Mixture of Anthraquinone Sulfo-Derivatives as an Inexpensive Organic Flow Battery Negolyte: Optimization of Battery Cell
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrolyte Preparation
2.2. Electrochemical Measurements
2.3. Battery Assembly
2.4. Flow Cell Experiments
2.5. NMR Spectroscopy and Other Characterization Techniques
3. Results and Discussions
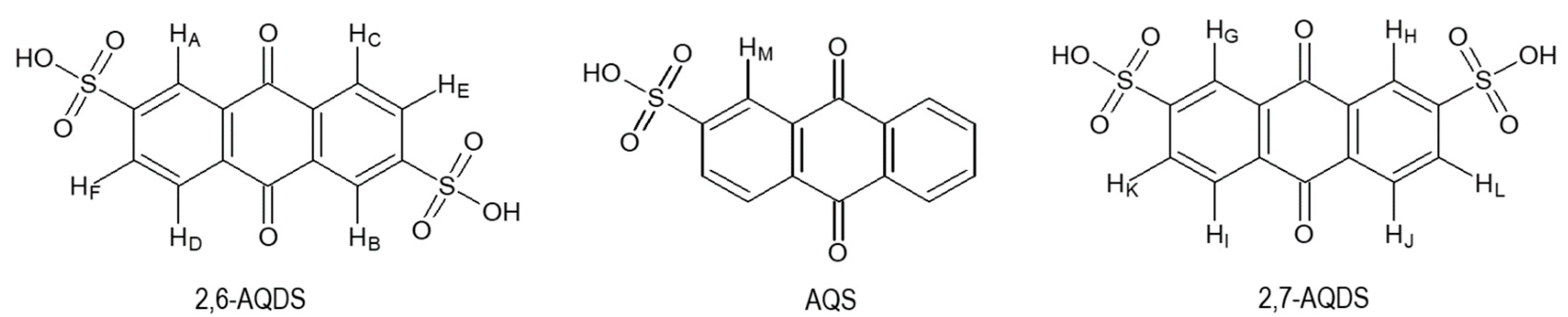
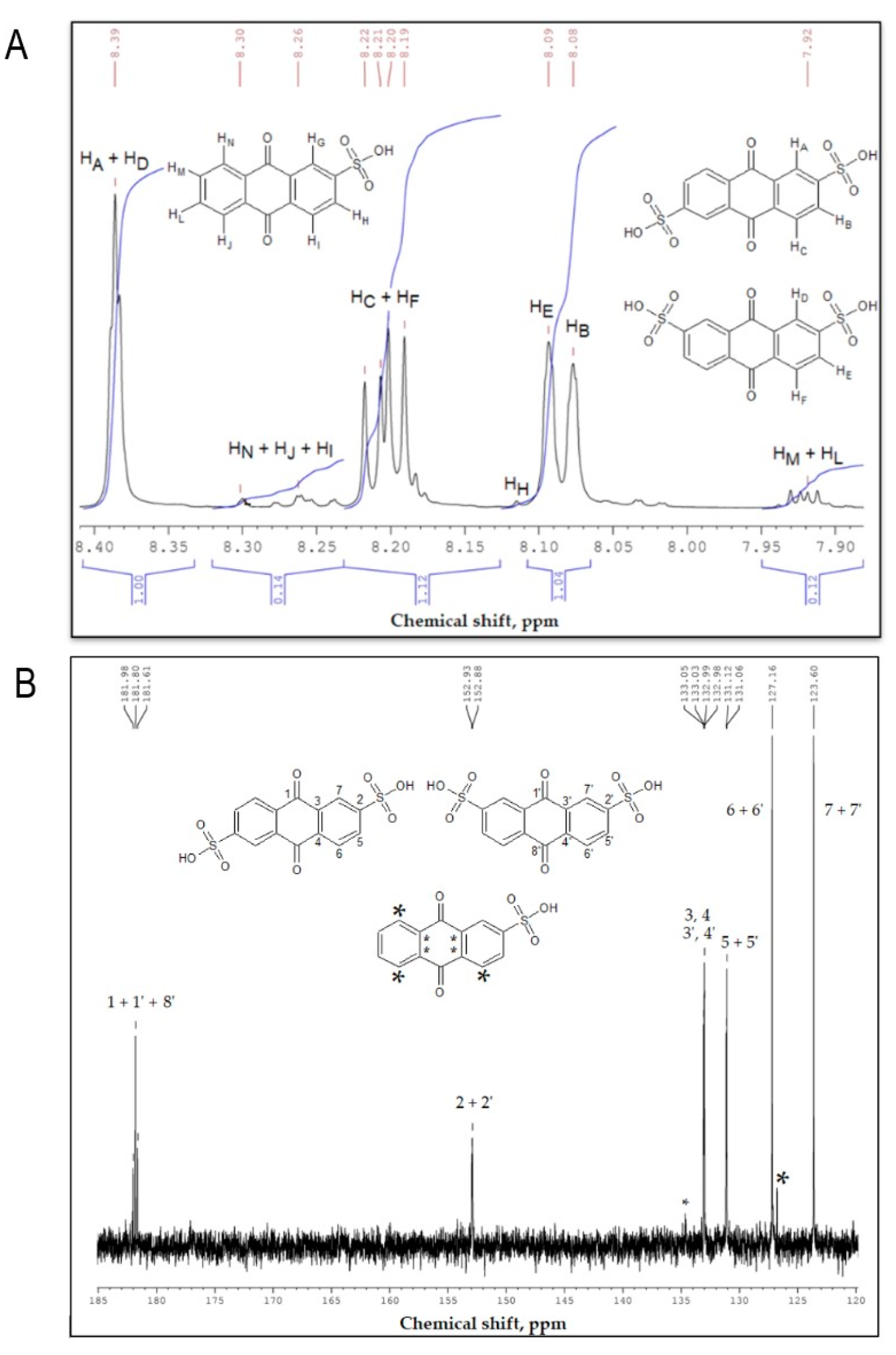
3.1. Composition of ASM
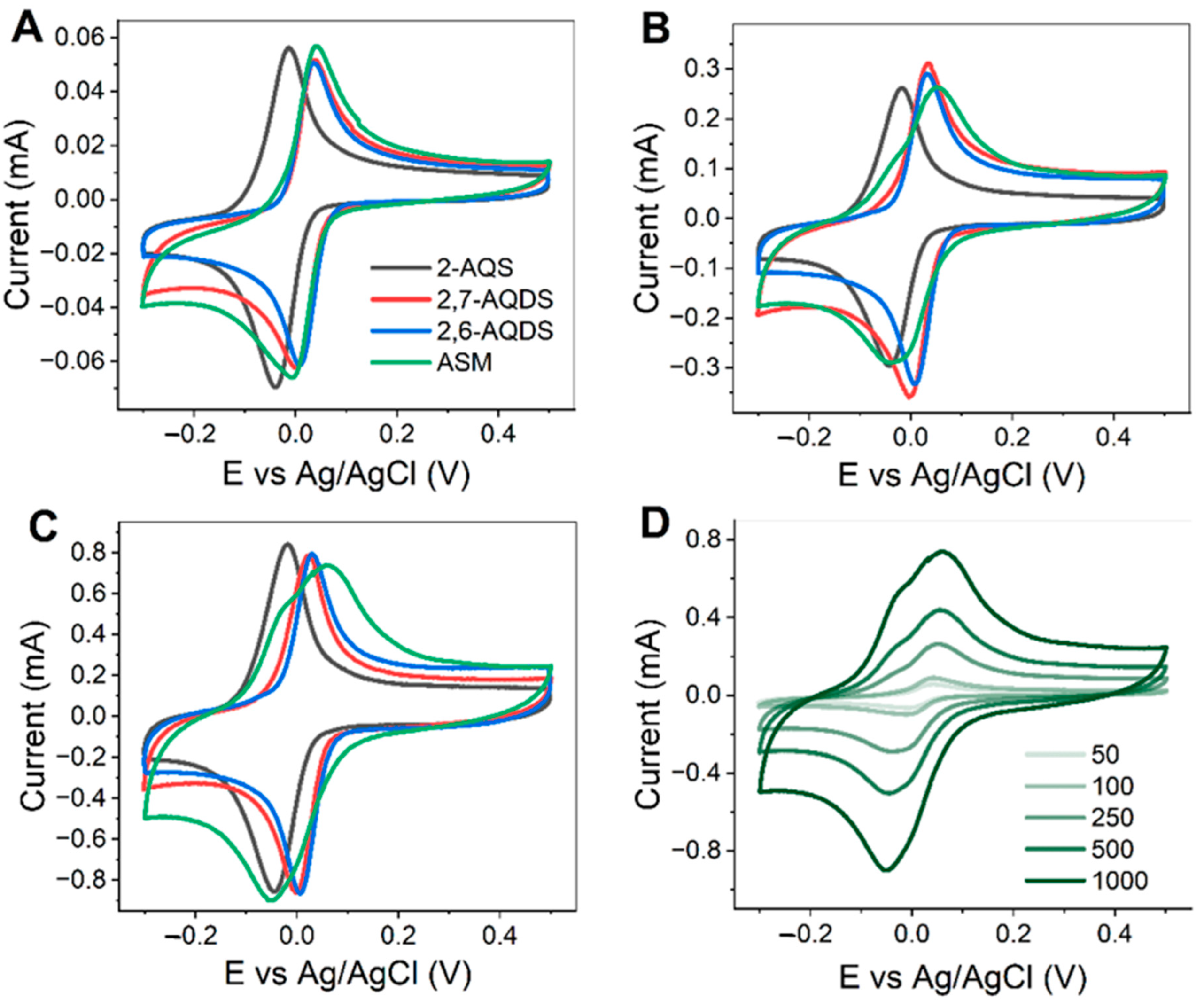
3.2. Electrochemical Behavior
3.3. Cell Optimization
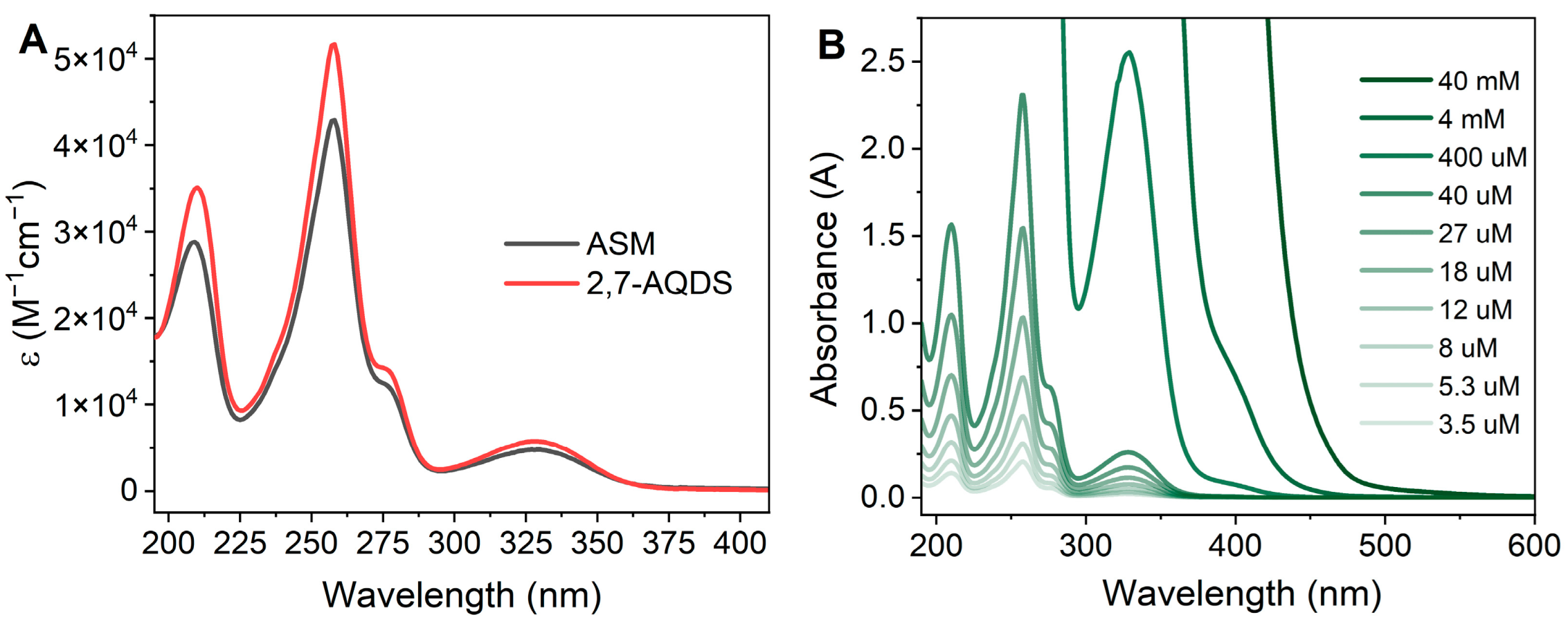
3.4. Comparison of ASM and 2,7-AQDS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RFB | redox flow battery |
| VRFB | vanadium redox flow battery |
| 2,7-AQDS | 9,10-anthraquinone-2,7-disulfonic acid |
| ABRFB | Anthraquinone-bromine |
| EE | energy efficiency |
| AQS | anthraquinone-2-sulfonic acid AQS |
| 2,6-AQDS | 9,10-anthraquinone-2,6-disulfonic acid |
| AS mixture | anthraquinone sulfonation mixture |
| CV | cycling voltammetry |
| SWV | square wave voltammetry |
| SHE | standard hydrogen electrode |
| SOC | state of charge |
| CE | coulombic (faradaic) efficiency |
| VE | voltaic efficiency |
| OCV | open circuit potential |
References
- Sánchez-Díez, E.; Ventosa, E.; Guarnieri, M.; Trovò, A.; Flox, C.; Marcilla, R.; Soavi, F.; Mazur, P.; Aranzabe, E.; Ferret, R. Redox Flow Batteries: Status and Perspective towards Sustainable Stationary Energy Storage. J. Power Sources 2021, 481, 228804. [Google Scholar] [CrossRef]
- Petrov, M.M.; Modestov, A.D.; Konev, D.V.; Antipov, A.E.; Loktionov, P.A.; Pichugov, R.D.; Kartashova, N.V.; Glazkov, A.T.; Abunaeva, L.Z.; Andreev, V.N.; et al. Redox Flow Batteries: Role in Modern Electric Power Industry and Comparative Characteristics of the Main Types. Russ. Chem. Rev. 2021, 90, 677–702. [Google Scholar] [CrossRef]
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Redox Flow Batteries for Energy Storage: Their Promise, Achievements and Challenges. Curr. Opin. Electrochem. 2019, 16, 117–126. [Google Scholar] [CrossRef]
- Winsberg, J.; Hagemann, T.; Janoschka, T.; Hager, M.D.; Schubert, U.S. Redox-Flow Batteries: From Metals to Organic Redox-Active Materials. Angew. Chemie. Int. Ed. 2017, 56, 686–711. [Google Scholar] [CrossRef]
