Microstructure Investigation of Polymer Electrolyte Fuel Cell Catalyst Layers Containing Perfluorosulfonated Ionomer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
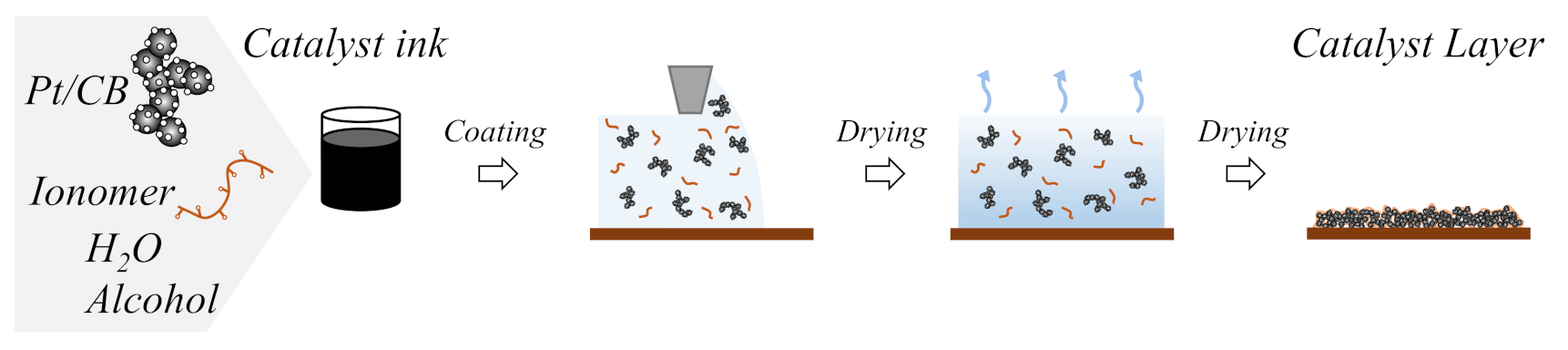
2.2. Catalyst Ink Preparation
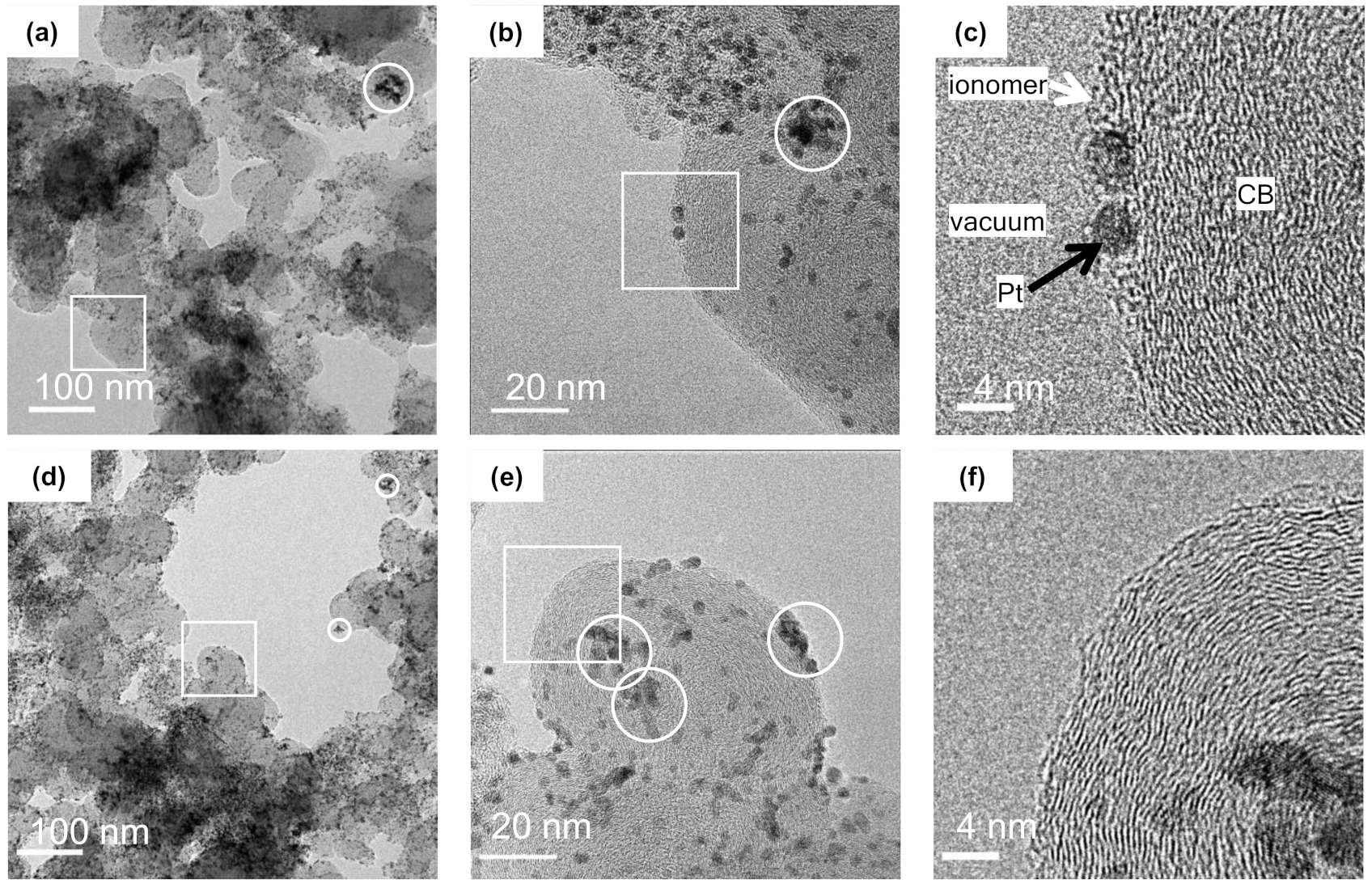
2.3. TEM Observations
2.4. X-Ray Scattering
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grot, W. Fluorinated Ionomers, 2nd ed.; William Andrew: Norwich, NY, USA, 2011; ISBN 13:978-1437744576. [Google Scholar]
- Kusoglu, A.; Weber, A.Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef] [PubMed]
- Holdcroft, S. Fuel Cell Catalyst Layers: A Polymer Science Perspective. Chem. Mater. 2014, 26, 381–393. [Google Scholar] [CrossRef]
- Wang, Y.; Diaz, D.F.R.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203. [Google Scholar] [CrossRef]
- Uemura, S.; Yoshida, T.; Koga, M.; Matsumoto, H.; Yang, X.; Shinohara, K.; Sasabe, T.; Hirai, S. Ink Degradation and Its Effects on the Crack Formation of Fuel Cell Catalyst Layers. J. Electrochem. Soc. 2019, 166, F89–F92. [Google Scholar] [CrossRef]
- Konosu, Y.; Koga, M.; Matsumoto, H.; Tokita, M.; Masunaga, H.; Hikima, T.; Sugimori, H.; Yoshida, T.; Shinohara, K.; Hirai, S. Time-Resolved Nanostructural Analysis of Catalyst Layer Formation Process by Synchrotron X-ray Scattering. ECS Trans. 2017, 80, 269–273. [Google Scholar] [CrossRef]
- Jomori, S.; Komatsubara, K.; Nonoyama, N.; Kato, M.; Yoshida, T. An Experimental Study of the Effects of Operational History on Activity Changes in a PEMFC. J. Electrochem. Soc. 2013, 160, F1067–F1073. [Google Scholar] [CrossRef]
