Reduced Low–Pressure Membrane Fouling by Inline Coagulation Pretreatment for a Colored River Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Water
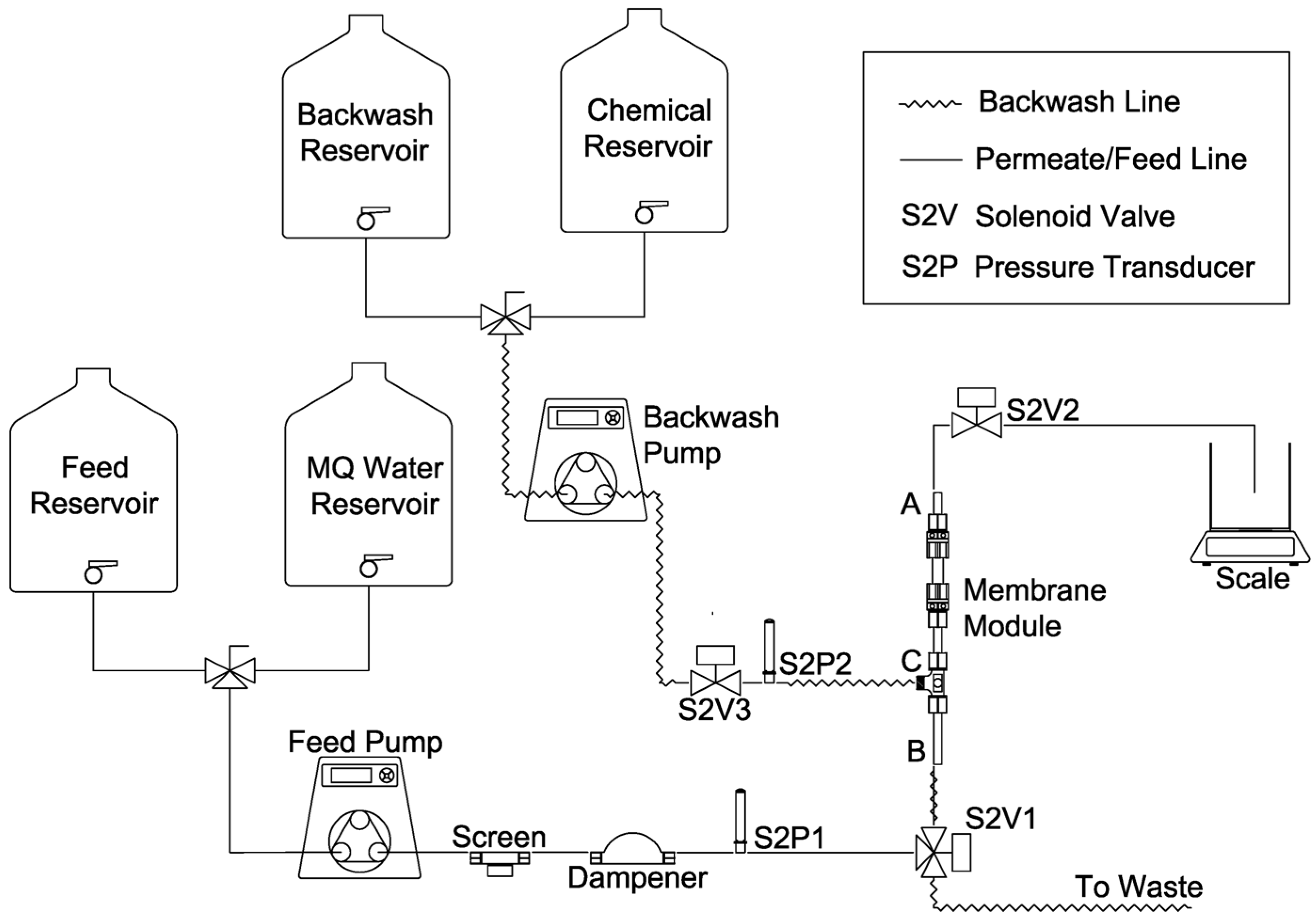
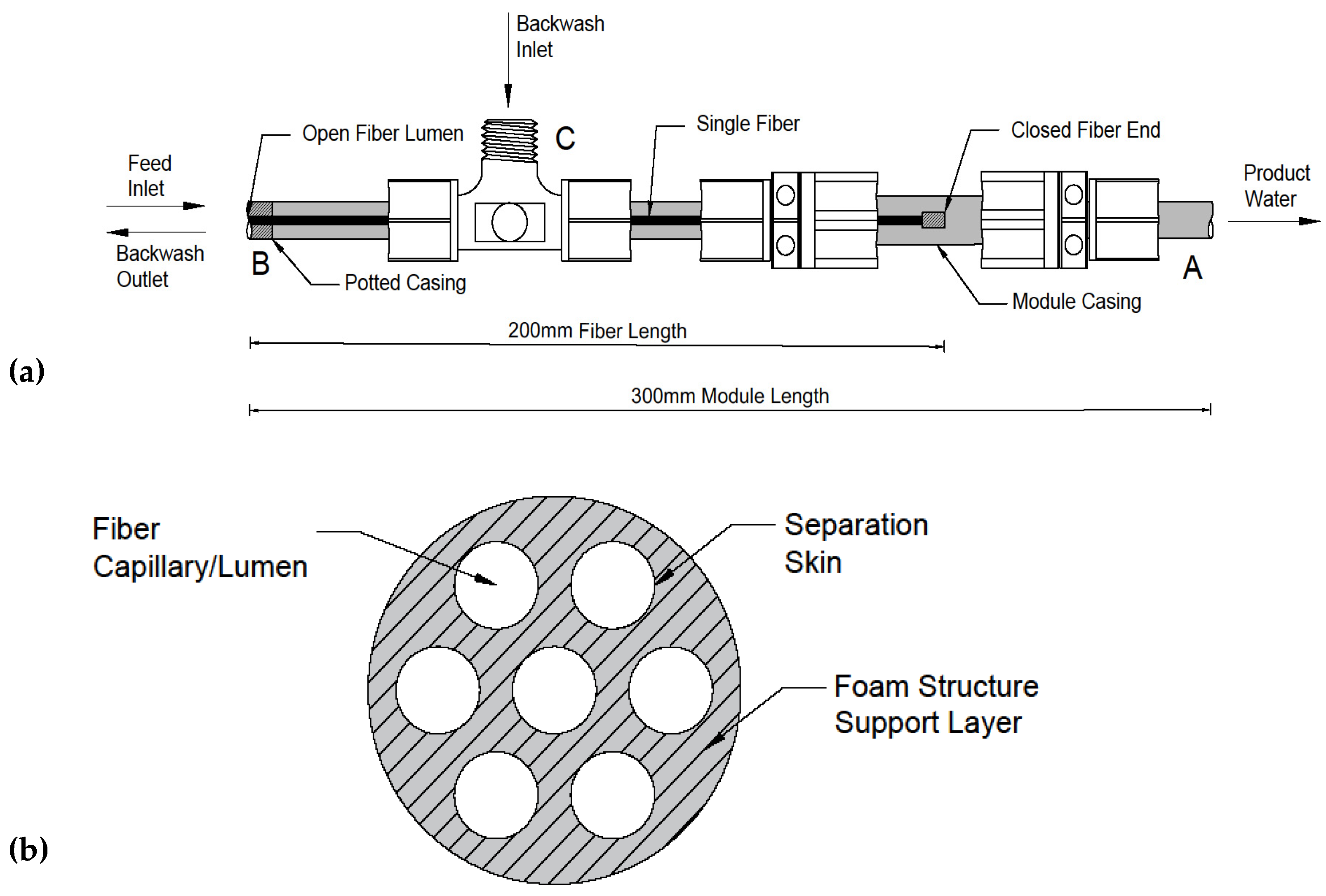
2.2. Filtration Apparatus and Membrane Module
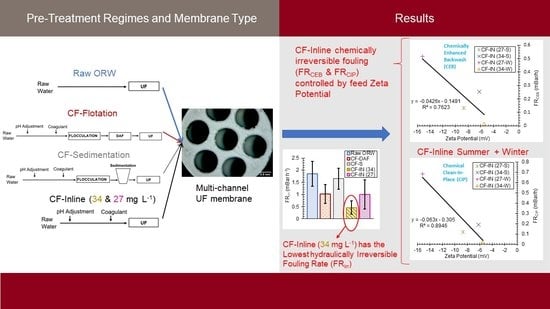
2.3. Pretreatment Methods
2.4. Multi–Day Filtration Test Protocol
2.5. Membrane Performance Measures
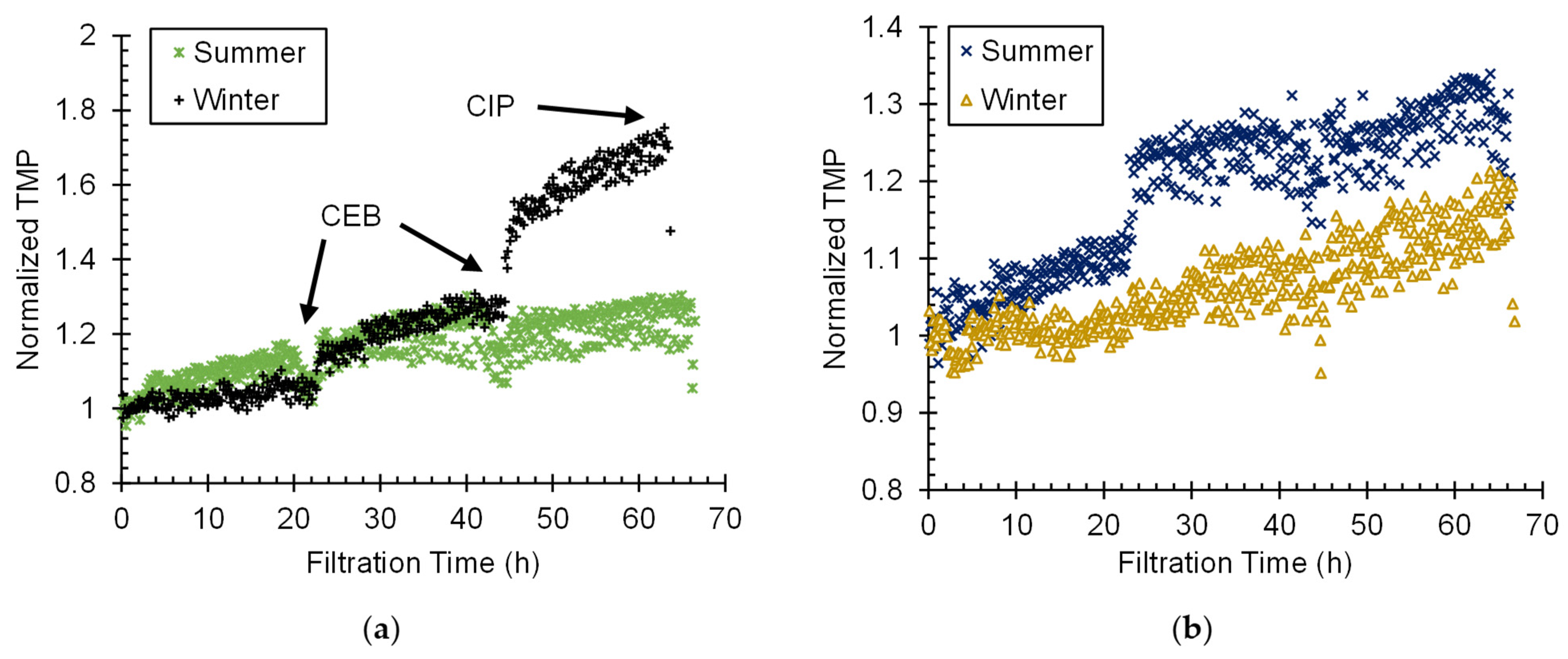
2.5.1. Normalized TMP (nTMP) Profiles
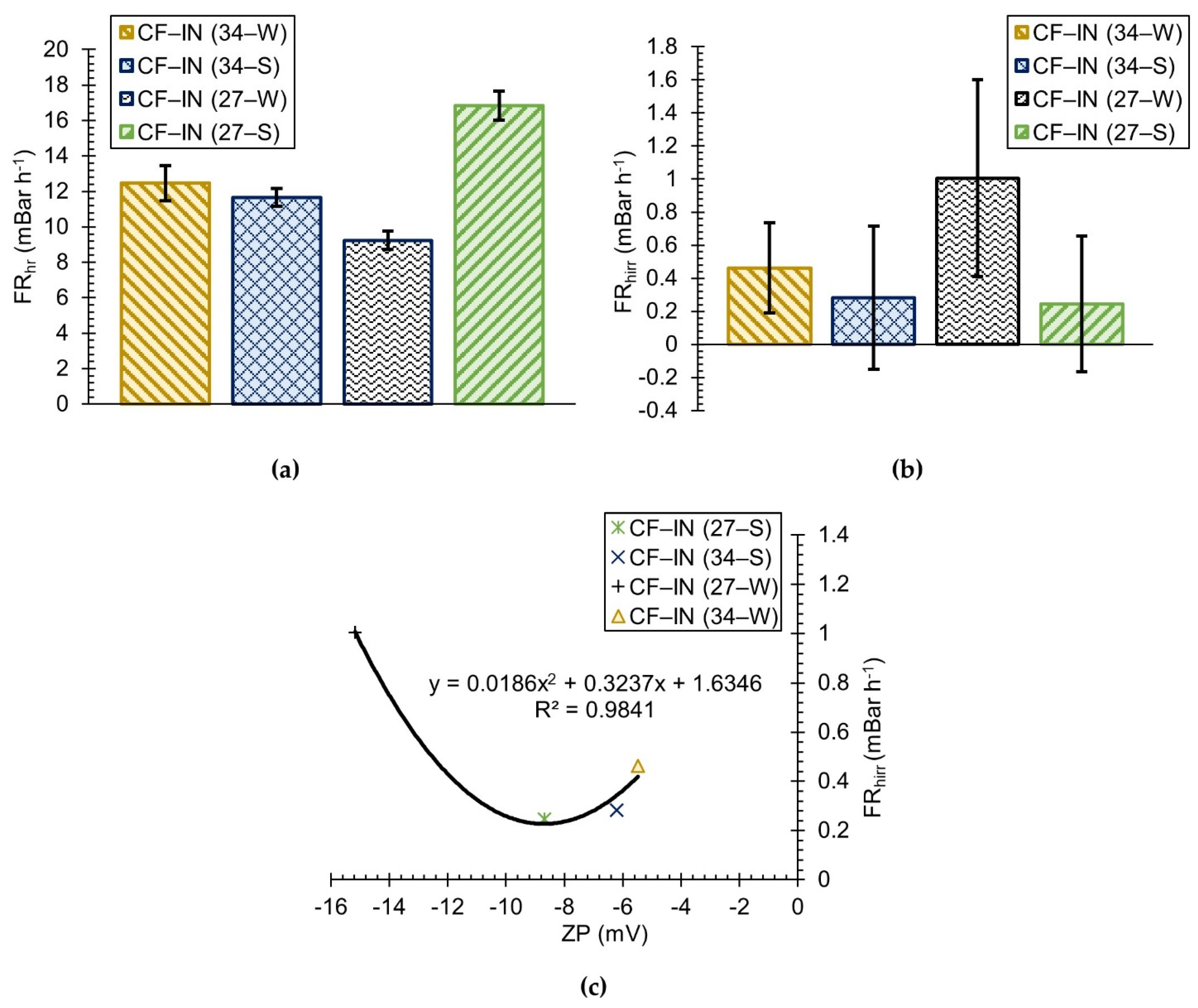
2.5.2. Fouling Rates
2.6. Analytical Methods
3. Results
3.1. Water Quality
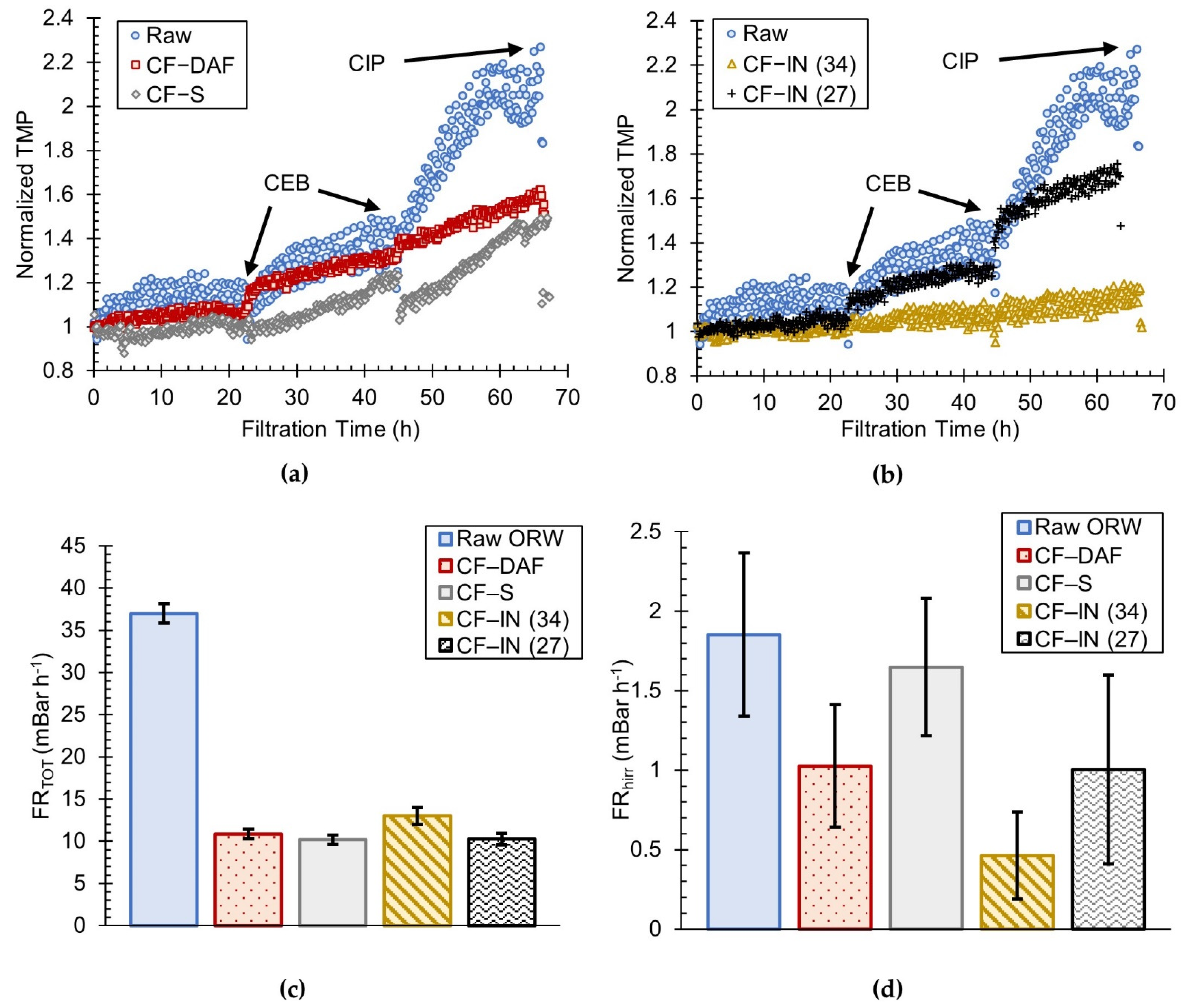
3.2. Comparison of CF–S, CF–DAF, and CF–IN Pretreatment during Winter Season
3.3. CF–IN Seasonality Assessment
4. Discussion
5. Conclusions
- For the winter water samples, CF–IN pretreatment at 34 mg L−1 could more effectively mitigate membrane fouling compared to CF–DAF and CF–S. This is in contrast to the findings of previous studies using the same highly colored river water but a different membrane type. This finding is an indication of the uniqueness of the interaction between membrane material/configuration and feed water quality and points to the necessity for preliminary testing when verifying the impact of pretreatment for a given membrane;
- The chemical reversibility of fouling for CF–IN tests seems to be strongly influenced by the ZP of the challenge waters. For CF–IN conditions with lower magnitude ZP, irreversibility of fouling following CEB and CIP was minimized;
- Distinct seasonal differences in CF–IN performance were observed. For the winter season, CF–IN pretreatment at a high dose led to reduced fouling, while for the summer season the low dose performed better. The better performance at the low dose is linked to the lower turbidity and HPO NOM concentrations in the summer water. The effect of these intra–annual variations should be the subject of future research;
- CF–S pretreatment of the highly colored ORW resulted in a similar level of fouling as CF–DAF using the h–PES TMC UF membrane. This was despite the fact that CF–DAF exhibited preferential removal of DOC and HPO organics (as measured by SUVA).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malkoske, T.A.; Bérubé, P.R.; Andrews, R.C. Coagulation/flocculation prior to low pressure membranes in drinking water treatment: A review. Environ. Sci. Water Res. Technol. 2020, 6, 2993–3023. [Google Scholar] [CrossRef]
