Phosphorus Recovery by Adsorption from the Membrane Permeate of an Anaerobic Membrane Bioreactor Digesting Waste-Activated Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactor Setup and Operating Conditions
2.2. Analytical Methods for Sludge and Membrane Permeate
2.3. Phosphate Desorption from the Adsorbent
2.4. Ex Situ Membrane Cleaning
3. Results and Discussion
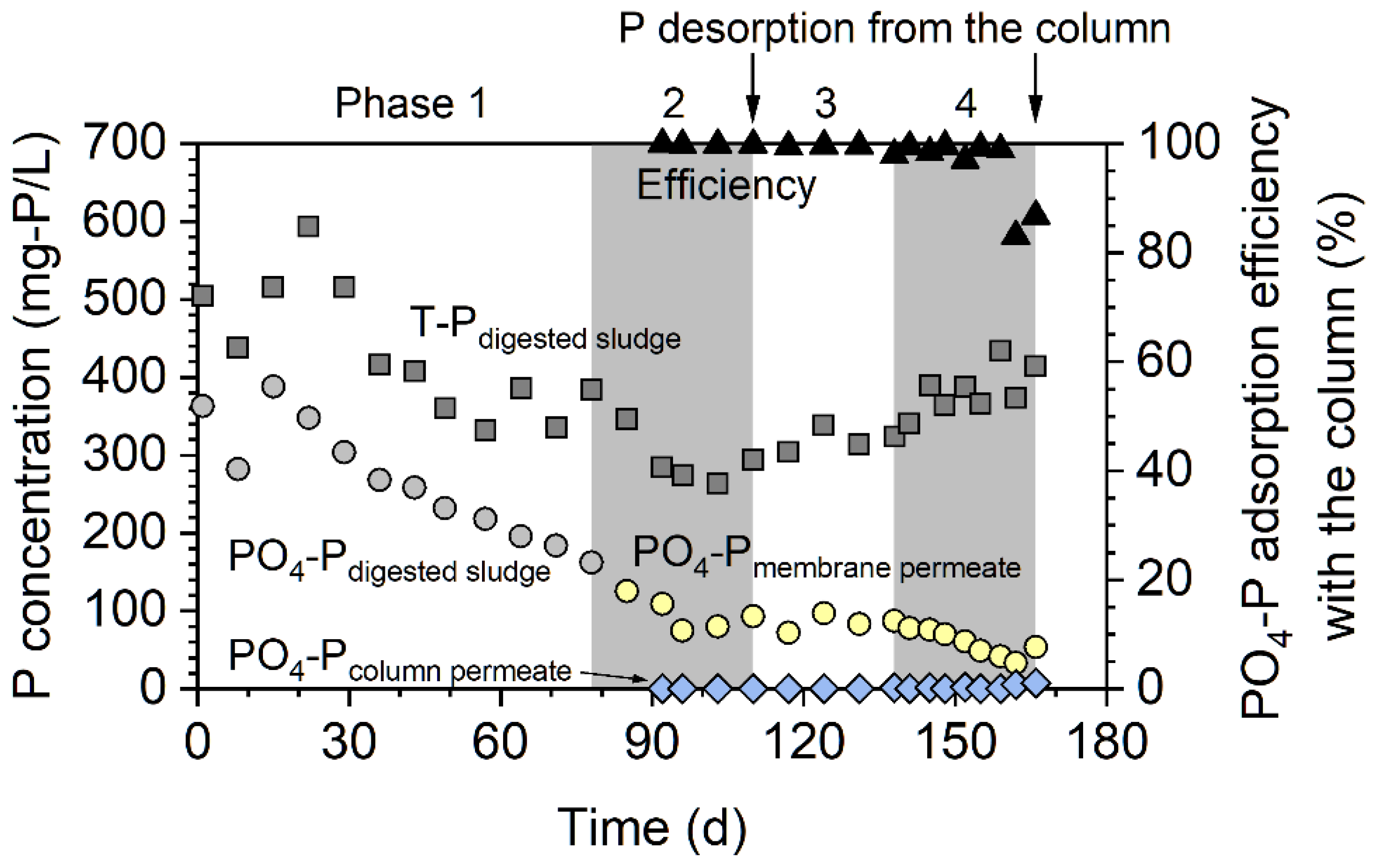
3.1. Phosphorus Adsorption and Recovery
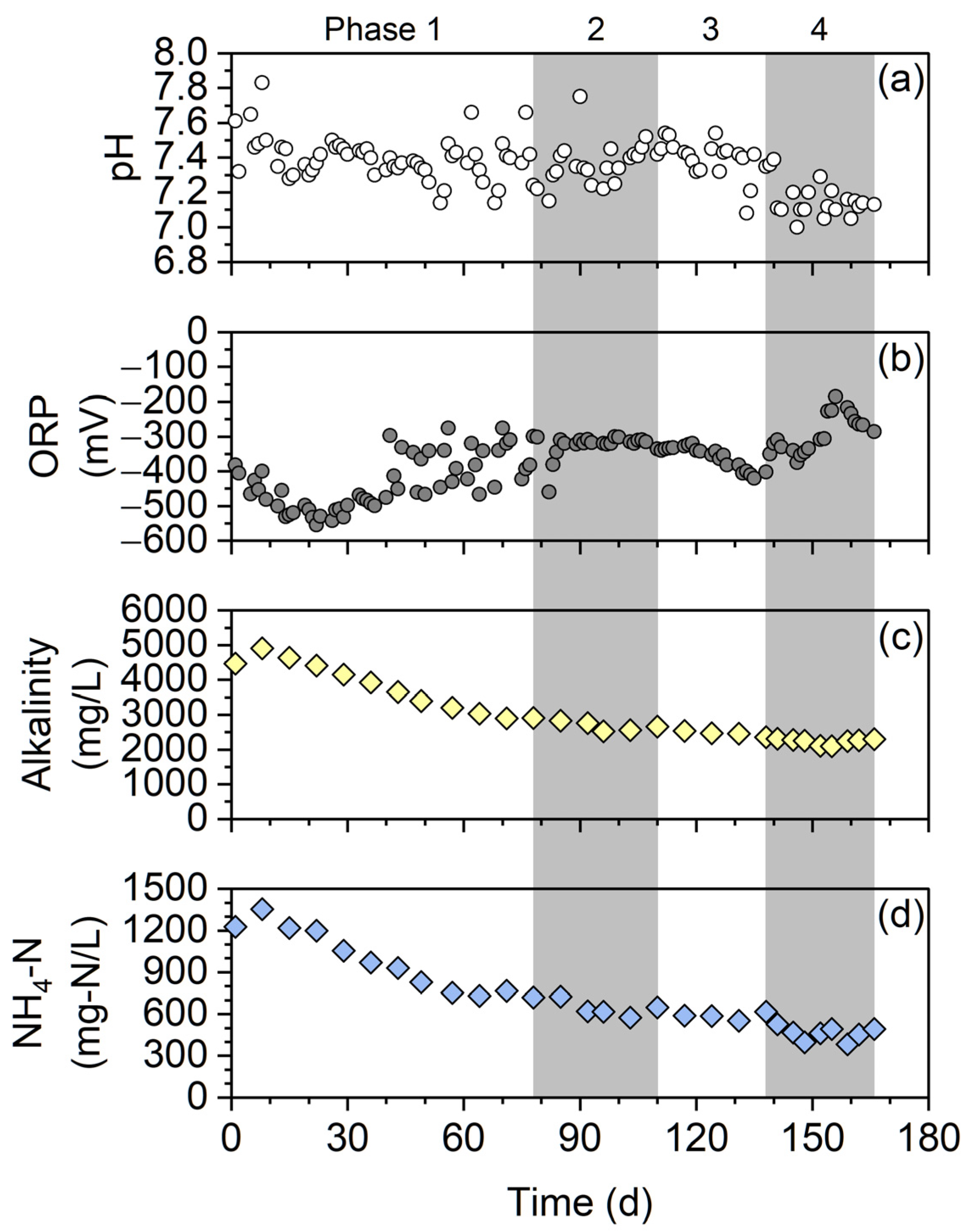
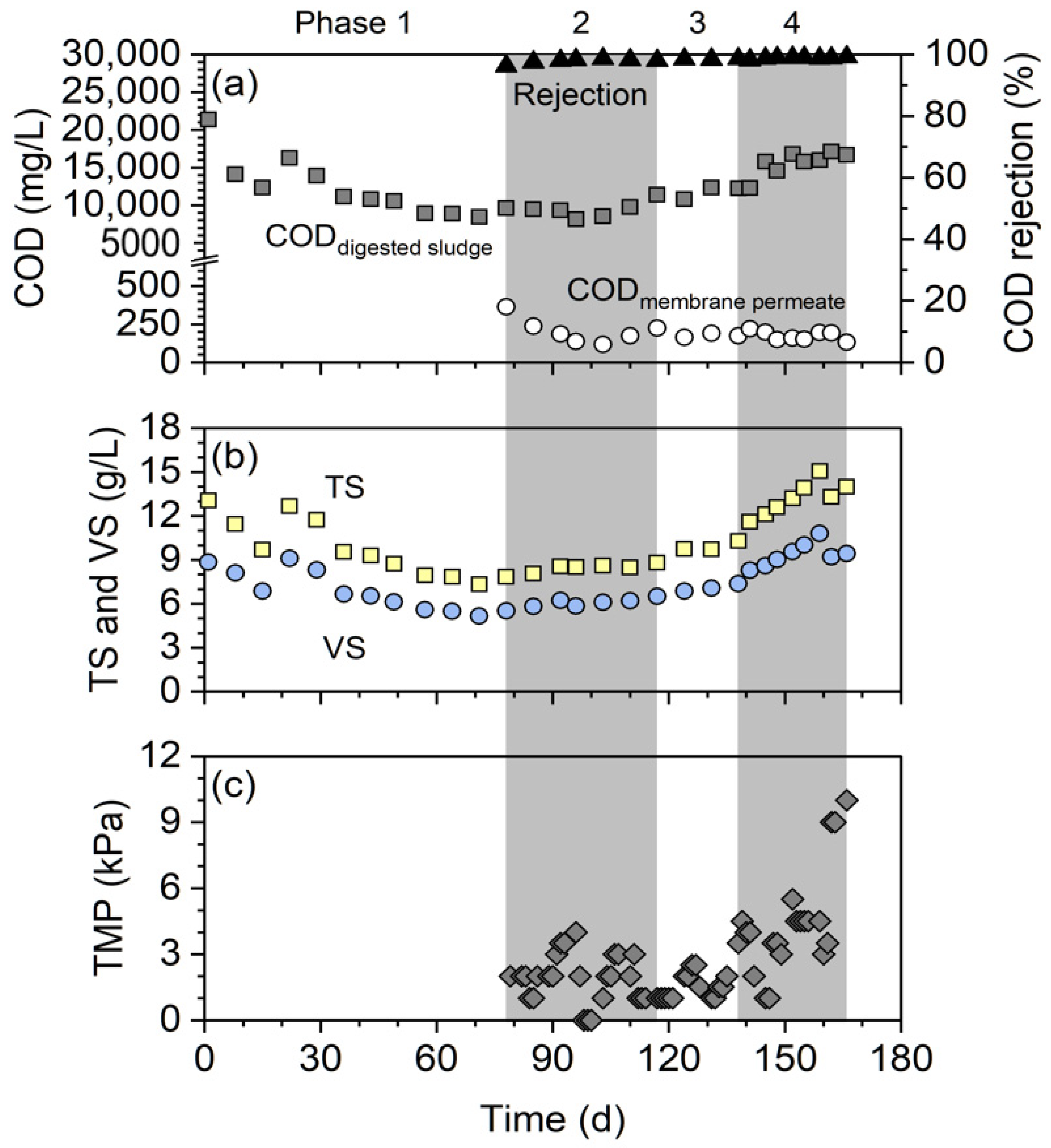
3.2. Performance of Digestion and Membrane Separation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Life’s Bottleneck: Sustaining the World’s Phosphorus for a Food Secure Future. Annu. Rev. Environ. Resour. 2014, 39, 161–188. [Google Scholar] [CrossRef]
- Ministry of Land, Infrastructure, Transport and Tourism. Available online: https://www.mlit.go.jp/common/000113958.pdf (accessed on 8 December 2021). (In Japanese).
