Hydroxytyrosol Enrichment of Olive Leaf Extracts via Membrane Separation Processes
Abstract
1. Introduction
2. Materials and Methods
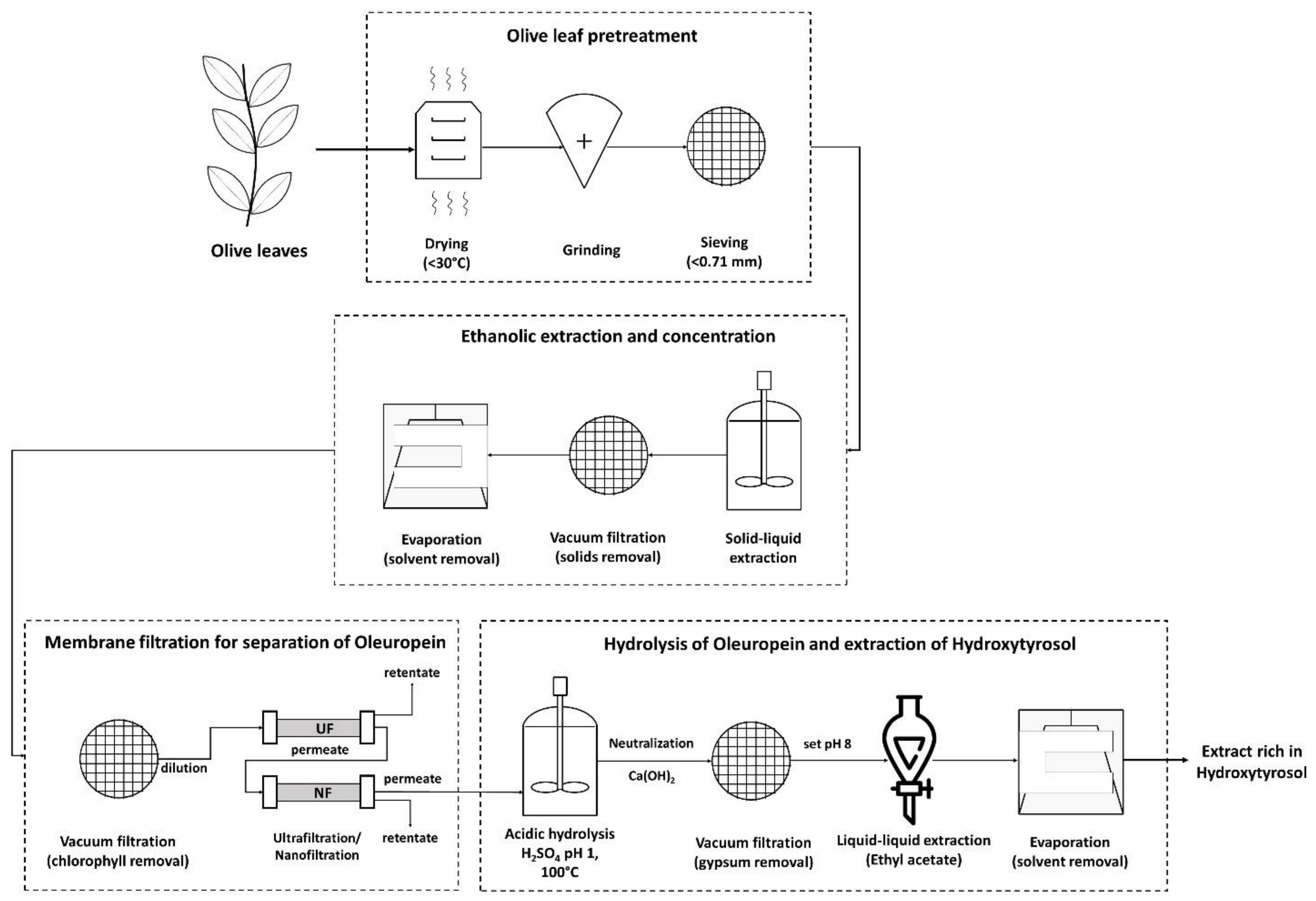
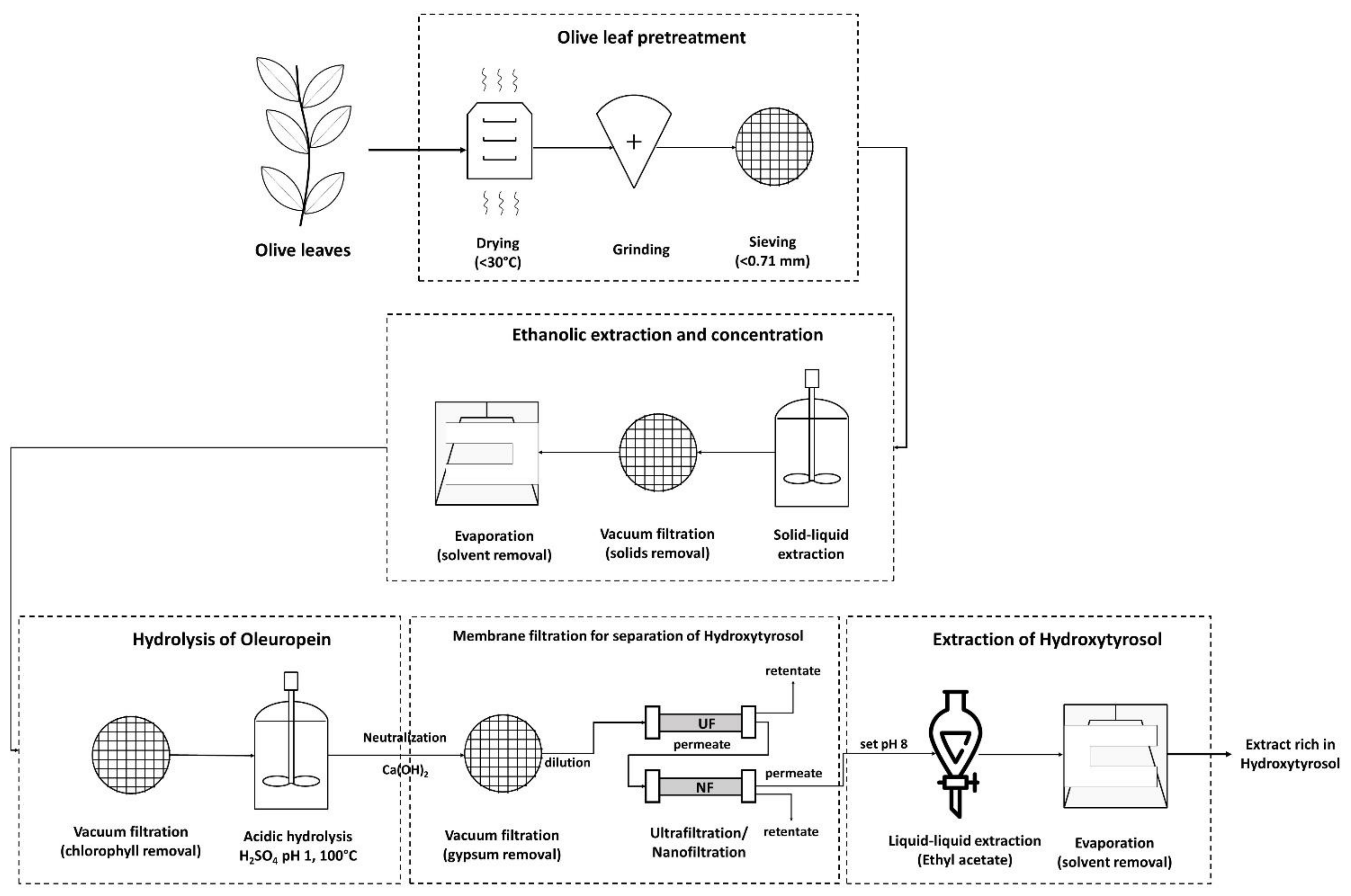
2.1. Pretreatment
2.2. Physicochemical Processes
2.3. Analyses
3. Results and Discussion
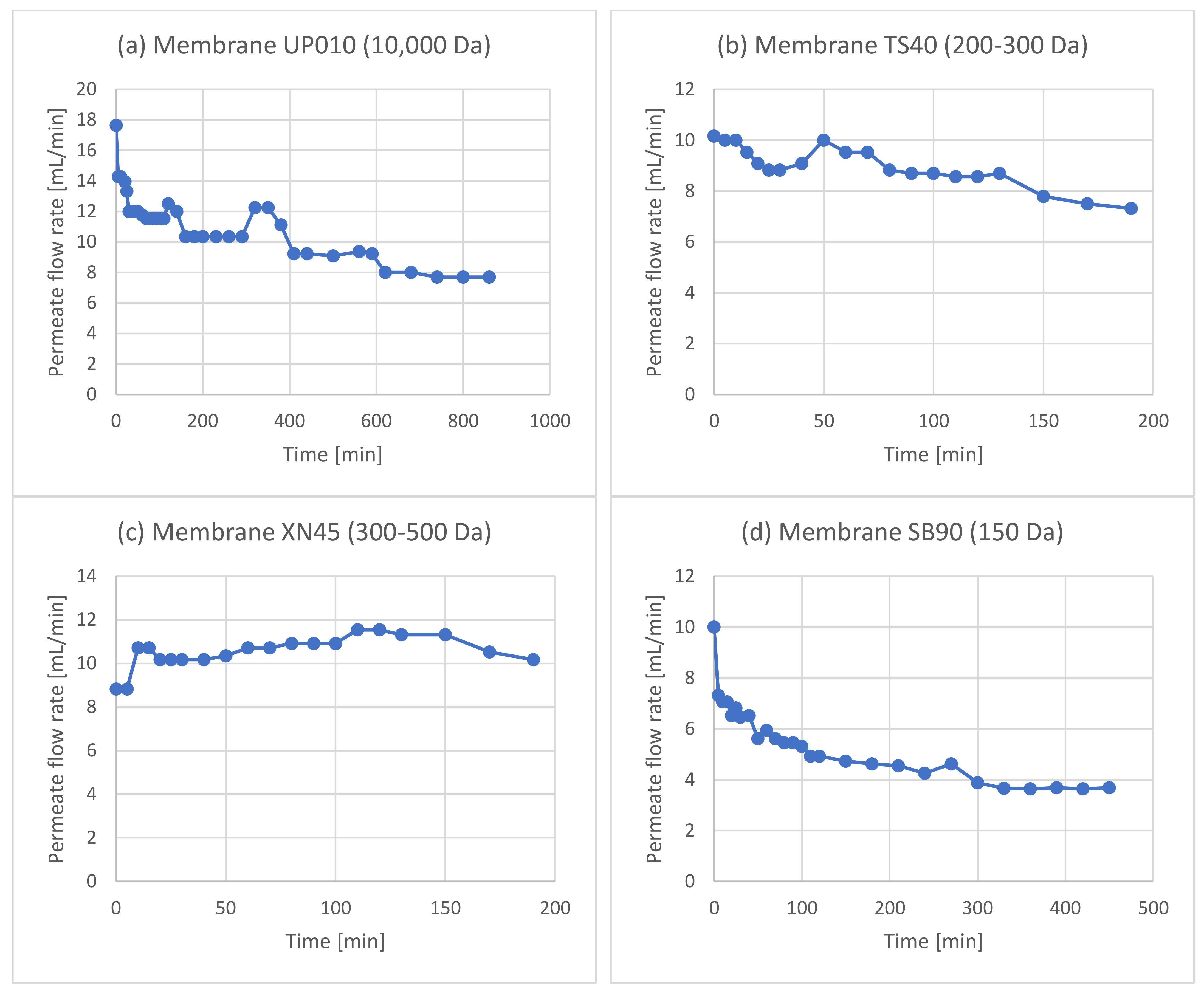
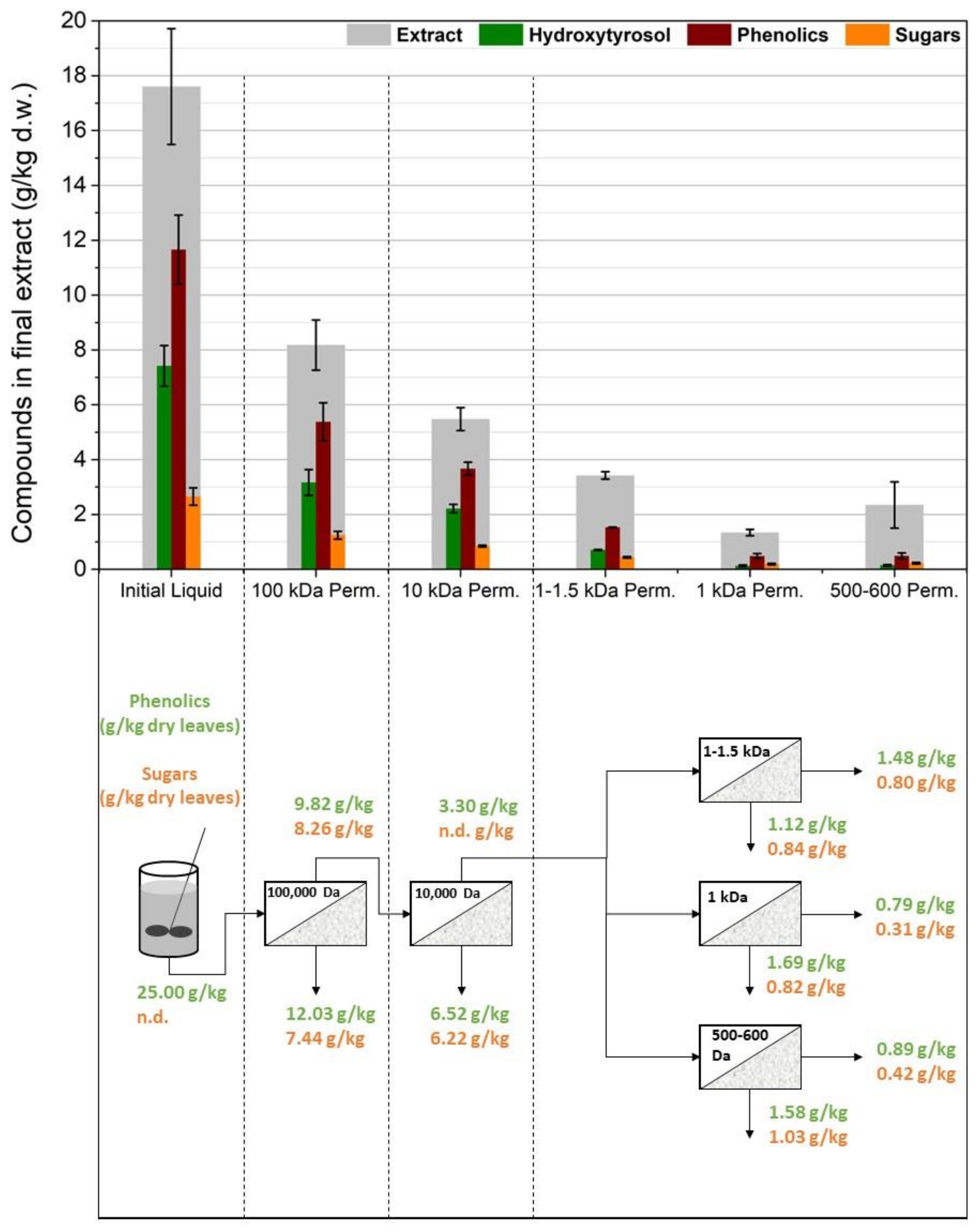
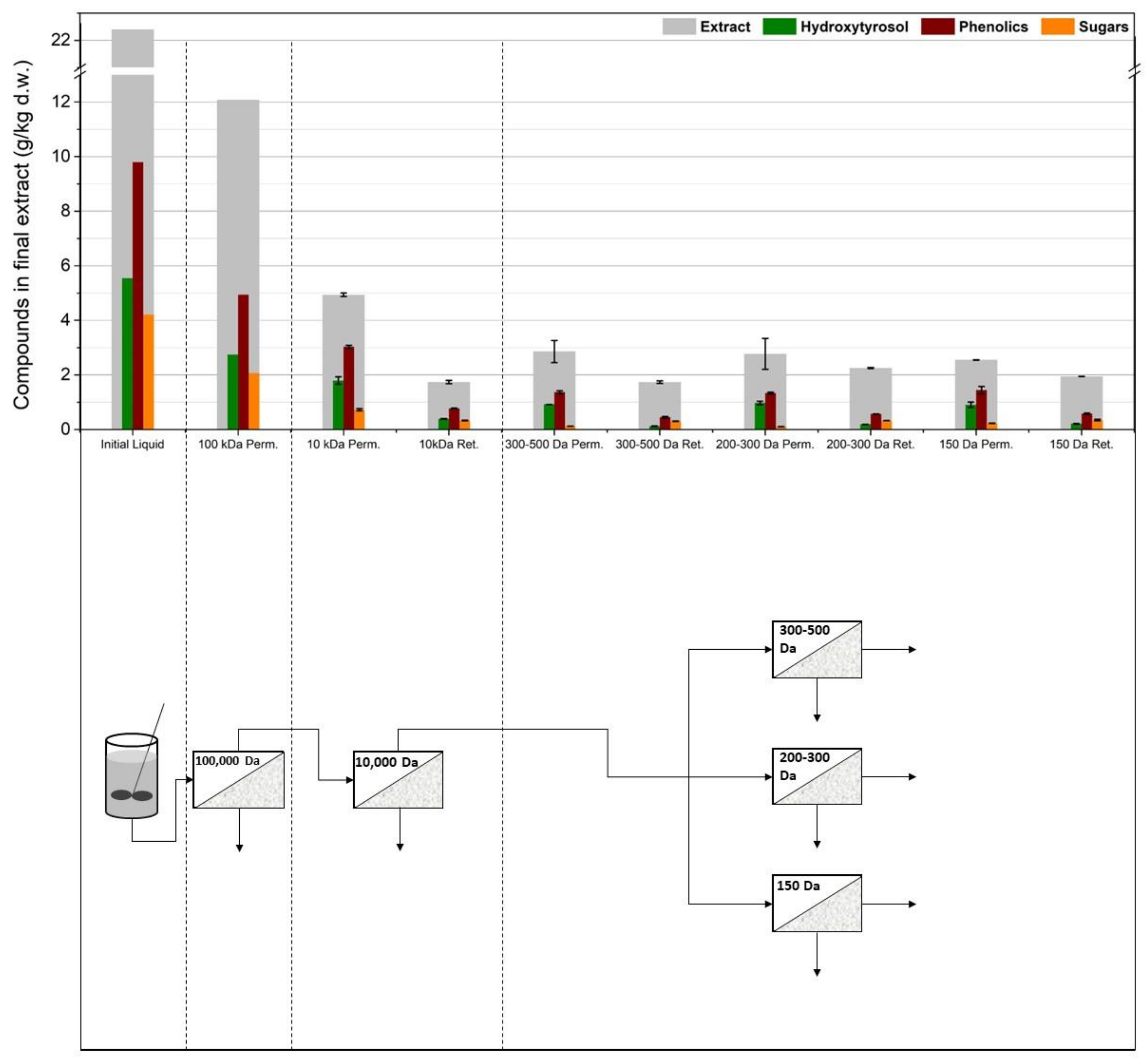
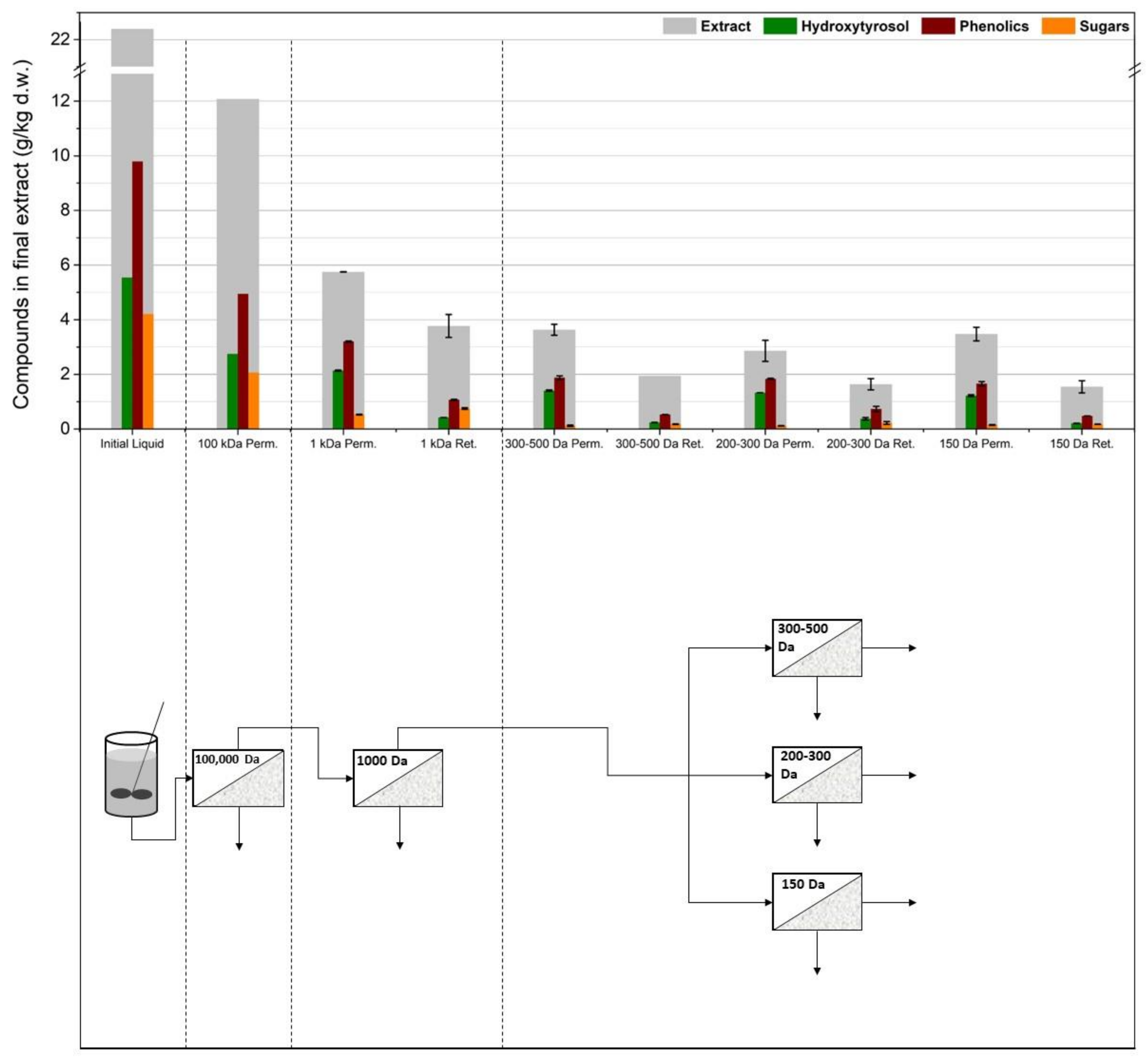
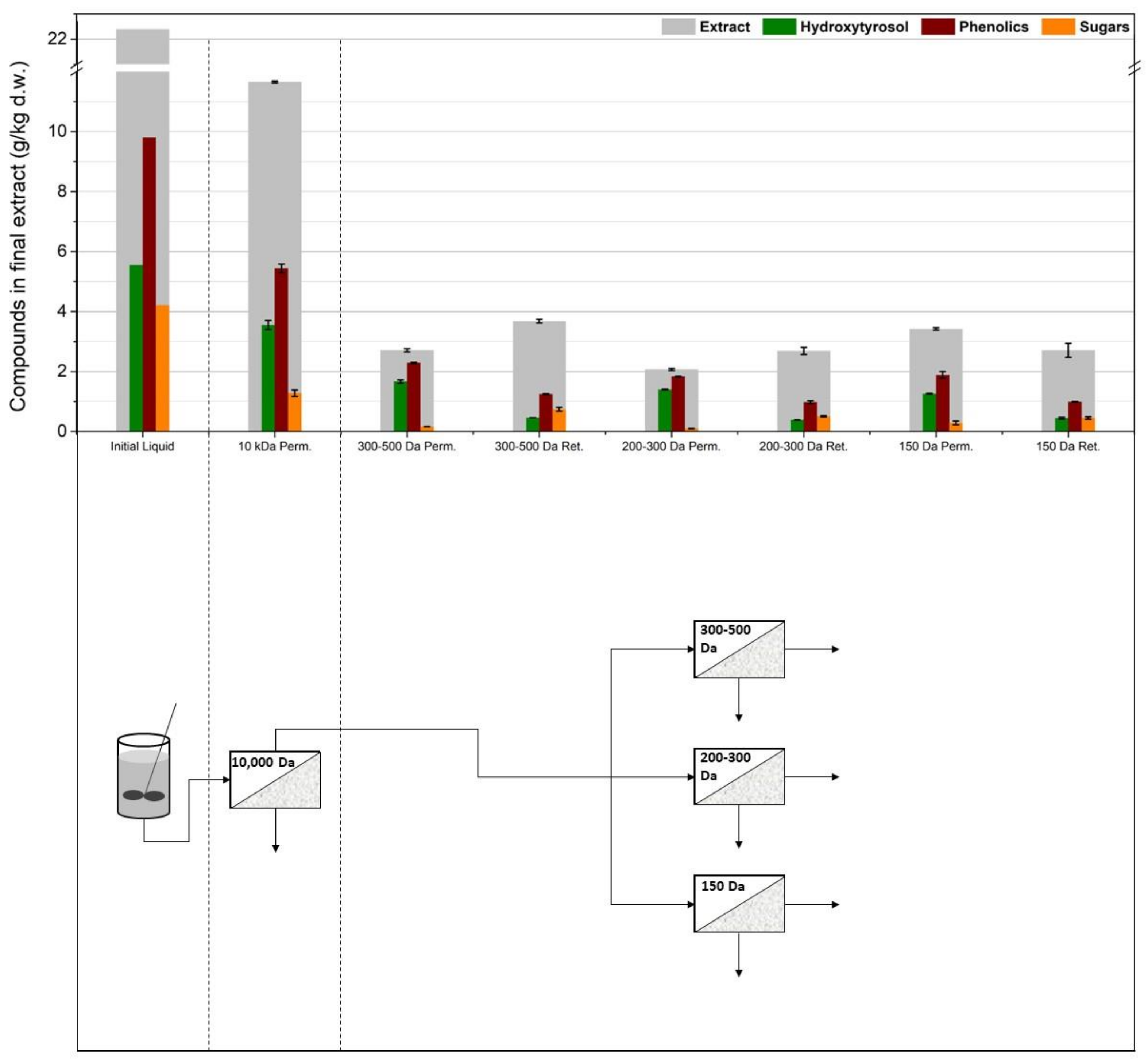
3.1. Membrane Filtration after Solid–Liquid Extraction
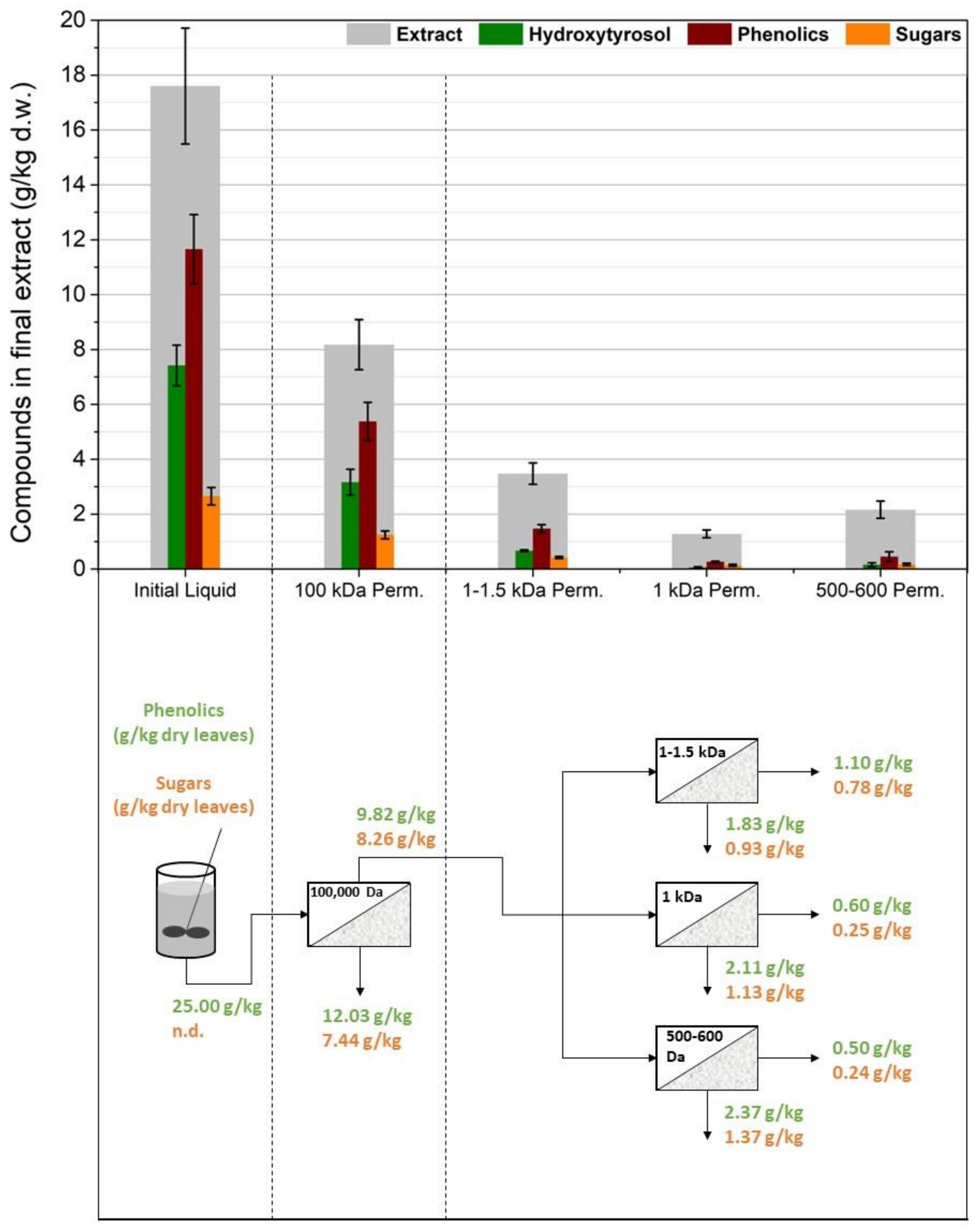
3.2. Membrane Filtration after Acid Hydrolysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Cellulose acetate | CA |
| Gallic acid equivalents | GAE |
| Molecular weight cut-off | MWCO |
| Nanofiltration | NF |
| Polyethersulfone | PES |
| Polyvinylidene fluoride | PVDF |
| Polysulfone | PSUH |
| Trifluoroacetic acid | TFA |
| Ultrafiltration | UF |
Appendix A
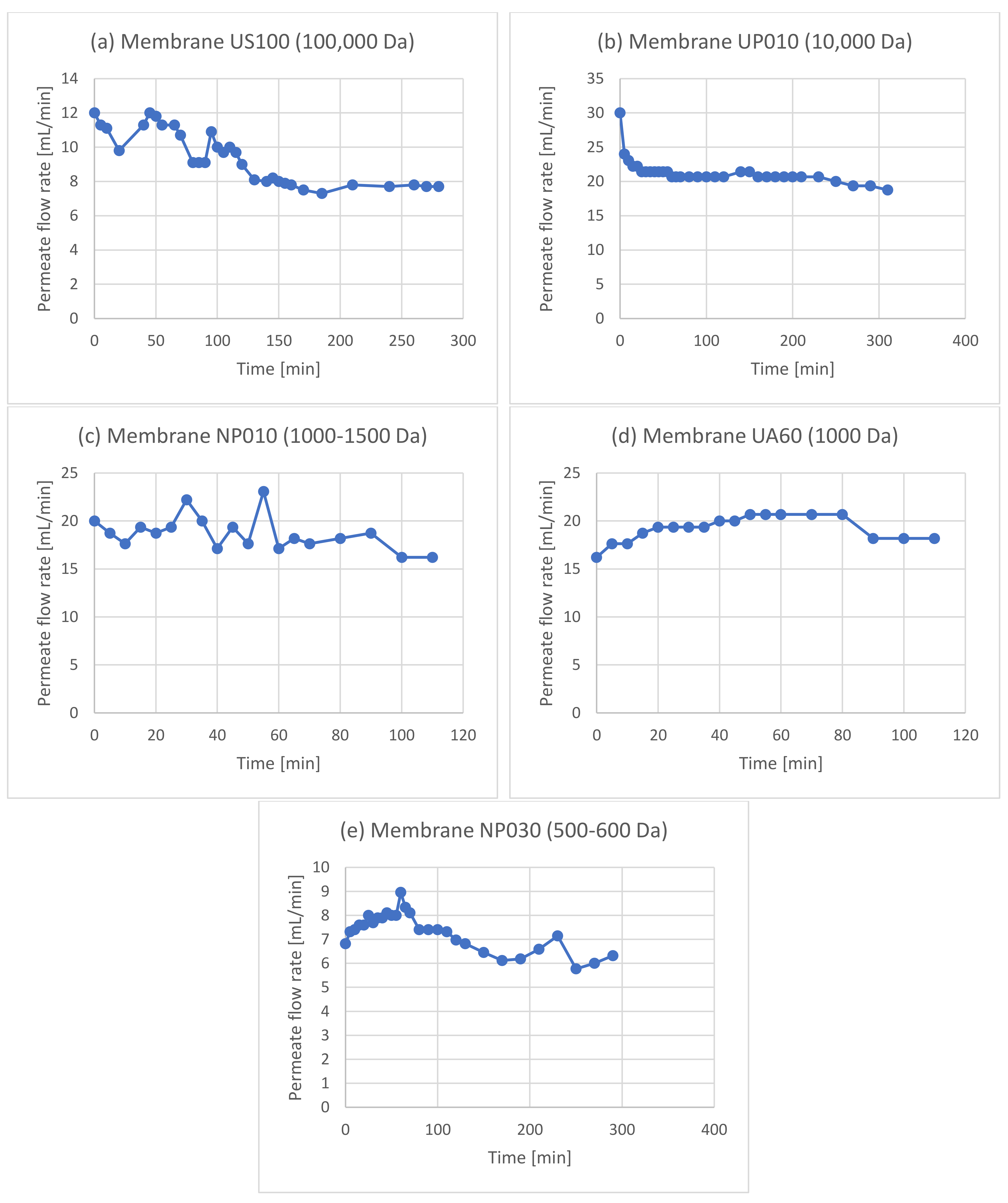
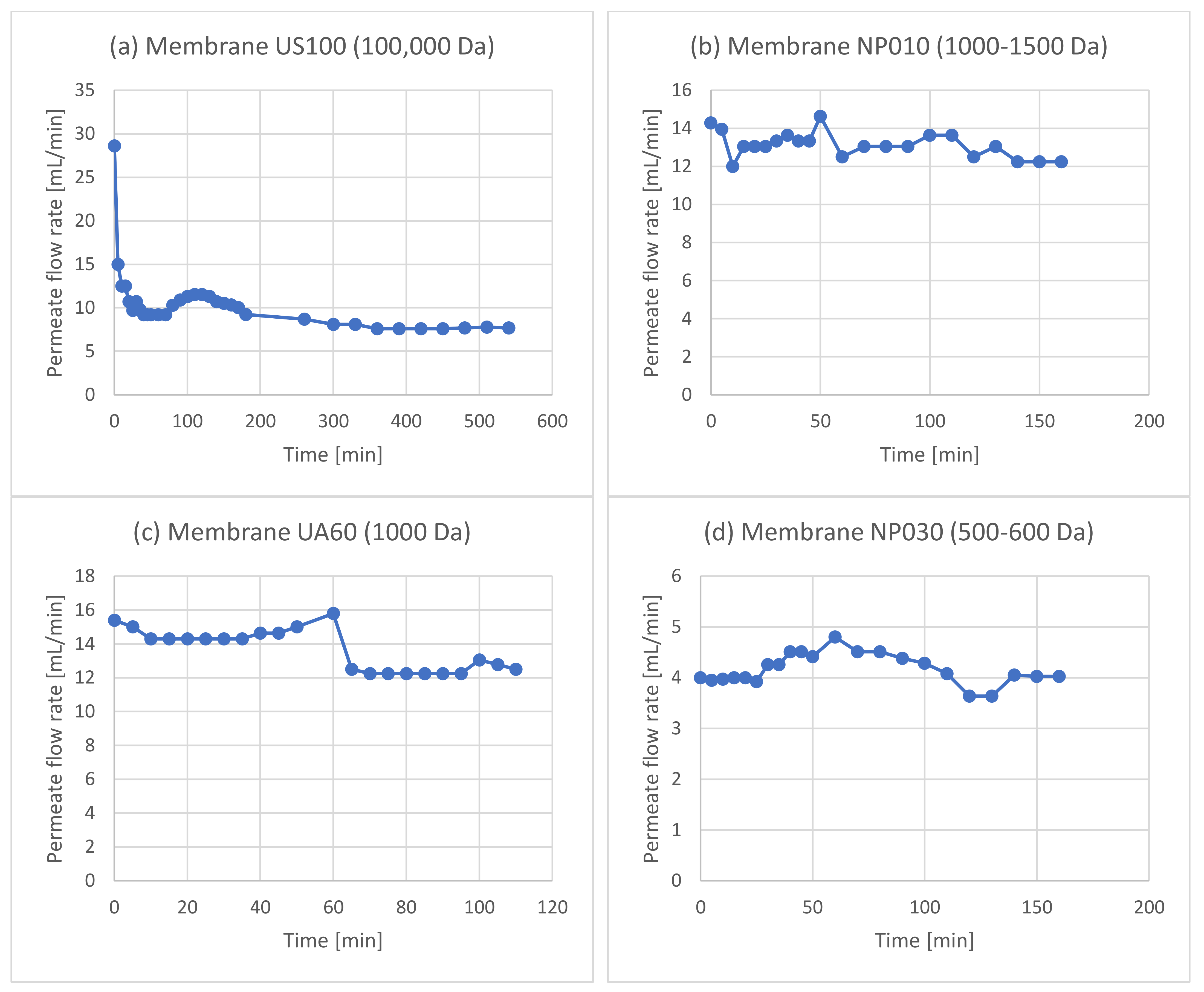
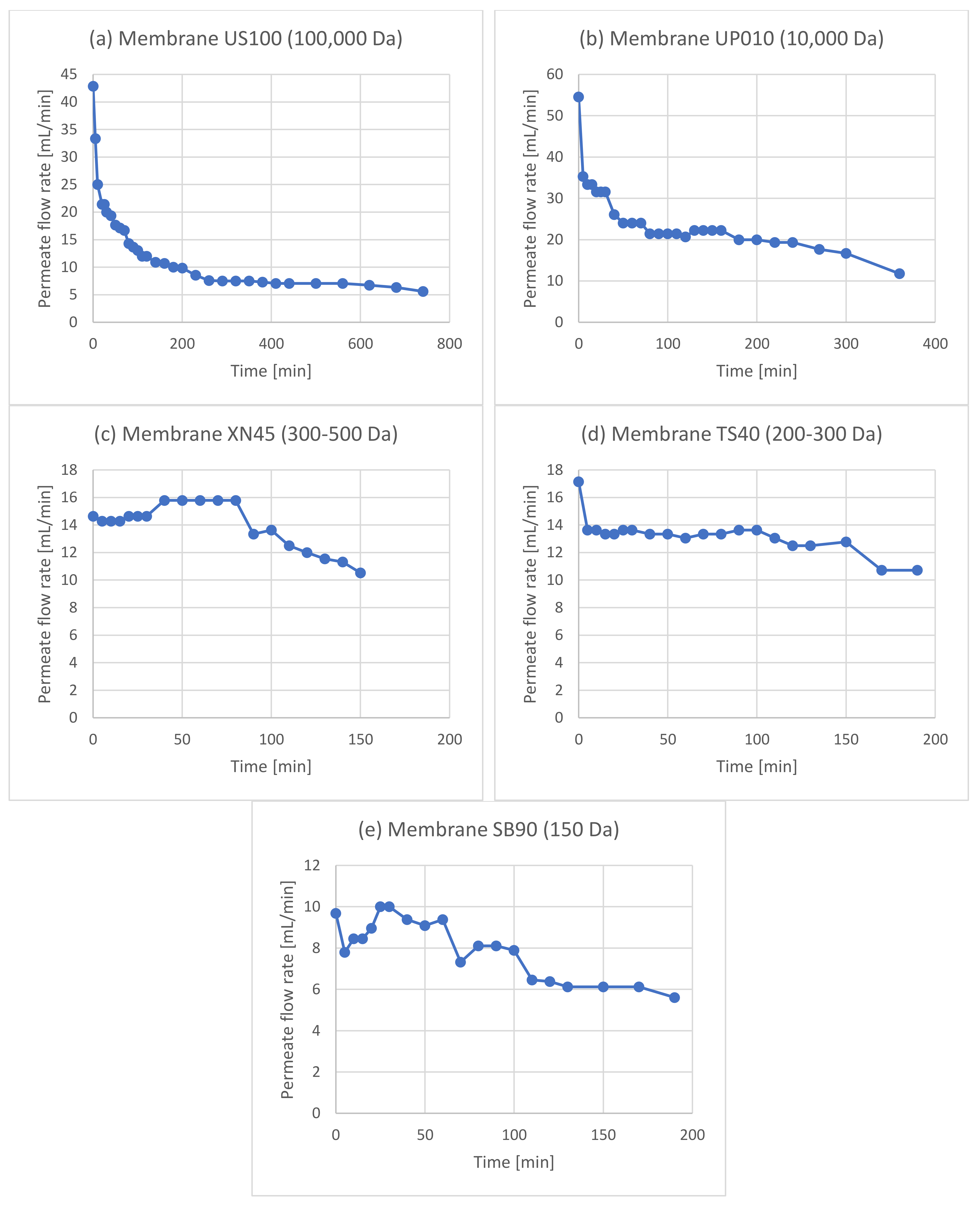
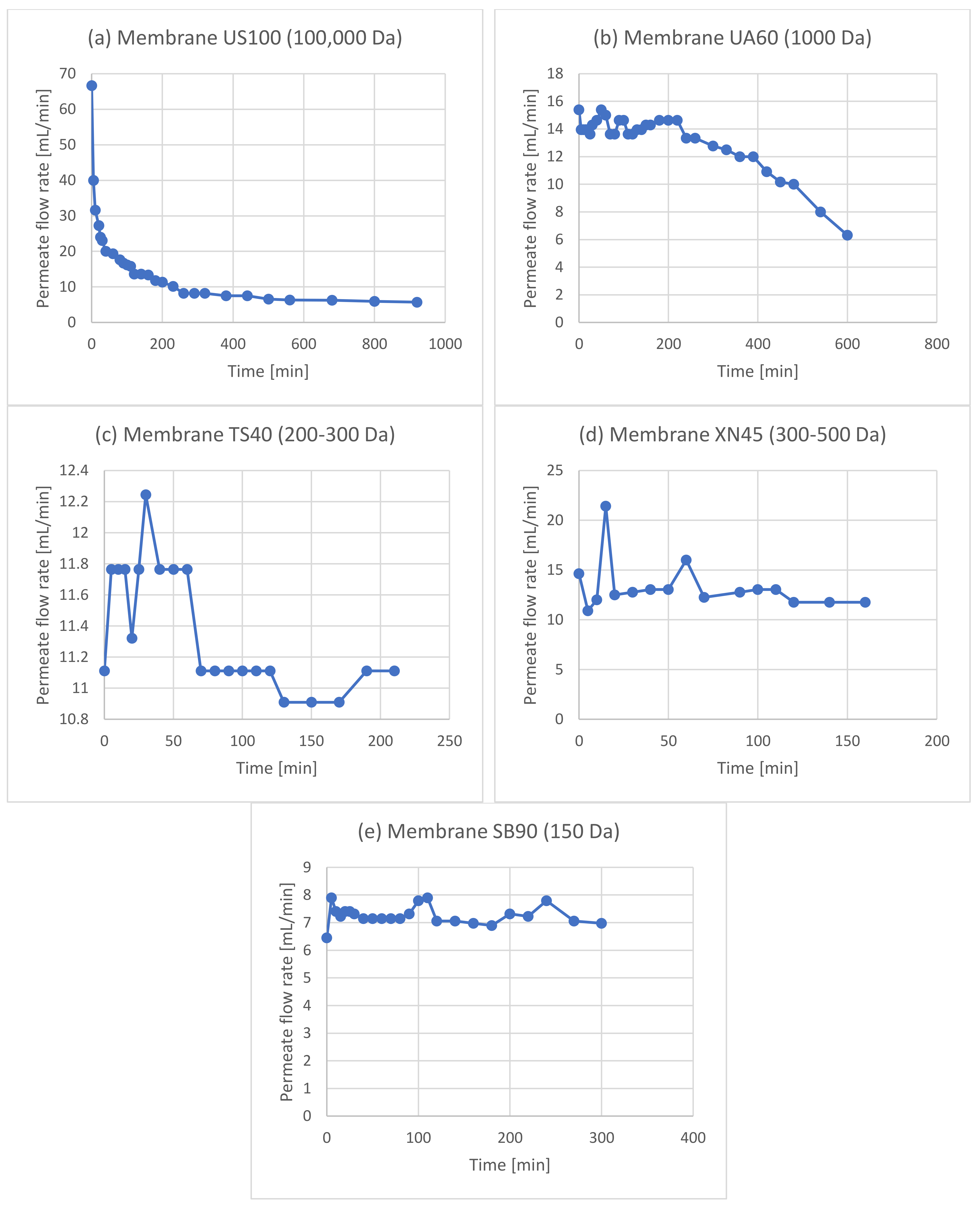
| Experiment | Membrane | Rejection of Phenols | Rejection of Sugars |
|---|---|---|---|
| 1.1 | US100 | 54% | 53% |
| UP010 | 32% | 32% | |
| NP010 | 58% | 48% | |
| UA60 | 87% | 77% | |
| NP030 | 87% | 73% | |
| 1.2 | US100 | 54% | 53% |
| NP010 | 73% | 66% | |
| UA60 | 95% | 89% | |
| NP030 | 92% | 87% | |
