Mitochondrial Cristae Architecture and Functions: Lessons from Minimal Model Systems
Abstract
1. Introduction
2. Mitochondrial Cristae: Dynamics Bioenergetic Compartments
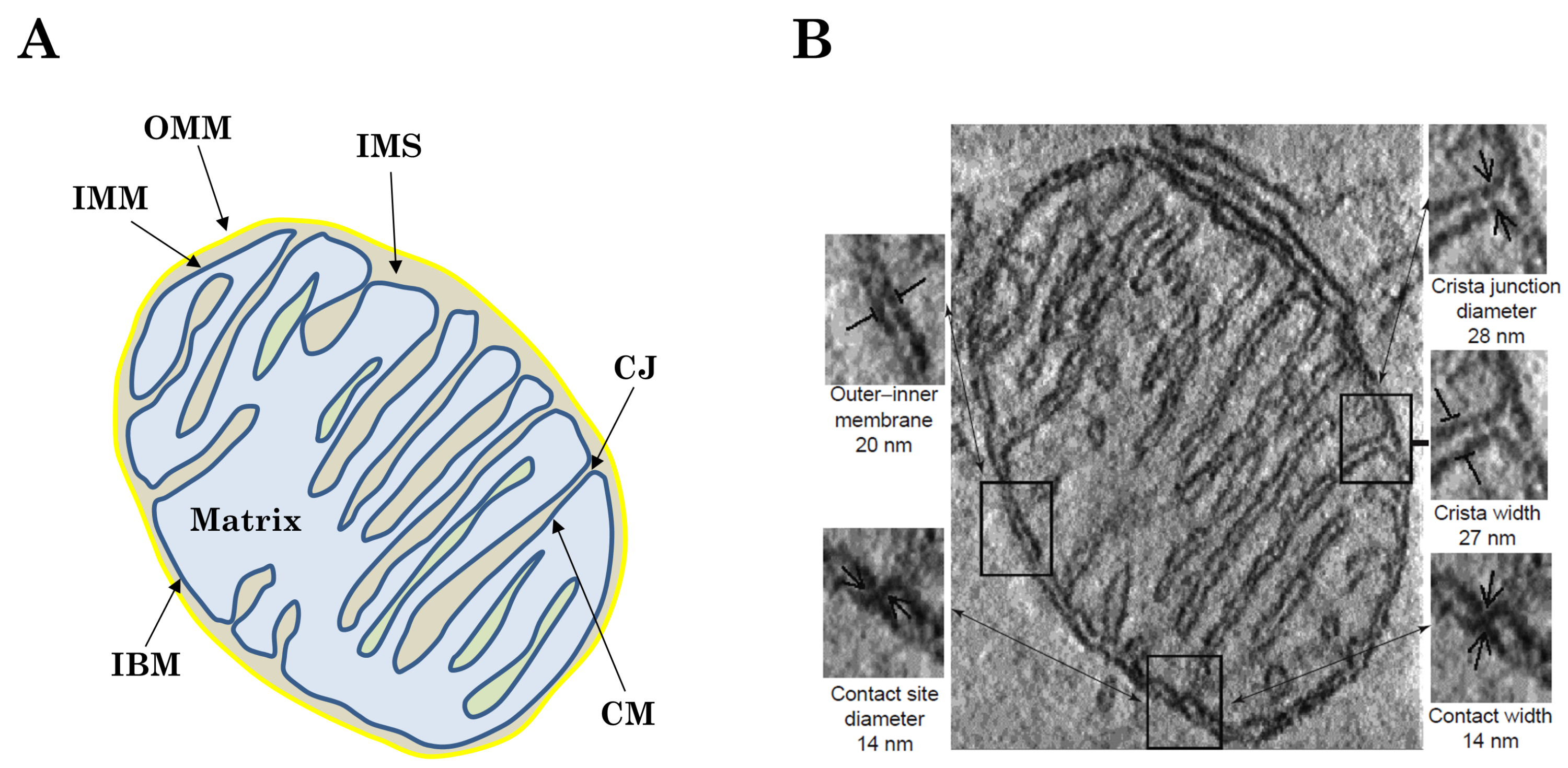
2.1. Morphology and Structure-Function Relationship of Cristae
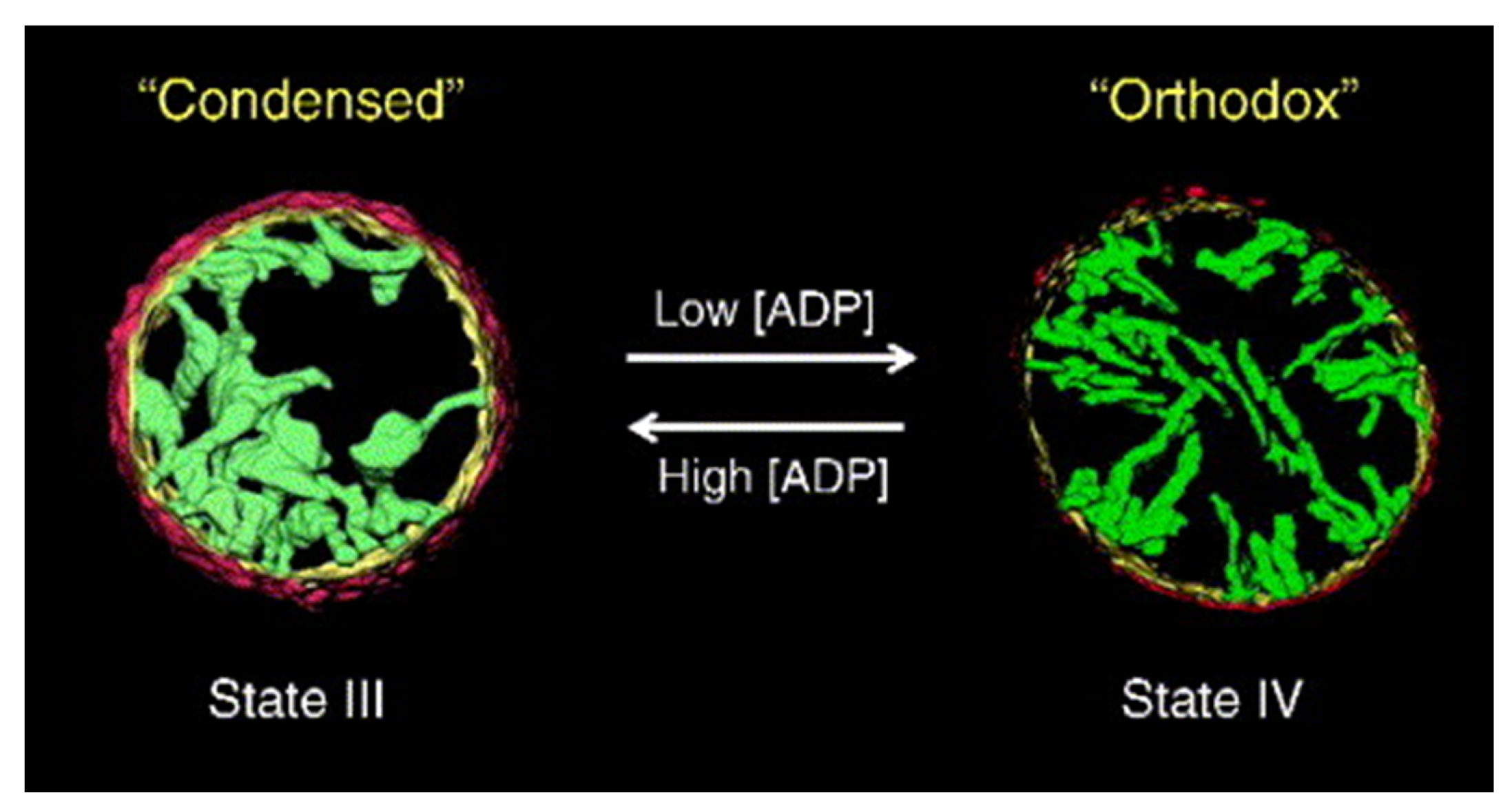
2.2. Plasticity of Cristae
3. Lipids of Mitochondria: Focus on the IMM
3.1. Presentation of the Main IMM Lipids
3.2. IMM Lipid Shape Matters
4. Physicochemical Properties of Only-IMM Lipid Systems
4.1. Interactions of CL with Other Phospholipids
4.2. Mechanical Properties of Only-IMM Lipid Systems
4.3. Non-Specific Regulatory Roles of IMM Lipids
5. The Intricate Link between Lipid Composition and Cristae Organization Revealed by Minimal Model Systems
5.1. Role of CL in Cristae Biogenesis, Morphology and Dynamics
5.2. Cone-Shaped Lipid Sorting within the IMM: Cristae Curvature and Leaflet Asymmetry
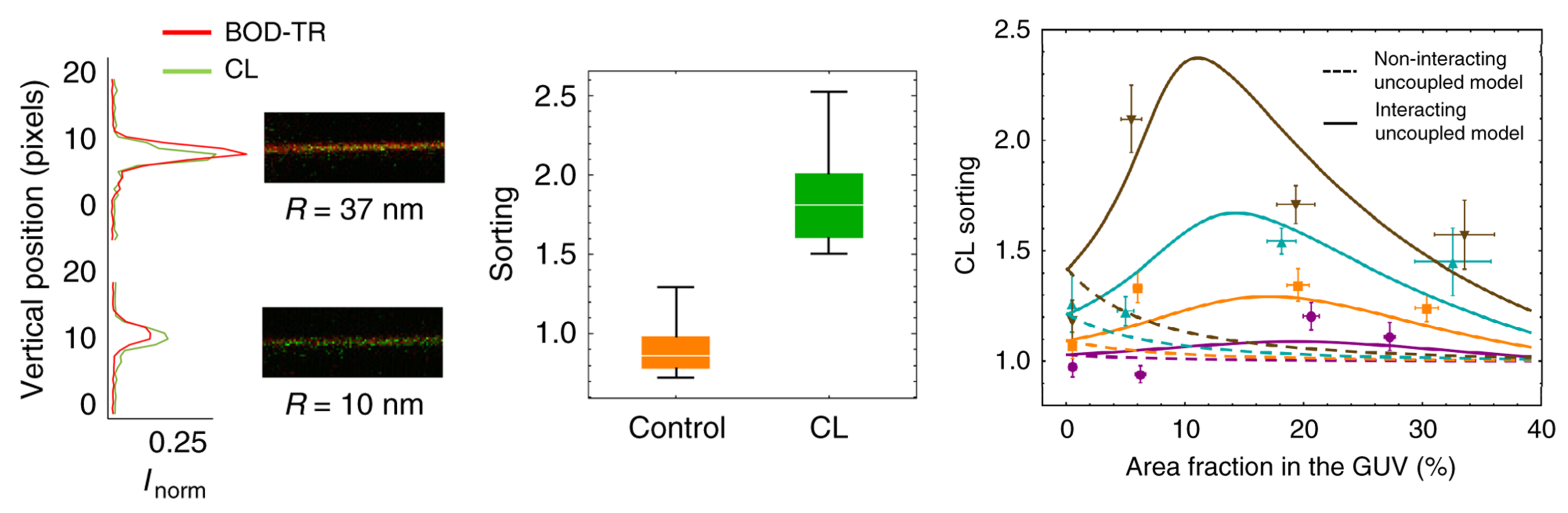
5.2.1. CL Enrichment Inside Cristae: The Role of Membrane Curvature
5.2.2. CL Asymmetric Distribution between Cristae Leaflet: Really More CL on the Matrix Side?
5.2.3. The PE Case
5.3. Lateral Membrane Organization within Cristae-Like Membranes
5.3.1. Is Lateral Compartmentalization Detected in Only-Lipid Systems?
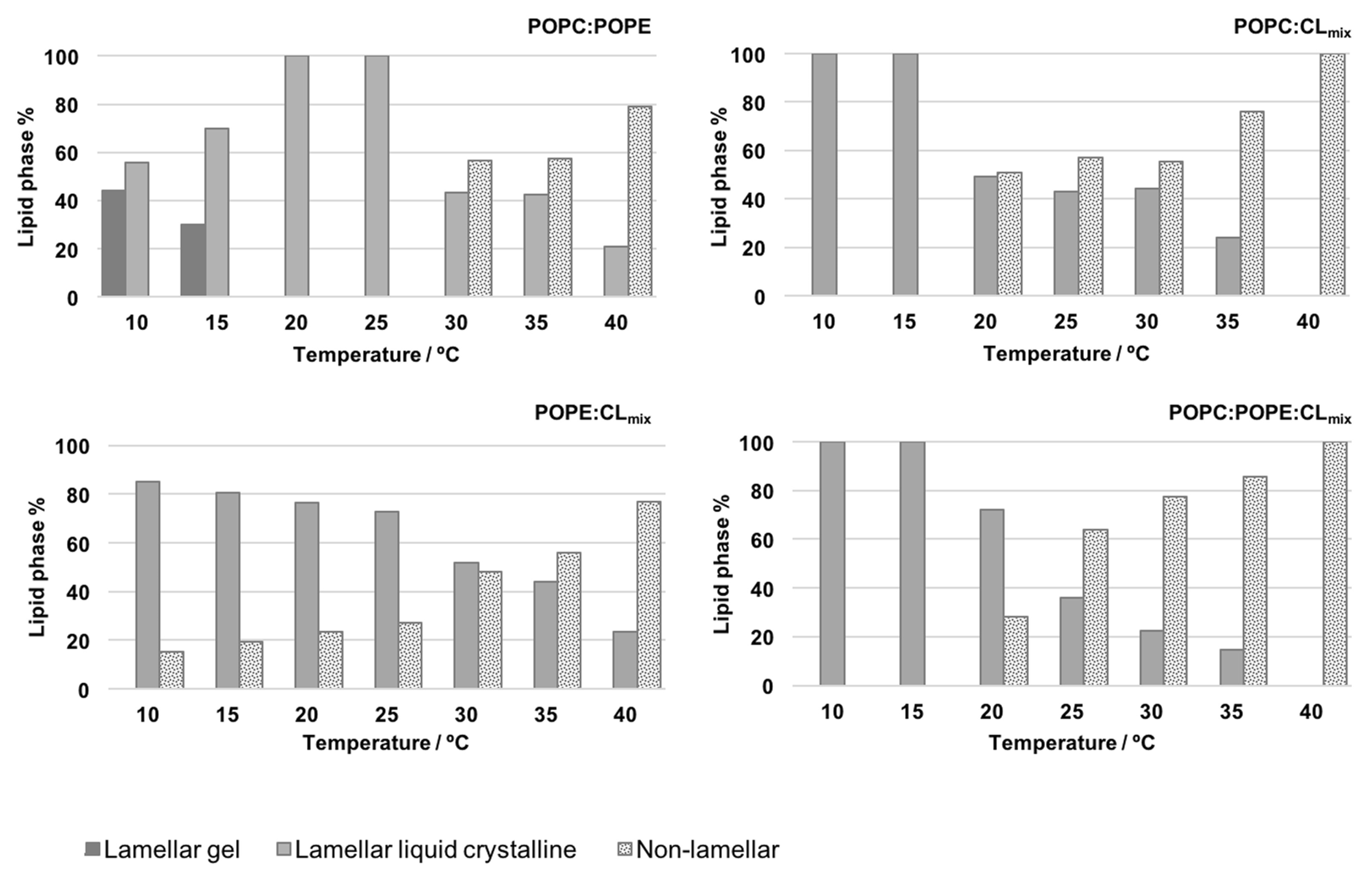
5.3.2. Possible Lamellar/Nonlamellar Phase Coexistence
6. Discussion: Lessons from Minimal Models for Lipid Functional Implications
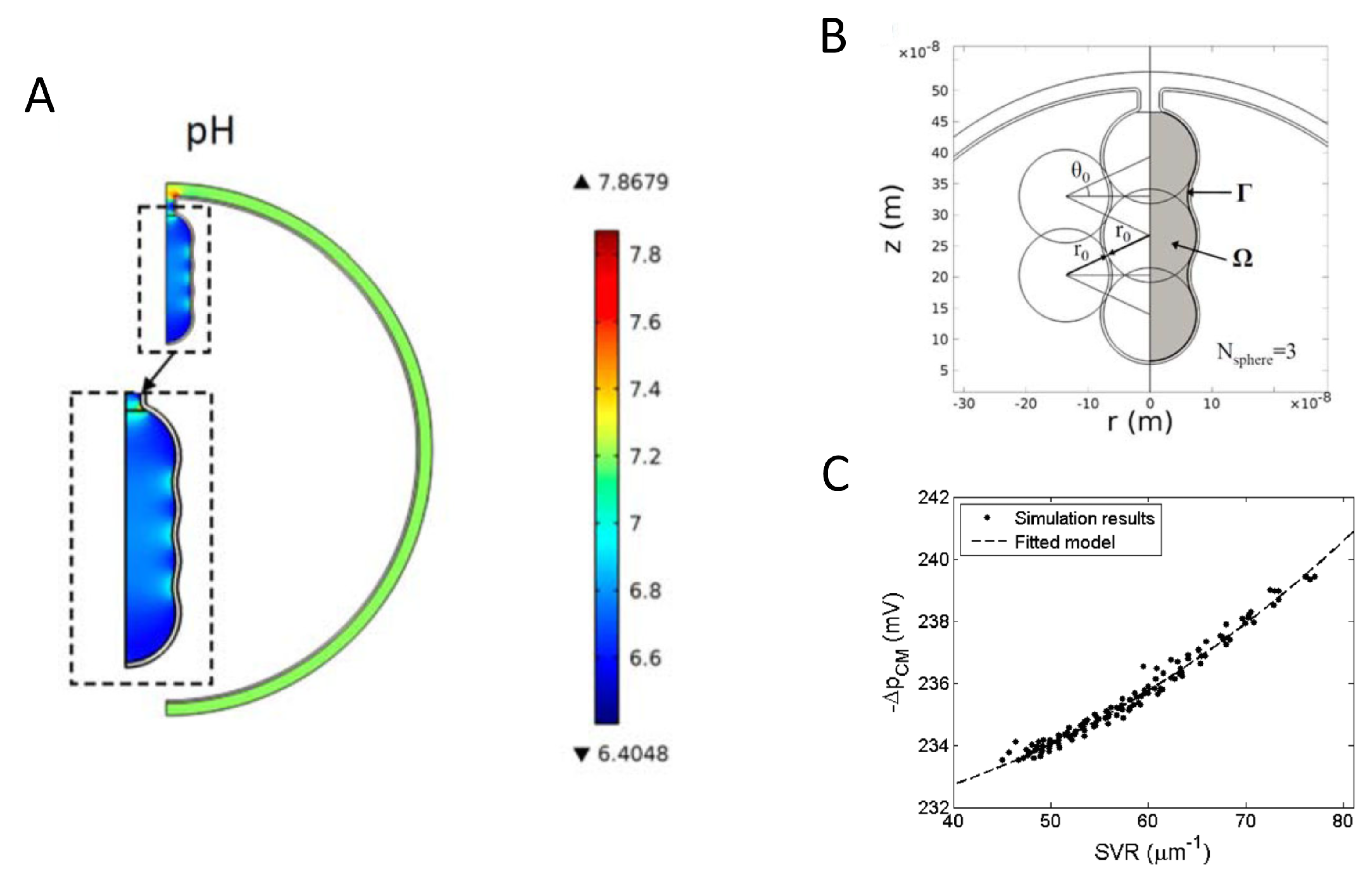
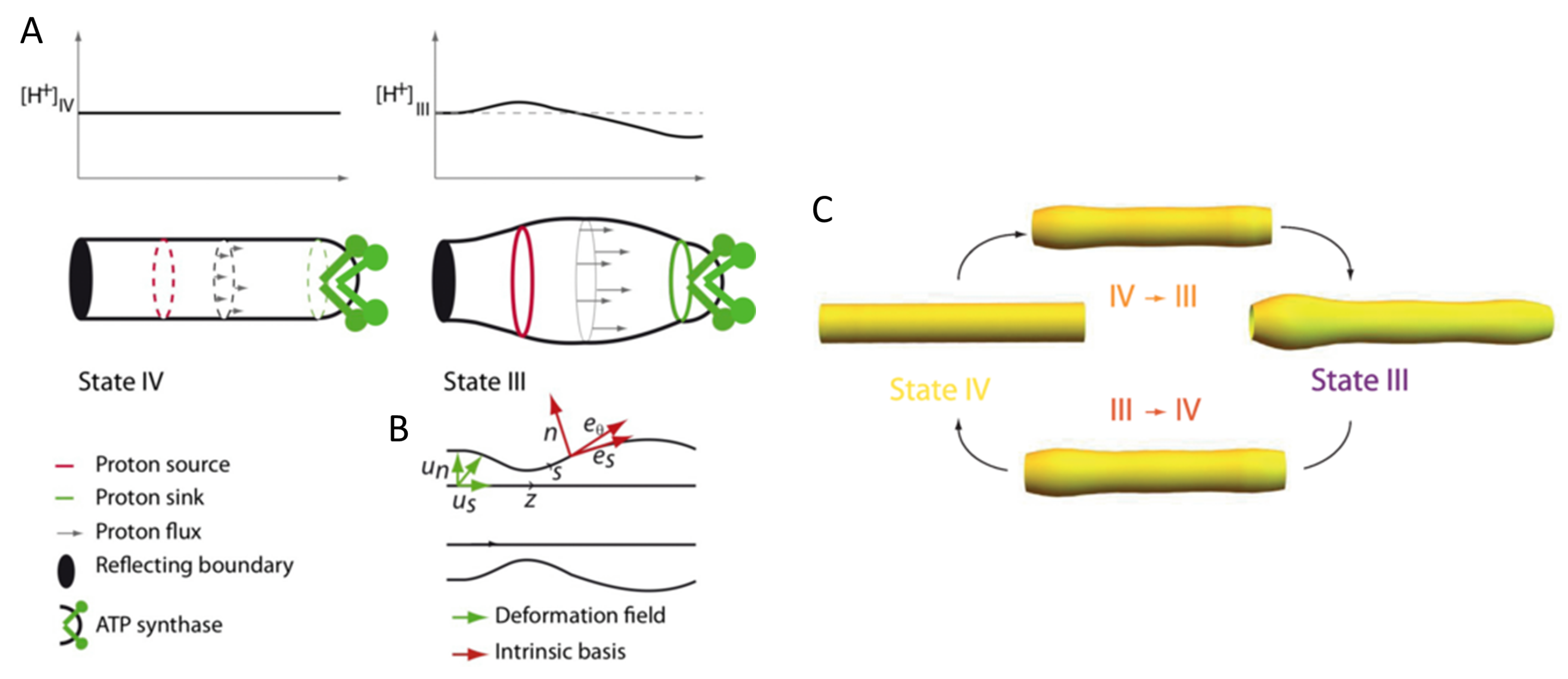
6.1. Cristae Plasticity: CL as a Sensor of Proton and Calcium Concentrations for the Modulation of Membrane Properties
6.2. Impact of CL Enrichment in OXPHOS Functioning: To Facilitate Proton Circuit along the Membrane
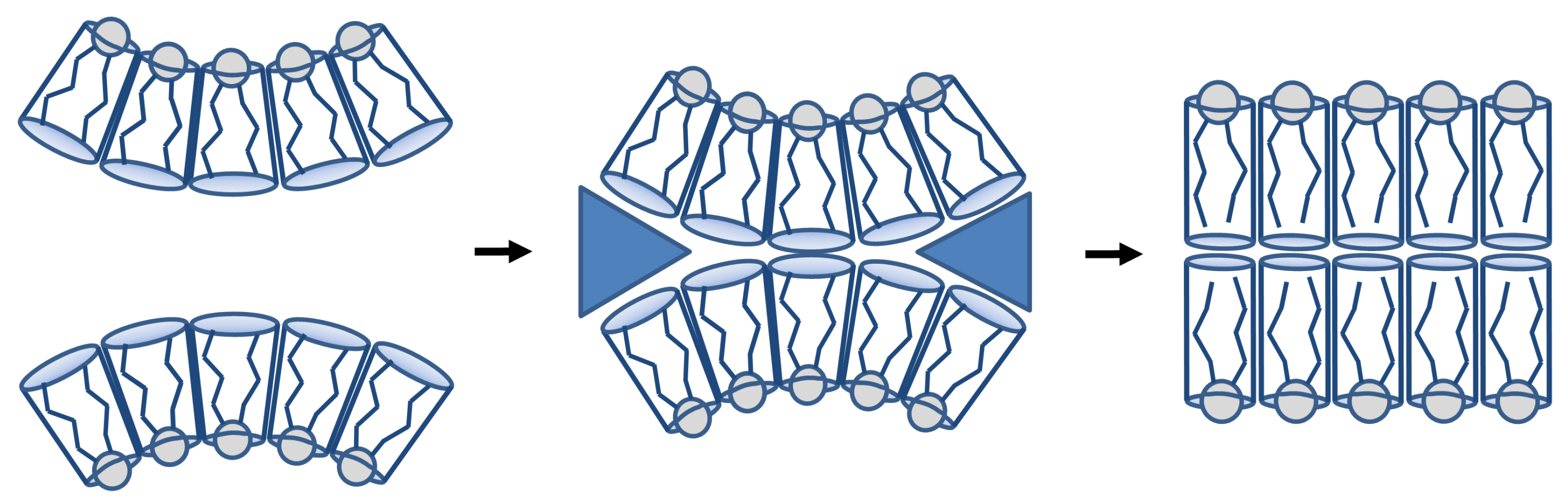
6.3. Role of Lipid Lateral Heterogeneity in ATP Synthase Functioning
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| OXPHOS | Oxidative phosphorylation |
| IMM | Inner mitochondrial membrane |
| MICOS | Mitochondrial contact site and cristae organizing System |
| OPA1 | GTPase optic atrophy 1 |
| OMM | Outer mitochondrial membrane |
| IBM | Inner boundary membrane |
| CM | Cristae membrane |
| IMS | Inner mitochondrial space |
| CJs | Cristae junctions |
| NADH | Nicotinamide adenine dinucleotide hydrogen |
| FADH_2 | Flavin adenine dinucleotide hydroquinone form |
| PMF | Proton-motive force |
| FF-ATP synthase | F-type ATP synthase |
| CL | Cardiolipin |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PI | Phosphatidylinositol |
| DOPE | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine |
| EPC | Egg phosphatidylcholine |
| POPC | 1-palmitoyl-2-oleoyl-glycero-3-phosphocholine |
| POPE | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DPPE | 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine |
| SOPC | 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| GUV | Giant unilamellar vesicle |
| PS | Phosphatidylserine |
| PG | Phosphatidylglycerol |
| Cyt c | Cytochrome c |
| DCCD-BPF | Dicyclohexylcarbodiimide-binding protein of the sector |
| NAO | Nonyl acridine orange |
| SMP | Submitochondrial particle |
| LUV | Large unilamellar vesicle |
| TTAPE-Me | 1,1,2,2-tetrakis[4-(2-trimethylammonioethoxy)-phenyl]ethene |
| HACD1 | 3-hydroxyacyl-CoA dehydratase 1 |
References
- Vafai, S.; Mootha, V. Mitochondrial disorders as windows into an ancient organelle. Nature 2012, 491, 374–383. [Google Scholar] [CrossRef]
- Pánek, T.; Eliáš, M.; Vancová, M.; Lukeš, J.; Hashimi, H. Returning to the Fold for Lessons in Mitochondrial Crista Diversity and Evolution. Curr. Biol. 2020, 30, R575–R588. [Google Scholar] [CrossRef] [PubMed]
- Kondadi, A.K.; Anand, R.; Reichert, A.S. Cristae Membrane Dynamics—A Paradigm Change. Trends Cell Biol. 2020, 30, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Zick, M.; Rabl, R.; Reichert, A.S. Cristae formation—linking ultrastructure and function of mitochondria. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2009, 1793, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, S.; Enriquez, J.A.; Scorrano, L. Mitochondrial Cristae: Where Beauty Meets Functionality. Trends Biochem. Sci. 2016, 41, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Colina-Tenorio, L.; Horten, P.; Pfanner, N.; Rampelt, H. Shaping the mitochondrial inner membrane in health and disease. J. Intern. Med. 2020, 287. [Google Scholar] [CrossRef]
- Glancy, B.; Kim, Y.; Katti, P.; Willingham, T.B. The Functional Impact of Mitochondrial Structure Across Subcellular Scales. Front. Physiol. 2020, 11, 1462. [Google Scholar] [CrossRef] [PubMed]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef]