- Peters, C.D.; Rantissi, T.; Gitis, V.; Hankins, N.P. Retention of natural organic matter by ultrafiltration and the mitigation of membrane fouling through pre-treatment, membrane enhancement, and cleaning—A review. J. Water Process Eng. 2021, 44, 102374. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Microfiltration membrane processes: A review of research trends over the past decade. J. Water Process Eng. 2019, 32, 100941. [Google Scholar] [CrossRef]
- Li, K.; Wen, G.; Li, S.; Chang, H.; Shao, S.; Huang, T.; Li, G.; Liang, H. Effect of pre-oxidation on low pressure membrane (LPM) for water and wastewater treatment: A review. Chemosphere 2019, 231, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, Y.; Liu, T.; Chu, B. Cost-effective polymer-based membranes for drinking water purification. Giant 2022, 10, 100099. [Google Scholar] [CrossRef]
- Kinser, K. Membrane Filtration: Past, Present, and Future. J. Am. Water Works Assoc. 2021, 113, 72–75. [Google Scholar] [CrossRef]
- Amjad, H.; Khan, Z.; Tarabara, V.V. Fractal structure and permeability of membrane cake layers: Effect of coagulation–flocculation and settling as pretreatment steps. Sep. Purif. Technol. 2015, 143, 40–51. [Google Scholar] [CrossRef]
- Pearce, G.K. UF/MF Membrane Water Treatment: Principles and Design, 1st ed.; Water Treatment Academy: Bangkok, Thailand, 2011; 387p, ISBN 616-90836-3-8. [Google Scholar]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. Water Treatment: Principles and Design, 3rd ed.; McGraw–Hill: New York, NY, USA, 2012. [Google Scholar]
- Hatt, J.W.; Germain, E.; Judd, S.J. Precoagulation–microfiltration for wastewater reuse. Water Res. 2011, 45, 6471–6478. [Google Scholar] [CrossRef]
- Sun, W.; Liu, J.; Chu, H.; Dong, B. Pretreatment and membrane hydrophilic modification to reduce membrane fouling. Membranes 2013, 3, 226–241. [Google Scholar] [CrossRef]
- Kimura, K.; Tanaka, K.; Watanabe, Y. Microfiltration of different surface waters with/without coagulation: Clear correlations between membrane fouling and hydrophilic biopolymers. Water Res. 2014, 49, 434–443. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Arhin, S.G.; Banadda, N.; Komakech, A.J.; Kabenge, I.; Wanyama, J. Membrane fouling control in low pressure membranes: A review on pretreatment techniques for fouling abatement. Environ. Eng. Res. 2016, 21, 109–120. [Google Scholar] [CrossRef]
- Schulz, M.; Soltani, A.; Zheng, X.; Ernst, M. Effect of inorganic colloidal water constituents on combined low–pressure membrane fouling with natural organic matter (NOM). J. Membr. Sci. 2016, 507, 154–164. [Google Scholar] [CrossRef]
