Effects of High Salinity on Alginate Fouling during Ultrafiltration of High-Salinity Organic Synthetic Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Membrane Filtration Experiments
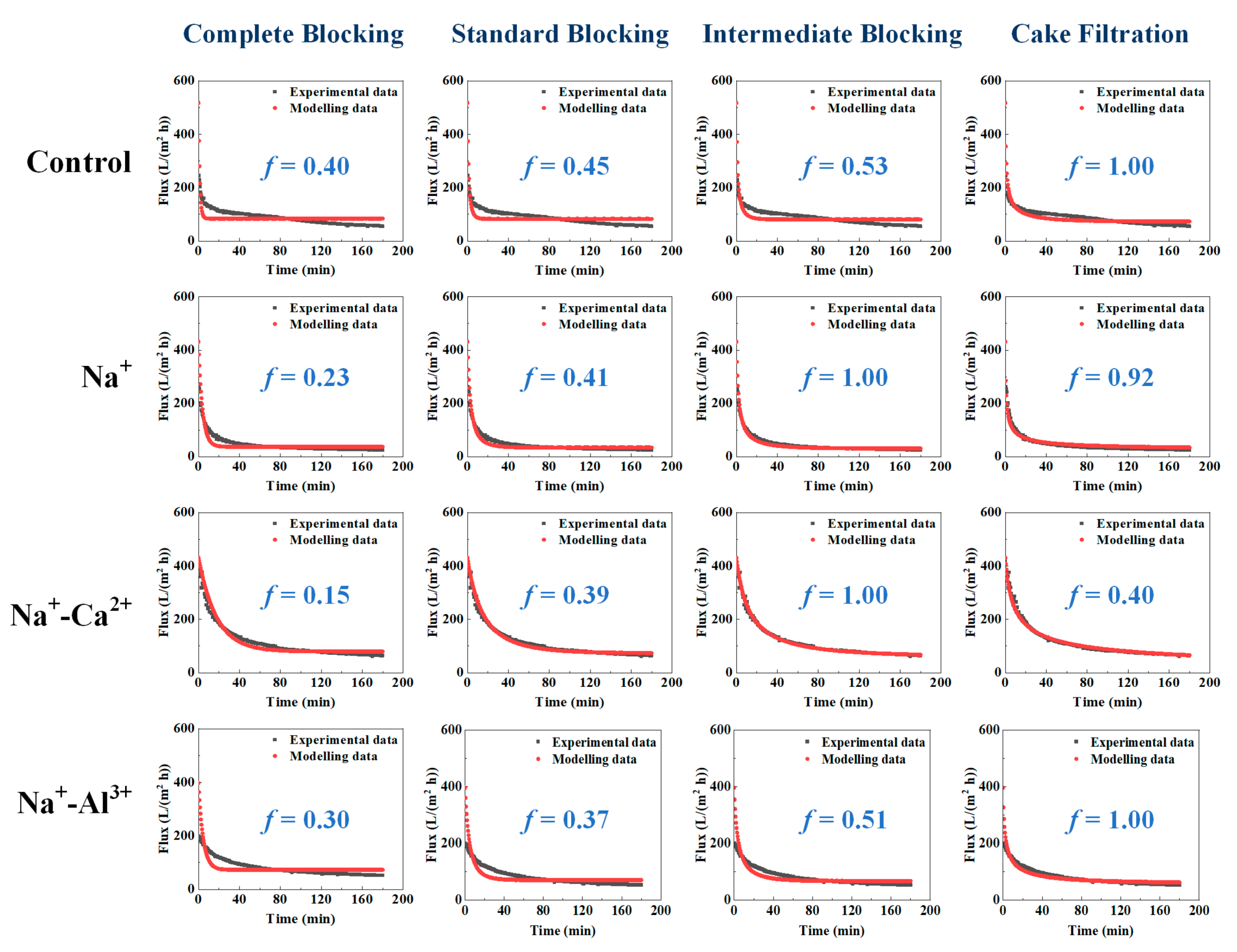
2.3. Modelling of Membrane Filtration
- (i)
- The fouled membrane was rinsed using deionized water for around 2 min to remove the reversible fouling layer;
- (ii)
- The value of Rm + Rir was then determined from Equation (1) by filtering deionized water through the rinsed membrane;
- (iii)
- The values of Rr and Rir were then obtained respectively from Equation (2), based on the determined Rt, Rm, and Rm + Rir values.
