Recent Advances of Polymeric Membranes in Tackling Plasticization and Aging for Practical Industrial CO2/CH4 Applications—A Review
Abstract
:1. Introduction
2. Commercial Polymeric Membrane for Gas Separation
2.1. Cellulose Acetates (CA)
2.2. Polysulfones (PSF)
2.3. Polyimides (PIs)
2.4. Polycarbonates (PCs)
2.5. Transport Mechanism for Gas Separation Membrane
3. Challenges for Gas-Separation Membrane
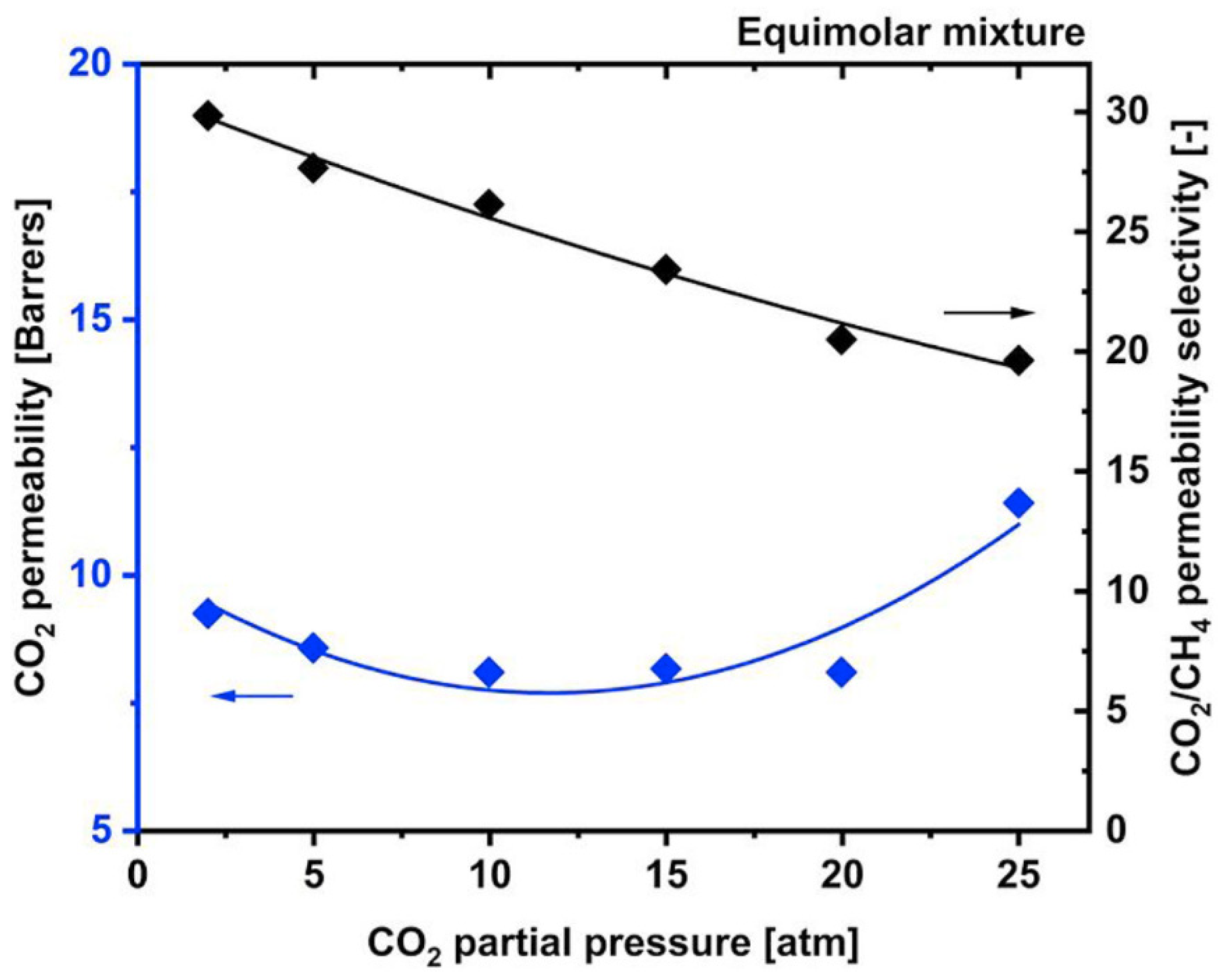
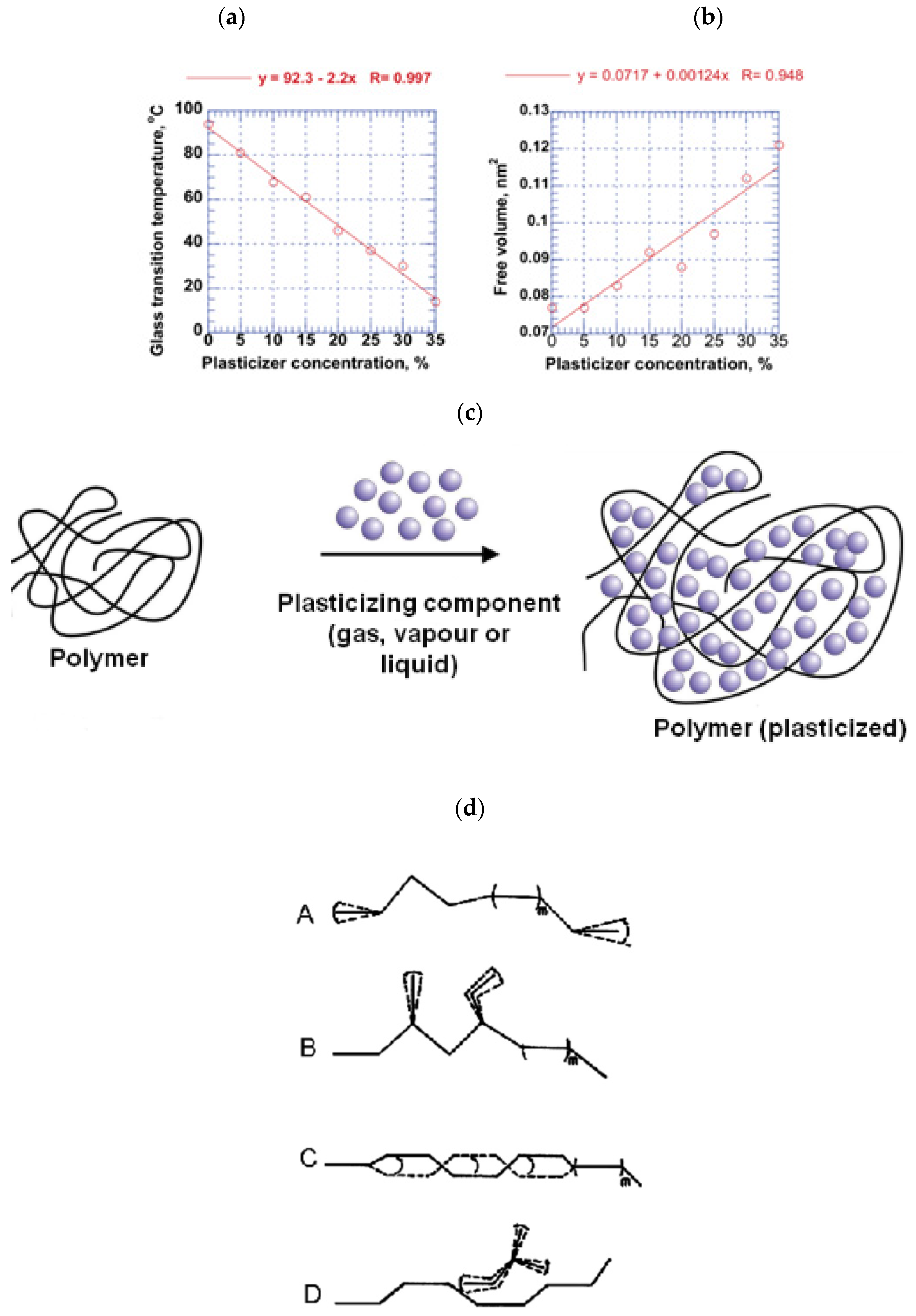
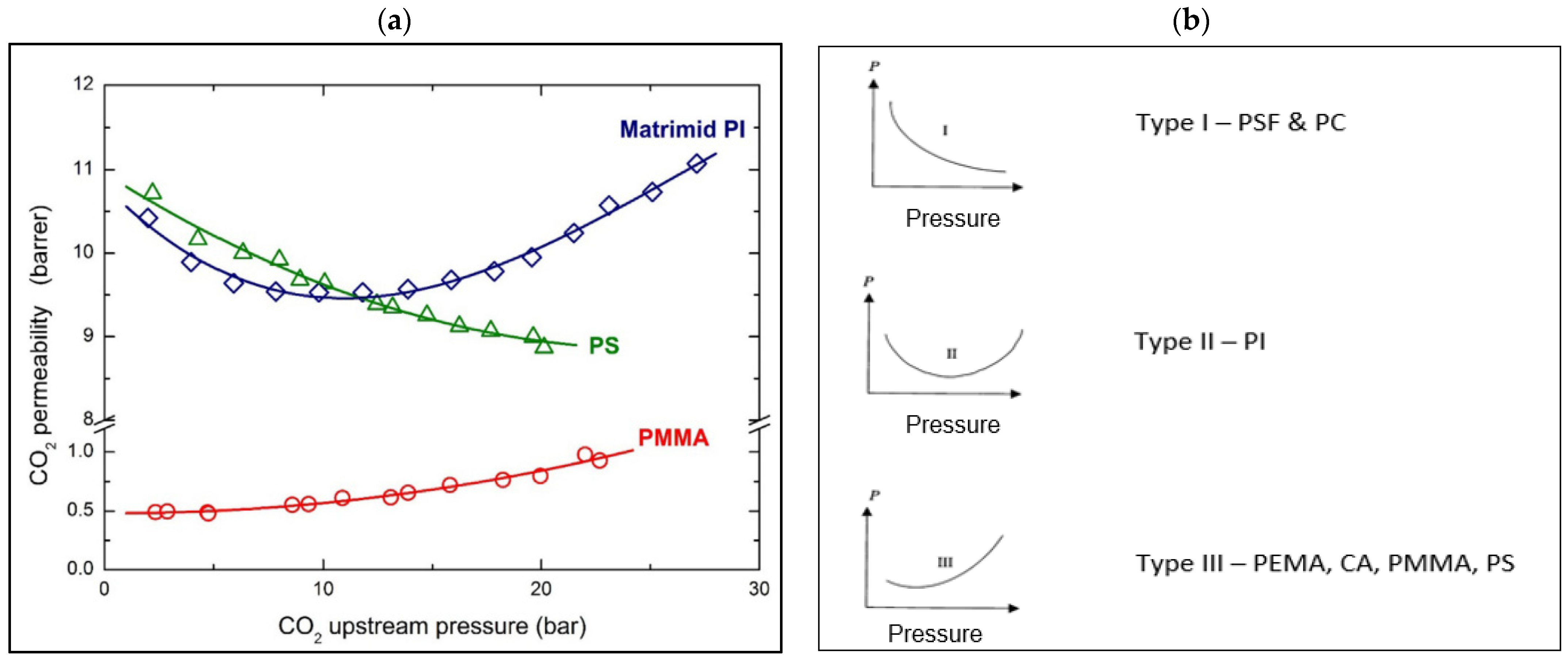
3.1. Plasticization Phenomena
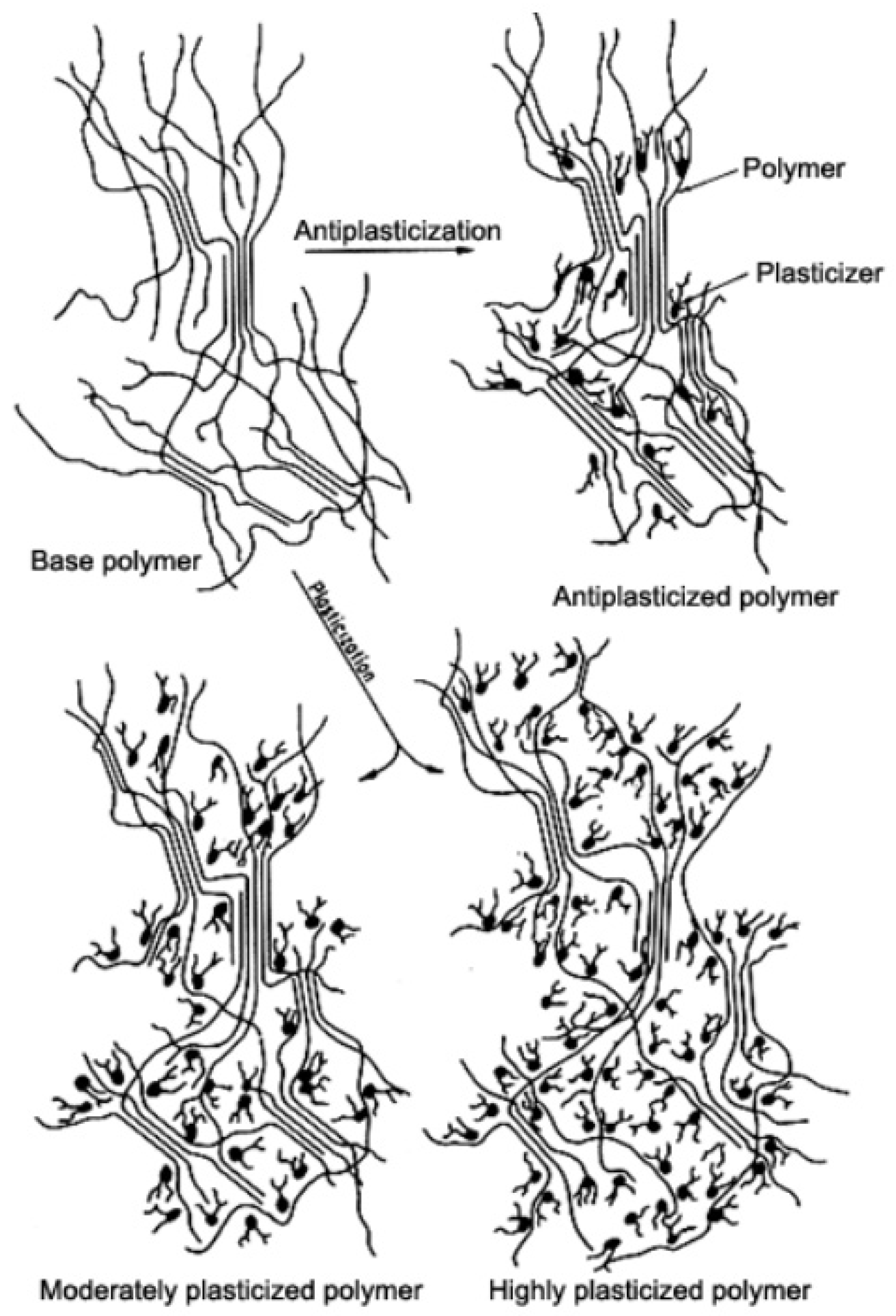
Plasticizers
3.2. Competitive Sorption
3.3. Polymer Aging
4. Strategies to Improve Polymeric Membranes towards Plasticization- and Aging-Resistance
4.1. Polymer Modification via Cross-Linking
4.1.1. Physical Crosslinking
4.1.2. Chemical Cross-Linking
Incorporation of CO2 Philic Groups
Incorporation of Bulky Groups
Functionalization Using Bromination
Sulfonation
Lithiation
Esterification
4.1.3. Thermal Crosslinking and Thermally Rearranged (TR) Polymers
PBOs, PBTs and PBIs
Template Polymerization
TR Polymers
4.1.4. Ultraviolet (UV) Radiation Cross-Linking
4.1.5. Surface Modification
4.2. Polymer Blending
4.3. Facilitated Transport Membranes (FTMs)
4.4. Mixed-Matrix Membranes (MMMs)
4.5. New Polymer
4.5.1. High Free-Volume Membrane/Super Glassy Polymer
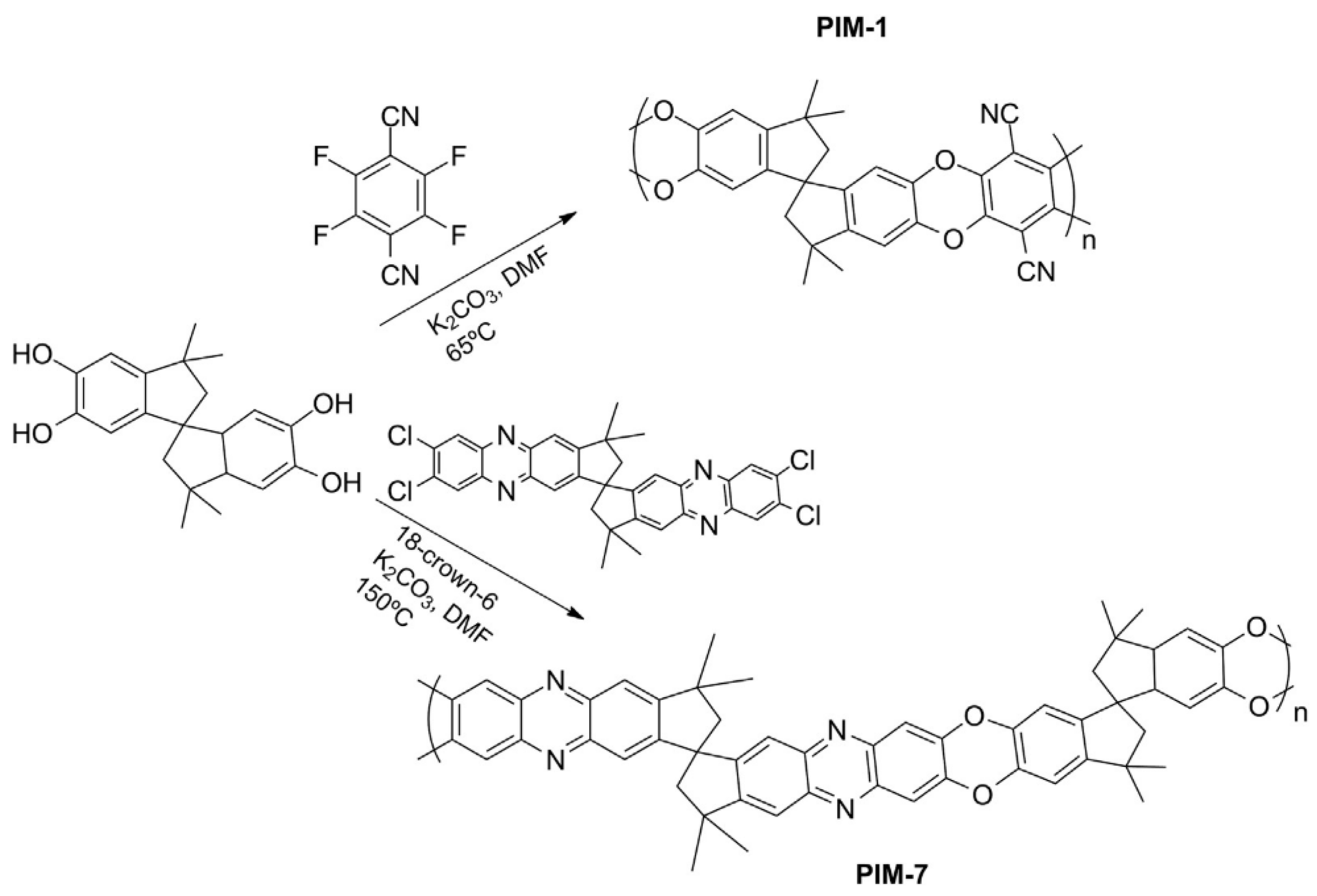
PIMs
PEO and PEG
PTMSP
Perfluorinated Polymer
5. Future Direction
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Global Greenhouse Gas Emissions Data; US Environmental Protection Agency. 2021. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 18 September 2021).
- Faramawy, S.; Zaki, T.; Sakr, A.A. Natural gas origin, composition, and processing: A review. J. Nat. Gas Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Li, P.; Hosseini, S.S.; Zhang, M.; Deng, L.; Xiang, D.; Cao, B. Approaches to Suppress CO2-Induced Plasticization of Polyimide Membranes in Gas. Processes 2019, 7, 51. [Google Scholar]
- Kargari, A.; Rezaeinia, S. State-of-the-art mod fi cation of polymeric membranes by PEO and PEG for carbon dioxide separation: A review of the current status and future perspectives. J. Ind. Eng. Chem. 2019, 84, 1–22. [Google Scholar] [CrossRef]
- Adewole, J.K.; Ahmad, A.L.; Ismail, S.; Leo, C.P. International Journal of Greenhouse Gas Control Current challenges in membrane separation of CO2 from natural gas: A review. Int. J. Greenh. Gas Control 2013, 17, 46–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas Control 2013, 12, 84–107. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mukhtar, H.B.; Shariff, A.M.; Mannan, H.A. A Review: Development of Polymeric Blend Membrane for Removal of CO2 from Natural Gas. Int. J. Eng. Technol. IJET-IJENS 2013, 13, 53–60. [Google Scholar]
- Bernardo, P.; Clarizia, G. 30 Years of Membrane Technology for Gas Separation. Chem. Eng. Trans. 2013, 32, 1999–2004. [Google Scholar]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Iarikov, D.D.; Ted Oyama, S. Review of CO2/CH4 Separation Membranes. In Membrane Science and Technology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 14, pp. 91–115. [Google Scholar]
- Russo, F.; Galiano, F.; Iulianelli, A.; Basile, A.; Figoli, A. Biopolymers for sustainable membranes in CO2 separation: A review. Fuel Process. Technol. 2021, 213, 106643. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Sheng, M. Membrane technology for CO2 capture: From pilot-scale investigation of two-stage plant to actual design. J. Memb. Sci. 2021, 624, 119137. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane gas separation: A review/state of the art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Kentish, S.E.; Scholes, C.A.; Stevens, G.W. Carbon Dioxide Separation through Polymeric Membrane Systems for Flue Gas Applications. Recent Patents Chem. Eng. 2008, 1, 52–66. [Google Scholar] [CrossRef]
- Schmeling, N.; Konietzny, R.; Sieffert, D.; Rölling, P.; Staudt, C. Functionalized copolyimide membranes for the separation of gaseous and liquid mixtures. Beilstein J. Org. Chem. 2010, 6, 789–800. [Google Scholar] [CrossRef]
- Low, Z.; Budd, P.M.; Mckeown, N.B.; Patterson, D.A. Gas Permeation Properties, Physical Aging, and Its Mitigation in High Free Volume Glassy Polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ho, W.S.W. Polymeric membranes for CO2 separation and capture. J. Memb. Sci. 2021, 628, 119244. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Materials selection guidelines for membranes that remove CO2 from gas mixtures. J. Mol. Struct. 2005, 739, 57–74. [Google Scholar] [CrossRef]
- Sridhar, S.; Smitha, B.; Aminabhavi, T.M. Separation of carbon dioxide from natural gas mixtures through polymeric membranes—A review. Sep. Purif. Rev. 2007, 36, 113–174. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Memb. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Yeo, Z.Y.; Chew, T.L.; Zhu, P.W.; Mohamed, A.R.; Chai, S.P. Conventional processes and membrane technology for carbon dioxide removal from natural gas: A review. J. Nat. Gas Chem. 2012, 21, 282–298. [Google Scholar] [CrossRef]
- Ma, X.-H.; Yang, S.-Y. Polyimide Gas Separation Membranes; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128126400. [Google Scholar]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Plasticization and Swelling in Polymeric Membranes in CO2 Removal from Natural Gas. Chem. Eng. Technol. 2016, 39, 1604–1616. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef] [Green Version]
- Du, N.; Canada, C.; Park, H.B.; Dal-cin, M.; Canada, C.; Guiver, M.D. Advances in high permeability polymeric membrane materials for CO2 separations. Energy Environ. Sci. 2012, 5, 7306. [Google Scholar] [CrossRef] [Green Version]
- Favvas, E.P.; Katsaros, F.K.; Papageorgiou, S.K.; Sapalidis, A.A.; Mitropoulos, A.C. A review of the latest development of polyimide based membranes for CO2 separations. React. Funct. Polym. 2017, 120, 104–130. [Google Scholar] [CrossRef]
- Lozano, E.; Pra, P.; Tejerina, F.; Herna, A. Effect of Fractional Free Volume and T g on Gas Separation Through Membranes Made with Different Glassy Polymers. J. Appl. Polym. Sci. 2008, 107, 1039–1046. [Google Scholar]
- Ahmad, M.Z. Synthesis and Characterization of Polyimide-Based Mixed Matrix Membranes for CO2/CH4 Separation/Mohd Zamidi Ahmad; University of Chemistry and Technology Prague: Prague, Czech Republic, 2018. [Google Scholar]
