Effect of Operating Conditions on Membrane Fouling in Pilot-Scale MBRs: Filaments Growth, Diminishing Dissolved Oxygen and Recirculation Rate of the Activated Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Configuration and Operation of Pilot-Scale MBR Treatment Systems
2.2. Filament Index (FI) Determination
2.3. Determination of Effluent Quality Parameters
2.4. Measurement of SMP Concentration
2.5. Determination of Particle Size Distribution
3. Results and Discussion
3.1. Fouling Examination
3.1.1. Effect of Filaments’ Development on Membrane Fouling—Correlation of TMP with FI and Temperature
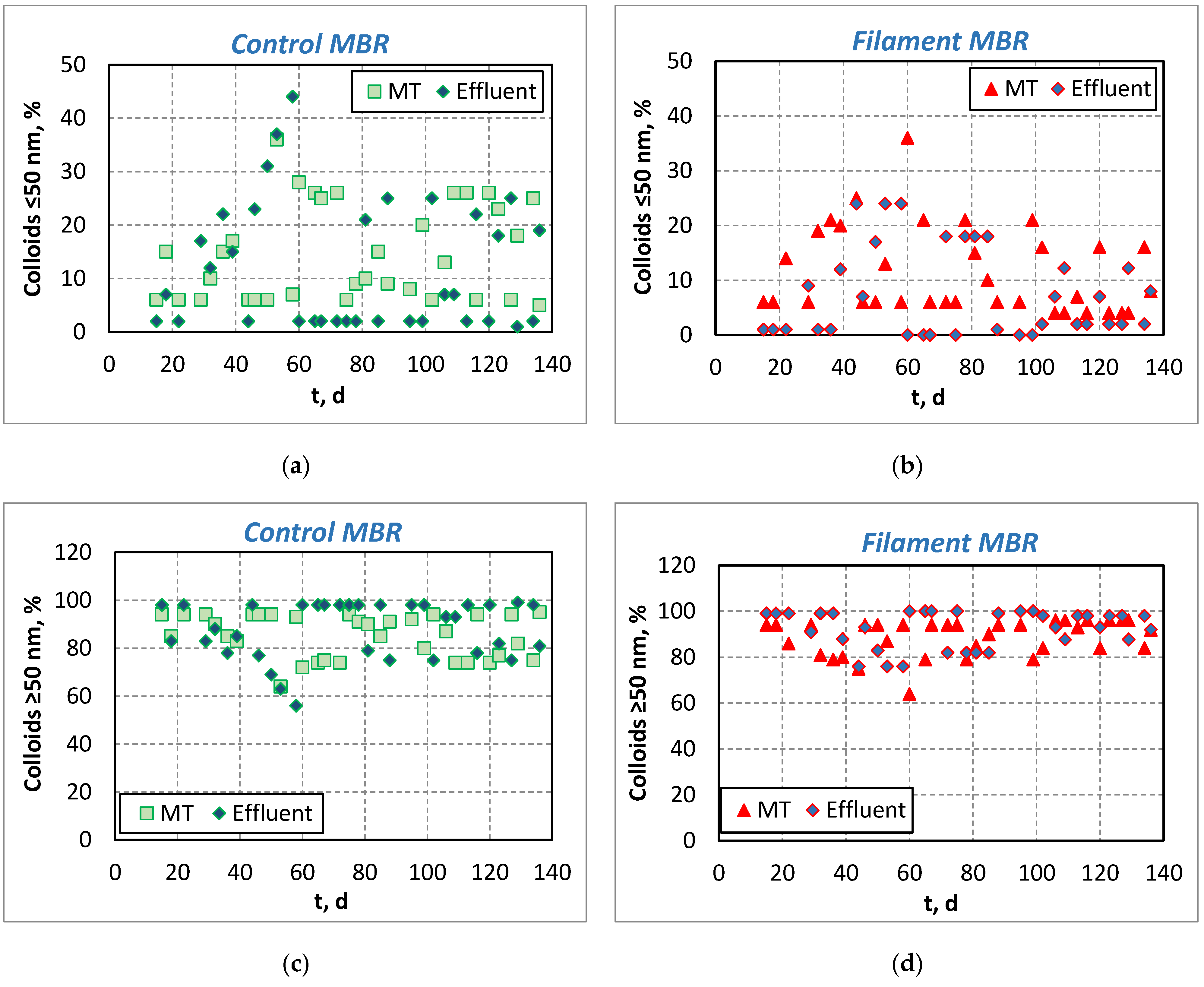
3.1.2. Investigation of Foulants—Evolution of SMP and Colloids
3.2. Removal of Organics and Nutrients
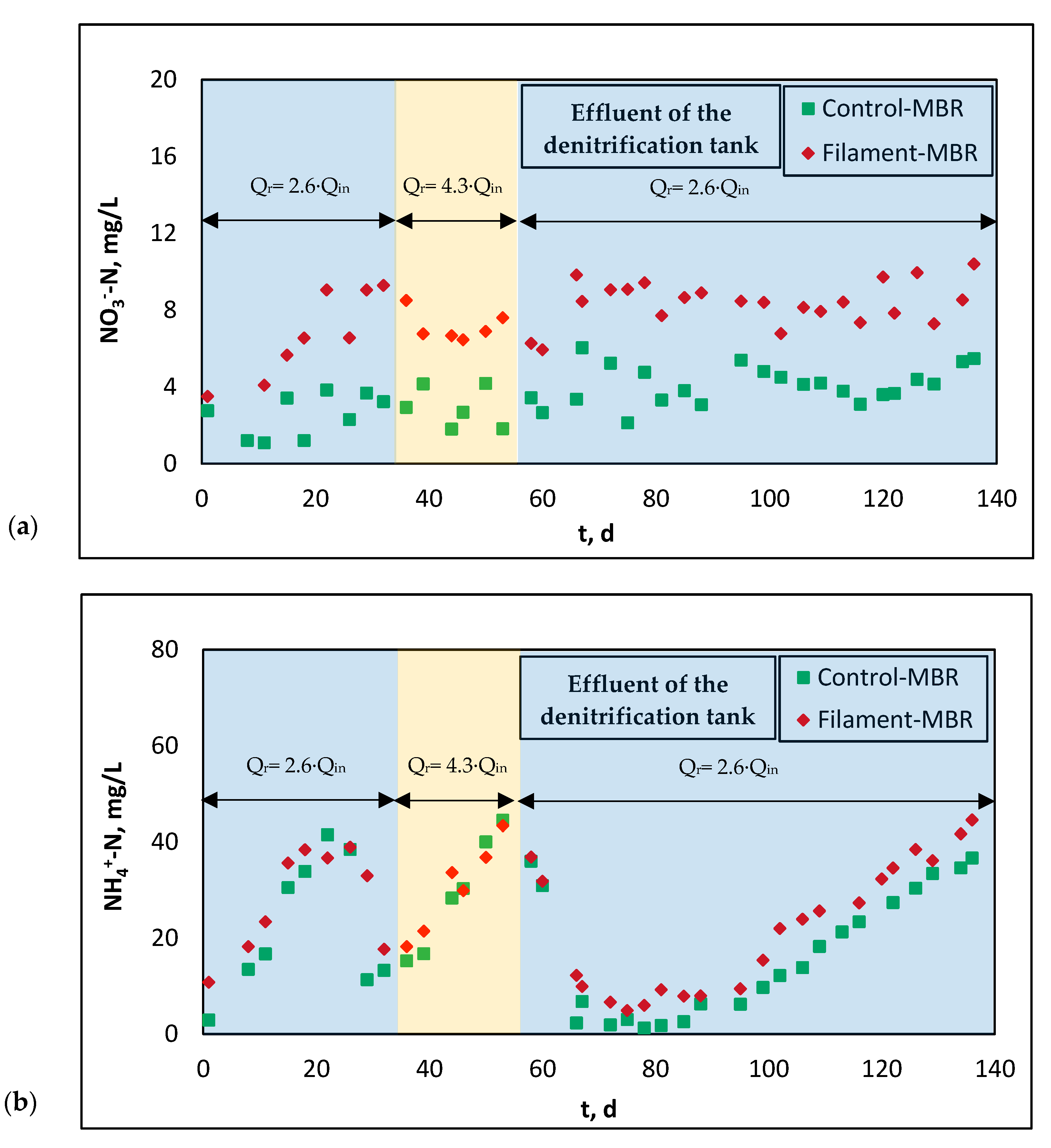
3.2.1. Nitrification and Denitrification—Effect of DO Diminishing in Recirculated Sludge Flow
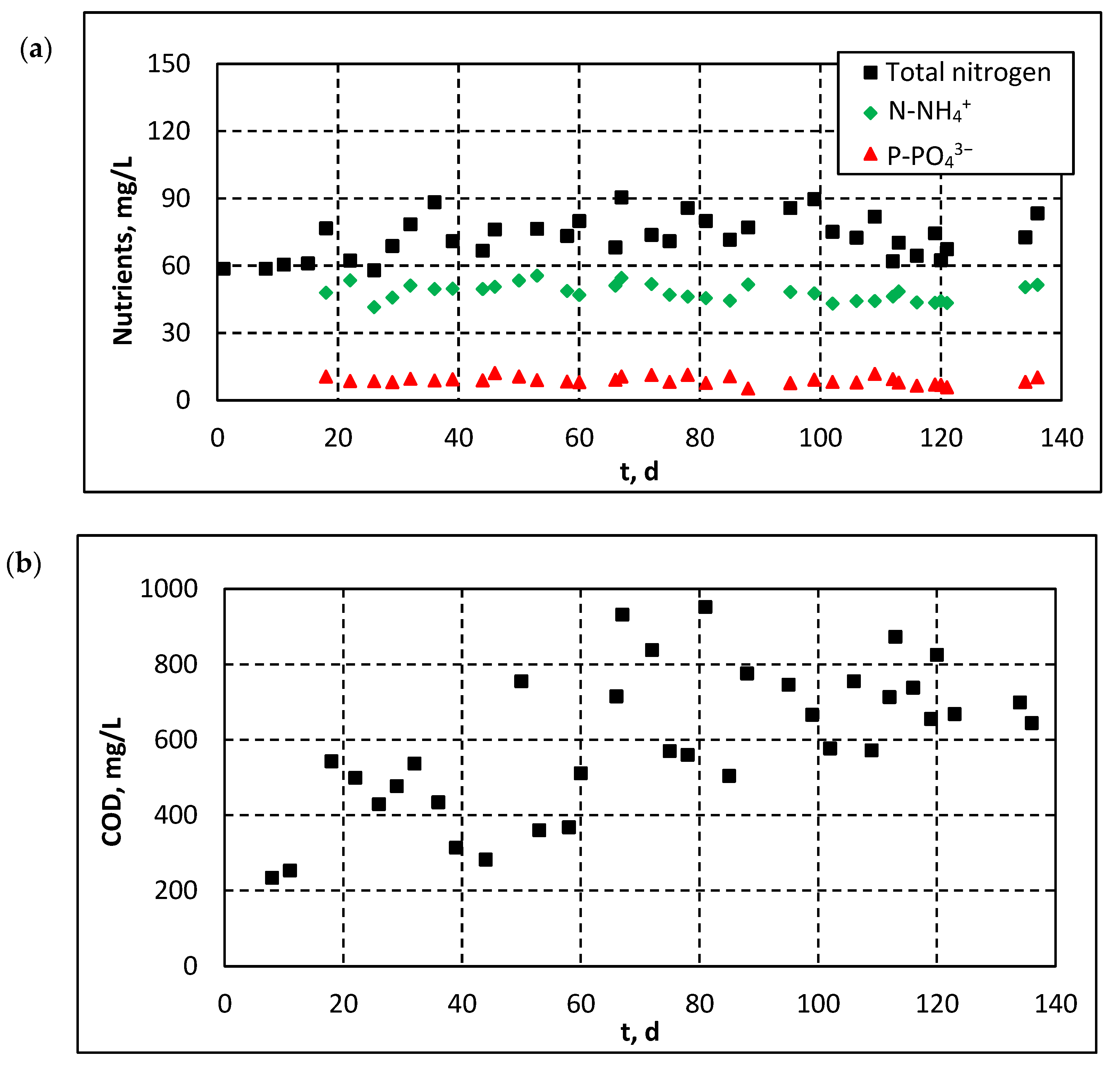
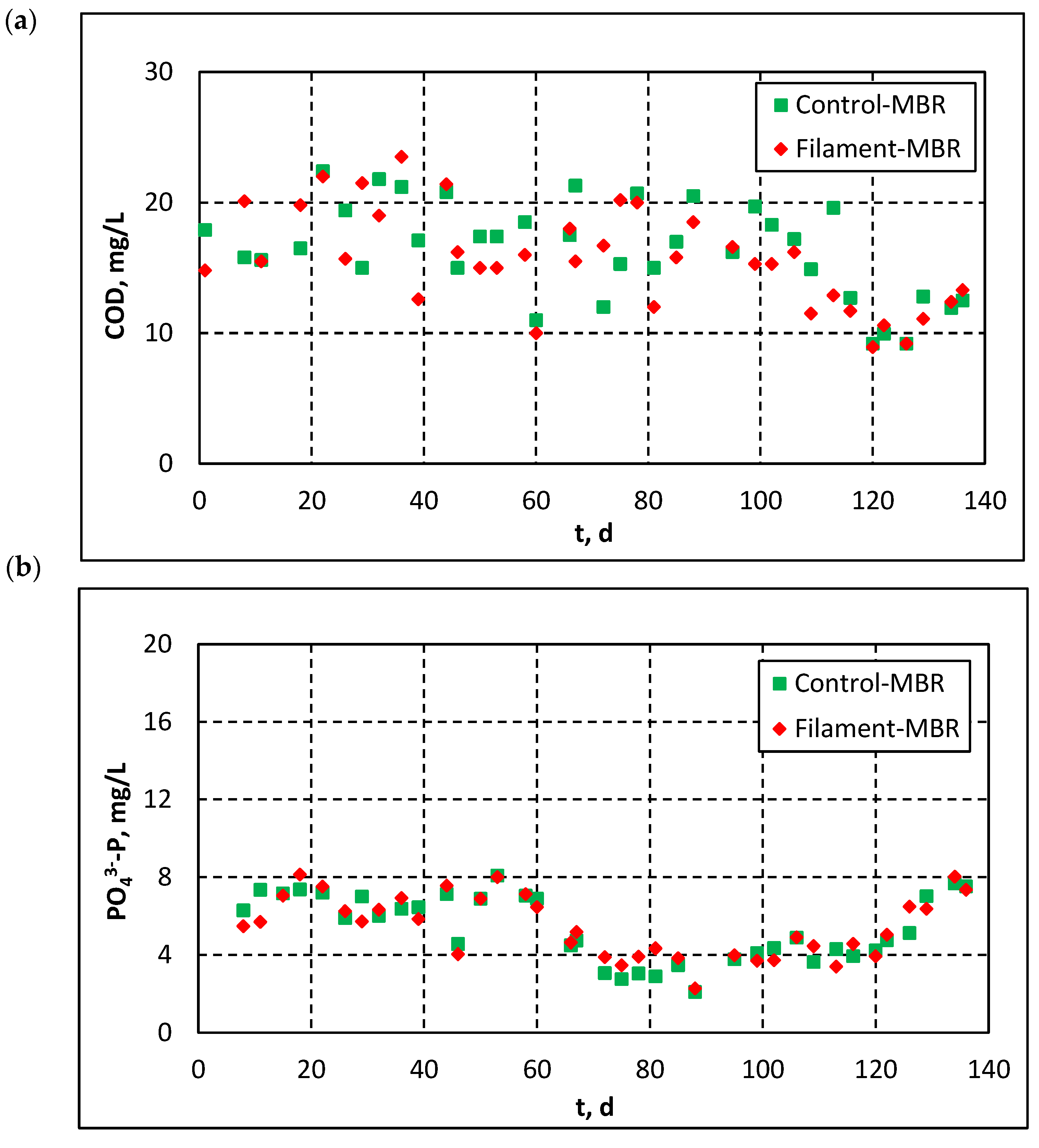
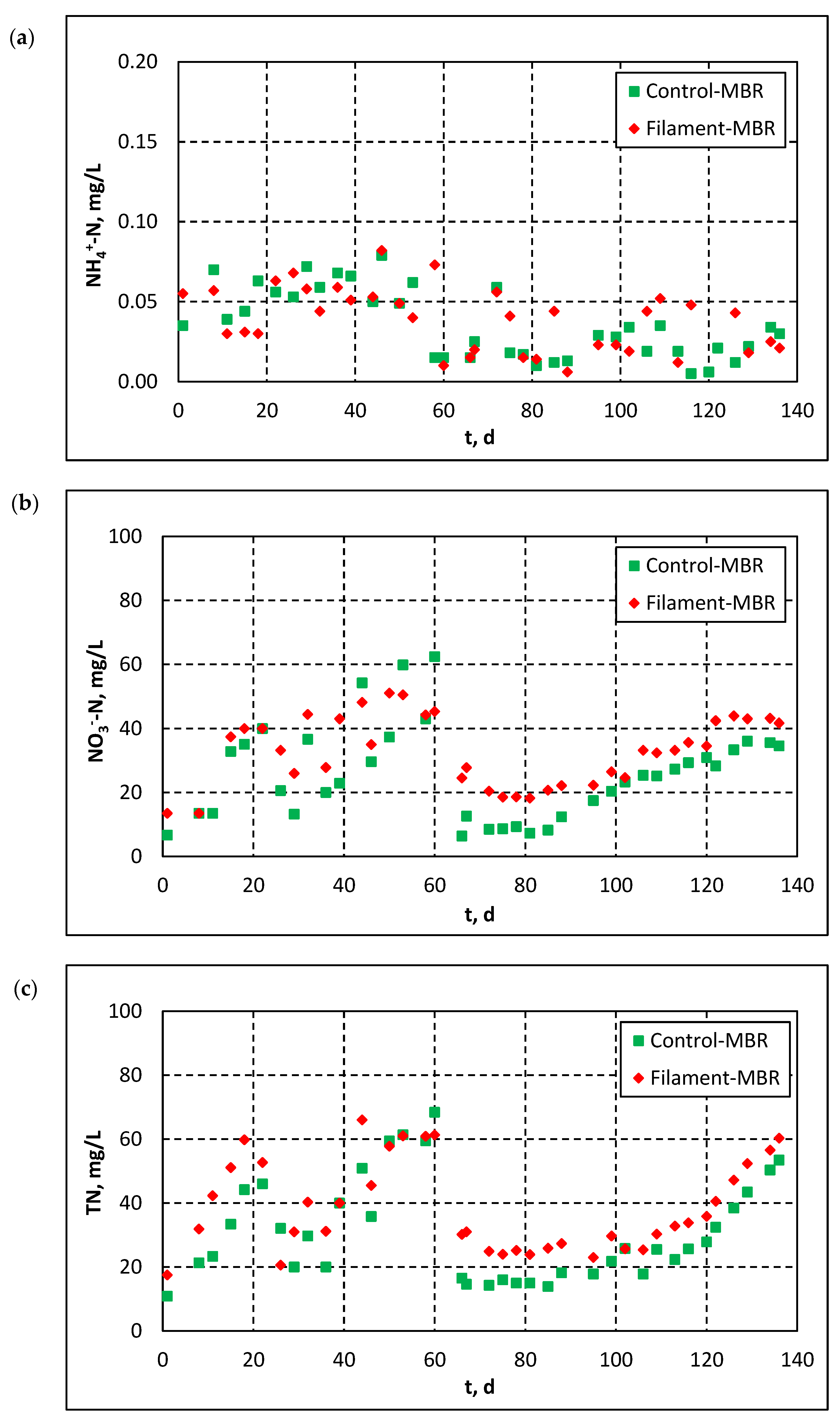
