Demonstration and Assessment of Purification Cascades for the Separation and Valorization of Hemicellulose from Organosolv Beechwood Hydrolyzates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Beechwood Hydrolyzates
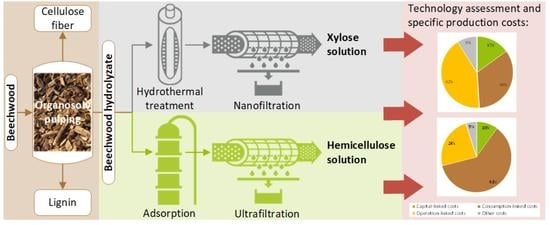
2.2. Purification Cascade 1
2.2.1. Hydrothermal Treatment
2.2.2. Nanofiltration
2.3. Purification Cascade 2
2.3.1. Adsorption
2.3.2. Ultrafiltration
2.4. Analytical Methods
2.4.1. High-Performance Liquid Chromatography
2.4.2. Gas Chromatography
2.4.3. UV/VIS Spectroscopy
2.5. Process Assessment
2.5.1. Flowsheet Simulation
2.5.2. Costing
3. Results and Discussion
3.1. Purification Cascade 1
3.1.1. Hydrothermal Treatment
3.1.2. Nanofiltration
3.2. Purification Cascade 2
3.2.1. Adsorption
3.2.2. Ultrafiltration
3.3. Process Assessment
3.3.1. Mass Balance
3.3.2. Energy Balance
3.3.3. Costing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate change 2014 mitigation of climate change. In Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Edenhofer, O., Pichs-Madruga, R., Sokona, Y., Farahani, E., Kadner, S., Seyboth, K., Adler, A., Baum, I., Brunner, S., Eickemeier, P., et al., Eds.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Fatih Demirbas, M. Biorefineries for biofuel upgrading: A critical review. Appl. Energy 2009, 86, S151–S161. [Google Scholar] [CrossRef]
- Michels, J. ‘‘Lignocellulose Biorefinery—Phase 1”—Final Scientific and Technical Report of All Project Partners. 2009. Available online: http://www.fnr-server.de/ftp/pdf/berichte/22014406.pdf (accessed on 10 June 2020).
- Michels, J. ‘‘Lignocellulose Biorefinery—Phase 2”—Final Scientific and Technical Report of All Project Partners. 2014. Available online: http://edok01.tib.uni-hannover.de/edoks/e01fb15/837304261.pdf (accessed on 10 June 2020).
- Sixta, H. Handbook of Pulp; Wiley-VCH Verlag GmbH: Weinheim, Germany; Chichester, UK, 2008; ISBN 9783527619887. [Google Scholar]
- Rößiger, B.; Röver, R.; Unkelbach, G.; Pufky-Heinrich, D. Production of bio-phenols for industrial application: Scale-up of the base-catalyzed depolymerization of lignin. Green Sustain. Chem. 2017, 7, 193–202. [Google Scholar] [CrossRef] [Green Version]
- Willför, S.; Sundberg, A.; Pranovich, A.; Holmbom, B. Polysaccharides in some industrially important hardwood species. Wood Sci. Technol. 2005, 39, 601–617. [Google Scholar] [CrossRef]
- Nitzsche, R.; Budzinski, M.; Gröngröft, A. Techno-economic assessment of a wood-based biorefinery concept for the production of polymer-grade ethylene, organosolv lignin and fuel. Bioresour. Technol. 2016, 200, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Söderqvist Lindblad, M.; Albertsson, A.-C.; Ranucci, E.; Laus, M.; Giani, E. Biodegradable polymers from renewable sources: Rheological characterization of hemicellulose-based hydrogels. Biomacromolecules 2005, 6, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Hartman, J.; Albertsson, A.-C.; Lindblad, M.S.; Sjöberg, J. Oxygen barrier materials from renewable sources: Material properties of softwood hemicellulose-based films. J. Appl. Polym. Sci. 2006, 100, 2985–2991. [Google Scholar] [CrossRef]
- Gröndahl, M.; Eriksson, L.; Gatenholm, P. Material properties of plasticized hardwood xylans for potential application as oxygen barrier films. Biomacromolecules 2004, 5, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.U.; Oliveira, R.C.; Buckeridge, M.S. Seed storage hemicelluloses as wet-end additives in papermaking. Carbohydr. Polym. 2003, 52, 367–373. [Google Scholar] [CrossRef]