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Kim, S.; Jung, H.Y.; Yang, J.H.; Kim, H.T. A Review of Vanadium Electrolytes for Vanadium Redox Flow Batteries. Renew. Sustain. Energy Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wei, L.; Wu, M.C.; Bai, B.F.; Zhao, T.S. Chloride ions as an electrolyte additive for high performance vanadium redox flow batteries. Appl. Energy 2021, 289, 116690. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Tang, L.; Liu, T.; Huang, J.; Peng, S.; Yang, X. Investigations on physicochemical properties and electrochemical performance of sulfate-chloride mixed acid electrolyte for vanadium redox flow battery. J. Power Sources 2019, 434, 226719. [Google Scholar] [CrossRef]
- Di Noto, V.; Vezzu, K.; Crivellaro, G.; Pagot, G.; Sun, C.; Meda, L.; Rutkowska, I.A.; Kulesza, P.J.; Zawodzinski, T.A. A general electrochemical formalism for vanadium redox flow batteries. Electrochim. Acta 2022, 408, 139937. [Google Scholar] [CrossRef]
- Schmidt, O.; Melchior, S.; Hawkes, A.; Staffell, I. Projecting the Future Levelized Cost of Electricity Storage Technologies. Joule 2019, 3, 81–100. [Google Scholar] [CrossRef]
- Minke, C.; Kunz, U.; Turek, T. Techno-Economic Assessment of Novel Vanadium Redox Flow Batteries with Large-Area Cells. J. Power Sources 2017, 361, 105–114. [Google Scholar] [CrossRef]
- Ciotola, A.; Fuss, M.; Colombo, S.; Poganietz, W.R. The Potential Supply Risk of Vanadium for the Renewable Energy Transition in Germany. J. Energy Storage 2021, 33, 102094. [Google Scholar] [CrossRef]
- Wedege, K.; Dražević, E.; Konya, D.; Bentien, A. Organic Redox Species in Aqueous Flow Batteries: Redox Potentials, Chemical Stability and Solubility. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kwabi, D.G.; Ji, Y.; Aziz, M.J. Electrolyte Lifetime in Aqueous Organic Redox Flow Batteries: A Critical Review. Chem. Rev. 2020, 120, 6467–6489. [Google Scholar] [CrossRef] [PubMed]
- Er, S.; Suh, C.; Marshak, M.P.; Aspuru-Guzik, A. Computational Design of Molecules for an All-Quinone Redox Flow Battery. Chem. Sci. 2015, 6, 885–893. [Google Scholar] [CrossRef]
- Huskinson, B.; Marshak, M.P.; Suh, C.; Er, S.; Gerhardt, M.R.; Galvin, C.J.; Chen, X.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. A Metal-Free Organic-Inorganic Aqueous Flow Battery. Nature 2014, 505, 195–198. [Google Scholar] [CrossRef]
- Chen, Q.; Gerhardt, M.R.; Hartle, L.; Aziz, M.J. A Quinone-Bromide Flow Battery with 1 W/Cm 2 Power Density. J. Electrochem. Soc. 2016, 163, A5010–A5013. [Google Scholar] [CrossRef]
- Chen, Q.; Eisenach, L.; Aziz, M.J. Cycling Analysis of a Quinone-Bromide Redox Flow Battery. J. Electrochem. Soc. 2016, 163, A5057–A5063. [Google Scholar] [CrossRef]