- Lam, B.; Wei, M.; Zhu, L.; Luo, S.; Guo, R.; Morisato, A.; Alexandridis, P.; Lin, H. Cellulose triacetate doped with ionic liquids for membrane gas separation. Polymer 2016, 89, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. Gr. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Yong, W.F.; Chung, T.S.; Weber, M.; Maletzko, C. New polyethersulfone (PESU) hollow fiber membranes for CO2 capture. J. Memb. Sci. 2018, 552, 305–314. [Google Scholar] [CrossRef]
- Julian, H.; Wenten, I.G. Polysulfone membranes for CO2/CH4 separation: State of the art. IOSR J. Eng. 2012, 2, 484–495. [Google Scholar] [CrossRef]
- Lokhandwala, K.A.; Baker, R.W. Natural Gas Processing with Membranes. Ind. Eng. Chem. Res. 2008, 1, 2109–2121. [Google Scholar]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Buonomenna, M.G. Membrane Separation of CO2 from Natural Gas. Recent Patents Mater. Sci. 2017, 10, 26–49. [Google Scholar] [CrossRef]
- Adewole, J.K.; Ahmad, A.L.; Sultan, A.S.; Ismail, S. Model-based analysis of polymeric membranes performance in high pressure CO2 removal from natural gas. J. Polym. Res. 2015, 22, 32. [Google Scholar] [CrossRef]
- Genduso, G.; Wang, Y.; Ghanem, B.S.; Pinnau, I. Permeation, sorption, and diffusion of CO2–CH4 mixtures in polymers of intrinsic microporosity: The effect of intrachain rigidity on plasticization resistance. J. Memb. Sci. 2019, 584, 100–109. [Google Scholar] [CrossRef]
- Brunetti, A.; Tocci, E.; Cersosimo, M.; Sung, J.; Hee, W.; Geun, J.; Moo, Y.; Drioli, E.; Barbieri, G. Mutual influence of mixed-gas permeation in thermally rearranged poly (benzoxazole- co -imide) polymer membranes. J. Memb. Sci. 2019, 580, 202–213. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Norman, N.L.; Anthony, G.F.; Ho, W.S.; Matsuura, T. .Advanced Membrane Technology and Applications; Wiley: Hoboken, NJ, USA, 2008; ISBN 9780471731672. [Google Scholar]
- Alaslai, N.; Ghanem, B.; Alghunaimi, F.; Litwiller, E.; Pinnau, I. Pure- and mixed-gas permeation properties of highly selective and plasticization resistant hydroxyl-diamine-based 6FDA polyimides for CO2/CH4 separation. J. Memb. Sci. 2016, 505, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Mulder, M. Basic Principles of Membrane Technology; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1996. [Google Scholar]
- Lu, H.T.; Liu, L.; Kanehashi, S.; Scholes, C.A.; Kentish, S.E. The impact of toluene and xylene on the performance of cellulose triacetate membranes for natural gas sweetening. J. Memb. Sci. 2018, 555, 362–368. [Google Scholar] [CrossRef]
- Mazyan, W.; Ahmadi, A.; Ahmed, H.; Hoorfar, M. Market and technology assessment of natural gas processing: A review. J. Nat. Gas Sci. Eng. 2016, 30, 487–514. [Google Scholar] [CrossRef]
- Ricci, E.; Di, E.; Degli, M.; Liu, L.; Mensitieri, G.; Fabbri, P.; Kentish, S.E.; Grazia, M.; Angelis, D. Towards a systematic determination of multicomponent gas separation with membranes: The case of CO2/CH4 in cellulose acetates. J. Memb. Sci. 2021, 628, 119226. [Google Scholar] [CrossRef]
- McKeen, L.W. The Effect of Temperature and Other factors on Plastics and Elastomers; Elsevier: Amsterdam, The Netherlands, 2008; pp. 213–241. [Google Scholar]
- Visser, T. Mixed Gas Plasticization Phenomena in Asymmetric Membranes. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2006. [Google Scholar]
- Genduso, G.; Pinnau, I. Quantification of sorption, diffusion, and plasticization properties of cellulose triacetate films under mixed-gas CO2/CH4 environment. J. Memb. Sci. 2020, 610, 118269. [Google Scholar] [CrossRef]
- Serbanescu, O.S.; Voicu, S.I.; Thakur, V.K. Polysulfone functionalized membranes: Properties and challenges. Mater. Today Chem. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Scholes, C.A.; Kentish, S.E.; Stevens, G.W. Effects of minor components in carbon dioxide capture using polymeric gas separation membranes. Sep. Purif. Rev. 2009, 38, 1–44. [Google Scholar] [CrossRef]
- Wong, K.K.; Jawad, Z.A. A review and future prospect of polymer blend mixed matrix membrane for CO2 separation. J. Polym. Res. 2019, 26, 289. [Google Scholar] [CrossRef]
- Rezac, M.E.; Sorensen, E.T.; Beckham, H.W. Transport properties of crosslinkable polyimide blends. J. Memb. Sci. 1997, 136, 249–259. [Google Scholar] [CrossRef]
- Sabu, T.; Anil, K.S.; Runcy, W.; Soney, C.G. Transport Properties of Polymeric Membranes; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Freeman, B.; Yampolskii, Y.; Pinnau, I. Materials Science of Membranes for Gas and Vapor Separation Materials; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kanehashi, S.; Nagai, K. Analysis of dual-mode model parameters for gas sorption in glassy polymers. J. Membr. Sci. 2005, 253, 117–138. [Google Scholar] [CrossRef]
- Miandoab, E.S.; Kentish, S.E.; Scholes, C.A. Modelling competitive sorption and plasticization of glassy polymeric membranes used in biogas upgrading. J. Memb. Sci. 2021, 617, 118643. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 1138–1148. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Guo, Z.; Wu, H.; Zhu, K.; Yang, L.; Younas, M.; Jiang, Z. Plasticization- and aging-resistant membranes with venation-like architecture for efficient carbon capture. J. Memb. Sci. 2020, 609, 118215. [Google Scholar] [CrossRef]
- Wypych, G. (Ed.) Handbook of Plasticizer, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2017; ISBN 9781895198973. [Google Scholar]
- Minelli, M.; Oradei, S.; Fiorini, M.; Sarti, G.C. CO2 plasticization effect on glassy polymeric membranes. Polymer 2019, 163, 29–35. [Google Scholar] [CrossRef]
- Sridhar, S.; Bee, S.; Bhargava, S. Membrane-based Gas Separation: Principle, Applications and Future Potential. Chem. Eng. Dig. 2014, 1, 1–25. [Google Scholar]
- Genduso, G.; Ghanem, B.S.; Pinnau, I. Experimental mixed-gas permeability, sorption and diffusion of CO2–CH4 mixtures in 6FDA-mPDA polyimide membrane: Unveiling the effect of competitive sorption on permeability selectivity. Membranes 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Freeman, B.D.; Pinnau, I. Gas and Liquid Separations Using Membranes: An Overview. In Advanced Materials for Membrane Separations; ACS Publications: Washington, DC, USA, 2004; pp. 1–23. [Google Scholar]
