Open Pore Ultrafiltration Hollow Fiber Membrane Fabrication Method via Dual Pore Former with Dual Dope Solution Phase
Abstract
1. Introduction
2. Materials & Methods
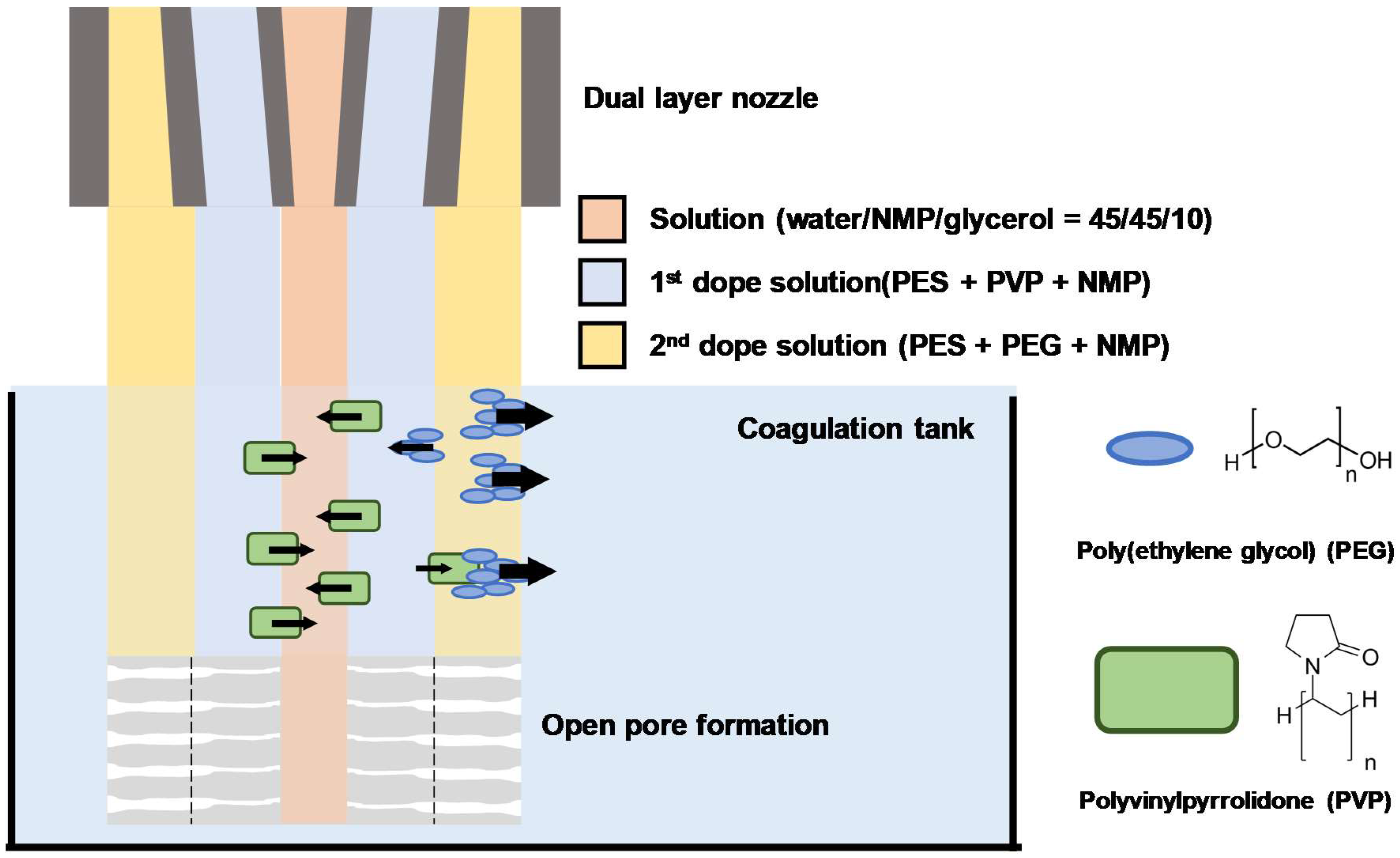
2.1. Dual-Layer Hollow-Fiber Membranes (DHF) Fabrication
2.1.1. Preparation of Recipe and Configuration of Dual-Layer
2.1.2. DHF Spinning Process Preparation
2.1.3. HFs Membrane Mini-Module Organization and Assembly
2.2. Membrane Characterization
2.2.1. HF Membrane Morphology
2.2.2. Porosity of HF Membranes
2.2.3. Pure Water Permeability (PWP) Test
2.2.4. Mean Pore Size Calculation
2.3. Membrane Performance Test
2.3.1. Bovine Serum Albumin (BSA) Leakage
2.3.2. Urea Clearance: An Application in Hemodialysis
3. Results and Discussion
3.1. Morphology of DHF and CHF Membrane via SEM
3.2. Membrane Properties and BSA Retention: In Comparison with CHF
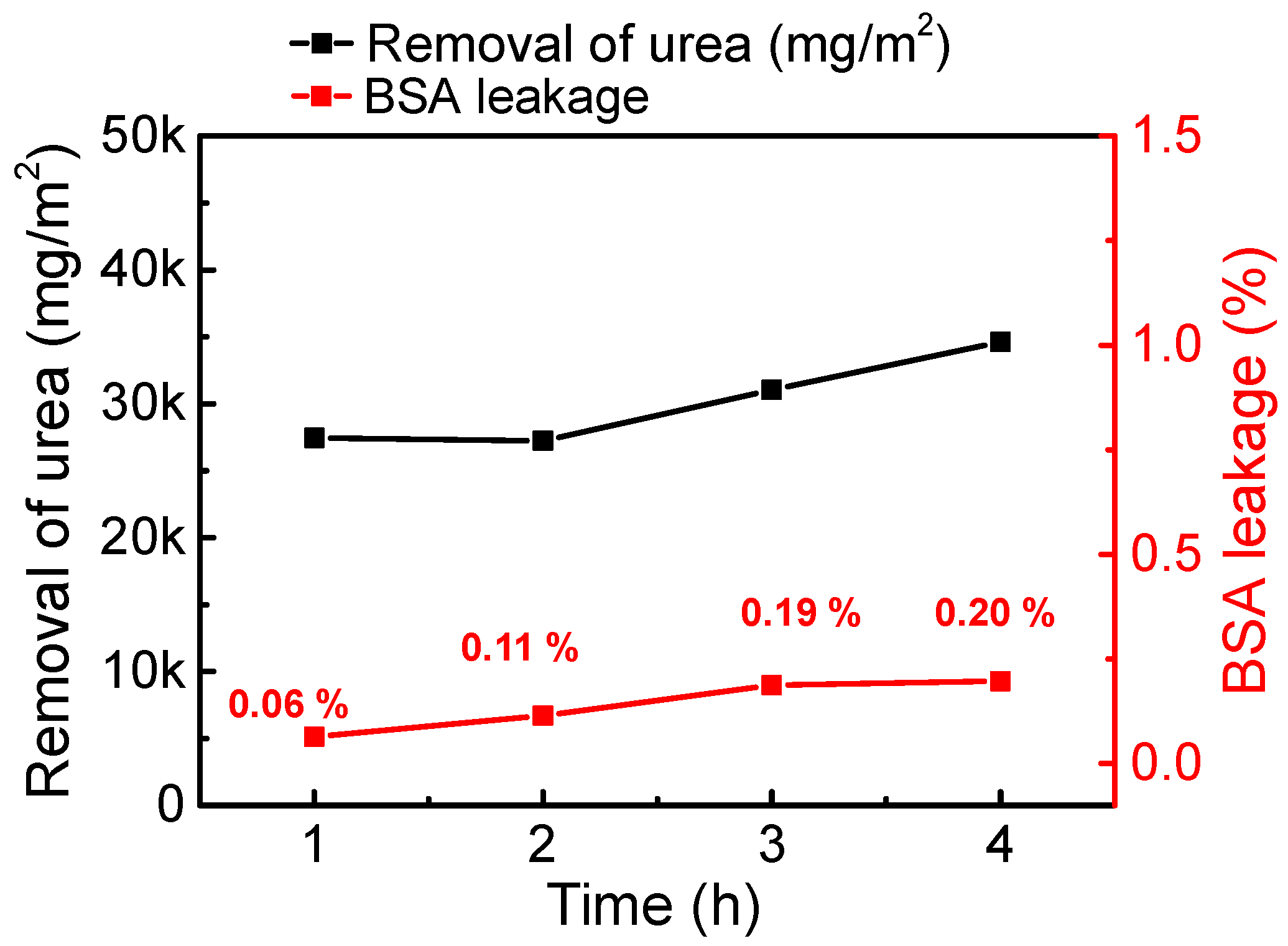
3.3. Urea Clearance by the Studied Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pendergast, M.T.M.; Hoek, E.M.V. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Jang, K.; Lim, J.; Lee, J.; Alayande, A.B.; Jung, B.; Kim, I.S. Fabrication of nanocomposite forward osmosis hollow fiber membrane for low reverse salt flux by modification of active layer via co-extrusion with graphene oxide. Desalin. Water Treat. 2020, 183, 121–130. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Jang, K.; Her, N.; Kim, C.S.; Kim, S.W.; Kim, I.S. Fabrication of hollow fiber membranes with different inner diameters for enhanced uremic toxins removal in hemodialysis: Exploring from high-flux to high molecular weight retention onset classes. J. Membr. Sci. 2022, 663, 121065. [Google Scholar] [CrossRef]