- Jermann, D.; Pronk, W.; Ka, R.; Halbeisen, M.; Boller, M. Influence of interactions between NOM and particles on UF fouling mechanisms. Water Res. 2008, 42, 3870–3878. [Google Scholar] [CrossRef] [PubMed]
- Amy, G. Fundamental understanding of organic matter fouling of membranes. Desalination 2008, 231, 44–51. [Google Scholar] [CrossRef]
- Buisson, H.; Lee, N.; Amy, G.; Croue, J. Identification and understanding of fouling in low–pressure membrane (MF/UF) filtration by natural organic. Water Res. 2004, 38, 4511–4523. [Google Scholar] [CrossRef]
- Fan, L.; Harris, J.L.; Roddick, F.A.; Booker, N.I.C.A. Influence of the Characterstics of Natural Organic Matter on the Fouling of Microfiltration Membranes. Water Res. 2001, 35, 4455–4463. [Google Scholar] [CrossRef]
- Ehsani, M.; Doan, H.; Lohi, A. A comprehensive review of membrane fouling and cleaning methods with emphasis on ultrasound–assisted fouling control processes. Korean J. Chem. Eng. 2021, 38, 1531–1555. [Google Scholar] [CrossRef]
- Porcelli, N.; Judd, S. Chemical cleaning of potable water membranes: The cost benefit of optimisation. Water Res. 2010, 44, 1389–1398. [Google Scholar] [CrossRef]
- Lin, J.C.-T.; Lee, D.-J.; Huang, C. Membrane Fouling Mitigation: Membrane Cleaning. Sep. Sci. Technol. 2010, 45, 858–872. [Google Scholar] [CrossRef]
- Miller, D.J.; Kasemset, S.; Paul, D.R.; Freeman, B.D. Comparison of membrane fouling at constant flux and constant transmembrane pressure conditions. J. Membr. Sci. 2014, 454, 505–515. [Google Scholar] [CrossRef]
- Lee, E.K.; Chen, V.; Fane, A.G. Natural organic matter (NOM) fouling in low pressure membrane filtration—Effect of membranes and operation modes. Desalination 2008, 218, 257–270. [Google Scholar] [CrossRef]
- Farahbakhsh, K.; Svrcek, C.; Guest, R.K.; Smith, D.W. A review of the impact of chemical pretreatment on low–pressure water treatment membranes. J. Environ. Eng. Sci. 2004, 3, 237–253. [Google Scholar] [CrossRef]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.; Han, Z.; Li, G. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Kumar, R.; Ismail, A.F. Fouling control on microfiltration/ultrafiltration membranes: Effects of morphology, hydrophilicity, and charge. J. Appl. Polym. Sci. 2015, 132, 42042. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Hilal, N.; Leo, C.P. A review on the applicability of integrated/hybrid membrane processes in water treatment and desalination plants. Desalination 2015, 363, 2–18. [Google Scholar] [CrossRef]