| 2.1 | US100 | 54% | 53% |
| UP010-P | 44% | 41% | |
| XN45-P | 55% | 83% | |
| TS40-P | 56% | 85% | |
| SB90-P | 52% | 68% | |
| 2.2 | US100 | 50% | 51% |
| UA60 | 35% | 74% | |
| XN45 | 41% | 77% | |
| TS40 | 43% | 78% | |
| SB90 | 48% | 72% | |
| 2.3 | UP010 | 44% | 70% |
| XN45 | 58% | 87% | |
| TS40 | 66% | 92% | |
| SB90 | 65% | 77% |
References
- Schütz, K.; Muks, E.; Carle, R.; Schieber, A. Quantitative determination of phenolic compounds in artichoke-based dietary supplements and pharmaceuticals by high-performance liquid chromatography. J. Agric. Food Chem. 2006, 54, 8812–8817. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Cao, G. Analysis of botanicals and dietary supplements for antioxidant capacity: A review. J. AOAC Int. 2000, 83, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Hanif, R.; Qiao, L.; Shiff, S.J.; Rigas, B. Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell cycle changes in colon adenocarcinoma cell lines by a prostaglandin-independent pathway. J. Lab. Clin. Med. 1997, 130, 576–584. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging Role of Phenolic Compounds as Natural Food Additives in Fish and Fish Products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Cottaz, A.; Bouarab, L.; De Clercq, J.; Oulahal, N.; Degraeve, P.; Joly, C. Potential of incorporation of antimicrobial plant phenolics into polyolefin-based food contact materials to produce active packaging by melt-blending: Proof of concept with isobutyl-4-hydroxybenzoate. Front. Chem. 2019, 7, 148. [Google Scholar] [CrossRef]