- Hansen, N.M.L.; Plackett, D. Sustainable films and coatings from hemicelluloses: A review. Biomacromolecules 2008, 9, 1493–1505. [Google Scholar] [CrossRef]
- Abejón, R. A bibliometric study of scientific publications regarding hemicellulose valorization during the 2000–2016 period: Identification of alternatives and hot topics. ChemEngineering 2018, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Delidovich, I.; Leonhard, K.; Palkovits, R. Cellulose and hemicellulose valorisation: An integrated challenge of catalysis and reaction engineering. Energy Environ. Sci. 2014, 7, 2803. [Google Scholar] [CrossRef]
- Naidu, D.S.; Hlangothi, S.P.; John, M.J. Bio-based products from xylan: A review. Carbohydr. Polym. 2018, 179, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Jong, E.; Stichnothe, H.; Bell, G.; Jörgensen, H. Bio-Based Chemicals: A 2020 Update; IEA Bioenergy: Vienna, Austria, 2020; ISBN 978-1-910154-69-4. [Google Scholar]
- Cheng, M.-H.; Dien, B.S.; Lee, D.K.; Singh, V. Sugar production from bioenergy sorghum by using pilot scale continuous hydrothermal pretreatment combined with disk refining. Bioresour. Technol. 2019, 289, 121663. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Deng, A.; Ren, J.; Liu, C.; Lu, Q.; Zhong, L.; Peng, F.; Sun, R. Catalytic hydrothermal pretreatment of corncob into xylose and furfural via solid acid catalyst. Bioresour. Technol. 2014, 158, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Köchermann, J.; Mühlenberg, J.; Klemm, M. Kinetics of hydrothermal furfural production from organosolv hemicellulose and d -xylose. Ind. Eng. Chem. Res. 2018, 57, 14417–14427. [Google Scholar] [CrossRef]
- Nitzsche, R.; Köchermann, J.; Gröngröft, A.; Kraume, M. Nanofiltration of organosolv hemicellulose hydrolyzate—Influence of hydrothermal pretreatment and membrane characteristics on filtration performance. Ind. Eng. Chem. Res. 2021, 60, 916–930. [Google Scholar] [CrossRef]
- van Zandvoort, I.; Wang, Y.; Rasrendra, C.B.; van Eck, E.R.H.; Bruijnincx, P.C.A.; Heeres, H.J.; Weckhuysen, B.M. Formation, molecular structure, and morphology of humins in biomass conversion: Influence of feedstock and processing conditions. ChemSusChem 2013, 6, 1745–1758. [Google Scholar] [CrossRef]
- Hu, X.; Kadarwati, S.; Wang, S.; Song, Y.; Hasan, M.M.; Li, C.-Z. Biomass-derived sugars and furans: Which polymerize more during their hydrolysis? Fuel Processing Technol. 2015, 137, 212–219. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Zereshki, S. Reverse osmosis alternative: Energy implication for sugar industry. Chem. Eng. Processing Process Intensif. 2008, 47, 1075–1080. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Tahmasebi, K.; Kerendi, S.H. Sugar syrup concentration using reverse osmosis membranes. Eng. Life Sci. 2004, 4, 187–190. [Google Scholar] [CrossRef]
- Gautam, A.; Menkhaus, T.J. Performance evaluation and fouling analysis for reverse osmosis and nanofiltration membranes during processing of lignocellulosic biomass hydrolysate. J. Membr. Sci. 2014, 451, 252–265. [Google Scholar] [CrossRef]
- Fane, A.G.; Xi, W.; Rong, W. Interface Science and Technology; Elsevir: Amsterdam, The Netherlands, 2006; pp. 109–132. [Google Scholar] [CrossRef]
- Fane, A.G.; Fell, C.J.D. A review of fouling and fouling control in ultrafiltration. Desalination 1987, 62, 117–136. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, C.; Wei, J. Separation of acetic acid from monosaccharides by NF and RO membranes: Performance comparison. J. Membr. Sci. 2013, 429, 243–251. [Google Scholar] [CrossRef]
