cancer and immune regulation and translational potential in cancer therapy. J. Exp. Clin. Cancer Res. 2022, 41, 286. [Google Scholar] [CrossRef]
- Sadu, L.; Krishnan, R.H.; Akshaya, R.L.; Das, U.R.; Satishkumar, S.; Selvamurugan, N. Exosomes in bone remodeling and breast cancer bone metastasis. Prog. Biophys. Mol. Biol. 2022, 175, 120–130. [Google Scholar] [CrossRef]
- Yi, X.; Chen, J.; Huang, D.; Feng, S.; Yang, T.; Li, Z.; Wang, X.; Zhao, M.; Wu, J.; Zhong, T. Current perspectives on clinical use of exosomes as novel biomarkers for cancer diagnosis. Front. Oncol. 2022, 12, 966981. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Lu, J.; Ng, I.O. Exosomes and cancer—Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Hassanpour, M.; Rezaie, J. Engineered extracellular vesicles: A novel platform for cancer combination therapy and cancer immunotherapy. Life Sci. 2022, 308, 120935. [Google Scholar] [CrossRef] [PubMed]
- Karami Fath, M.; Azami, J.; Jaafari, N.; Akbari Oryani, M.; Jafari, N.; Karim Poor, A.; Azargoonjahromi, A.; Nabi-Afjadi, M.; Payandeh, Z.; Zalpoor, H.; et al. Exosome application in treatment and diagnosis of B-cell disorders: Leukemias, multiple sclerosis, and arthritis rheumatoid. Cell Mol. Biol. Lett. 2022, 27, 74. [Google Scholar] [CrossRef]
- Pan, S.; Chen, Y.; Yan, J.; Li, F.; Chen, X.; Xu, X.; Xing, H. The emerging roles and mechanisms of exosomal non-coding RNAs in the mutual regulation between adipose tissue and other related tissues in obesity and metabolic diseases. Front. Endocrinol. 2022, 13, 975334. [Google Scholar] [CrossRef]
- Ormazabal, V.; Nair, S.; Carrión, F.; Mcintyre, H.D.; Salomon, C. The link between gestational diabetes and cardiovascular diseases: Potential role of extracellular vesicles. Cardiovasc. Diabetol. 2022, 21, 174. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Vats, R.; Yadav, P.; Bhardwaj, R. Exosomics in oral cancer diagnosis, prognosis, and therapeutics—An emergent and imperative non-invasive natural nanoparticle-based approach. Crit. Rev. Oncol. Hematol. 2022, 178, 103799. [Google Scholar] [CrossRef] [PubMed]
- Hosseinikhah, S.M.; Gheybi, F.; Moosavian, S.A.; Shahbazi, M.A.; Jaafari, M.R.; Sillanpää, M.; Kesharwani, P.; Alavizadeh, S.H.; Sahebkar, A. Role of exosomes in tumour growth, chemoresistance and immunity: State-of-the-art. J. Drug Target, 2022; in press. [Google Scholar] [CrossRef]
- Khan, F.H.; Reza, M.J.; Shao, Y.F.; Perwez, A.; Zahra, H.; Dowlati, A.; Abbas, A. Role of exosomes in lung cancer: A comprehensive insight from immunomodulation to theragnostic applications. Biochim. Biophys. Acta. Rev. Cancer 2022, 1877, 188776. [Google Scholar] [CrossRef]
- Zelli, V.; Compagnoni, C.; Capelli, R.; Corrente, A.; Di Vito Nolfi, M.; Zazzeroni, F.; Alesse, E.; Tessitore, A. Role of exosomal microRNAs in cancer therapy and drug resistance mechanisms: Focus on hepatocellular carcinoma. Front. Oncol. 2022, 12, 940056. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Luo, B. Effects of Exosomal Viral Components on the Tumor Microenvironment. Cancers 2022, 14, 3552. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Li, L.; He, D.; Chi, J.; Li, Q.; Wu, Y.; Zhao, Y.; Zhang, S.; Wang, L.; et al. Engineered extracellular vesicles and their mimetics for cancer immunotherapy. J. Control. Release 2022, 349, 679–698. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Nandi, D.; Sarkar, K.; Venkataraman, P.; Ramkumar, K.M.; Ranjan, P.; Janardhanan, R. Exosomes as Theranostic Targets: Implications for the Clinical Prognosis of Aggressive Cancers. Front. Mol. Biosci. 2022, 9, 890768. [Google Scholar] [CrossRef] [PubMed]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, Z.; Zhang, M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J. Transl. Med. 2022, 20, 291. [Google Scholar] [CrossRef]
- Di Bella, M.A. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology 2022, 11, 804. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, Y.; Yashiro, R. Extracellular Vesicles as Novel Drug-Delivery Systems through Intracellular Communications. Membranes 2022, 12, 550. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Tang, Y.; Cheng, Y.C. Exosomes as Targeted Delivery Drug System: Advances in Exosome Loading, Surface Functionalization and Potential for Clinical Application. Curr. Drug Deliv. 2022; in press. [Google Scholar] [CrossRef]
- Wang, H.; You, Y.; Zhu, X. The Role of Exosomes in the Progression and Therapeutic Resistance of Hematological Malignancies. Front. Oncol. 2022, 12, 887518. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Song, H.; Kim, N.H.; Kim, J.H. The role of extracellular vesicles in animal reproduction and diseases. J. Anim. Sci. Biotechnol. 2022, 13, 62. [Google Scholar] [CrossRef]
- Lee, C.; Han, J.; Jung, Y. Pathological Contribution of Extracellular Vesicles and Their MicroRNAs to Progression of Chronic Liver Disease. Biology 2022, 1, 637. [Google Scholar] [CrossRef] [PubMed]
- Asemani, Y.; Najafi, S.; Ezzatifar, F.; Zolbanin, N.M.; Jafari, R. Recent highlights in the immunomodulatory aspects of Treg cell-derived extracellular vesicles: Special emphasis on autoimmune diseases and transplantation. Cell Biosci. 2022, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, X.; Zhang, J. Exosome-Based Nanoplatforms: The Emerging Tools for Breast Cancer Therapy. Front. Oncol. 2022, 12, 898605. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Dai, X. Emerging Roles of Extracellular Non-Coding RNAs in Vascular Diseases. J. Cardiovasc. Transl. Res. 2022, 15, 492–499. [Google Scholar] [CrossRef]
