The Evolution of the Use of Extracorporeal Membrane Oxygenation in Respiratory Failure
Abstract
:1. Background on ECMO for ARDS
2. ECMO in the Modern Era
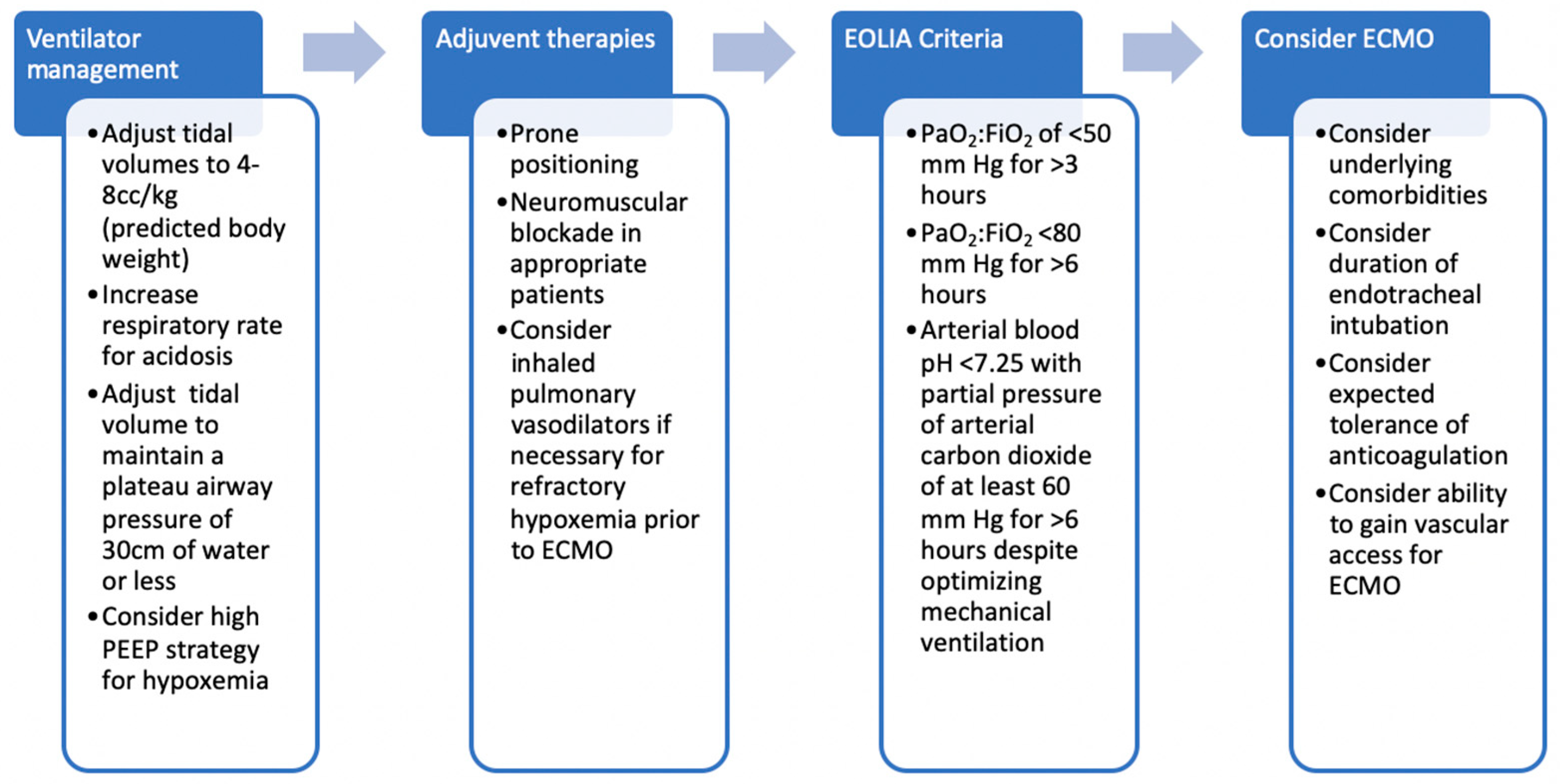
3. Criteria for ECMO
4. Complications
5. Consideration of Extracorporeal Carbon Dioxide Removal (ECCO2R) in Moderate ARDS
6. Ventilator Management
7. Anticoagulation
8. Blood Transfusion
9. Extubation and Mobilization
10. Evolving Applications of ECMO in ARDS
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zapol, W.M.; Snider, M.T.; Hill, J.D.; Fallat, R.J.; Bartlett, R.H.; Edmunds, L.H.; Morris, A.H.; Peirce, E.C., II; Thomas, A.N.; Proctor, H.J.; et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979, 242, 2193–2196. [Google Scholar] [CrossRef]
- Hill, J.D.; O’Brien, T.G.; Murray, J.J.; Dontigny, L.; Bramson, M.L.; Osborn, J.J.; Gerbode, F. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N. Engl. J. Med. 1972, 286, 629–634. [Google Scholar] [CrossRef]
- Combes, A.; Bacchetta, M.; Brodie, D.; Müller, T.; Pellegrino, V. Extracorporeal membrane oxygenation for respiratory failure in adults. Curr. Opin. Crit. Care 2012, 18, 99–104. [Google Scholar] [CrossRef] [PubMed]
- The Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar]
- Peek, G.J.; Mugford, M.; Tiruvoipati, R.; Wilson, A.; Allen, E.; Thalanany, M.M.; Hibbert, C.L.; Truesdale, A.; Clemens, F.; Cooper, N.; et al. CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 2009, 374, 1351–1363. [Google Scholar] [CrossRef]
- Davies, A.; Jones, D.; Gattas, D. Extracorporeal Membrane Oxygenation for ARDS Due to 2009 Influenza A(H1N1)—Reply. JAMA 2010, 303, 941–942. [Google Scholar] [CrossRef]
- Davies, A.; Jones, D.; Bailey, M.; Beca, J.; Bellomo, R.; Blackwell, N.; Forrest, P.; Gattas, D.; Granger, E.; Herkes, R.; et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009, 302, 1888–1895. [Google Scholar]
- Noah, M.A.; Peek, G.J.; Finney, S.J.; Griffiths, M.J.; Harrison, D.A.; Grieve, R.; Sadique, M.Z.; Sekhon, J.S.; McAuley, D.F.; Firmin, R.K.; et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011, 306, 1659–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zangrillo, A.; Biondi-Zoccai, G.; Landoni, G.; Frati, G.; Patroniti, N.; Pesenti, A.; Pappalardo, F. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: A systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit. Care 2013, 17, R30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combes, A.; Hajage, D.; Capellier, G.; Demoule, A.; Lavoué, S.; Guervilly, C.; Da Silva, D.; Zafrani, L.; Tirot, P.; Veber, B.; et al. EOLIA Trial Group, REVA, and ECMONet. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2018, 378, 1965–1975. [Google Scholar] [CrossRef]
