Application of Coagulation–Membrane Rotation to Improve Ultrafiltration Performance in Drinking Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
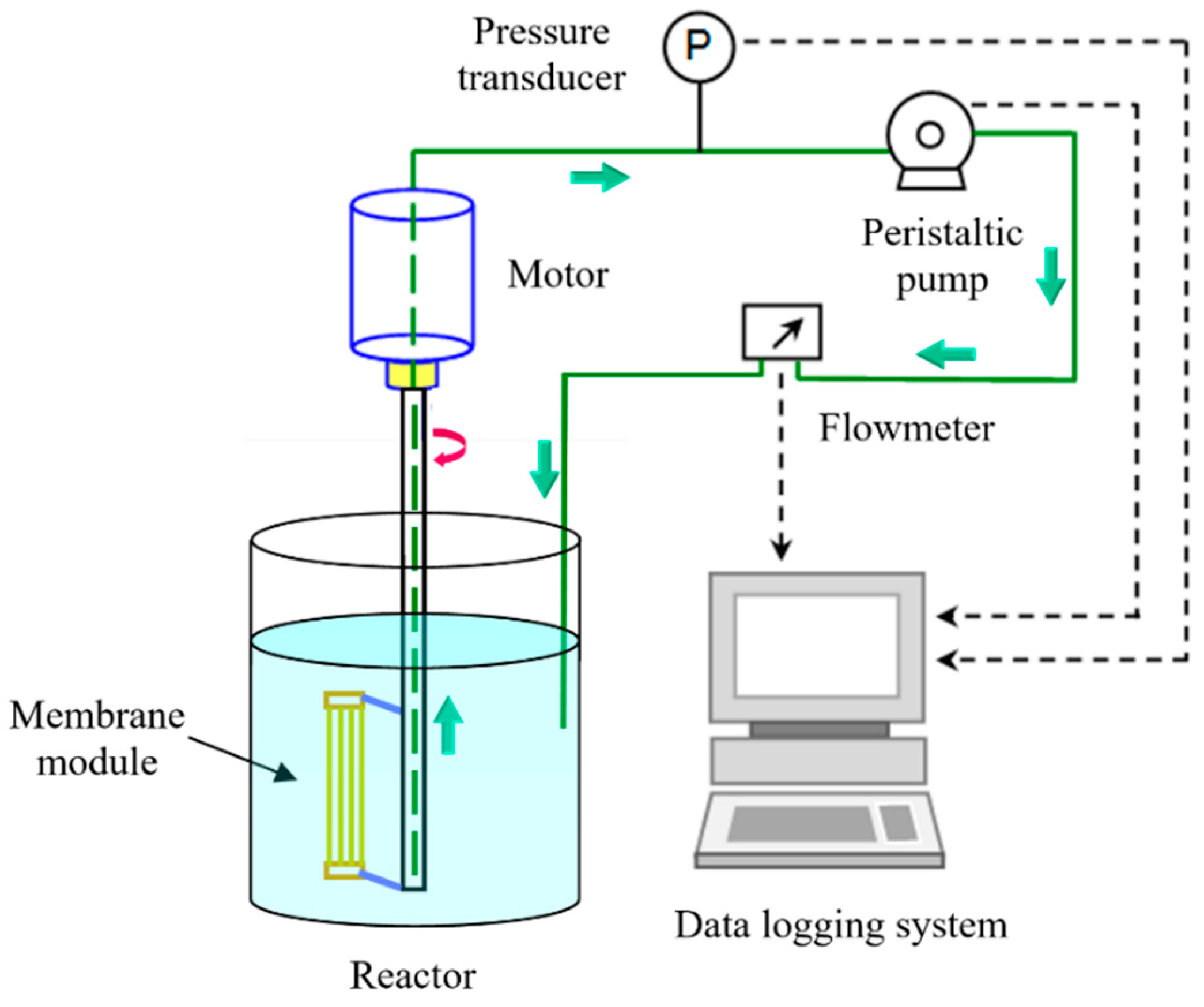
2.1. Experimental Setup and Operation Procedures
2.2. Hollow Fibre Membrane Module
2.3. Feed Solutions and Coagulants
2.4. Membrane Filtration Analysis
2.5. Theory of Membrane Rotation
2.6. Analytical Methods
3. Results and Discussion
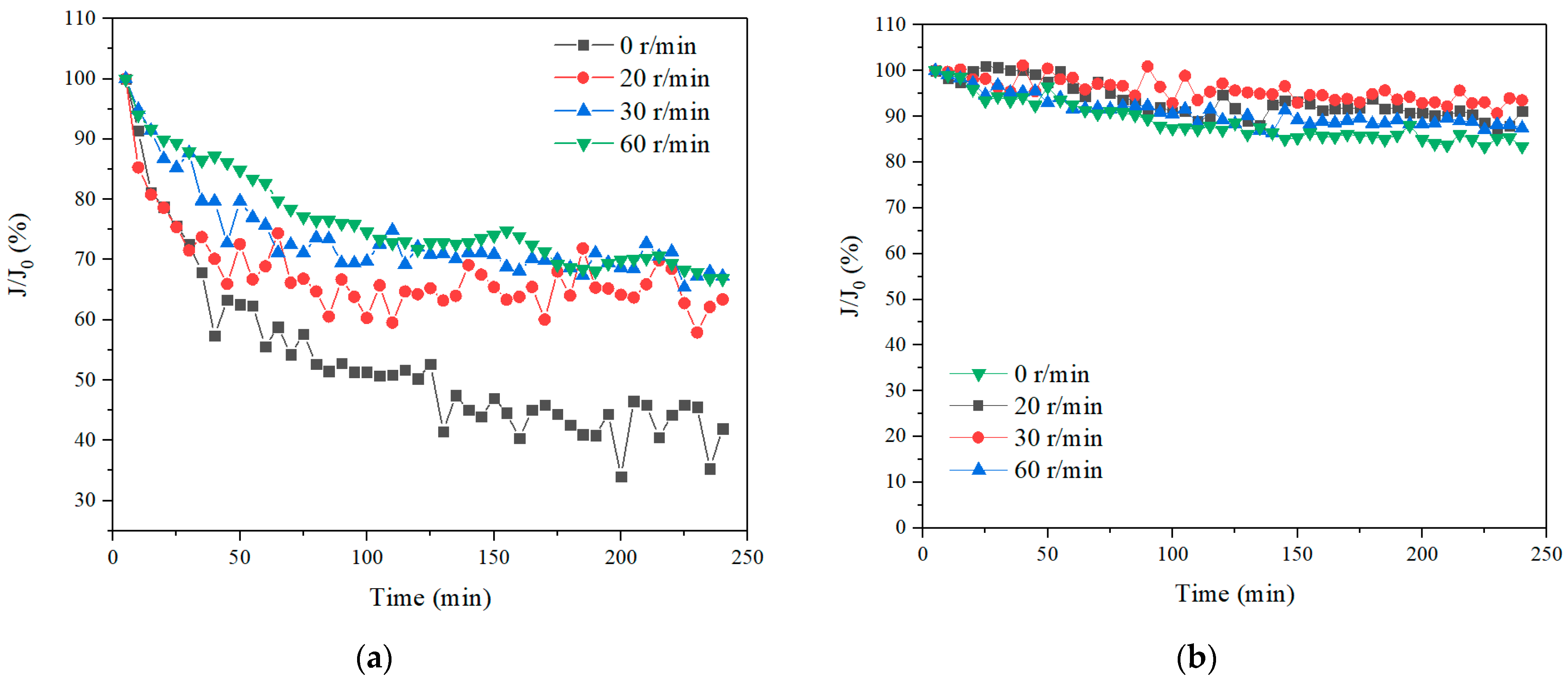
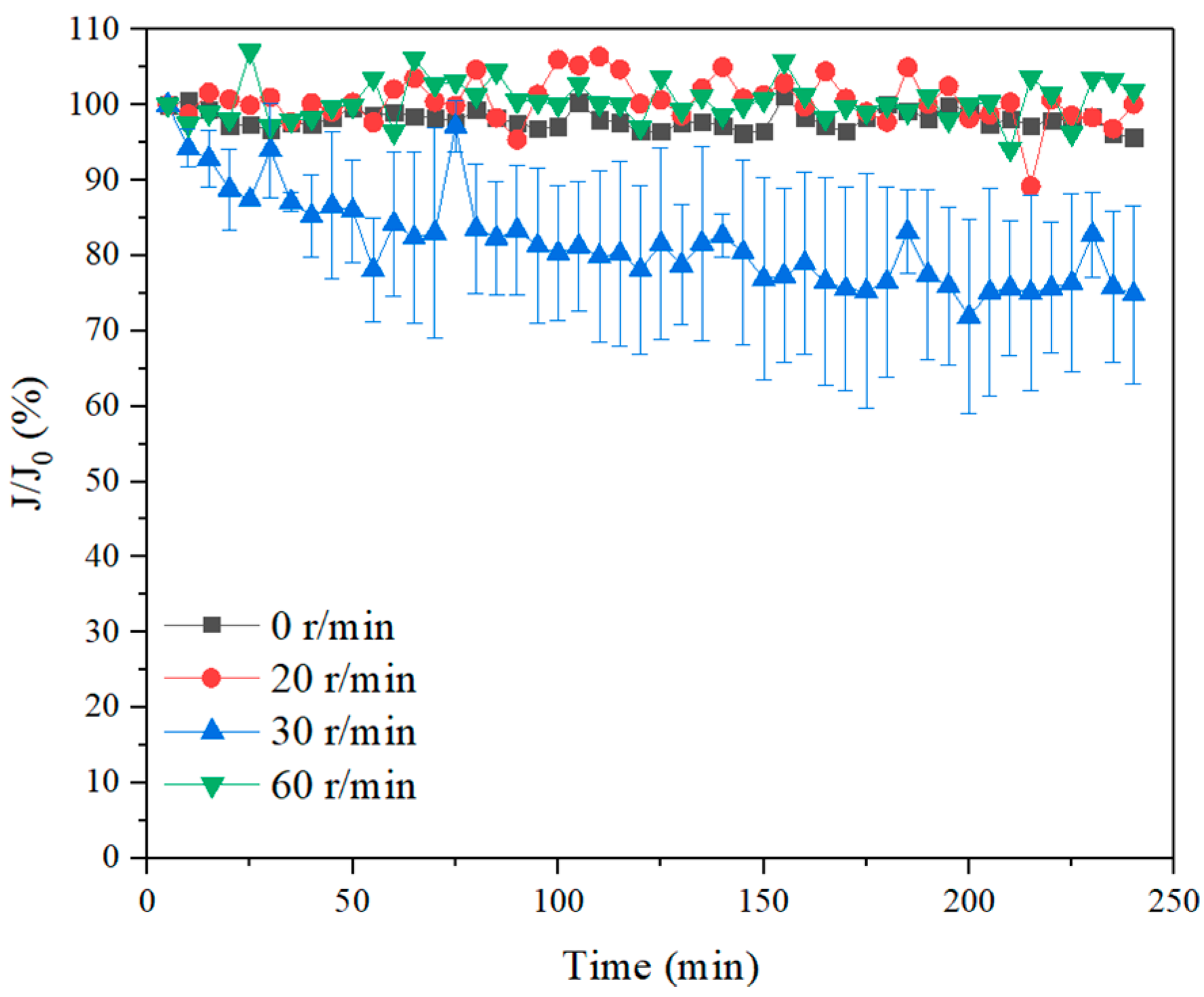
3.1. Effect of Membrane Rotation to Improve UF Performance
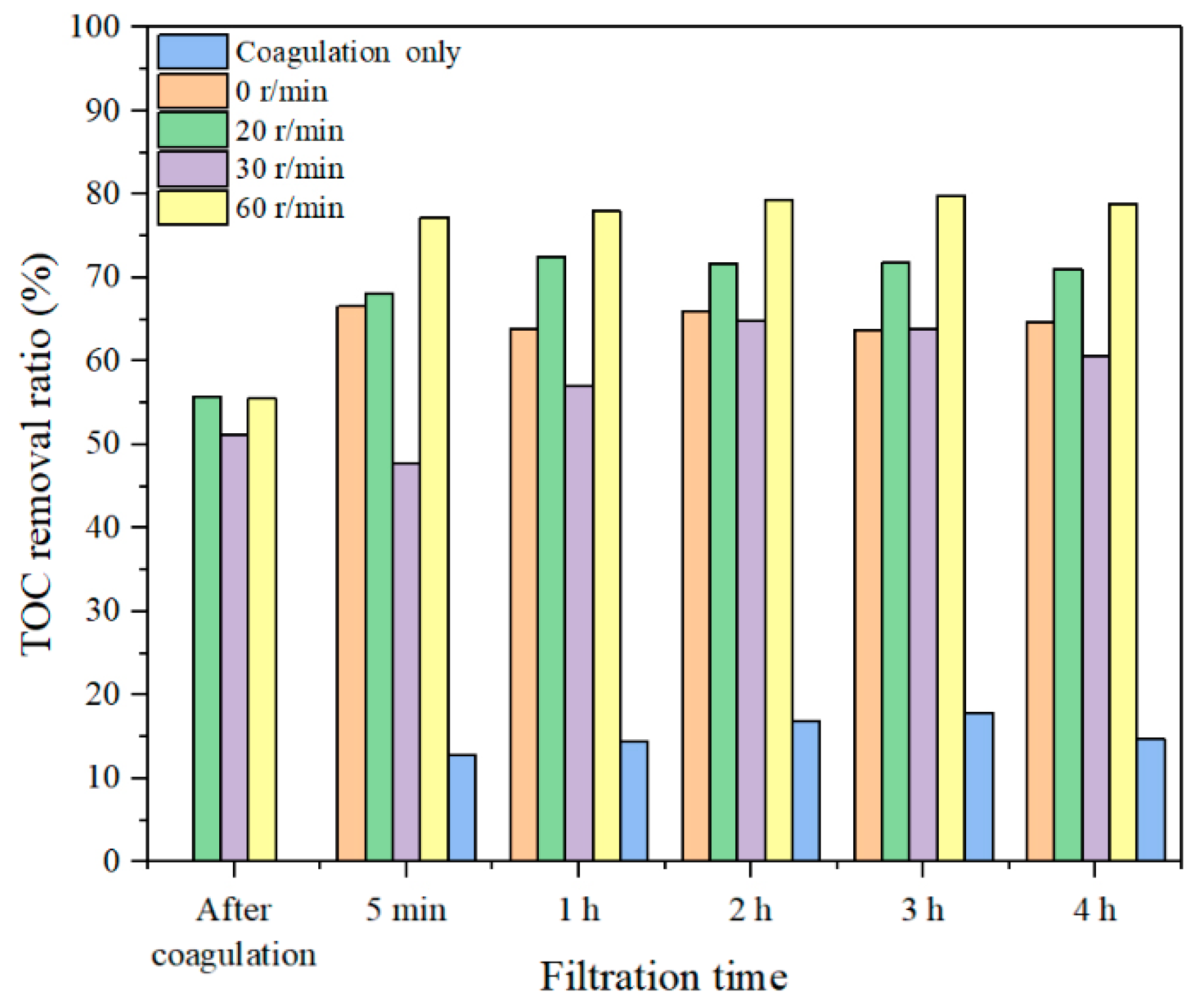
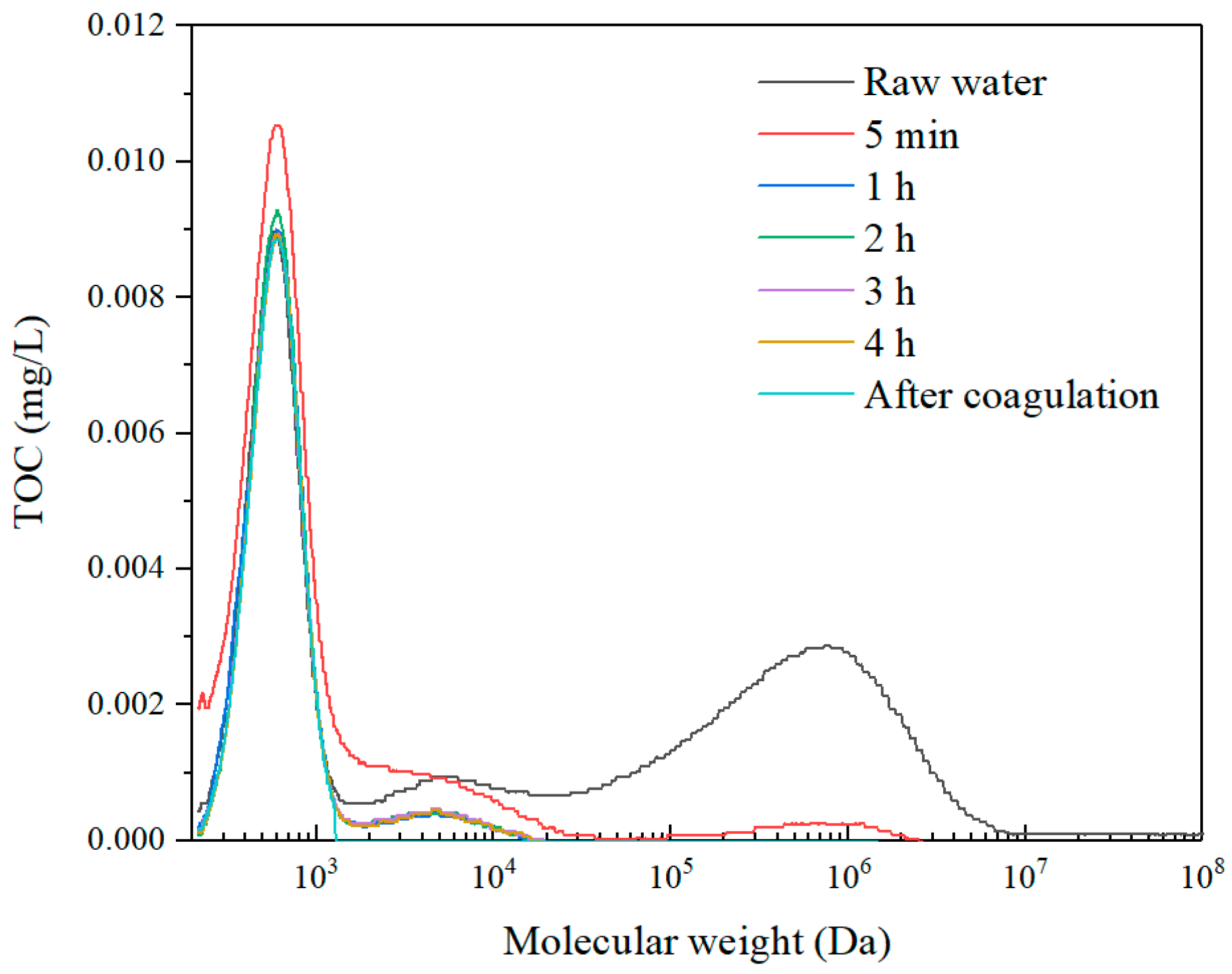
3.2. Effect of Coagulation–Membrane Rotation to Improve UF Performance
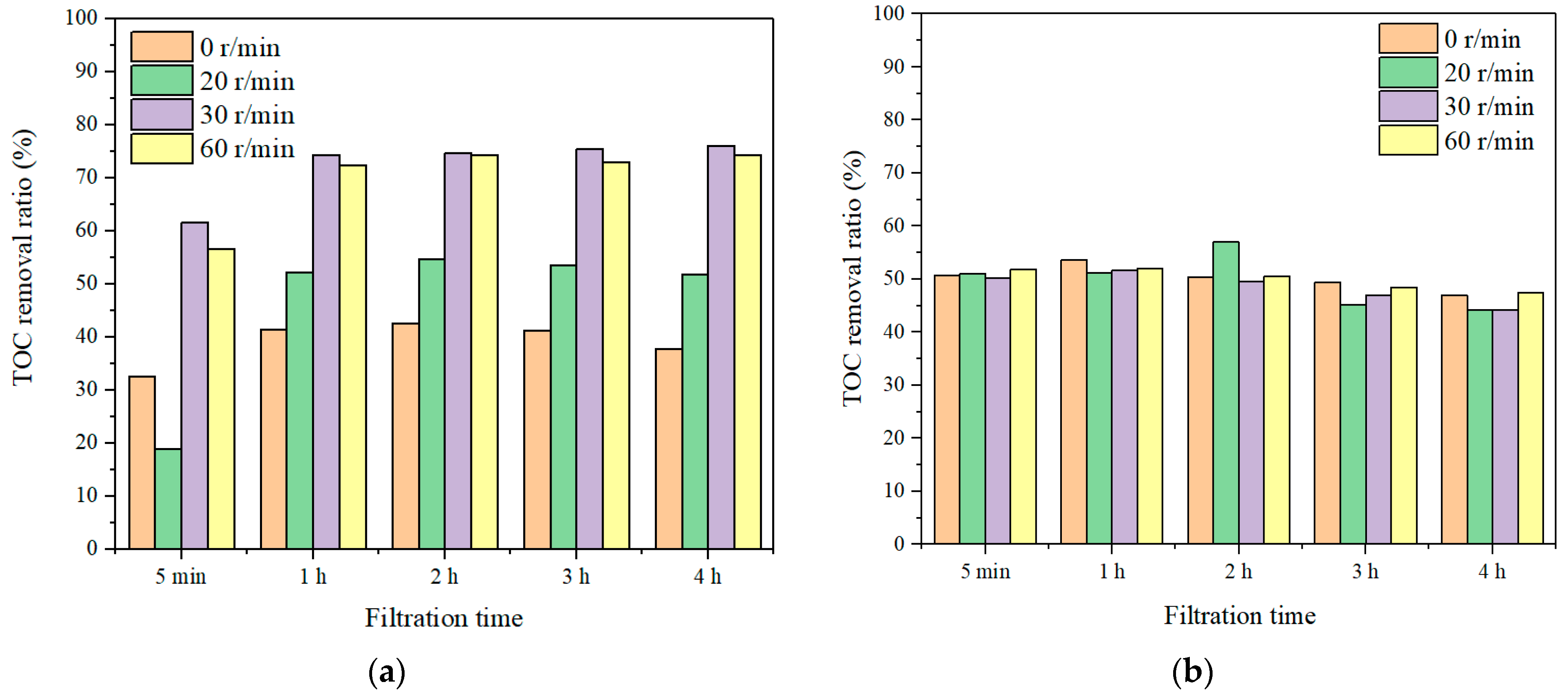
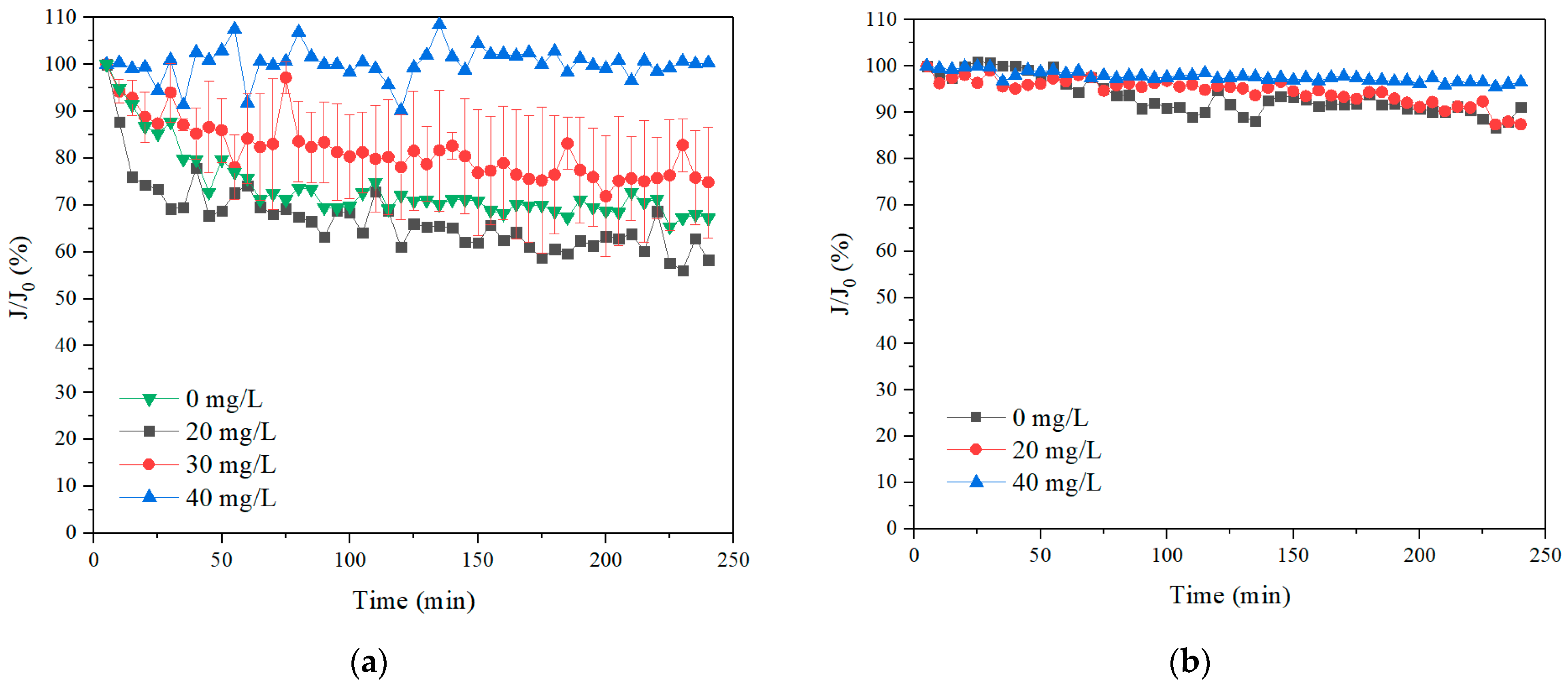
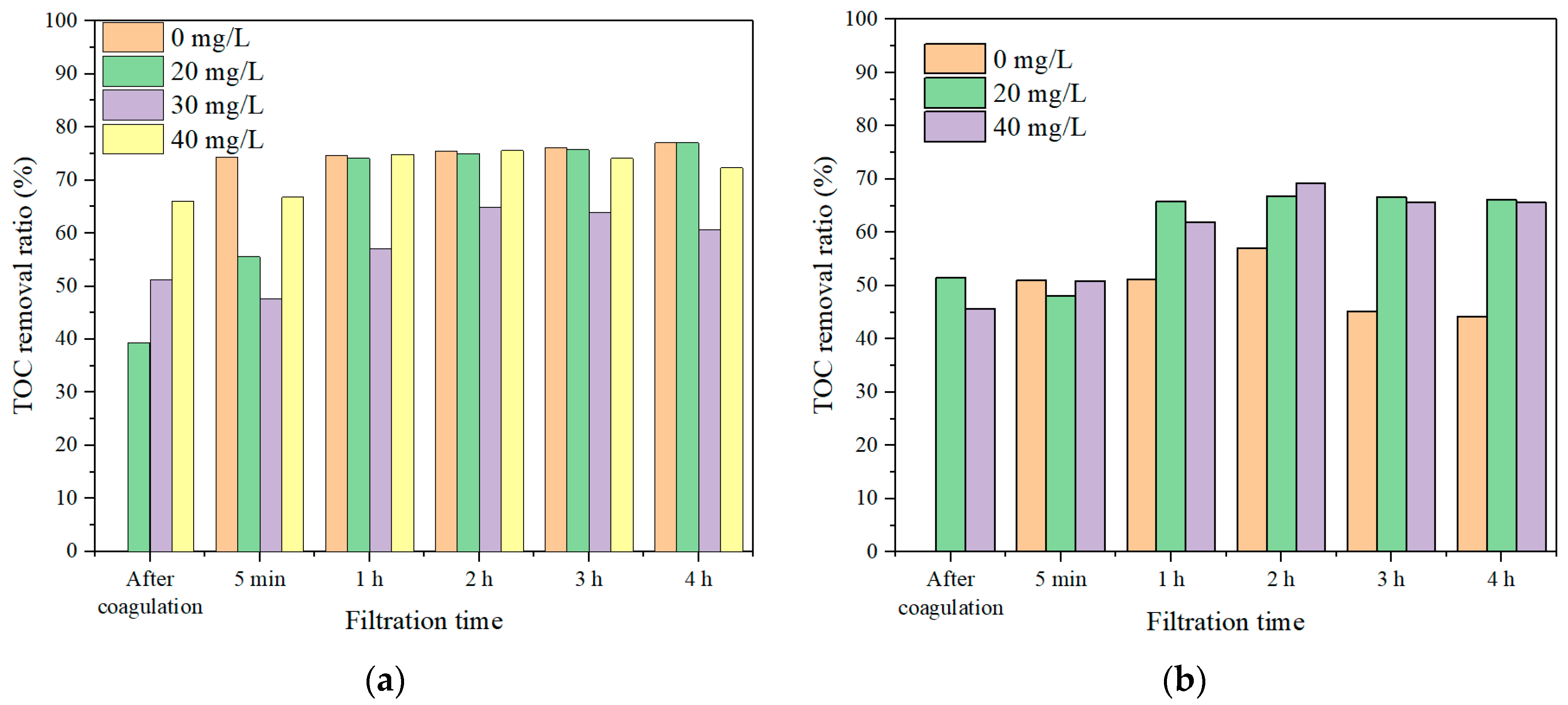
3.2.1. Effect of Coagulation Dosages on Coagulation–Membrane Rotation Performance
3.2.2. Effect of Rotation Speeds on Coagulation–Membrane Rotation Performance
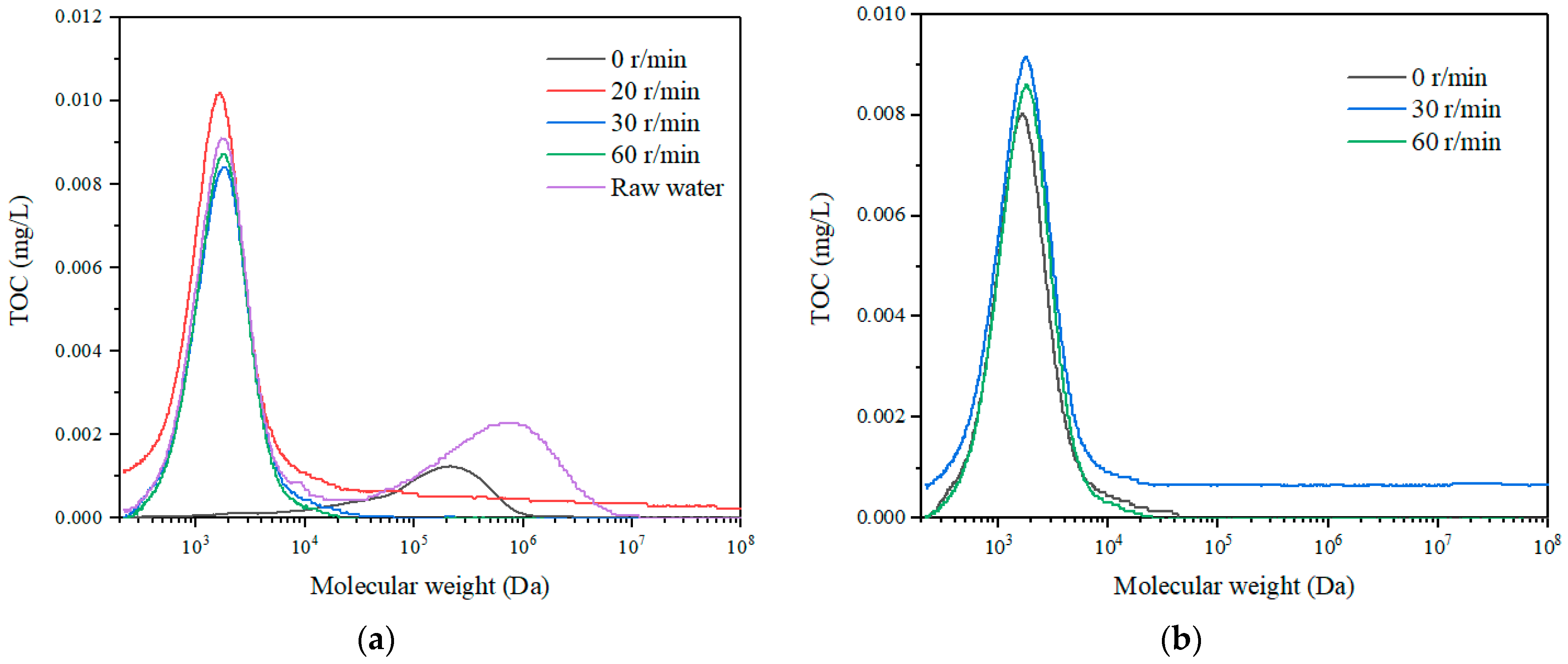
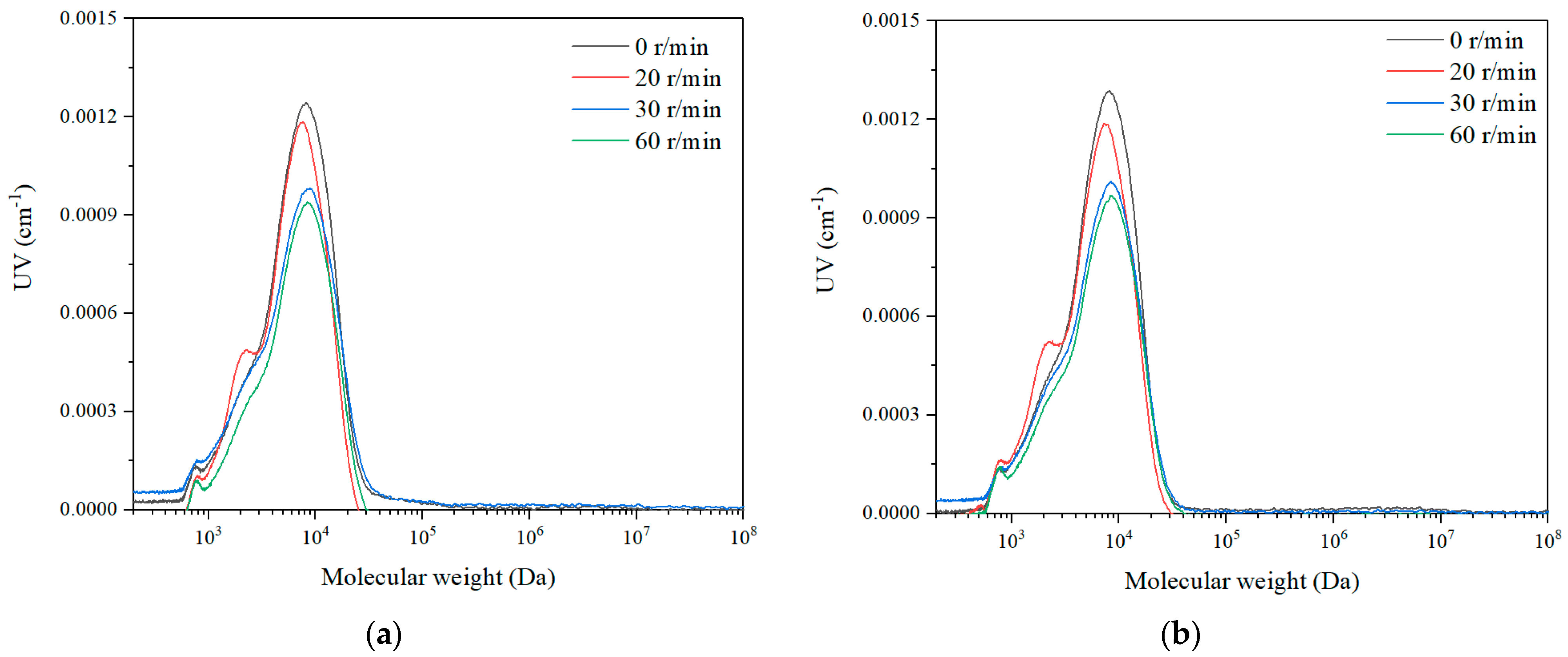
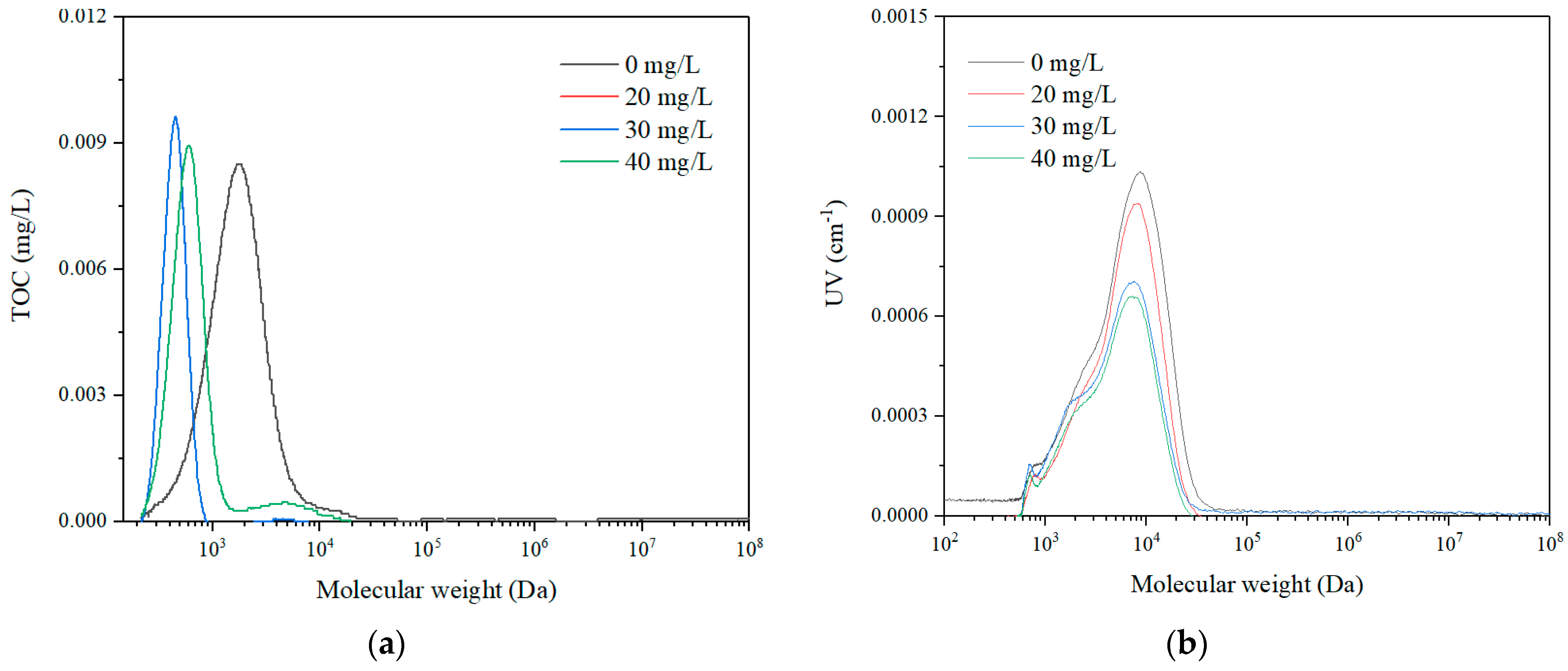
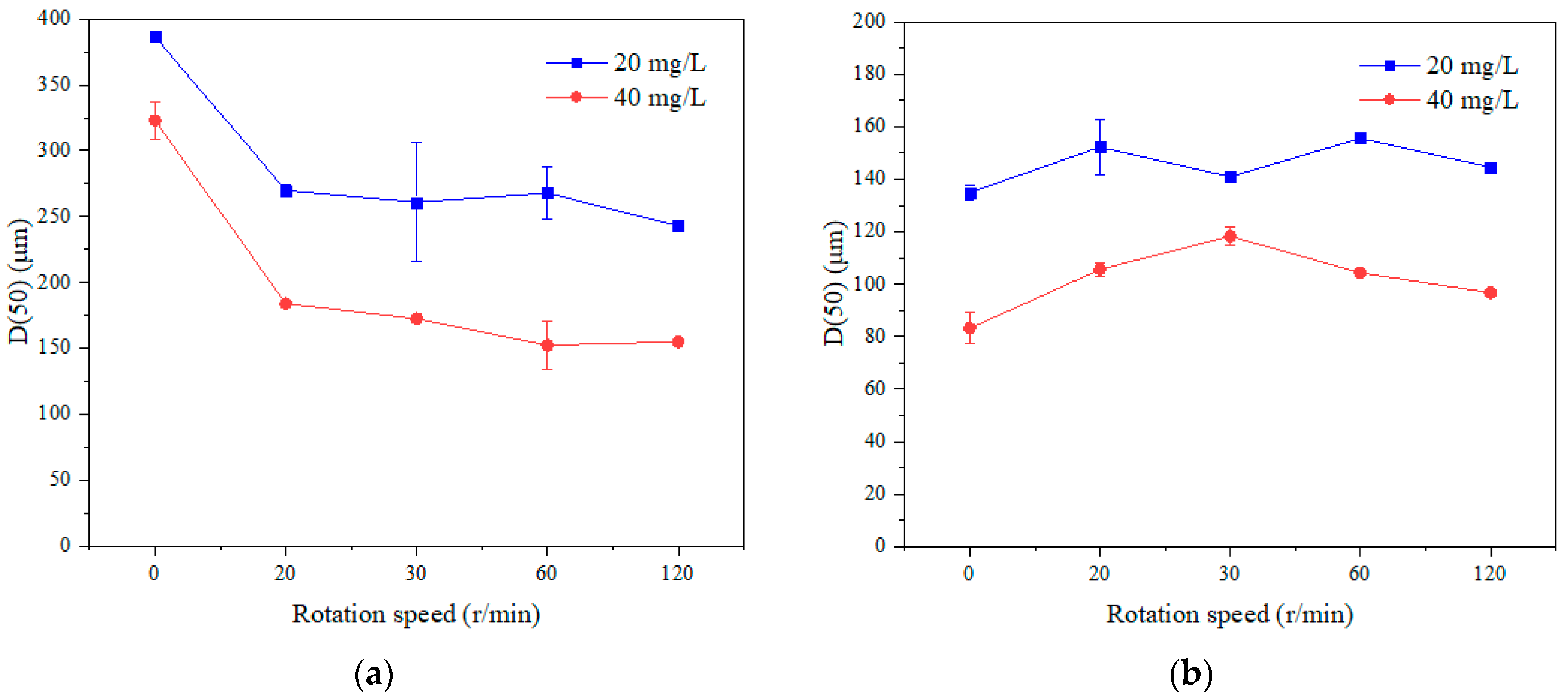
3.2.3. Particle Size Distribution
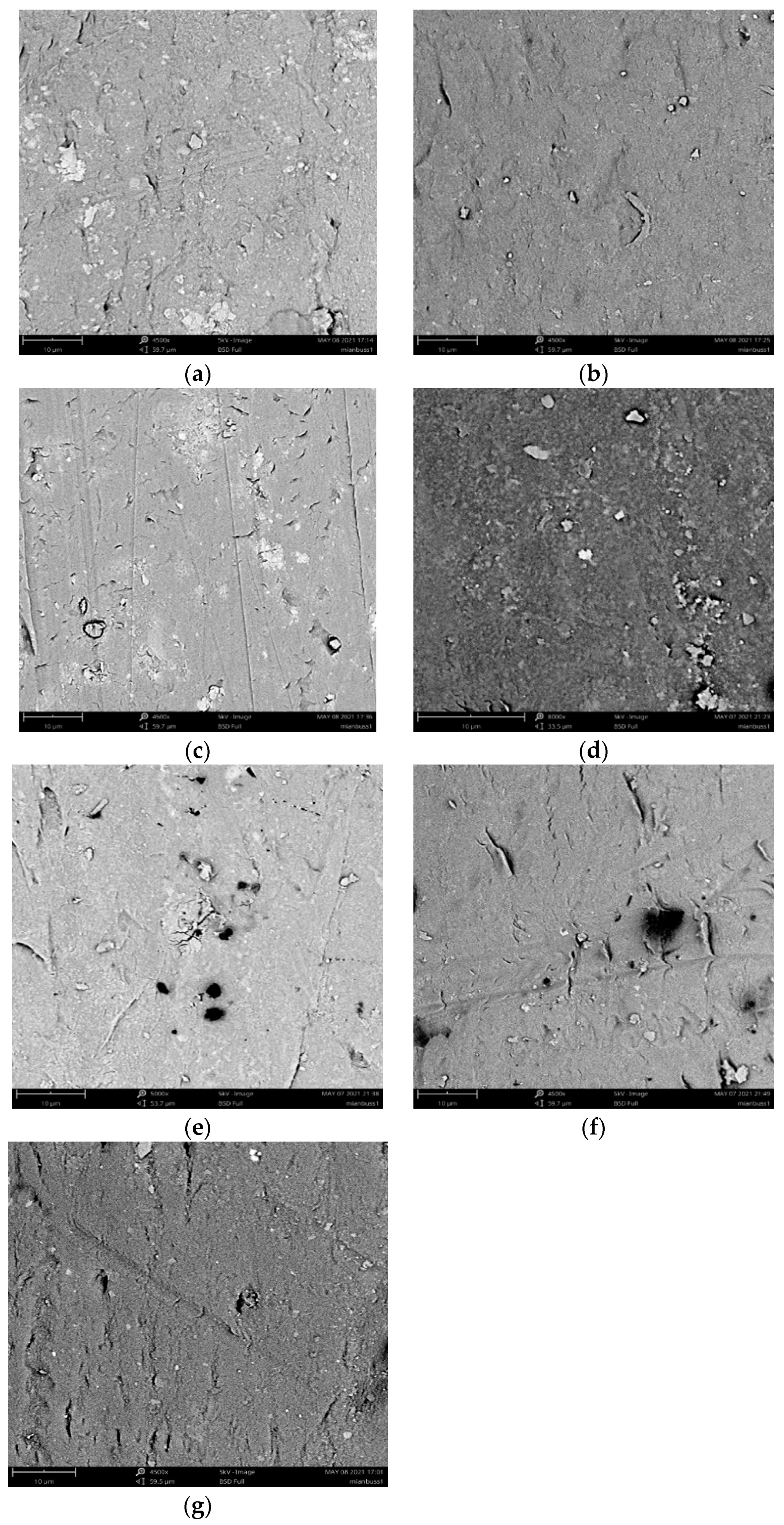
3.2.4. Membrane Surface Morphology Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Modi, A.; Bellare, J. Efficient removal of 2,4-dichlorophenol from contaminated water and alleviation of membrane fouling by high flux polysulfone-iron oxide/graphene oxide composite hollow fiber membranes. J. Water Process Eng. 2020, 33, 101113. [Google Scholar] [CrossRef]