- Bazzoni, R.; Tanasi, I.; Turazzi, N.; Krampera, M. Update on the Role and Utility of Extracellular Vesicles in Hematological Malignancies. Stem Cells 2022, 40, 619–629. [Google Scholar] [CrossRef]
- Liu, K.; Gao, X.; Kang, B.; Liu, Y.; Wang, D.; Wang, Y. The Role of Tumor Stem Cell Exosomes in Cancer Invasion and Metastasis. Front. Oncol. 2022, 12, 836548. [Google Scholar] [CrossRef]
- Kumari, M.; Anji, A. Small but Mighty-Exosomes, Novel Intercellular Messengers in Neurodegeneration. Biology 2022, 11, 413. [Google Scholar] [CrossRef]
- Al Halawani, A.; Mithieux, S.M.; Yeo, G.C.; Hosseini-Beheshti, E.; Weiss, A.S. Extracellular Vesicles: Interplay with the Extracellular Matrix and Modulated Cell Responses. Int. J. Mol. Sci. 2022, 23, 3389. [Google Scholar] [CrossRef]
- Xu, K.; Jin, Y.; Li, Y.; Huang, Y.; Zhao, R. Recent Progress of Exosome Isolation and Peptide Recognition-Guided Strategies for Exosome Research. Front. Chem. 2022, 10, 844124. [Google Scholar] [CrossRef]
- Zhao, K.; Li, X.; Shi, Y.; Lu, Y.; Qiu, P.; Deng, Z.; Yao, W.; Wang, J. Exosomes in the tumor microenvironment of cholangiocarcinoma: Current status and future perspectives. J. Transl. Med. 2022, 20, 117. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, D.; Song, Y.; He, R.; Wang, T. Research Progress in the Application of Exosomes in Immunotherapy. Front. Immunol. 2022, 13, 731516. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Kaviani, M.; Soleimanian, S.; Azarpira, N.; Asvar, Z.; Pakbaz, S. Stem Cell-Derived Exosome as Potential Therapeutics for Microbial Diseases. Front. Microbiol. 2022, 12, 786111. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Wrzecińska, M.; Czerniawska-Piątkowska, E.; Kupczyński, R. Exosomes—Spectacular role in reproduction. Biomed. Pharmacother. 2022, 148, 112752. [Google Scholar] [CrossRef]
- Xue, D.; Han, J.; Liang, Z.; Jia, L.; Liu, Y.; Tuo, H.; Peng, Y. Current Perspectives on the Unique Roles of Exosomes in Drug Resistance of Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2022, 9, 99–112. [Google Scholar] [CrossRef]
- Dehkordi, N.R.; Dehkordi, N.R.; Farjoo, M.H. Therapeutic properties of stem cell-derived exosomes in ischemic heart disease. Eur. J. Pharmacol. 2022, 920, 174839. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tan, W.F.; Yang, M.Q.; Li, J.Y.; Geller, D.A. The therapeutic potential of exosomes derived from different cell sources in liver diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 322, G397–G404. [Google Scholar] [CrossRef]
- Whittle, K.; Kao, S.; Clarke, S.; Grau, G.E.R.; Hosseini-Beheshti, E. Exploring the role of extracellular vesicles and their protein cargo in lung cancer metastasis: A review. Crit. Rev. Oncol. Hematol. 2022, 171, 103603. [Google Scholar] [CrossRef]
- Zhang, H.; Xing, J.; Dai, Z.; Wang, D.; Tang, D. Exosomes: The key of sophisticated cell-cell communication and targeted metastasis in pancreatic cancer. Cell Commun. Signal. 2022, 20, 9. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Zheng, W.; Xiang, Z.; Ye, C.S.; Yin, Q.Q.; Wang, S.H.; Xu, C.A.; Wu, W.H.; Hui, T.C.; Wu, Q.Q.; et al. Clinical implications of exosome-derived noncoding RNAs in liver. Lab. Investig. 2022, 102, 464–473. [Google Scholar] [CrossRef]
- Brown, P.A. Differential and targeted vesiculation: Pathologic cellular responses to elevated arterial pressure. Mol. Cell Biochem. 2022, 477, 1023–1040. [Google Scholar] [CrossRef]
- Gholami Farashah, M.S.; Javadi, M.; Mohammadi, A.; Soleimani Rad, J.; Shakouri, S.K.; Roshangar, L. Bone marrow mesenchymal stem cell’s exosomes as key nanoparticles in osteogenesis and bone regeneration: Specific capacity based on cell type. Mol. Biol. Rep. 2022; in press. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nagaya, S.; Yuyama, K.; Kotani, A.; Igarashi, Y.; Okazaki, T. Ceramide Metabolism Regulated by Sphingomyelin Synthase 2 Is Associated with Acquisition of Chemoresistance via Exosomes in Human Leukemia Cells. Int. J. Mol. Sci. 2022, 23, 10648. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, F.; Carpinteiro, A.; Edwards, M.J.; Wilson, G.C.; Keitsch, S.; Soddemann, M.; Wilker, B.; Kleuser, B.; Becker, K.A.; Müller, C.P.; et al. Stress induces major depressive disorder by a neutral sphingomyelinase 2-mediated accumulation of ceramide-enriched exosomes in the blood plasma. J. Mol. Med. 2022, 100, 1493–1508. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.B.; Chen, S.; Jordan-Javed, F.; Parent, C.A. Ceramide-rich microdomains facilitate nuclear envelope budding for non-conventional exosome formation. Nat. Cell Biol. 2022, 24, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Habibi, J.; DeMarco, V.G.; Hulse, J.L.; Hayden, M.R.; Whaley-Connell, A.; Hill, M.A.; Sowers, J.R.; Jia, G. Inhibition of sphingomyelinase attenuates diet—Induced increases in aortic stiffness. J. Mol. Cell Cardiol. 2022, 167, 32–39. [Google Scholar] [CrossRef]
- Melero-Fernandez de Mera, R.M.; Villaseñor, A.; Rojo, D.; Carrión-Navarro, J.; Gradillas, A.; Ayuso-Sacido, A.; Barbas, C. Ceramide Composition in Exosomes for Characterization of Glioblastoma Stem-Like Cell Phenotypes. Front. Oncol. 2022, 11, 788100. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Fukushima, M.; Mauer, A.S.; Liao, C.Y.; Ferris, A.; Dasgupta, D.; Heppelmann, C.J.; Vanderboom, P.M.; Saraswat, M.; Pandey, A.; et al. A Comparative Proteomic Analysis of Extracellular Vesicles Associated With Lipotoxicity. Front. Cell Dev. Biol. 2021, 9, 735001. [Google Scholar] [CrossRef] [PubMed]
