Abstract
PT-2385 is currently regarded as a potent and selective inhibitor of hypoxia-inducible factor-2α (HIF-2α), with potential antineoplastic activity. However, the membrane ion channels changed by this compound are obscure, although it is reasonable to assume that the compound might act on surface membrane before entering the cell´s interior. In this study, we intended to explore whether it and related compounds make any adjustments to the plasmalemmal ionic currents of pituitary tumor (GH3) cells and human 13-06-MG glioma cells. Cell exposure to PT-2385 suppressed the peak or late amplitude of delayed-rectifier K+ current (IK(DR)) in a time- and concentration-dependent manner, with IC50 values of 8.1 or 2.2 µM, respectively, while the KD value in PT-2385-induced shortening in the slow component of IK(DR) inactivation was estimated to be 2.9 µM. The PT-2385-mediated block of IK(DR) in GH3 cells was little-affected by the further application of diazoxide, cilostazol, or sorafenib. Increasing PT-2385 concentrations shifted the steady-state inactivation curve of IK(DR) towards a more hyperpolarized potential, with no change in the gating charge of the current, and also prolonged the time-dependent recovery of the IK(DR) block. The hysteretic strength of IK(DR) elicited by upright or inverted isosceles-triangular ramp voltage was decreased during exposure to PT-2385; meanwhile, the activation energy involved in the gating of IK(DR) elicitation was noticeably raised in its presence. Alternatively, the presence of PT-2385 in human 13-06-MG glioma cells effectively decreased the amplitude of IK(DR). Considering all of the experimental results together, the effects of PT-2385 on ionic currents demonstrated herein could be non-canonical and tend to be upstream of the inhibition of HIF-2α. This action therefore probably contributes to down-streaming mechanisms through the changes that it or other structurally resemblant compounds lead to in the perturbations of the functional activities of pituitary cells or neoplastic astrocytes, in the case that in vivo observations occur.
1. Introduction
Hypoxia-inducible factor-1α (HIF-1α) has been reported to be expressed in pituitary tumors [1,2,3,4,5,6,7,8,9,10]. Alternatively, HIF-2α has also been reported to be expressed in pituitary tumors [11]. HIF-2α antagonists such as PT-2385 ((S)-3-((2,2-difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1H-inden-4-yl)oxy)-5-fluorobenzoniltrile) are increasingly being proposed as a potential effective treatment of choice for HIF-2α-related tumors and metabolic disorders [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. As a potent inhibitor of HIF-2α, PT-2385 was thought to selectively disrupt the heterodimerization of HIF-2α with HIF-1β [12,14,21]. It is very potent in impeding cancer cell growth, proliferation, and tumor angiogenesis, particularly in clear-cell renal cell carcinoma [12,13,14,20,21,22]. A report has recently revealed that roxadustat (FG-4592), a small molecule recognized to be an inhibitor of HIF prolyl hydroxylase, was effective in perturbing the amplitude and gating of voltage-gated K+ currents [27]. However, whether or how PT-2385 is capable of modulating the strength of plasmalemmal K+ currents is uncertain.
Voltage-gated K+ (KV) channels are well-recognized as having essential roles in determining the membrane excitability associated with delayed-rectifier KV channels, for example KV3 (KCNC) or KV2 (KCNB) channels, and they are expressed ubiquitously in neuroendocrine or endocrine cells [28,29,30,31,32,33,34]. A cause-and-effect relationship regarding the activity of KV3 or KV2 channels and the magnitude of delayed-rectifier K+ currents (IK(DR)) is correlated with action potential firing in many cell types and has increasingly been demonstrated [29,30,31,32,35,36,37,38,39,40,41,42,43,44]. KV channels of the KV3.1–KV3.2 types have also been regarded to be the main determinants of IK(DR) identified in pituitary cells, including pituitary tumor (GH3) cells [28,30,31]. The biophysical properties of IK(DR) in GH3 cells are unique in being manifested by a positively shifted voltage dependency as well as by a rapid deactivation rate [27,30,31,35,41,45]. The activity of HIF-1α has recently been reported to regulate the proliferation and invasiveness of oral cancer cells through the Kv3.4 channel [23]. Alternatively, PT-2385 was reported to have rapid effects on the impairment of hypoxic ventilator control [46]. Our understanding of whether PT-2385 or other related compounds cause any adjustments with regard to the amplitude, gating, and hysteresis of these types of K+ currents (e.g., IK(DR)) remains largely incomplete, although recent studies have demonstrated a possible link between the expression of HIF and the regulation of KV-channel activity [23,47,48,49,50].
In view of the foregoing considerations, the following attempts were undertaken to determine how PT-2385 and other relevant compounds could result in adjustments to the ionic currents in pituitary tumor (GH3) cells. Moreover, some researchers have performed studies to demonstrate the effectiveness of this compound in suppressing the growth and invasiveness of gliomas [18,19]. Therefore, we decided to investigate the possible actions of PT-2385 on IK(DR) in the GH3 cells of pituitary and human 13-06-MG glioma cells. Findings from the present observations tempt us to reflect that the IK(DR) inherent in different cell types could be an additional non-canonical target through which PT-2385 can act to influence the functional activities of the cells involved.
2. Materials and Methods
2.1. Chemicals and Solutions
PT-2385 (PT, PT2385, (S)-3-((2,2-difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1H-inden-4-yl)oxy)-5-fluorobenzoniltrile, C17H12F3NO4S) was acquired from MedChemExpress (Asia Bioscience, Taipei, Taiwan). Diazoxide (Diaz), tetrodotoxin (TTX), and tetraethylammonium chloride (TEA) were from Sigma-Aldrich (Merck, Taipei, Taiwan). Sorafenib (SOR) was purchased from Selleck (Asia Bioscience, Taipei, Taiwan), and cilostazol (Cil) from Tocris (Union Biomed, Taipei, Taiwan). PT-2385 was dissolved as 20 mM stock solution in dimethyl sulfoxide and was diluted by extracellular solution to achieve the final concentrations. Unless stated otherwise, we obtained fetal bovine and calf serum, L-glutamine, trypsin/EDTA, and the culture media (e.g., Ham’s F12-12 medium) from HyCloneTM (Thermo Fisher; Level Biotech, Tainan, Taiwan). Additionally, other chemicals were acquired at the best available quality, of analytical grade.
The normal Tyrode’s solution contained (in mM): NaCl 136.5, KCl 5.4, CaCl2 1.8, MgCl2 0.53, glucose 5.5, and 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) 5.5, and the pH was adjusted to 7.4 with NaOH. For measurements of IK(DR) across the cell membrane, the cells were placed in Ca2+-free Tyrode’s solution in order to preclude the contamination of voltage-gated Ca2+ currents and Ca2+-activated K+ currents. In whole-cell recordings, we filled the pipet with internal solution, which contained: KH2PO4 1, K-aspartate 140, MgCl2 1, Na2ATP 3, EGTA 0.1, Na2GTP 0.1, and HEPES 5 (in mM), and the pH was adjusted to 7.4 with KOH. To measure IK(DR) in 13-06-MG glioma cells, the high K+-bathing solution contained: KCl 145, MgCl2 0.53, and HEPES-KOH 5 (in mM and pH 7.4). All solutions were prepared by de-mineralized water with the water purification system Milli-Q® (Merck, Tainan, Taiwan). During experimental days, the bathing or backfilling solution and culture medium were filtered by using an Acrodisc® syringe filter with 0.2-µm Supor® nylon membrane (Pall; Bio-Check, Tainan, Taiwan).
2.2. Cell Preparations
The preparation of GH3 or 13-06-MG is reported in the Supplementary Materials. References [51,52] are cited in the Supplementary Materials.
2.3. Electrophysiological Measurements
GH3 or 13-06-MG cells were harvested and rapidly transferred to a customized chamber shortly before the electrical recordings. The chamber was positioned on the stage of an inverted microscope. Cells were kept for immersion in normal Tyrode’s solution at 20–25 °C; the composition of this solution is described above. Patch-clamp recordings were undertaken under whole-cell mode with either an RK-400 (Biologic, Echirolles, France) or an AxoClamp 2B amplifier (Molecular Devices; Kim Forest, Tainan, Taiwan) [52,53]. Patch electrodes with tip resistance of 3–5 MΩ were made from Kimax-51 capillaries (#34500 (1.5–1.8 mm in outer diameter); Dogger, Tainan, Taiwan), using either a PP-830 vertical puller (Narishige, Tokyo, Japan) or a P-97 horizontal puller (Sutter, Novato, CA), and their tips were then fire-polished with MF-83 microforge (Narishige). The signals, which comprised voltages and current tracings, were stored online at 10 kHz in a touchscreen computer (ASUSPRO-BU401LG, ASUS, Tainan, Taiwan) equipped with Digidata 1440A interface (Molecular Devices), controlled by pCLAMP 10.7 software (Molecular Devices). The potentials were revised for the liquid–liquid junction potential that appeared when the composition of the pipette solution was different from the solution of the bath.
2.4. Data Analyses
To determine the concentration-dependent inhibitory effect of PT-2385 on IK(DR), ionic current (i.e., IK(DR)) was evoked by a depolarizing pulse (1-s duration) from −50 to +50 mV, and current amplitudes (i.e., peak or late IK(DR)) taken with or without the addition of varying PT-2385 concentrations were measured at the beginning or end of test potential. The IK(DR) in the control period (i.e., PT-2385 was not present) was considered to be 1.0, and the values obtained with different concentrations of PT-2385 were then compared and analyzed. The PT-2385 concentration yielding a 50% reduction (i.e., IC50) of peak or late IK(DR) was evaluated with goodness-of-fit assessments by using the 3-parameter logistic model (i.e., a modified form of sigmoidal Hill equation). That is,
where [PT] is the PT-2385 concentration applied, IC50 is the PT-2385 concentration required for 50% inhibition of peak or late IK(DR), and nH is the Hill coefficient; moreover, maximal inhibition (i.e., 1 − a) was approximated from the equation. This equation converged reliably to produce the best-fit line and parameter estimates.