- Kumari, P.; Modi, A.; Bellare, J. Enhanced flux and antifouling property on municipal wastewater of polyethersulfone hollow fiber membranes by embedding carboxylated multi-walled carbon nanotubes and a vitamin E derivative. Sep. Purif. Technol. 2020, 235, 116199. [Google Scholar] [CrossRef]
- Modi, A.; Bellare, J. Zeolitic imidazolate framework-67/carboxylated graphene oxide nanosheets incorporated polyethersulfone hollow fiber membranes for removal of toxic heavy metals from contaminated water. Sep. Purif. Technol. 2020, 249, 117160. [Google Scholar] [CrossRef]
- Huang, W.W.; Wang, L.; Zhou, W.Z.; Lv, W.G.; Hu, M.L.; Chu, H.Q.; Dong, B.Z. Effects of combined ozone and PAC pretreatment on ultrafiltration membrane fouling control and mechanisms. J. Membr. Sci. 2017, 533, 378–389. [Google Scholar] [CrossRef]
- Huang, W.W.; Lv, W.G.; Zhou, W.Z.; Hu, M.L.; Dong, B.Z. Investigation of the fouling behaviors correlating to water characteristics during the ultrafiltration with ozone treatment. Sci. Total Environ. 2019, 676, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Z.; Liu, T.; Crawshaw, J.; Liu, T.; Graham, N. Ultrafiltration and nanofiltration membrane fouling by natural organic matter: Mechanisms and mitigation by pre-ozonation and pH. Water Res. 2018, 139, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, T.; Chu, H.Q.; Zhang, W.Z.; Huang, W.W.; Dong, B.Z.; Wu, D.J.; Chen, F.Y. Natural organic matter separation by forward osmosis: Performance and mechanisms. Water Res. 2021, 191, 116829. [Google Scholar] [CrossRef]
- Liu, J.X.; Fan, Y.Q.; Sun, Y.H.; Wang, Z.H.; Zhao, D.S.; Li, T.; Dong, B.Z.; Tang, C.Y.Y. Modelling the critical roles of zeta potential and contact angle on colloidal fouling with a coupled XDLVO-collision attachment approach. J. Membr. Sci. 2021, 623, 119048. [Google Scholar] [CrossRef]