- Aly, S. Pre-Treatment Evaluation Prior to Ultrafiltration in Secondary Effluent Treatment for Water Reuse. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2015. [Google Scholar]
- Thompson, M.A. Membrane filtration of high turbidity sources. Water Supply 2001, 1, 325–330. [Google Scholar] [CrossRef]
- Côté, P.; Mourato, D.; Güngerich, C.; Russell, J.; Houghton, E. Immersed membrane filtration for the production of drinking water: Case studies. Desalination 1998, 117, 181–188. [Google Scholar] [CrossRef]
- Schneider, O.; Freeman, S.M. Recent advances in MF/UF membrane treatment. J. N. Engl. Water Works Assoc. 2005, 119, 98–104. [Google Scholar]
- Zydney, A.L.; Zeman, L.J. Microfiltration and Ultrafiltration: Principles and Applications, 1st ed.; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Moon, J.; Kang, M.-S.; Lim, J.-L.; Kim, C.-H.; Park, H.-D. Evaluation of a low–pressure membrane filtration for drinking water treatment: Pretreatment by coagulation/sedimentation for the MF membrane. Desalination 2009, 247, 271–284. [Google Scholar] [CrossRef]
- Fabris, R.; Lee, E.K.; Chow, C.W.K.; Chen, V.; Drikas, M. Pre-treatments to reduce fouling of low pressure micro-filtration (MF) membranes. J. Membr. Sci. 2007, 289, 231–240. [Google Scholar] [CrossRef]
- Huang, H.; Schwab, K.; Jacangelo, J.G. Pretreatment for low pressure membranes in water treatment: A review. Environ. Sci. Technol. 2009, 43, 3011–3019. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Gong, W.; Li, G. Performance evaluation of water treatment ultrafiltration pilot plants treating algae–rich reservoir water. Desalination 2008, 221, 345–350. [Google Scholar] [CrossRef]
- Ma, B.; Ding, Y.; Wang, B.; Qi, Z.; Bai, Y.; Liu, R.; Liu, H.; Qu, J. Influence of sedimentation with pre-coagulation on ultrafiltration membrane fouling performance. Sci. Total Environ. 2020, 708, 134671. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, T.; Qiu, K.; Ma, B.; Wu, R. Membrane fouling performance of Fe-based coagulation–ultrafiltration process: Effect of sedimentation time. Environ. Res. 2021, 195, 110756. [Google Scholar] [CrossRef]
- American Water Works Association. Water Quality & Treatment: A Handbook on Drinking Water, 6th ed.; Edzwald, J.K., Ed.; McGraw-Hill: New York, NY, USA, 2011. [Google Scholar]
- Edzwald, J.K.; Haarhoff, J. Dissolved Air Flotation for Water Clarification; McGraw-Hill: New York, NY, USA, 2012. [Google Scholar]