- Liu, Y.; Liu, Z.; Morisato, A.; Bhuwania, N.; Chinn, D.; Koros, J. Natural gas sweetening using a cellulose triacetate hollow fiber membrane illustrating controlled plasticization benefits. J. Memb. Sci. 2020, 601, 117910. [Google Scholar] [CrossRef]
- Bos, A.; Pünt, I.G.M.; Wessling, M.; Strathmann, H. CO2-induced plasticization phenomena in glassy polymers. J. Memb. Sci. 1999, 155, 67–78. [Google Scholar] [CrossRef]
- Ismail, A.F.; Lorna, W. Penetrant-induced plasticization phenomenon in glassy polymers for gas separation membrane. Sep. Purif. Technol. 2002, 23, 173–194. [Google Scholar] [CrossRef]
- Ahmad, F.; Lau, K.K.; Shariff, A.M.; Yeong, Y.F. Temperature and pressure dependence of membrane permeance and its effect on process economics of hollow fiber gas separation system. J. Memb. Sci. 2013, 430, 44–55. [Google Scholar] [CrossRef]
- Ricci, E.; Benedetti, F.M.; Dose, M.E.; De Angelis, M.G.; Freeman, B.D.; Paul, D.R. Competitive sorption in CO2/CH4 separations: The case of HAB-6FDA polyimide and its TR derivative and a general analysis of its impact on the selectivity of glassy polymers at multicomponent conditions. J. Memb. Sci. 2020, 612, 118374. [Google Scholar] [CrossRef]
- Neyertz, S.; Brown, D. Single- and mixed-gas sorption in large-scale molecular models of glassy bulk polymers. Competitive sorption of a binary CH4/N2 and a ternary CH4/N2/CO2 mixture in a polyimide membrane. J. Memb. Sci. 2020, 614, 118478. [Google Scholar] [CrossRef]
- He, Z.; Wang, K. The “ideal selectivity” vs. “true selectivity” for permeation of gas mixture in nanoporous membranes. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 323. [Google Scholar]
- Huang, Y.; Paul, D.R. Effect of temperature on physical aging of thin glassy polymer films. Macromolecules 2005, 38, 10148–10154. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Mizrahi Rodriguez, K.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Yong, W.F.; Kwek, K.H.A.; Liao, K.S.; Chung, T.S. Suppression of aging and plasticization in highly permeable polymers. Polymer 2015, 77, 377–386. [Google Scholar] [CrossRef]
- Yavari, M.; Le, T.; Lin, H. Physical aging of glassy per fl uoropolymers in thin fi lm composite membranes. Part I. Gas transport properties. J. Memb. Sci. 2017, 525, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Houben, H.J.M.; Borneman, Z.; Nijmeijer, K. Plasticization behavior of crown-ether containing polyimide membranes for the separation of CO2. Sep. Purif. Technol. 2020, 255, 117307. [Google Scholar] [CrossRef]
- Visser, T.; Koops, G.H.; Wessling, M. On the subtle balance between competitive sorption and plasticization effects in asymmetric hollow fiber gas separation membranes. J. Memb. Sci. 2005, 252, 265–277. [Google Scholar] [CrossRef]
- Mazinani, S.; Ramezani, R.; Molelekwa, G.F.; Darvishmanesh, S.; Di, R.; Bruggen, B. Van Der Plasticization suppression and CO2 separation enhancement of Matrimid through homogeneous blending with a new high performance polymer. J. Memb. Sci. 2019, 574, 318–324. [Google Scholar] [CrossRef]
- Lau, C.H.; Nguyen, P.T.; Hill, M.R.; Thornton, A.W.; Konstas, K.; Doherty, C.M.; Mulder, R.J.; Bourgeois, L.; Liu, A.C.Y.; Sprouster, D.J.; et al. Ending aging in super glassy polymer membranes. Angew. Chemie-Int. Ed. 2014, 53, 5322–5326. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Liu, C.; Tran, H. High Selectivity Membranes for Hydrogen Sulfide and Carbon Dioxide Removal from Natural Gas. U.S. Patent 16/533,297, 11 February 2021. [Google Scholar]
- Clarizia, G.; Tasselli, F.; Bernardo, P. Effect of Physical Aging on Gas Transport in Asymmetric Polyimide Hollow Fibers Prepared by Triple-Orifice Spinneret. Polymers 2020, 12, 441. [Google Scholar] [CrossRef] [Green Version]
- Budd, P.M.; McKeown, N.B. Highly permeable polymers for gas separation membranes. Polym. Chem. 2010, 1, 63–68. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Sadrzadeh, M.; Matsuura, T. Thermally stable polymers for advanced high-performance gas separation membranes. Prog. Energy Combust. Sci. 2018, 66, 1–41. [Google Scholar] [CrossRef]
- Adewole, J.K.; Sultan, A.S. Polymeric Membranes for Natural Gas Processing: Polymer Synthesis and Membrane Gas Transport Properties. In Functional Polymers, Polymers and Polymeric Composites; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Ramachandran, R.; Jung, D.; Spokoyny, A.M. Cross-linking dots on metal oxides. NPG Asia Mater. 2019, 11, 9–12. [Google Scholar] [CrossRef] [Green Version]
- Achoundong, C.S.K.; Bhuwania, N.; Burgess, S.K.; Karvan, O.; Johnson, J.R.; Koros, W.J. Silane Modi fi cation of Cellulose Acetate Dense Films as Materials for Acid Gas Removal. Macromolecules 2013, 46, 5584–5594. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Soltani, S.M. Microporous and Mesoporous Materials Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Zhao, S.; Chen, Z.; Huang, H.; Guo, R.; Chen, Y. Microporous polyimides containing bulky tetra- o -isopropyl and naphthalene groups for gas separation membranes. J. Memb. Sci. 2019, 585, 282–288. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Q.; Liang, J.; Zhang, Y.; Jin, J. Adamantane-grafted polymer of intrinsic microporosity with fi nely tuned interchain spacing for improved CO2 separation performance. Sep. Purif. Technol. 2020, 233, 116008. [Google Scholar] [CrossRef]
- Rahmani, M.; Kazemi, A.; Talebnia, F.; Gamali, P.A. Fabrication and characterization of brominated separation: Application of response surface methodology (RSM). e-Polymers 2016, 16, 481–492. [Google Scholar] [CrossRef]
- Guiver, M.D.; Robertson, G.P.; Yoshikawa, M. Functionalized Polysulfones: Methods for Chemical Modification and Membrane Applications. In Membrane Formation and Modification; ACS Publications: Washington, DC, USA, 2000. [Google Scholar]
- Koros, W.J.; Wallace, D.; Wind, J.D.; Miller, S.J.; Staudt-Bickel, C. Crosslinked and Crosslinkable Hollow Fiber Membrane and Method of Making Same Utility. U.S. Patent 2205/0268783 A1, 10 June 2005. [Google Scholar]
- Zhang, C.; Li, P.; Cao, B. Decarboxylation crosslinking of polyimides with high CO2/CH4 separation performance and plasticization resistance. J. Memb. Sci. 2017, 528, 206–216. [Google Scholar] [CrossRef]
- Kratochvil, A.M.; Koros, W.J. Decarboxylation-Induced Cross-Linking of a Polyimide for Enhanced CO2 Plasticization Resistance. Macromolecules 2008, 41, 7920–7927. [Google Scholar] [CrossRef]