- Cullis, P.; De Kruijff, B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1979, 559, 399–420. [Google Scholar] [CrossRef]
- Shearman, G.C.; Ces, O.; Templer, R.H.; Seddon, J.M. Inverse lyotropic phases of lipids and membrane curvature. J. Phys. Condens. Matter 2006, 18, S1105–S1124. [Google Scholar] [CrossRef] [PubMed]
- Burger, K.N. Greasing Membrane Fusion and Fission Machineries. Traffic 2000, 1, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Frolov, V.; Shnyrova, A.; Zimmerberg, J. Lipid Polymorphisms and Membrane Shape. Cold Spring Harb. Perspect. Biol. 2011, 3, a004747. [Google Scholar] [CrossRef]
- Roger, A.J.; Muñoz-Gómez, S.A.; Kamikawa, R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017, 27, R1177–R1192. [Google Scholar] [CrossRef]
- Raven, J.A. Determinants, and implications, of the shape and size of thylakoids and cristae. J. Plant Physiol. 2021, 257, 153342. [Google Scholar] [CrossRef] [PubMed]
- Frey, T.G.; Manella, C.A. The internal structure of mitochondria. Trends Biochem. Sci. 2000, 25, 319–324. [Google Scholar] [CrossRef]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef] [PubMed]
- Schlame, M. Protein crowding in the inner mitochondrial membrane. Biochim. Biophys. Acta (BBA) Bioenerg. 2021, 1862, 148305. [Google Scholar] [CrossRef] [PubMed]
- Busch, K.B. Inner mitochondrial membrane compartmentalization: Dynamics across scales. Int. J. Biochem. Cell Biol. 2020, 120, 105694. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P. Coupling of Phosphorylation to Electron and Hydrogen Transfer by a Chemi-Osmotic type of Mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Gejl, K.; Hey-Mogensen, M.; Holmberg, H.C.; Suetta, C.; Krustrup, P.; Elemans, C.; Ortenblad, N. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J. Physiol. 2016, 595. [Google Scholar] [CrossRef] [PubMed]
- Acehan, D.; Xu, Y.; Stokes, D.; Schlame, M. Comparison of lymphoblast mitochondria from normal subjects and patients with Barth syndrome using electron microscopic tomography. Lab. Investig. A J. Tech. Methods Pathol. 2007, 87, 40–48. [Google Scholar] [CrossRef]
- Siegmund, S.E.; Grassucci, R.; Carter, S.D.; Barca, E.; Farino, Z.J.; Juanola-Falgarona, M.; Zhang, P.; Tanji, K.; Hirano, M.; Schon, E.A.; et al. Three-Dimensional Analysis of Mitochondrial Crista Ultrastructure in a Patient with Leigh Syndrome by In Situ Cryoelectron Tomography. iScience 2018, 6, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Prola, A.; Blondelle, J.; Vandestienne, A.; Piquereau, J.; Denis, R.G.P.; Guyot, S.; Chauvin, H.; Mourier, A.; Maurer, M.; Henry, C.; et al. Cardiolipin content controls mitochondrial coupling and energetic efficiency in muscle. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Palade, G.E. The fine structure of mitochondria. Anat. Rec. 1952, 114, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Mannella, C.A. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2006, 1763, 542–548. [Google Scholar] [CrossRef]
- Ikon, N.; Ryan, R.O. Cardiolipin and mitochondrial cristae organization. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Cabrera, R.; Mehrotra, A.; Rigoni, G.; Soriano, M. Who and how in the regulation of mitochondrial cristae shape and function. Biochem. Biophys. Res. Commun. 2018, 500, 94–101. [Google Scholar] [CrossRef]
- Blum, T.B.; Hahn, A.; Meier, T.; Davies, K.M.; Kühlbrandt, W. Dimers of mitochondrial ATP synthase induce membrane curvature and self-assemble into rows. Proc. Natl. Acad. Sci. USA 2019, 116, 4250–4255. [Google Scholar] [CrossRef]
- Renken, C.; Siragusa, G.; Perkins, G.; Washington, L.; Nulton, J.; Salamon, P.; Frey, T.G. A thermodynamic model describing the nature of the crista junction: A structural motif in the mitochondrion. J. Struct. Biol. 2002, 138, 137–144. [Google Scholar] [CrossRef]
- Ponnuswamy, A.; Nulton, J.; Mahaffy, J.M.; Salamon, P.; Frey, T.G.; Baljon, A.R.C. Modeling tubular shapes in the inner mitochondrial membrane. Phys. Biol. 2005, 2, 73–79. [Google Scholar] [CrossRef]
- Ghochani, M.; Nulton, J.; Salamon, P.; Frey, T.; Rabinovitch, A.; Baljon, A. Tensile Forces and Shape Entropy Explain Observed Crista Structure in Mitochondria. Biophys. J. 2010, 99, 3244–3254. [Google Scholar] [CrossRef] [PubMed]
- Gilkerson, R.W.; Selker, J.M.; Capaldi, R.A. The cristal membrane of mitochondria is the principal site of oxidative phosphorylation. FEBS Lett. 2003, 546, 355–358. [Google Scholar] [CrossRef]
- Vogel, F.; Bornhövd, C.; Neupert, W.; Reichert, A.S. Dynamic subcompartmentalization of the mitochondrial inner membrane. J. Cell Biol. 2006, 175, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Song, D.H.; Park, J.; Maurer, L.; Lu, W.; Philbert, M.; Sastry, A. Biophysical significance of the inner mitochondrial membrane structure on the electrochemical potential of mitochondria. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2013, 88, 062723. [Google Scholar] [CrossRef] [PubMed]