- Abesekara, M.S.; Chau, Y. Recent advances in surface modification of micro- and nano-scale biomaterials with biological membranes and biomolecules. Front. Bioeng. Biotechnol. 2022, 10, 972790. [Google Scholar] [CrossRef]
- Canning, P.; Alwan, A.; Khalil, F.; Zhang, Y.; Opara, E.C. Perspectives and Challenges on the Potential Use of Exosomes in Bioartificial Pancreas Engineering. Ann. Biomed. Eng. 2022, 50, 1177–1186. [Google Scholar] [CrossRef]
- Sundaram, T.S.; Giromini, C.; Rebucci, R.; Pistl, J.; Bhide, M.; Baldi, A. Role of omega-3 polyunsaturated fatty acids, citrus pectin, and milk-derived exosomes on intestinal barrier integrity and immunity in animals. J. Anim. Sci. Biotechnol. 2022, 13, 40. [Google Scholar] [CrossRef]
- Fang, Z.; Ding, Y.; Xue, Z.; Li, P.; Li, J.; Li, F. Roles of exosomes as drug delivery systems in cancer immunotherapy: A mini-review. Discov. Oncol. 2022, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Sheta, M.; Fujii, M.; Calderwood, S.K. Cancer extracellular vesicles, tumoroid models, and tumor microenvironment. Semin. Cancer Biol. 2022, 86, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, Y.; Cao, X. The exosomes in tumor immunity. Oncoimmunology 2015, 4, e1027472. [Google Scholar] [CrossRef] [PubMed]
- Szeliski, K.; Drewa, T.; Pokrywczyńska, M. Small extracellular vesicles as a multicomponent biomarker platform in urinary tract carcinomas. Front. Mol. Biosci. 2022, 9, 916666. [Google Scholar] [CrossRef]
- Shang, X.; Fang, Y.; Xin, W.; You, H. The Application of Extracellular Vesicles Mediated miRNAs in Osteoarthritis: Current Knowledge and Perspective. J. Inflamm. Res. 2022, 15, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, Y.; Ma, L.; Chen, Y.; Liu, J.; Guo, Y.; Yu, T.; Zhang, L.; Zhu, L.; Shu, Y. Role of exosomal non-coding RNAs from tumor cells and tumor-associated macrophages in the tumor microenvironment. Mol. Ther. 2022, 30, 3133–3154. [Google Scholar] [CrossRef]
- Huang, Z.; Keramat, S.; Izadirad, M.; Chen, Z.S.; Soukhtanloo, M. The Potential Role of Exosomes in the Treatment of Brain Tumors, Recent Updates and Advances. Front. Oncol. 2022, 12, 869929. [Google Scholar] [CrossRef]
- Wang, W.; Hao, L.P.; Song, H.; Chu, X.Y.; Wang, R. The Potential Roles of Exosomal Non-Coding RNAs in Hepatocellular Carcinoma. Front. Oncol. 2022, 12, 790916. [Google Scholar] [CrossRef]
- Javadi, M.; Rad, J.S.; Farashah, M.S.G.; Roshangar, L. An Insight on the Role of Altered Function and Expression of Exosomes and MicroRNAs in Female Reproductive Diseases. Reprod. Sci. 2022, 29, 1395–1407. [Google Scholar] [CrossRef]
- Weaver, A.M.; Patton, J.G. Argonautes in Extracellular Vesicles: Artifact or Selected Cargo? Cancer Res. 2020, 80, 379–381. [Google Scholar] [CrossRef]
- O’Grady, T.; Njock, M.S.; Lion, M.; Bruyr, J.; Mariavelle, E.; Galvan, B.; Boeckx, A.; Struman, I.; Dequiedt, F. Sorting and packaging of RNA into extracellular vesicles shape intracellular transcript levels. BMC Biol. 2022, 20, 72. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, L.; Zou, W.; Chen, X.; Roizman, B.; Zhou, G.G. hnRNPA2B1 Associated with Recruitment of RNA into Exosomes Plays a Key Role in Herpes Simplex Virus 1 Release from Infected Cells. J. Virol. 2020, 94, e00367-20. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Gutiérrez-Vázquez, C.; Sánchez-Cabo, F.; Pérez-Hernández, D.; Vázquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sánchez-Madrid, F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013, 4, 2980. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.; Ruelcke, J.E.; Lewis, A.; Bond, C.S.; Fox, A.H.; Bharti, V.; Wani, S.; Cloonan, N.; Lai, A.; Margolin, D.; et al. Caveolin-1-driven membrane remodelling regulates hnRNPK-mediated exosomal microRNA sorting in cancer. Clin. Transl. Med. 2021, 11, e381. [Google Scholar] [CrossRef]
- Wozniak, A.L.; Adams, A.; King, K.E.; Dunn, W.; Christenson, L.K.; Hung, W.T.; Weinman, S.A. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J. Cell Biol. 2020, 219, e201912074. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Su, S.; Patil, D.P.; Liu, H.; Gan, J.; Jaffrey, S.R.; Ma, J. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat. Commun. 2018, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, L.; Giurato, G.; Cicchini, C.; Montaldo, C.; Mancone, C.; Tarallo, R.; Battistelli, C.; Alonzi, T.; Weisz, A.; Tripodi, M. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016, 17, 799–808. [Google Scholar] [CrossRef]
- Hao, S.; Bai, O.; Yuan, J.; Qureshi, M.; Xiang, J. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol. Immunol. 2006, 3, 205–211. [Google Scholar] [PubMed]
- Staubach, S.; Bauer, F.N.; Tertel, T.; Börger, V.; Stambouli, O.; Salzig, D.; Giebel, B. Scaled preparation of extracellular vesicles from conditioned media. Adv. Drug Deliv. Rev. 2021, 177, 113940. [Google Scholar] [CrossRef] [PubMed]