- Lopes, J.; Gonçalves, I.; Nunes, C.; Teixeira, B.; Mendes, R.; Ferreira, P.; Coimbra, M.A. Potato peel phenolics as additives for developing active starch-based films with potential to pack smoked fish fillets. Food Packag. Shelf Life 2021, 28, 100644. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green alternative methods for the extraction of antioxidant bioactive compounds from winery wastes and by-products: A review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total phenolic content and antioxidant capacity of agri-food waste and by-products. Ital. J. Anim. Sci. 2019, 18, 336–341. [Google Scholar] [CrossRef]
- Kabir, F.; Tow, W.W.; Hamauzu, Y.; Katayama, S.; Tanaka, S.; Nakamura, S. Antioxidant and cytoprotective activities of extracts prepared from fruit and vegetable wastes and by-products. Food Chem. 2015, 167, 358–362. [Google Scholar] [CrossRef]
- Taylor, G. Biofuels and the biorefinery concept. Energy Policy 2008, 36, 4406–4409. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A.S. Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Zagklis, D.P.; Paraskeva, C.A. Preliminary design of a phenols purification plant. J. Chem. Technol. Biotechnol. 2020, 95, 373–383. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Papageorgiou, C.S.; Paraskeva, C.A. Technoeconomic Analysis of the Recovery of Phenols from Olive Mill Wastewater through Membrane Filtration and Resin Adsorption/Desorption. Sustainability 2021, 13, 2376. [Google Scholar] [CrossRef]