- Westermann, B. Westermann BMitochondrial fusion and fission in cell life and death. Nat. Reviews. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Hyde, B.; Shirihai, O.S. Mitochondrial fusion, fission and autophagy as a quality control axis: The bioenergetic view. Biochim. Biophys. Acta (BBA) Bioenerg. 2008, 1777, 1092–1097. [Google Scholar] [CrossRef]
- Piquereau, J.; Caffin, F.; Novotova, M.; Prola, A.; Garnier, A.; Mateo, P.; Fortin, D.; Huynh, L.H.; Nicolas, V.; Alavi, M.V.; et al. Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc. Res. 2012, 94, 408–417. [Google Scholar] [CrossRef]
- Hackenbrock, C.R. Ultrastructural bases for metabolically linked mechanical activity in mitochondria: I. Reversible Ultrastructural Changes with Change in Metabolic Steady State in Isolated Liver Mitochondria. J. Cell Biol. 1966, 30, 269–297. [Google Scholar] [CrossRef] [PubMed]
- Chvanov, M. Metabolic Control of Elastic Properties of the Inner Mitochondrial Membrane. J. Phys. Chem. B 2006, 110, 22903–22909. [Google Scholar] [CrossRef]
- Almendro-Vedia, V.G.; Natale, P.; Mell, M.; Bonneau, S.; Monroy, F.; Joubert, F.; López-Montero, I. Nonequilibrium fluctuations of lipid membranes by the rotating motor protein F1F0-ATP synthase. Proc. Natl. Acad. Sci. USA 2017, 114, 11291–11296. [Google Scholar] [CrossRef]
- Huang, X.; Fan, J.; Li, L.; Liu, H.; Wu, R.; Wu, Y.; Wei, L.; Mao, H.; Lal, A.; Xi, P.; et al. Fast, long-term, super-resolution imaging with Hessian structured illumination microscopy. Nat. Biotechnol. 2018, 36, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Stephan, T.; Roesch, A.; Riedel, D.; Jakobs, S. Live-cell STED nanoscopy of mitochondrial cristae. Sci. Rep. 2019, 9, 12419. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Taki, M.; Sato, Y.; Tamura, Y.; Yaginuma, H.; Okada, Y.; Yamaguchi, S. A photostable fluorescent marker for the superresolution live imaging of the dynamic structure of the mitochondrial cristae. Proc. Natl. Acad. Sci. USA 2019, 116, 15817–15822. [Google Scholar] [CrossRef]
- Wolf, D.M.; Segawa, M.; Kondadi, A.K.; Anand, R.; Bailey, S.T.; Reichert, A.S.; van der Bliek, A.M.; Shackelford, D.B.; Liesa, M.; Shirihai, O.S. Individual cristae within the same mitochondrion display different membrane potentials and are functionally independent. EMBO J. 2019, 38, e101056. [Google Scholar] [CrossRef] [PubMed]
- Segawa, M.; Wolf, D.M.; Hultgren, N.W.; Williams, D.S.; van der Bliek, A.M.; Shackelford, D.B.; Liesa, M.; Shirihai, O.S. Quantification of cristae architecture reveals time-dependent characteristics of individual mitochondria. Life Sci. Alliance 2020, 3. [Google Scholar] [CrossRef] [PubMed]
- Kondadi, A.K.; Anand, R.; Hänsch, S.; Urbach, J.; Zobel, T.; Wolf, D.M.; Segawa, M.; Liesa, M.; Shirihai, O.S.; Weidtkamp-Peters, S.; et al. Cristae undergo continuous cycles of membrane remodelling in a MICOS-dependent manner. EMBO Rep. 2020, 21, e49776. [Google Scholar] [CrossRef]
- Osman, C.; Voelker, D.R.; Langer, T. Making heads or tails of phospholipids in mitochondria. J. Cell Biol. 2011, 192, 7–16. [Google Scholar] [CrossRef]
- Daum, G.; Vance, J.E. Import of lipids into mitochondria. Prog. Lipid Res. 1997, 36, 103–130. [Google Scholar] [CrossRef]
- Mejia, E.M.; Hatch, G.M. Mitochondrial phospholipids: Role in mitochondrial function. J. Bioenerg. Biomembr. 2016, 48, 99–112. [Google Scholar] [CrossRef]
- Kates, M.; Syz, J.Y.; Gosser, D.; Haines, T.H. pH-dissociation characteristics of cardiolipin and its 2′-deoxy analogue. Lipids 1993, 28, 877–882. [Google Scholar] [CrossRef]
- Lewis, R.N.; McElhaney, R.N. The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 2069–2079. [Google Scholar] [CrossRef]
- Sathappa, M.; Alder, N.N. The ionization properties of cardiolipin and its variants in model bilayers. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, G.; Sparr, E. Ionization Constants pKa of Cardiolipin. PLoS ONE 2013, 8, 1–6. [Google Scholar] [CrossRef]
- Kooijman, E.; Swim, L.; Graber, Z.; Tyurina, Y.; Bayır, H.; Kagan, V. Magic angle spinning 31P NMR spectroscopy reveals two essentially identical ionization states for the cardiolipin phosphates in phospholipid liposomes. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859, 61–68. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Gruner, S.M. Intrinsic curvature hypothesis for biomembrane lipid composition: A role for nonbilayer lipids. Proc. Natl. Acad. Sci. USA 1985, 82, 3665–3669. [Google Scholar] [CrossRef]
