Hyper Cross-Linked Polymers as Additives for Preventing Aging of PIM-1 Membranes
Abstract
:1. Introduction
2. Materials and Methods
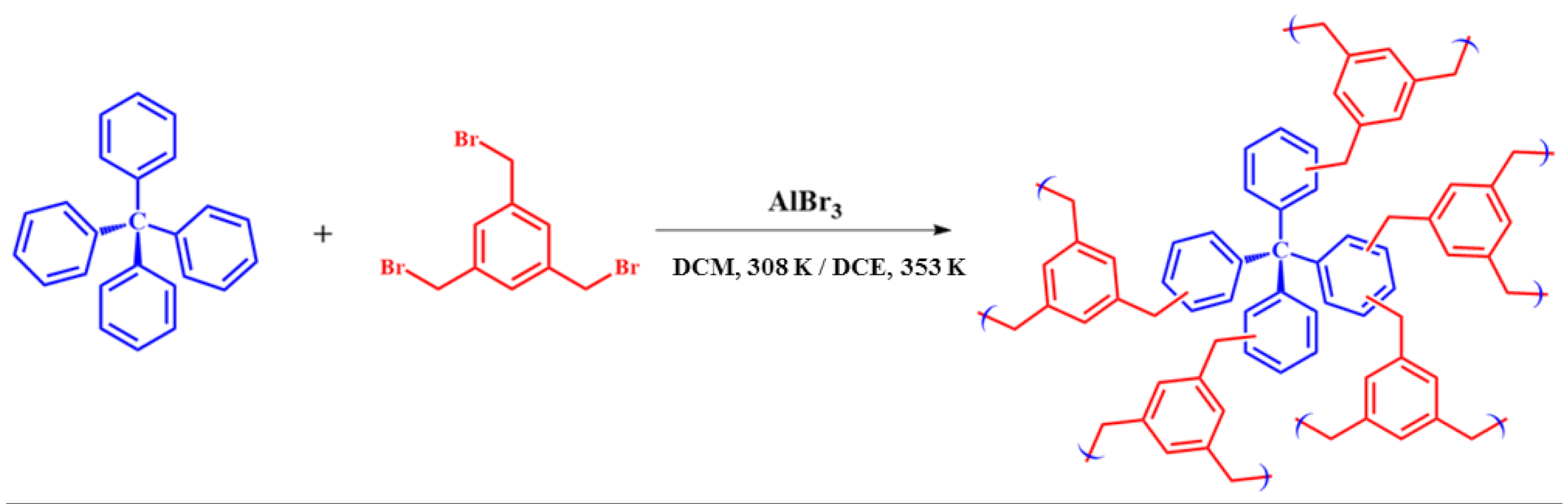
2.1. Materials Preparation
2.2. Membranes Preparation
2.3. Membranes Characterizations
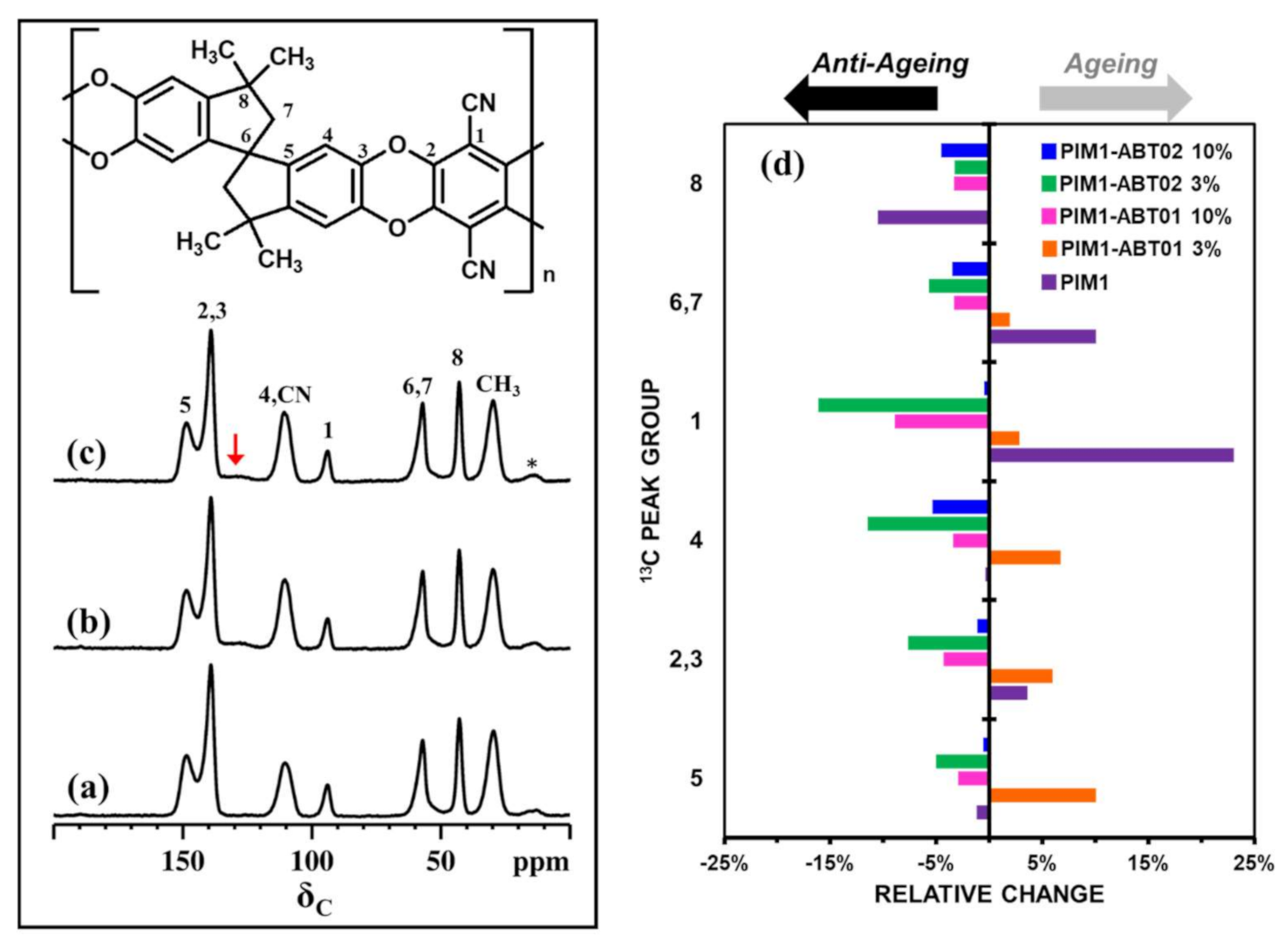
3. Results
3.1. Characterization of the HCPs
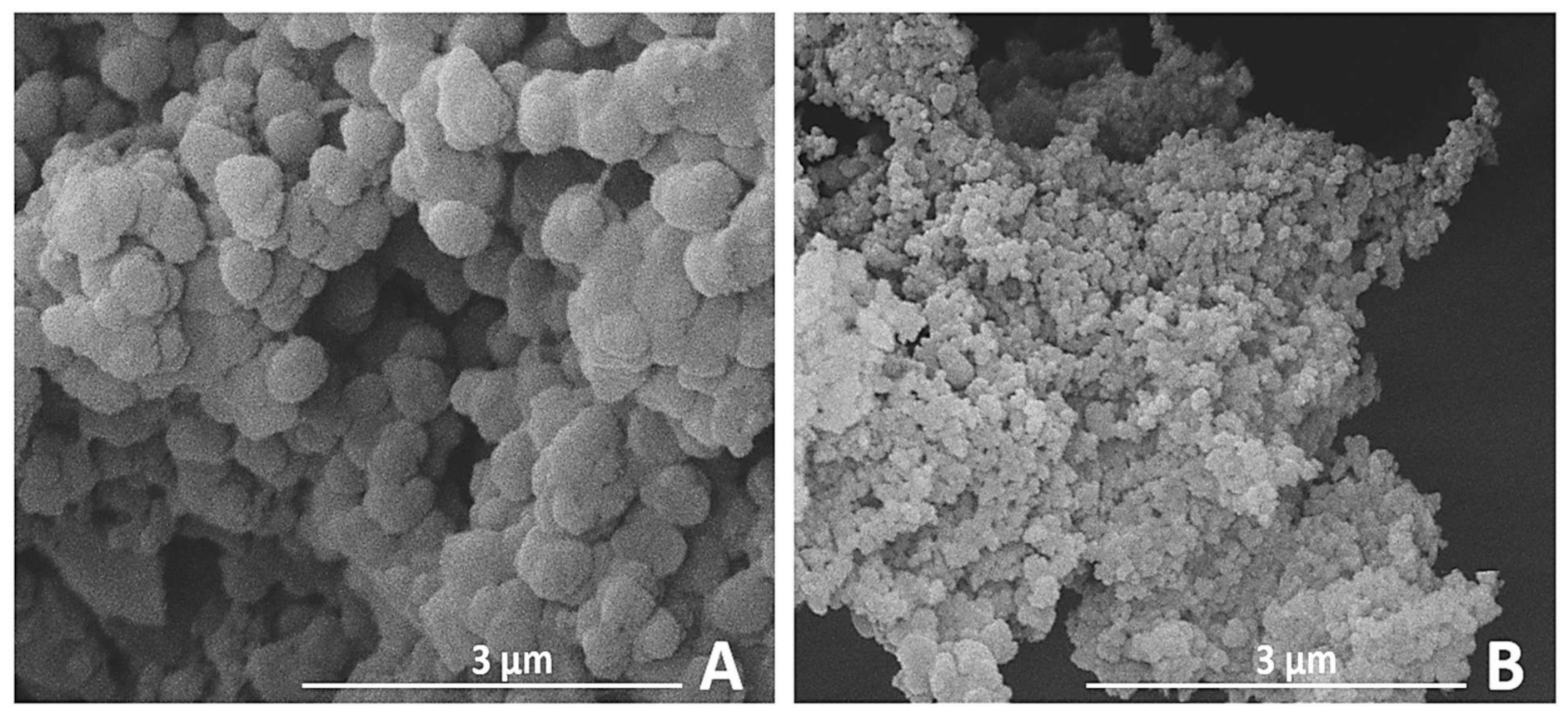
3.1.1. SEM
3.1.2. N2 Physisorption Analysis
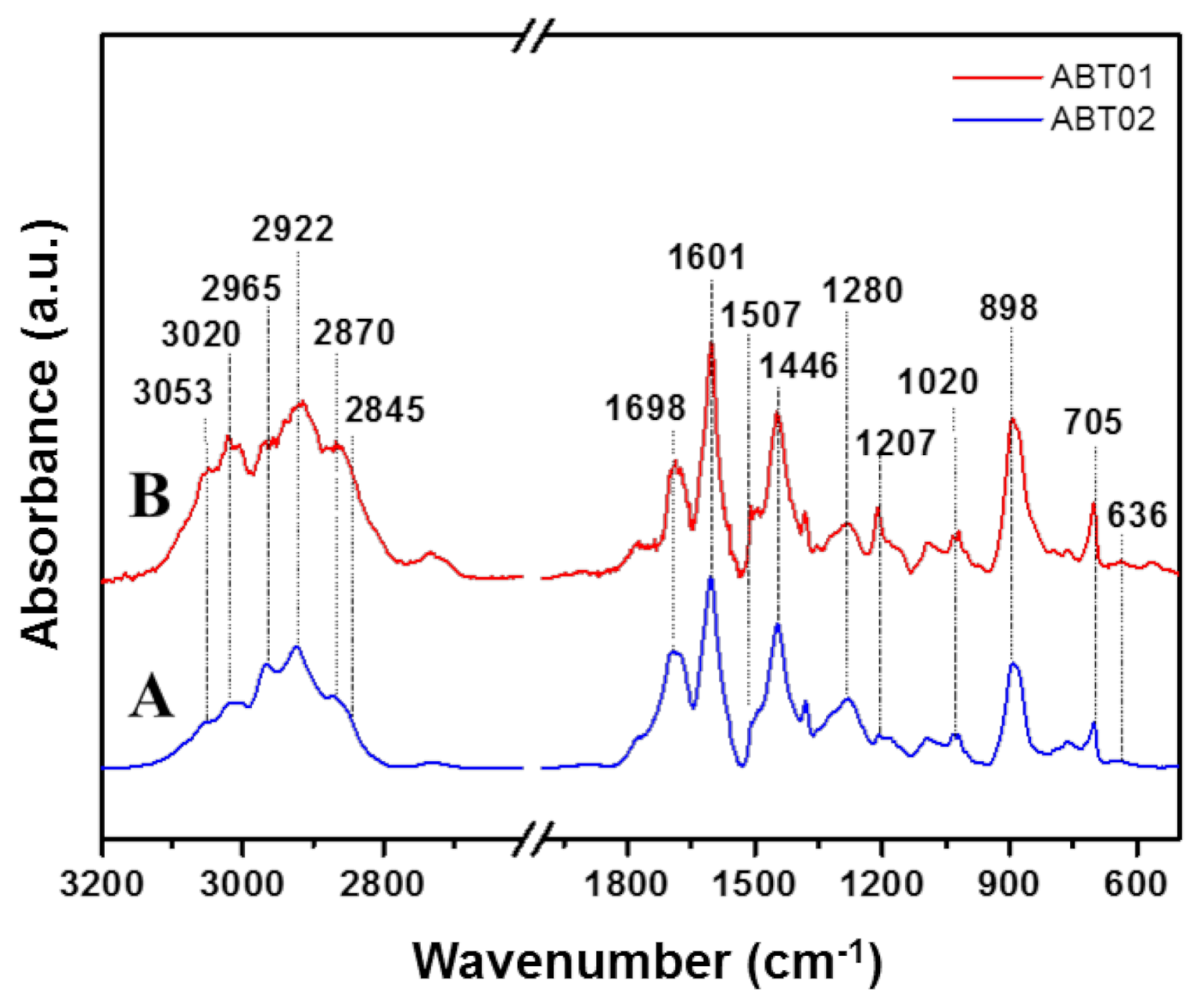
3.1.3. FT-IR Spectroscopy
3.1.4. SS-NMR Spectroscopy
3.2. Membranes Characterization
3.2.1. Permeability Measurements
3.2.2. SS-NMR 13C T1 Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Credence Research. Gas Separation Membranes Market by Type, By Application—Growth Future Prospects and Competitive Analysis, 2016–2024; Credence Research: San Jose, CA, USA, 2017. [Google Scholar]
- Abanades, J.; Arias, B.; Lyngfelt, A.; Mattisson, T.; Wiley, D.; Li, H.; Ho, M.; Mangano, E.; Brandani, S. Emerging CO2 capture systems. Int. J. Greenh. Gas Control. 2015, 40, 126–166. [Google Scholar] [CrossRef] [Green Version]
- Ho, M.T.; Allinson, G.W.; Wiley, D.E. Reducing the Cost of CO2 Capture from Flue Gases Using Membrane Technology. Ind. Eng. Chem. Res. 2008, 47, 1562–1568. [Google Scholar] [CrossRef]
- Duong, S. White Paper: Cost Effective Alternative to Distillation for Olefin Purification and Extraction. Available online: https://www.imtexmembranes.com/cost-effective-alternative-to-distillation-for-olefin-purification-and-extraction (accessed on 1 January 2019).