- Wind, J.D.; Staudt-bickel, C.; Paul, D.R.; Koros, W.J. The Effects of Crosslinking Chemistry on CO2 Plasticization of Polyimide Gas Separation Membranes. Ind. Eng. Chem. Res. 2002, 41, 6139–6148. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, J.; Tian, Z.; Liu, X.; Cao, B.; Li, P. Molecular Design of Troger’s Base-Based Polymers Containing Spirobichroman Structure for Gas Separation. Ind. Eng. Chem. Res. 2017, 56, 12783–12788. [Google Scholar] [CrossRef]
- Hu, L.; Cheng, J.; Li, Y.; Liu, J.; Zhou, J.; Cen, K. In-situ grafting to improve polarity of polyacrylonitrile hollow fiber-supported polydimethylsiloxane membranes for CO2 separation. J. Colloid Interface Sci. 2018, 510, 12–19. [Google Scholar] [CrossRef]
- Lin, W.; Chung, T. Gas permeability, diffusivity, solubility, and aging characteristics of 6FDA-durene polyimide membranes. J. Membr. Sci. 2001, 186, 183–193. [Google Scholar] [CrossRef]
- Niwa, M.; Kawakami, H.; Kanamori, T.; Shinbo, T.; Kaito, A.; Nagaoka, S. Gas Separation of Asymmetric 6FDA Polyimide Membrane with Oriented Surface Skin Layer. Macromolecules 2001, 34, 9039–9044. [Google Scholar] [CrossRef]
- Suhaimi, N.H.; Yeong, Y.F.; Ch’ng, C.W.M.; Jusoh, N. Tailoring CO2/CH4 Separation Performance of Mixed Matrix Membranes by Using ZIF-8 Particles Functionalized with Di ff erent Amine Groups. Polymers 2019, 11, 2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Li, L.; Hou, M.; Xue, J.; Liu, Y.; Pan, Z.; Song, C.; Wang, T. Enhanced CO2 permeability of thermal crosslinking membrane via sulfonation/desulfonation of phenolphthalein-based cardo poly (arylene ether ketone). J. Memb. Sci. 2020, 598, 117824. [Google Scholar] [CrossRef]
- Largier, T.; Huang, F.; Kahn, W.; Cornelius, C.J. Poly (phenylene) synthesized using diels-alder chemistry and its sulfonation: Sulfonate group complexation with metal counter-ions, physical properties, and gas transport. J. Memb. Sci. 2019, 572, 320–331. [Google Scholar] [CrossRef]
- Liu, C.; Tran, H. Polyimide Blend Membrane for Gas Separations. U.S. Patent 9,308,499 B1, 12 April 2016. [Google Scholar]
- Xu, R.; Li, L.; Jin, X.; Hou, M.; He, L.; Lu, Y.; Song, C.; Wang, T. Thermal crosslinking of a novel membrane derived from phenolphthalein- based cardo poly (arylene ether ketone) to enhance CO2/CH4 separation performance and plasticization resistance. J. Memb. Sci. 2019, 586, 306–317. [Google Scholar] [CrossRef]
- Qiu, W.; Chen, C.; Xu, L.; Cui, L.; Paul, D.R.; Koros, W.J. Sub- T g Cross-Linking of a Polyimide Membrane for Enhanced CO2 Plasticization Resistance for Natural Gas Separation. Macromolecules 2011, 44, 6046–6056. [Google Scholar] [CrossRef]
- Chen, C.; Miller, S.J.; Koros, W.J. Characterization of Thermally Cross-Linkable Hollow Fiber Membranes for Natural Gas Separation. Ind. Eng. Chem. Res. 2013, 52, 1015–1022. [Google Scholar] [CrossRef]
- Deng, L.; Xue, Y.; Yan, J.; Hon, C.; Cao, B.; Li, P. Oxidative crosslinking of copolyimides at sub-T g temperatures to enhance resistance against CO2 -induced plasticization. J. Memb. Sci. 2019, 583, 40–48. [Google Scholar] [CrossRef]
- Du, N.; Cin, M.M.D.; Pinnau, I.; Nicalek, A.; Robertson, G.P.; Guiver, M.D. Azide-based Cross-Linking of Polymers of Intrinsic Microporosity (PIMs) for Condensable Gas Separation. Macromol. Rapid Commun. 2011, 32, 631–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Galizia, M.; Gleason, K.L.; Scholes, C.A.; Paul, D.R.; Benny, D. Influence of toluene on CO2 and CH4 gas transport properties in thermally rearranged (TR) polymers based on 3,3′-dihydroxy-4,4′-diamino-biphenyl (HAB) and 2,2′-bis-(3,4-dicarboxyphenyl) hexafluotopropane dianhydride (6FDA). J. Memb. Sci. 2016, 514, 282–293. [Google Scholar] [CrossRef] [Green Version]
- Scholes, C.A.; Dong, G.; Sung, J.; Jin, H.; Lee, J.; Moo, Y. Permeation and separation of SO2, H2S and CO2 through thermally rearranged (TR) polymeric membranes. Sep. Purif. Technol. 2017, 179, 449–454. [Google Scholar] [CrossRef]
- Lee, J.; Sung, J.; Kim, J.F.; Jin, H.; Park, H. Densification-induced hollow fiber membranes using crosslinked thermally rearranged (XTR) polymer for CO2 capture. J. Memb. Sci. 2019, 573, 393–402. [Google Scholar] [CrossRef]
- Brunetti, A.; Cersosimo, M.; Sung, J.; Dong, G.; Fontananova, E.; Moo, Y.; Drioli, E.; Barbieri, G. Thermally rearranged mixed matrix membranes for CO2 separation: An aging study. Int. J. Greenh. Gas Control 2017, 61, 16–26. [Google Scholar] [CrossRef]
- Li, F.Y.; Xiao, Y.; Ong, Y.K.; Chung, T. UV-Rearranged PIM-1 Polymeric Membranes for Advanced Hydrogen Purifi cation and Production. Adv. Energy Mater. 2012, 2, 1456–1466. [Google Scholar] [CrossRef]
- Altun, V.; Remigy, J.; Vankelecom, I.F.J. UV-cured polysulfone-based membranes: E ff ect of co-solvent addition and evaporation process on membrane morphology and SRNF performance. J. Memb. Sci. 2017, 524, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Djoko, T.; Puji, D.; Ryan, I. Enhancement of separation performance of nano hybrid PES–TiO2 membrane using three combination e ff ects of ultraviolet irradiation, ethanol-acetone immersion, and thermal annealing process for CO2 removal. J. Environ. Chem. Eng. 2018, 6, 2865–2873. [Google Scholar]
- Park, C.; Chang, B.; Kim, J.; Moo, Y. UV-crosslinked poly (PEGMA-co-MMA-co-BPMA) membranes: Synthesis, characterization, and CO2/N2 and CO2/CO separation. J. Memb. Sci. 2019, 587, 117167. [Google Scholar] [CrossRef]
- Sazanova, T.S.; Otvagina, K.V.; Vorotyntsev, I.V. The contributions of supramolecular organization to mechanical properties of chitosan and chitosan copolymers with synthetic polymers according to atomic force microscopy. Polym. Test. 2018, 68, 350–358. [Google Scholar] [CrossRef]
- Sazanova, T.S.; Otvagina, K.V.; Kryuchkov, S.S.; Zarubin, D.M.; Fukina, D.G.; Vorotyntsev, A.V.; Vorotyntsev, I.V. Revealing the surface effect on gas transport and mechanical properties in nonporous polymeric membranes in terms of surface free energy. Langmuir 2020, 36, 12911–12921. [Google Scholar] [CrossRef]
- Vasagar, V.; Hassan, M.K.; Khraisheh, M. Membrane surface modification and functionalization. Membranes 2021, 11, 877. [Google Scholar] [CrossRef]
- SpecialChem Polyethersulfone (PES)—Complete Guide on High-Temperature Engineering Polymer. Available online: https://omnexus.specialchem.com/selection-guide/polyethersulfone-pes-thermoplastic (accessed on 24 January 2021).