The relationships either between the normalized amplitude and the conditioning potential (i.e., the quasi-steady-state inactivation curve of IK(DR)) or between the instantaneous current and the upsloping end of ITRV (i.e., instantaneous current-voltage [I-V] relationship of the current) were constructed and then fitted with a Boltzmann function in the following form:
In this equation, Imax is the maximal amplitude of IK(DR) activated in response to step or ramp depolarization, V1/2 is the voltage at which there is half-maximal inactivation or activation of the current, q is the apparent gating charge, F is the Faraday constant, R is the ideal gas constant, and T is the absolute temperature.
The free energy involved in the gating of IK(DR) (∆G0) was evaluated on the assumption that there is a 2-state (i.e., in an equilibrium between closed (resting) and open states) gating model existing in the KV channel. The ∆G0 for IK(DR) activation at 0 mV would be equal to q × F × V1/2 [54,55,56,57]. The standard errors of ∆G0 (i.e., ) were calculated according to:
where σq or represents the standard error in q or V1/2, respectively.
2.5. Statistical Analyses
Linear or nonlinear curve fitting to experimental data sets presented herein was performed using either the Microsoft Solver function embedded in Excel® 2016 (Microsoft) or the OriginPro 2021 program (OriginLab®; Scientific Formosa, Kaohsiung, Taiwan) [58]. Values of experimental measurements were presented as the mean ± standard error of mean (SEM) and sample sizes (n) indicative of the cell number which the experimental results were collected from, and the error bars were hence plotted as the SEM. The Student’s t test (paired or unpaired) was initially employed; however, as the statistical difference among different groups was necessarily evaluated, we performed either analysis of variance (ANOVA)-1 or ANOVA-2 (with or without repeated measure) followed by Duncan’s post hoc test. Differences of mean values between groups were considered significant at p < 0.05.
3. Results
3.1. Effect of PT-2385 on Delayed-Rectifier K+ Current (IK(DR)) Measured from Pituitary Tumor (GH3) Cells
In this study, we initially exploited the whole-cell configuration of standard patch-clamp technique to assess the perturbations of PT-2385 in the ionic currents of GH3 cells. In order to record the currents of IK(DR), cells were bathed in Ca2+-free Tyrode’s solution containing 0.5 mM CdCl2 and 1 µM tetrodotoxin (TTX) while the recording pipet was backfilled with K+-containing internal solution. TTX or CdCl2 was used to block voltage-gated Na+ or Ca2+ currents, respectively. As the whole-cell mode was established, the examined cell was maintained at the potential of −50 mV in voltage clamp mode and the depolarizing step was subsequently applied (1-s duration) to +50 mV for the elicitation of IK(DR) [27,31,32,59]. Of interest, 1 min after cell exposure to PT-2385, the amplitude of IK(DR) evoked by the 1-s-long step depolarization from –50 to +50 mV became progressively decreased (Figure 1A). For example, at the level of +50 mV, the addition of 3 µM PT-2385 was found to diminish the peak or late amplitude of IK(DR) to 609 ± 63 or 318 ± 41 pA (n = 8, p < 0.05), respectively, from control values of 818 ± 72 or 529 ± 51 pA (n = 8). After washout of the agent, the peak or late amplitude of the current returned to 807 ± 69 or 517 ± 48 pA (n = 8, p < 0.05). In the continued presence of 3 µM PT-2385, the further addition of 10 mM tetraethylammonium chloride (TEA) almost fully abolished current amplitude. Additionally, in the presence of 3 µM PT-2385, the slow-component value in the inactivation time constant (τinact(S)) of IK(DR) activated by a 1-s depolarizing pulse to +50 mV was rapidly decreased to 786 ± 119 msec (n = 8, p < 0.05) from a control value of 1514 ± 217 msec (n = 8); however, a minimal change in fast component of current inactivation was seen in its presence. The results strongly suggest that PT-2385-mediated inhibition of IK(DR) in GH3 cells is accompanied by an enhancement of the current inactivation rate (Figure 1A).
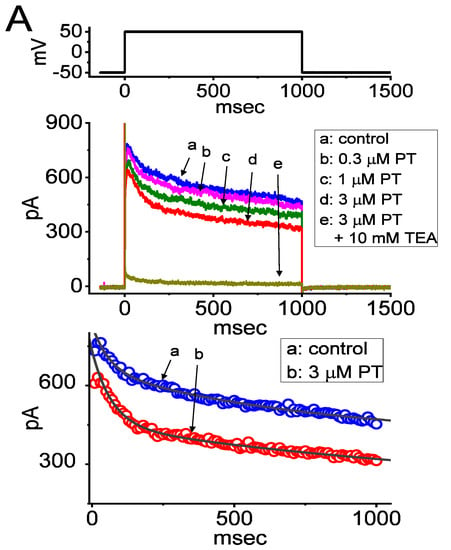
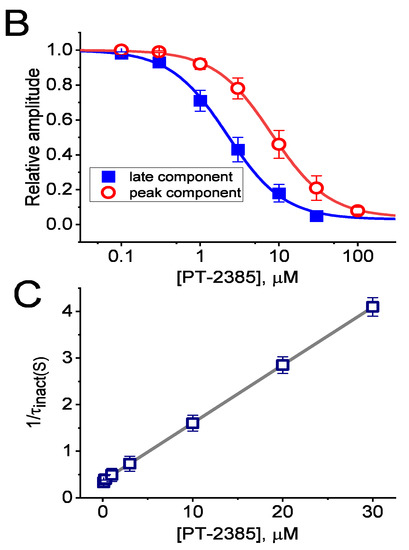
Figure 1.
Inhibitory effect of PT-2385 (PT) on delayed-rectifier K+ current (IK(DR)) measured from pituitary tumor (GH3) cells. Whole-cell patch-clamp experiments were undertaken via experimented cells in Ca2+-free Tyrode’s solution containing 1 µM tetrodotoxin (TTX) and 0.5 mM CdCl2, while the recording pipet was filled with K+-containing solution. In the upper part of (A), representative IK(DR) tracings were obtained for the control (i.e., PT-2385 was not present, (a)), and during cell exposure to 0.3 µM PT-2385 (b), 1 µM PT-2385 (c), 3 µM PT-2385 (d), or 3 µM PT-2385 plus 10 mM tetraethylammonium chloride (TEA). The voltage-clamp profile is illustrated in the uppermost part. In the lower part of (A), the time courses of current inactivation in the absence (a) and presence (b) of 3 µM PT-2385 were well approximated by a 2-exponential decay (indicated in gray smooth line). The values of fast or slow components (i.e., τinact(S)) in the inactivation time constants of IK(DR) obtained in the absence and presence of 3 µM PT-2385 were 63 or 1514 msec, and 61 or 786 msec, respectively. (B) The concentration–response curve of PT-2385-induced block of peak or late IK(DR) occurring in GH3 cells. The continuous line represents the best fit to the modified Hill equation, as elaborated in the Materials and Methods. The IC50 values for PT-2385-induced inhibition of peak or late IK(DR) were reliably estimated to be 8.1 or 2.2 µM, respectively. Each point represents the mean ± SEM (n = 9–12). (C) Relationship of the PT-2385 concentration as a function of the slow component in the inactivation rate constant (1/τinact(S)) (mean ± SEM; n = 8 for each point). Of note, the value of 1/τinact(S) is directly proportional to the PT-2385 concentration. Based on a minimal reaction scheme elaborated in the text, the value of k+1* or k−1 was estimated to be 0.124 s−1µM−1 or 0.362 s−1, respectively; hence, the KD value (k−1/k+1*, i.e., dissociation constant) turned out to be 0.036 µM (or 36 nM), a value which is closely similar to the IC50 value required for its inhibitory effect on late IK(DR).
The correlation between the concentration of PT-2385 and the relative amplitude of peak or late IK(DR) was further determined and established. In these experiments, we held the examined cell in a voltage clamp at −50 mV, and the depolarizing pulse (1-s duration) to +50 mV was applied, while current amplitudes in the presence of varying PT-2385 concentrations were taken at the start or end-pulse of step depolarization. As illustrated in Figure 1B, the presence of various concentrations of PT-2385 differentially suppressed the peak and late amplitudes of IK(DR) in a concentration-dependent manner. By virtue of a non-linear least-squares fit to the experimental data, the value of the IC50 (i.e., concentration required from a half-maximal inhibition) for the inhibitory effect of PT-2385 on peak or late IK(DR) was yielded as 8.1 or 2.2 µM, respectively. These results reflect that PT-2385 can exercise a depressant action on IK(DR) in these cells, and that the late component of IK(DR) activated by step depolarization was subject to being inhibited to a higher extent than the peak (or transient) component of the current.
3.2. Kinetic Estimate of IK(DR) Block by PT-2385
During cell exposure to PT-2385, apart from the decreased IK(DR) amplitude, the inactivation rate of the current responding to step depolarization tended to become raised. In this regard, we extended the study to estimate the inactivation kinetics of PT-2385-mediated inhibition of IK(DR) activated by a long depolarizing pulse in situations where cells were exposed to varying PT-2385 concentrations. The concentration dependence of the IK(DR)-inactivation rate caused by adding this compound was constructed and is hence illustrated in Figure 1C. The experimental observations demonstrated that its effects on IK(DR) resulted in a concentration-dependent increase in the rate of current inactivation. Consequently, the effect on IK(DR) identified in GH3 cells can be reasonably explained by a state-dependent blocking mechanism through which this compound is allowed to preferentially bind to the open or open-inactivated state of the KV channels. The reaction scheme for this viewpoint is thus simply
Alternatively, the dynamical system yielding three equations becomes
where α or β is called the kinetic constant for the opening or closing of the KV channel, respectively; k+1* or k−1 is that for blocking (i.e., depending on the PT-2385 concentration) or unblocking by adding PT-2385; [B] is the PT-2385 concentration used; and, C, O, or O·[B] indicates the closed (or resting), open, or open-blocked state of the KV channel, respectively.