- Sjöman, E.; Mänttäri, M.; Nyström, M.; Koivikko, H.; Heikkilä, H. Xylose recovery by nanofiltration from different hemicellulose hydrolyzate feeds. J. Membr. Sci. 2008, 310, 268–277. [Google Scholar] [CrossRef]
- Weng, Y.-H.; Wei, H.-J.; Tsai, T.-Y.; Lin, T.-H.; Wei, T.-Y.; Guo, G.-L.; Huang, C.-P. Separation of furans and carboxylic acids from sugars in dilute acid rice straw hydrolyzates by nanofiltration. Bioresour. Technol. 2010, 101, 4889–4894. [Google Scholar] [CrossRef]
- Qi, B.; Luo, J.; Chen, X.; Hang, X.; Wan, Y. Separation of furfural from monosaccharides by nanofiltration. Bioresour. Technol. 2011, 102, 7111–7118. [Google Scholar] [CrossRef]
- Murthy, G.; Sridhar, S.; Sunder, M.; Shankaraia, B.; Ramakrishna, M. Concentration of xylose reaction liquor by nanofiltration for the production of xylitol sugar alcohol. Sep. Purif. Technol. 2005, 44, 205–211. [Google Scholar] [CrossRef]
- Heinonen, J.; Sanlaville, Q.; Niskakoski, H.; Tamper, J.; Sainio, T. Separation and recovery of lignin from hydrolysates of lignocellulose with a polymeric adsorbent. Sep. Purif. Technol. 2017, 186, 125–134. [Google Scholar] [CrossRef]
- Nitzsche, R.; Gröngröft, A.; Kraume, M. Separation of lignin from beech wood hydrolysate using polymeric resins and zeolites –Determination and application of adsorption isotherms. Sep. Purif. Technol. 2019, 209, 491–502. [Google Scholar] [CrossRef]
- Gütsch, J.S.; Sixta, H. Regeneration of spent activated charcoals used for lignin removal from prehydrolysis-kraft prehydrolyzates. Ind. Eng. Chem. Res. 2012, 51, 8624–8630. [Google Scholar] [CrossRef]
- Louchouarn, P.; Opsahl, S.; Benner, R. Isolation and quantification of dissolved lignin from natural waters using solid-phase extraction and GC/MS. Anal. Chem. 2000, 72, 2780–2787. [Google Scholar] [CrossRef]
- Ranjan, R.; Thust, S.; Gounaris, C.E.; Woo, M.; Floudas, C.A.; Keitz, M.v.; Valentas, K.J.; Wei, J.; Tsapatsis, M. Adsorption of fermentation inhibitors from lignocellulosic biomass hydrolyzates for improved ethanol yield and value-added product recovery. Microporous Mesoporous Mater. 2009, 122, 143–148. [Google Scholar] [CrossRef]
- Persson, T.; Jönsson, A.-S. Fouling of ultrafiltration membranes during isolation of hemicelluloses in the forest industry. Sch. Res. Exch. 2009, 2009, 1–7. [Google Scholar] [CrossRef]
- Koivula, E.; Kallioinen, M.; Sainio, T.; Antón, E.; Luque, S.; Mänttäri, M. Enhanced membrane filtration of wood hydrolysates for hemicelluloses recovery by pretreatment with polymeric adsorbents. Bioresour. Technol. 2013, 143, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.-G.; Jia, N.; Zhu, J.-F.; Li, S.-M.; Peng, F.; Sun, R.-C. Isolation and characterization of hemicelluloses extracted by hydrothermal pretreatment. Bioresour. Technol. 2012, 114, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Peng, P.; Xu, F.; Sun, R.-C. Fractional purification and bioconversion of hemicelluloses. Biotechnol. Adv. 2012, 30, 879–903. [Google Scholar] [CrossRef]
- Persson, T.; Jönsson, A.-S. Isolation of hemicelluloses by ultrafiltration of thermomechanical pulp mill process water—Influence of operating conditions. Chem. Eng. Res. Des. 2010, 88, 1548–1554. [Google Scholar] [CrossRef]
- Al Manasrah, M.; Kallioinen, M.; Ilvesniemi, H.; Mänttäri, M. Recovery of galactoglucomannan from wood hydrolysate using regenerated cellulose ultrafiltration membranes. Bioresour. Technol. 2012, 114, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, H.; Arkell, A.; Jönsson, A.-S. Membrane performance during ultrafiltration of a high-viscosity solution containing hemicelluloses from wheat bran. Sep. Purif. Technol. 2011, 83, 144–150. [Google Scholar] [CrossRef]