- Ng, K.K.; Lin, C.F.; Panchangam, S.C.; Hong, P.K.A.; Yang, P.Y. Reduced membrane fouling in a novel bio-entrapped membrane reactor for treatment of food and beverage processing wastewater. Water Res. 2011, 45, 4269–4278. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, B.T.; Avis, K.E. Membrane filtration of pharmaceutical solutions. Am. J. Hosp. Pharm. 1993, 50, 1921–1936. [Google Scholar] [CrossRef]
- Jang, K.; Hwang, D.K.; Auxilia, F.M.; Jang, J.; Song, H.; Oh, B.Y.; Kim, Y.; Nam, J.; Park, J.W.; Jeong, S.; et al. Sub-10-nm Co3O4 nanoparticles/graphene composites as high-performance anodes for lithium storage. Chem. Eng. J. 2017, 309, 15–21. [Google Scholar] [CrossRef]
- Jang, J.; Kang, Y.; Han, J.H.; Jang, K.; Kim, C.M.; Kim, I.S. Developments and future prospects of reverse electrodialysis for salinity gradient power generation: Influence of ion exchange membranes and electrodes. Desalination 2020, 491, 114540–114553. [Google Scholar] [CrossRef]
- Strathmann, H.; Giorno, L.; Drioli, E. An Introduction to Membrane Science and Technology; Consiglio Nazionale Delle Ricerche (CNR-ITM) at University of Calabria: Rende, Italy, 2006; ISBN 978-3-527-32451-4. [Google Scholar]
- Lee, M.; Gan, Y.; Yang, C.; Ren, C.; Xue, X. Fabrication and accelerated long-term stability test of asymmetrical hollow fiber-supported thin film oxygen separation membrane. J. Membr. Sci. 2022, 655, 120600–120608. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Xu, L.; Zeng, F.; Hou, D.; Wang, J. Optimizing stretching conditions in fabrication of PTFE hollow fiber membrane for performance improvement in membrane distillation. J. Membr. Sci. 2018, 550, 126–135. [Google Scholar] [CrossRef]