- Bogatcheva, N.V.; Coleman, M.E. Conditioned Medium of Mesenchymal Stromal Cells: A New Class of Therapeutics. Biochemistry 2019, 84, 1375–1389. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Xu, W.M.; Li, A.; Chen, J.J.; Sun, E.J. Research Development on Exosome Separation Technology. J. Membr. Biol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Crescitelli, R.; Lässer, C.; Lötvall, J. Isolation and characterization of extracellular vesicle subpopulations from tissues. Nat. Protoc. 2021, 16, 1548–1580. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.S.; Vaz, M.; Henriques, A.G. A review on comparative studies addressing exosome isolation methods from body fluids. Anal. Bioanal. Chem. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, P.; Kim, D.H.; Liu, B.F.; Demirci, U. Towards Microfluidic-Based Exosome Isolation and Detection for Tumor Therapy. Nano Today 2021, 37, 101066. [Google Scholar] [CrossRef] [PubMed]
- Bari, S.M.I.; Hossain, F.B.; Nestorova, G.G. Advances in Biosensors Technology for Detection and Characterization of Extracellular Vesicles. Sensors 2021, 21, 7645. [Google Scholar] [CrossRef]
- Gebeyehu, A.; Kommineni, N.; Meckes, D.G., Jr.; Sachdeva, M.S. Role of Exosomes for Delivery of Chemotherapeutic Drugs. Crit. Rev. Ther. Drug Carr. Syst. 2021, 38, 53–97. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.M.; Xia, S.J.; Lu, R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie 2021, 76, 61–67. [Google Scholar] [CrossRef]
- Armstrong, J.P.; Holme, M.N.; Stevens, M.M. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano 2017, 11, 69–83. [Google Scholar] [CrossRef]
- Choi, H.; Yim, H.; Park, C.; Ahn, S.H.; Ahn, Y.; Lee, A.; Yang, H.; Choi, C. Targeted Delivery of Exosomes Armed with Anti-Cancer Therapeutics. Membranes 2022, 12, 85. [Google Scholar] [CrossRef]
- Yuan, F.; Li, Y.M.; Wang, Z. Preserving extracellular vesicles for biomedical applications: Consideration of storage stability before and after isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The impact of storage on extracellular vesicles: A systematic study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, H.; Mou, Y.; Li, L.; Jin, W. Functions and clinical applications of exosomes in pancreatic cancer. Mol. Biol. Rep. 2022, 49, 11037–11048. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Yang, Y.; Tian, X. Crosstalk between Pancreatic Cancer Cells and Cancer-Associated Fibroblasts in the Tumor Microenvironment Mediated by Exosomal MicroRNAs. Int. J. Mol. Sci. 2022, 23, 9512. [Google Scholar] [CrossRef]
- Pan, Y.; Tang, H.; Li, Q.; Chen, G.; Li, D. Exosomes and their roles in the chemoresistance of pancreatic cancer. Cancer Med. 2022; in press. [Google Scholar] [CrossRef]
- Xu, W.X.; Wang, D.D.; Zhao, Z.Q.; Zhang, H.D.; Yang, S.J.; Zhang, Q.; Li, L.; Zhang, J. Exosomal microRNAs shuttling between tumor cells and macrophages: Cellular interactions and novel therapeutic strategies. Cancer Cell Int. 2022, 22, 190. [Google Scholar] [CrossRef]
- Tang, J.; He, J.; Feng, C.; Tu, C. Exosomal MiRNAs in Osteosarcoma: Biogenesis and Biological Functions. Front. Pharmacol. 2022, 13, 902049. [Google Scholar] [CrossRef]
- Kumar, V.B.S.; Anjali, K. Tumour generated exosomal miRNAs: A major player in tumour angiogenesis. Biochim. Biophys Acta. Mol. Basis Dis. 2022, 1868, 166383. [Google Scholar] [CrossRef]
- Li, C.; Zhou, T.; Chen, J.; Li, R.; Chen, H.; Luo, S.; Chen, D.; Cai, C.; Li, W. The role of Exosomal miRNAs in cancer. J. Transl. Med. 2022, 20, 6. [Google Scholar] [CrossRef]
- Chen, X.; Feng, J.; Chen, W.; Shao, S.; Chen, L.; Wan, H. Small extracellular vesicles: From promoting pre-metastatic niche formation to therapeutic strategies in breast cancer. Cell Commun. Signal. 2022, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Baldasici, O.; Pileczki, V.; Cruceriu, D.; Gavrilas, L.I.; Tudoran, O.; Balacescu, L.; Vlase, L.; Balacescu, O. Breast Cancer-Delivered Exosomal miRNA as Liquid Biopsy Biomarkers for Metastasis Prediction: A Focus on Translational Research with Clinical Applicability. Int. J. Mol. Sci. 2022, 23, 9371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jiang, Z.; Tang, D. The value of exosome-derived noncoding RNAs in colorectal cancer proliferation, metastasis, and clinical applications. Clin. Transl. Oncol. 2022, 24, 2305–2318. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.E. Extracellular vesicles in cancer therapy. Semin. Cancer Biol. 2022, 86, 296–309. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, S.; Li, Z.; Yang, Q.; Li, B.; Zhu, S.; Wang, L.; Wu, J.; Yuan, J.; Wang, C.; et al. Breast cancer-released exosomes trigger cancer-associated cachexia to promote tumor progression. Adipocyte 2019, 8, 31–45. [Google Scholar] [CrossRef]
- Kalita-de Croft, P.; Sharma, S.; Sobrevia, L.; Salomon, C. Extracellular vesicle interactions with the external and internal exposome in mediating carcinogenesis. Mol. Asp. Med. 2022, 87, 101039. [Google Scholar] [CrossRef]
- Pandian, S.R.K.; Vijayakumar, K.K.; Kunjiappan, S.; Babkiewicz, E.; Maszczyk, P. Emerging role of exosomes in hematological malignancies. Clin. Exp. Med. 2022; in press. [Google Scholar] [CrossRef]
- Mavroeidi, P.; Vetsi, M.; Dionysopoulou, D.; Xilouri, M. Exosomes in Alpha-Synucleinopathies: Propagators of Pathology or Potential Candidates for Nanotherapeutics? Biomolecules 2022, 12, 957. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, S. Exosomes from WSSV-infected shrimp contain viral components that mediate virus infection. J. Gen. Virol. 2022; in press. [Google Scholar] [CrossRef]
- Huda, M.N.; Nurunnabi, M. Potential Application of Exosomes in Vaccine Development and Delivery. Pharm. Res. 2022, 39, 2635–2671. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Regulation of Extracellular Vesicle-Mediated Immune Responses against Antigen-Specific Presentation. Vaccines 2022, 10, 1691. [Google Scholar] [CrossRef]