- Khataee, A.; Wedege, K.; Dražević, E.; Bentien, A. Differential PH as a Method for Increasing Cell Potential in Organic Aqueous Flow Batteries. J. Mater. Chem. A 2017, 5, 21875–21882. [Google Scholar] [CrossRef]
- Lee, W.; Permatasari, A.; Kwon, B.W.; Kwon, Y. Performance Evaluation of Aqueous Organic Redox Flow Battery Using Anthraquinone-2,7-Disulfonic Acid Disodium Salt and Potassium Iodide Redox Couple. Chem. Eng. J. 2019, 358, 1438–1445. [Google Scholar] [CrossRef]
- Lee, W.; Permatasari, A.; Kwon, Y. Neutral PH Aqueous Redox Flow Batteries Using an Anthraquinone-Ferrocyanide Redox Couple. J. Mater. Chem. C 2020, 8, 5727–5731. [Google Scholar] [CrossRef]
- Cao, J.; Zhu, Z.; Xu, J.; Tao, M.; Chen, Z. Nitrogen-Doped Porous Graphene as a Highly Efficient Cathodic Electrocatalyst for Aqueous Organic Redox Flow Battery Application. J. Mater. Chem. A 2017, 5, 7944–7951. [Google Scholar] [CrossRef]
- Yang, B.; Hoober-Burkhardt, L.; Krishnamoorthy, S.; Murali, A.; Prakash, G.K.S.; Narayanan, S.R. High-Performance Aqueous Organic Flow Battery with Quinone-Based Redox Couples at Both Electrodes. J. Electrochem. Soc. 2016, 163, A1442–A1449. [Google Scholar] [CrossRef]
- Gerhardt, M.R.; Tong, L.; Gómez-Bombarelli, R.; Chen, Q.; Marshak, M.P.; Galvin, C.J.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. Anthraquinone Derivatives in Aqueous Flow Batteries. Adv. Energy Mater. 2017, 7, 1601488. [Google Scholar] [CrossRef]
- Geysens, P.; Evers, J.; Dehaen, W.; Fransaer, J.; Binnemans, K. Enhancing the Solubility of 1,4-Diaminoanthraquinones in Electrolytes for Organic Redox Flow Batteries through Molecular Modification. RSC Adv. 2020, 10, 39601–39610. [Google Scholar] [CrossRef]
- Xia, L.; Huo, W.; Gao, H.; Zhang, H.; Chu, F.; Liu, H.; Tan, Z. Intramolecular Hydrogen Bonds Induced High Solubility for Efficient and Stable Anthraquinone Based Neutral Aqueous Organic Redox Flow Batteries. J. Power Sources 2021, 498, 229896. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Chu, D. An Organic Electroactive Material for Flow Batteries. Electrochim. Acta 2016, 190, 737–743. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Y.; Qian, Y.; Zhang, L.; Ye, J.; Zhang, X.; Zhao, Y. Anthraquinone-Based Anode Material for Aqueous Redox Flow Batteries Operating in Nondemanding Atmosphere. J. Power Sources 2021, 501, 229984. [Google Scholar] [CrossRef]
- Kwabi, D.G.; Lin, K.; Ji, Y.; Kerr, E.F.; Goulet, M.A.; De Porcellinis, D.; Tabor, D.P.; Pollack, D.A.; Aspuru-Guzik, A.; Gordon, R.G.; et al. Alkaline Quinone Flow Battery with Long Lifetime at PH 12. Joule 2018, 2, 1894–1906. [Google Scholar] [CrossRef]