- Apel, P. Track etching technique in membrane technology. Radiat. Meas. 2001, 34, 559–566. [Google Scholar] [CrossRef]
- Sa-nguanruksa, J.; Rujiravanit, R.; Supaphol, P.; Tokura, S. Porous polyethylene membranes by template-leaching technique: Preparation and characterization. Polym. Test. 2004, 23, 91–99. [Google Scholar] [CrossRef]
- Steele, B.C.H. Interfacial reactions associated with ceramic ion transport membranes. Solid State Ion. 1995, 75, 157–165. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P. Preparation and characterizations of a new PS/TiO2 hybrid membranes by sol–gel process. Polymer 2006, 47, 2683–2688. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S. Sea Water Demineralization by Means of an Osmotic Membrane. In Saline Water Conversion-II; American Chemical Society: Washington, DC, USA, 1963; Chapter 9; pp. 117–132. ISBN 978-0-841-20039-5. [Google Scholar]
- Ismail, N.; Venault, A.; Mikkola, J.P.; Bouyer, D.; Drioli, E.; Kiadeh, N.T.H. Investigating the potential of membranes formed by the vapor induced phase separation process. J. Membr. Sci. 2020, 597, 117601–117635. [Google Scholar] [CrossRef]
- Tsai, H.A.; Kuo, C.Y.; Lin, J.H.; Wang, D.M.; Deratani, A.; Pochat-Bohatier, C.; Lee, K.R.; Lai, J.Y. Morphology control of polysulfone hollow fiber membranes via water vapor induced phase separation. J. Membr. Sci. 2006, 278, 390–400. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Yang, D.; Jian, X. Preparation and characterization of polypiperazine amide/PPESK hollow fiber composite nanofiltration membrane. J. Membr. Sci. 2007, 301, 85–92. [Google Scholar] [CrossRef]