3.2.2. Influent and Effluent Quality Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| ASP | Activated Sludge Process |
| COD | Chemical Oxygen Demand |
| DO | Dissolved Oxygen |
| EPS | Extracellular Polymeric Substances |
| F/M | Food to Microorganisms |
| FI | Filament Index |
| MBR | Membrane Bio-Reactor |
| MLSS | Mixed Liquor Suspended Solids |
| Qin | influent rate |
| Qout | filtration rate |
| Qr | recirculation rate |
| SCADA | Supervisory Control and Data Acquisition |
| SMP | Soluble Microbial Products |
| SMPc | carbohydrate fraction of SMP |
| SMPp | protein fraction of SMP |
| SRT | Solids Retention Time |
| TMP | Trans-Membrane Pressure |
| TN | Total Nitrogen |
References
- Nam, K.; Heo, S.; Rhee, G.; Kim, M.; Yoo, C. Dual-objective optimization for energy-saving and fouling mitigation in MBR plants using AI-based influent prediction and an integrated biological-physical model. J. Membr. Sci. 2021, 626, 119208. [Google Scholar] [CrossRef]
- Yin, X.; Li, X.; Hua, Z.; Ren, Y. The growth process of the cake layer and membrane fouling alleviation mechanism in a MBR assisted with the self-generated electric field. Water Res. 2020, 171, 115452. [Google Scholar] [CrossRef] [PubMed]