- Teixeira, M.R.; Rosa, M.J. Comparing dissolved air flotation and conventional sedimentation to remove cyanobacterial cells of Microcystis aeruginosa. Part I: The key operating conditions. Sep. Purif. Technol. 2006, 52, 84–94. [Google Scholar] [CrossRef]
- Ren, L.; Liu, C.; Meng, T.; Sun, Y. Effects of micro–flocculation pretreatment on the ultrafiltration membrane fouling caused by different dissolved organic matters in treated wastewater. J. Water Reuse Desalin. 2021, 11, 597–609. [Google Scholar] [CrossRef]
- Long, Y.; Yu, G.; Dong, L.; Xu, Y.; Lin, H.; Deng, Y.; You, X.; Yang, L.; Liao, B.-Q. Synergistic fouling behaviors and mechanisms of calcium ions and polyaluminum chloride associated with alginate solution in coagulation-ultrafiltration (UF) process. Water Res. 2021, 189, 116665. [Google Scholar] [CrossRef]
- Teng, J.; Chen, Y.; Ma, G.; Hong, H.; Sun, T.; Liao, B.-Q.; Lin, H. Membrane fouling by alginate in polyaluminum chloride (PACl) coagulation/microfiltration process: Molecular insights. Sep. Purif. Technol. 2020, 236, 116294. [Google Scholar] [CrossRef]
- Lebeau, T.; Lelièvre, C.; Buisson, H.; Cléret, D.; Van de Venter, L.W.; Côté, P. Immersed membrane filtration for the production of drinking water: Combination with PAC for NOM and SOCs removal. Desalination 1998, 117, 219–231. [Google Scholar] [CrossRef]
- Ding, Q.; Yamamura, H.; Murata, N.; Aoki, N.; Yonekawa, H.; Hafuka, A.; Watanabe, Y. Characteristics of meso-particles formed in coagulation process causing irreversible membrane fouling in the coagulation–microfiltration water treatment. Water Res. 2016, 101, 127–136. [Google Scholar] [CrossRef]
- Keucken, A.; Heinicke, G.; Persson, K.M.; Köhler, S.J. Combined Coagulation and Ultrafiltration Process to Counteract Increasing NOM in Brown Surface Water. Water 2017, 9, 697. [Google Scholar] [CrossRef]
- Jung, C.W.; Kang, L.S. Application of combined coagulation–ultrafiltration membrane process for water treatment. Korean J. Chem. Eng. 2003, 20, 855–861. [Google Scholar] [CrossRef]
- Naim, R.; Epsztein, R.; Felder, A.; Heyer, M.; Heijnen, M.; Gitis, V. Rethinking the role of in-line coagulation in tertiary membrane filtration of municipal effluents. Sep. Purif. Technol. 2014, 125, 11–20. [Google Scholar] [CrossRef]
- Ratajczak, M.J.; Exall, K.N.; Huck, P.M. Factors affecting coagulation as a pretreatment to ultrafiltration membranes. Water Qual. Res. J. 2012, 47, 103–116. [Google Scholar] [CrossRef]