- Crowder, M.L.; Gooding, C.H. Spiral wound, hollow fiber membrane modules: A new approach to higher mass transfer efficiency. J. Membr. Sci. 1997, 137, 17–29. [Google Scholar] [CrossRef]
- Sewerin, T.; Elshof, M.G.; Matencio, S.; Boerrigter, M.; Yu, J.; de Grooth, J. Advances and Applications of Hollow Fiber Nanofiltration Membranes: A Review. Membranes 2021, 11, 890. [Google Scholar] [CrossRef]
- Gebru, K.A.; Das, C. Effects of solubility parameter differences among PEG, PVP and CA on the preparation of ultrafiltration membranes: Impacts of solvents and additives on morphology, permeability and fouling performances. Chin. J. Chem. Eng. 2017, 25, 911–923. [Google Scholar] [CrossRef]
- Malik, T.; Razzaq, H.; Razzaque, S.; Nawaz, H.; Siddiqa, A.; Siddiq, M.; Qaisar, S. Design and synthesis of polymeric membranes using water-soluble pore formers: An overview. Polym. Bull. 2019, 76, 4879–4901. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Ghalamchi, L.; Vatanpour, V.; Khataee, A.; Yousefpoor, M. Novel polymeric additives in the preparation and modification of polymeric membranes: A comprehensive review. J. Ind. Eng. Chem. 2022, 109, 100–124. [Google Scholar] [CrossRef]