- McGowan, R.; Sally, Á.; McCabe, A.; Moran, B.M.; Finn, K. Circulating Nucleic Acids as Novel Biomarkers for Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 2027. [Google Scholar] [CrossRef] [PubMed]
- Preethi, K.A.; Selvakumar, S.C.; Ross, K.; Jayaraman, S.; Tusubira, D.; Sekar, D. Liquid biopsy: Exosomal microRNAs as novel diagnostic and prognostic biomarkers in cancer. Mol. Cancer 2022, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liang, Q.; Xu, Z.; Cai, Y.; Peng, B.; Li, J.; Zhang, W.; Kang, F.; Hong, Q.; Yan, Y.; et al. Current Understanding of Exosomal MicroRNAs in Glioma Immune Regulation and Therapeutic Responses. Front. Immunol. 2022, 12, 813747. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, H.; Hampton, D.; Marques de Menezes, E.G.; Deng, X.; Montoya, J.G.; Anderson, J.; Norris, P.J. Comparative Analysis of Extracellular Vesicles in Patients with Severe and Mild Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Immunol. 2022, 13, 841910. [Google Scholar] [CrossRef] [PubMed]
- Giloteaux, L.; O’Neal, A.; Castro-Marrero, J.; Levine, S.M.; Hanson, M.R. Cytokine profiling of extracellular vesicles isolated from plasma in myalgic encephalomyelitis/chronic fatigue syndrome: A pilot study. J. Transl. Med. 2020, 18, 387. [Google Scholar] [CrossRef]
- Eguchi, A.; Fukuda, S.; Kuratsune, H.; Nojima, J.; Nakatomi, Y.; Watanabe, Y.; Feldstein, A.E. Identification of actin network proteins, talin-1 and filamin-A, in circulating extracellular vesicles as blood biomarkers for human myalgic encephalomyelitis/chronic fatigue syndrome. Brain. Behav. Immun. 2020, 84, 106–114. [Google Scholar] [CrossRef]
- Valencia, J.; Ferreira, M.; Merino-Torres, J.F.; Marcilla, A.; Soriano, J.M. The Potential Roles of Extracellular Vesicles as Biomarkers for Parkinson’s Disease: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 11508. [Google Scholar] [CrossRef]
- Sepúlveda, D.; Cisternas-Olmedo, M.; Arcos, J.; Nassif, M.; Vidal, R.L. Contribution of Autophagy-Lysosomal Pathway in the Exosomal Secretion of Alpha-Synuclein and Its Impact in the Progression of Parkinson’s Disease. Front. Mol. Neurosci. 2022, 15, 805087. [Google Scholar] [CrossRef]
- Li, K.L.; Huang, H.Y.; Ren, H.; Yang, X.L. Role of exosomes in the pathogenesis of inflammation in Parkinson’s disease. Neural. Regen. Res. 2022, 17, 1898–1906. [Google Scholar] [CrossRef]
- Ouerdane, Y.; Hassaballah, M.Y.; Nagah, A.; Ibrahim, T.M.; Mohamed, H.A.H.; El-Baz, A.; Attia, M.S. Exosomes in Parkinson: Revisiting Their Pathologic Role and Potential Applications. Pharmaceuticals 2022, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, B.; Wang, J.; Xu, L.; Yu, S.; Fu, J.; Yan, X.; Su, J. Aβ and Tau Regulate Microglia Metabolism via Exosomes in Alzheimer’s Disease. Biomedicines 2022, 10, 1800. [Google Scholar] [CrossRef]
- Chen, P.C.; Wu, D.; Hu, C.J.; Chen, H.Y.; Hsieh, Y.C.; Huang, C.C. Exosomal TAR DNA-binding protein-43 and neurofilaments in plasma of amyotrophic lateral sclerosis patients: A longitudinal follow-up study. J. Neurol. Sci. 2020, 418, 117070. [Google Scholar] [CrossRef] [PubMed]
- Younas, N.; Fernandez Flores, L.C.; Hopfner, F.; Höglinger, G.U.; Zerr, I. A new paradigm for diagnosis of neurodegenerative diseases: Peripheral exosomes of brain origin. Transl. Neurodegener. 2022, 11, 28. [Google Scholar] [CrossRef]
- Mustapic, M.; Eitan, E.; Werner, J.K., Jr.; Berkowitz, S.T.; Lazaropoulos, M.P.; Tran, J.; Goetzl, E.J.; Kapogiannis, D. Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes. Front. Neurosci. 2017, 11, 278. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Elahi, F.M.; Mustapic, M.; Kapogiannis, D.; Pryhoda, M.; Gilmore, A.; Gorgens, K.A.; Davidson, B.; Granholm, A.C.; Ledreux, A. Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J. 2019, 33, 5082–5088. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Kovac, A.; Korff, A.; Cook, T.J.; Ginghina, C.; Bullock, K.M.; Yang, L.; Stewart, T.; Zheng, D.; Aro, P.; et al. CNS tau efflux via exosomes is likely increased in Parkinson’s disease but not in Alzheimer’s disease. Alzheimers. Dement. 2016, 12, 1125–1131. [Google Scholar] [CrossRef]
- Blijdorp, C.J.; Hartjes, T.A.; Wei, K.Y.; van Heugten, M.H.; Bovée, D.M.; Budde, R.P.J.; van de Wetering, J.; Hoenderop, J.G.J.; van Royen, M.E.; Zietse, R.; et al. Nephron mass determines the excretion rate of urinary extracellular vesicles. J. Extracell. Vesicles 2022, 11, e12181. [Google Scholar] [CrossRef]
- Tomiyama, E.; Fujita, K.; Nonomura, N. Urinary Extracellular Vesicles: Ultracentrifugation Method. Methods Mol. Biol. 2021, 2292, 173–181. [Google Scholar] [CrossRef]
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 2022, 21, 56. [Google Scholar] [CrossRef]
- Ebert, B.; Rai, A.J. Isolation and Characterization of Amniotic Fluid-Derived Extracellular Vesicles for Biomarker Discovery. Methods Mol. Biol. 2019, 1885, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Kim, H.; McGee, L.; Johnson, A.E.; Talwar, S.; Marugan, J.; Southall, N.; Hu, X.; Lal, M.; Mondal, D.; et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: A drug repurposing strategy for advanced cancer. Sci. Rep. 2018, 8, 8161. [Google Scholar] [CrossRef] [PubMed]
- Urabe, F.; Kosaka, N.; Sawa, Y.; Yamamoto, Y.; Ito, K.; Yamamoto, T.; Kimura, T.; Egawa, S.; Ochiya, T. miR-26a regulates extracellular vesicle secretion from prostate cancer cells via targeting SHC4, PFDN4, and CHORDC1. Sci. Adv. 2020, 6, eaay3051. [Google Scholar] [CrossRef] [PubMed]