- Le Moal, M.; Gascuel-Odoux, C.; Menesguen, A.; Souchon, Y.; Etrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019, 651, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.H.; Luo, J.H.; Xie, H.H.; Yang, H.; Chen, S.P.; Liu, J.Y.; Zhang, R.N.; Li, Y.Y. Species, fractions, and characterization of phosphorus in sewage sludge: A critical review from the perspective of recovery. Sci. Total Environ. 2021, 786, 147437. [Google Scholar] [CrossRef] [PubMed]
- Mayer, B.K.; Baker, L.A.; Boyer, T.H.; Drechsel, P.; Gifford, M.; Hanjra, M.A.; Parameswaran, P.; Stoltzfus, J.; Westerhoff, P.; Rittmann, B.E. Total Value of Phosphorus Recovery. Environ. Sci. Technol. 2016, 50, 6606–6620. [Google Scholar] [CrossRef]
- Raheem, A.; Sikarwar, V.S.; He, J.; Dastyar, W.; Dionysiou, D.D.; Wang, W.; Zhao, M. Opportunities and challenges in sustainable treatment and resource reuse of sewage sludge: A review. Chem. Eng. J. 2018, 337, 616–641. [Google Scholar] [CrossRef]
- Gonzalez, A.; Hendriks, A.T.W.M.; van Lier, J.B.; de Kreuk, M. Pre-treatments to enhance the biodegradability of waste activated sludge: Elucidating the rate limiting step. Biotechnol. Adv. 2018, 36, 1434–1469. [Google Scholar] [CrossRef] [Green Version]
- Appels, L.; Baeyens, J.; Degreve, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zhou, S.Q.; Dai, L.L.; Dai, X.H. The transformation of phosphorus fractions in high-solid sludge by anaerobic digestion combined with the high temperature thermal hydrolysis process. Bioresour. Technol. 2020, 309, 123314. [Google Scholar] [CrossRef]
- Mazzini, S.; Borgonovo, G.; Scaglioni, L.; Bedussi, F.; D’Imporzano, G.; Tambone, F.; Adani, F. Phosphorus speciation during anaerobic digestion and subsequent solid/liquid separation. Sci. Total Environ. 2020, 731, 139284. [Google Scholar] [CrossRef]
- Yu, B.H.; Xiao, X.M.; Wang, J.W.; Hong, M.; Deng, C.; Li, Y.Y.; Liu, J.Y. Enhancing phosphorus recovery from sewage sludge using anaerobic-based processes: Current status and perspectives. Bioresour. Technol. 2021, 341, 125899. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, A.M.; Ozgun, H.; Dereli, R.K.; Isik, O.; Ozcan, O.Y.; van Lier, J.B.; Ozturk, I.; Ersahin, M.E. Anaerobic membrane bioreactors for sludge digestion: Current status and future perspectives. Crit. Rev. Environ. Sci. 2020, 51, 2119–2157. [Google Scholar] [CrossRef]
- Cheng, H.; Li, Y.M.; Hu, Y.S.; Guo, G.Z.; Cong, M.; Xiao, B.Y.; Li, Y.Y. Bioenergy recovery from methanogenic co-digestion of food waste and sewage sludge by a high-solid anaerobic membrane bioreactor (AnMBR): Mass balance and energy potential. Bioresour. Technol. 2021, 326, 124754. [Google Scholar] [CrossRef] [PubMed]
- Hafuka, A.; Mimura, K.; Ding, Q.; Yamamura, H.; Satoh, H.; Watanabe, Y. Performance of anaerobic membrane bioreactor during digestion and thickening of aerobic membrane bioreactor excess sludge. Bioresour. Technol. 2016, 218, 476–479. [Google Scholar] [CrossRef]
- Hafuka, A.; Mashiko, R.; Odashima, R.; Yamamura, H.; Satoh, H.; Watanabe, Y. Digestion performance and contributions of organic and inorganic fouling in an anaerobic membrane bioreactor treating waste activated sludge. Bioresour. Technol. 2019, 272, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Heronemus, E.; Gamage, K.H.H.; Hettiarachchi, G.M.; Parameswaran, P. Efficient recovery of phosphorus and sulfur from Anaerobic Membrane Bioreactor (AnMBR) permeate using chemical addition of iron and evaluation of its nutrient availability for plant uptake. Sci. Total Environ. 2021, 783, 146850. [Google Scholar] [CrossRef]
- Zhao, Q.; Tian, J.Z.; Zhang, K.F.; Wang, H.B.; Li, M.; Meng, S.J.; Mu, R.M.; Liu, L.; Yin, M.M.; Li, J.J.; et al. Phosphate recovery from the P-enriched brine of AnMBR-RO-IE treating municipal wastewater via an innovated phosphorus recovery batch reactor with nano-sorbents. Chemosphere 2021, 284, 131259. [Google Scholar] [CrossRef]
- Fang, L.P.; Wu, B.L.; Lo, I.M.C. Fabrication of silica-free superparamagnetic ZrO2@Fe3O4 with enhanced phosphate recovery from sewage: Performance and adsorption mechanism. Chem. Eng. J. 2017, 319, 258–267. [Google Scholar] [CrossRef]
- Saifuddin, M.; Bae, J.; Kim, K.S. Role of Fe, Na and Al in Fe-Zeolite-A for adsorption and desorption of phosphate from aqueous solution. Water Res. 2019, 158, 246–256. [Google Scholar] [CrossRef]
- Furuya, K.; Hafuka, A.; Kuroiwa, M.; Satoh, H.; Watanabe, Y.; Yamamura, H. Development of novel polysulfone membranes with embedded zirconium sulfate-surfactant micelle mesostructure for phosphate recovery from water through membrane filtration. Water Res. 2017, 124, 521–526. [Google Scholar] [CrossRef]
- Pitakteeratham, N.; Hafuka, A.; Satoh, H.; Watanabe, Y. High efficiency removal of phosphate from water by zirconium sulfate-surfactant micelle mesostructure immobilized on polymer matrix. Water Res. 2013, 47, 3583–3590. [Google Scholar] [CrossRef] [PubMed]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- DR3900 Laboratory Spectrophotometer for Water Analysis. Available online: https://www.hach.com/spectrophotometers/dr3900-laboratory-spectrophotometer-for-water-analysis/family-downloads?productCategoryId=35547203834 (accessed on 27 December 2021).