- Oemer, G.; Koch, J.; Wohlfarter, Y.; Alam, M.T.; Lackner, K.; Sailer, S.; Neumann, L.; Lindner, H.H.; Watschinger, K.; Haltmeier, M.; et al. Phospholipid Acyl Chain Diversity Controls the Tissue-Specific Assembly of Mitochondrial Cardiolipins. Cell Rep. 2020, 30, 4281–4291.e4. [Google Scholar] [CrossRef] [PubMed]
- Pennington, E.R.; Funai, K.; Brown, D.A.; Shaikh, S.R. The role of cardiolipin concentration and acyl chain composition on mitochondrial inner membrane molecular organization and function. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2019, 1864, 1039–1052. [Google Scholar] [CrossRef]
- Siegel, D.P.; Epand, R.M. The mechanism of lamellar-to-inverted hexagonal phase transitions in phosphatidylethanolamine: Implications for membrane fusion mechanisms. Biophys. J. 1997, 73, 3089–3111. [Google Scholar] [CrossRef]
- Zimmerberg, J. Membrane biophysics. Curr. Biol. 2006, 16, R272–R276. [Google Scholar] [CrossRef]
- Kollmitzer, B.; Heftberger, P.; Rappolt, M.; Pabst, G. Monolayer spontaneous curvature of raft-forming membrane lipids. Soft Matter 2013, 9, 10877–10884. [Google Scholar] [CrossRef]
- Ortiz, A.; Killian, J.A.; Verkleij, A.J.; Wilschut, J. Membrane Fusion and the Lamellar-to-Inverted-Hexagonal Phase Transition in Cardiolipin Vesicle Systems Induced by Divalent Cations. Biophys. J. 1999, 77, 2003–2014. [Google Scholar] [CrossRef]
- Chen, Y.F.; Tsang, K.Y.; Chang, W.F.; Fan, Z.A. Differential dependencies on [Ca2+] and temperature of the monolayer spontaneous curvatures of DOPE, DOPA and cardiolipin: Effects of modulating the strength of the inter-headgroup repulsion. Soft Matter 2015, 11, 4041–4053. [Google Scholar] [CrossRef]
- Nichols-Smith, S.; Teh, S.Y.; Kuhl, T. Thermodynamic and mechanical properties of model mitochondrial membranes. Biochim. Biophys. Acta 2004, 1663, 82–88. [Google Scholar] [CrossRef]
- Òscar, D.; Sanz, F.; Montero, M.T.; Hernández-Borrell, J. Thermodynamic and structural study of the main phospholipid components comprising the mitochondrial inner membrane. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 213–221. [Google Scholar] [CrossRef]
- Sennato, S.; Bordi, F.; Cametti, C.; Coluzza, C.; Desideri, A.; Rufini, S. Evidence of Domain Formation in Cardiolipin—Glycerophospholipid Mixed Monolayers. A Thermodynamic and AFM Study. J. Phys. Chem. B 2005, 109, 15950–15957. [Google Scholar] [CrossRef]
- Róg, T.; Martinez-Seara, H.; Munck, N.; Orešič, M.; Karttunen, M.; Vattulainen, I. Role of Cardiolipins in the Inner Mitochondrial Membrane: Insight Gained through Atom-Scale Simulations. J. Phys. Chem. B 2009, 113, 3413–3422. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, M.; Maliniak, A. Mechanical Properties of Coarse-Grained Bilayers Formed by Cardiolipin and Zwitterionic Lipids. J. Chem. Theory Comput. 2010, 6, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, M.; Maliniak, A. Molecular Dynamics Simulations of Cardiolipin Bilayers. J. Phys. Chem. B 2008, 112, 11655–11663. [Google Scholar] [CrossRef] [PubMed]
- Khalifat, N.; Fournier, J.B.; Angelova, M.I.; Puff, N. Lipid packing variations induced by pH in cardiolipin-containing bilayers: The driving force for the cristae-like shape instability. Biochim. Biophys. Acta (BBA) Biomembr. 2011, 1808, 2724–2733. [Google Scholar] [CrossRef]
- Pöyry, S.; Róg, T.; Karttunen, M.; Vattulainen, I. Mitochondrial Membranes with Mono- and Divalent Salt: Changes Induced by Salt Ions on Structure and Dynamics. J. Phys. Chem. B 2009, 113, 15513–15521. [Google Scholar] [CrossRef]
- Wilson, B.A.; Ramanathan, A.; Lopez, C.F. Cardiolipin-Dependent Properties of Model Mitochondrial Membranes from Molecular Simulations. Biophys. J. 2019, 117, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, W. Elastic Properties of Lipid Bilayers: Theory and Possible Experiments. Z. FüR Naturforschung 1973, 28, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Unsay, J.D.; Cosentino, K.; Subburaj, Y.; García-Sáez, A.J. Cardiolipin Effects on Membrane Structure and Dynamics. Langmuir 2013, 29, 15878–15887. [Google Scholar] [CrossRef]
- Pan, J.; Cheng, X.; Sharp, M.; Ho, C.S.; Khadka, N.; Katsaras, J. Structural and mechanical properties of cardiolipin lipid bilayers determined using neutron spin echo, small angle neutron and X-ray scattering, and molecular dynamics simulations. Soft Matter 2015, 11, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Boscia, A.L.; Treece, B.W.; Mohammadyani, D.; Klein-Seetharaman, J.; Braun, A.R.; Wassenaar, T.A.; Klösgen, B.; Tristram-Nagle, S. X-ray structure, thermodynamics, elastic properties and MD simulations of cardiolipin/dimyristoylphosphatidylcholine mixed membranes. Chem. Phys. Lipids 2014, 178, 1–10. [Google Scholar] [CrossRef]