- Gao, K.; Li, T.; Zhao, Q.Q.; Liu, W.; Liu, J.X.; Song, Y.L.; Chu, H.Q.; Dong, B.Z. UF fouling behavior of allelopathy of extracellular organic matter produced by mixed algae co-cultures. Sep. Purif. Technol. 2021, 261, 118297. [Google Scholar] [CrossRef]
- Long, Y.; Yu, G.Y.; Dong, L.; Xu, Y.C.; Lin, H.J.; Deng, Y.; You, X.J.; Yang, L.N.; Liao, B.Q. Synergistic fouling behaviors and mechanisms of calcium ions and polyaluminum chloride associated with alginate solution in coagulation-ultrafiltration (UF) process. Water Res. 2021, 189, 116665. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Sun, C.; Huang, T.L.; Li, G.B.; Liang, H. Membrane fouling in an integrated adsorption-UF system: Effects of NOM and adsorbent properties. Environ. Sci. Water Res. 2020, 6, 78–86. [Google Scholar] [CrossRef]
- Su, X.; Li, W.D.; Palazzolo, A.; Ahmed, S. Permeate flux increase by colloidal fouling control in a vibration enhanced reverse osmosis membrane desalination system. Desalination 2019, 453, 22–36. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, Y.J.; Mao, Z.M.; Tan, W.S.; Tan, Y.Z.; Chew, J.W.; Chong, T.H.; Fane, A.G. Spacer vibration for fouling control of submerged flat sheet membranes. Sep. Purif. Technol. 2019, 210, 719–728. [Google Scholar] [CrossRef]
- Kola, A.; Ye, Y.; Ho, A.; Le-Clech, P.; Chen, V.K. Application of low frequency transverse vibration on fouling limitation in submerged hollow fibre membranes. J. Membr. Sci. 2012, 409, 54–65. [Google Scholar] [CrossRef]
- Pourbozorg, M.; Li, T.; Law, A.W.K. Effect of turbulence on fouling control of submerged hollow fibre membrane filtration. Water Res. 2016, 99, 101–111. [Google Scholar] [CrossRef]