- Nitzsche, R.; Gröngröft, A.; Goj, I.; Kraume, M. Ultrafiltration of beechwood hydrolysate for concentrating hemicellulose sugars and removal of lignin—Parameter estimation using statistical methods and multiobjective optimization. Ind. Eng. Chem. Res. 2020, 59, 7875–7887. [Google Scholar] [CrossRef]
- Schulze, P.; Seidel-Morgenstern, A.; Lorenz, H.; Leschinsky, M.; Unkelbach, G. Advanced process for precipitation of lignin from ethanol organosolv spent liquors. Bioresour. Technol. 2016, 199, 128–134. [Google Scholar] [CrossRef]
- Thuvander, J.; Jönsson, A.-S. Extraction of galactoglucomannan from thermomechanical pulp mill process water by microfiltration and ultrafiltration—Influence of microfiltration membrane pore size on ultrafiltration performance. Chem. Eng. Res. Des. 2016, 105, 171–176. [Google Scholar] [CrossRef]
- Stenius, P. Surface Chemistry and Charge of Cellulosic Fibres; Marie Curie Initial Training Network: Helsinki/Espoo, Finland, 2011. [Google Scholar]
- Koivula, E.; Kallioinen, M.; Preis, S.; Testova, L.; Sixta, H.; Mänttäri, M. Evaluation of various pretreatment methods to manage fouling in ultrafiltration of wood hydrolysates. Sep. Purif. Technol. 2011, 83, 50–56. [Google Scholar] [CrossRef]
- Arkell, A.; Olsson, J.; Wallberg, O. Process performance in lignin separation from softwood black liquor by membrane filtration. Chem. Eng. Res. Des. 2014, 92, 1792–1800. [Google Scholar] [CrossRef]
- Natelson, R.H.; Wang, W.-C.; Roberts, W.L.; Zering, K.D. Technoeconomic analysis of jet fuel production from hydrolysis, decarboxylation, and reforming of camelina oil. Biomass Bioenergy 2015, 75, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Wooley, R.; Putsche, V. Development of an ASPEN PLUS Physical Property Database for Biofuels Components; National Renewable Energy Laboratory: Middletown, DE, USA, 1996.
- Aden, A.; Ruth, M.; Ibsen, K.; Jechura, J.; Neeves, K.; Sheehan, J.; Wallace, B. Lignocellulosic Biomass to Ethanol Process Design and Economics Utilizing CoCurrent Dilute Acid Pre hydrolysis and Enzymatic Hydrolysis for Corn Stover; National Renewable Energy Laboratory: Middletown, DE, USA, 2002; Technical Report NREL/TP-510-32438.
- Nitzsche, R.; Gröngröft, A.; Köchermann, J.; Meisel, K.; Etzold, H.; Verges, M.; Leschinsky, M.; Bachmann, J.; Saake, B.; Torkler, S.; et al. Platform and fine chemicals from woody biomass—Demonstration and assessment of a novel biorefinery. Biomass Convers. Biorefinery 2020, 48, 793. [Google Scholar] [CrossRef]
- Takkellapati, S.; Li, T.; Gonzalez, M.A. An overview of biorefinery derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Environ. Policy 2018, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Kajaste, R. Chemicals from biomass—Managing greenhouse gas emissions in biorefinery production chains—A review. J. Clean. Prod. 2014, 75, 1–10. [Google Scholar] [CrossRef]
- Ahsan, L.; Jahan, M.S.; Ni, Y. Recovering/concentrating of hemicellulosic sugars and acetic acid by nanofiltration and reverse osmosis from prehydrolysis liquor of kraft based hardwood dissolving pulp process. Bioresour. Technol. 2014, 155, 111–115. [Google Scholar] [CrossRef]
- VDI 6025; Betriebswirtschaftliche Berechnungen für Investitionsgüter und Anlagen. Verein Deutscher Ingenieure: Alexisbad, Germany, 2012.