- Puri, P.S. Chapter Commercial Applications of Membranes in Gas Separations. In Membrane Engineering for the Treatment of Gases: Gas-Separation Problems with Membranes; Royal Society of Chemistry (RSC): Cambridge, UK, 2011; pp. 215–244. [Google Scholar]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.; Jubin, R.; Choate, B. Materials for Separation Technology: Energy and Emission Reduction Opportunities; U.S. Department of Energy: Washington, DC, USA, 2005. [Google Scholar]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Gür, T.M. Permselectivity of zeolite filled polysulfone gas separation membranes. J. Membr. Sci. 1994, 93, 283–289. [Google Scholar] [CrossRef]
- Süer, M.G.; Baç, N.; Yilmaz, L. Gas permeation characteristics of polymer-zeolite mixed matrix membranes. J. Membr. Sci. 1994, 91, 77–86. [Google Scholar] [CrossRef]
- Hussain, M.; König, A. Mixed-Matrix Membrane for Gas Separation: Polydimethylsiloxane Filled with Zeolite. Chem. Eng. Technol. 2012, 35, 561–569. [Google Scholar] [CrossRef]
- Zarshenas, K.; Raisi, A.; Aroujalian, A. Mixed matrix membrane of nano-zeolite NaX/poly (ether-blockamide) for gas separation applications. J. Membr. Sci. 2016, 510, 270–283. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.; Janiak, C.; Sumby, C. Mixed-Matrix Membranes. Angew. Chem. 2017, 56, 9292–9310. [Google Scholar] [CrossRef] [PubMed]
- Nejad, M.; Asghari, M.; Afsari, M. Investigation of Carbon Nanotubes in Mixed Matrix Membranes for Gas Separation: A Review. ChemBioEng Rev. 2016, 3, 276–298. [Google Scholar] [CrossRef]