- Goligher, E.C.; Tomlinson, G.; Hajage, D.; Wijeysundera, D.N.; Fan, E.; Jüni, P.; Brodie, D.; Slutsky, A.S.; Combes, A. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome and Posterior Probability of Mortality Benefit in a Post Hoc Bayesian Analysis of a Randomized Clinical Trial. JAMA 2018, 320, 2251–2259. [Google Scholar] [CrossRef]
- Munshi, L.; Walkey, A.; Goligher, E.; Pham, T.; Uleryk, E.M.; Fan, E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Lancet Respir Med. 2019, 7, 163–172. [Google Scholar] [CrossRef]
- Abrams, D.; Ferguson, N.D.; Brochard, L.; Fan, E.; Mercat, A.; Combes, A.; Pellegrino, V.; Schmidt, M.; Slutsky, A.; Brodie, D. ECMO for ARDS: From salvage to standard of care? Lancet Respir. Med. 2019, 7, 108–110. [Google Scholar] [CrossRef]
- Brodie, D.; Slutsky, A.; Combes, A. Extracorporeal Life Support for Adults with Respiratory Failure and Related Indications: A Review. JAMA 2019, 322, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Agerstrand, C.L.; Bacchetta, M.D.; Brodie, D. ECMO for adult respiratory failure: Current use and evolving applications. ASAIO 2014, 60, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Fanelli, V.; Pham, T.; Ranieri, V.M. Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: The SUPERNOVA study. Intensive Care Med. 2019, 45, 592–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lower tidal volume strategy (3 mL/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS The prospective randomized Xtravent-study. Intensive Care Med. 2013, 39, 847–856. [CrossRef] [PubMed] [Green Version]
- Brenner, K.; Abrams, D.C.; Agerstrand, C.L.; Brodie, D. Extracorporeal carbon dioxide removal for refractory status asthmaticus: Experience in distinct exacerbation phenotypes. Perfusion 2014, 29, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Tipograf, Y.; Salna, M.; Minko, E.; Grogan, E.L.; Agerstrand, C.; Sonett, J.; Brodie, D.; Bacchetta, M. Outcomes of Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation. Ann. Thorac. Surg. 2019, 107, 1456–1463. [Google Scholar] [CrossRef] [Green Version]
- Abrams, D.C.; Brenner, K.; Burkart, K.M.; Agerstrand, C.L.; Thomashow, B.M.; Bacchetta, M.; Brodie, D. Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2013, 10, 307–314. [Google Scholar] [CrossRef]
- Schmidt, M.; Pham, T.; Arcadipane, A.; Agerstrand, C.; Ohshimo, S.; Pellegrino, V.; Vuylsteke, A.; Guervilly, C.; McGuinness, S.; Pierard, S.; et al. Mechanical Ventilation Management during Extracorporeal Membrane Oxygenation for Acute Respiratory Distress Syndrome. An International Multicenter Prospective Cohort. Am. J. Respir. Crit. Care Med. 2019, 200, 1002–1012. [Google Scholar] [CrossRef]