2.4. Other Measurements
3. Results
3.1. Effects of High Salinity on the Properties of Alginates
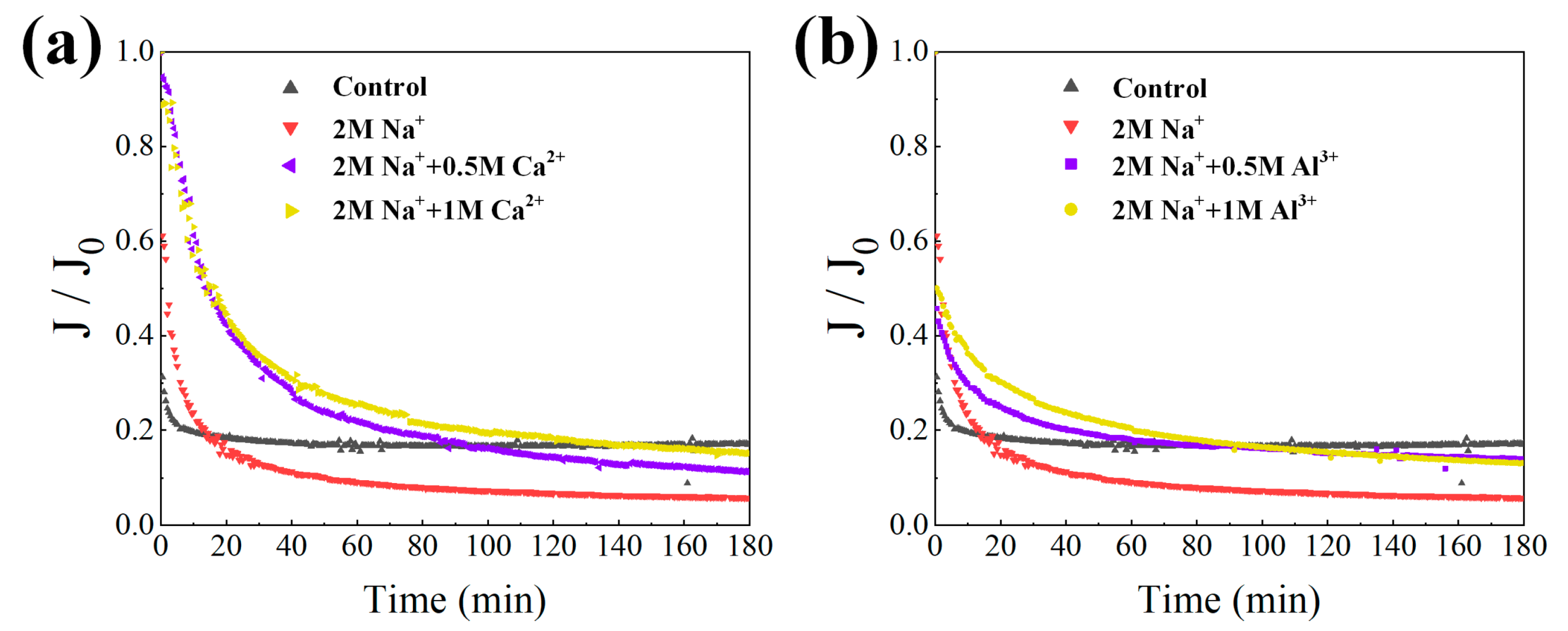
3.2. Effects of High Salinity on Membrane Fouling Propensity
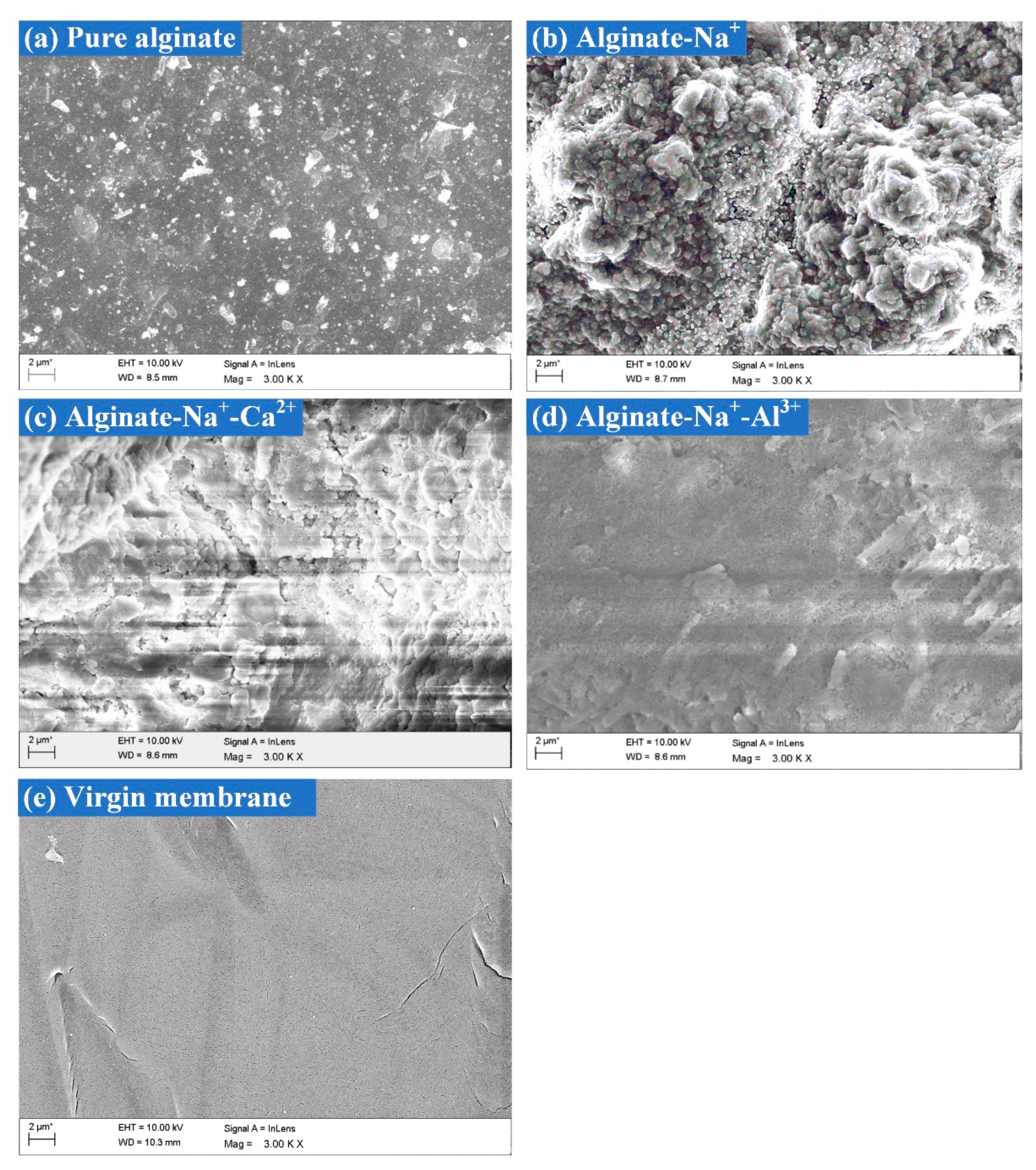
3.3. Effects of High Salinity on Membrane Fouling Morphology
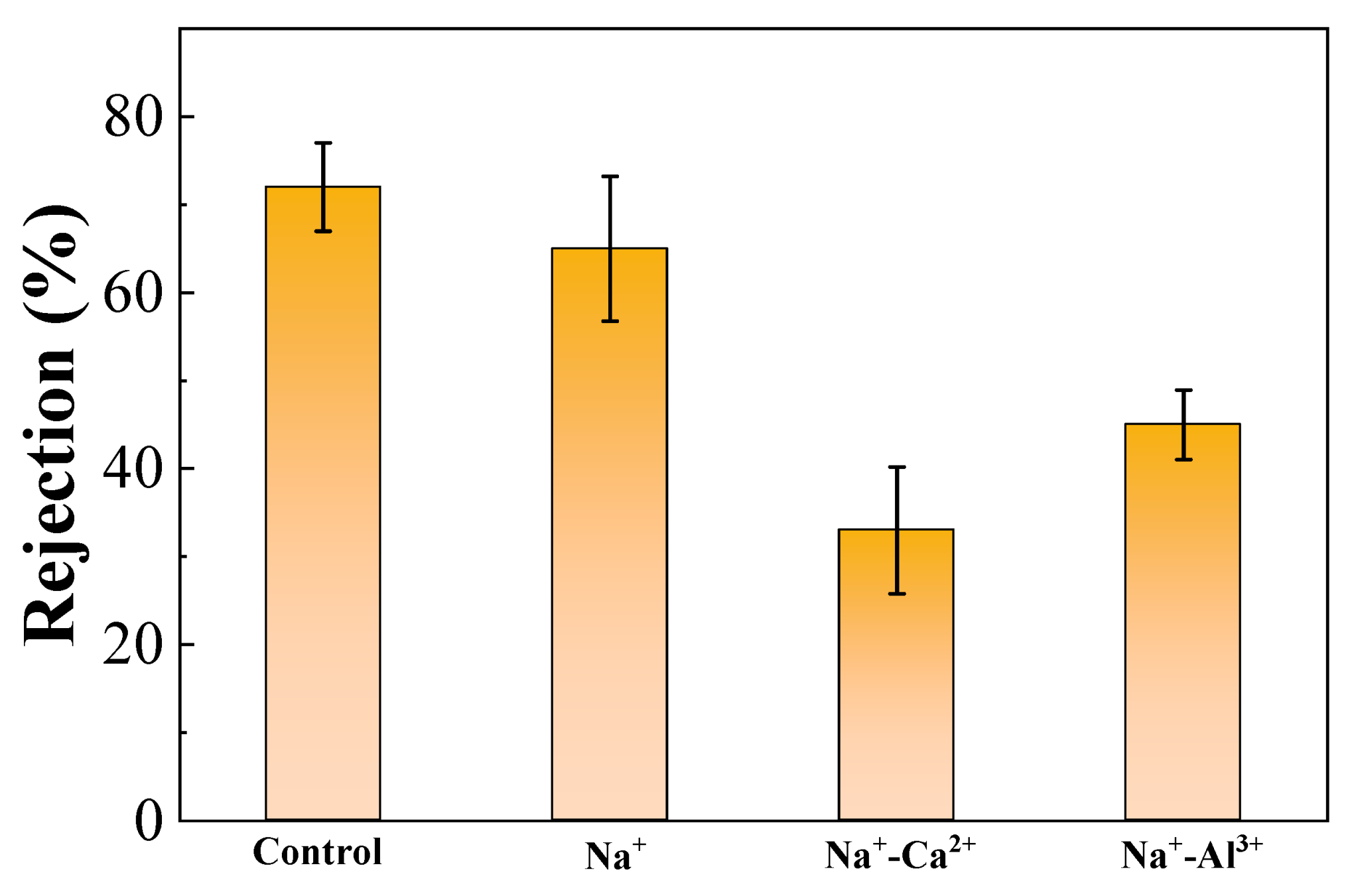
3.4. Effects of High Salinity on Membrane Rejection
4. Discussion
5. Conclusions
- (i)
- Compared with pure alginate, membrane fouling was enhanced by the presence of Na+, while decreased fouling development was observed when Ca2+ and Al3+ were further added on the basis of Na+, but entirely differentiated fouling morphology and foulant properties were found with the cases of Na+-Ca2+ and Na+-Al3+.
- (ii)
- The membrane rejection rate for organics was generally weakened by high salinity environments; however, the synergistic effects of Na+ and Ca2+ further worsened permeate quality because of significant defects and cracks appearing on the fouling layer.
- (iii)
- Ca2+ enlarged the molecule size of the alginate due to the strong coordination effects of Ca2+ with carboxyl groups to bridge alginate chains together, while negligible binding affinity with the alginate was found for Al3+ addition.