- Asgarpour, K.; Shojaei, Z.; Amiri, F.; Ai, J.; Mahjoubin-Tehran, M.; Ghasemi, F.; ArefNezhad, R.; Hamblin, M.R.; Mirzaei, H. Exosomal microRNAs derived from mesenchymal stem cells: Cell-to-cell messages. Cell Commun. Signal. 2020, 18, 149. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gu, J.; Yang, O.; Wang, J.; Wang, Y.; Kong, J. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal miRNA-29c Decreases Cardiac Ischemia/Reperfusion Injury Through Inhibition of Excessive Autophagy via the PTEN/Akt/mTOR Signaling Pathway. Circ. J. 2020, 84, 1304–1311. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhou, X.; Zhang, M.; Cui, L.; Li, B.; Liu, Y.; Su, R.; Sun, K.; Hu, Y.; Yang, F.; et al. Mesenchymal stem cell-derived exosomes protect against liver fibrosis via delivering miR-148a to target KLF6/STAT3 pathway in macrophages. Stem Cell Res. Ther. 2022, 13, 330. [Google Scholar] [CrossRef]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Jeng, F.S.; Chang, C.W.; Yang, B.H.; Liu, R.S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 594. [Google Scholar] [CrossRef]
- Lee, B.C.; Kang, I.; Yu, K.R. Therapeutic Features and Updated Clinical Trials of Mesenchymal Stem Cell (MSC)-Derived Exosomes. J. Clin. Med. 2021, 10, 711. [Google Scholar] [CrossRef]
- Le, M.N.; Fan, Z.H. Exosome isolation using nanostructures and microfluidic devices. Biomed. Mater. 2021, 16, 022005. [Google Scholar] [CrossRef]
- Wu, D.; Yan, J.; Shen, X.; Sun, Y.; Thulin, M.; Cai, Y.; Wik, L.; Shen, Q.; Oelrich, J.; Qian, X.; et al. Profiling surface proteins on individual exosomes using a proximity barcoding assay. Nat. Commun. 2019, 10, 3854. [Google Scholar] [CrossRef]
- Koster, H.J.; Rojalin, T.; Powell, A.; Pham, D.; Mizenko, R.R.; Birkeland, A.C.; Carney, R.P. Surface enhanced Raman scattering of extracellular vesicles for cancer diagnostics despite isolation dependent lipoprotein contamination. Nanoscale 2021, 13, 14760–14776. [Google Scholar] [CrossRef] [PubMed]
- Nizamudeen, Z.; Markus, R.; Lodge, R.; Parmenter, C.; Platt, M.; Chakrabarti, L.; Sottile, V. Rapid and accurate analysis of stem cell-derived extracellular vesicles with super resolution microscopy and live imaging. Biochim. Biophys. Acta. Mol. Cell Res. 2018, 1865, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Riazanski, V.; Mauleon, G.; Lucas, K.; Walker, S.; Zimnicka, A.M.; McGrath, J.L.; Nelson, D.J. Real time imaging of single extracellular vesicle pH regulation in a microfluidic cross-flow filtration platform. Commun. Biol. 2022, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Martins, Á.M.; Ramos, C.C.; Freitas, D.; Reis, C.A. Glycosylation of Cancer Extracellular Vesicles: Capture Strategies, Functional Roles and Potential Clinical Applications. Cells 2021, 10, 109. [Google Scholar] [CrossRef]
- Willms, E.; Cabañas, C.; Mäger, I.; Wood, M.J.A.; Vader, P. Extracellular Vesicle Heterogeneity: Subpopulations, Isolation Techniques, and Diverse Functions in Cancer Progression. Front. Immunol. 2018, 9, 738. [Google Scholar] [CrossRef]
- Jang, S.C.; Economides, K.D.; Moniz, R.J.; Sia, C.L.; Lewis, N.; McCoy, C.; Zi, T.; Zhang, K.; Harrison, R.A.; Lim, J.; et al. ExoSTING, an extracellular vesicle loaded with STING agonists, promotes tumor immune surveillance. Commun. Biol. 2021, 4, 497. [Google Scholar] [CrossRef]
- Lewis, N.D.; Sia, C.L.; Kirwin, K.; Haupt, S.; Mahimkar, G.; Zi, T.; Xu, K.; Dooley, K.; Jang, S.C.; Choi, B.; et al. Exosome Surface Display of IL12 Results in Tumor-Retained Pharmacology with Superior Potency and Limited Systemic Exposure Compared with Recombinant IL12. Mol. Cancer Ther. 2021, 20, 523–534. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Benecke, L.; Coray, M.; Umbricht, S.; Chiang, D.; Figueiró, F.; Muller, L. Exosomes: Small EVs with Large Immunomodulatory Effect in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 3600. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Martin, P.; Fogarty, B.; Brown, A.; Schurman, K.; Phipps, R.; Yin, V.P.; Lockman, P.; Bai, S. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 2015, 32, 2003–20014. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, J.; Hu, D.; Xie, F.; Yang, T.; Li, Z.; Wang, X.; Xiao, Y.; Zhong, J.; Jiang, Y.; et al. Advances in Biological Function and Clinical Application of Small Extracellular Vesicle Membrane Proteins. Front. Oncol. 2021, 11, 675940. [Google Scholar] [CrossRef]
- Ter-Ovanesyan, D.; Norman, M.; Lazarovits, R.; Trieu, W.; Lee, J.H.; Church, G.M.; Walt, D.R. Framework for rapid comparison of extracellular vesicle isolation methods. eLife 2021, 10, e70725. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef]
- Tzaridis, T.; Bachurski, D.; Liu, S.; Surmann, K.; Babatz, F.; Gesell Salazar, M.; Völker, U.; Hallek, M.; Herrlinger, U.; Vorberg, I.; et al. Extracellular Vesicle Separation Techniques Impact Results from Human Blood Samples: Considerations for Diagnostic Applications. Int. J. Mol. Sci. 2021, 22, 9211. [Google Scholar] [CrossRef] [PubMed]
- Liangsupree, T.; Multia, E.; Riekkola, M.L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Q.; Deng, Y.; Chen, M.; Yang, C. Improving Isolation of Extracellular Vesicles by Utilizing Nanomaterials. Membranes 2021, 12, 55. [Google Scholar] [CrossRef]
- Wang, F.; Cerione, R.A.; Antonyak, M.A. Isolation and characterization of extracellular vesicles produced by cell lines. STAR Protoc. 2021, 2, 100295. [Google Scholar] [CrossRef]