- Mannan, H.A.; Mukhtar, H.; Shaharun, M.S.; Othman, M.R.; Murugesan, T. Polysulfone/poly (ether sulfone) blended membranes for CO2 separation. J. Appl. Polym. Sci. 2016, 133, 42946. [Google Scholar]
- Mosleh, S.; Mozdianfard, M.R.; Hemmati, M.; Khanbabaei, G. Synthesis and characterization of rubbery/glassy blend membranes for CO2/CH4 gas separation. J. Polym. Res. 2016, 23, 120. [Google Scholar] [CrossRef]
- Kojabad, M.E.; Babaluo, A.; Tavakoli, A. A novel semi-mobile carrier facilitated transport membrane containing aniline/poly (ether-block-amide) for CO2/N2 separation: Molecular simulation and experimental study. Sep. Purif. Technol. 2021, 266, 118494. [Google Scholar] [CrossRef]
- Tu, Z.; Liu, P.; Zhang, X.; Shi, M.; Zhang, Z.; Luo, S. Highly-selective separation of CO2 from N2 or CH4 in task-specific ionic liquid membranes: Facilitated transport and salting-out effect. Sep. Purif. Technol. 2021, 254, 117621. [Google Scholar] [CrossRef]
- Kunalan, S.; Dey, K.; Kumar, P.; Velachi, V.; Kumar, P.; Palanivelu, K.; Jayaraman, N. Efficient facilitated transport PETIM dendrimer-PVA-PEG/PTFE composite flat-bed membranes for selective removal of CO2. J. Memb. Sci. 2021, 622, 119007. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Liu, J.; Xu, Q.; Yin, J. Highly selective separation of CO2/N2 using [Emim][Tf2N] supported ionic liquid membranes prepared by supercritical fluid deposition. J. Supercrit. Fluids 2021, 170, 105139. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Kim, J.H.; Nam, S.Y. Piperidinium functionalized poly(2,6 dimethyl 1,4 phenylene oxide) based polyionic liquid/ionic liquid (PIL/IL) composites for CO2 separation. J. Ind. Eng. Chem. 2021, 99, 81–89. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, W.; Tu, Z.; Peng, L.; Wu, Y.; Hu, X. Supported Ionic Liquid Membranes with Dual-Site Interaction Mechanism for Efficient Separation of CO2. ACS Sustain. Chem. Eng 2019, 7, 10792–10799. [Google Scholar] [CrossRef]
- Zhang, X.; Tu, Z.; Li, H.; Li, L.; Wu, Y.; Hu, X. Supported protic-ionic-liquid membranes with facilitated transport mechanism for the selective separation of CO2. J. Memb. Sci. 2017, 527, 60–67. [Google Scholar] [CrossRef]
- Zhang, X.; Tu, Z.; Li, H.; Huang, K.; Hu, X. Selective separation of H2S and CO2 from CH4 by supported ionic liquid membranes. J. Memb. Sci. 2017, 543, 282–287. [Google Scholar] [CrossRef]
- Halder, K.; Munir, M.; Grünauer, J.; Shishatskiy, S.; Abetz, C. Blend membranes of ionic liquid and polymers of intrinsic microporosity with improved gas separation characteristics. J. Memb. Sci. 2017, 539, 368–382. [Google Scholar] [CrossRef]
- Klemm, A.; Lee, Y.Y.; Mao, H.; Gurkan, B. Facilitated Transport Membranes With Ionic Liquids for CO2 Separations. Front. Chem. 2020, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, Z.; Zhao, D. Mixed Matrix Membranes for Natural Gas Upgrading: Current Status and Opportunities. Ind. Eng. Chem. Res. 2018, 57, 4139–4169. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F.; Sanip, S.M.; Ng, B.C.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Alberto, M.; Bhavsar, R.; Luque-alled, J.M.; Vijayaraghavan, A.; Budd, P.M.; Gorgojo, P. Impeded physical aging in PIM-1 membranes containing graphene-like fi llers. J. Memb. Sci. 2018, 563, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Aqilah, N.; Fauzan, B.; Mukhtar, H.; Nasir, R. Composite amine mixed matrix membranes for high- pressure CO2–CH4 separation: Synthesis, characterization and performance evaluation. R. Soc. Open Sci. 2020, 7, 200795. [Google Scholar]
- Chen, X.Y.; Tien-binh, N.; Romero, A.; Patón, A.; Sanchez-silva, L.; Valverde, J.L.; Kaliaguine, S.; Rodrigue, D. Gas Separation Properties of Mixed Matrix Membranes Based on Polyimide and Graphite Oxide Graphical abstract Keywords. J. Membr. Sci. Res. 2020, 6, 58–69. [Google Scholar]
- Natarajan, P.; Sasikumar, B.; Elakkiya, S.; Arthanareeswaran, G.; Ismail, A.F.; Youravong, W.; Yuliwati, E. Pillared cloisite 15A as an enhancement filler in polysulfone mixed matrix membranes for CO2/N2 and O2/N2 gas separation. J. Nat. Gas Sci. Eng. 2021, 86, 103720. [Google Scholar] [CrossRef]
- Roilo, D.; Checchetto, R. Gas Transport Properties and Free Volume Structure of Polymer Nanocomposite Membranes. Ph.D. Thesis, University of Trento, Trento, Italy, 2017. [Google Scholar]
- Padbury, R. Bulk Property Modification of Fiber Forming Polymers Using Vapor Phase; North Carolina State University: Raleigh, NC, USA, 2013. [Google Scholar]