- Alayande, A.B.; Obaid, M.; Yu, H.W.; Kim, I.S. High-flux ultrafiltration membrane with open porous hydrophilic structure using dual pore formers. Chemosphere 2019, 227, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.L.; Sarif, M.; Ismail, S. Development of an integrally skinned ultrafiltration membrane for wastewater treatment: Effect of different formulations of PSF/NMP/PVP on flux and rejection. Desalination 2005, 179, 257–263. [Google Scholar] [CrossRef]
- Hamid, N.A.A.; Ismail, A.F.; Matsuura, T.; Zularisam, A.W.; Lau, W.J.; Yuliwati, E.; Abdullah, M.S. Morphological and separation performance study of pollysulfon/titanium dioxide (PSF/TiO2) ultrafiltration membranes for humic acid removal. Desalination 2011, 273, 85–92. [Google Scholar] [CrossRef]
- Stevens, L.A.; Shastri, S.; Levey, A.S. Assessment of Renal Function. In Comprehensive Clinical Nephrology, 4th ed.; Floege, J., Johnson, R.J., Feehally, J., Eds.; Mosby: Maryland Heights, MO, USA, 2010; Chapter 9; ISBN 978-0-323-05876-6. [Google Scholar]
- Kirsch, A.H.; Lyko, R.; Nilsson, L.-G.; Beck, W.; Amdahl, M.; Lechner, R.; Schneider, A.; Wanner, C.; Rosenkranz, A.R.; Krieter, D.H. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol. Dial. Transplant. 2017, 32, 165–172. [Google Scholar] [CrossRef]
- Kalam, M.A.; Alshamsan, A.; Alkholief, M.; Alsarra, I.A.; Ali, R.; Haq, N.; Anwer, M.K.; Shakeel, F. Solubility measurement and various solubility parameters of glipizide in different neat solvents. ACS Omega 2020, 5, 1708–1716. [Google Scholar] [CrossRef]
- Marino, T.; Russo, F.; Figoli, A. The formation of polyvinylidene fluoride membranes with tailored properties via vapour/non-solvent induced phase separation. Membranes 2018, 8, 71. [Google Scholar] [CrossRef]