- Rawicz, W.; Olbrich, K.; McIntosh, T.; Needham, D.; Evans, E. Effect of Chain Length and Unsaturation on Elasticity of Lipid Bilayers. Biophys. J. 2000, 79, 328–339. [Google Scholar] [CrossRef]
- Tiberti, M.; Antonny, B.; Gautier, R. The transbilayer distribution of polyunsaturated phospholipids determines their facilitating effect on membrane deformation. Soft Matter 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Boyd, K.; Alder, N.; May, E. Buckling Under Pressure: Curvature Based Lipid Segregation and Stability Modulation in Cardiolipin Containing Bilayers. Langmuir 2017, 33. [Google Scholar] [CrossRef]
- Beltrán-Heredia, E.; Tsai, F.C.; Salinas-Almaguer, S.; Cao, F.J.; Bassereau, P.; Monroy, F. Membrane curvature induces cardiolipin sorting. Commun. Biol. 2019, 2, 225. [Google Scholar] [CrossRef] [PubMed]
- Rand, R.; Sengupta, S. Cardiolipin forms hexagonal structures with divalent cations. Biochim. Biophys. Acta (BBA) Biomembr. 1972, 255, 484–492. [Google Scholar] [CrossRef]
- Cullis, P.; Verkleij, A.; Ververgaert, P. Polymorphic phase behaviour of cardiolipin as detected by 31P NMR and freeze-fracture techniques. Effects of calcium, dibucaine and chlorpromazine. Biochim. Biophys. Acta (BBA) Biomembr. 1978, 513, 11–20. [Google Scholar] [CrossRef]
- de Kruijff, B.; Verkleij, A.; Leunissen-Bijvelt, J.; van Echteld, C.; Hille, J.; Rijnbout, H. Further aspects of the Ca2+-dependent polymorphism of bovine heart cardiolipin. Biochim. Biophys. Acta (BBA) Biomembr. 1982, 693, 1–12. [Google Scholar] [CrossRef][Green Version]
- Fox, C.A.; Ellison, P.; Ikon, N.; Ryan, R.O. Calcium-induced transformation of cardiolipin nanodisks. Biochim. Biophys. Acta (BBA) Biomembr. 2019, 1861, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Kaye, R.; Marsh, D. Induction of the lamellar-inverted hexagonal phase transition in cardiolipin by protons and monovalent cations. Biochim. Biophys. Acta (BBA) Biomembr. 1983, 734, 347–352. [Google Scholar] [CrossRef]
- Khalifat, N.; Puff, N.; Bonneau, S.; Fournier, J.B.; Angelova, M.I. Membrane Deformation under Local pH Gradient: Mimicking Mitochondrial Cristae Dynamics. Biophys. J. 2008, 95, 4924–4933. [Google Scholar] [CrossRef] [PubMed]
- Khalifat, N.; Rahimi, M.; Bitbol, A.F.; Seigneuret, M.; Fournier, J.B.; Puff, N.; Arroyo, M.; Angelova, M.I. Interplay of Packing and Flip-flop in Local Bilayer Deformation. How Phosphatidylglycerol Could Rescue Mitochondrial Function in a Cardiolipin-deficient Yeast Mutant. Biophys. J. 2014, 107, 879–890. [Google Scholar] [CrossRef]
- Fournier, J.B.; Khalifat, N.; Puff, N.; Angelova, M.I. Chemically Triggered Ejection of Membrane Tubules Controlled by Intermonolayer Friction. Phys. Rev. Lett. 2009, 102, 018102. [Google Scholar] [CrossRef] [PubMed]
- Bitbol, A.F.; Puff, N.; Sakuma, Y.; Imai, M.; Fournier, J.B.; Angelova, M.I. Lipid membrane deformation in response to a local pH modification: Theory and experiments. Soft Matter 2012, 8, 6073–6082. [Google Scholar] [CrossRef]
- Bitbol, A.F.; Fournier, J.B. Membrane properties revealed by spatiotemporal response to a local inhomogeneity. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Bitbol, A.F.; Fournier, J.B.; Angelova, M.; Puff, N. Dynamical membrane curvature instability controlled by intermonolayer friction. J. Physics. Condens. Matter Inst. Phys. J. 2011, 23, 284102. [Google Scholar] [CrossRef]
- Song, D.H.; Park, J.; Philbert, M.A.; Sastry, A.M.; Lu, W. Effects of local pH on the formation and regulation of cristae morphologies. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2014, 90, 022702. [Google Scholar] [CrossRef]
- Rieger, B.; Junge, W.; Busch, K.B. Lateral pH gradient between OXPHOS complex IV and F0F1 ATP-synthase in folded mitochondrial membranes. Nat. Commun. 2014, 5, 3103. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.; Bonneau, S.; Joubert, F.; Bitbol, A.F.; Berthoumieux, H. Mitochondrial cristae modeled as an out-of-equilibrium membrane driven by a proton field. Phys. Rev. E 2020, 102, 022401. [Google Scholar] [CrossRef] [PubMed]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Planas-Iglesias, J.; Dwarakanath, H.; Mohammadyani, D.; Yanamala, N.; Kagan, V.E.; Klein-Seetharaman, J. Cardiolipin Interactions with Proteins. Biophys. J. 2015, 109, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Musatov, A.; Sedlák, E. Role of cardiolipin in stability of integral membrane proteins. Biochimie 2017, 142, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Beales, P.A.; Bergstrom, C.L.; Geerts, N.; Groves, J.T.; Vanderlick, T.K. Single Vesicle Observations of the Cardiolipin—Cytochrome c Interaction: Induction of Membrane Morphology Changes. Langmuir 2011, 27, 6107–6115. [Google Scholar] [CrossRef]