- Khan, M.M.; Filiz, V.; Bengtson, G.; Shishatskiy, S.; Rahman, M.M.; Lillepaerg, J.; Abetz, V. Enhanced gas permeability by fabricating mixed matrix membranes of functionalized multiwalled carbon nanotubes and polymers of intrinsic microporosity (PIM). J. Membr. Sci. 2013, 436, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Sutrisna, P.D.; Hou, J.; Li, H.; Zhang, Y.; Chen, V. Improved operational stability of Pebax-based gas separation membranes with ZIF-8: A comparative study of flat sheet and composite hollow fibre membranes. J. Membr. Sci. 2017, 524, 266–279. [Google Scholar] [CrossRef]
- Li, T.; Pan, Y.; Peinemann, K.V.; Lai, Z. Carbon dioxide selective mixed matrix composite membrane containing ZIF-7 nanofillers. J. Membr. Sci. 2013, 425, 235–242. [Google Scholar] [CrossRef]
- Bushell, A.F.; Budd, P.M.; Attfield, M.P.; Jones, J.T.; Hasell, T.; Cooper, A.I.; Bernanrdo, P.; Bazzarelli, F.; Clarizia, G.; Jansen, J. Nanoporous organic polymer/cage composite membranes. Angew. Chem. 2013, 52, 1253–1256. [Google Scholar] [CrossRef]
- Şen, D.; Kalıpçılar, H.; Yilmaz, L. Development of polycarbonate based zeolite 4A filled mixed matrix gas separation membranes. J. Membr. Sci. 2007, 303, 194–199. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, J. POC/PIM-1 mixed-matrix membranes for water desalination: A molecular simulation study. J. Membr. Sci. 2020, 608, 118173. [Google Scholar] [CrossRef]
- Yu, G.; Li, Y.; Wang, Z.; Liu, T.X.; Zhu, G.; Zou, X. Mixed matrix membranes derived from nanoscale porous organic frameworks for permeable and selective CO2 separation. J. Membr. Sci. 2019, 591, 117343. [Google Scholar] [CrossRef]
- Begni, F.; Paul, G.; Lasseuguette, E.; Mangano, E.; Bisio, C.; Ferrari, M.C.; Gatti, G. Synthetic Saponite Clays as Additives for Reducing Aging Effects in PIM1 Membranes. ACS Appl. Polym. Mater. 2020, 2, 3481–3490. [Google Scholar] [CrossRef]