- Gkotsis, P.; Zouboulis, A.; Mitrakas, M. Using Additives for Fouling Control in a Lab-Scale MBR; Comparing the Anti-Fouling Potential of Coagulants, PAC and Bio-Film Carriers. Membranes 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, A.M.; Pagilla, K.; Heijnen, J.J.; van Loosdrecht, M.C. Filamentous bulking sludge—A critical review. Water Res. 2004, 38, 793–817. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhang, H.; Yang, F.; Li, Y.; Xiao, J.; Zhang, X. Effect of filamentous bacteria on membrane fouling in submerged membrane bioreactor. J. Membr. Sci. 2006, 272, 161–168. [Google Scholar] [CrossRef]
- Meng, F.; Yang, F. Fouling mechanisms of deflocculated sludge, normal sludge, and bulking sludge in membrane bioreactor. J. Membr. Sci. 2007, 305, 48–56. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Ohandja, D.-G.; Yang, F.; Wong, F.-S.; Chua, H.-C. Impact of filamentous bacteria on properties of activated sludge and membrane-fouling rate in a submerged MBR. Sep. Purif. Technol. 2008, 59, 238–243. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, P.; Wang, Q.; Wu, Z.; Zhou, Q.; Yang, D. Effective control of membrane fouling by filamentous bacteria in a submerged membrane bioreactor. Chem. Eng. J. 2010, 158, 608–615. [Google Scholar] [CrossRef]
- Banti, D.C.; Karayannakidis, P.D.; Samaras, P.; Mitrakas, M.G. An innovative bioreactor set-up that reduces membrane fouling by adjusting the filamentous bacterial population. J. Membr. Sci. 2017, 542, 430–438. [Google Scholar] [CrossRef]
- Gkotsis, P.; Lemonidis, G.; Mitrakas, M.; Pentedimos, A.; Kostoglou, M.; Zouboulis, A. Quantifying the Effect of COD to TN Ratio, DO Concentration and Temperature on Filamentous Microorganisms’ Population and Trans-Membrane Pressure (TMP) in Membrane Bio-Reactors (MBR). Processes 2020, 8, 1514. [Google Scholar] [CrossRef]