The blocking (forward, k+1*) or unblocking (backward, k−1) rate constant was estimated based on the slow component in the inactivation time constants (τinact(S)) of IK(DR) during cell exposure to varying PT-2385 concentrations (Figure 1C). The relationship can be satisfied by
In this equation, k+1* or k−1 was respectively derived from the slope or from the y-axis intercept at [B] = 0 (i.e., the point at which the line crosses the y-axis) of the linear regression at which the reciprocal time constants of the current (1/τinact(S)) versus the PT-2385 concentration was interpolated. The resultant relationship between 1/τinact(S) and [B] was found in the straight line through experimental data points with a correlation coefficient of 0.95 (Figure 1C). The blocking or unblocking rate constant was thereafter yielded to be 0.124 s−1µM−1 or 0.362 s−1, respectively. According to the evolving rate constants, dividing k−1 by k+1* gave a dissociation constant (KD) of 2.9 µM during cell exposure to PT-2385, a value that was noted to be close to the effective IC50 (2.2. µM) of this compound needed for inhibition of late IK(DR).
3.3. Comparison among the Effects of PT-2385 (PT), PT-2385 Plus Diazoxide (Diaz), and PT-2385 Plus Cilostazol (Cil), or PT-2385 Plus Sorafenib (SOR) on the Amplitude of IK(DR)
We continued to evaluate and then compare the effect of PT-2385 and PT-2385 plus different related compounds (e.g., Diaz, Cil, SOR) on IK(DR) magnitude. As summarized in Figure 2, the amplitude of IK(DR) was effectively inhibited by the addition of 3 µM PT-2385. However, as cells were continually exposed to PT-2385 (3 µM), the subsequent addition of either diazoxide (Diaz, 30 µM), cilostazol (Cil, 10 µM), or sorafenib (SOR, 10 µM) did not result in any further modifications to the PT-2385-mediated block of peak or late IK(DR). Diaz or Cil has been reported to activate ATP-sensitive K+ (KATP) channels or large-conductance Ca2+-activated K+ (BKCa) channels, respectively [34,60,61], while SOR was previously recognized to suppress the activity of tyrosine kinase [62]. Additionally, the presence of Diaz (30 µM), Cil (10 µM), or SOR (10 µM) alone was not noted to have significant effects on the IK(DR) amplitude observed in GH3 cells. Under this circumstance, it is reasonable to assume that the PT-2835-mediated block of IK(DR) in GH3 cells tends to be independent of either the inhibition of KATP or BKCa channels, or the stimulation of tyrosine-kinase activity.
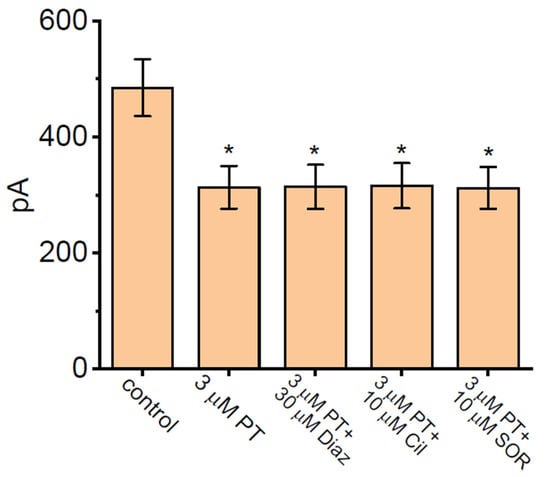
Figure 2.
Comparisons among the effects of PT-2385 (PT), PT-2385 plus diazoxide (Diaz), PT-2385 plus cilostazol (Cil), and PT-2385 plus sorafenib (SOR) on IK(DR) amplitude in GH3 cells (mean ± SEM; n = 7 for each bar). The whole experiments were performed in cells bathed in Ca2+-free Tyrode’s solution and the recording pipet was filled with K+-enriched internal solution. The IK(DR) amplitude activated by a 1-s depolarizing step from −50 to +50 mV was taken at the end-pulse of command potential. In this set of experiments on PT-2385 plus tested compounds (e.g., Diaz, Cil, and SOR), each tested compound was subsequently added to the bath in the continued presence of PT-2385 (3 µM). * Significantly different from control (p < 0.05).
3.4. Inhibitory Effect of PT-2385 on Mean Current versus Voltage (I-V) Relationship of IK(DR)
In another separate set of experiments, we voltage-clamped at –50 mV and then applied a series of command voltage pulses (1-s duration) from –60 to +50 mV in 10 mV increments to the examined GH3 cells. Under this voltage-clamp protocol, a family of IK(DR) was robustly elicited, and the currents were manifested by an outwardly directed rectifying property with a small relaxation in the time course of current inactivation [31,41,45,59,62]. Of note, after 1 min of cell exposure to 1 µM PT-2385, the IK(DR) magnitude became reduced, particularly at the potentials ranging between 0 and +50 mV (Figure 3A). Figure 3B respectively illustrates the mean I-V relationships of IK(DR) measured at the start (initial peak) and end-pulse (late or sustained) of each potential obtained in the control period (upper panel) or during exposure to 1 µM PT-2385 (lower panel). For example, at the level of +50 mV, PT-2385 at a concentration of 1 µM led to decreased peak amplitude from 803 ± 62 to 667 ± 46 pA (n = 8, p < 0.05), while, at the same level of voltage pulse, this compound at the same concentration reduced the late IK(DR) from 635 ± 61 to 381 ± 41 pA (n = 8, p < 0.05). After the agent was removed, the peak or late amplitude of IK(DR) was back to 786 ± 58 or 611 ± 57 pA (n = 7, p < 0.05). It is conceivable, therefore, that the potency of the PT-2385-mediated block of late or sustained IK(DR) activated by different step depolarizations (i.e., the potentials ranging between 0 and −50 mV) was higher than that for its blocking on the instantaneous peak component of the current.
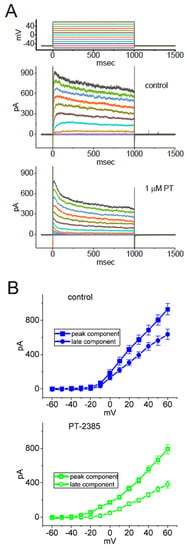
Figure 3.
Effect of PT-2385 on mean the current versus voltage (I-V) relationship of IK(DR) in GH3 cells. In these experiments, cell membrane potentials were held at −50 mV, and the depolarizing command pulse to a series of voltage steps (1-s duration) ranging between −60 and +50 mV in 10-mV increments (as indicated in the uppermost of (A)) was thereafter applied to evoke the currents. (A) Representative current traces in the control period (upper image) and during exposure to 1 µM PT-2385 (lower image). The current traces indicated in different colors with or without the presence of PT-2385 correspond to the potential ones in the uppermost part of (A). (B) Mean I-V relationships of IK(DR) taken before (upper image, blue-filled symbols) or after (lower image, green open symbols) 1 µM PT-2385. The experimental data points in (B) were collected at the start (square symbols) or end-pulse (circle symbols) of the 1-s depolarizing voltage command. Each point represents the mean ± SEM (n = 8).
3.5. The Steady-State Inactivation Curve of IK(DR) during Exposure to PT-2385
When cells were exposed to varying concentrations of PT-2385, the IK(DR) activated via membrane depolarization displayed an evident peak, followed by an exponential relaxation to a steady-state level. Hence, we also investigated the quasi-steady-state inactivation curve of IK(DR) achieved in the presence of PT-2385 by exploiting a two-step voltage protocol. In this set of current-recording experiments, we placed cells in Ca2+-free Tyrode’s solution, and then filled up the pipet with K+-enriched solution during the measurements. Once a whole-cell configuration was established, we delivered a two-pulse protocol to the examined cells in situations where varying PT-2385 concentrations were present. According to the least-squares minimization procedure, the best-fitting parameters (i.e., V1/2 and q) for the IK(DR)-inactivation curve by a test of goodness of fit were convergently obtained in the presence of 1 or 3 µM PT-2385. Consequently, we constructed the normalized amplitude of IK(DR) (i.e., I/Imax) against the conditioning command potentials (Figure 4A,B), and the continuous sigmoidal curve by adjusting the line to minimize the vertical deviations between the data points, and the line was approximately fitted with a single Boltzmann isotherm, as detailed in Materials and Methods. The values of V1/2 during exposure to 1 µM and 3 µM PT-2385 were calculated to be −34.7 ± 1.8 and −41.3 ± 1.8 mV (n = 8), respectively, while the q values (i.e., apparent gating charge) were 2.0 ± 0.3 and 2.1 ± 0.3 e (n = 8), respectively. These experimental results showed that during exposure to varying PT-2385 concentrations (i.e., 1 and 3 µM), the V1/2 value of the IK(DR)-inactivation curve in GH3 cells was modified, despite its ineffectiveness in changing the apparent gating charge of the current (i.e., the steepness of the inactivation curve).
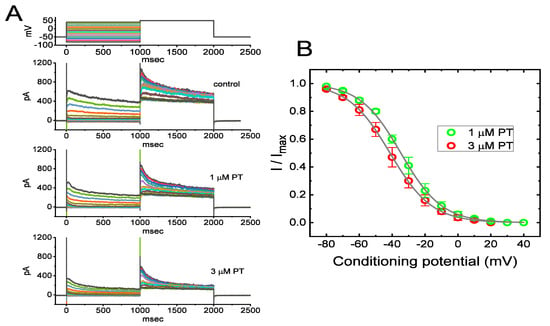
Figure 4.