- Lau, C.H.; Konstas, K.; Doherty, C.M.; Smith, S.J.; Hou, R.; Wang, H.; Carta, M.; Yoon, H.; Park, J.; Freeman, R.-M.; et al. Tailoring molecular interactions between microporous polymers in high performance mixed matrix membranes for gas separations. Nanoscale 2020, 12, 17405–17410. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.J.D.; Lau, C.H.; Mardel, J.I.; Kitchin, M.; Konstas, K.; Ladewig, B.P.; Hill, M.R. Physical aging in glassy mixed matrix membranes; tuning particle interaction for mechanically robust nanocomposite films. J. Mater. Chem. A. 2016, 4, 10627–10634. [Google Scholar] [CrossRef]
- Lau, C.H.; Konstas, K.; Thornton, A.W.; Liu, A.C.; Mudie, S.; Kennedy, D.F.; Howard, S.C.; Hill, A.J.; Hill, M. Gas Separation Mem-branes Loaded with Porous Aromatic Frameworks that Improve with Age. Angew. Chem. 2015, 54, 2669–2673. [Google Scholar] [CrossRef]
- Khdhayyer, M.; Bushell, A.F.; Budd, P.M.; Attfield, M.P.; Jiang, D.; Burrows, A.D.; Esposito, E.; Bernardo, E.; Monteleone, M.; Fuoco, A.; et al. Mixed matrix membranes based on MIL-101 metal−organic frameworks in polymer of intrinsic microporosity PIM-1. Sep. Purif. Technol. 2019, 212, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Alberto, M.; Bhavsar, R.; Luque-Alled, J.M.; Vijayaraghavan, A.; Budd, P.M.; Gorgojo, P. Impeded physical aging in PIM-1 membranes containing graphene-like fillers. J. Membr. Sci. 2018, 563, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Turner, S.R. Hypercrosslinked Polymers: A Review. Polym. Rev. 2017, 58, 1–41. [Google Scholar] [CrossRef]