- Abrams, D.; Schmidt, M.; Pham, T.; Beitler, J.R.; Fan, E.; Goligher, E.C.; McNamee, J.J.; Patroniti, N.; Wilcox, M.E.; Combes, A.; et al. Mechanical Ventilation for Acute Respiratory Distress Syndrome during Extracorporeal Life Support. Research and Practice. Am. J. Respir. Crit. Care Med. 2020, 201, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Marhong, J.D.; Munshi, L.; Detsky, M.; Telesnicki, T.; Fan, E. Mechanical ventilation during extracorporeal life support (ECLS): A systematic review. Intensive Care Med. 2015, 41, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Bembea, M.M.; Annich, G.; Rycus, P.; Oldenburg, G.; Berkowitz, I.; Pronovost, P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: An international survey. Pediatr. Crit. Care Med. 2013, 14, e77–e84. [Google Scholar] [CrossRef]
- Agerstrand, C.L.; Burkart, K.M.; Abrams, D.C.; Bacchetta, M.D.; Brodie, D. Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann. Thorac. Surg. 2015, 99, 590–595. [Google Scholar] [CrossRef]
- Hébert, P.C.; Wells, G.; Blajchman, M.A.; Marshall, J.; Martin, C.; Pagliarello, G.; Tweeddale, M.; Schweitzer, I.; Yetisir, E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N. Engl. J. Med. 1999, 340, 409–417. [Google Scholar] [CrossRef]
- Doyle, A.J.; Richardson, C.; Sanderson, B.; Wong, K.; Wyncoll, D.; Camporota, L.; Barrett, N.A.; Hunt, B.J.; Retter, A. Restrictive Transfusion Practice in Adults Receiving Venovenous Extracorporeal Membrane Oxygenation: A Single-Center Experience. Crit. Care Explor. 2020, 2, e0077. [Google Scholar] [CrossRef]
- Xia, J.; Gu, S.; Li, M.; Liu, D.; Huang, X.; Yi, L.; Wu, L.; Fan, G.; Zhan, Q. Spontaneous breathing in patients with severe acute respiratory distress syndrome receiving prolonged extracorporeal membrane oxygenation. BMC Pulm. Med. 2019, 19, 237. [Google Scholar] [CrossRef]
- Cho, H.J.; Heinsar, S.; Jeong, I.S.; Shekar, K.; Li Bassi, G.; Jung, J.S.; Suen, J.Y.; Fraser, J.F. ECMO use in COVID-19: Lessons from past respiratory virus outbreaks-a narrative review. Crit. Care 2020, 24, 301. [Google Scholar] [CrossRef]
- Alshahrani, M.S.; Sindi, A.; Alshamsi, F.; Al-Omari, A.; El Tahan, M.; Alahmadi, B.; Zein, A.; Khatani, N.; Al-Hameed, F.; Alamri, S.; et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann. Intensive Care 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Cai, Z.; Xianyu, Y.; Yang, B.X.; Song, T.; Yan, Q. Prognosis when using extracorporeal membrane oxygenation (ECMO) for critically ill COVID-19 patients in China: A retrospective case series. Crit. Care 2020, 24, 148. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.; Xiong, J.; Feng, Z.; Shi, Y. Extracorporeal membrane oxygenation (ECMO): Does it have a role in the treatment of severe COVID-19? Int. J. Infect. Dis. 2020, 94, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, K.; Antognini, D.; Combes, A.; Paden, M.; Zakhary, B.; Ogino, M.; MacLaren, G.; Brodie, D.; Shekar, K. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir. Med. 2020, 8, 518–526. [Google Scholar] [CrossRef] [Green Version]