Effect of PT-2385 on the quasi-steady-state inactivation curve of IK(DR) in GH3 cells. These experiments were undertaken using a 2-step voltage-clamp protocol (as indicated in the inset). (A) Representative IK(DR) traces obtained in the control (i.e., PT-2385 was not present, upper image) and in the presence of 1 µM PT-2385 (middle image) or 3 µM PT-2385 (lower image). The uppermost part shows the voltage-clamp protocol applied. (B) Steady-state inactivation curve of IK(DR) obtained during exposure to 1 µM PT-2385 (○) or 3 µM PT-2385 (○) (mean ± SEM; n = 8 for each point). The continuous lines overlaid on the data points were well-fitted by the Boltzmann equation detailed in Materials and Methods.
3.6. PT-2385 on the Recovery of IK(DR) Block Measured from GH3 Cells
We also investigated the recovery from IK(DR) block by PT-2385 by the use of another two-step voltage-clamp profile. The voltage-clamp protocol applied consists of an initial (i.e., the first conditioning) depolarizing step from −50 to +50 mV, sufficiently long to ensure the block to reach a steady-state level; subsequently, following different inter-pulse intervals, a second command pulse was applied at the same potential as the first conditioning one (Figure 5A). As the ratios (second current/first current, or relative amplitude) of the peak amplitude of IK(DR) activated by the first and the second conditioning pulse were derived for a measure of recovery from IK(DR) block in the presence of PT-2385, the value of the recovery time constant was constructed and hence plotted as a function of the inter-pulse interval (Figure 5B). The time course for the recovery of IK(DR) block in the presence of 1 or 3 µM PT-2385 was noted to be described by a single-exponential function. By minimizing the squares of the deviations, the time constant for current recovery in the presence of 1 µM PT-2385 was estimated to be 0.59 ± 0.04 s (n = 7), significantly smaller than the value of 0.83 ± 0.06 s (n = 7, p < 0.05) which was obtained during exposure to 3 µM PT-2385. The experimental observations thus tempted us to imply that the slowing of IK(DR)-current recovery caused by adding PT-2385 would result from its preferential block in the open or open-inactivated state of the KV channel.
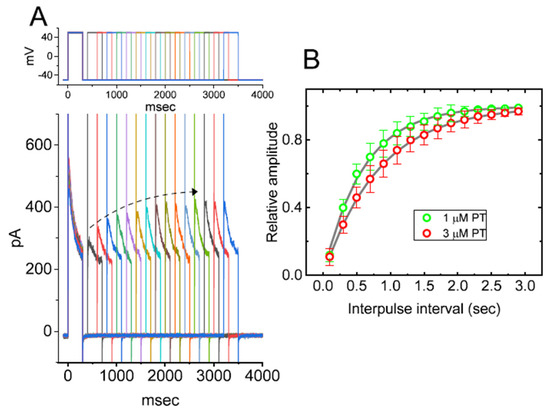
Figure 5.
Recovery from IK(DR) block caused by PT-2385. In these recording experiments, we kept cells to be bathed in Ca2+-free Tyrode’s solution, while the pipet was filled with K+-containing solution. The examined GH3-cell was depolarized from −50 to +50 mV with a duration of 300 msec, and the different interpulse durations were then delivered. (A) Representative IK(DR) traces in the presence of 1 µM or 3 µM PT-2385. The dashed curved arrow in (A) indicates an exponential rise as a function of the interpulse interval, while the uppermost part denotes the voltage-clamp protocol applied. (B) Time course of recovery from IK(DR) block taken in the presence of 1 µM PT-2385 (○) or 3 µM PT-2385 (○). The relative amplitude of IK(DR) was measured as a ratio of the first peak amplitude divided by the second one. The recovery time course (indicated in gray line) during exposure to 1 or 3 µM PT-2385 was noted to display an exponential rise, with a time constant of 0.59 or 0.83 s, respectively. Each point is the mean ± SEM (n = 7 for each point).
3.7. Effect of PT-2385 on the Hysteretic Behavior of IK(DR) Triggered by Isosceles-Triangular Ramp Voltage (ITRV) with Varying Durations
Previous studies have demonstrated the capability of IK(DR) strength to influence either varying patterns of bursting firing or action potential configurations in many types of electrically excitable cells [31,37,38,40,41,42,63,64,65]. Therefore, we continued to determine whether and how the presence of PT-2385 could adjust the IK(DR) strength activated in response to long-lasting ITRV with varying durations. In this set of experiments, in the absence or presence of PT-2385, we held the examined cell at −50 mV, and an upsloping (forward) limb from −80 to +60 mV followed by a downsloping (backward) limb back to −80 mV (i.e., upright ITRV) with varying durations (between 0.4 and 3.2 s) were thereafter applied (Figure 6A,B). Under this experimental condition, the voltage-dependent hysteresis of IK(DR) in response to upright ITRV with varying durations was observed. Of additional interest, during cell exposure to 1 or 3 µM PT-2385, the strength of current responding to both rising and falling limbs of upright ITRV was progressively decreased with increasing the PT-2385 concentrations (Figure 6A). For example, as the duration of isosceles-triangular ramp pulse applied was set at 3.2 s (i.e., the ramp speed of ±87.5 mV/s), the value of ∆area (i.e., the difference in area encircled by the curve in the forward and backward direction) for voltage-dependent hysteresis in the control period (i.e., PT-2385 was not present) was 3.18 ± 0.41 mV·nA (n = 8), while at the duration of 3.2 s (i.e., ramp speed at ±87.5 mV/s), the value of ∆area (indicated in shaded area) in the presence of 3 µM PT-2385 was significantly reduced to 1.18 ± 0.25 mV·nA (n = 8, p < 0.05) (Figure 6C). Findings from these data led us to propose that there was the occurrence of voltage-dependent hysteresis for IK(DR) activation in response to upright ITRV in GH3 cells [66], and that the hysteretic strength of the current was noticeably reduced with increasing PT-2385 concentration.
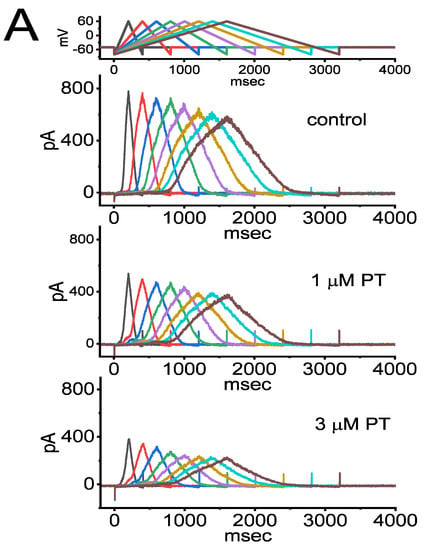
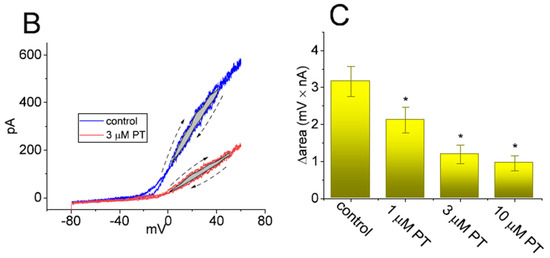
Figure 6.
Effect of PT-2385 on IK(DR) activated in response to upright isosceles-triangular ramp voltage (ITRV) with varying durations (from 0.4 to 3.2 s), utilized to mimic different depolarizing or repolarizing slopes of bursting patterns in electrically excitable cells. Cells were bathed in Ca2+-free Tyrode’s solution containing 1 µM TTX and 0.5 mM CdCl2, and the pipet was filled with K+-enriched solution. (A) Representative current traces activated by the uppermost voltage-clamp ITRV profile, which were obtained in the absence (upper) and presence of 1 µM PT-2385 (middle) or 3 µM PT-2385. Of note, there was a mild decline in peak IK(DR) activated by ITRV with increasing ramp-pulse durations. The uppermost part shows the voltage-clamp profile delivered. In (B), the effect of PT-2385 (3 µM) on voltage-dependent hysteresis (i.e., the relationship of forward or backward IK(DR) versus membrane voltage) activated in response to ITRV with a duration of 3.2 s (or with a ramp speed of ±87.5 mV/s) is illustrated. Current trace in blue color is the control, while that in red was taken after exposure to 3 µM PT-2385. Dashed arrows in (B) indicate the direction of IK(DR) over which time goes during the elicitation by ITRV. (C) Effect of PT-2385 (1, 3, and 10 µM) on the ∆area (i.e., area under the curve activated during the upsloping and downsloping end of ITRV) of voltage-dependent hysteresis elicited by ITRV with a ramp speed of ±87.5 mV/s (mean ± SEM; n = 7 for each bar). The shaded area indicates the curve activated during the upsloping and downsloping limb of the upright ITRV. Of note, there was an emergence of voltage-dependent hysteresis for IK(DR) activated by ITRV, and the presence of PT-2385 was concentration-dependently able to cause a progressive reduction in the ∆area of voltage hysteresis of the current. * Significantly different from control (i.e., PT-2385 was not present, and currents were evoked by ITRV with ramp speed of ±87.5 mV/s) (p < 0.05).
3.8. Effect of PT-2385 on the Activation Energy Required for IK(DR) Elicitation by ITRV
In another set of experiments, we ascertained whether PT-2385 could perturb any modifications in the free energy during IK(DR) elicitation caused by ITRV. As illustrated in Figure 7A, in the absence or presence of 3 µM PT-2385, the instantaneous (or transient) activation curve of the current elicited by the upsloping (forward) end of ITRV with a ramp speed of 87.5 mV/s was obtained. Thereafter, the Boltzmann isotherm (detailed in Materials and Methods) was performed with a test of goodness to fit to experimental data points in order to estimate the values of q and V1/2 for such instantaneous activation curves of ITRV-induced IK(DR). Based on the values of q and V1/2 during the upsloping end of upright ITRV, the free energy entailed in the gating of IK(DR) activation at 0 mV (∆G0) in the absence or presence of varying PT-2385 concentrations (i.e., ∆G0 = q × F × V1/2) was derived and is thereafter illustrated in Figure 7B. Of note, as the PT-2385 concentration was raised, the activation energy (∆G0 value) required for IK(DR) elicitation was progressively augmented during upright ITRV with a duration of 3.6 s. For example, as GH3 cells were exposed to 3 µM PT-2385, the ∆G0 value was significantly elevated to 2.98 ± 0.14 kJ/mol from a control value of 1.98 ± 0.07 kJ/mol (n = 7, p < 0.05).