- Neyroud, A.S.; Chiechio, R.M.; Moulin, G.; Ducarre, S.; Heichette, C.; Dupont, A.; Budzynski, M.; Even-Hernandez, P.; Faro, M.J.L.; Yefimova, M.; et al. Diversity of Extracellular Vesicles in Human Follicular Fluid: Morphological Analysis and Quantification. Int. J. Mol. Sci. 2022, 23, 11676. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Cai, Q.; Jin, H. Effective methods for isolation and purification of extracellular vesicles from plants. J. Integr. Plant Biol. 2021, 63, 2020–2030. [Google Scholar] [CrossRef]
- Chen, B.Y.; Sung, C.W.; Chen, C.; Cheng, C.M.; Lin, D.P.; Huang, C.T.; Hsu, M.Y. Advances in exosomes technology. Clin. Chim. Acta. 2019, 493, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.T.; Nishimura, T.; Suetsugu, S. Ultracentrifugal separation, characterization, and functional study of extracellular vesicles derived from serum-free cell culture. STAR Protoc. 2021, 2, 100625. [Google Scholar] [CrossRef] [PubMed]
- Campos-Silva, C.; Cáceres-Martell, Y.; Sánchez-Herrero, E.; Sandúa, A.; Beneitez-Martínez, A.; González, Á.; Provencio, M.; Romero, A.; Jara-Acevedo, R.; Yáñez-Mó, M.; et al. A simple immunoassay for extracellular vesicle liquid biopsy in microliters of non-processed plasma. J. Nanobiotechnol. 2022, 20, 72. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Jeyaraj, M.; Qasim, M.; Kim, J.H. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells 2019, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—Efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, P.; Zhang, T.; Xu, Z.; Huang, X.; Wang, R.; Du, L. Review on Strategies and Technologies for Exosome Isolation and Purification. Front. Bioeng. Biotechnol. 2022, 9, 811971. [Google Scholar] [CrossRef]
- Kaddour, H.; Tranquille, M.; Okeoma, C.M. The Past, the Present, and the Future of the Size Exclusion Chromatography in Extracellular Vesicles Separation. Viruses 2021, 13, 2272. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.S.K.; Upton, F.M.; Rees, E.; Limb, C.; Jiao, L.R.; Krell, J.; Frampton, A.E. Size-Exclusion Chromatography as a Technique for the Investigation of Novel Extracellular Vesicles in Cancer. Cancers 2020, 12, 3156. [Google Scholar] [CrossRef] [PubMed]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Logozzi, M.; Di Raimo, R.; Mizzoni, D.; Fais, S. Immunocapture-based ELISA to characterize and quantify exosomes in both cell culture supernatants and body fluids. Methods Enzymol. 2020, 645, 155–180. [Google Scholar] [CrossRef] [PubMed]
- Isaksson, G.L.; Nielsen, M.B.; Hinrichs, G.R.; Krogstrup, N.V.; Zachar, R.; Stubmark, H.; Svenningsen, P.; Madsen, K.; Bistrup, C.; Jespersen, B.; et al. Proteinuria is accompanied by intratubular complement activation and apical membrane deposition of C3dg and C5b-9 in kidney transplant recipients. Am. J. Physiol. Renal. Physiol. 2022, 322, F150–F163. [Google Scholar] [CrossRef]
- Yousif, G.; Qadri, S.; Parray, A.; Akhthar, N.; Shuaib, A.; Haik, Y. Exosomes Derived Neuronal Markers: Immunoaffinity Isolation and Characterization. Neuromol. Med. 2022, 24, 339–351. [Google Scholar] [CrossRef]
- Wang, Y.T.; Cai, M.D.; Sun, L.L.; Hua, R.N. A Rapid and Facile Separation-Detection Integrated Strategy for Exosome Profiling Based on Boronic Acid-Directed Coupling Immunoaffinity. Anal. Chem. 2021, 93, 16059–16067. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Paisrisarn, P.; Yanagida, T.; Konakade, Y.; Nakamura, Y.; Nagashima, K.; Musa, M.; Thiodorus, I.A.; Takahashi, H.; Naganawa, T.; et al. Molecular profiling of extracellular vesicles via charge-based capture using oxide nanowire microfluidics. Biosens. Bioelectron. 2021, 194, 113589. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, J.; Ji, X.; Tan, Z.; Lubman, D.M. Column-based Technology for CD9-HPLC Immunoaffinity Isolation of Serum Extracellular Vesicles. J. Proteome Res. 2021, 20, 4901–4911. [Google Scholar] [CrossRef]
- Paulmurugan, R.; Liu, Y.; Sukumar, U.K.; Kanada, M.; Massoud, T.F. BRET Sensors for Imaging Membrane Integrity of Microfluidically Generated Extracellular Vesicles. Methods Mol. Biol. 2022, 2525, 227–238. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Amin Mahdian, S.M.; Ebrahimi, M.S.; Taghizadieh, M.; Vosough, M.; Sadri Nahand, J.; Hosseindoost, S.; Vousooghi, N.; Javar, H.A.; Larijani, B.; et al. Microfluidics for detection of exosomes and microRNAs in cancer: State of the art. Mol. Ther. Nucleic Acids 2022, 28, 758–791. [Google Scholar] [CrossRef]
- Abreu, C.M.; Costa-Silva, B.; Reis, R.L.; Kundu, S.C.; Caballero, D. Microfluidic platforms for extracellular vesicle isolation, analysis and therapy in cancer. Lab. Chip. 2022, 22, 1093–1125. [Google Scholar] [CrossRef]
- Diaz-Armas, G.G.; Cervantes-Gonzalez, A.P.; Martinez-Duarte, R.; Perez-Gonzalez, V.H. Electrically driven microfluidic platforms for exosome manipulation and characterization. Electrophoresis 2022, 43, 327–339. [Google Scholar] [CrossRef]
- Shirejini, S.Z.; Inci, F. The Yin and Yang of exosome isolation methods: Conventional practice, microfluidics, and commercial kits. Biotechnol. Adv. 2022, 54, 107814. [Google Scholar] [CrossRef]