- Bergstrom, C.L.; Beales, P.A.; Lv, Y.; Vanderlick, T.K.; Groves, J.T. Cytochrome c causes pore formation in cardiolipin-containing membranes. Proc. Natl. Acad. Sci. USA 2013, 110, 6269–6274. [Google Scholar] [CrossRef]
- Pennington, R.; Sullivan, E.; Fix, A.; Dadoo, S.; Zeczycki, T.; DeSantis, A.; Schlattner, U.; Coleman, R.; Chicco, A.; Brown, D.; et al. Proteolipid domains form in biomimetic and cardiac mitochondrial vesicles and are regulated by cardiolipin concentration but not monolyso-cardiolipin. J. Biol. Chem. 2018, 293, 15933–15946. [Google Scholar] [CrossRef]
- Acehan, D.; Malhotra, A.; Xu, Y.; Ren, M.; Stokes, D.; Schlame, M. Cardiolipin Affects the Supramolecular Organization of ATP Synthase in Mitochondria. Biophys. J. 2011, 100, 2184–2192. [Google Scholar] [CrossRef] [PubMed]
- Gasanov, S.E.; Kim, A.A.; Yaguzhinsky, L.S.; Dagda, R.K. Non-bilayer structures in mitochondrial membranes regulate ATP synthase activity. Biochim. Biophys. Acta (BBA) Biomembr. 2018, 1860, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Bazan, S.; Mileykovskaya, E.; Mallampalli, V.; Heacock, P.; Sparagna, G.; Dowhan, W. Cardiolipin-dependent Reconstitution of Respiratory Supercomplexes from Purified Saccharomyces cerevisiae Complexes III and IV. J. Biol. Chem. 2012, 288. [Google Scholar] [CrossRef]
- Szeto, H. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br. J. Pharmacol. 2014, 171. [Google Scholar] [CrossRef]
- Cooke, I.R.; Deserno, M. Coupling between Lipid Shape and Membrane Curvature. Biophys. J. 2006, 91, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Baoukina, S.; Ingólfsson, H.; Marrink, S.; Tieleman, D. Curvature-Induced Sorting of Lipids in Plasma Membrane Tethers. Adv. Theory Simul. 2018, 1, 1800034. [Google Scholar] [CrossRef]
- Elías-Wolff, F.; Lindén, M.; Lyubartsev, A.P.; Brandt, E.G. Curvature sensing by cardiolipin in simulated buckled membranes. Soft Matter 2019, 15, 792–802. [Google Scholar] [CrossRef]
- Kamal, M.M.; Mills, D.; Grzybek, M.; Howard, J. Measurement of the membrane curvature preference of phospholipids reveals only weak coupling between lipid shape and leaflet curvature. Proc. Natl. Acad. Sci. USA 2009, 106, 22245–22250. [Google Scholar] [CrossRef]
- Tian, A.; Baumgart, T. Sorting of Lipids and Proteins in Membrane Curvature Gradients. Biophys. J. 2009, 96, 2676–2688. [Google Scholar] [CrossRef]
- Sorre, B.; Callan-Jones, A.; Manzi, J.; Goud, B.; Prost, J.; Bassereau, P.; Roux, A. Nature of curvature coupling of amphiphysin with membranes depends on its bound density. Proc. Natl. Acad. Sci. USA 2012, 109, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Prévost, C.; Zhao, H.; Manzi, J.; Lemichez, E.; Lappalainen, P.; Callan-Jones, A.; Bassereau, P. IRSp53 senses negative membrane curvature and phase separates along membrane tubules. Nat. Commun. 2015, 6, 8529. [Google Scholar] [CrossRef] [PubMed]
- Sorre, B.; Callan-Jones, A.; Manneville, J.B.; Nassoy, P.; Joanny, J.F.; Prost, J.; Goud, B.; Bassereau, P. Curvature-driven lipid sorting needs proximity to a demixing point and is aided by proteins. Proc. Natl. Acad. Sci. USA 2009, 106, 5622–5626. [Google Scholar] [CrossRef] [PubMed]
- Callan-Jones, A.; Bassereau, P. Curvature-driven membrane lipid and protein distribution. Curr. Opin. Solid State Mater. Sci. 2013, 17, 143–150. [Google Scholar] [CrossRef]
- Krebs, J.; Hauser, H.; Carafoli, E. Asymmetric distribution of phospholipids in the inner mitochondrial membrane of beef heart mitochondria. J. Biol. Chem. 1979, 254, 5308–5316. [Google Scholar] [CrossRef]
- Harb, J.S.; Comte, J.; Gautheron, D.C. Asymmetrical orientation of phospholipids and their interactions with marker enzymes in pig heart mitochondrial inner membrane. Arch. Biochem. Biophys. 1981, 208, 305–318. [Google Scholar] [CrossRef]
- Cheneval, D.; Müller, M.; Toni, R.; Ruetz, S.; Carafoli, E. Adriamycin as a probe for the transversal distribution of cardiolipin in the inner mitochondrial membrane. J. Biol. Chem. 1985, 260, 13003–13007. [Google Scholar] [CrossRef]
- Petit, J.M.; Huet, O.; Gallet, P.F.; Maftah, A.; Ratinaud, M.H.; Julien, R. Direct analysis and significance of cardiolipin transverse distribution in mitochondrial inner membranes. Eur. J. Biochem. 1994, 220, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Gallet, P.F.; Petit, J.M.; Maftah, A.; Zachowski, A.; Julien, R. Asymmetrical distribution of cardiolipin in yeast inner mitochondrial membrane triggered by carbon catabolite repression. Biochem. J. 1997, 324, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Gallet, P.F.; Zachowski, A.; Julien, R.; Fellmann, P.; Devaux, P.F.; Maftah, A. Transbilayer movement and distribution of spin-labelled phospholipids in the inner mitochondrial membrane. Biochim. Biophys. Acta (BBA) Biomembr. 1999, 1418, 61–70. [Google Scholar] [CrossRef][Green Version]