- (iv)
- Na+-Al3+ triggered more irreversible fouling formation, probably making the membranes difficult to clean and thus increasing operational cost.
- (v)
- This study gained more insights into the current treatment of high-salinity wastewater using membrane-based techniques.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Safavi, M.; Mohammadi, T. High-salinity water desalination using VMD. Chem. Eng. J. 2009, 149, 191–195. [Google Scholar] [CrossRef]
- Mannina, G.; Cosenza, A.; di Trapani, D.; Capodici, M.; Viviani, G. Membrane bioreactors for treatment of saline wastewater contaminated by hydrocarbons (diesel fuel): An experimental pilot plant case study. Chem. Eng. J. 2016, 291, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Acquah, I.; Liu, H.; Li, W.; Tan, S. A critical review on saline wastewater treatment by membrane bioreactor (MBR) from a microbial perspective. Chemosphere 2019, 220, 1150–1162. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, H.; Bañuelos, G.; Yan, B.; Zhou, Q.; Yu, X.; Cheng, X. Constructed wetlands for saline wastewater treatment: A review. Ecol. Eng. 2017, 98, 275–285. [Google Scholar] [CrossRef]
- He, H.; Chen, Y.; Li, X.; Cheng, Y.; Yang, C.; Zeng, G. Influence of salinity on microorganisms in activated sludge processes: A review. Int. Biodeterior. Biodegrad. 2017, 119, 520–527. [Google Scholar] [CrossRef]
- Wang, J.; Cahyadi, A.; Wu, B.; Pee, W.; Fane, A.G.; Chew, J.W. The roles of particles in enhancing membrane filtration: A review. J. Membr. Sci. 2020, 595, 117570. [Google Scholar] [CrossRef]
- Song, W.; Li, Z.; Ding, Y.; Liu, F.; You, H.; Qi, P.; Wang, F.; Li, Y.; Jin, C. Performance of a novel hybrid membrane bioreactor for treating saline wastewater from mariculture: Assessment of pollutants removal and membrane filtration performance. Chem. Eng. J. 2018, 331, 695–703. [Google Scholar] [CrossRef]
- Foglia, A.; Akyol, Ç.; Frison, N.; Katsou, E.; Eusebi, A.L.; Fatone, F. Long-term operation of a pilot-scale anaerobic membrane bioreactor (AnMBR) treating high salinity low loaded municipal wastewater in real environment. Sep. Purif. Technol. 2020, 236, 116279. [Google Scholar] [CrossRef]
- Mei, X.; Ding, Y.; Li, P.; Xu, L.; Wang, Y.; Guo, Z.; Shen, W.; Yang, Y.; Wang, Y.; Xiao, Y.; et al. A novel system for zero-discharge treatment of high-salinity acetonitrile-containing wastewater: Combination of pervaporation with a membrane-aerated bioreactor. Chem. Eng. J. 2020, 384, 123338. [Google Scholar] [CrossRef]
- You, X.; Zhang, J.; Shen, L.; Li, R.; Xu, Y.; Zhang, M.; Hong, H.; Yang, L.; Ma, Y.; Lin, H. Thermodynamic mechanisms of membrane fouling during filtration of alginate solution in coagulation-ultrafiltration (UF) process in presence of different ionic strength and iron(III) ion concentration. J. Membr. Sci. 2021, 635, 119532. [Google Scholar] [CrossRef]
- Long, Y.; Yu, G.; Dong, L.; Xu, Y.; Lin, H.; Deng, Y.; You, X.; Yang, L.; Liao, B.Q. Synergistic fouling behaviors and mechanisms of calcium ions and polyaluminum chloride associated with alginate solution in coagulation-ultrafiltration (UF) process. Water Res. 2021, 189, 116665. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Rao, L.; Zhang, J.; Shen, L.; Xu, Y.; You, X.; Liao, B.-Q.; Lin, H. Novel in-situ electroflotation driven by hydrogen evolution reaction (HER) with polypyrrole (PPy)-Ni-modified fabric membrane for efficient oil/water separation. J. Membr. Sci. 2021, 635, 119502. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Lin, H.; Zhao, L.; Shen, L.; Li, R.; Xu, Y.; Hong, H.; He, Y. Membrane fouling caused by biological foams in a submerged membrane bioreactor: Mechanism insights. Water Res. 2020, 181, 115932. [Google Scholar] [CrossRef]