- Zamani, F.; Law, A.W.K.; Fane, A.G. Hydrodynamic analysis of vibrating hollow fibre membranes. J. Membr. Sci. 2013, 429, 304–312. [Google Scholar] [CrossRef]
- Pourbozorg, M.; Li, T.; Law, A.W.K. Fouling of submerged hollow fiber membrane filtration in turbulence: Statistical dependence and cost-benefit analysis. J. Membr. Sci. 2017, 521, 43–52. [Google Scholar] [CrossRef]
- Ma, B.W.; Xue, W.J.; Bai, Y.H.; Liu, R.P.; Chen, W.; Liu, H.J.; Qu, J.H. Enhanced alleviation of ultrafiltration membrane fouling by regulating cake layer thickness with pre-coagulation during drinking water treatment. J. Membr. Sci. 2020, 596, 117732. [Google Scholar] [CrossRef]
- Shi, W.; Benjamin, M.M. Membrane interactions with NOM and an adsorbent in a vibratory shear enhanced filtration process (VSEP) system. J. Membr. Sci. 2008, 312, 23–33. [Google Scholar] [CrossRef]
- Li, T.; Law, A.W.K.; Cetin, M.; Fane, A.G. Fouling control of submerged hollow fibre membranes by vibrations. J. Membr. Sci. 2013, 427, 230–239. [Google Scholar] [CrossRef]
- Li, T.; Law, A.W.K.; Jiang, Y.S.; Harijanto, A.K.; Fane, A.G. Fouling control of submerged hollow fibre membrane bioreactor with transverse vibration. J. Membr. Sci. 2016, 505, 216–224. [Google Scholar] [CrossRef]
- Ruigomez, I.; Vera, L.; Gonzalez, E.; Rodriguez-Sevilla, J. Pilot plant study of a new rotating hollow fibre membrane module for improved performance of an anaerobic submerged MBR. J. Membr. Sci. 2016, 514, 105–113. [Google Scholar] [CrossRef]
- Li, T.; Law, A.W.K.; Fane, A.G. Submerged hollow fibre membrane filtration with transverse and longitudinal vibrations. J. Membr. Sci. 2014, 455, 83–91. [Google Scholar] [CrossRef]