- Tsyurupa, M.; Davankov, V. Hypercrosslinked polymers: Basic principle of preparing the new class of polymeric materials. React. Funct. Polym. 2002, 53, 193–203. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, B.; Wood, C.D. Solution-processable hypercrosslinked polymers by low cost strategies: A promising platform for gas storage and separation. J. Mater. Chem. A. 2016, 4, 15072–15080. [Google Scholar] [CrossRef]
- Hou, R.; O’Loughlin, R.; Ackroyd, J.; Liu, Q.; Doherty, C.M.; Wang, H.; Hill, M.R.; Smith, S.J.D. Greatly Enhanced Gas Selectivity in Mixed-Matrix Membranes through Size-Controlled Hyper-cross-linked Polymer Additives. Ind. Eng. Chem. Res. 2020, 59, 13773–13782. [Google Scholar] [CrossRef]
- Lau, C.H.; Mulet, X.; Konstas, K.; Doherty, C.M.; Sani, M.-A.; Separovic, F.; Hill, M.R.; Wood, C.D. Hypercrosslinked Additives for Ageless Gas-Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 1998–2001. [Google Scholar] [CrossRef]
- Mitra, T.; Bhavsar, R.S.; Adams, D.; Budd, P.M.; Cooper, A.I. PIM-1 mixed matrix membranes for gas separations using cost-effective hypercrosslinked nanoparticle fillers. Chem. Commun. 2016, 52, 5581–5584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budd, P.M.; McKeown, N.B.; Ghanem, B.S.; Msayib, K.J.; Fritsch, D.; Starannikova, L.; Belov, N.; Sanfirova, O.; Yampolskii, Y.; Shantarovich, V. Gas permeation parameters and other physicochemical properties of a polymer of intrinsic microporosity: Polybenzodioxane PIM-1. J. Membr. Sci. 2008, 325, 851–860. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, With Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef] [Green Version]