- Abdulhamid, M.A.; Genduso, G.; Wang, Y.; Ma, X.; Pinnau, I. Plasticization-Resistant Carboxyl-Functionalized 6FDA-Polyimide of Intrinsic Microporosity (PIM–PI) for Membrane-Based Gas Separation. Ind. Eng. Chem. Res. 2020, 59, 5247–5256. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Swaidan, R.; Belmabkhout, Y.; Zhu, Y.; Litwiller, E.; Jouiad, M.; Pinnau, I.; Han, Y. Synthesis and Gas Transport Properties of Hydroxyl-Functionalized Polyimides with Intrinsic Microporosity. Macromolecules 2012, 45, 3841–3849. [Google Scholar] [CrossRef]
- Karimi, S.; Firouzfar, E.; Khoshchehreh, M.R. Journal of Petroleum Science and Engineering Assessment of gas separation properties and CO2 plasticization of polysulfone/polyethylene glycol membranes. J. Pet. Sci. Eng. 2019, 173, 13–19. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Peinemann, K. Nanostructured membrane material designed for carbon dioxide separation. J. Membr. Sci. 2010, 350, 124–129. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K. Pebax®/polyethylene glycol blend thin film composite membranes for CO2 separation: Performance with mixed gases. Sep. Purif. Technol. 2008, 62, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, M.; Iwanaga, H.; Hara, Y.; Iwamoto, M. Gas Permeabilities of Cellulose Nitrate/Poly (ethylene Glycol) Blend Membranes. J. Appl. Polym. Sci. 1982, 27, 2387–2393. [Google Scholar] [CrossRef]
- Okamoto, K.; Fujii, M.; Okamyo, S.; Suzuki, H.; Tanaka, K.; Kita, H. Gas Permeation Properties of Poly(ether imide) Segmented Copolymers. Macromolecules 1995, 28, 6950–6956. [Google Scholar] [CrossRef]
- Yoshino, M.; Ito, K.; Kita, H.; Okamoto, K. Effects of Hard-Segment Polymers on CO2/N2 Gas- Separation Properties of Poly (ethylene oxide) -Segmented. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1707–1715. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, Y.; Yin, J.; Zhang, C.; Feng, G.; Zhang, Y.; Meng, J. Tuning the micro-phase separation of the PES-g-PEG comb-like copolymer membrane for efficient CO2 separation. Sep. Purif. Technol. 2021, 265, 118465. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, J.P.; Jang, E.; Lee, K.B.; Kang, Y.S.; Kim, J.H. CO2-philic PBEM-g-POEM comb copolymer membranes: Synthesis, characterization and CO2/N2 separation. J. Memb. Sci. 2016, 502, 191–201. [Google Scholar] [CrossRef]
- Bazhenov, S.D.; Borisov, I.L.; Bakhtin, D.S.; Rybakova, A.N.; Khotimskiy, V.S.; Molchanov, S.P.; Volkov, V. V High-permeance crosslinked PTMSP thin-film composite membranes as supports for CO2 selective layer formation. Green Energy Environ. 2016, 1, 235–245. [Google Scholar] [CrossRef]
- Feng, H.; Hong, T.; Mahurin, S.M.; Mays, J.W.; Sokolov, A.P.; Kang, N.; Saito, T. Gas separation mechanism of CO2 selective amidoxime-poly(1-trimethylsilyl-1-propyne) membranes. Polym. Chem. 2017, 8, 3341–3350. [Google Scholar] [CrossRef]
- Yampolskii, Y.; Belov, N.; Alentiev, A. Perfluorinated polymers as materials of membranes for gas and vapor separation. J. Memb. Sci. 2020, 598, 117779. [Google Scholar] [CrossRef]
- Chiang, H.; Fang, M.; Okamoto, Y. Mechanical, optical and gas transport properties of poly (perfluoro-2- methylene-4-methyl-1, 3-dioxolane) membrane containing perfluoropolyether as a plasticizer. J. Fluor. Chem. 2020, 236, 109572. [Google Scholar] [CrossRef]
- Baker, R.W.; Pinnau, I.; He, Z.; Amo, K.D.; Costa, A.R.D.; Daniels, R. Carbon Dioxide Gas Separation Using Organic-Vapor Resistant Membranes. U.S. Patent 5,572,680 B2, 3 June 2003. [Google Scholar]
- Baker, R.W.; Pinnau, I.; He, Z.; Amo, K.D.; Costa, A.R.D.; Daniels, R. Nitrogen Gas Separation Using Organic Vapor Resistant Membrane. U.S. Patent 6579341 B2, 17 June 2003. [Google Scholar]

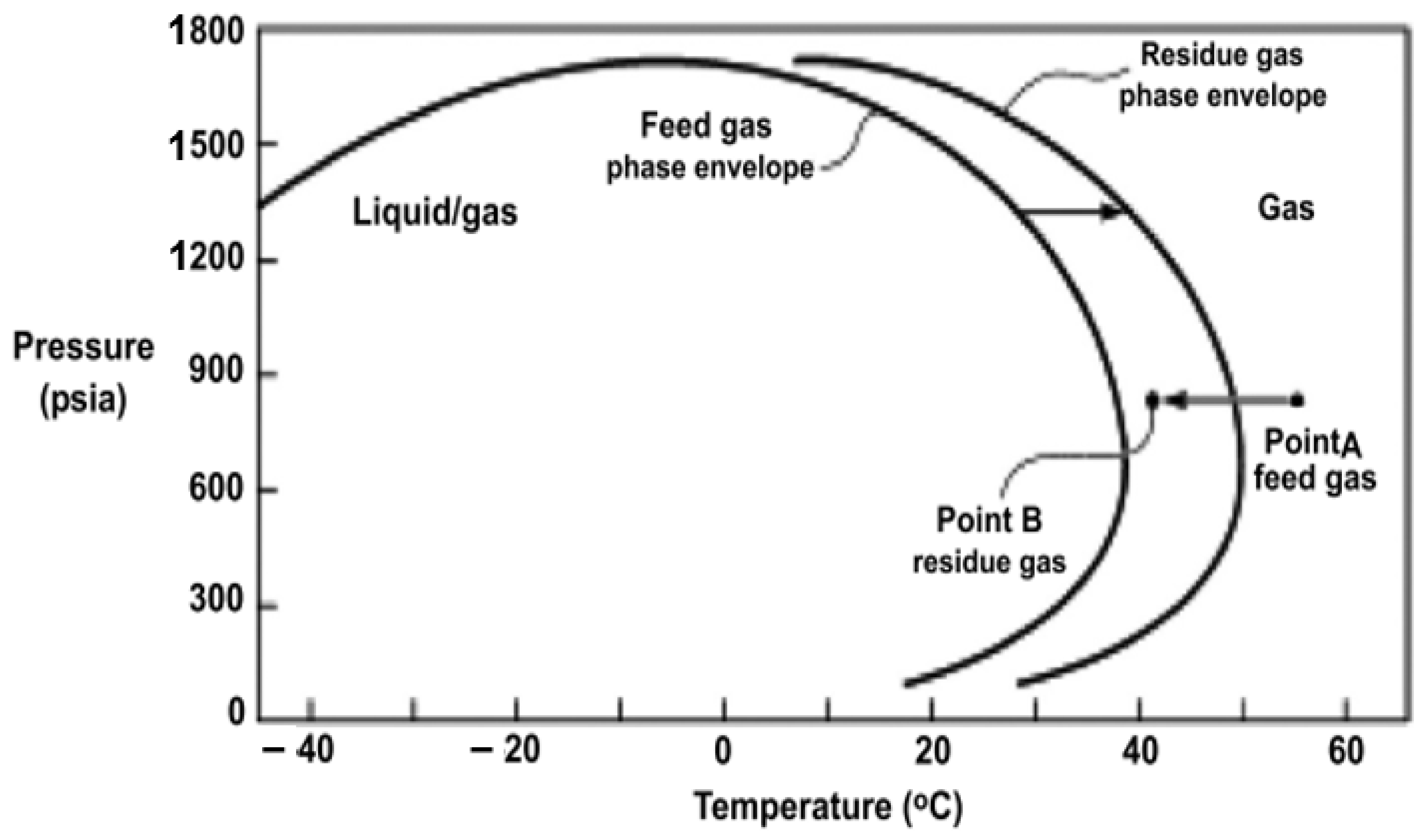


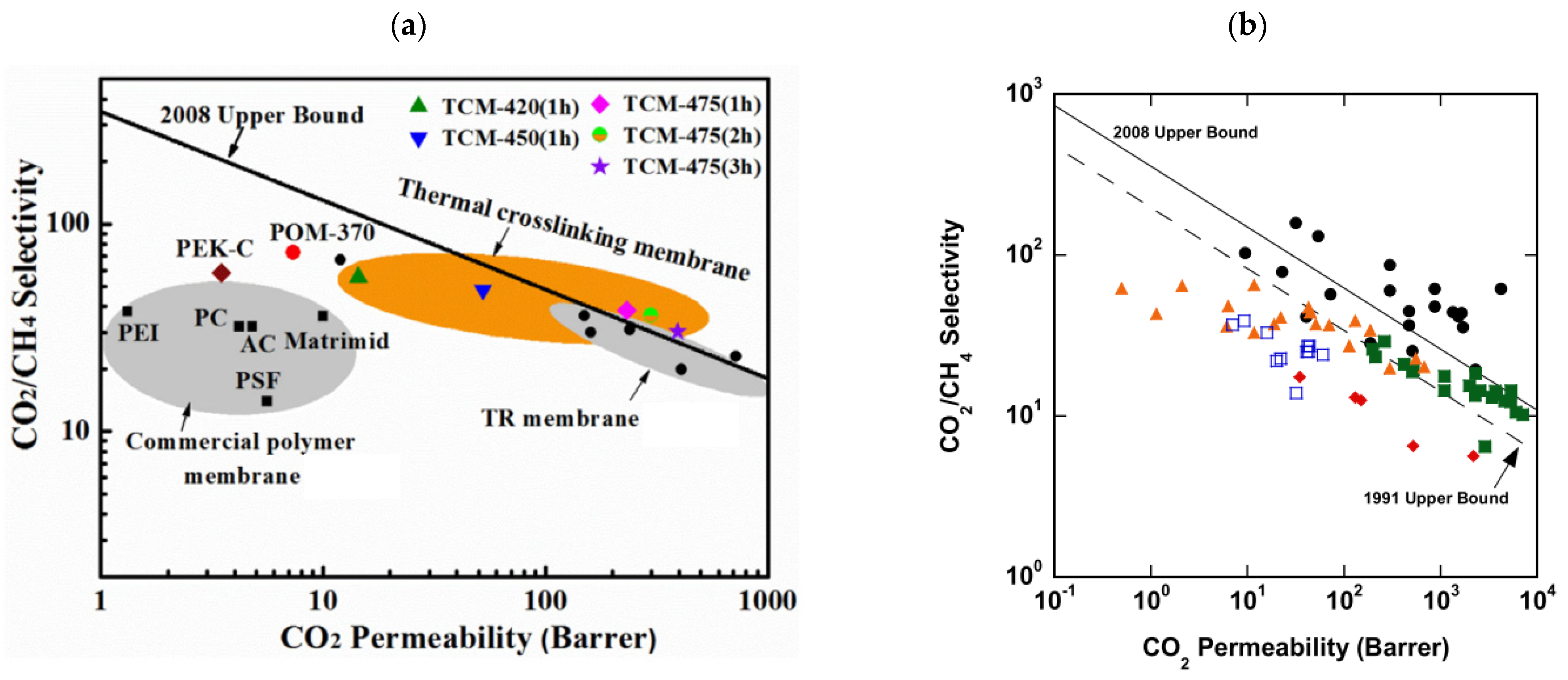
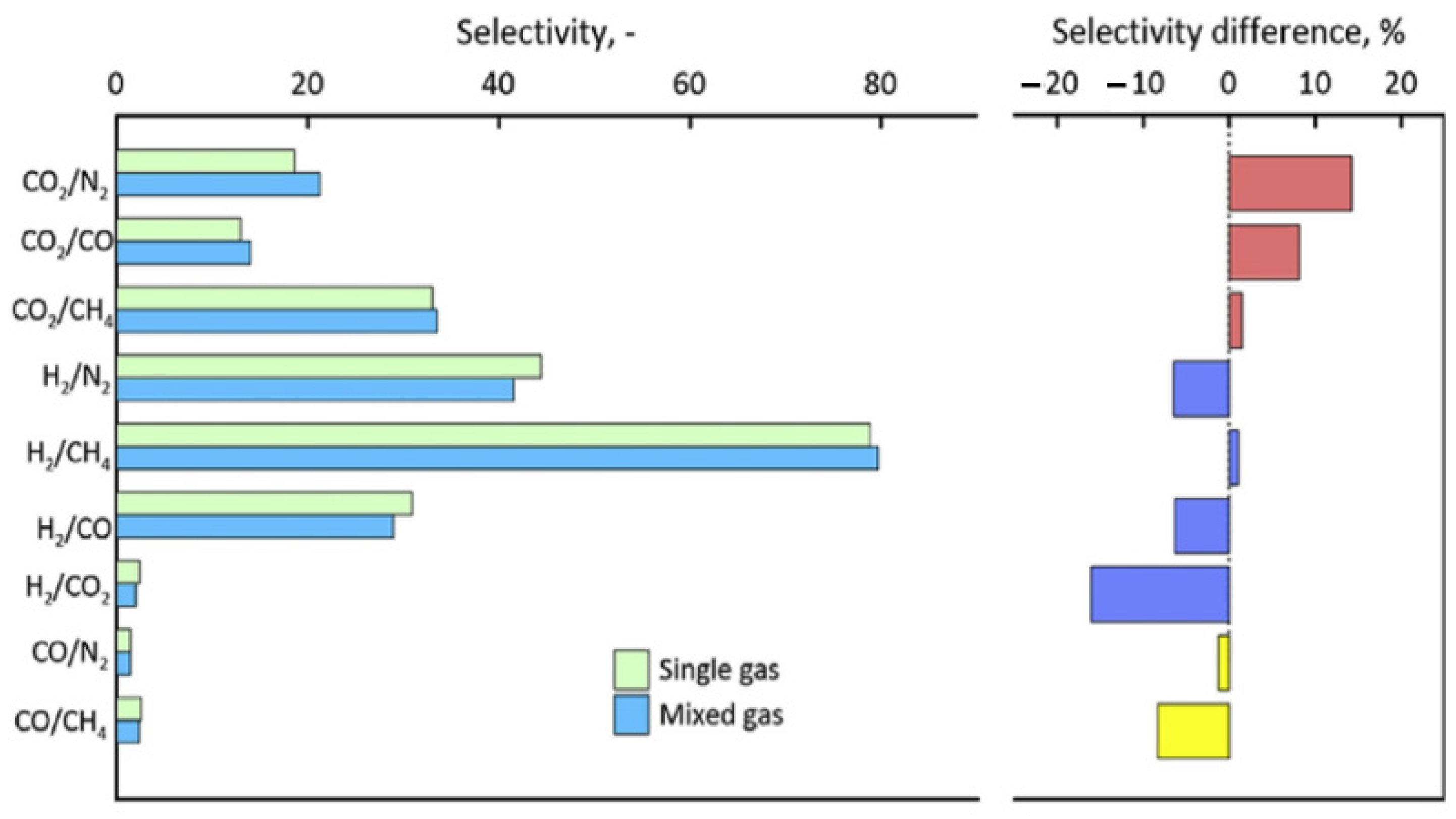
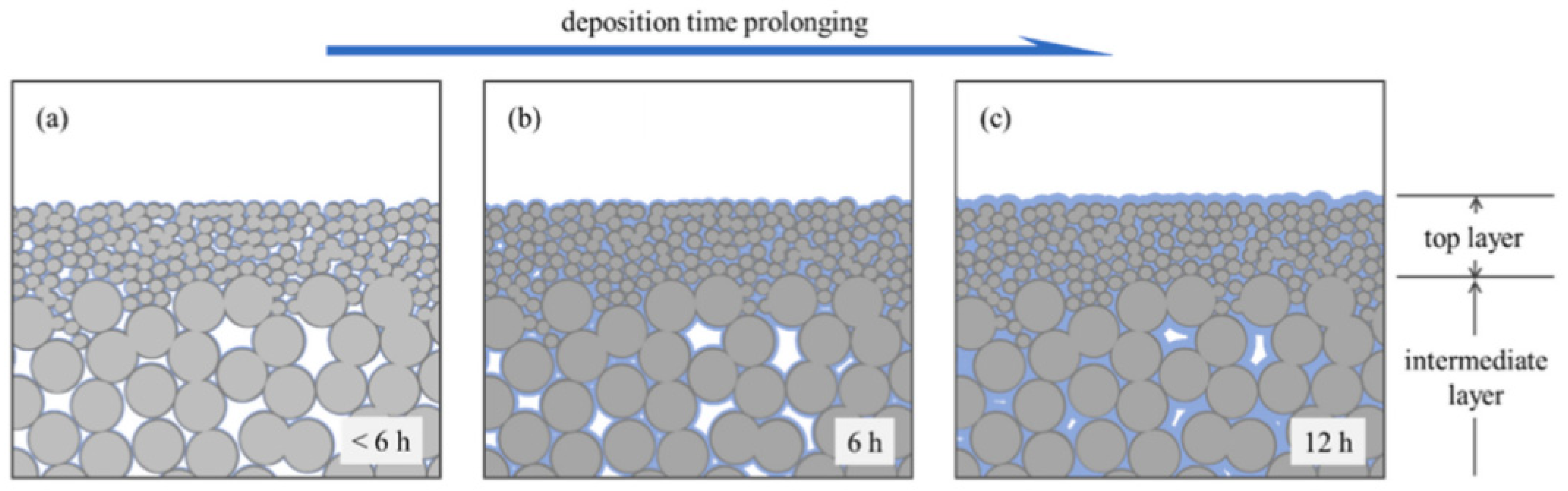
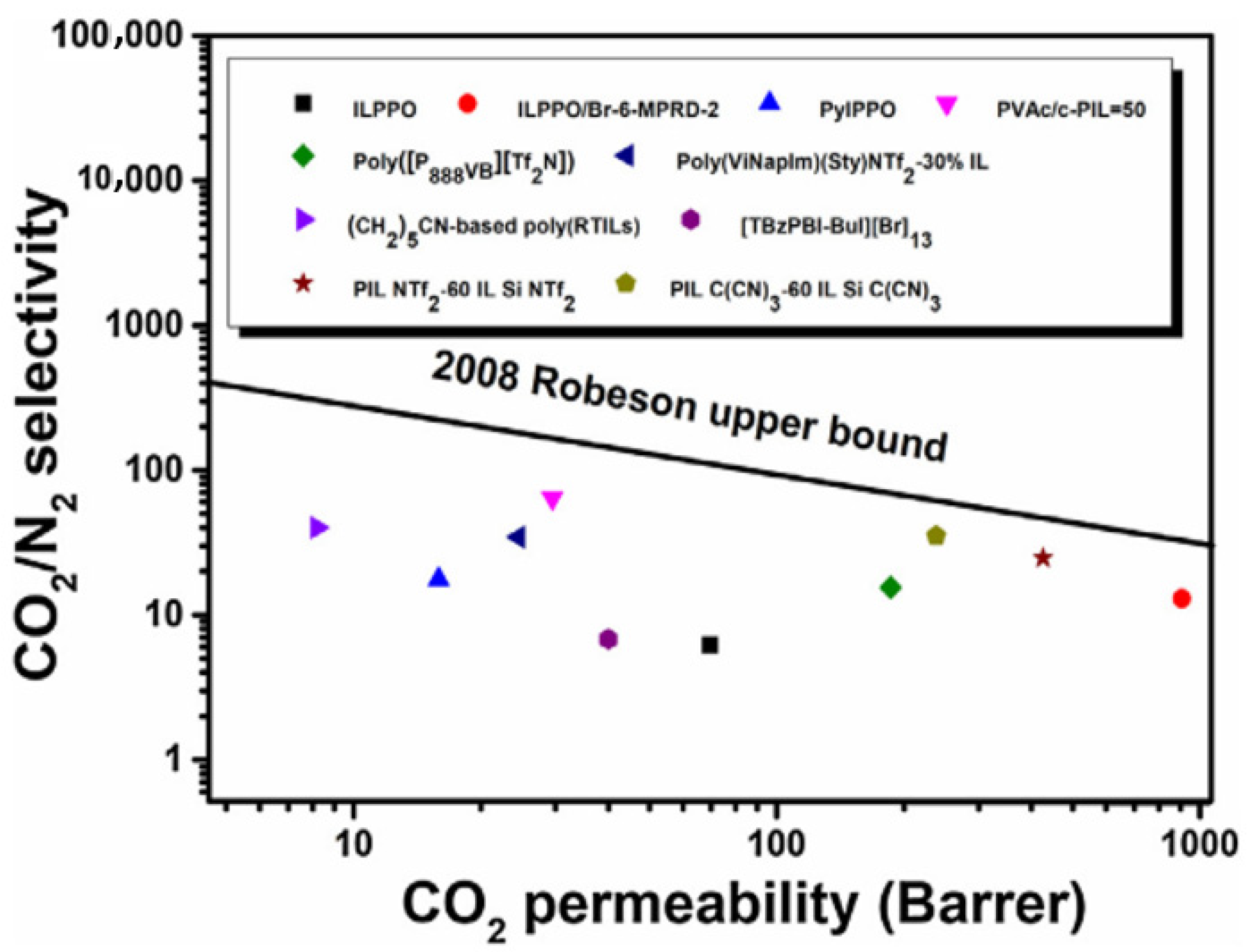
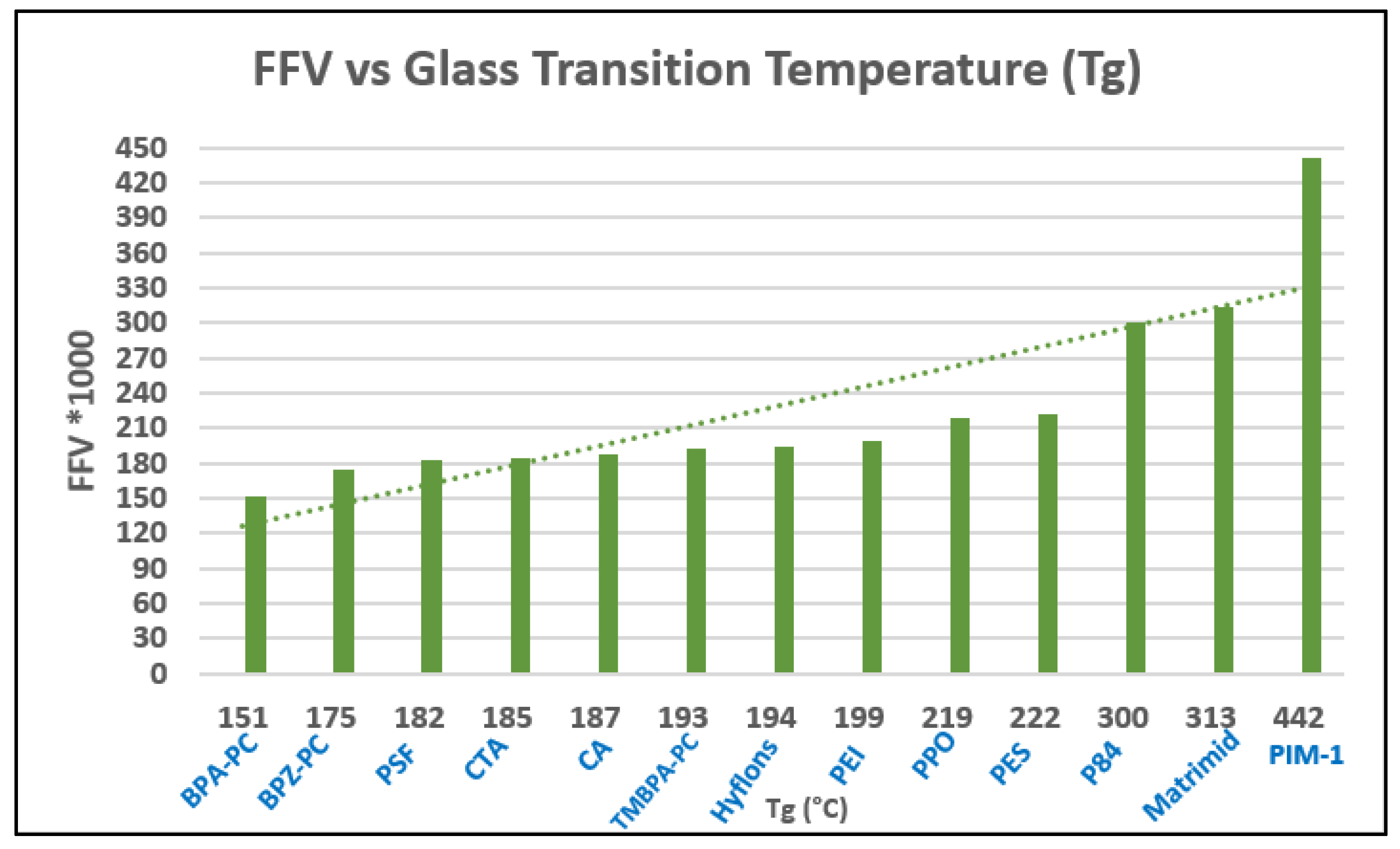
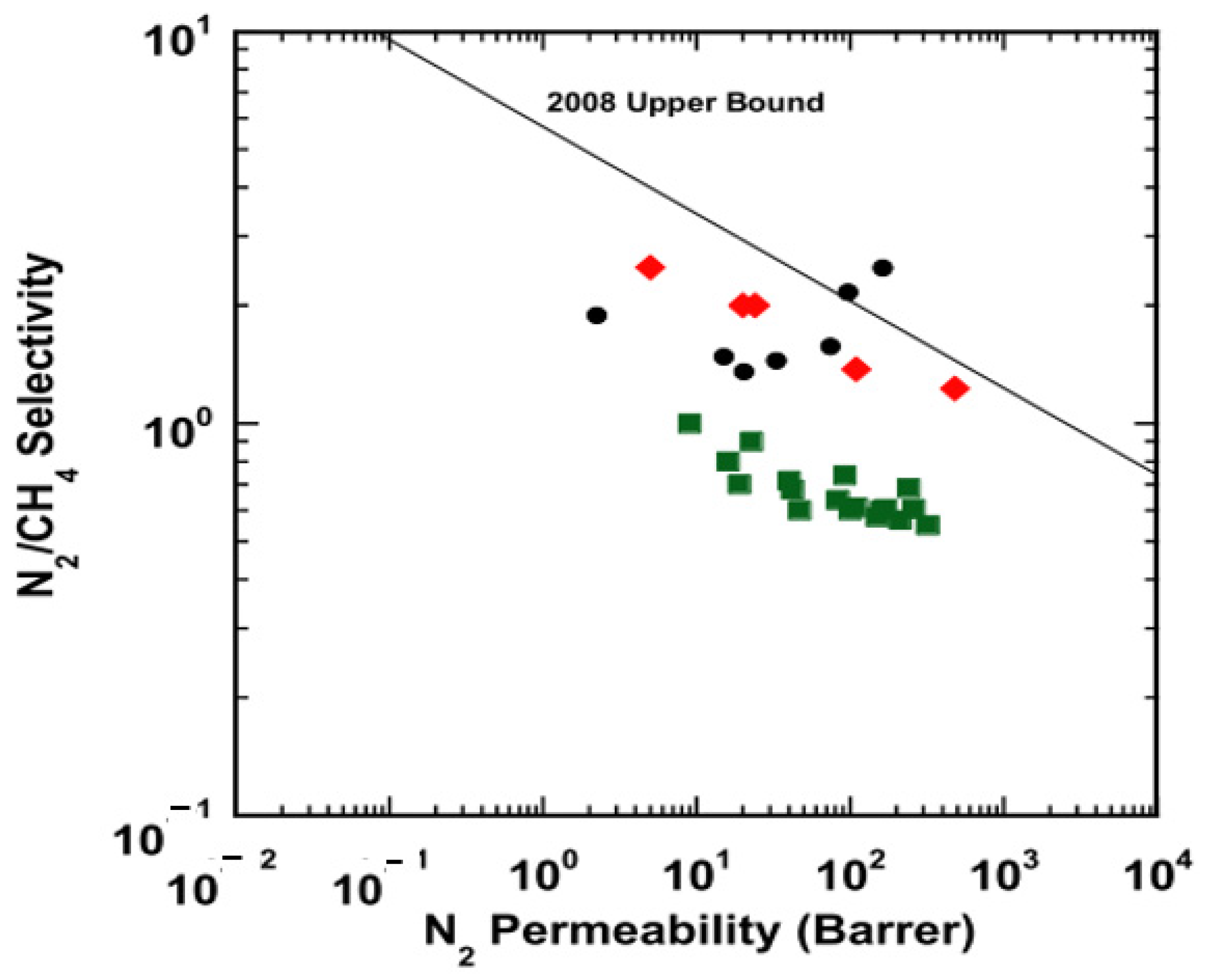
| Producer | CO2 Separation Process |
|---|---|
| IGS (Generon membrane) DIVEX GLOBAL (Helipur membrane) | CO2/H2 CO2 from N2/Ar/O2 |
| UOP—A Honeywell Company (-) | CO2/H2 |
| AIRRANE Co, Ltd. (Hollow fiber polysulfone (PSF)-based membrane) | CO2 from CH4/H2/N2 |
| CYNARA (Cellulose triacetate (CTA) based) | CO2 from natural gas |
| MEDAL (Polyaramide based) GRACE, SEPAREX (Cellulose acetate (CA) MTR (Perfluoro polymers based) PBI Performance Products, Inc. (Hollow fiber Celazole®PBI based) | CO2/H2 |
| AIR Liquide S.A. (-) | CO2 from biogas/natural gas |
| EVONIK Industries AG (Sepuran® membranes) Compact Membranes Systems, Inc. (Fluoropolymer based) | CO2 from biogas CO2/CH4, CO2/N2 |
| No. | Polymer Name | Tg (°C) | Chemical Structure |
|---|---|---|---|
| (a) | Cellulose & Cellulose Tri-Acetate (CTA) | 185 |  |
| (b) | Polysulfone (PSF) | 182 | 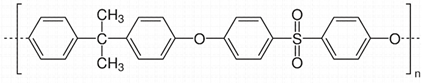 |
| (c) | Polyethersulfone (PES) | 222 | 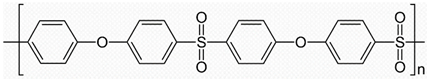 |