- Kim, J.; Shin, J.; Kim, H.; Lee, J.Y.; Yoon, M.H.; Won, S.; Lee, B.C.; Song, K.G. Membrane fouling control using a rotary disk in a submerged anaerobic membrane sponge bioreactor. Bioresour. Technol. 2014, 172, 321–327. [Google Scholar] [CrossRef]
- Belfort, G.; Davis, R.H.; Zydney, A.L. The Behavior of Suspensions and Macromolecular Solutions in Cross-Flow Microfiltration. J. Membr. Sci. 1994, 96, 1–58. [Google Scholar] [CrossRef]
- Chew, J.W.; Kilduff, J.; Belfort, G. The behavior of suspensions and macromolecular solutions in crossflow microfiltration: An update. J. Membr. Sci. 2020, 601, 117865. [Google Scholar] [CrossRef]
- Ding, A.; Liang, H.; Li, G.B.; Derlon, N.; Szivak, I.; Morgenroth, E.; Pronk, W. Impact of aeration shear stress on permeate flux and fouling layer properties in a low pressure membrane bioreactor for the treatment of grey water. J. Membr. Sci. 2016, 510, 382–390. [Google Scholar] [CrossRef]
- Wray, H.E.; Andrews, R.C.; Berube, P.R. Surface shear stress and membrane fouling when considering natural water matrices. Desalination 2013, 330, 22–27. [Google Scholar] [CrossRef]
- Huang, H.; Lee, N.; Young, T.; Gary, A.; Lozier, J.C.; Jacangelo, J.G. Natural organic matter fouling of low-pressure, hollow-fiber membranes: Effects of NOM source and hydrodynamic conditions. Water Res. 2007, 41, 3823–3832. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, K.M.; Yang, H.Y.; Li, K.; Liu, X.F.; Wang, Z.H.; Zhou, Q.Q.; Li, G.B.; Liang, H. The relationship between size-segregated particles migration phenomenon and combined membrane fouling in ultrafiltration processes: The significance of shear stress. J. Taiwan Inst. Chem. Eng. 2019, 96, 45–52. [Google Scholar] [CrossRef]