- Hadrich, F.; Chamkha, M.; Sayadi, S. Protective effect of olive leaves phenolic compounds against neurodegenerative disorders: Promising alternative for Alzheimer and Parkinson diseases modulation. Food Chem. Toxicol. 2022, 159, 112752. [Google Scholar] [CrossRef]
- Topuz, S.; Bayram, M. Oleuropein extraction from leaves of three olive varieties (Olea europaea L.): Antioxidant and antimicrobial properties of purified oleuropein and oleuropein extracts. J. Food Process. Preserv. 2022, 46, e15697. [Google Scholar] [CrossRef]
- Omar, S.H. Oleuropein in olive and its pharmacological effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef]
- Wichers, H.J.; Soler-rivas, C.; Espı, J.C. Review Oleuropein and related compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Borjan, D.; Leitgeb, M.; Knez, Ž.; Hrnčič, M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules 2020, 25, 5946. [Google Scholar] [CrossRef] [PubMed]
- Fava, G.; Di Mauro, M.D.; Spampinato, M.; Biondi, D.; Gambera, G.; Centonze, G.; Maggiore, R.; D’Antona, N. Hydroxytyrosol Recovery From Olive Mill Wastewater: Process Optimization and Development of a Pilot Plant. Clean Soil Air Water 2017, 45, 1600042. [Google Scholar] [CrossRef]
- Bonetti, A.; Venturini, S.; Ena, A.; Faraloni, C. Innovative method for recovery and valorization of hydroxytyrosol from olive mill wastewaters. Water Sci. Technol. 2016, 74, 73–86. [Google Scholar] [CrossRef]