| (d) | Poly trimethyl phenylene ethersulfone (TPES) | NA |  |
| (e) | Polyetherimide—PEI (Ultem®) (BPADA-PPD) [45] | 199 | 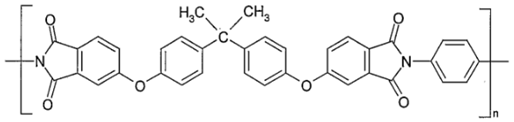 |
| (f) | Polyimide—PI (Matrimide® 5218) (BTDA-DAPI) | 313 | 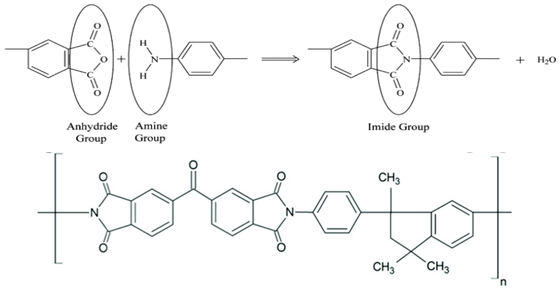 |
| (g) | Polyamide-imide—PAI (Torlon) | 281 |  |
| (h) | 6FDA-based polyimide | 348 | 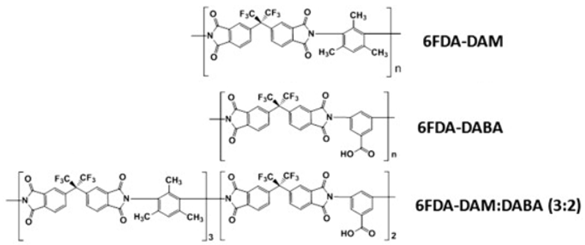 |
| (i) | Polycarbonate Bisphenol Z & A (BPZ-PC & BPA-PC) | 175 & 151 |  |
| (j) | Polymer of intrinsic microporosity-1 (PIM-1) | 442 | 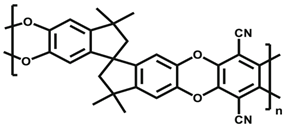 |
| (k) | Hyflon | 194 |  |
| No | Counterbalance Effect | Permeability | Selectivity | Tg | FFV |
|---|---|---|---|---|---|
| 1 | Plasticization |  |  |  |  |
| 2 | Competitive sorption |  |  |  |  |
| 3 | Aging |  |  |  |  |
| 4 | Net Effect |  |  |  |  |
| No. | Modification Routes | Improvement Strategies/Reaction Schemes |
|---|---|---|
| 1 | Incorporation of CO2-philic groups into the polymer to improve polarity towards CO2. Oxygen functionalities can improve sorption properties. The interaction can be via hydrogen bonding or electrostatic. | Incorporation of various Lewis bases/pendant polar groups such as ether, hydroxyl, carboxylic and carbonyl oxygen promotes physical interaction with CO2 (electronegativity) due to higher polarity, thus producing higher CO2 solubility. It can produce extra hydrogen bonding that further alters the pore size of the membranes, resulting in higher activation energy for CO2 and CH4. The incorporation of micropores from the incorporation of polar groups have enhanced the permeability of CO2.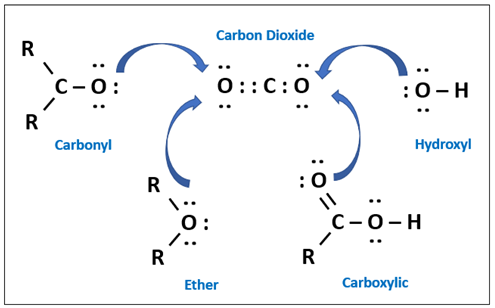 |
| 2 | Incorporation of bulky groups to improve chain stiffness and limit chain packing |  Bulky hexafluoro-substituted carbon (–C(CF3)2) groups in dianhydride structure of 6FDA-based PIs increased chain stiffness, hence increasing selectivity while inhibiting chain packing since the bulky group can serve as a molecular spacer which can increase the permeability and reduce aging.  Bulky diamines help disrupt chain packing while increasing free volume. This contributes to the enhancement in permeability, while the aromatic rings are responsible for chain rigidity for better selectivity [25] and aging resistance. Amines strongly and selectively bind the CO2 via chemisorption leading to higher heat of adsorption [86]. Bulky tetra-o-isopropyl and naphthalene groups are introduced to membranes, resulting in high FFV values and enhanced polymeric backbone rigidity. The disturbed chain packing leads to high gas permeabilities [87] and aging resistance. Rigid, bulky and diamond-like structure Adamantane is grafted into the membrane main chain and side chain through a simple acyl chloride-substitution reaction to adjust the chain packing. D-spacing of the membranes could be finely tuned by adjusting the mole ratio of grafted adamantane moiety. The resultant membrane CO2 permeability is enhanced significantly [88]. |
| 3 | Functionalization using bromination to increase FFV and thus permeability. | 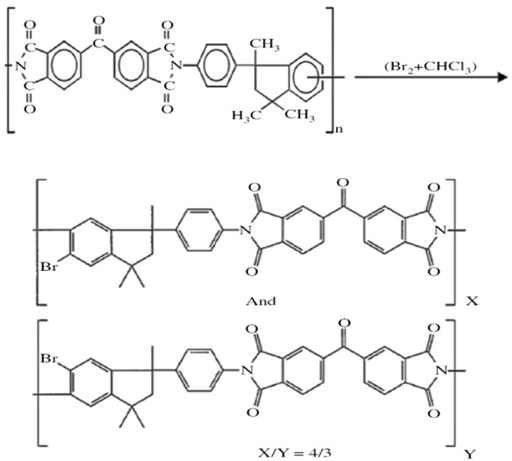 Brominated Matrimid 5218 membranes were much more permeable due to the higher FFV of the brominated membranes [89]. |
| 4 | Sulfonation to improve rigidity and FFV | 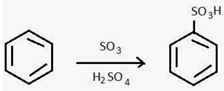 Sulfonation reaction successfully enhanced the microvoids, promoted interchain hydrogen bonding by the SO3H group and boosted the rigidity. As the degree of sulfonation increased, gas permeability increased by 3.2-fold [83] and improved aging resistance. |
| 5 | Lithiation to improve solubility, FFV and chain rigidity | 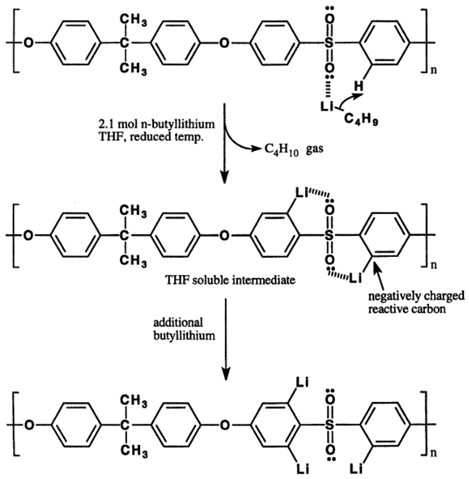 Lithiation is performed in PSF due to the availability of strong electron-withdrawing effect and capability of the lone pairs of electrons on the sulfone oxygen atoms to combine with the lithiation agent. Butyllithium substitutes the ortho-sulfone hydrogen atoms with lithium atoms so that the aromatic carbon atoms to which lithium is attached nominally have a negative charge, which can improve its performance and enhance attraction towards CO2 [90]. |
| 6 | Esterification by diols or polyols | 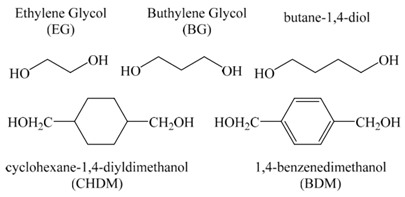 Common diol cross-linkers can be introduced through carboxylic acid or sulfonic acid groups to form ester bonds, linking the two polymer chains [3]. Esterification, which involves the reaction between acid and alcohol, serves as a function to create limited rotational ability [91]. |
| Polymer | Carrier | Condition | Gas Pair | Separation Performance | Neat | References |
|---|---|---|---|---|---|---|