- Hao, L.; Liss, S.; Liao, B. Influence of COD:N ratio on sludge properties and their role in membrane fouling of a submerged membrane bioreactor. Water Res. 2016, 89, 132–141. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Sue, W. Filamentous bacteria in a foaming membrane bioreactor. J. Membr. Sci. 2009, 342, 42–49. [Google Scholar] [CrossRef]
- Eikelboom, D.H. Process Control of Activated Sludge Plants by Microscopic Investigation, 1st ed.; IWA Publishing: Zutphen, The Netherlands, 2000. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Hartree, E. Determination of protein: A modification of the lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- Pajdak-Stós, A.; Fiałkowska, E. The influence of temperature on the effectiveness of filamentous bacteria removal from activated sludge by rotifers. Water Environ. Res. 2012, 84, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Peng, Y.; Wang, S.; Yang, X.; Wang, Z.; Zhu, A. Stable limited filamentous bulking through keeping the competition between floc-formers and filaments in balance. Bioresour. Technol. 2012, 103, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Banti, D.; Mitrakas, M.; Fytianos, G.; Tsali, A.; Samaras, P. Combined Effect of Colloids and SMP on Membrane Fouling in MBRs. Membranes 2020, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Banti, D.C.; Samaras, P.; Tsioptsias, C.; Zouboulis, A.; Mitrakas, M.; Dimitra, B.C.; Petros, S.; Costas, T.; Anastasios, Z.; Manassis, M. Mechanism of SMP aggregation within the pores of hydrophilic and hydrophobic MBR membranes and aggregates detachment. Sep. Purif. Technol. 2018, 202, 119–129. [Google Scholar] [CrossRef]
- Christensen, M.; Niessen, W.; Sørensen, N.B.; Hansen, S.H.; Jørgensen, M.K.; Nielsen, P.H. Sludge fractionation as a method to study and predict fouling in MBR systems. Sep. Purif. Technol. 2018, 194, 329–337. [Google Scholar] [CrossRef]
- Tan, T.W.; Ng, H.Y. Influence of mixed liquor recycle ratio and dissolved oxygen on performance of pre-denitrification submerged membrane bioreactors. Water Res. 2008, 42, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
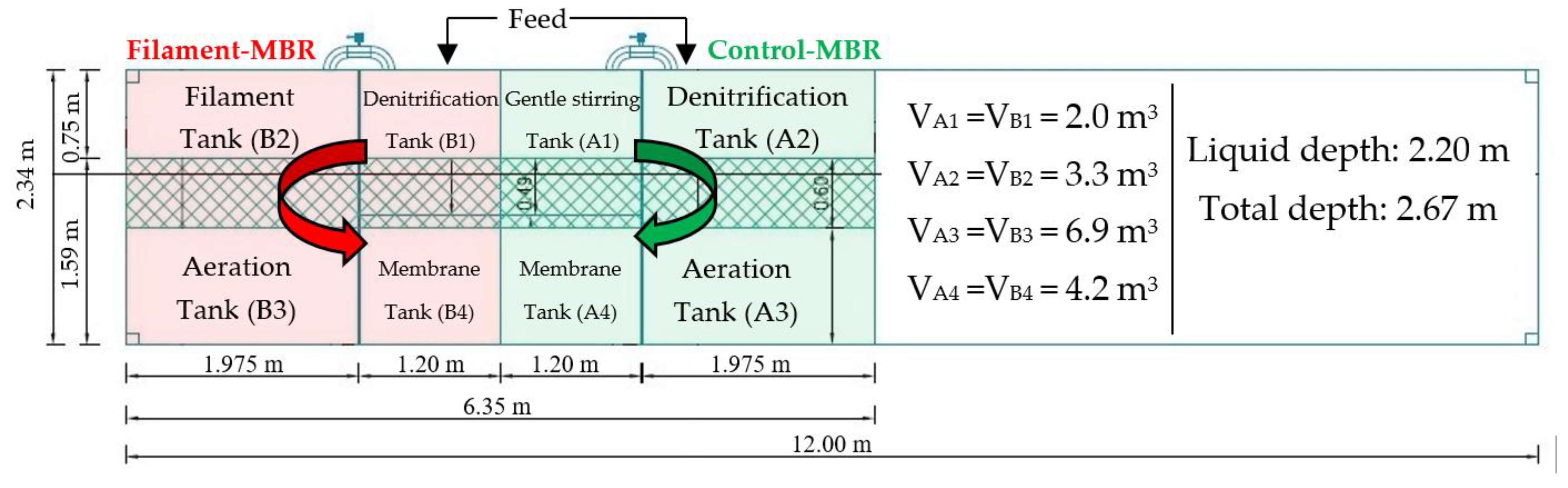
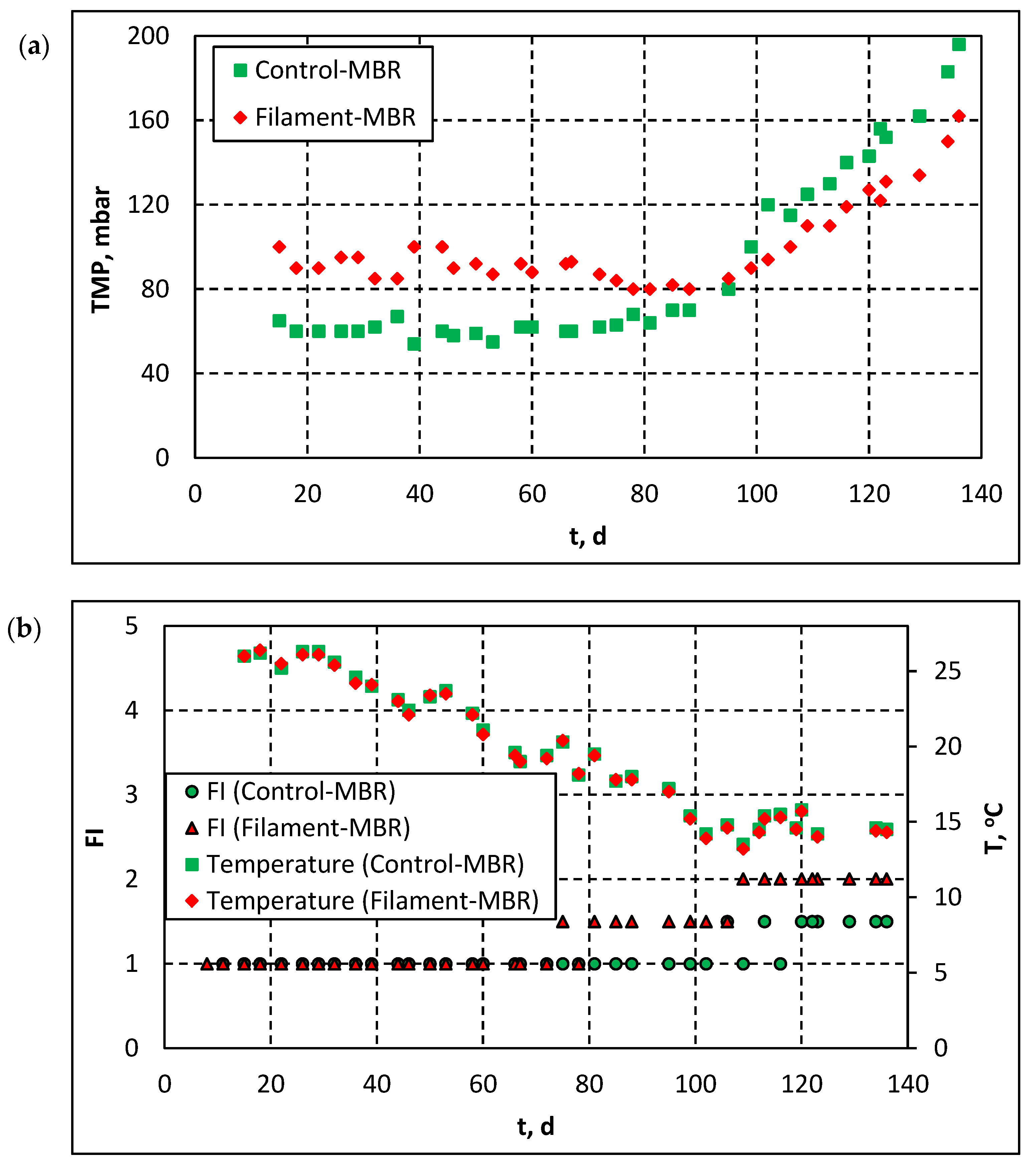
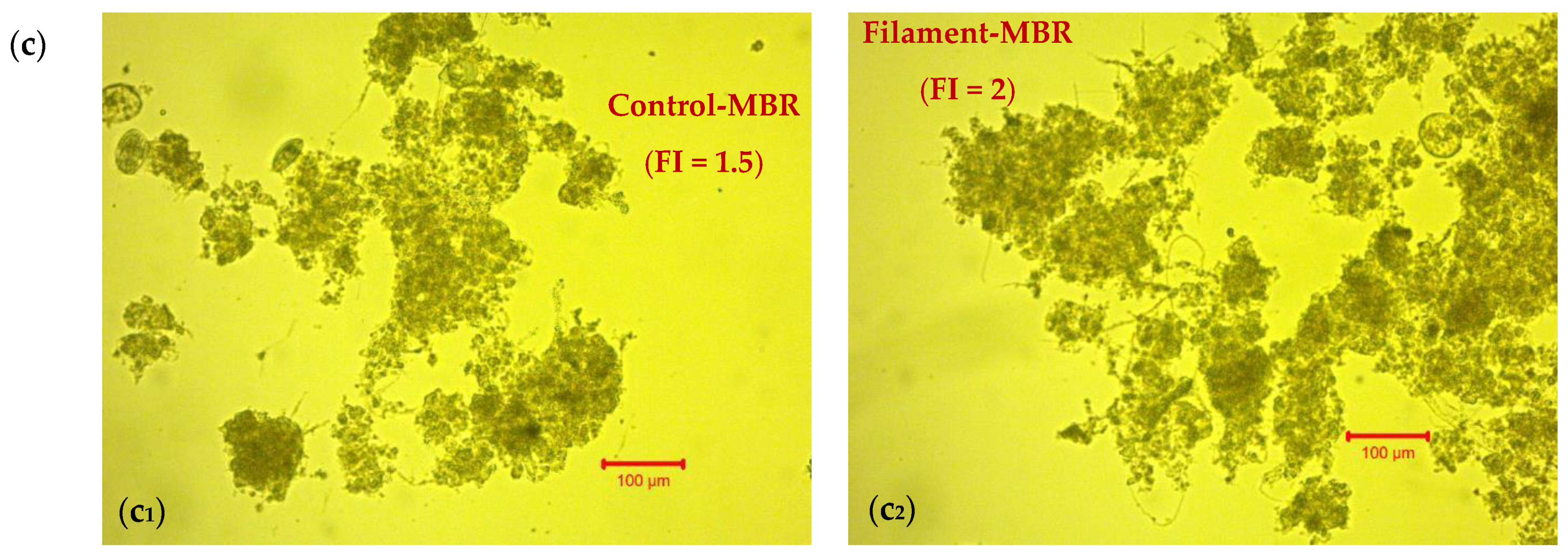