- Cai, W.; Han, J.; Zhang, X.; Liu, Y. Formation mechanisms of emerging organic contaminants during on-line membrane cleaning with NaOCl in MBR. J. Hazard. Mater. 2020, 386, 121966. [Google Scholar] [CrossRef]
- Weinberger, M.E.; Kulozik, U. Pulsatile crossflow improves microfiltration fractionation of cells and proteins. J. Membr. Sci. 2021, 629, 119295. [Google Scholar] [CrossRef]
- Li, X.; Tang, G.; Zhang, D.; Wu, L.; Lu, S.; Zhang, Y.; Cao, X.; Cheng, W.; Feng, J.; Yan, W.; et al. Fouling control in ultrafiltration of secondary effluent using polyaniline/TiO2 adsorption and subsequent treatment of desorption eluate using electrochemical oxidation. Chem. Eng. J. 2020, 382, 122915. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, Y.H.; Lee, C.H.; Kim, H.S.; Kim, H.S. Effect of different physical conditions on fouling control in in-situ chemical cleaning in place (CIP) for flat sheet membranes fouled by secondary effluents. Chem. Eng. J. 2016, 302, 128–136. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, H.; Qu, F.; Ding, A.; Chang, H.; Liu, B.; Tang, X.; Wu, D.; Li, G. Fabrication of Mn oxide incorporated ceramic membranes for membrane fouling control and enhanced catalytic ozonation of p-chloronitrobenzene. Chem. Eng. J. 2017, 308, 1010–1020. [Google Scholar] [CrossRef]
- Wang, J.; Wu, B.; Chew, J.W. Membrane fouling mitigation by fluidized granular activated carbon: Effect of fiber looseness and impact on irreversible fouling. Sep. Purif. Technol. 2020, 242, 116764. [Google Scholar] [CrossRef]
- You, X.; Teng, J.; Chen, Y.; Long, Y.; Yu, G.; Shen, L.; Lin, H. New insights into membrane fouling by alginate: Impacts of ionic strength in presence of calcium ions. Chemosphere 2020, 246, 125801. [Google Scholar] [CrossRef]
- Hao, Y.; Moriya, A.; Maruyama, T.; Ohmukai, Y.; Matsuyama, H. Effect of metal ions on humic acid fouling of hollow fiber ultrafiltration membrane. J. Membr. Sci. 2011, 376, 247–253. [Google Scholar] [CrossRef]
- Hao, Y.; Moriya, A.; Ohmukai, Y.; Matsuyama, H.; Maruyama, T. Effect of metal ions on the protein fouling of hollow-fiber ultrafiltration membranes. Sep. Purif. Technol. 2013, 111, 137–144. [Google Scholar] [CrossRef]
- van de Ven, W.J.C.; Sant, K.v.t.; Pünt, I.G.M.; Zwijnenburg, A.; Kemperman, A.J.B.; van der Meer, W.G.J.; Wessling, M. Hollow fiber dead-end ultrafiltration: Influence of ionic environment on filtration of alginates. J. Membr. Sci. 2008, 308, 218–229. [Google Scholar] [CrossRef]
- Meng, S.; Winters, H.; Liu, Y. Ultrafiltration behaviors of alginate blocks at various calcium concentrations. Water Res. 2015, 83, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Gao, Z.; Yu, S.; Lv, M.; Shi, Y.; Wang, J. New insights into membrane fouling formation during ultrafiltration of organic wastewater with high salinity. J. Membr. Sci. 2021, 635, 119446. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.W. Uncertainty of preset-order kinetic equations in description of biosorption data. Bioresour. Technol. 2008, 99, 3309–3312. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Engineers; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Zhang, Y.; Ma, F.; Li, G.-B. Fouling of ultrafiltration membrane by algal-rich water: Effect of kalium, calcium, and aluminum. J. Colloid Interface Sci. 2013, 405, 22–27. [Google Scholar] [CrossRef]