- Kamińska, G.; Pronk, W.; Traber, J. Effect of coagulant dose and backflush pressure on NOM membrane fouling in inline coagulation–ultrafiltration. Desalin. Water Treat. 2020, 199, 188–197. [Google Scholar] [CrossRef]
- Peleato, N.M.; Legge, R.L.; Andrews, R.C. Characterization of UF foulants and fouling mechanisms when applying low in-line coagulant pre-treatment. Water Res. 2017, 126, 1–11. [Google Scholar] [CrossRef]
- Wray, H.E.; Andrews, R.C.; Bérubé, P.R. Ultrafiltration organic fouling control: Comparison of air-sparging and coagulation. J. Am. Water Works Assoc. 2014, 106, E76–E85. [Google Scholar] [CrossRef]
- Tabatabai, S.A.A.; Kennedy, M.D.; Amy, G.L.; Schippers, J.C. Optimizing inline coagulation to reduce chemical consumption in MF/UF systems. Desalin. Water Treat. 2009, 6, 94–101. [Google Scholar] [CrossRef]
- Braghetta, A.; Jacangelo, J.G.; Chellam, S.; Michael, L.; Utne, B.A.; Jacangelo, J.G.; Chellam, S.; Utne, B.A. DAF pretreatment: Its effect on MF performance. J. Am. Water Works Assoc. 1997, 89, 90–101. [Google Scholar] [CrossRef]
- Jang, N.-Y.; Watanabe, Y.; Minegishi, S. Performance of ultrafiltration membrane process combined with coagulation/sedimentation. Water Sci. Technol. 2005, 51, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Qu, J.; Gregory, J. Pre-coagulation on the submerged membrane fouling in nano-scale: Effect of sedimentation process. Chem. Eng. J. 2015, 262, 676–682. [Google Scholar] [CrossRef]
- Goh, Y.T.; Harris, J.L.; Roddick, F.A. Reducing the effect of cyanobacteria in the microfiltration of secondary effluent. Water Sci. Technol. 2010, 62, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- do Amaral, P.A.P.; Coral, L.A.; Nagel-Hassemer, M.E.; Belli, T.J.; Lapolli, F.R. Association of dissolved air flotation (DAF) with microfiltration for cyanobacterial removal in water supply. Desalin. Water Treat. 2013, 51, 1664–1671. [Google Scholar] [CrossRef]
- Walker, S.; Narbaitz, R.M. Hollow fiber ultrafiltration of Ottawa River water: Floatation versus sedimentation pre-treatment. Chem. Eng. J. 2016, 288, 228–237. [Google Scholar] [CrossRef]
- Xu, B.; Narbaitz, R.M. Improved membrane pretreatment of high hydrophobic natural organic matter (NOM) waters by floatation. J. Membr. Sci. 2016, 518, 120–130. [Google Scholar] [CrossRef]
- Ladouceur, J.D.; Narbaitz, R.M. Comparing sedimentation, flotation, and inline pre-treatment for low–pressure membrane fouling reduction. J. Environ. Eng. USA, 2023, in press.