- Gwak, H.; Park, S.; Kim, J.; Lee, J.D.; Kim, I.S.; Kim, S.I.; Hyun, K.A.; Jung, H.I. Microfluidic chip for rapid and selective isolation of tumor-derived extracellular vesicles for early diagnosis and metastatic risk evaluation of breast cancer. Biosens. Bioelectron. 2021, 192, 113495. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, K. Plant-derived extracellular vesicles as oral drug delivery carriers. J. Control. Release 2022, 350, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.B.; Abdull Razis, A.F.; Ooi, J.; Chan, K.W.; Ismail, N.; Foo, J.B. Theragnostic Applications of Mammal and Plant-Derived Extracellular Vesicles: Latest Findings, Current Technologies, and Prospects. Molecules 2022, 27, 3941. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, E.L.; Fuentes, F.B.; Felmer, R.N.; Yeste, M.; Arias, M.E. Extracellular vesicles in mammalian reproduction: A review. Zygote 2022, 30, 440–463. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, D.; Menjivar, N.; Gebremedhn, S. Current knowledge and the future potential of extracellular vesicles in mammalian reproduction. Reprod. Fertil. Dev. 2021, 34, 174–189. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, K.; Hu, H.; Xing, X.; Huang, X.; Gao, H. Extracellular vesicles: Their functions in plant-pathogen interactions. Mol. Plant Pathol. 2022, 23, 760–771. [Google Scholar] [CrossRef]
- Díez-Sainz, E.; Milagro, F.I.; Riezu-Boj, J.I.; Lorente-Cebrián, S. Effects of gut microbiota-derived extracellular vesicles on obesity and diabetes and their potential modulation through diet. J. Physiol. Biochem. 2022, 78, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe. 2018, 24, 637–652.e8. [Google Scholar] [CrossRef]
- Suri, K.; D’Souza, A.; Huang, D.; Bhavsar, A.; Amiji, M. Bacterial extracellular vesicle applications in cancer immunotherapy. Bioact. Mater. 2022, 22, 551–566. [Google Scholar] [CrossRef]
- Mishra, S.; Amatya, S.B.; Salmi, S.; Koivukangas, V.; Karihtala, P.; Reunanen, J. Microbiota and Extracellular Vesicles in Anti-PD-1/PD-L1 Therapy. Cancers 2022, 4, 5121. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, H.; Han, Y.; Hu, Y.; Geng, Z.; Su, J. Bacterial extracellular vesicles-based therapeutic strategies for bone and soft tissue tumors therapy. Theranostics 2022, 12, 6576–6594. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, Q.; Haesebrouck, F.; Van Hoecke, L.; Vandenbroucke, R.E. The tremendous biomedical potential of bacterial extracellular vesicles. Trends Biotechnol. 2022, 40, 1173–1194. [Google Scholar] [CrossRef]
- Janda, M.; Robatzek, S. Extracellular vesicles from phytobacteria: Properties, functions and uses. Biotechnol. Adv. 2022, 58, 107934. [Google Scholar] [CrossRef] [PubMed]
- Mustajab, T.; Kwamboka, M.S.; Choi, D.A.; Kang, D.W.; Kim, J.; Han, K.R.; Han, Y.; Lee, S.; Song, D.; Chwae, Y.J. Update on Extracellular Vesicle-Based Vaccines and Therapeutics to Combat COVID-19. Int. J. Mol. Sci. 2022, 23, 11247. [Google Scholar] [CrossRef]
- Goubran, H.; Seghatchian, J.; Sabry, W.; Ragab, G.; Burnouf, T. Platelet and extracellular vesicles in COVID-19 infection and its vaccines. Transfus. Apher. Sci. 2022, 61, 103459. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.H.; Thapa, N.; Kim, B.J.; Lee, J.O.; Jang, Y.N.; Chwae, Y.J.; Kim, J. Possibility of exosome-based coronavirus disease 2019 vaccine (Review). Mol. Med. Rep. 2022, 25, 26. [Google Scholar] [CrossRef]
- Burgos-Ravanal, R.; Campos, A.; Díaz-Vesga, M.C.; González, M.F.; León, D.; Lobos-González, L.; Leyton, L.; Kogan, M.J.; Quest, A.F.G. Extracellular Vesicles as Mediators of Cancer Disease and as Nanosystems in Theranostic Applications. Cancers 2021, 13, 3324. [Google Scholar] [CrossRef] [PubMed]
- Negahdaripour, M.; Vakili, B.; Nezafat, N. Exosome-based vaccines and their position in next generation vaccines. Int. Immunopharmacol. 2022, 113, 109265. [Google Scholar] [CrossRef]
- Dyball, L.E.; Smales, C.M. Exosomes: Biogenesis, targeting, characterization and their potential as "Plug & Play" vaccine platforms. Biotechnol. J. 2022, 17, e2100646. [Google Scholar] [CrossRef]
- Parveen, S.; Subramanian, K. Emerging Roles of Extracellular Vesicles in Pneumococcal Infections: Immunomodulators to Potential Novel Vaccine Candidates. Front. Cell Infect. Microbiol. 2022, 12, 836070. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Mukherjee, S.; Ali, S.; Bhardwaj, U.; Choudhary, R.K.; Balakrishnan, S.; Naseem, A.; Mir, S.A.; Banawas, S.; Alaidarous, M.; et al. Pioneer Role of Extracellular Vesicles as Modulators of Cancer Initiation in Progression, Drug Therapy, and Vaccine Prospects. Cells 2022, 11, 490. [Google Scholar] [CrossRef] [PubMed]
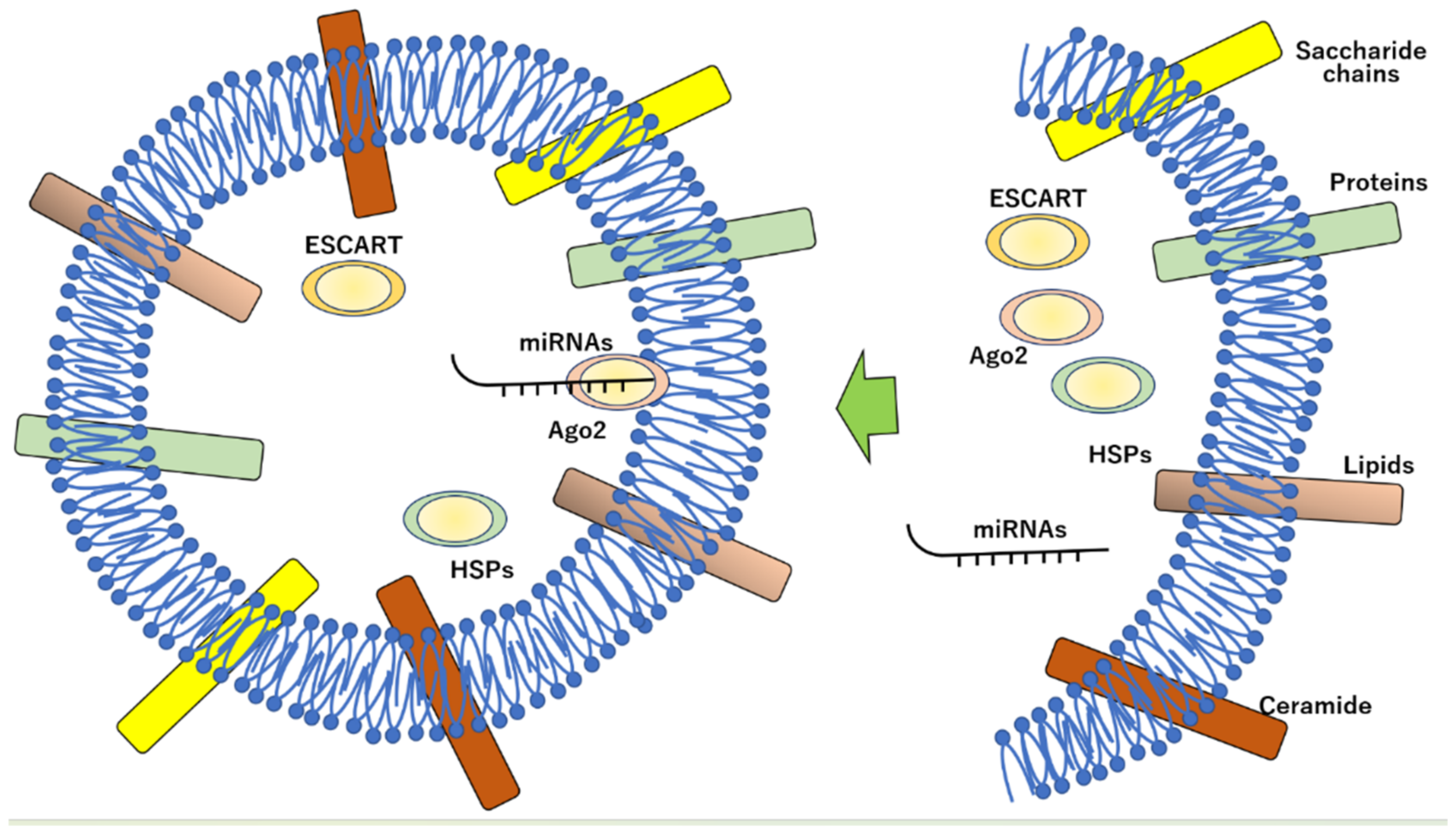
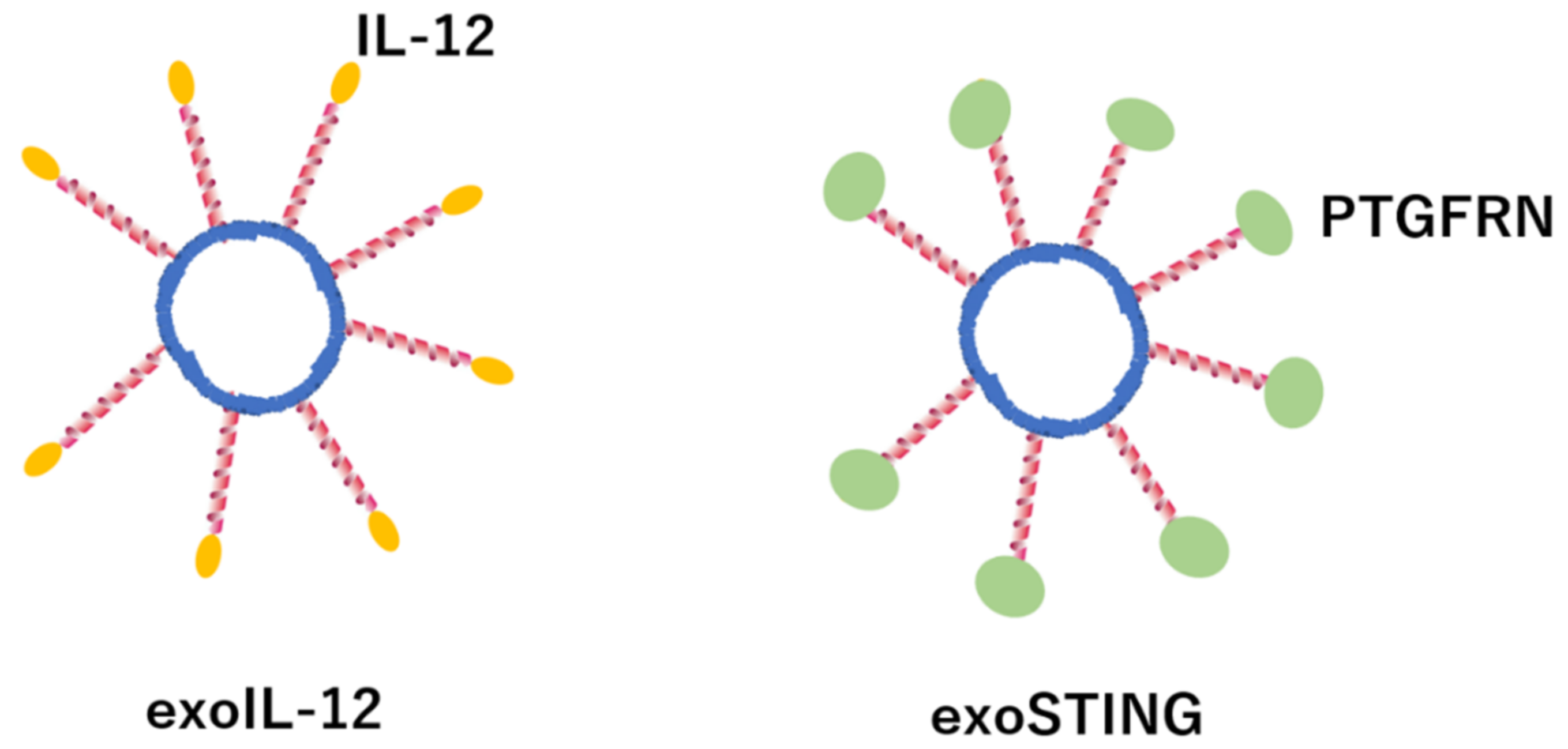
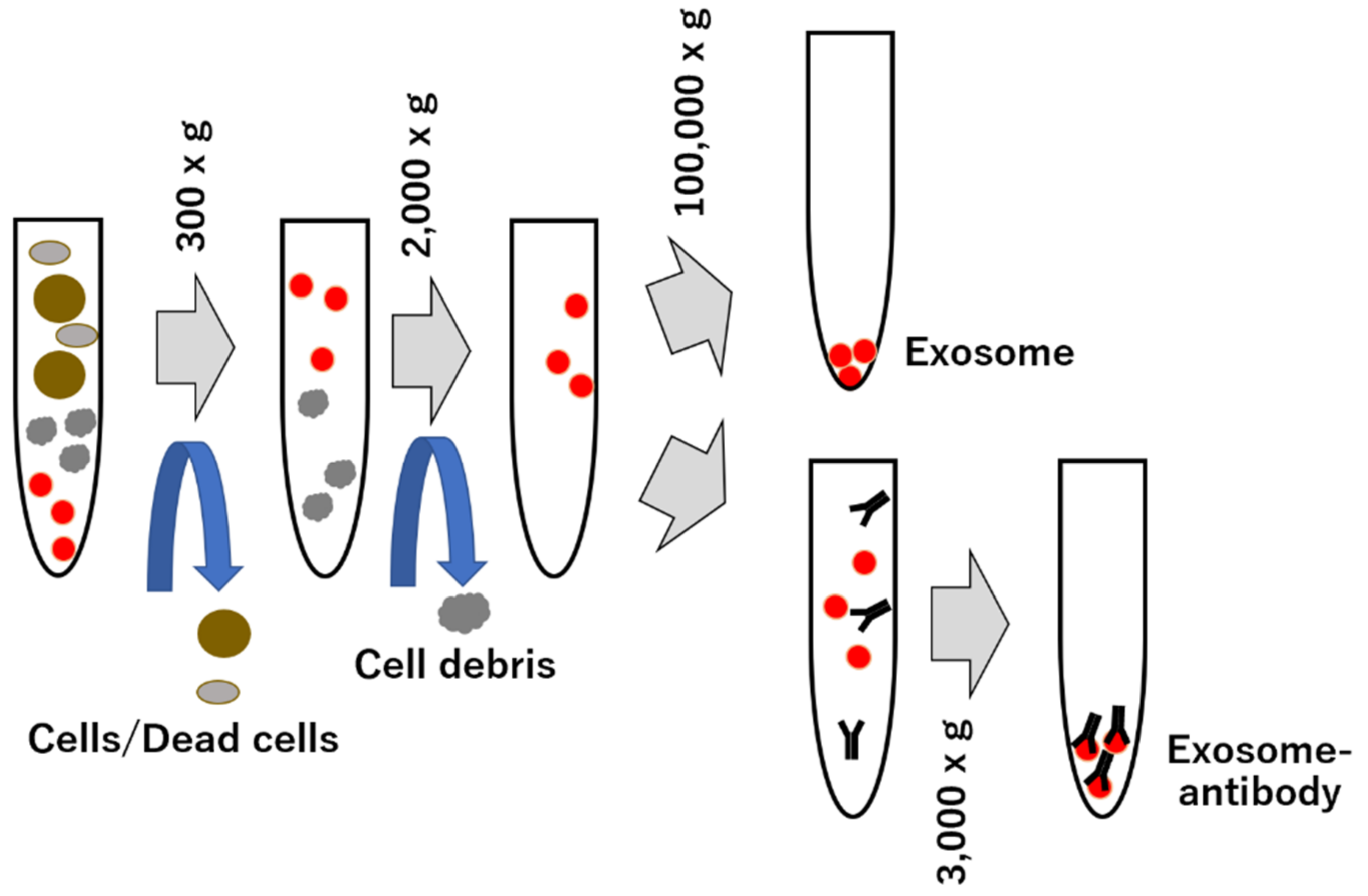
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaka, Y.; Yashiro, R. Advances in Purification, Modification, and Application of Extracellular Vesicles for Novel Clinical Treatments. Membranes 2022, 12, 1244. https://doi.org/10.3390/membranes12121244
Matsuzaka Y, Yashiro R. Advances in Purification, Modification, and Application of Extracellular Vesicles for Novel Clinical Treatments. Membranes. 2022; 12(12):1244. https://doi.org/10.3390/membranes12121244
Chicago/Turabian StyleMatsuzaka, Yasunari, and Ryu Yashiro. 2022. "Advances in Purification, Modification, and Application of Extracellular Vesicles for Novel Clinical Treatments" Membranes 12, no. 12: 1244. https://doi.org/10.3390/membranes12121244
APA StyleMatsuzaka, Y., & Yashiro, R. (2022). Advances in Purification, Modification, and Application of Extracellular Vesicles for Novel Clinical Treatments. Membranes, 12(12), 1244. https://doi.org/10.3390/membranes12121244