- Saluja, H.; Mehanna, A.; Panicucci, R.; Atef, E. Hydrogen bonding: Between strengthening the crystal packing and improving solubility of three haloperidol derivatives. Molecules 2016, 21, 719. [Google Scholar] [CrossRef]
- Chang, S.; Fane, A.G. The effect of fibre diameter on filtration and flux distribution-relavance to submerged hollow fibre modules. J. Membr. Sci. 2001, 184, 221–231. [Google Scholar] [CrossRef]
- Lee, M.; Wu, Z.; Wang, R.; Li, K. Micor-structured alumina hollow fiber membranes–potential applications in wastewater treatment. J. Membr. Sci. 2014, 461, 39–48. [Google Scholar] [CrossRef]
- Mansur, S.; Othman, M.H.D.; Ismail, A.F.; Zainol Abidin, M.N.; Said, N.; Sean, G.P.; Hasbullah, H.; Sheikh Abdul Kadir, S.H.; Kamal, F. Study on the Effect of Spinning Conditions on the Performance of PSf/PVP Ultrafiltration Hollow Fiber Membrane. Malays. J. Fundam. Appl. Sci. 2018, 14, 343–347. [Google Scholar] [CrossRef]
- An, Z.; Xu, R.; Dai, F.; Xue, G.; He, X.; Zhao, Y.; Chen, L. PVDF/PVDF-g-PACMO Blend Hollow Fiber Membranes for Hemodialysis: Preparation, Characterization, and Performance. RSC Adv. 2017, 7, 26593–26600. [Google Scholar] [CrossRef]
- Abidin, M.N.Z.; Goh, P.S.; Said, N.; Ismail, A.F.; Othman, M.H.D.; Abdullah, M.S.; Ng, B.C.; Hasbullah, H.; Sheikh Abdul Kadir, S.H.; Kamal, F.; et al. Polysulfone/Amino-Silanized Poly(Methyl Methacrylate) Dual Layer Hollow Fiber Membrane for Uremic Toxin Separation. Sep. Purif. Technol. 2020, 236, 116216. [Google Scholar] [CrossRef]
- de Fierro, A.B.; Voigt, M.; Storr, M.; Krause, B. MCO membranes: Enhanced selectivity in high-flux class. Sci. Rep. 2015, 5, 18448–18454. [Google Scholar] [CrossRef]