- Abrams, D.; Lorusso, R.; Vincent, J.L.; Brodie, D. ECMO during the COVID-19 pandemic: When is it unjustified? Crit. Care 2020, 24, 507. [Google Scholar] [CrossRef]
- Barbaro, R.P.; MacLaren, G.; Boonstra, P.S.; Iwashyna, T.J.; Slutsky, A.S.; Fan, E.; Bartlett, R.H.; Tonna, J.E.; Hyslop, R.; Fanning, J.J.; et al. Extracorporeal Life Support Organization. Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020, 396, 1071–1078. [Google Scholar] [CrossRef]
- Schmidt, M.; Hajage, D.; Lebreton, G.; Monsel, A.; Voiriot, G.; Levy, D.; Baron, E.; Beurton, A.; Chommeloux, J.; Meng, P.; et al. Groupe de Recherche Clinique en REanimation et Soins intensifs du Patient en Insuffisance Respiratoire aiguE (GRC-RESPIRE) Sorbonne Université; Paris-Sorbonne ECMO-COVID investigators. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: A retrospective cohort study. Lancet Respir. Med. 2020, 8, 1121–1131. [Google Scholar]
- Diaz, R.A.; Graf, J.; Zambrano, J.M.; Ruiz, C.; Espinoza, J.A.; Bravo, S.I.; Salazar, P.A.; Bahamondes, J.C.; Castillo, L.B.; Gajardo, A.I.; et al. National Advisory Commission for Adult ECMO. ECMO for COVID-19-Associated Severe ARDS in Chile: A Nationwide Incidence and Cohort Study. Am. J. Respir. Crit. Care Med. 2021. [Google Scholar] [CrossRef]
- Lebreton, G.; Schmidt, M.; Ponnaiah, M.; Folliguet, T.; Para, M.; Guihaire, J.; Lansac, E.; Sage, E.; Cholley, B.; Mégarbane, B.; et al. Paris ECMO-COVID-19 investigators. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: A multicentre cohort study. Lancet Respir. Med. 2021. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feldhaus, D.; Brodie, D.; Lemaitre, P.; Sonett, J.; Agerstrand, C. The Evolution of the Use of Extracorporeal Membrane Oxygenation in Respiratory Failure. Membranes 2021, 11, 491. https://doi.org/10.3390/membranes11070491
Feldhaus D, Brodie D, Lemaitre P, Sonett J, Agerstrand C. The Evolution of the Use of Extracorporeal Membrane Oxygenation in Respiratory Failure. Membranes. 2021; 11(7):491. https://doi.org/10.3390/membranes11070491
Chicago/Turabian StyleFeldhaus, Danielle, Daniel Brodie, Philippe Lemaitre, Joshua Sonett, and Cara Agerstrand. 2021. "The Evolution of the Use of Extracorporeal Membrane Oxygenation in Respiratory Failure" Membranes 11, no. 7: 491. https://doi.org/10.3390/membranes11070491
APA StyleFeldhaus, D., Brodie, D., Lemaitre, P., Sonett, J., & Agerstrand, C. (2021). The Evolution of the Use of Extracorporeal Membrane Oxygenation in Respiratory Failure. Membranes, 11(7), 491. https://doi.org/10.3390/membranes11070491