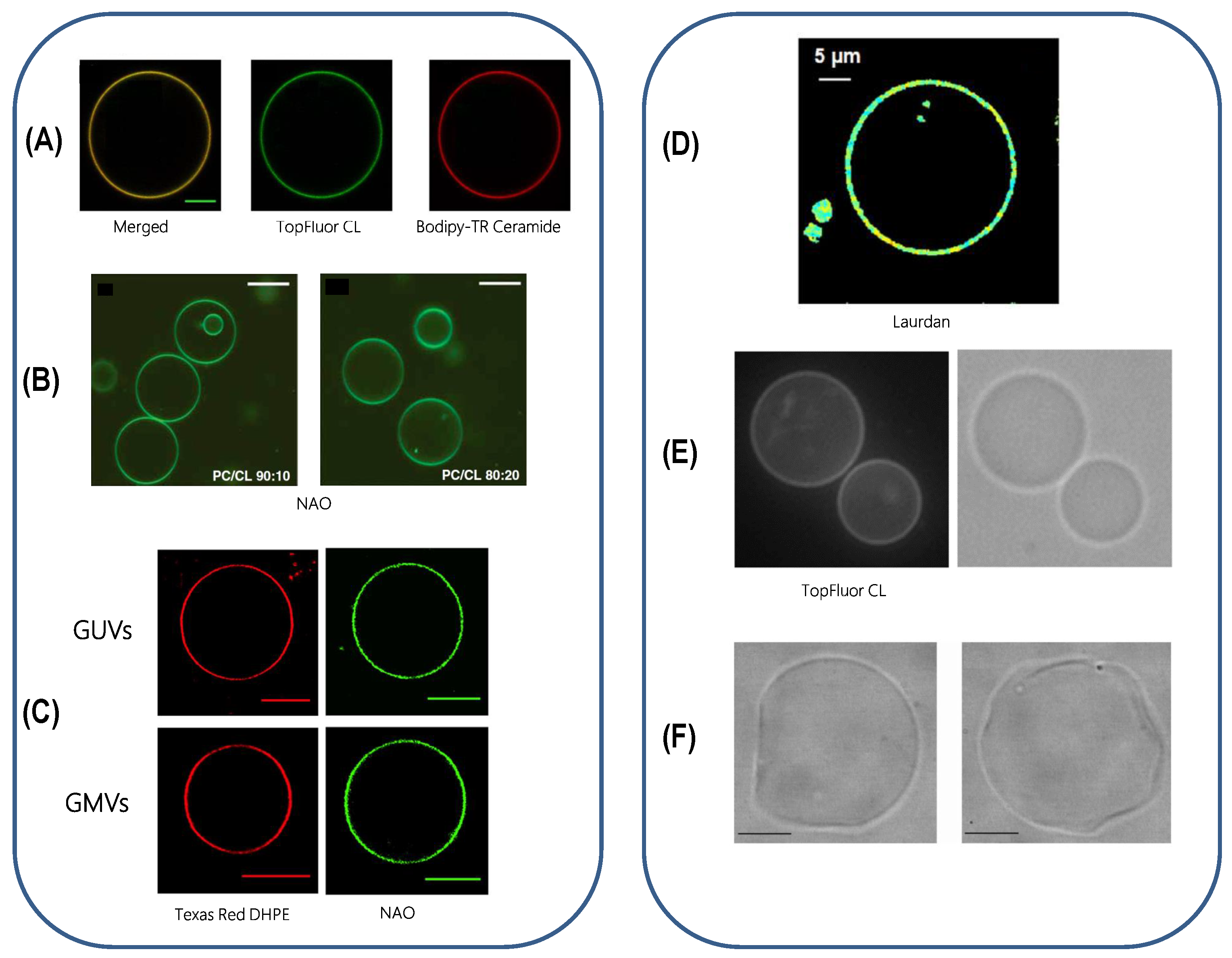
- Jacobson, J.; Duchen, M.; Heales, S. Intracellular distribution of the fluorescent dye nonyl acridine orange responds to the mitochondrial membrane potential: Implications for assays of cardiolipin and mitochondrial mass. J. Neurochem. 2002, 82, 224–233. [Google Scholar] [CrossRef]
- Gohil, V.; Gvozdenovic-Jeremic, J.; Schlame, M.; Greenberg, M. Binding of 10-N-nonyl acridine orange to cardiolipin-deficient yeast cells: Implications for assay of cardiolipin. Anal. Biochem. 2005, 343, 350–352. [Google Scholar] [CrossRef]
- Kagan, V.; Chu, C.; Tyurina, Y.; Cheikhi, A.; Bayir, H. Cardiolipin asymmetry, oxidation and signaling. Chem. Phys. Lipids 2013, 179. [Google Scholar] [CrossRef] [PubMed]
- Schlattner, U.; Tokarska-Schlattner, M.; Rousseau, D.; Boissan, M.; Mannella, C.; Epand, R.; Lacombe, M.L. Mitochondrial cardiolipin/phospholipid trafficking: The role of membrane contact site complexes and lipid transfer proteins. Chem. Phys. Lipids 2014, 179, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Mukhopadhyay, R.; Wingreen, N. A Curvature-Mediated Mechanism for Localization of Lipids to Bacterial Poles. PLoS Comput. Biol. 2006, 2, e151. [Google Scholar] [CrossRef]
- Elmer-Dixon, M.M.; Hoody, J.; Steele, H.B.B.; Becht, D.C.; Bowler, B.E. Cardiolipin Preferentially Partitions to the Inner Leaflet of Mixed Lipid Large Unilamellar Vesicles. J. Phys. Chem. B 2019, 123, 9111–9122. [Google Scholar] [CrossRef] [PubMed]
- Tasseva, G.; Bai, H.D.; Davidescu, M.; Haromy, A.; Michelakis, E.; Vance, J.E. Phosphatidylethanolamine Deficiency in Mammalian Mitochondria Impairs Oxidative Phosphorylation and Alters Mitochondrial Morphology*. J. Biol. Chem. 2013, 288, 4158–4173. [Google Scholar] [CrossRef]
- Baker, C.; Basu Ball, W.; Pryce, E.; Gohil, V. Specific Requirements of Non-bilayer Phospholipids in Mitochondrial Respiratory Chain Function and Formation. Mol. Biol. Cell 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, J.; Kennelly, J.; Wan, S.; Vance, J.; Vance, D.; Jacobs, R. The Critical Role of Phosphatidylcholine and Phosphatidylethanolamine Metabolism in Health and Disease. Biochim. Biophys. Acta (BBA) Biomembr. 2017, 1859. [Google Scholar] [CrossRef]
- Heden, T.D.; Johnson, J.M.; Ferrara, P.J.; Eshima, H.; Verkerke, A.R.P.; Wentzler, E.J.; Siripoksup, P.; Narowski, T.M.; Coleman, C.B.; Lin, C.T.; et al. Mitochondrial PE potentiates respiratory enzymes to amplify skeletal muscle aerobic capacity. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef]
- Bashkirov, P.V.; Chekashkina, K.V.; Akimov, S.A.; Kuzmin, P.I.; Frolov, V.A. Variation of lipid membrane composition caused by strong bending. Biochem. (Moscow) Suppl. Ser. A Membr. Cell Biol. 2011, 5, 205–211. [Google Scholar] [CrossRef]
- Wang, H.Y.; Bharti, D.; Levental, I. Membrane Heterogeneity Beyond the Plasma Membrane. Front. Cell Dev. Biol. 2020, 8, 1186. [Google Scholar] [CrossRef] [PubMed]
- Mileykovskaya, E.; Dowhan, W. Visualization of Phospholipid Domains inEscherichia coli by Using the Cardiolipin-Specific Fluorescent Dye 10-N-Nonyl Acridine Orange. J. Bacteriol. 2000, 182, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Koppelman, C.M.; Den Blaauwen, T.; Duursma, M.C.; Heeren, R.M.A.; Nanninga, N. Escherichia coli Minicell Membranes Are Enriched in Cardiolipin. J. Bacteriol. 2001, 183, 6144–6147. [Google Scholar] [CrossRef] [PubMed]
- Kawai, F.; Shoda, M.; Harashima, R.; Sadaie, Y.; Hara, H.; Matsumoto, K. Cardiolipin Domains in Bacillus subtilis Marburg Membranes. J. Bacteriol. 2004, 186, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- Romantsov, T.; Helbig, S.; Culham, D.E.; Gill, C.; Stalker, L.; Wood, J.M. Cardiolipin promotes polar localization of osmosensory transporter ProP in Escherichia coli. Mol. Microbiol. 2007, 64, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Mileykovskaya, E. Subcellular localization of Escherichia coli osmosensory transporter ProP: Focus on cardiolipin membrane domains. Mol. Microbiol. 2007, 64, 1419–1422. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Huang, K.C.; Wingreen, N.S. Lipid Localization in Bacterial Cells through Curvature-Mediated Microphase Separation. Biophys. J. 2008, 95, 1034–1049. [Google Scholar] [CrossRef]
- Renner, L.D.; Weibel, D.B. Cardiolipin microdomains localize to negatively curved regions of Escherichia coli membranes. Proc. Natl. Acad. Sci. USA 2011, 108, 6264–6269. [Google Scholar] [CrossRef]