- Hamza, M.; Sayadi, S. The Possibility of Recovering of Hydroxytyrosol from Olive Milling Wastewater by Enzymatic Bioconversion. In Products from Olive Tree; Boskou, D., Clodoveo, M.L., Eds.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Fernandez-Bolanos, J.; Lopez, O.; Fernandez-Bolanos, J.; Rodriguez-Gutierrez, G. Hydroxytyrosol and Derivatives: Isolation, Synthesis, and Biological Properties. Curr. Org. Chem. 2008, 12, 442–463. [Google Scholar] [CrossRef]
- Capasso, R.; Evidente, A.; Avolio, S.; Solla, F. A highly convenient synthesis of hydroxytyrosol and its recovery from agricultural waste waters. J. Agric. Food Chem. 1999, 47, 1745–1748. [Google Scholar] [CrossRef]
- Papageorgiou, C.S.; Lyri, P.; Xintaropoulou, I.; Diamantopoulos, I.; Zagklis, D.P.; Paraskeva, C.A. High-Yield Production of a Rich-in-Hydroxytyrosol Extract from Olive (Olea europaea) Leaves. Antioxidants 2022, 11, 1042. [Google Scholar] [CrossRef]
- Mudimu, O.A.; Peters, M.; Brauner, F.; Braun, G. Overview of membrane processes for the recovery of polyphenols from olive mill wastewater olive mill wastewater. Am. J. Environ. Sci. 2012, 8, 195–201. [Google Scholar] [CrossRef][Green Version]
- Zagklis, D.P.; Vavouraki, A.I.; Kornaros, M.E.; Paraskeva, C.A. Purification of olive mill wastewater phenols through membrane filtration and resin adsorption/desorption. J. Hazard. Mater. 2015, 285, 69–76. [Google Scholar] [CrossRef]