- Shen, X.; Gao, B.Y.; Huang, X.; Bu, F.; Yue, Q.Y.; Li, R.H.; Jin, B. Effect of the dosage ratio and the viscosity of PAC/PDMDAAC on coagulation performance and membrane fouling in a hybrid coagulation-ultrafiltration process. Chemosphere 2017, 173, 288–298. [Google Scholar] [CrossRef]
- Teng, J.H.; Shen, L.G.; Xu, Y.C.; Chen, Y.F.; Wu, X.L.; He, Y.M.; Chen, J.R.; Lin, H.J. Effects of molecular weight distribution of soluble microbial products (SMPs) on membrane fouling in a membrane bioreactor (MBR): Novel mechanistic insights. Chemosphere 2020, 248, 126013. [Google Scholar] [CrossRef] [PubMed]
- Fallahianbijan, F.; Emami, P.; Hillsley, J.M.; Motevalian, S.P.; Conde, B.C.; Reilly, K.; Zydney, A.L. Effect of membrane pore structure on fouling behavior of glycoconjugate vaccines. J. Membr. Sci. 2021, 619, 118797. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Y.L.; Gui, B.; Gao, K.; Zhao, Q.Q.; Qu, R.X.; Liu, T.D.; Hoffmann, M.; Staaks, C.; Dong, B.Z. Application of coagulation-ultrafiltration-nanofiltration in a pilot study for Tai Lake water treatment. Water Environ. Res. 2020, 92, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Genkin, G.; Waite, T.D.; Fane, A.G.; Chang, S. The effect of vibration and coagulant addition on the filtration performance of submerged hollow fibre membranes. J. Membr. Sci. 2006, 281, 726–734. [Google Scholar] [CrossRef]
- Al Akoum, O.; Jaffrin, M.Y.; Ding, L.H.; Paullier, P.; Vanhoutte, C. An hydrodynamic investigation of microfiltration and ultrafiltration in a vibrating membrane module. J. Membr. Sci. 2002, 197, 37–52. [Google Scholar] [CrossRef]
- Gao, K.; Li, T.; Liu, J.X.; Dong, B.Z.; Chu, H.Q. Ultrafiltration membrane fouling performance by mixtures with micromolecular and macromolecular organics. Environ. Sci. Water Res. 2019, 5, 277–286. [Google Scholar] [CrossRef]