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; McGraw-Hill: Boston, MA, USA, 2004; ISBN 9780071240444. [Google Scholar]
- Chauvel, A.; Fouriner, G.; Raimbault, C. Manual of Process Economic Evaluation—New, Revised and Expanded Edition; Editions Technip: Paris, France, 2001; ISBN 9782710808367. [Google Scholar]
- Chemiewirtschaft in Zahlen 2020; Verband der Chemischen Industrie e. V.: Frankfurt am Main, Germany, 2020.
- Turton, R.; Shaeiwitz, J.A.; Bhattacharyya, D.; Whiting, W.B. Analysis, Synthesis, and Design of Chemical Processes, 5th ed.; Prentice Hall: Boston, MA, USA, 2018; ISBN 9780134177403. [Google Scholar]
- Kwon, D.Y.; Vigneswaran, S.; Fane, A.G.; Aim, R.B. Experimental determination of critical flux in cross-flow microfiltration. Sep. Purif. Technol. 2000, 19, 169–181. [Google Scholar] [CrossRef]
- Bacchin, P.; Aimar, P.; Field, R. Critical and sustainable fluxes: Theory, experiments and applications. J. Membr. Sci. 2006, 281, 42–69. [Google Scholar] [CrossRef] [Green Version]
- Weng, Y.-H.; Wei, H.-J.; Tsai, T.-Y.; Chen, W.-H.; Wei, T.-Y.; Hwang, W.-S.; Wang, C.-P.; Huang, C.-P. Separation of acetic acid from xylose by nanofiltration. Sep. Purif. Technol. 2009, 67, 95–102. [Google Scholar] [CrossRef]
- Nilsson, M.; Trägårdh, G.; Östergren, K. The influence of pH, salt and temperature on nanofiltration performance. J. Membr. Sci. 2008, 312, 97–106. [Google Scholar] [CrossRef]
- Sharma, R.R.; Agrawal, R.; Chellam, S. Temperature effects on sieving characteristics of thin-film composite nanofiltration membranes: Pore size distributions and transport parameters. J. Membr. Sci. 2003, 223, 69–87. [Google Scholar] [CrossRef]
- Montané, D.; Nabarlatz, D.; Martorell, A.; Torné-Fernández, V.; Fierro, V. Removal of lignin and associated impurities from xylo-oligosaccharides by activated carbon adsorption. Ind. Eng. Chem. Res. 2006, 45, 2294–2302. [Google Scholar] [CrossRef]
- Asikkala, J.; Tamminen, T.; Argyropoulos, D.S. Accurate and reproducible determination of lignin molar mass by acetobromination. J. Agric. Food Chem. 2012, 60, 8968–8973. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Hatakeyama, H. Lignin structure, properties, and application. Adv. Polym. Sci. 2010, 232. [Google Scholar] [CrossRef]
- Kallioinen, M.; Koivula, E.; Puro, L.; Mänttäri, M. Influence of operating temperature on fractionation in ultrafiltration of wood-based hydrolysate. In Proceedings of the Nordic Wood Biorefinery Conference, Stockholm, Sweden, 22–24 March 2011. [Google Scholar]
- Puro, L.; Kallioinen, M.; Mänttäri, M.; Nyström, M. Evaluation of behavior and fouling potential of wood extractives in ultrafiltration of pulp and paper mill process water. J. Membr. Sci. 2011, 368, 150–158. [Google Scholar] [CrossRef]
- Arkell, A.; Krawczyk, H.; Thuvander, J.; Jönsson, A.-S. Evaluation of membrane performance and cost estimates during recovery of sodium hydroxide in a hemicellulose extraction process by nanofiltration. Sep. Purif. Technol. 2013, 118, 387–393. [Google Scholar] [CrossRef]
- Larsson, E. Preconcentration of Dilute Acid Hydrolyzate by Membrane Filtration; Lund University: Lund, Sweden, 2009; pp. 1–6. [Google Scholar]
- United States. Environmental Protection Agency. Treatability Manual Volume IV—Cost Estimating; Office of Research and Development, U.S. Environmental Protection Agency: Washington, DC, USA, 1980.