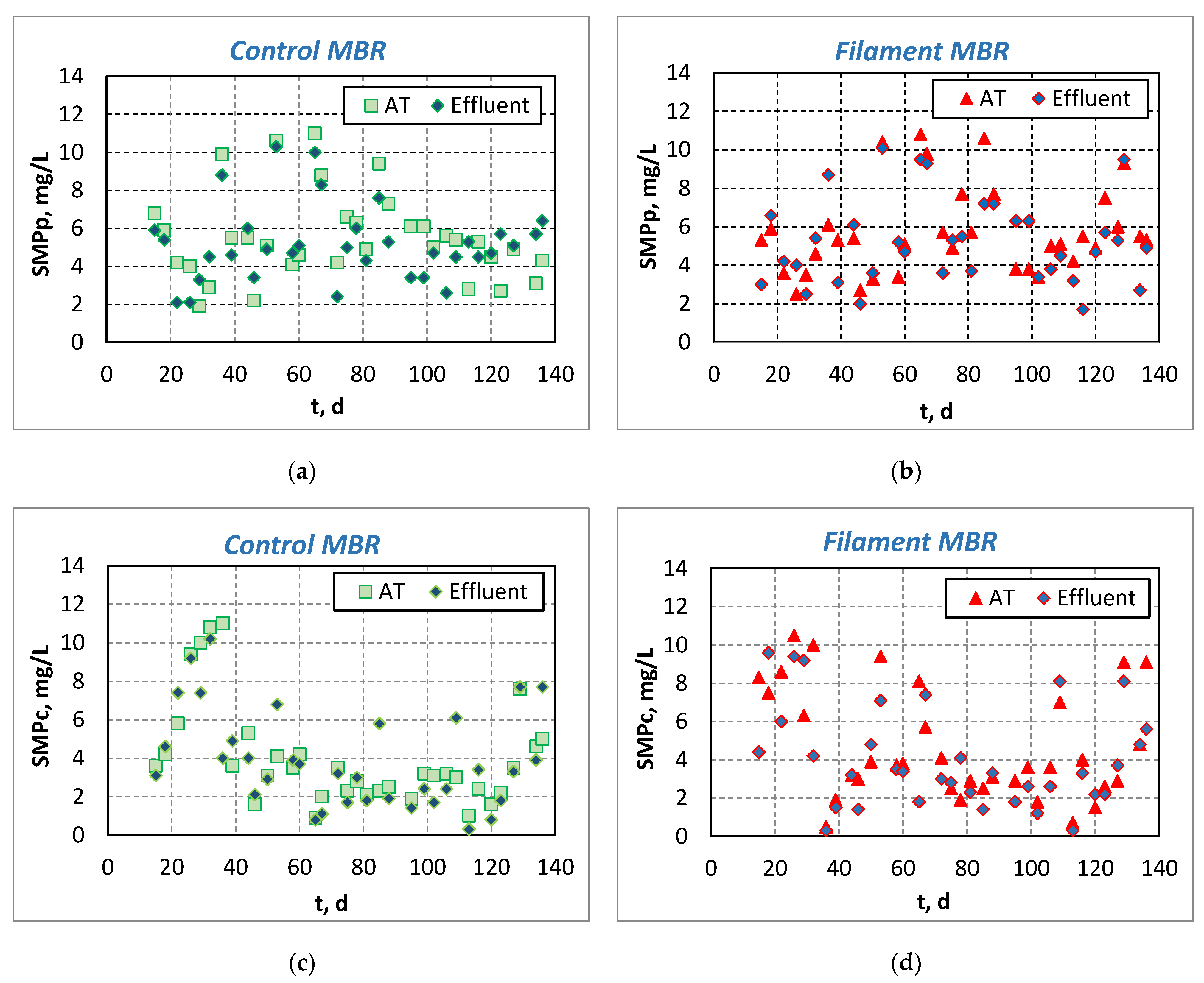
| Operating conditions | |
| Inlet flow rate | Qin = 700 L/h |
| Outlet (permeate) flow rate | Qout = 700 L/h |
| Recirculation rate | Qr = 1800 L/h and 3000 L/h |
| Flux | J = 13.5 Lm−2h−1 |
| DO concentration | Aeration tanks: DO = 2.5 ± 0.5 mg/L |
| Filament tank: DO = 0.5 ± 0.3 mg/L | |
| Denitrification tanks: DO < 0.2 mg/L | |
| MLSS concentration | MLSS = 6500 mg/L |
| Membrane characteristics | |
| Module type | NADIR® UP150 (Microdyn-Nadir GmbH) |
| Construction material | Polyethersulfone |
| Configuration | Flat sheet |
| Pore size | 0.04 μm |
| Membrane area | 52 m2 |
| Maximum TMP filtration limit | 400 mbar |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkotsis, P.; Banti, D.; Pritsa, A.; Mitrakas, M.; Samaras, P.; Peleka, E.; Zouboulis, A. Effect of Operating Conditions on Membrane Fouling in Pilot-Scale MBRs: Filaments Growth, Diminishing Dissolved Oxygen and Recirculation Rate of the Activated Sludge. Membranes 2021, 11, 490. https://doi.org/10.3390/membranes11070490