| Pebax | Aniline molecule (50 wt%) | 7 bar, 25 °C | CO2, N2 Binary CO2, N2 (20%, 80%) | α = 92.5; PCO2 = 151 Barrer α = 68; PCO2 = 123 Barrer | α = 22.2; PCO2 = 75 Barrer α =19; PCO2 = 83 Barrer | [122] |
| PES | [DMAPAH][F]- Fluorion-based protic ionic liquids (FPILs) | 0.1 bar, 40 °C (water content 40%) | CO2, N2 CO2, CH4 | α = 774; PCO2 = 2572 Barrer α = 387; PCO2 = 2572 Barrer | - | [123] |
| PVA-PEG-GA (Glutaraldehyde) | (PETIM)– Amine-rich poly(ether imine) | 2 bar, 30 °C | CO2, N2 | α = 56; PCO2 = 207.7 Barrer | - | [124] |
| γ-Al2O3 | [Emim] [Tf2N]-ionic liquid | 1.5 bar, 30 °C | CO2, N2 | α = 27; PCO2 = 23 GPU | - | [125] |
| ILPPO | (Br-6-MPRD)– 1-bromohexyl-1 methylpiperidinium bromide | 1 bar, 25 °C | CO2, N2 CO2, O2 | α = 12.94; PCO2 = 907 Barrer α = 14.9; PCO2 = 907 Barrer | α = 6.21; PCO2 = 70 Barrer α = 5.15; PCO2 = 70 Barrer | [126] |
| PES | ([bmim][PhO])1-butyl-3-methylimidazolium phenolate | 0.1 bar, 40 °C | CO2, N2 | α = 135; PCO2 = 2020 Barrer (Dry) α = 127; PCO2 = 2540 Barrer (Wet) | - | [3] |
| PES | [DMAPAH] [MOAc] | 0.1 bar, 40 °C | CO2, N2 | α = 66; PCO2 = 1391 Barrer | - | [128] |
| PVDF | [Bmim][Ac] [Bmim][BF4] | 0.1 bar, 30 °C | H2S/CH4 H2S/CO2 H2S/CH4 H2S/CO2 | α = 142; PH2S = 5279 Barrer α = 11.9; PCO2 = 443 Barrer α = 40; PH2S = 3708 Barrer α = 3.5; PCO2 = 1056 Barrer | [129] | |
| PIM-COP | [C6mim][Tf2N] embedded | - | CO2, N2 | α = 30; PCO2 = 800 Barrer | α = 19; PCO2 = 7440 Barrer | [130] |
| Polymer | Inorganic Filler | Condition | Gas Pair | Separation Performance | Neat | References |
|---|---|---|---|---|---|---|
| PIM-1 | Graphene-like fillers | 2 bar, 25 °C | Binary CO2, CH4 (50%, 50%) | PCO2 = (3.5 ± 0.6) × 103 Barrer α = 22.9 ± 1.1 | PCO2 = (2.0 ± 0.7) × 103 Barrer α = 30.0 ± 4.7 | [134] |
| PES | Carbon Molecular Sieve (CMS) and diethanolamine (DEA) | 30 bar, 25 °C | CO2, CH4 | PCO2 = ~300 GPU α = 16.04 | PCO2 = ~140 GPU α = 8.15 | [135] |
| MATRIMID 5218 | AMP-RGO (Ascorbic acid multiphase reduced graphene oxide) | 10 bar, 30 °C | CO2, CH4 | PCO2 = 10.7 Barrer α = 79.8 | - | [136] |
| PI | ZIF-95 | 3 bar, 30 °C | CO2, CH4 | PCO2 = 23.2 ± 0.38 Barrer α = 58 | PCO2 = 5.7 ± 0.11 Barrer α = 33 | [125] |
| PSF | Cloisite 15A | 4 bar, 25 °C | CO2, N2 | PCO2 = 72.04 GPU α = 518.34 | PCO2 = 21.68 GPU α = 22.58 | [137] |
| Polymer | Chemical Structure |
|---|---|
| Hyflon AD60 | 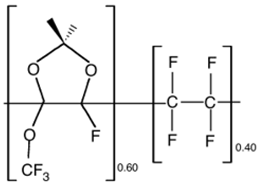 |
| Hyflon AD80 | 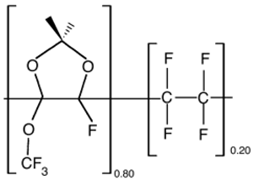 |
| Cytop | 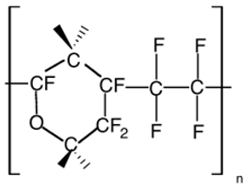 |
| Strategies | Achievement/Advantages | Issues/Enhancement Needed/Proposed |
|---|---|---|
| Polymer Modifcation via Cross-linking | Manage to improve performance and plasticization and aging resistance | To reduce complexity and improve on mechanical strength |
| Polymer Blending | Improvement within the average of the selected polymer performance accompanied by reduction in one aspect. | To improve the blended polymer performance further via cross-linking |
| FTMs | Manage to improve performance for low-pressure applications | To improve for high-pressure applications, to resolve the water/solvent evaporation and hydrocarbon fouling issues |
| MMMs | Manage to improve polymeric membrane performance and mechanical strength within the inorganic properties | To improve on sealing, homogeneity and agglomeration |
| New Polymer | Successfully improve on permeability and plasticization resistance to a great extent | To improve on selectivity and aging |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadirkhan, F.; Goh, P.S.; Ismail, A.F.; Wan Mustapa, W.N.F.; Halim, M.H.M.; Soh, W.K.; Yeo, S.Y. Recent Advances of Polymeric Membranes in Tackling Plasticization and Aging for Practical Industrial CO2/CH4 Applications—A Review. Membranes 2022, 12, 71. https://doi.org/10.3390/membranes12010071
Kadirkhan F, Goh PS, Ismail AF, Wan Mustapa WNF, Halim MHM, Soh WK, Yeo SY. Recent Advances of Polymeric Membranes in Tackling Plasticization and Aging for Practical Industrial CO2/CH4 Applications—A Review. Membranes. 2022; 12(1):71. https://doi.org/10.3390/membranes12010071
Chicago/Turabian StyleKadirkhan, Farahdila, Pei Sean Goh, Ahmad Fauzi Ismail, Wan Nurul Ffazida Wan Mustapa, Mohd Hanif Mohamad Halim, Wei Kian Soh, and Siew Yean Yeo. 2022. "Recent Advances of Polymeric Membranes in Tackling Plasticization and Aging for Practical Industrial CO2/CH4 Applications—A Review" Membranes 12, no. 1: 71. https://doi.org/10.3390/membranes12010071
APA StyleKadirkhan, F., Goh, P. S., Ismail, A. F., Wan Mustapa, W. N. F., Halim, M. H. M., Soh, W. K., & Yeo, S. Y. (2022). Recent Advances of Polymeric Membranes in Tackling Plasticization and Aging for Practical Industrial CO2/CH4 Applications—A Review. Membranes, 12(1), 71. https://doi.org/10.3390/membranes12010071