- Jalmar, O.; García-Sáez, A.J.; Berland, L.; Gonzalvez, F.; Petit, P.X. Giant unilamellar vesicles (GUVs) as a new tool for analysis of caspase-8/Bid-FL complex binding to cardiolipin and its functional activity. Cell Death Dis. 2010, 1, e103. [Google Scholar] [CrossRef]
- Kawai, C.; Ferreira, J.C.; Baptista, M.S.; Nantes, I.L. Not Only Oxidation of Cardiolipin Affects the Affinity of Cytochrome c for Lipid Bilayers. J. Phys. Chem. B 2014, 118, 11863–11872. [Google Scholar] [CrossRef]
- Cheniour, M.; Brewer, J.; Bagatolli, L.; Marcillat, O.; Granjon, T. Evidence of proteolipid domain formation in an inner mitochondrial membrane mimicking model. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861. [Google Scholar] [CrossRef] [PubMed]
- Tomšiè, N.; Babnik, B.; Lombardo, D.; Mavčič, B.; Kandušer, M.; Iglič, A.; Kralj-Iglič, V. Shape and Size of Giant Unilamellar Phospholipid Vesicles Containing Cardiolipin. J. Chem. Inf. Model. 2005, 45, 1676–1679. [Google Scholar] [CrossRef] [PubMed]
- Domènech, O.; Redondo, L.; Picas, L.; Morros, A.; Montero, M.T.; Hernández-Borrell, J. Atomic force microscopy characterization of supported planar bilayers that mimic the mitochondrial inner membrane. J. Mol. Recognit. 2007, 20, 546–553. [Google Scholar] [CrossRef]
- Lopes, S.; Ivanova, G.; de Castro, B.; Gameiro, P. Revealing cardiolipins influence in the construction of a significant mitochondrial membrane model. Biochim. Biophys. Acta (BBA) Biomembr. 2018, 1860, 2465–2477. [Google Scholar] [CrossRef] [PubMed]
- Jouhet, J. Importance of the hexagonal lipid phase in biological membrane organization. Front. Plant Sci. 2013, 4, 494. [Google Scholar] [CrossRef] [PubMed]
- Schlame, M.; Ren, M. Barth syndrome, a human disorder of cardiolipin metabolism. FEBS Lett. 2006, 580, 5450–5455. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, T.; Nishijima, M.; Suzuki, K.; Akamatsu, Y. Mitochondrial dysfunction of a cultured Chinese hamster ovary cell mutant deficient in cardiolipin. J. Biol. Chem. 1993, 268, 22914–22919. [Google Scholar] [CrossRef]
- Basu Ball, W.; Neff, J.K.; Gohil, V.M. The role of nonbilayer phospholipids in mitochondrial structure and function. FEBS Lett. 2018, 592, 1273–1290. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.J.; Balaska, D.; Cheng, W.H. The ups and downs of mitochondrial calcium signalling in the heart. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 856–864. [Google Scholar] [CrossRef]
- Mannella, C.A. Consequences of Folding the Mitochondrial Inner Membrane. Front. Physiol. 2020, 11, 536. [Google Scholar] [CrossRef]
- Heberle, J.; Riesle, J.; Thiedemann, G.; Oesterhelt, D.; Dencher, N.A. Proton migration along the membrane surface and retarded surface to bulk transfer. Nature 1994, 370, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.J. Chemiosmotic Coupling: Protons fast and slow. Curr. Biol. 1995, 5, 25–27. [Google Scholar] [CrossRef]
- Yoshinaga, M.Y.; Kellermann, M.Y.; Valentine, D.L.; Valentine, R.C. Phospholipids and glycolipids mediate proton containment and circulation along the surface of energy-transducing membranes. Prog. Lipid Res. 2016, 64, 1–15. [Google Scholar] [CrossRef]
- Morelli, A.; Ravera, S.; Calzia, D.; Panfoli, I. An update of the chemiosmotic theory as suggested by possible proton currents inside the coupling membrane. Open Biol. 2019, 9, 180221. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.; Meyrat, A.; Stoldt, S.; Santiago, R.; Wenzel, D.; Jakobs, S.; von Ballmoos, C.; Ott, M. Kinetic coupling of the respiratory chain with ATP synthase, but not proton gradients, drives ATP production in cristae membranes. Proc. Natl. Acad. Sci. USA 2020, 117, 2412–2421. [Google Scholar] [CrossRef] [PubMed]
- Haines, T.H.; Dencher, N.A. Cardiolipin: A proton trap for oxidative phosphorylation. FEBS Lett. 2002, 528. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joubert, F.; Puff, N. Mitochondrial Cristae Architecture and Functions: Lessons from Minimal Model Systems. Membranes 2021, 11, 465. https://doi.org/10.3390/membranes11070465
Joubert F, Puff N. Mitochondrial Cristae Architecture and Functions: Lessons from Minimal Model Systems. Membranes. 2021; 11(7):465. https://doi.org/10.3390/membranes11070465
Chicago/Turabian StyleJoubert, Frédéric, and Nicolas Puff. 2021. "Mitochondrial Cristae Architecture and Functions: Lessons from Minimal Model Systems" Membranes 11, no. 7: 465. https://doi.org/10.3390/membranes11070465
APA StyleJoubert, F., & Puff, N. (2021). Mitochondrial Cristae Architecture and Functions: Lessons from Minimal Model Systems. Membranes, 11(7), 465. https://doi.org/10.3390/membranes11070465