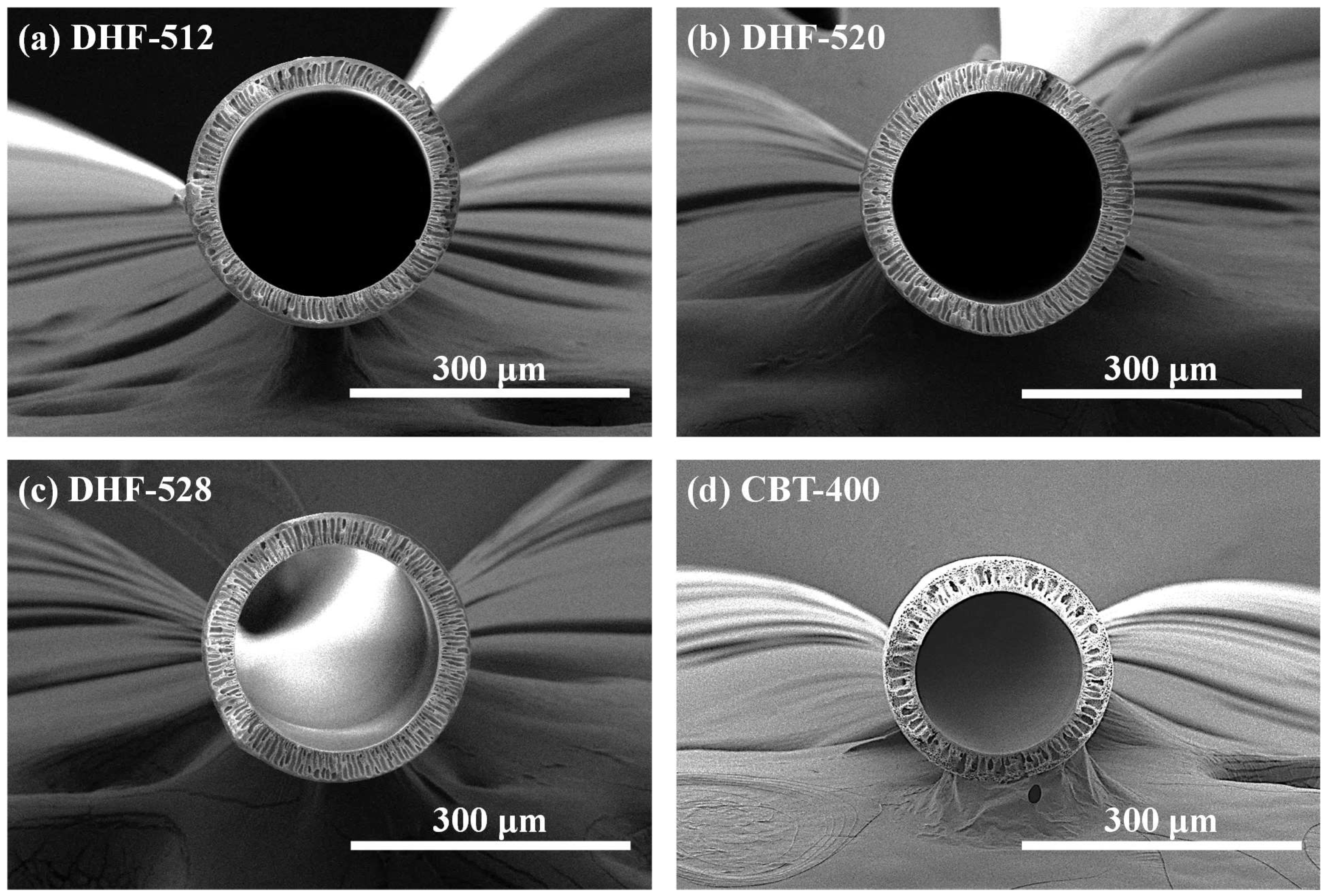

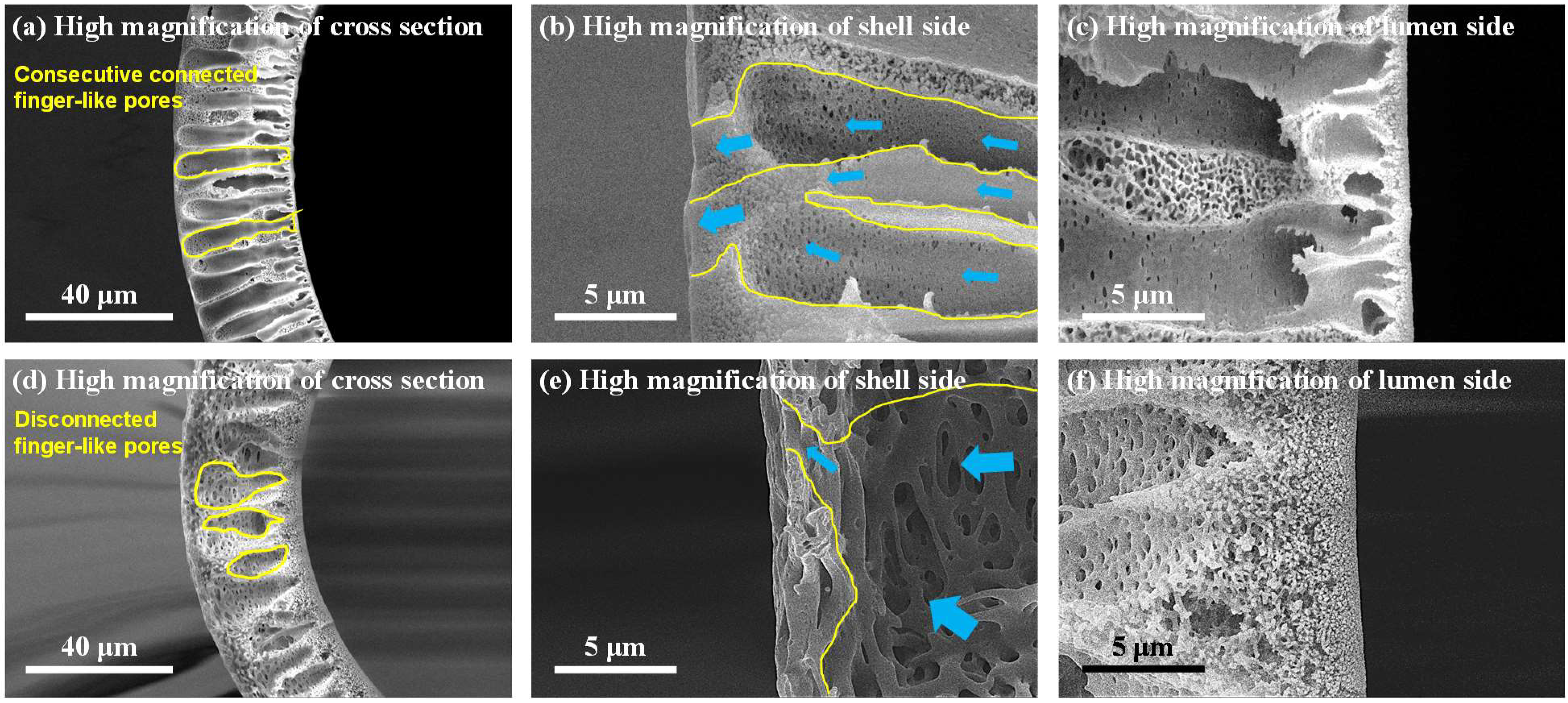
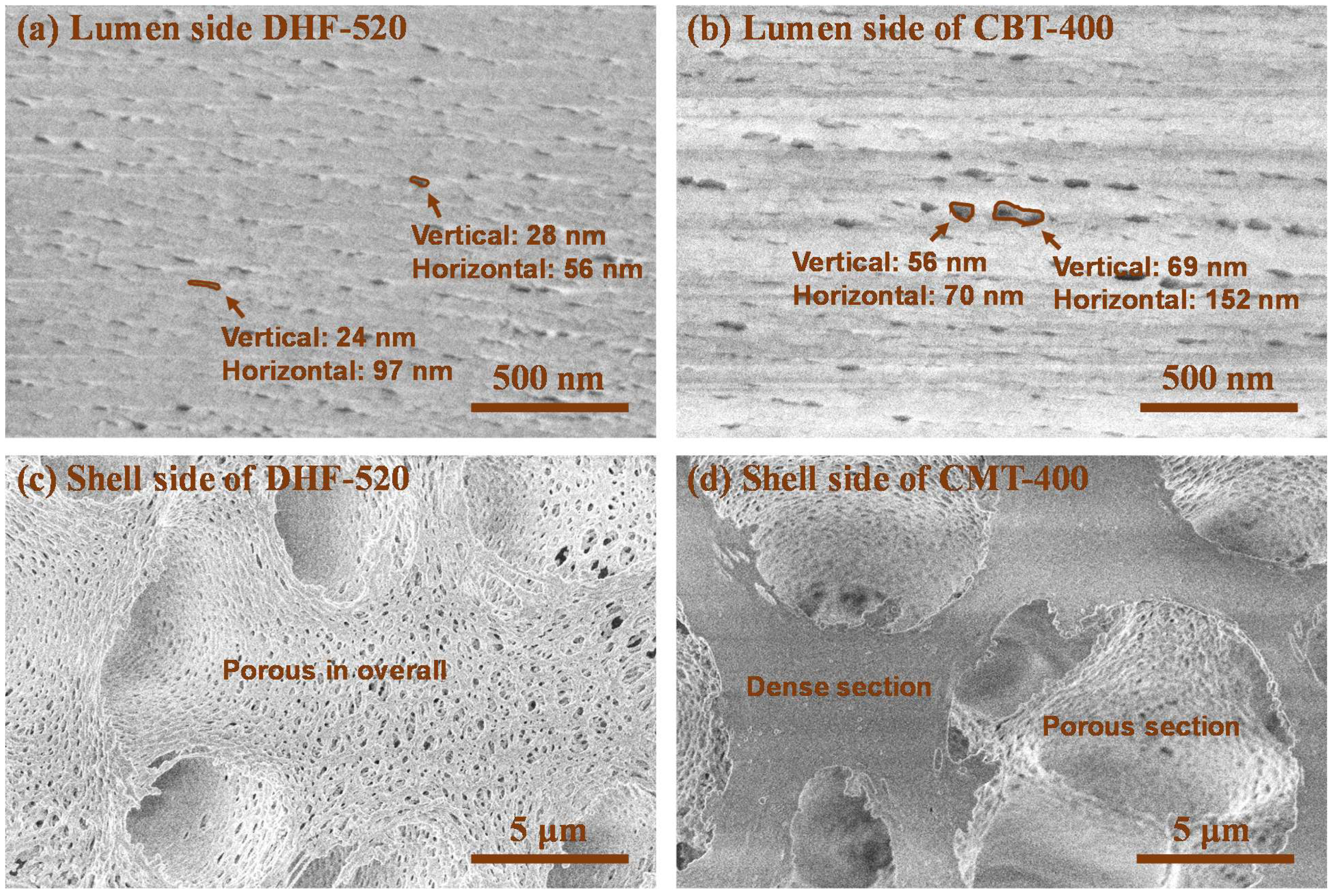

| Classification | Water-Soluble Pore Former (WSP, wt.%) | N-Methyl-2-pyrrolidone (NMP, wt.%) | Poly-Ether Sulfone (PES, wt.%) | ||
|---|---|---|---|---|---|
| DHF-512 | 1st dope | PVP | 5 | 81 | 14 |
| 2nd dope | PEG | 12 | 74 | ||
| DHF-520 | 1st dope | PVP | 5 | 81 | |
| 2nd dope | PEG | 20 | 66 | ||
| DHF-528 | 1st dope | PVP | 5 | 81 | |
| 2nd dope | PEG | 28 | 58 | ||
| Factors | Values |
|---|---|
| Bore solution supply (mL/min) | 0.6–0.8 |
| 1st dope solution supply (R.P.M.) | 1–1.5 |
| 2nd dope solution supply (R.P.M.) | 1–1.5 |
| Air gap (cm) | 20–30 |
| Take-up winder speed (m/min) | 12–15 |
| Coagulation tank temperature (°C) | 25 |
| Nozzle temperature (°C) | 40–55 |
| Dope tank temperature (°C) | 40–55 |
| Classification | Molecular Weight (M.W.) | Hansen Solubility Parameter (, MPa1/2) |
|---|---|---|
| Water | 18.02 g/moL | 47.80 [31] |
| N-methyl-2-pyrrolidone (NMP) | 99.13 g/moL | 22.90 [32] |
| Polyvinylpyrrolidone k-30 (PVP) | 40,000 Dalton | 21.57 [33] |
| Polyethylene glycol (PEG) | 400 Dalton | 18.90 [31] |
| Classification | WSP (wt.%) | Outer Diameter (μm) | Thickness (μm) | Mean Pore Size (nm) | Porosity (%) | Pure Water Permeability (L/m2·h·bar) | BSA Leakage (%) | Urea Clearance (mL/min) | |
|---|---|---|---|---|---|---|---|---|---|
| PVP | PEG | ||||||||
| DHF-512 | 5 | 12 | 286.9 ± 8.3 | 34.5 ± 2.6 | 26.8 | 92.0 ± 1.0 | 188.0 ± 14.5 | 0.06 ± 0.02 | None |
| DHF-520 | 5 | 20 | 298.1 ± 6.1 | 33.2 ± 0.5 | 27.4 | 93.8 ± 1.2 | 213.5 ± 4.0 | 0.17 ± 0.08 | 257.6 * |