- Pokhrel, S.P.; Milke, M.W.; Bello-Mendoza, R.; Buitron, G.; Thiele, J. Use of solid phosphorus fractionation data to evaluate phosphorus release from waste activated sludge. Waste Manag. 2018, 76, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Ucisik, A.S.; Henze, M. Biological hydrolysis and acidification of sludge under anaerobic conditions: The effect of sludge type and origin on the production and composition of volatile fatty acids. Water Res. 2008, 42, 3729–3738. [Google Scholar] [CrossRef] [PubMed]
- Bacelo, H.; Pintor, A.M.A.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 2020, 381, 122566. [Google Scholar] [CrossRef]
- Huang, W.Y.; Zhang, Y.M.; Li, D. Adsorptive removal of phosphate from water using mesoporous materials: A review. J. Environ. Manag. 2017, 193, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.O.; Jung, J.T. Performance of membrane-coupled organic acid fermentor for the resources recovery form municipal sewage sludge. Water Sci. Technol. 2007, 55, 245–252. [Google Scholar] [CrossRef]
- Rajagopal, R.; Masse, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- Wang, Z.W.; Ma, J.X.; Tang, C.Y.Y.; Kimura, K.; Wang, Q.Y.; Han, X.M. Membrane cleaning in membrane bioreactors: A review. J. Membr. Sci. 2014, 468, 276–307. [Google Scholar] [CrossRef]



| Phase | Operational Mode | HRT (d) | SRT (d) | Filtration Flux (LMH) | Column Flow Rate (mL/h) |
|---|---|---|---|---|---|
| 1 | CSTR | 60 | 60 | - | - |
| 2 | AnMBR | 65 | 100 | 0.33 | 1.98 |
| 3 | AnMBR | 45 | 100 | 0.79 | 4.12 |
| 4 | AnMBR | 19 | 100 | 2.08 | 9.11 |
| Parameters (Unit) | Values ± Standard Deviations |
|---|---|
| TS (g/L) | 9.2 ± 2.8 |
| VS (g/L) | 7.3 ± 2.2 |
| VS/TS (%) | 79 ± 3 |
| T-COD (g/L) | 12.5 ± 3.8 |
| NH4-N (mg-N/L) | 13.5 ± 10.8 |
| T-P (mg-P/L) | 208 ± 64 |
| PO4-P (mg-P/L) | 14.0 ± 9.2 |
| Phase | Organic Loading Rate (g-VS/L/d) | Biogas Yield (L/g-VSinput) | Biogas Production Rate (L/L/d) | CH4 Content (%) |
|---|---|---|---|---|
| 1 | 0.12 | 0.35 | 0.043 | - 1 |
| 2 | 0.17 | 0.34 | 0.057 | 79.7 ± 2.4 |
| 3 | 0.25 | 0.28 | 0.071 | 80.8 ± 2.1 |
| 4 | 0.47 | 0.26 | 0.123 | 81.0 ± 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafuka, A.; Kimura, K. Phosphorus Recovery by Adsorption from the Membrane Permeate of an Anaerobic Membrane Bioreactor Digesting Waste-Activated Sludge. Membranes 2022, 12, 99. https://doi.org/10.3390/membranes12010099
Hafuka A, Kimura K. Phosphorus Recovery by Adsorption from the Membrane Permeate of an Anaerobic Membrane Bioreactor Digesting Waste-Activated Sludge. Membranes. 2022; 12(1):99. https://doi.org/10.3390/membranes12010099
Chicago/Turabian StyleHafuka, Akira, and Katsuki Kimura. 2022. "Phosphorus Recovery by Adsorption from the Membrane Permeate of an Anaerobic Membrane Bioreactor Digesting Waste-Activated Sludge" Membranes 12, no. 1: 99. https://doi.org/10.3390/membranes12010099
APA StyleHafuka, A., & Kimura, K. (2022). Phosphorus Recovery by Adsorption from the Membrane Permeate of an Anaerobic Membrane Bioreactor Digesting Waste-Activated Sludge. Membranes, 12(1), 99. https://doi.org/10.3390/membranes12010099