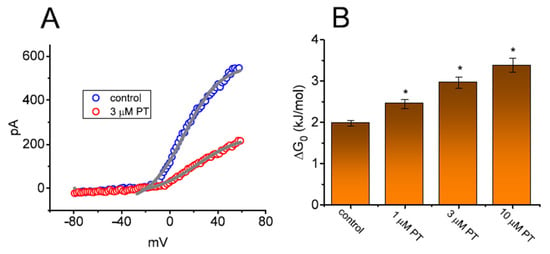
Figure 7.
Effect of PT-2385 on the change in free energy at 0 mV (∆G0) required for elicitation of IK(DR) by ramp-up depolarization. (A) Representative instantaneous current traces elicited by the upsloping limb of upright ITRV at a duration of 3.2 s (or with ramp speed of ±87.5 mV/s). The continuous gray lines achieved with or without PT-2385 addition were least-squares fitted to the Boltzmann equation. The values of V1/2 or q (apparent gating charge) activated by upright ITRV with a duration of 3.2 s in the absence (○) and presence (○) of 3 µM PT-2385 were estimated to be +12.3 mV or 1.7 e and +23.0 mV or 1.3 e, respectively. (B) The relationship of the free energy level at 0 mV (∆G0) versus the different PT-2385 concentrations (mean ± SEM; n = 7 for each bar). The estimation of DG0 is elaborated under Materials and Methods. Of note, increasing PT-2385 concentrations resulted in higher values of ∆G0 identified in GH3 cells. * Significantly different from control (p < 0.05).
3.9. Effect of PT-2385 on the Hysteresis Activated during Inverted ITRV
We continued to determine whether there is hysteresis of IK(DR) elicited by inverted ITRV at varying durations. As demonstrated in Figure 8A, in keeping with the above-stated results from upright ITRV, the hysteretic strength of the current by inverted ITRV in the absence and presence of PT-2385 was clearly observed in combination with a gradual decline in peak IK(DR) with decreasing ramp speed. As shown in Figure 8B, the relationship of the instantaneous current versus membrane potential was constructed during inverted ITRV with ramp speed of ±87.5 mV/s. Our experimental observations demonstrate the effectiveness of PT-2385 in diminishing the inverted ITRV-induced strength of voltage-dependent hysteresis; meanwhile, the hysteretic magnitude activated by inverted ITRV tends to be stronger than that by upright ITRV with the same ramp speed.
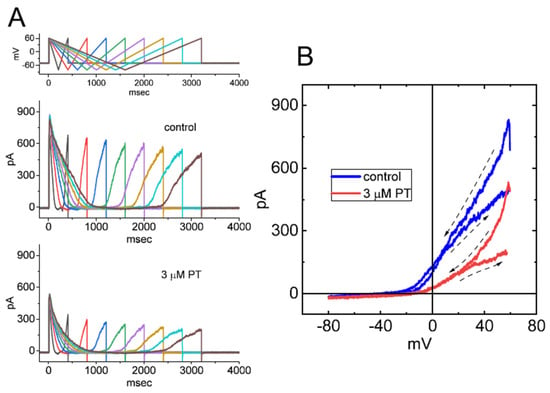
Figure 8.
Effect of PT-2385 on hysteresis of IK(DR) activated by inverted ITRV in GH3 cells. (A) Representative current traces obtained in the absence (upper image) and presence of 3 µM PT-2385 (lower image). Current traces were activated by inverted ITRV at varying durations (indicated in the uppermost part). (B) Representative hysteresis of IK(DR) elicited by inverted ITRV with a ramp speed of ±87.5 mV/s. Dashed arrows in (B) indicate the direction of IK(DR) over which time goes during the elicitation by ITRV. Of note, the hysteretic behavior of IK(DR) in response to inverted ITRV was still observed and was greater than that by upright ITRV, while cell exposure to 3 µM PT-2385 decreased the ∆area of voltage-dependent hysteresis evoked by inverted ITRV.
3.10. Ability of PT-2385 to Inhibit IK(DR) Amplitude in Human 13-06-MG Glioma Cells
PT-2385 has been recently reported to inhibit the proliferation of glioma cells [18,19]. In a final stage of experiments, we thus intended to explore whether the presence of PT-2385 was able to modulate ionic currents in other types of neoplastic cells (e.g., malignant glioma cells). As illustrated in Figure 9, in whole-cell current recordings, the magnitude of ramp pulse-induced IK(DR) in 13-06-MG glioma cells was robustly detected, as described previously [67]. After 1 min of cell exposure to PT-2385, the amplitude of IK(DR) was progressively decreased. For example, at the level of +80 mV, the addition of 3 µM PT-2385 significantly decreased the current amplitude from 1221 ± 228 to 732 ± 187 pA (n = 7, p < 0.05). After washout of the compound, the amplitude returned to 1217 ± 207 pA (n = 7, p < 0.05). Therefore, similar to the GH3 cells stated above, the IK(DR) existing in 13-06-MG cells was noted to be sensitive to inhibition by PT-2385. Apart from the inhibition of HIF-2α, PT-2385-induced changes in malignant behaviors of glioma [18,19] could, to some extent, be closely associated with its inhibitory action of IK(DR).
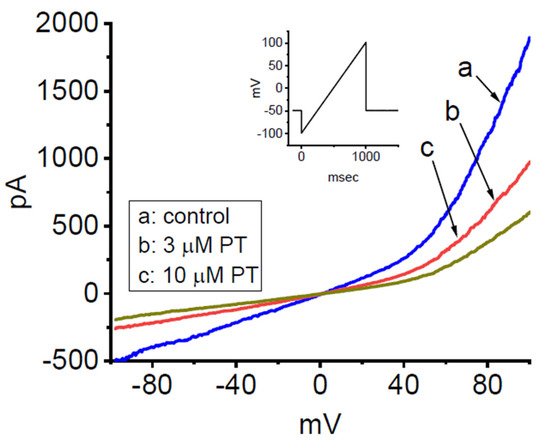
Figure 9.
Inhibitory effect of PT-2385 on IK(DR) recorded from human 13-06-MG glioma cells. In these experiments, we kept cells to be bathed in high-K+, Ca2+-free solution and filled up the recording pipet by using a K+-enriched solution. The examined cell was voltage-clamped at −50 mV, and an upsloping ramp pulse from −100 to +100 mV for a duration of 1 s was then applied to evoke IK(DR). Inset shows the voltage protocol applied. (a) control (i.e., PT-2385 was not present); (b) 3 µM PT-2385; and (c) 10 µM PT-2385.
4. Discussion
In the present study, cell exposure to PT-2385 was able to decrease the amplitude of IK(DR) and raise the inactivation time course of the current. A concentration-dependent inhibition of peak or late IK(DR), with respectively effective IC50 values of 8.1 or 2.2 µM, was observed as GH3 cells were exposed to different PT-2385 concentrations. The value of the dissociation constant (KD) derived from quantitative estimate of the inactivation time course of IK(DR) was yielded to be 2.8 µM. Moreover, cell exposure to this compound not only decreased the magnitude of voltage-dependent hysteresis for IK(DR) in reply to upright or inverted isosceles-triangular ramp voltage (ITRV), but also increased free energy involved in the gating IK(DR) elicitation. It could also decrease the amplitude of IK(DR) in human 13-06-MG glioma cells. Collectively, the PT-2385-mediated effectiveness in the modifications of ionic currents described herein tends to be independent of an interaction with HIF-2α and it could thus be a confounding factor affecting neuronal or endocrine function.
Perhaps more noticeable with respect to the issue of the magnitude in the PT-2385-mediated block of IK(DR) observed in GH3 cells is that the rising phase of the current activated in response to rapid membrane depolarization remained little-altered in its presence; however, the addition of this compound had the propensity to accelerate IK(DR) inactivation in a time-, state-, and concentration-dependent fashion. This could thus be interpreted to mean that PT-2385 is able to reach the blocking site in situations where the KV channel favorably resides in the open or open-inactivated state. Consequently, this property is reasonably explained by the first-order binding scheme (i.e., ), as demonstrated above [30,59]. Pertinent to such reaction scheme is that open-blocked KV channels are not closed unless the PT-2385 dissociates from the binding site. Meanwhile, the time-dependent block produced by PT-2385 reflects that it preferentially binds to and blocks an open state (or conformation) of the channel with a KD value of 2.9 µM observed in GH3 cells, a value which is close to the IC50 value (i.e., 2.2 µM) entailed for its inhibitory effect on late IK(DR), not on peak IK(DR).
The magnitude of IK(DR) reduced by PT-2385, concomitantly with a considerable rise in current inactivation in response to long sustained depolarization, may be the cause of its perturbing effects on membrane excitability in varying types of electrically excitable cells (e.g., neurons and neuroendocrine or endocrine cells). Because the recovery from current inactivation in the presence of PT-2385 observed in this study also became slowed, a duration greater than 3 s was required for current magnitude to recover completely. The steady-state inactivation curve of IK(DR) was more hyperpolarized as the PT-2385 concentration was increased. As a consequence, a PT-2385-perturbed block of IK(DR) will even become pronounced when a train of action potentials occurs, since the IK(DR) magnitude, especially at the occurrence of transient resurgent K+ currents, is decreased as a function of firing frequency [32,40,41,42].