- Liu, T.; Graham, N.; Yu, W.Z. Evaluation of a novel composite chitosan-graphene oxide membrane for NOM removal during water treatment. J. Environ. Chem. Eng. 2021, 9, 105716. [Google Scholar] [CrossRef]
- Ma, B.W.; Wu, S.Q.; Wang, B.D.; Qi, Z.L.; Bai, Y.H.; Liu, H.J.; Qu, J.H.; Wu, R.J. Influence of floc dynamic protection layer on alleviating ultrafiltration membrane fouling induced by humic substances. J. Environ. Sci. 2020, 90, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.R.; de Pinho, M.N.; Elimelech, M. Mechanisms of colloidal natural organic matter fouling in ultrafiltration. J. Membr. Sci. 2006, 281, 716–725. [Google Scholar] [CrossRef]
- Raspati, G.S.; Meyn, T.; Leiknes, T. Analysis of Membrane and Cake Layer Resistances in Coagulation: Constant Flux Dead-End Microfiltration of NOM. Separ. Sci. Technol. 2013, 48, 2252–2262. [Google Scholar] [CrossRef]
- Ma, B.W.; Yu, W.Z.; Liu, H.J.; Qu, J.H. Effect of low dosage of coagulant on the ultrafiltration membrane performance in feedwater treatment. Water Res. 2014, 51, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.Z.; Gui, B.; Liu, J.X.; Wang, Z.H.; Tan, K.T. Analysis of organic foulants in the coagulation-microfiltration process for the treatment of Taihu Lake. Environ. Technol. 2019, 40, 3364–3370. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Watanabe, Y.; Tambo, N.; Ozawa, G. Removal of humic substances by UF and NF membrane systems. Water Sci. Technol. 1999, 40, 121–129. [Google Scholar] [CrossRef]
- Takata, K.; Yamamoto, K.; Bian, R.; Watanabe, Y. Removal of humic substances with vibratory shear enhanced processing membrane filtration. Desalination 1998, 117, 273–282. [Google Scholar] [CrossRef]