- Paul, G.; Begni, F.; Melicchio, A.; Golemme, G.; Bisio, C.; Marchi, D.; Cossi, M.; Marchese, L.; Gatti, G. Hy-per-Cross-Linked Polymers for the Capture of Aromatic Volatile Compounds. ACS Appl. Polym. Mater. 2020, 2, 647–658. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Jia, X.; Zhang, B.; Zhang, H.; Zhang, A.; Zhang, Q. Hypercrosslinked polymers: Controlled preparation and effective adsorption of aniline. J. Mater. Sci. 2016, 51, 8579–8592. [Google Scholar] [CrossRef]
- Socrate, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley & Sons Ltd.: West Sussex, UK, 2004. [Google Scholar]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Meyers, R.A., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2000. [Google Scholar]
- Tsyurupa, M.P.; Blinnikova, Z.K.; Davidovich, Y.A.; Lyubimov, S.E.; Naumkin, A.V.; Davankov, V.A. On the nature of ‘‘func-tional groups’’ in non-functionalized hypercrosslinked polystyrenes. React. Funct. Polym. 2012, 72, 973–982. [Google Scholar] [CrossRef]
- Olah, G.A.; Kobayashi, S.; Tashiro, M. Aromatic Substitution. XXX.1 23Friedel-Crafts Benzylation of Benzene and Toluene with Benzyl and Substituted Benzyl Halides. J. Am. Chem. Soc. 1972, 21, 94. [Google Scholar]
- Errahali, M.; Gatti, G.; Tei, L.; Paul, G.; Rolla, G.A.; Canti, L.; Fraccarollo, A.; Cossi, M.; Comotti, A.; Sozzani, P.; et al. Microporous Hyper Cross-Linked Aromatic Polymers Designed for Methane and Carbon Dioxide Adsorption. J. Phys. Chem. C 2014, 118, 28699–28710. [Google Scholar] [CrossRef]
- Van der Made, A.W.; Van der Made, R.H. A Convenient Procedure for Bromomethylation of Aromatic Compounds. Selective Mono-, Bis-, or Trisbromomethylation. J. Org. Chem. 1993, 58, 1262–1263. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Swaidan, R.; Ghanem, B.; Pinnau, I. Fine-Tuned Intrinsically Ultramicroporous Polymers Redefine the Permeability/Selectivity Upper Bounds of Membrane-Based Air and Hydrogen Separations. ACS Macro Lett. 2015, 4, 947–951. [Google Scholar] [CrossRef] [Green Version]