- Hu, B.; Luo, J.; Hu, M.; Yuan, B.; Liu, T.L. A PH-Neutral, Metal-Free Aqueous Organic Redox Flow Battery Employing an Ammonium Anthraquinone Anolyte. Angew. Chem. 2019, 131, 16782–16789. [Google Scholar] [CrossRef]
- Caram, J.A.; Banera, M.J.; Suárez, J.F.M.; Mirífico, M.V. Electrochemical Behaviour of Anthraquinone Dyes in Non Aqueous Solvent Solution: Part I. Medium Effect on the Electrochemical Behaviour. Electrochim. Acta 2017, 249, 431–445. [Google Scholar] [CrossRef]
- Wu, M.; Jing, Y.; Wong, A.A.; Fell, E.M.; Jin, S.; Tang, Z.; Gordon, R.G.; Aziz, M.J. Extremely Stable Anthraquinone Negolytes Synthesized from Common Precursors. Chem 2020, 6, 1432–1442. [Google Scholar] [CrossRef]
- Guiheneuf, S.; Lê, A.; Godet-Bar, T.; Chancelier, L.; Fontmorin, J.M.; Floner, D.; Geneste, F. Behaviour of 3,4-Dihydroxy-9,10-Anthraquinone-2-Sulfonic Acid in Alkaline Medium: Towards a Long-Cycling Aqueous Organic Redox Flow Battery. ChemElectroChem 2021, 8, 2526–2533. [Google Scholar] [CrossRef]
- Ruan, W.; Mao, J.; Chen, Q. Redox Flow Batteries toward More Soluble Anthraquinone Derivatives. Curr. Opin. Electrochem. 2021, 29, 100748. [Google Scholar] [CrossRef]
- Lima, A.R.F.; Pereira, R.C.; Azevedo, J.; Mendes, A.; Sérgio Seixas de Melo, J. On the Path to Aqueous Organic Redox Flow Batteries: Alizarin Red S Alkaline Negolyte. Performance Evaluation and Photochemical Studies. J. Mol. Liq. 2021, 336, 116364. [Google Scholar] [CrossRef]
- Preger, Y.; Johnson, M.R.; Biswas, S.; Anson, C.W.; Root, T.W.; Stahl, S.S. Anthraquinone-Mediated Fuel Cell Anode with an Off-Electrode Heterogeneous Catalyst Accessing High Power Density When Paired with a Mediated Cathode. ACS Energy Lett. 2020, 5, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.M.; Zhu, X.; Logan, B.E. AQDS Immobilized Solid-Phase Redox Mediators and Their Role during Bioelectricity Generation and RR2 Decolorization in Air-Cathode Single-Chamber Microbial Fuel Cells. Bioelectrochemistry 2017, 118, 123–130. [Google Scholar] [CrossRef]
- Santos, M.S.S.; Peixoto, L.; Azevedo, J.; Monteiro, R.A.R.; Dias-Ferreira, C.; Alves, M.M.; Mendes, A. Microbially-Charged Electrochemical Fuel for Energy Storage in a Redox Flow Cell. J. Power Sources 2020, 445, 227307. [Google Scholar] [CrossRef]
- Wei, Z.; Almakrami, H.; Lin, G.; Agar, E.; Liu, F. An Organic-Inorganic Hybrid Photoelectrochemical Storage Cell for Improved Solar Energy Storage. Electrochim. Acta 2018, 263, 570–575. [Google Scholar] [CrossRef]