- Ostolska, I.; Wiśniewska, M. Application of the zeta potential measurements to explanation of colloidal Cr2O3 stability mechanism in the presence of the ionic polyamino acids. Colloid Polym. Sci. 2014, 292, 2453–2464. [Google Scholar] [CrossRef] [Green Version]
- Carneiro-da-Cunha, M.G.; Cerqueira, M.A.; Souza, B.W.S.; Teixeira, J.A.; Vicente, A.A. Influence of concentration, ionic strength and pH on zeta potential and mean hydrodynamic diameter of edible polysaccharide solutions envisaged for multinanolayered films production. Carbohydr. Polym. 2011, 85, 522–528. [Google Scholar] [CrossRef] [Green Version]
- Idota, Y.; Kogure, Y.; Kato, T.; Yano, K.; Arakawa, H.; Miyajima, C.; Kasahara, F.; Ogihara, T. Relationship between physical parameters of various metal ions and binding affinity for alginate. Biol. Pharm. Bull. 2016, 39, 1893–1896. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Ma, J.; Tang, C.Y.; Kimura, K.; Wang, Q.; Han, X. Membrane cleaning in membrane bioreactors: A review. J. Membr. Sci. 2014, 468, 276–307. [Google Scholar] [CrossRef]
- Jarusutthirak, C.; Mattaraj, S.; Jiraratananon, R. Influence of inorganic scalants and natural organic matter on nanofiltration membrane fouling. J. Membr. Sci. 2007, 287, 138–145. [Google Scholar] [CrossRef]


| Resistance | Control | Na+ | Na+-Ca2+ | Na+-Al3+ | ||
|---|---|---|---|---|---|---|
| 0.5 M Ca2+ | 1.0 M Ca2+ | 0.5 M Al3+ | 1.0 M Al3+ | |||
| Rm (×1012 m−1) | 1.61 | 2.00 | 1.20 | 1.71 | 1.47 | 1.83 |
| Rr (×1012 m−1) | 7.58 | 40.73 | 9.33 | 9.53 | 6.09 | 9.24 |
| Rir (×1012 m−1) | 0.18 | 2.47 | 0.09 | 0.02 | 2.94 | 2.86 |
| Rt (×1012 m−1) | 9.37 | 45.20 | 10.62 | 11.26 | 10.50 | 13.90 |
| Salts | Best Fitting Model | Kinetic Rate | Back-Transport Flux (J*, L/(m2 h)) |
|---|---|---|---|
| Control | Cake filtration | kD = 5.1 × 10−6 min m−2 | 72.93 |
| Na+-Al3+ | Cake filtration | kD = 4.1 × 10−6 min m−2 | 66.12 |
| Na+ | Intermediate blocking | kC = 0.0011 m−1 | 30.63 |
| Na+-Ca2+ | Intermediate blocking | kC = 0.0002 m−1 | 59.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, W.; Chen, Q.; Zhang, J.; Li, Y.; Xie, W.; Wang, J. Effects of High Salinity on Alginate Fouling during Ultrafiltration of High-Salinity Organic Synthetic Wastewater. Membranes 2021, 11, 590. https://doi.org/10.3390/membranes11080590
Cai W, Chen Q, Zhang J, Li Y, Xie W, Wang J. Effects of High Salinity on Alginate Fouling during Ultrafiltration of High-Salinity Organic Synthetic Wastewater. Membranes. 2021; 11(8):590. https://doi.org/10.3390/membranes11080590
Chicago/Turabian StyleCai, Weiwei, Qiuying Chen, Jingyu Zhang, Yan Li, Wenwen Xie, and Jingwei Wang. 2021. "Effects of High Salinity on Alginate Fouling during Ultrafiltration of High-Salinity Organic Synthetic Wastewater" Membranes 11, no. 8: 590. https://doi.org/10.3390/membranes11080590
APA StyleCai, W., Chen, Q., Zhang, J., Li, Y., Xie, W., & Wang, J. (2021). Effects of High Salinity on Alginate Fouling during Ultrafiltration of High-Salinity Organic Synthetic Wastewater. Membranes, 11(8), 590. https://doi.org/10.3390/membranes11080590