Gkotsis P, Banti D, Pritsa A, Mitrakas M, Samaras P, Peleka E, Zouboulis A. Effect of Operating Conditions on Membrane Fouling in Pilot-Scale MBRs: Filaments Growth, Diminishing Dissolved Oxygen and Recirculation Rate of the Activated Sludge. Membranes. 2021; 11(7):490. https://doi.org/10.3390/membranes11070490
Chicago/Turabian StyleGkotsis, Petros, Dimitra Banti, Anastasia Pritsa, Manassis Mitrakas, Petros Samaras, Efrosini Peleka, and Anastasios Zouboulis. 2021. "Effect of Operating Conditions on Membrane Fouling in Pilot-Scale MBRs: Filaments Growth, Diminishing Dissolved Oxygen and Recirculation Rate of the Activated Sludge" Membranes 11, no. 7: 490. https://doi.org/10.3390/membranes11070490
APA StyleGkotsis, P., Banti, D., Pritsa, A., Mitrakas, M., Samaras, P., Peleka, E., & Zouboulis, A. (2021). Effect of Operating Conditions on Membrane Fouling in Pilot-Scale MBRs: Filaments Growth, Diminishing Dissolved Oxygen and Recirculation Rate of the Activated Sludge. Membranes, 11(7), 490. https://doi.org/10.3390/membranes11070490