- Konstantinos, B.; Petrotos, K.; Lellis, T.; Kokkora, M.; Gkoutsidis, P. Purification of Olive Mill Wastewater Using Microfiltration Membrane Technology. J. Membr. Sep. Technol. 2014, 3, 50–55. [Google Scholar] [CrossRef]
- Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Cassano, A. Olive mill wastewater polyphenol-enriched fractions by integrated membrane process: A promising source of antioxidant, hypolipidemic and hypoglycaemic compounds. Antioxidants 2020, 9, 602. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mater. 2013, 248–249, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Zagklis, D.P.; Papageorgiou, C.S.; Paraskeva, C.A. 18—Valorization of phenolic extracts from Olea europaea L. by membrane operations. In Membrane Engineering in the Circular Economy; Iulianelli, A., Cassano, A., Conidi, C., Petrotos, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 495–524. ISBN 978-0-323-85253-1. [Google Scholar]
- Lozada, G.S.L.; López, A.I.G.; Martínez-Férez, A.; Ochando-Pulido, J.M. On the modeling and optimization of two-phase olive-oil washing wastewater treatment and polyphenols recovery by ceramic tubular microfiltration membranes. J. Environ. Manag. 2022, 316, 115227. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Corpas-Martínez, J.R.; Vellido-Perez, J.A.; Martinez-Ferez, A. Optimization of polymeric nanofiltration performance for olive-oil-washing wastewater phenols recovery and reclamation. Sep. Purif. Technol. 2020, 236, 116261. [Google Scholar] [CrossRef]