- Dien, B.S.; Nichols, N.N.; Bothast, R.J. Recombinant Escherichia coli engineered for production of L-lactic acid from hexose and pentose sugars. J. Ind. Microbiol. Biotechnol. 2001, 27, 259–264. [Google Scholar] [CrossRef]
- Nolleau, V.; Preziosi-Belloy, L.; Delgenes, J.P.; Navarro, J.M. Xylitol production from xylose by two yeast strains: Sugar tolerance. Curr. Microbiol. 1993, 27, 191–197. [Google Scholar] [CrossRef]
- F.O. Licht. World Ethanol & Biofuels Report; F.O. Licht: London, UK, 2018. [Google Scholar]
- Mitsubishi Chemical Corporation. Product Data Sheet—SEPABEADSTM SP700. Available online: https://www.diaion.com/en/products/synthetic_adsorbents/data_sheet_sp/pdf/SP700_Product_data_sheet.pdf (accessed on 29 April 2021).
- Jönsson, A.-S.; Wallberg, O. Cost estimates of kraft lignin recovery by ultrafiltration. Desalination 2009, 237, 254–267. [Google Scholar] [CrossRef]
- Dimitriou, I.; García-Gutiérrez, P.; Elder, R.H.; Cuéllar-Franca, R.M.; Azapagic, A.; Allen, R.W.K. Carbon dioxide utilisation for production of transport fuels: Process and economic analysis. Energy Environ. Sci. 2015, 8, 1775–1789. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Energy Statistics—Prices of Natural Gas and Electricity. 2018. Available online: https://ec.europa.eu/eurostat/data/database (accessed on 2 September 2021).






| Components | Concentration (g/L) | MW (g/mol) | pKa | ||
|---|---|---|---|---|---|
| BWH 1 | BWH 2 | BWH 3 | |||
| Oligomeric hemicellulose | 4.0 | 10.3 | 22.8 | 300–10,000 a,b | 12–13 c,d |
| Glucose | 0.6 | 1.7 | 2.8 | 180.16 e | 12.46 e |
| Xylose | 6.0 | 13.3 | 34.2 | 150.13 e | 12.14 e |
| 5-HMF | 0.4 | 0.6 | 0.8 | 126.11 d | n/a |
| Furfural | 0.5 | 0.1 | n.d. | 96.08 d | n/a |
| Acetic acid | 4.1 | 3.8 | 3.9 | 60.05 e | 4.756 e |
| Lignin | 1.6 | 3.2 | 4.8 | 500–1000 a,b | 3–10 c,f |
| Parameter | Value | Unit |
|---|---|---|
| Average cost of capital | 4 | % p.a. |
| Assessment period | 20 | Years |
| Maintenance, repairs | 5 | % of FCI p.a. a |
| Administration | 20 | % of labor costs p.a. a |
| Insurance, uncertainties | 1.5 | % of FCI p.a. a |
| Labor costs | 28.5 | EUR/h b |
| UF/NF membrane | 30 | €/m² c |
| Membrane housing | 50 | €/m² c |
| Adsorbent material | 5100 | €/m³ c |
| HTBWH 1.1 | HTBWH 1.2 | HTBWH 1.3 | |
|---|---|---|---|
| Pressure (MPa) | 16.2 | 13.5 | 12.9 |
| Temperature (°C) | 180 | 174 | 180 |
| pH (-) | 2.6 | 1.7 | 1.7 |
| Oligomeric hemicellulose (g/L) | 1.5 | 0.0 | 0.0 |
| Xylose (g/L) | 8.2 | 10.3 | 9.2 |
| Furfural (g/L) | 0.7 | n/a | 1.3 |
| Purification Cascadeand Main Streams | Mass Input | Mass Output | Energy Efficiency | (Production) Costs |
|---|---|---|---|---|
| [t/h] | [%] | [EUR/t] | ||
| Purification cascade 1 | ||||
| Beechwood hydrolyzate 3 | 32.3 (10.3 MW) | 0.0 | ||
| Wastewater | 0.0 | 24.5 | 2.5 a | |
| Xylose solution (C = 200 g/L) | 0.0 | 7.8 (8.0 MW) | 59 | 73.7 c |