- Li, W.; Fu, H.C.; Li, L.; Cabán-Acevedo, M.; He, J.H.; Jin, S. Integrated Photoelectrochemical Solar Energy Conversion and Organic Redox Flow Battery Devices. Angew. Chem. Int. Ed. 2016, 55, 13104–13108. [Google Scholar] [CrossRef]
- Darling, R.M. Techno-economic analyses of several redox flow batteries using levelized cost of energy storage Curr. Opin. Chem. Eng. 2022, 37, 100855. [Google Scholar] [CrossRef]
- Sun, C.; Znahg, H. Review of the Development of First-Generation Redox Flow Batteries: Iron-Chromium System. ChemSusChem 2021, 15, e202101798. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, V.; Milshtein, J.D.; Barton, J.L.; Carney, T.J.; Darling, R.M.; Brushett, F.R. Estimating the Cost of Organic Battery Active Materials: A Case Study on Anthraquinone Disulfonic Acid. Transl. Mater. Res. 2018, 5, 034001. [Google Scholar] [CrossRef]
- Crossley, M.L. The separation of mono-β-, 2,6- and 2,7-sulfonic acids of anthraquinone. J. Am. Chem. Soc. 1915, 37, 2178–2181. [Google Scholar] [CrossRef]
- Anello, L.G.; Berenbaum, M.B.; Peterson, J.O.; Sukornick, B.; Sogn, A.W. Sulfonation of anthraquinone in sulfur dioxide solvent. US Patent US4124606A, 7 November 1978. [Google Scholar]
- Gregory, T.D.; Perry, M.L.; Albertus, P. Cost and Price Projections of Synthetic Active Materials for Redox Flow Batteries. J. Power Sources 2021, 499, 229965. [Google Scholar] [CrossRef]
- Fontmorin, J.-M.; Guiheneuf, S.; Godet-Bar, T.; Floner, D.; Geneste, F. How anthraquinones can enable aqueous organic redox flow batteries to meet the needs of industrialization. Curr. Opin. Colloid Interface Sci. 2022, 101624. [Google Scholar] [CrossRef]
- Mazúr, P.; Charvát, J.; Mrlík, J.; Pocedič, J.; Akrman, J.; Kubáč, L.; Řeháková, B.; Kosek, J. Evaluation of Electrochemical Stability of Sulfonated Anthraquinone-Based Acidic Electrolyte for Redox Flow Battery Application. Molecules 2021, 26, 2484. [Google Scholar] [CrossRef]
- Rogers, D.G. Process of Sulphonating Anthraquinone and Its Derivatives. U.S. Patent US1963383A, 19 June 1934. [Google Scholar]
- Pichugov, R.D.; Konev, D.V.; Petrov, M.M.; Antipov, A.E.; Loktionov, P.A.; Abunaeva, L.Z.; Usenko, A.A.; Vorotyntsev, M.A. Electrolyte Flow Field Variation: A Cell for Testing and Optimization of Membrane Electrode Assembly for Vanadium Redox Flow Batteries. Chempluschem 2020, 85, 1919–1927. [Google Scholar] [CrossRef]
- Modestov, A.D.; Konev, D.V.; Antipov, A.E.; Vorotyntsev, M.A. Hydrogen-Bromate Flow Battery: Can One Reach Both High Bromate Utilization and Specific Power? J. Solid State Electrochem. 2019, 23, 3075–3088. [Google Scholar] [CrossRef]