- Tundis, R.; Conidi, C.; Loizzo, M.R.; Sicari, V.; Romeo, R.; Cassano, A. Concentration of Bioactive Phenolic Compounds in Olive Mill Wastewater by Direct Contact Membrane Distillation. Molecules 2021, 26, 1808. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Josefsson, B.; Uppström, L.; Östling, G. Automatic spectrophotometric procedures for the determination of the total amount of dissolved carbohydrates in sea water. Deep Sea Res. Oceanogr. Abstr. 1972, 19, 385–395. [Google Scholar] [CrossRef]
- Geens, J.; Van der Bruggen, B.; Vandecasteele, C. Characterisation of the solvent stability of polymeric nanofiltration membranes by measurement of contact angles and swelling. Chem. Eng. Sci. 2004, 59, 1161–1164. [Google Scholar] [CrossRef]
- Rezzadori, K.; Penha, F.M.; Proner, M.C.; Zin, G.; Petrus, J.C.C.; Di Luccio, M. Impact of Organic Solvents on Physicochemical Properties of Nanofiltration and Reverse-Osmosis Membranes. Chem. Eng. Technol. 2019, 42, 2700–2708. [Google Scholar] [CrossRef]

| Membrane | Membrane Type | Membrane Material | Permeability (LMH/bar) | MWCO (Da) |
|---|---|---|---|---|
| US100 | UF | Polysulfone (PSUH) | >100 | 100,000 |
| UP010 | UF | Polyethersulfone (PES) | >50 | 10,000 |
| NP010 | NF | Polyethersulfone (PES) | >5 | 1000–1500 |
| UA60 | NF | Thin-Film Polypiperazine | 76.5–136 (LMH) | 1000 |