| Purification cascade 2 | ||||
| Beechwood hydrolyzate 3 | 32.3 (10.3 MW) | 0.0 | ||
| Process water | 12.2 | 0.0 | 0.15 a | |
| Ethanol | 0.2 (1.7 MW) | 0.0 | 550 b | |
| Wastewater | 0.0 | 37.1 | 2.5 a | |
| Hemicellulose solution (C = 200 g/L) | 0.0 | 7.6 (7.0 MW) | 26 | 135.1 c |
| Process Utility | Purification Cascade 1 | Purification Cascade 2 | Costs |
|---|---|---|---|
| [MW] | |||
| Electrical power | 0.3 | 0.2 | 150 EUR/MWh a |
| Cool water | 0.0 | 7.2 | 0.043 EUR/t b |
| Low-pressure steam | 0.0 | 8.8 | 25 EUR/t b |
| High-pressure steam | 1.5 | 0.0 | 25 EUR/t b |
| Purification Cascade 1 | Purification Cascade 2 | ||
|---|---|---|---|
| Fixed-capital investments | [kEUR] | 9415 | 10,962 |
| Capital-linked costs | [kEUR/a] | 696 | 809 |
| Depreciation | [kEUR/a] | 473 | 550 |
| Interest | [kEUR/a] | 223 | 259 |
| Consumption-linked costs | [kEUR/a] | 1543 | 5002 |
| Raw Material | [kEUR/a] | - | - |
| Auxiliary and operating material | [kEUR/a] | 51 | 1080 |
| Energy Supply | [kEUR/a] | 1001 | 3180 |
| Disposal costs | [kEUR/a] | 491 | 742 |
| Operation-linked costs | [kEUR/a] | 1906 | 1984 |
| Labor Costs | [kEUR/a] | 1436 | 1436 |
| Maintenance | [kEUR/a] | 471 | 548 |
| Other costs | [kEUR/a] | 428 | 452 |
| Administration | [kEUR/a] | 287 | 287 |
| Insurance | [kEUR/a] | 94 | 110 |
| Uncertainties | [kEUR/a] | 47 | 55 |
| Purification Cascade 1 | Purification Cascade 2 | ||
|---|---|---|---|
| Capital-linked costs | [EUR/tproduct] | 11.2 | 13.3 |
| Consumption-linked costs | [EUR/tproduct] | 24.9 | 81.9 |
| Operation-linked costs | [EUR/tproduct] | 30.7 | 32.5 |
| Other costs | [EUR/tproduct] | 6.9 | 7.4 |
| Production costs | [EUR/tproduct] | 73.7 | 135.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nitzsche, R.; Etzold, H.; Verges, M.; Gröngröft, A.; Kraume, M. Demonstration and Assessment of Purification Cascades for the Separation and Valorization of Hemicellulose from Organosolv Beechwood Hydrolyzates. Membranes 2022, 12, 82. https://doi.org/10.3390/membranes12010082
Nitzsche R, Etzold H, Verges M, Gröngröft A, Kraume M. Demonstration and Assessment of Purification Cascades for the Separation and Valorization of Hemicellulose from Organosolv Beechwood Hydrolyzates. Membranes. 2022; 12(1):82. https://doi.org/10.3390/membranes12010082
Chicago/Turabian StyleNitzsche, Roy, Hendrik Etzold, Marlen Verges, Arne Gröngröft, and Matthias Kraume. 2022. "Demonstration and Assessment of Purification Cascades for the Separation and Valorization of Hemicellulose from Organosolv Beechwood Hydrolyzates" Membranes 12, no. 1: 82. https://doi.org/10.3390/membranes12010082
APA StyleNitzsche, R., Etzold, H., Verges, M., Gröngröft, A., & Kraume, M. (2022). Demonstration and Assessment of Purification Cascades for the Separation and Valorization of Hemicellulose from Organosolv Beechwood Hydrolyzates. Membranes, 12(1), 82. https://doi.org/10.3390/membranes12010082