- White, D.M.; Garland, D.S.; Narr, J.; Woolard, C.R. Natural organic matter and DBP formation potential in Alaskan water supplies. Water Res. 2003, 37, 939–947. [Google Scholar] [CrossRef]
- Goslan, E.H.; Voros, S.; Banks, J.; Wilson, D.; Hillis, P.; Campbell, A.T.; Parsons, S.A. A model for predicting dissolved organic carbon distribution in a reservoir water using fluorescence spectroscopy. Water Res. 2004, 38, 783–791. [Google Scholar] [CrossRef]
- Irvine, E.; Welch, D.; Smith, A.; Rachwal, T. Nanofiltration for colour removal—8 years’ operational experience in Scotland. Water Sci. Technol. Water Supply 2019, 11, 10. [Google Scholar]
- Klante, C.; Larson, M.; Persson, K.M. Brownification in Lake Bolmen, Sweden, and its relationship to natural and human-induced changes. J. Hydrol. Reg. Stud. 2021, 36, 100863. [Google Scholar] [CrossRef]
- Sillanpaa, M. Natural Organic Matter in Water, 1st ed.; Butterworth–Heinemann: Oxford, UK, 2015. [Google Scholar]
- Dang, H.T.; Narbaitz, R.M.; Matsuura, T. Evaluation of Apparatus for Membrane Cleaning Tests. J. Environ. Eng. 2010, 136, 1161–1170. [Google Scholar] [CrossRef]
- Johansson, L.; Temnerud, J.; Abrahamsson, J.; Kleja, D.B. Variation in Organic Matter and Water Color in Lake Mälaren during the Past 70 Years. AMBIO 2010, 39, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Tanaka, K.; Watanabe, Y. Confirmation of the correlation between membrane fouling in microfiltration and biopolymer concentrations in various Japanese surface waters. Water Sci. Technol. Water Supply 2015, 15, 288–293. [Google Scholar] [CrossRef]
- Inge GmbH. Inge Product Series: Dizzer XL Series Modules. Process and Design Guidelines; Inge GmbH: Greifenberg, Germany, 2020; 66p. [Google Scholar]
- Gonzalez-Galvis, J.P.; Narbaitz, R.M. Large batch bench–scale dissolved air flotation system (LB–DAF) for drinking water treatability tests. Environ. Sci. Water Res. Technol. 2020, 6, 1004–1017. [Google Scholar] [CrossRef]
- Tang, C.K.; Peleato, N.M.; Bérubé, P.R.; Andrews, R.C. Impact of low coagulant dosages on protein fouling of ultrafiltration membranes. J. Water Process Eng. 2019, 31, 100801. [Google Scholar] [CrossRef]
- Waterman, D. Bench Scale Analysis of Ultrafiltration Membranes for Investigation of Fouling by Natural Organic Matter in Surface Waters. Master’s Thesis, University of Ottawa, Ottawa, ON, Canada, 2008; 199p. [Google Scholar]
- Konieczny, K.; Rajca, M.; Sakol, D.; Plonka, J.; Bodzek, M. Coagulation–ultrafiltration system for river water treatment. Desalination 2009, 240, 2–6. [Google Scholar] [CrossRef]
- Choi, K.Y.J.; Dempsey, B.A. In-line coagulation with low–pressure membrane filtration. Water Res. 2004, 38, 4271–4281. [Google Scholar] [CrossRef]
- Ayache, C.; Pidou, M.; Croué, J.P.; Labanowski, J.; Poussade, Y.; Tazi-Pain, A.; Keller, J.; Gernjak, W. Impact of effluent organic matter on low–pressure membrane fouling in tertiary treatment. Water Res. 2013, 47, 2633–2642. [Google Scholar] [CrossRef]
- Le Clech, P.; Jefferson, B.; Chang, I.S.; Judd, S.J. Critical flux determination by the flux-step method in a submerged membrane bioreactor. J. Membr. Sci. 2003, 227, 81–93. [Google Scholar] [CrossRef]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Howe, K.J.; Marwah, A.; Chiu, K.-P.; Adham, S.S. Effect of Coagulation on the Size of MF and UF Membrane Foulants. Environ. Sci. Technol. 2006, 40, 7908–7913. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.A.; Anderson, W.B.; Huck, P.M. In-line coagulation assessment for ultrafiltration fouling reduction to treat secondary effluent for water reuse. Water Sci. Technol. 2021, 83, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Karanfil, T.; Erdogan, I.; Schlautman, M. Selecting Filter Membranes for Measuring DOC and UV254. J. Am. Water Works Assoc. 2003, 95, 86–100. [Google Scholar] [CrossRef]
- American Public Health Association, Water Environment Federation, American Water Works Association. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; Clesceri, L., Greenberg, A., Eaton, A., Eds.; American Public Health Association, Water Environment Federation, American Water Works Association: Washington, DC, USA, 2012. [Google Scholar]
- Wang, Y.; Ma, H.; Wang, X.; Ju, L.; Tian, L.; Qi, H.; Yu, H.; He, G.; Li, J. Study on the operation performance and floc adhesion mechanism of dissolved air flotation equipment. Environ. Sci. Pollut. Res. 2022, 29, 54219–54233. [Google Scholar] [CrossRef] [PubMed]
- Croft, J. Natural Organic Matter Characterization of Different Source and Treated Waters; Implications for Membrane Fouling Control. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2012. [Google Scholar]