- Harms, S.; Rätzke, K.; Faupel, F.; Chaukura, N.; Budd, P.M.; Egger, W.; Ravelli, L. Aging and Free Volume in a Polymer of Intrinsic Microporosity (PIM-1). J. Adhes. 2012, 88, 608–619. [Google Scholar] [CrossRef]




 ) N2 permeability on t0; (
) N2 permeability on t0; (  ) N2 permeability on tf; (
) N2 permeability on tf; (  ) CO2 permeability on t0; (
) CO2 permeability on t0; (  ) CO2 permeability on tf (850 days).
) CO2 permeability on tf (850 days).
 ) N2 permeability on t0; (
) N2 permeability on t0; (  ) N2 permeability on tf; (
) N2 permeability on tf; (  ) CO2 permeability on t0; (
) CO2 permeability on t0; (  ) CO2 permeability on tf (850 days).
) CO2 permeability on tf (850 days).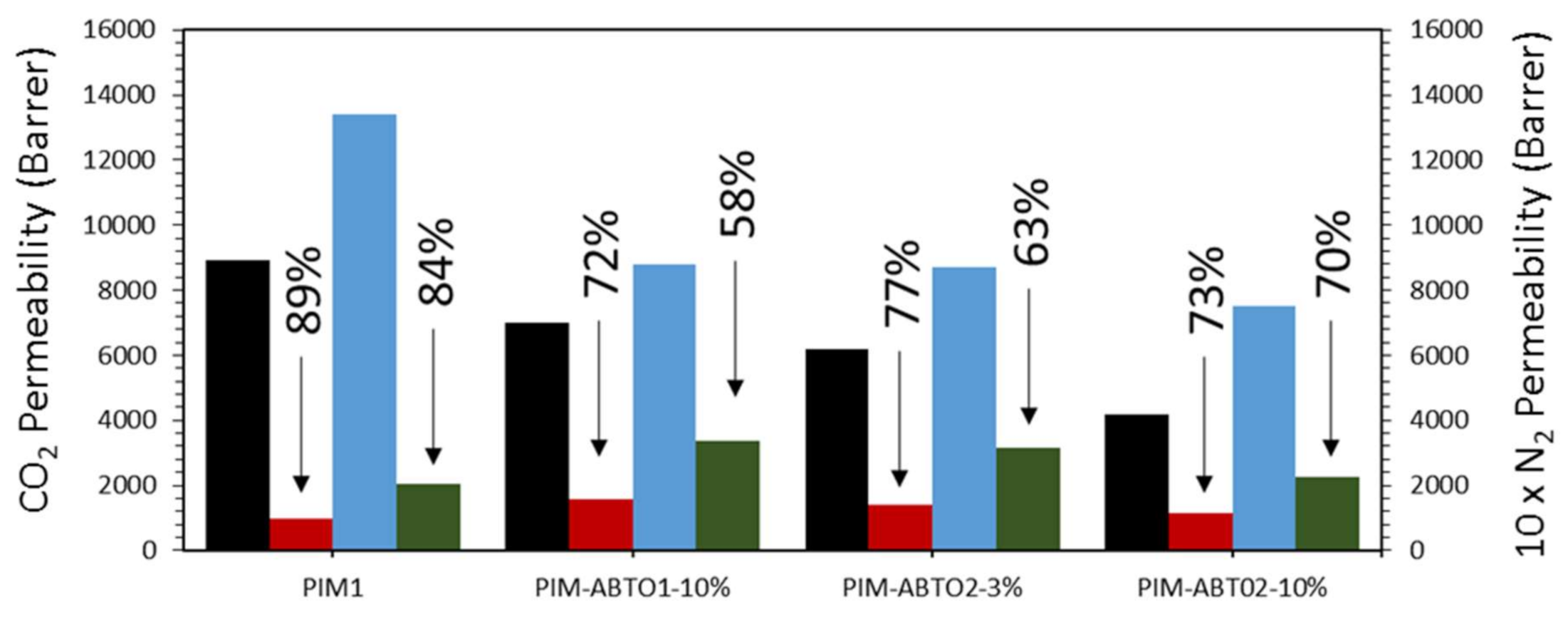

| Sample | SSABET (m2/g) | VTot (cc/g) | Vmicro (cc/g) | Vmeso(cc/g) 20 < Å < 100 | ||
|---|---|---|---|---|---|---|
| Total | <7Å | 7 < Å < 20 | ||||
| ABT01 | 823 | 0.52 | 0.28 | 0.08 | 0.20 | 0.24 |
| ABT02 | 990 | 0.61 | 0.35 | 0.10 | 0.25 | 0.26 |
| Band Positions (cm−1) | Assignments [39,40] |
|---|---|
| 3053 | νAs Aromatic C-H |
| 3020 | νS Aromatic C-H |
| 2965 | νAs Aliphatic C-H (-CH3) |
| 2922 | νAs Aliphatic C-H (-CH2-) |
| 2870 | νS Aliphatic C-H (-CH3) |
| 2845 | νS Aliphatic C-H (-CH2-) |
| 1280 | ν skeletal -C-C- |
| 1700–1210 | Collective stretching vibrations of poly-substituted benzene rings |
| 900–700 | Collective bending vibrations of poly-substituted benzene rings |