- Loktionov, P.; Bocharova, A.; Konev, D.; Modestov, A.; Pichugov, R.; Petrov, M.; Antipov, A. Two-Membrane Acid-Base Flow Battery with Hydrogen Electrodes for Neutralization-to-Electrical Energy Conversion. ChemSusChem 2021, 14, 4583–4592. [Google Scholar] [CrossRef]
- Petrov, M.M.; Konev, D.V.; Kuznetsov, V.V.; Antipov, A.E.; Glazkov, A.T.; Vorotyntsev, M.A. Electrochemically Driven Evolution of Br-Containing Aqueous Solution Composition. J. Electroanal. Chem. 2019, 836, 125–133. [Google Scholar] [CrossRef]
- Yao, Y.; Lei, J.; Shi, Y.; Ai, F.; Lu, Y.C. Assessment Methods and Performance Metrics for Redox Flow Batteries. Nat. Energy 2021, 6, 582–588. [Google Scholar] [CrossRef]
- Goulet, M.-A.; Aziz, M. J Flow Battery Molecular Reactant Stability Determined by Symmetric Cell Cycling Methods. J. Electrochem. Soc. 2018, 165, A1466. [Google Scholar] [CrossRef]
- Bien, H.-S.; Stawitz, J.; Wunderlich, K. Anthraquinone Dyes and Intermediates. Ullmann’s Encycl. Ind. Chem. 2000, 513–578. [Google Scholar] [CrossRef]
- Wiberg, C.; Carney, T.J.; Brushett, F.; Ahlberg, E.; Wang, E. Dimerization of 9,10-anthraquinone-2,7-Disulfonic acid (AQDS). Electrochim. Acta 2019, 317, 478–485. [Google Scholar] [CrossRef]
- Carney, T.J.; Collins, S.J.; Moore, J.S.; Brushett, F.R. Concentration-Dependent Dimerization of Anthraquinone Disulfonic Acid and Its Impact on Charge Storage. Chem. Mater. 2017, 29, 4801–4810. [Google Scholar] [CrossRef]
- Yang, Z.; Tong, L.; Tabor, D.P.; Beh, E.S.; Goulet, M.A.; De Porcellinis, D.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. Alkaline Benzoquinone Aqueous Flow Battery for Large-Scale Storage of Electrical Energy. Adv. Energy Mater. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Mao, J.; Ruan, W.; Chen, Q. Understanding the Aqueous Solubility of Anthraquinone Sulfonate Salts: The Quest for High Capacity Electrolytes of Redox Flow Batteries. J. Electrochem. Soc. 2020, 167, 070522. [Google Scholar] [CrossRef]
- Tong, L.; Chen, Q.; Wong, A.A.; Gómez-Bombarelli, R.; Aspuru-Guzik, A.; Gordon, R.G.; Aziz, M.J. UV-Vis Spectrophotometry of Quinone Flow Battery Electrolyte for: In Situ Monitoring and Improved Electrochemical Modeling of Potential and Quinhydrone Formation. Phys. Chem. Chem. Phys. 2017, 19, 31684–31691. [Google Scholar] [CrossRef]