Recent pharmacokinetic studies have shown that after being administered orally at twice-per-day dose of 800 mg, the plasma level of PT-2385 in patients with clear cell renal cell carcinoma was reported to reach around 3000–5000 ng/mL (or 7.8–13.0 µM) [20]. Therefore, it is possible that the used concentration of PT-2385 in this study tends to be achievable in the plasma of treated patients. Any changes in the amplitude, gating, and hysteresis of IK(DR) caused by PT-2385 depend not simply on the PT-2385 concentration, but also on the preexisting resting potential and varying bursting patterns of excitable cells. From this perspective, the observed effects by PT-2385 in this study are likely to arise to the extent of clinically achievable concentrations.
There seems to be a divergence in the IC50 value between the PT-2385-induced block of late IK(DR) (around 2.2 µM) and its effect on HIF-2α inhibition (around 27 nM) [22]. The plausible interpretation for this difference is unknown at present; however, it could be due to the varying experimental maneuvers used in the study. In terms of investigations on HIF-2α inhibition, surface-membrane components in host cells could have mostly been lysed and removed; consequently, PT-2385 could readily reach HIF-2α inside the cells [13,14,22]. However, in the present observations, intact cells were used to investigate different types of membrane ionic currents in GH3 cells or 13-06-MG glioma cells.
Under hypoxic conditions along with possible changes in HIF expression, the activity of ATP-dependent K+ (KATP) channels in GH3 cells or 13-06-MG glioma cells could be elevated during exposure to PT-2385 [47,48,49,50]. The presence of PT-2385 was previously demonstrated to counteract the adverse effect of sorafenib (SOR), known to suppress the activity of tyrosine kinases, in hepatocellular carcinoma [16]. However, in our whole-cell current recording, the intracellular solution contained 3 mM ATP, a value known to adequately suppress KATP-channel activity [60,68]. However, the PT-2385-mediated inhibition of IK(DR) observed in GH3 cells was little -affected by the subsequent application of either 30 µM diazoxide (Diaz) or 10 µM cilostazol (Cil). Diaz or Cil were respectively reported to be stimulators of KATP channels or large-conductance Ca2+-activated K+ (BKCa) channels [34,60,61]. Moreover, in the continued presence of PT-2385, the subsequent addition of sorafenib (SOR), an inhibitor of tyrosine kinase [62], failed to modify PT-2385-induced block of IK(DR) in GH3 cells. As such, a PT-induced reduction in whole-cell IK(DR) strength depicted in recent study is unlikely to be linked either to inhibition of KATP- or BKCa-channel activity, or to stimulation of tyrosine-kinase activity, although these channels or enzymes are functionally active in pituitary cells [60,61,62].
It needs to be emphasized that as it is administered, PT-2385 needs to enter cytosol or even the nucleus before being able interact with HIF-2α or the HIF-α/HIF-β complex [12,13,14,21]. In other words, despite being small in size, the molecules must pass through cell-surface membrane before entering the cell interior and then reaching the target. In this regard, it is reasonable to assume that the perturbations to plasmalemmal ionic currents caused by PT-2385 would precede its subsequent actions through the inhibition of HIF-2α. The extent of PT-2385-mediated modifications to ionic currents (e.g., IK(DR)) is closely linked to the inhibition of HIF-2α, followed by changes in proliferation of different types of neoplastic cells [12,13,14,16,17,26].
The perturbations by PT-2385 of membrane ionic currents shown here tend to be acute in onset. Hence, the actions could be unlinked to its interaction with the activity of HIF-2α, as stated recently [9,12,14,25]. Both the steady-state inactivation curve of IK(DR) and recovery from current inactivation were demonstrated in the presence of PT-2385. The actions of this compound on the magnitude or kinetic gating of IK(DR) are thought to occur either through its preferential binding to the open state of the KV channel or through an interaction with the channel’s open conformation.
The phenomenon of the voltage-dependent hysteresis of ionic currents has been proposed to play roles in affecting the electrical behavior of electrically excitable cells. Consistent with previous observations on other types of ionic currents [45,52,57,66], the IK(DR) present in GH3 cells underwent hysteresis in which there is likely to be a mode shift, because the voltage sensitivity of the gating charge movement is thought to depend on the previous state of the channel [57,66]. Moreover, we further evaluated the possible perturbation by PT-2385 of the instantaneous and non-equilibrium property inherent in IK(DR) activated by upright or inverted ITRV. The results found PT-2385′s effectiveness in decreasing the ∆area of hysteresis for IK(DR) elicited by ITRV. Moreover, the presence of PT-2385 could increase the ∆G0 value for IK(DR) activation in response to an instantaneous upsloping ramp pulse. In this regard, any modifications of IK(DR) caused by PT-2385 depend not only on the PT-2385 concentration used but also various factors that include the pre-existing resting potential or varying firing patterns of action potentials.
It is important to mention that a possible link between HIF and regulation of KV-channel activity has been demonstrated [23,47,48,49,50]. Another study [69] showed the ability of HIF-1 to regulate KV1.2 channels in rat PC12 cells. It is possible that HIF-2α regulates KV-channel activity directly. Whether PT-2385 exercises an inhibitory effect on IK(DR) in GH3 or 13-05-MG cells where HIF-2α is knocked down remains to be further investigated.
All in all, in this study, we hitherto revealed the unidentified action of PT-2385 through which it could modify the amplitude, gating, and hysteretic behavior of IK(DR). The modifications to the magnitude, gating, and voltage-dependent hysteresis of IK(DR) caused by the presence of PT-2385 are not simply a bystander effect, and may potentially converge to act on the functional activities of neurons and endocrine or neuroendocrine cells if similar in vivo observations occur. To what extent such direct actions on IK(DR) contribute to the rapid impairment in hypoxic ventilatory drive [62] remains to be further resolved.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/membranes11080636/s1, The preparation of GH3 or 13-06-MG is reported in the Supplementary Materials. References [51,52] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, H.-T.H., Y.-C.L. and S.-N.W.; methodology, S.-N.W.; software, S.-N.W.; validation, G.-L.L., Y.-C.L. and S.-N.W.; formal analysis, S.-N.W.; investigation, H.-T.H., G.-L.L., Y.-C.L. and S.-N.W.; resources, H.-T.H., Y.-C.L. and S.-N.W.; data curation, S.-N.W.; writing—review and editing, Y.-C.L. and S.-N.W.; visualization, Y.-C.L. and S.-N.W.; supervision, Y.-C.L. and S.-N.W.; project administration, Y.-C.L. and S.-N.W.; funding acquisition, H.-T.H., Y.-C.L. and S.-N.W. All authors have read and agreed to the published version of the manuscript.
Funding
This study was in part supported by a grant from Ministry of Science and Technology (MOST-108-2314-B-006-094), Taiwan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are indebted to Shin-Yen Chao, Tzu-Hsien Chuang, Zi-Han Gao, and Sih-Wei Lee for their assistance in the present study.
Conflicts of Interest
The authors declare no conflict of interests that are directly relevant to this study. The content and writing of the paper are solely the responsibility of the authors.