| 636 | ν aliphatic C-Br (–CH2Br) |
| Filler | % wt | Permeability CO2 (Barrer) (±5%) | Selectivity CO2/N2 |
|---|---|---|---|
| ABT01 | 0 | 13,400 | 15 |
| 3 | 14,700 | 18 | |
| 10 | 8800 | 13 | |
| ABT02 | 0 | 13,400 | 15 |
| 3 | 8690 | 14 | |
| 10 | 7500 | 18 |
| Membrane | PCO2 (Barrer) | PN2 (Barrer) | Selectivity CO2/N2 | |||
|---|---|---|---|---|---|---|
| t0 | tf | t0 | tf | t0 | tf | |
| PIM-1 | 13,400 | 2040 | 890 | 100 | 15 | 21 |
| PIM1-ABT01-10% | 8800 | 3390 | 700 | 160 | 13 | 21 |
| PIM1-ABT02-3% | 8690 | 3170 | 620 | 140 | 14 | 22 |
| PIM1-ABT02-10% | 7500 | 2270 | 420 | 110 | 18 | 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begni, F.; Lasseuguette, E.; Paul, G.; Bisio, C.; Marchese, L.; Gatti, G.; Ferrari, M.-C. Hyper Cross-Linked Polymers as Additives for Preventing Aging of PIM-1 Membranes. Membranes 2021, 11, 463. https://doi.org/10.3390/membranes11070463
Begni F, Lasseuguette E, Paul G, Bisio C, Marchese L, Gatti G, Ferrari M-C. Hyper Cross-Linked Polymers as Additives for Preventing Aging of PIM-1 Membranes. Membranes. 2021; 11(7):463. https://doi.org/10.3390/membranes11070463
Chicago/Turabian StyleBegni, Federico, Elsa Lasseuguette, Geo Paul, Chiara Bisio, Leonardo Marchese, Giorgio Gatti, and Maria-Chiara Ferrari. 2021. "Hyper Cross-Linked Polymers as Additives for Preventing Aging of PIM-1 Membranes" Membranes 11, no. 7: 463. https://doi.org/10.3390/membranes11070463
APA StyleBegni, F., Lasseuguette, E., Paul, G., Bisio, C., Marchese, L., Gatti, G., & Ferrari, M.-C. (2021). Hyper Cross-Linked Polymers as Additives for Preventing Aging of PIM-1 Membranes. Membranes, 11(7), 463. https://doi.org/10.3390/membranes11070463