| DHF-528 | 5 | 28 | 284.6 ± 4.8 | 33.8 ± 0.7 | 33.2 | 92.2 ± 0.6 | 296.2 ± 5.7 | 1.74 ± 0.03 | None |
| CHF | No information | 244.3 ± 1.8 | 31.2 ± 1.1 | 31.7 | 85.6 ± 2.0 | 248.0 ± 19.9 | 2.16 ± 0.19 | 281.9 [30] ** | |
| Classification | DHF-520 | CHF |
|---|---|---|
| Inner diameter (ID) (m) | 2.316 × 10−4 | 2.443 × 10−4 |
| Effective area of HF (m2) | 1.819 × 10−4 | 1.429 × 10−4 |
| Required number of fibers | ≈9896 fibers | ≈12,599 fibers |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, K.; Nguyen, T.-T.; Yi, E.; Kim, C.S.; Kim, S.W.; Kim, I.S. Open Pore Ultrafiltration Hollow Fiber Membrane Fabrication Method via Dual Pore Former with Dual Dope Solution Phase. Membranes 2022, 12, 1140. https://doi.org/10.3390/membranes12111140
Jang K, Nguyen T-T, Yi E, Kim CS, Kim SW, Kim IS. Open Pore Ultrafiltration Hollow Fiber Membrane Fabrication Method via Dual Pore Former with Dual Dope Solution Phase. Membranes. 2022; 12(11):1140. https://doi.org/10.3390/membranes12111140
Chicago/Turabian StyleJang, Kyunghoon, Thanh-Tin Nguyen, Eunsung Yi, Chang Seong Kim, Soo Wan Kim, and In S. Kim. 2022. "Open Pore Ultrafiltration Hollow Fiber Membrane Fabrication Method via Dual Pore Former with Dual Dope Solution Phase" Membranes 12, no. 11: 1140. https://doi.org/10.3390/membranes12111140
APA StyleJang, K., Nguyen, T.-T., Yi, E., Kim, C. S., Kim, S. W., & Kim, I. S. (2022). Open Pore Ultrafiltration Hollow Fiber Membrane Fabrication Method via Dual Pore Former with Dual Dope Solution Phase. Membranes, 12(11), 1140. https://doi.org/10.3390/membranes12111140