| NP030 | NF | Polyethersulfone (PES) | >1 | 500–600 |
| XN45 | NF | Thin-Film Polypiperazine | 47.6–73.1 (LMH) | 300–500 |
| TS50 | NF | Piperazine | 300 | |
| TS40 | NF | Thin-Film Polypiperazine | 40.8–61.2 (LMH) | 200–300 |
| SB90 | NF | Cellulose Acetate (CA) | 44.2–69.7 (LMH) | 150 |
| X-20 | RO | Thin-Film Polyamide | 47.6–71.4 (LMH) | <150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papageorgiou, C.S.; Lymberopoulos, S.; Bakas, P.; Zagklis, D.P.; Sygouni, V.; Paraskeva, C.A. Hydroxytyrosol Enrichment of Olive Leaf Extracts via Membrane Separation Processes. Membranes 2022, 12, 1027. https://doi.org/10.3390/membranes12111027
Papageorgiou CS, Lymberopoulos S, Bakas P, Zagklis DP, Sygouni V, Paraskeva CA. Hydroxytyrosol Enrichment of Olive Leaf Extracts via Membrane Separation Processes. Membranes. 2022; 12(11):1027. https://doi.org/10.3390/membranes12111027
Chicago/Turabian StylePapageorgiou, Costas S., Stathis Lymberopoulos, Panagiotis Bakas, Dimitris P. Zagklis, Varvara Sygouni, and Christakis A. Paraskeva. 2022. "Hydroxytyrosol Enrichment of Olive Leaf Extracts via Membrane Separation Processes" Membranes 12, no. 11: 1027. https://doi.org/10.3390/membranes12111027
APA StylePapageorgiou, C. S., Lymberopoulos, S., Bakas, P., Zagklis, D. P., Sygouni, V., & Paraskeva, C. A. (2022). Hydroxytyrosol Enrichment of Olive Leaf Extracts via Membrane Separation Processes. Membranes, 12(11), 1027. https://doi.org/10.3390/membranes12111027