- Ma, B.W.; Wang, B.D.; Hu, C.Z. Dynamic regulation of Al-based floc cake layers by spiral rotation during ultrafiltration in drinking water treatment. Environ. Res. 2021, 196, 110353. [Google Scholar] [CrossRef] [PubMed]

| No. | Name | Molecular Formula | Manufacturer |
|---|---|---|---|
| 1 | Sodium alginate | (C6H7NaO6)n | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) |
| 2 | Tannic acid | C76H52O46 | Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) |
| 3 | Polyaluminum chloride | AlClHO | Xiya Reagent (Chengdu, China) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Huang, W.; Liu, H.; Li, T.; Chi, N.; Chu, H.; Dong, B. Application of Coagulation–Membrane Rotation to Improve Ultrafiltration Performance in Drinking Water Treatment. Membranes 2021, 11, 643. https://doi.org/10.3390/membranes11080643
Yu H, Huang W, Liu H, Li T, Chi N, Chu H, Dong B. Application of Coagulation–Membrane Rotation to Improve Ultrafiltration Performance in Drinking Water Treatment. Membranes. 2021; 11(8):643. https://doi.org/10.3390/membranes11080643
Chicago/Turabian StyleYu, Hongjian, Weipeng Huang, Huachen Liu, Tian Li, Nianping Chi, Huaqiang Chu, and Bingzhi Dong. 2021. "Application of Coagulation–Membrane Rotation to Improve Ultrafiltration Performance in Drinking Water Treatment" Membranes 11, no. 8: 643. https://doi.org/10.3390/membranes11080643
APA StyleYu, H., Huang, W., Liu, H., Li, T., Chi, N., Chu, H., & Dong, B. (2021). Application of Coagulation–Membrane Rotation to Improve Ultrafiltration Performance in Drinking Water Treatment. Membranes, 11(8), 643. https://doi.org/10.3390/membranes11080643