Abbreviations
| Cil | cilostazol |
| Diaz | Diazoxide |
| ∆G0 | free energy involved in current activation at 0 mV |
| HIF | hypoxia-inducible factor |
| I-V | current versus voltage |
| IC50 | the concentration required for 50% inhibition |
| IK(DR) | delayed-rectifier K+ current |
| ITRV | isosceles-triangular ramp voltage |
| KATP | channel, ATP-sensitive K+ channel |
| KD | dissociation constant |
| KV | channel, voltage-gated K+ channel |
| SEM | standard error of mean |
| SOR | Sorafenib |
| TEA | tetraethylammonium chloride |
| τinact(S) | time constant in the slow component of current inactivation |
| TTX | tetrodotoxin |
References
- Vidal, S.; Horvath, E.; Kovacs, K.; Kuroki, T.; Lloyd, R.V.; Scheithauer, B.W. Expression of hypoxia-inducible factor-1alpha (HIF-1alpha) in pituitary tumours. Histol. Histopathol. 2003, 18. [Google Scholar]
- Kim, K.; Yoshida, D.; Teramoto, A. Expression of Hypoxia-Inducible Factor 1α and Vascular Endothelial Growth Factor in Pituitary Adenomas. Endocr. Pathol. 2005, 16, 115–122. [Google Scholar] [CrossRef]
- Yoshida, D.; Kim, K.; Noha, M.; Teramoto, A. Anti-Apoptotic Action by Hypoxia Inducible Factor 1-Alpha in Human Pituitary Adenoma Cell Line, HP-75 in Hypoxic Condition. J. Neuro Oncol. 2006, 78, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, D.; Kim, K.; Yamazaki, M.; Teramoto, A. Expression of Hypoxia-Inducible Factor 1α and Cathepsin D in Pituitary Adenomas. Endocr. Pathol. 2005, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, D.; Koketshu, K.; Nomura, R.; Teramoto, A. The CXCR4 antagonist AMD3100 suppresses hypoxia-mediated growth hormone production in GH3 rat pituitary adenoma cells. J. Neuro Oncol. 2010, 100, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Yoshida, D.; Teramoto, A. Stromal cell-derived factor-1 expression in pituitary adenoma tissues and upregulation in hypoxia. J. Neuro Oncol. 2009, 94, 173–181. [Google Scholar] [CrossRef]
- Lei, T.; Xiao, Z.; Liu, Q.; Zhao, B.; Wu, J. Hypoxia induces hemorrhagic transformation in pituitary adenomas via the HIF-1? signaling pathway. Oncol. Rep. 2011, 26, 1457–1464. [Google Scholar] [CrossRef]
- Zhang, C.; Qiang, Q.; Jiang, Y.; Hu, L.; Ding, X.; Lu, Y.; Hu, G. Effects of hypoxia inducible factor-1α on apoptotic inhibition and glucocorticoid receptor downregulation by dexamethasone in AtT-20 cells. BMC Endocr. Disord. 2015, 15, 24. [Google Scholar] [CrossRef]
- Kinali, B.; Senoglu, M.; Karadag, F.K.; Karadag, A.; Middlebrooks, E.H.; Oksuz, P.; Sandal, E.; Turk, C.; Diniz, G. Hypoxia-Inducible Factor 1α and AT-Rich Interactive Domain-Containing Protein 1A Expression in Pituitary Adenomas: Association with Pathological, Clinical, and Radiological Features. World Neurosurg. 2019, 121, e716–e722. [Google Scholar] [CrossRef]
- Lucia, K.; Wu, Y.; Garcia, J.M.; Barlier, A.; Buchfelder, M.; Saeger, W.; Renner, U.; Stalla, G.K.; Theodoropoulou, M. Hypoxia and the hypoxia inducible factor 1α activate protein kinase A by repressing RII beta subunit transcription. Oncogene 2020, 39, 3367–3380. [Google Scholar] [CrossRef]
- Tella, S.H.; Taïeb, D.; Pacak, K. HIF-2alpha: Achilles’ heel of pseudohypoxic subtype paraganglioma and other related conditions. Eur. J. Cancer 2017, 86, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hill, H.; Christie, A.; Kim, M.S.; Holloman, E.; Pavia-Jimenez, A.; Homayoun, F.; Ma, Y.; Patel, N.; Yell, P.; et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nat. Cell Biol. 2016, 539, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Wallace, E.M.; Rizzi, J.P.; Han, G.; Wehn, P.M.; Cao, Z.; Du, X.; Cheng, T.; Czerwinski, R.M.; Dixon, D.D.; Goggin, B.S.; et al. A Small-Molecule Antagonist of HIF2α Is Efficacious in Preclinical Models of Renal Cell Carcinoma. Cancer Res. 2016, 76, 5491–5500. [Google Scholar] [CrossRef]
- Martínez-Sáez, O.; Borau, P.G.; Alonso-Gordoa, T.; Molina-Cerrillo, J.; Grande, E. Targeting HIF-2 α in clear cell renal cell carcinoma: A promising therapeutic strategy. Crit. Rev. Oncol. 2017, 111, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yagai, T.; Luo, Y.; Liang, X.; Chen, T.; Wang, Q.; Sun, D.; Zhao, J.; Ramakrishnan, S.K.; Sun, L.; et al. Activation of intestinal hypoxia-inducible factor 2α during obesity contributes to hepatic steatosis. Nat. Med. 2017, 23, 1298–1308. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, L.; Chen, J.; Sun, Y.; Lin, H.; Jin, R.-A.; Tang, M.; Liang, X.; Cai, X. Increasing AR by HIF-2α inhibitor (PT-2385) overcomes the side-effects of sorafenib by suppressing hepatocellular carcinoma invasion via alteration of pSTAT3, pAKT and pERK signals. Cell Death Dis. 2017, 8, e3095. [Google Scholar] [CrossRef]
- Yoshino, H.; Nohata, N.; Miyamoto, K.; Yonemori, M.; Sakaguchi, T.; Sugita, S.; Itesako, T.; Kofuji, S.; Nakagawa, M.; Dahiya, R.; et al. PHGDH as a Key Enzyme for Serine Biosynthesis in HIF2α-Targeting Therapy for Renal Cell Carcinoma. Cancer Res. 2017, 77, 6321–6329. [Google Scholar] [CrossRef]
- Renfrow, J.J.; Soike, M.H.; Debinski, W.; Ramkissoon, S.H.; Mott, R.T.; Frenkel, M.B.; Sarkaria, J.N.; Lesser, G.J.; E Strowd, R. Hypoxia-inducible factor 2α: A novel target in gliomas. Futur. Med. Chem. 2018, 10, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Renfrow, J.J.; Soike, M.H.; West, J.L.; Ramkissoon, S.H.; Metheny-Barlow, L.; Mott, R.T.; Kittel, C.A., Jr.; D’Agostino, R.B.; Tatter, S.B.; Laxton, A.W.; et al. Attenuating hypoxia driven malignant behavior in glioblastoma with a novel hypoxia-inducible factor 2 alpha inhibitor. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.D.; Infante, J.R.; Lam, E.T.; Figlin, R.A.; Rini, B.I.; Brugarolas, J.; Zojwalla, N.J.; Lowe, A.M.; Wang, K.; Wallace, E.M.; et al. Phase I Dose-Escalation Trial of PT2385, a First-in-Class Hypoxia-Inducible Factor-2α Antagonist in Patients With Previously Treated Advanced Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2018, 36, 867–874. [Google Scholar] [CrossRef]
- Courtney, K.D.; Ma, Y.; de Leon, A.D.; Christie, A.; Xie, Z.; Woolford, L.; Singla, N.; Joyce, A.; Hill, H.; Madhuranthakam, A.J.; et al. HIF-2 Complex Dissociation, Target Inhibition, and Acquired Resistance with PT2385, a First-in-Class HIF-2 Inhibitor, in Patients with Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. 2020, 26, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Wehn, P.M.; Rizzi, J.P.; Dixon, D.D.; Grina, J.A.; Schlachter, S.T.; Wang, B.; Xu, R.; Yang, H.; Du, X.; Han, G.; et al. Design and Activity of Specific Hypoxia-Inducible Factor-2α (HIF-2α) Inhibitors for the Treatment of Clear Cell Renal Cell Carcinoma: Discovery of Clinical Candidate (S)-3-((2,2-Difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1H-inden-4-yl)oxy)-5-fluorobenzonitrile (PT2385). J. Med. Chem. 2018, 61, 9691–9721. [Google Scholar] [CrossRef]
- Qian, C.; Dai, Y.; Xu, X.; Jiang, Y. HIF-1α Regulates Proliferation and Invasion of Oral Cancer Cells through Kv3.4 Channel. Ann. Clin. Lab Sci. 2019, 49, 457–467. [Google Scholar] [PubMed]
- Schwartz, A.J.; Das, N.K.; Ramakrishnan, S.K.; Jain, C.; Jurkovic, M.T.; Wu, J.; Nemeth, E.; Lakhal-Littleton, S.; Colacino, J.A.; Shah, Y.M. Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J. Clin. Investig. 2018, 129, 336–348. [Google Scholar] [CrossRef]
- Hsu, T.-S.; Lin, Y.-L.; Wang, Y.-A.; Mo, S.-T.; Chi, P.-Y.; Lai, A.C.-Y.; Pan, H.-Y.; Chang, Y.-J.; Lai, M.-Z. HIF-2α is indispensable for regulatory T cell function. Nat. Commun. 2020, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Persson, C.U.; von Stedingk, K.; Fredlund, E.; Bexell, D.; Påhlman, S.; Wigerup, C.; Mohlin, S. ARNT-dependent HIF-2 transcriptional activity is not sufficient to regulate downstream target genes in neuroblastoma. Exp. Cell Res. 2020, 388, 111845. [Google Scholar] [CrossRef]
- Chang, W.-T.; Lo, Y.-C.; Gao, Z.-H.; Wu, S.-N. Evidence for the Capability of Roxadustat (FG-4592), an Oral HIF Prolyl-Hydroxylase Inhibitor, to Perturb Membrane Ionic Currents: An Unidentified yet Important Action. Int. J. Mol. Sci. 2019, 20, 6027. [Google Scholar] [CrossRef]
- Wulfsen, I.; Hauber, H.P.; Schiemann, D.; Bauer, C.K.; Schwarz, J.R. Expression of mRNA for voltage-dependent and inward-rectifying K channels in GH3/B6 cells and rat pituitary. J. Neuroendocr. 2001, 12, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-Y.; Tsai, Y.-C.; Wu, S.-N.; Liu, Y.-C. Tramadol-induced blockade of delayed rectifier potassium current in NG108-15 neuronal cells. Eur. J. Pain 2006, 10, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Lin, M.-W.; Lin, A.-A.; Peng, H.; Wu, S.-N. Evidence for state-dependent block of DPI 201-106, a synthetic inhibitor of Na+ channel inactivation, on delayed-rectifier K+ current in pituitary tumor (GH3) cells. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2008, 59, 409–423. [Google Scholar]
- Huang, C.-C.; Tsai, J.J.; Wu, S.N. Experimental and simulation studies on the mechanisms of levetiracetam-mediated inhibition of delayed-rectifier potassium current (KV3.1): Contribution to the firing of action potentials. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60, 37–47. [Google Scholar]