- Arenas, L.F.; Ponce de León, C.; Walsh, F.C. Engineering Aspects of the Design, Construction and Performance of Modular Redox Flow Batteries for Energy Storage. J. Energy Storage 2017, 11, 119–153. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Znahg, H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019, 43, 8739–8752. [Google Scholar] [CrossRef]
- Loktionov, P.; Kartashova, N.; Konev, D.; Abunaeva, L.; Antipov, A.; Ruban, E.; Terent’ev, A.; Gvozdik, N.; Lyange, M.; Usenko, A.; et al. Fluoropolymer impregnated graphite foil as a bipolar plates of vanadium flow battery. Int. J. Energy Res. 2021, 46, 10123–10132. [Google Scholar] [CrossRef]
- Modestov, A.; Kartashova, N.; Pichugov, R.; Petrov, M.; Antipov, A.; Abunaeva, L. Bromine Crossover in Operando Analysis of Proton Exchange Membranes in Hydrogen−Bromate Flow Batteries. Membranes 2022, 12, 815. [Google Scholar] [CrossRef] [PubMed]
- Gandomi, Y.A.; Aaron, D.S.; Houser, J.R.; Daugherty, M.C.; Clement, J.T.; Pezeshki, A.M.; Ertugrul, T.Y.; Moseley, D.P.; Mench, M.M. Critical Review—Experimental Diagnostics and Material Characterization Techniques Used on Redox Flow Batteries. J. Electrochem. Soc. 2018, 165, A970–A1010. [Google Scholar] [CrossRef]
- Lu, M.Y.; Deng, Y.M.; Yang, W.W.; Ye, M.; Jiao, Y.H.; Xu, Q. A Novel Rotary Serpentine Flow Field with Improved Electrolyte Penetration and Species Distribution for Vanadium Redox Flow Battery. Electrochim. Acta 2020, 361, 137089. [Google Scholar] [CrossRef]
- Houser, J.; Pezeshki, A.; Clement, J.T.; Aaron, D.; Mench, M.M. Architecture for Improved Mass Transport and System Performance in Redox Flow Batteries. J. Power Sources 2017, 351, 96–105. [Google Scholar] [CrossRef]
- Dennison, C.R.; Agar, E.; Akuzum, B.; Kumbur, E.C. Enhancing Mass Transport in Redox Flow Batteries by Tailoring Flow Field and Electrode Design. J. Electrochem. Soc. 2016, 163, A5163–A5169. [Google Scholar] [CrossRef]
- Huang, Z.; Mu, A.; Wu, L.; Wang, H. Vanadium Redox Flow Batteries: Flow Field Design and Flow Rate Optimization. J. Energy Storage 2021, 45, 103526. [Google Scholar] [CrossRef]
- Batchelor-McAuley, C.; Li, Q.; Dapin, S.M.; Compton, R.G. Voltammetric Characterization of DNA Intercalators across the Full PH Range: Anthraquinone-2,6-Disulfonate and Anthraquinone-2-Sulfonate. J. Phys. Chem. B 2010, 114, 4094–4100. [Google Scholar] [CrossRef]
- Gamboa-Valero, N.; Astudillo, P.D.; González-Fuentes, M.A.; Leyva, M.A.; Rosales-Hoz, M.D.J.; González, F.J. Hydrogen Bonding Complexes in the Quinone-Hydroquinone System and the Transition to a Reversible Two-Electron Transfer Mechanism. Electrochim. Acta 2016, 188, 602–610. [Google Scholar] [CrossRef]