- Kimura, K.; Maeda, T.; Yamamura, H.; Watanabe, Y. Irreversible membrane fouling in microfiltration membranes filtering coagulated surface water. J. Membr. Sci. 2008, 320, 356–362. [Google Scholar] [CrossRef]
- Maeng, S.K.; Timmes, T.C.; Kim, H.-C. Characteristics of flocs formed by polymer–only coagulation in water treatment and their impacts on the performance of downstream membrane separation. Environ. Technol. 2017, 38, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Black, A.P.; Hannah, S.A. Electrophoretic Studies of Turbidity Removal by Coagulation with Aluminum Sulfate. J. Am. Water Works Assoc. 1961, 53, 438–452. [Google Scholar] [CrossRef]
- Sharp, E.L.; Parsons, S.A.; Jefferson, B. Seasonal variations in natural organic matter and its impact on coagulation in water treatment. Sci. Total Environ. 2006, 363, 183–194. [Google Scholar] [CrossRef]
- Wang, J.; Pan, S.; Luo, D. Characterization of cake layer structure on the microfiltration membrane permeability by iron pre-coagulation. J. Environ. Sci. 2013, 25, 308–315. [Google Scholar] [CrossRef]
- Lerch, A.; Uhl, W.; Gimbel, R. CFD modelling of floc transport and coating layer build-up in single UF/MF membrane capillaries driven in inside-out mode. Water Supply 2007, 7, 37–47. [Google Scholar] [CrossRef]
- Arkhangelsky, E.; Lerch, A.; Uhl, W.; Gitis, V. Organic fouling and floc transport in capillaries. Sep. Purif. Technol. 2011, 80, 482–489. [Google Scholar] [CrossRef]
- Lorenzen, S.; Keiding, K.; Christensen, M.L. The effect of particle surface charge density on filter cake properties during dead-end filtration. Chem. Eng. Sci. 2017, 163, 155–166. [Google Scholar] [CrossRef]
- Kim, H.-C. Microfiltration of humic-rich water coagulated with cationic polymer: The effects of particle characteristics on the membrane performance. J. Membr. Sci. 2015, 475, 349–356. [Google Scholar] [CrossRef]
- Yamamura, H.; Okimoto, K.; Kimura, K.; Watanabe, Y. Influence of calcium on the evolution of irreversible fouling in microfiltration/ultrafiltration membranes. J. Water Supply Res. Technol.-Aqua 2007, 56, 425–434. [Google Scholar] [CrossRef]
- Yoo, S.S. Operating cost reduction of in-line coagulation/ultrafiltration membrane process attributed to coagulation condition optimization for irreversible fouling control. Water 2018, 10, 1076. [Google Scholar] [CrossRef]

| Characteristic | Value |
|---|---|
| Operation | Pressure driven inside–out |
| Filtration Flux, L m−2 h−1 | 80 |
| Backwash Flux, L m−2 h−1 | 300 |
| Filtration Cycle Duration, min | 30 |
| Backwash Cycle Duration, min | 2 |
| CEB Interval, day | 1 |
| CEB Chemicals | 0.05 N NaOH + 50 mg L−1 Cl− |
| CIP Interval, day | 3 |
| CIP Chemicals | 0.1 N NaOH + 200 mg L−1 Cl− |
| Parameter | Raw ORW | CF–DAF | CF–S | CF–IN (34 mg L−1) | CF–IN (27 mg L−1) | |||
|---|---|---|---|---|---|---|---|---|
| Winter | Summer | Winter | Winter | Winter | Summer | Winter | Summer | |
| Turbidity (NTU) | 4.88 ± 0.01 1 | 1.94 ± 0.01 | 0.591 ± 0.02 | 2.42 ± 0.01 | 9.11 ± 0.07 | 5.4 ± 0.01 | 8.15 ± 0.04 | 4.76 ± 0.03 |
| pH (unit) | 7.52 ± 0.02 | 7.70 ± 0.03 | 6.28 ± 0.02 | 6.08 ± 0.01 | 6.02 ± 0.01 | 5.99 ± 0.03 | 5.98 ± 0.03 | 6.05 ± 0.03 |
| UV254 (cm−1) | 0.276 ± 0.001 | 0.225 ± 0.001 | 0.042 ± 0.001 | 0.054 ± 0.001 | 0.051 ± 0.02 | 0.047 ± 0.01 | 0.058 ± 0.001 | 0.055 ± 0.001 |
| True Color (Pt. Co.) | 55.3 ± 0.5 | 32.3 ± 0.5 | 2 ± 0 | 2.3 ± 0.5 | 2 ± 0 | 2 ± 0 | 2.3 ± 0.3 | 3.3 ± 0.3 |
| Zeta Potential (mV) | −22 ± 0.8 | −19.3 ± 0.25 | −6.31 ± 0.20 | −7.23 ± 0.24 | −6.21 ± 0.22 | −5.48 ± 0.13 | −15.17 ± 0.37 | −8.69 ± 0.14 |
| DOC (mg L−1) | 6.64 ± 0.02 | 6.58 ± 0.01 | 2.27 ± 0.01 | 2.67 ± 0.01 | 2.29 ± 0.01 | 2.33 ± 0.01 | 2.55 ± 0.01 | 2.63 ± 0.01 |
| SUVA (L mg−1 m−1) | 4.16 | 3.41 | 1.85 | 2.03 | 2.21 | 1.99 | 2.26 | 2.08 |
| DOC Removal (%) | NA | NA | 65.81 | 59.79 | 65.51 | 64.59 | 61.6 | 60.03 |
| UV254 Removal (%) | NA | NA | 84.78 | 80.43 | 81.52 | 79.11 | 78.98 | 75.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladouceur, J.D.; Narbaitz, R.M. Reduced Low–Pressure Membrane Fouling by Inline Coagulation Pretreatment for a Colored River Water. Membranes 2022, 12, 1028. https://doi.org/10.3390/membranes12111028
Ladouceur JD, Narbaitz RM. Reduced Low–Pressure Membrane Fouling by Inline Coagulation Pretreatment for a Colored River Water. Membranes. 2022; 12(11):1028. https://doi.org/10.3390/membranes12111028
Chicago/Turabian StyleLadouceur, Joseph D., and Roberto M. Narbaitz. 2022. "Reduced Low–Pressure Membrane Fouling by Inline Coagulation Pretreatment for a Colored River Water" Membranes 12, no. 11: 1028. https://doi.org/10.3390/membranes12111028
APA StyleLadouceur, J. D., & Narbaitz, R. M. (2022). Reduced Low–Pressure Membrane Fouling by Inline Coagulation Pretreatment for a Colored River Water. Membranes, 12(11), 1028. https://doi.org/10.3390/membranes12111028