- Kaczmarek, L.K.; Zhang, Y. Kv3 Channels: Enablers of Rapid Firing, Neurotransmitter Release, and Neuronal Endurance. Physiol. Rev. 2017, 97, 1431–1468. [Google Scholar] [CrossRef]
- Fletcher, P.A.; Sherman, A.; Stojilkovic, S.S. Common and diverse elements of ion channels and receptors underlying electrical activity in endocrine pituitary cells. Mol. Cell. Endocrinol. 2018, 463, 23–36. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Yang, C.-J.; Lee, Y.-C.; Chen, P.-C.; Liu, Y.-C.; Wu, S.-N. The comprehensive electrophysiological study of curcuminoids on delayed-rectifier K + currents in insulin-secreting cells. Eur. J. Pharmacol. 2018, 819, 233–241. [Google Scholar] [CrossRef]
- Hernández-Pineda, R.; Chow, A.; Amarillo, Y.; Moreno, H.; Saganich, M.; De Miera, E.C.V.-S.; Hernández-Cruz, A.; Rudy, B. Kv3.1–Kv3.2 Channels Underlie a High-Voltage–Activating Component of the Delayed Rectifier K+ Current in Projecting Neurons From the Globus Pallidus. J. Neurophysiol. 1999, 82, 1512–1528. [Google Scholar] [CrossRef]
- Schultz, J.-H.; Volk, T.; Ehmke, H. Heterogeneity of Kv2.1 mRNA expression and delayed rectifier current in single isolated myocytes from rat left ventricle. Circ. Res. 2001, 88, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Baranauskas, G. Ionic Channel Function in Action Potential Generation: Current Perspective. Mol. Neurobiol. 2007, 35, 129–150. [Google Scholar] [CrossRef]
- Johnston, J.; Griffin, S.J.; Baker, C.; Skrzypiec, A.; Chernova, T.; Forsythe, I. Initial segment Kv2.2 channels mediate a slow delayed rectifier and maintain high frequency action potential firing in medial nucleus of the trapezoid body neurons. J. Physiol. 2008, 586, 3493–3509. [Google Scholar] [CrossRef] [PubMed]
- Bocksteins, E.; Van De Vijver, G.; Van Bogaert, P.-P.; Snyders, D.J. Kv3 channels contribute to the delayed rectifier current in small cultured mouse dorsal root ganglion neurons. Am. J. Physiol. Physiol. 2012, 303, C406–C415. [Google Scholar] [CrossRef]
- Liu, P.W.; Bean, B.P. Kv2 Channel Regulation of Action Potential Repolarization and Firing Patterns in Superior Cervical Ganglion Neurons and Hippocampal CA1 Pyramidal Neurons. J. Neurosci. 2014, 34, 4991–5002. [Google Scholar] [CrossRef] [PubMed]
- Labro, A.J.; Priest, M.F.; Lacroix, J.J.; Snyders, D.J.; Bezanilla, F. Kv3.1 uses a timely resurgent K+ current to secure action potential repolarization. Nat. Commun. 2015, 6, 10173. [Google Scholar] [CrossRef]
- Brown, M.R.; El-Hassar, L.; Zhang, Y.; Alvaro, G.; Large, C.H.; Kaczmarek, L.K. Physiological modulators of Kv3.1 channels adjust firing patterns of auditory brain stem neurons. J. Neurophysiol. 2016, 116, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Chao, R.-Y.; Cheng, C.-H.; Wu, S.-N.; Chen, P.-C. Defective trafficking of Kv2.1 channels in MPTP-induced nigrostriatal degeneration. J. Neurochem. 2017, 144, 483–497. [Google Scholar] [CrossRef]
- Di Lucente, J.; Nguyen, H.M.; Wulff, H.; Jin, L.-W.; Maezawa, I. The voltage-gated potassium channel Kv1.3 is required for microglial pro-inflammatory activation in vivo. Glia 2018, 66, 1881–1895. [Google Scholar] [CrossRef]
- Lu, T.-L.; Lu, T.-J.; Wu, S.-N. Inhibitory effective perturbations of cilobradine (dk-ah269), a blocker of hcn channels, on the amplitude and gating of both hyperpolarization-activated cation and delayed-rectifier potassium currents. Int. J. Mol. Sci. 2020, 21, 2416. [Google Scholar] [CrossRef]
- Cheng, X.; Prange-Barczynska, M.; Fielding, J.W.; Zhang, M.; Burrell, A.L.; Lima, J.D.; Eckardt, L.; Argles, I.L.; Pugh, C.W.; Buckler, K.J.; et al. Marked and rapid effects of pharmacological HIF-2α antagonism on hypoxic ventilatory control. J. Clin. Investig. 2020, 130, 2237–2251. [Google Scholar] [CrossRef]
- Raeis, V.; Philip-Couderc, P.; Roatti, A.; Habre, W.; Sierra, J.; Kalangos, A.; Beghetti, M.; Baertschi, A.J. Central venous hypoxemia is a determinant of human atrial atp-sensitive potassium channel expression: Evidence for a novel hypoxia-inducible factor 1alpha-forkhead box class o signaling pathway. Hypertension 2010, 55, 1186–1192. [Google Scholar] [CrossRef][Green Version]
- Zhang, C.C.; Sadek, H.A. Hypoxia and Metabolic Properties of Hematopoietic Stem Cells. Antioxid. Redox Signal. 2014, 20, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, W.; Park, B.M.; Lee, G.-J.; Kim, S.H. Hypoxia augments nahs-induced anp secretion via katp channel, hif-1α and ppar-γ pathway. Peptides 2019, 121, 170123. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, W.; Chen, W.; Wang, H.; Zhang, Y.; Yu, T. Mechanism of the hypoxia inducible factor 1/hypoxic response element pathway in rat myocardial ischemia/diazoxide post-conditioning. Mol. Med. Rep. 2020, 21, 1527–1536. [Google Scholar] [CrossRef]
- Huang, M.-H.; Liu, P.-Y.; Wu, S.-N. Characterization of Perturbing Actions by Verteporfin, a Benzoporphyrin Photosensitizer, on Membrane Ionic Currents. Front. Chem. 2019, 7, 566. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Lin, C.-L.; Fang, W.-Y.; Lőrinczi, B.; Szatmári, I.; Chang, W.-H.; Fülöp, F.; Wu, S.-N. Effective Activation by Kynurenic Acid and Its Aminoalkylated Derivatives on M-Type K+ Current. Int. J. Mol. Sci. 2021, 22, 1300. [Google Scholar] [CrossRef]
- Wu, S.-N.; Hsu, M.-C.; Liao, Y.-K.; Wu, F.-T.; Jong, Y.-J.; Lo, Y.-C. Evidence for inhibitory effects of flupirtine, a centrally acting analgesic, on delayed rectifier k(+) currents in motor neuron-like cells. Evid. Based Complement Altern. Med. 2012, 2012, 148403. [Google Scholar] [CrossRef] [PubMed]
- Yifrach, O.; MacKinnon, R. Energetics of Pore Opening in a Voltage-Gated K+ Channel. Cell 2002, 111, 231–239. [Google Scholar] [CrossRef]
- Wu, S.-N.; Yeh, C.-C.; Huang, H.-C.; Yang, W.-H. Cholesterol depletion with (2-hydroxypropyl)- beta-cyclodextrin modifies the gating of membrane electroporation-induced inward current in pituitary tumor gh3 cells: Experimental and analytical studies. Cell Physiol. Biochem. 2011, 28, 959–968. [Google Scholar] [CrossRef]
- Hsu, H.-T.; Lo, Y.-C.; Huang, Y.-M.; Tseng, Y.-T.; Wu, S.-N. Important modifications by sugammadex, a modified γ-cyclodextrin, of ion currents in differentiated NSC-34 neuronal cells. BMC Neurosci. 2017, 18, 6. [Google Scholar] [CrossRef]
- Villalba-Galea, C.A.; Chiem, A.T. Hysteretic Behavior in Voltage-Gated Channels. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Vivaudou, M. eeFit: A Microsoft Excel-embedded program for interactive analysis and fitting of experimental dose–response data. Biotech. 2019, 66, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-W.; Wang, Y.-J.; Liu, S.-I.; Lin, A.-A.; Lo, Y.-C.; Wu, S.-N. Characterization of aconitine-induced block of delayed rectifier K+ current in differentiated NG108-15 neuronal cells. Neuropharmacology 2008, 54, 912–923. [Google Scholar] [CrossRef]
- Wu, S.-N.; Li, H.-F.; Chiang, H.-T. Characterization of ATP-sensitive potassium channels functionally expressed in pituitary GH3 cells. J. Membr. Biol. 2000, 178, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-N.; Liu, S.-I.; Huang, M.-H. Cilostazol, an inhibitor of type 3 phosphodiesterase, stimulates large-conductance, calcium-activated potassium channels in pituitary gh3 cells and pheochromocytoma pc12 cells. Endocrinology 2004, 145, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Liu, P.-Y.; Lee, K.; Feng, Y.-H.; Wu, S.-N. Differential Inhibitory Actions of Multitargeted Tyrosine Kinase Inhibitors on Different Ionic Current Types in Cardiomyocytes. Int. J. Mol. Sci. 2020, 21, 1672. [Google Scholar] [CrossRef] [PubMed]
- Delmar, M.; Ibarra, J.; Davidenko, J.; Lorente, P.; Jalife, J. Dynamics of the background outward current of single guinea pig ventricular myocytes. Ionic mechanisms of hysteresis in cardiac cells. Circ. Res. 1991, 69, 1316–1326. [Google Scholar] [CrossRef]
- Kusters, J.M.A.M.; Cortes, J.M.; Van Meerwijk, W.P.M.; Ypey, D.L.; Theuvenet, A.P.R.; Gielen, C.C.A.M. Hysteresis and Bistability in a Realistic Cell Model for Calcium Oscillations and Action Potential Firing. Phys. Rev. Lett. 2007, 98, 098107. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-N.; Chen, B.-S.; Lin, M.-W.; Liu, Y.-C. Contribution of slowly inactivating potassium current to delayed firing of action potentials in NG108-15 neuronal cells: Experimental and theoretical studies. J. Theor. Biol. 2008, 252, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Villalba-Galea, C.A. Hysteresis in voltage-gated channels. Channels 2016, 11, 140–155. [Google Scholar] [CrossRef]
- Huang, M.H.; Huang, Y.-M.; Wu, S.-N. The inhibition by oxaliplatin, a platinum-based anti-neoplastic agent, of the activity of intermediate-conductance ca²⁺-activated k⁺ channels in human glioma cells. Cell Physiol. Biochem. 2015, 37, 1390–1406. [Google Scholar] [CrossRef]
- Wu, S.-N.; Wu, A.Z.; Sung, R.J. Identification of two types of ATP-sensitive K+ channels in rat ventricular myocytes. Life Sci. 2007, 80, 378–387. [Google Scholar] [CrossRef]
- Dong, Q.; Zhao, N.; Xia, C.-K.; Du, L.-L.; Fu, X.-X.; Du, Y.-M. Hypoxia induces voltage-gated K+ (Kv) channel expression in pulmonary arterial smooth muscle cells through hypoxia-inducible factor-1 (HIF-1). Bosn. J. Basic Med. Sci. 2012, 12, 158–163. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).