- Huskinson, B.; Marshak, M.P.; Gerhardt, M.R.; Aziz, M.J. Cycling of a Quinone-Bromide Flow Battery for Large-Scale Electrochemical Energy Storage. ECS Trans. 2014, 61, 27–30. [Google Scholar] [CrossRef]
- Li, G.; Jia, Y.; Zhang, S.; Li, X.; Li, J.; Li, L. The Crossover Behavior of Bromine Species in the Metal-Free Flow Battery. J. Appl. Electrochem. 2017, 47, 261–272. [Google Scholar] [CrossRef]
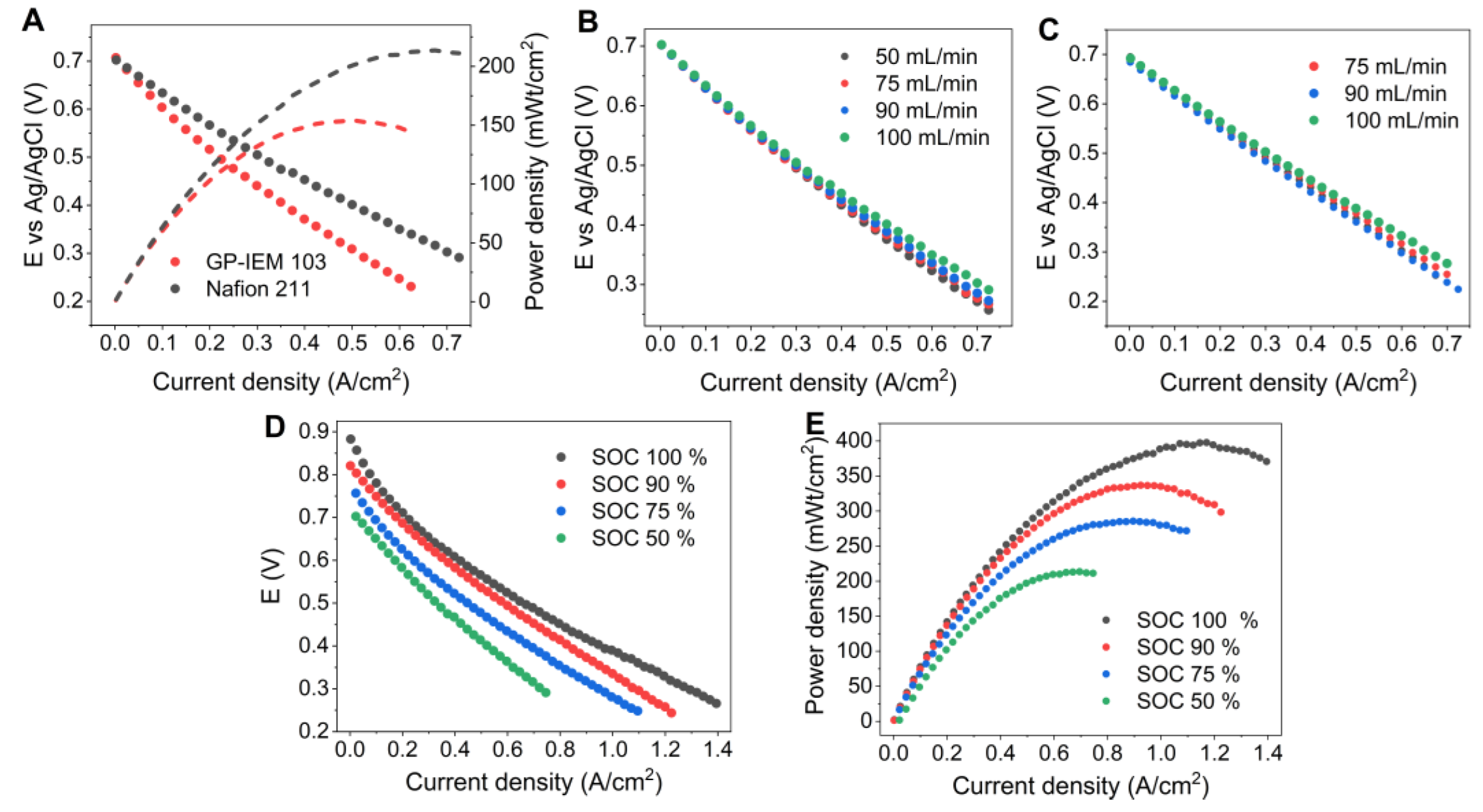
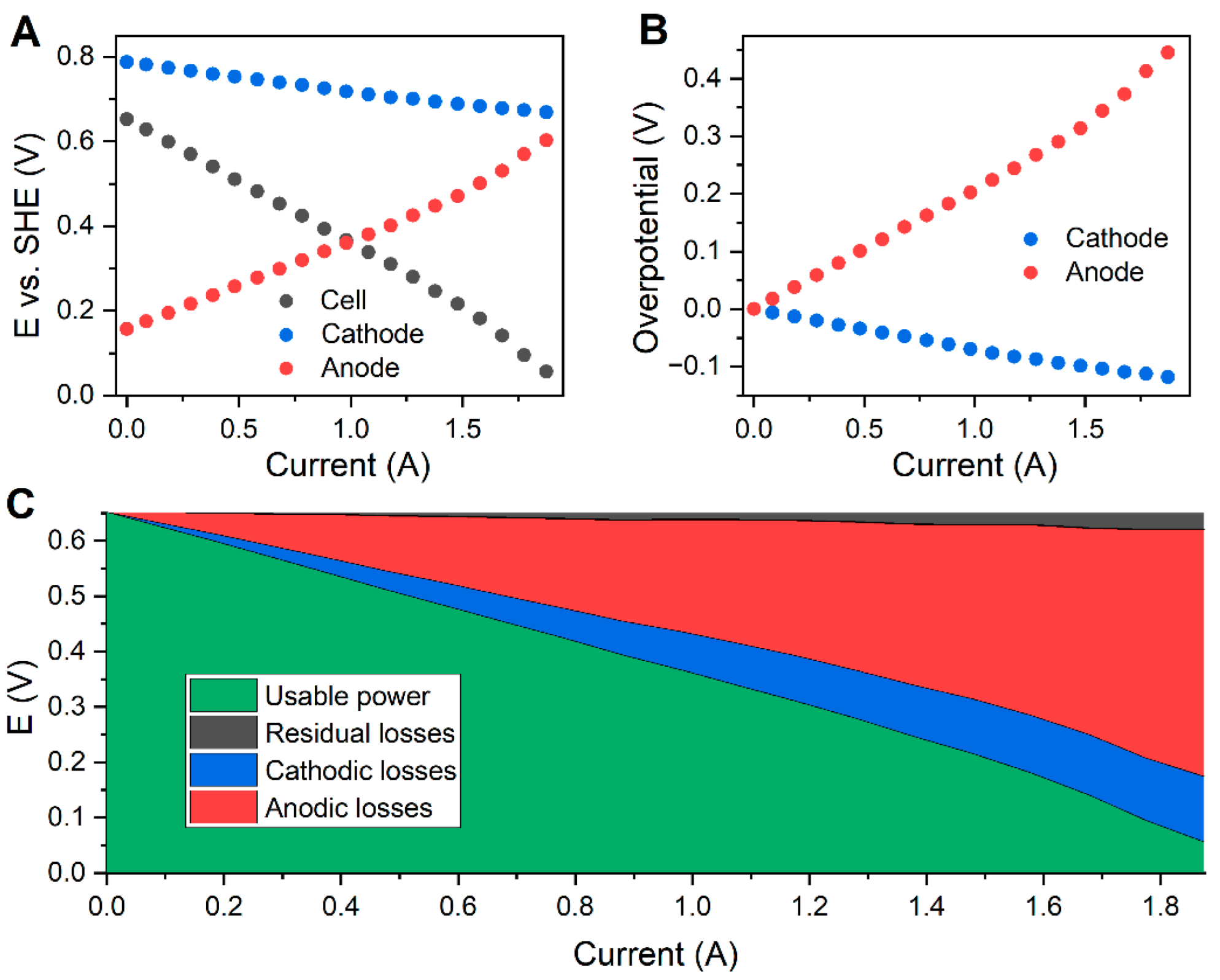
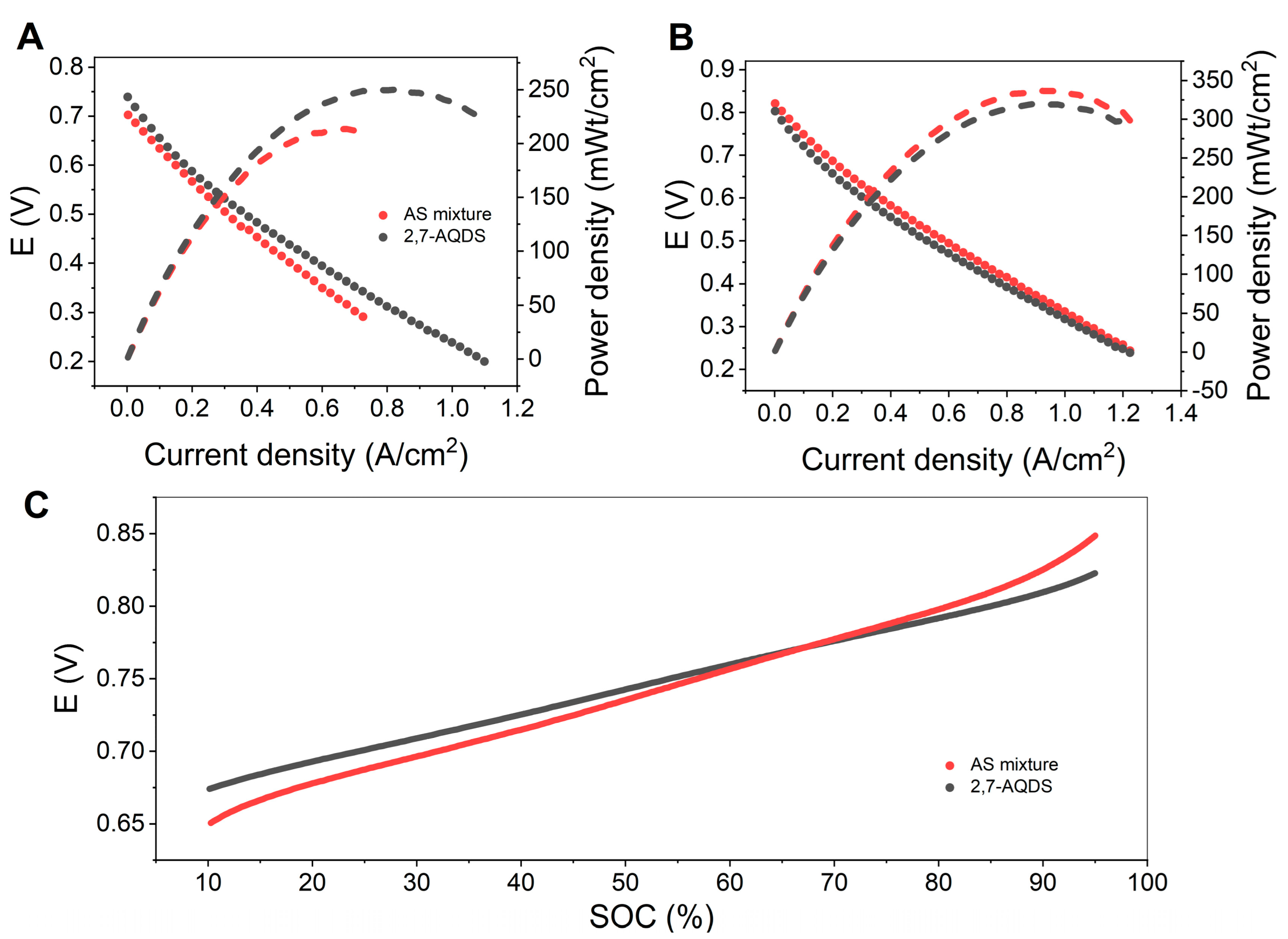
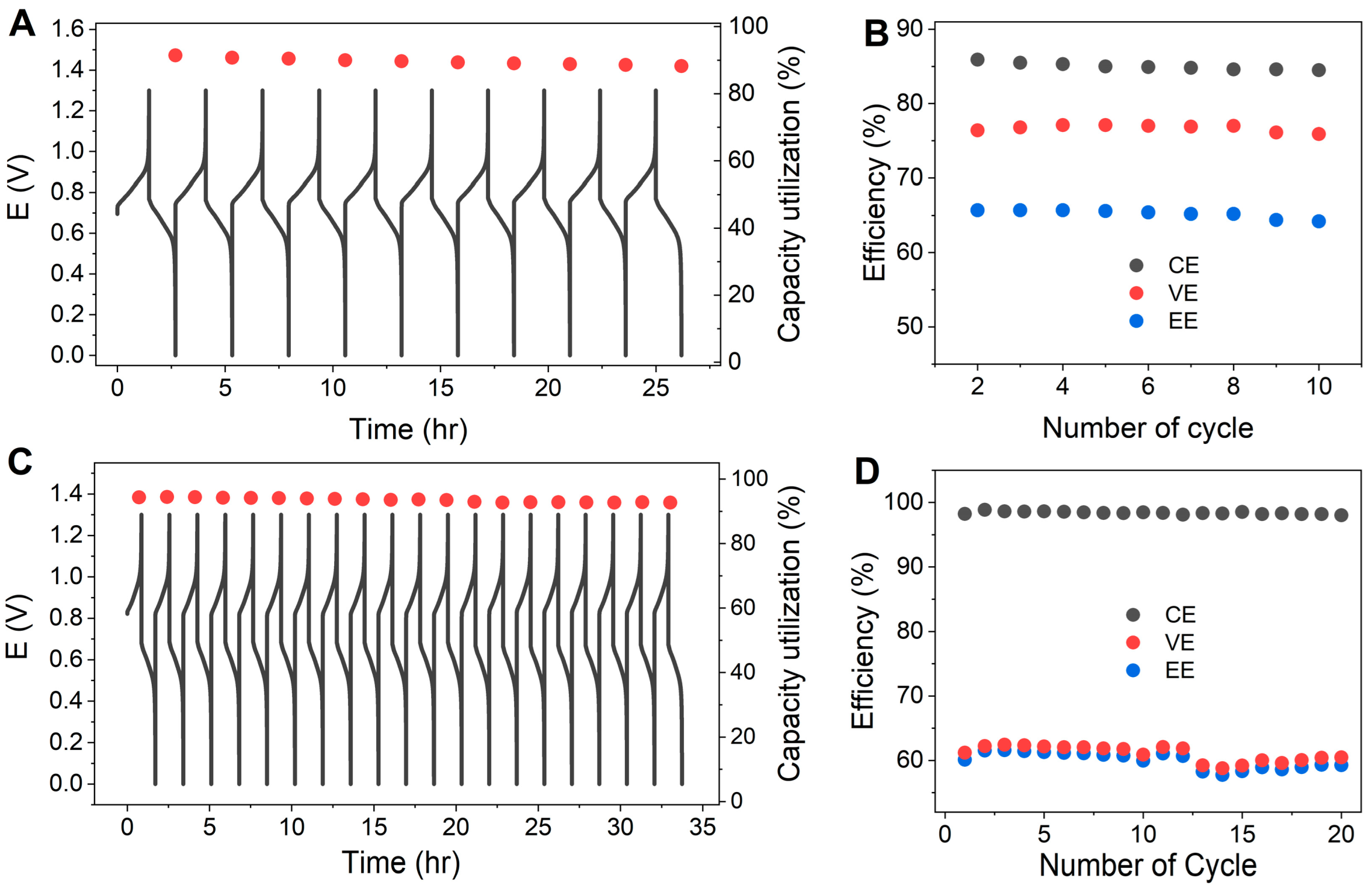
| Flow Rate [mL min−1] | Peak Power Density [mW cm−2] | |
|---|---|---|
| Serpentine | Flow-Through | |
| 50 | 185 | 194 |
| 75 | 190 | 201 |
| 90 | 182 | 202 |
| 100 | 200 | 214 |
| ASM | 2,7 AQDS | ||
|---|---|---|---|
| Nafion 211 (@ 0.1 A cm−2) | Nafion 117 (@ 0.2 A cm−2) | Nafion 211 (@ 0.1 A cm−2) | |
| Capacity Utilization, % | 90.7 | 93.5 | 91.2 |
| Capacity retention, % | 99.8 | 99.8 | 99.9 |
| Coulombic Efficiency, % | 85.9 | 98.5 | 94.2 |
| Voltaic Efficiency, % | 76.4 | 60.9 | 75.8 |
| Energy Efficiency, % | 65.7 | 60.0 | 71.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, M.; Chikin, D.; Abunaeva, L.; Glazkov, A.; Pichugov, R.; Vinyukov, A.; Levina, I.; Motyakin, M.; Mezhuev, Y.; Konev, D.; et al. Mixture of Anthraquinone Sulfo-Derivatives as an Inexpensive Organic Flow Battery Negolyte: Optimization of Battery Cell. Membranes 2022, 12, 912. https://doi.org/10.3390/membranes12100912
Petrov M, Chikin D, Abunaeva L, Glazkov A, Pichugov R, Vinyukov A, Levina I, Motyakin M, Mezhuev Y, Konev D, et al. Mixture of Anthraquinone Sulfo-Derivatives as an Inexpensive Organic Flow Battery Negolyte: Optimization of Battery Cell. Membranes. 2022; 12(10):912. https://doi.org/10.3390/membranes12100912
Chicago/Turabian StylePetrov, Mikhail, Dmitry Chikin, Lilia Abunaeva, Artem Glazkov, Roman Pichugov, Alexey Vinyukov, Irina Levina, Mikhail Motyakin, Yaroslav Mezhuev, Dmitry Konev, and et al. 2022. "Mixture of Anthraquinone Sulfo-Derivatives as an Inexpensive Organic Flow Battery Negolyte: Optimization of Battery Cell" Membranes 12, no. 10: 912. https://doi.org/10.3390/membranes12100912
APA StylePetrov, M., Chikin, D., Abunaeva, L., Glazkov, A., Pichugov, R., Vinyukov, A., Levina, I., Motyakin, M., Mezhuev, Y., Konev, D., & Antipov, A. (2022). Mixture of Anthraquinone Sulfo-Derivatives as an Inexpensive Organic Flow Battery Negolyte: Optimization of Battery Cell. Membranes, 12(10), 912. https://doi.org/10.3390/membranes12100912