- He, Q.; Suraweera, N.; Joy, D.C.; Keffer, D.L. Structure of the Ionomer Film in Catalyst Layers of Proton Exchange Membrane Fuel Cells. J. Phys. Chem. C 2013, 117, 25305–25316. [Google Scholar] [CrossRef]
- Ishikawa, H.; Sugawara, Y.; Inoue, G.; Kawase, M. Effects of Pt and ionomer ratios on the structure of catalyst layer: A theoretical model for polymer electrolyte fuel cells. J. Power Sources 2018, 374, 196–204. [Google Scholar] [CrossRef]
- Xie, J.; Garzon, F.; Zawodzinski, T.; Smith, W. Ionomer Segregation in Composite MEAs and Its Effect on Polymer Electrolyte Fuel Cell Performance. J. Electrochem. Soc. 2004, 151, A1084–A1093. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, H.; Ilavsky, J.; Stanciu, L.; Ho, D.; Justice, M.J.; Petrache, H.I.; Xie, J. Investigation of a Catalyst Ink Dispersion Using Both Ultra-Small-Angle X-ray Scattering and Cryogenic TEM. Langmuir 2010, 26, 19199–19208. [Google Scholar] [CrossRef]
- Lopez-Haro, M.; Guétaz, L.; Printemps, T.; Morin, A.; Escribano, S.; Jouneau, P.-H.; Bayle-Guillemaud, P.; Chandezon, F.; Gebel, G. Three-dimensional analysis of Nafion layers in fuel cell electrodes. Nat. Commun. 2014, 5, 5229. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Mashio, T.; Horibe, N.; Akizuki, K.; Ohma, A. Analysis of the Microstructure Formation Process and Its Influence on the Performance of Polymer Electrolyte Fuel-Cell Catalyst Layers. ChemElectroChem 2015, 2, 1560–1567. [Google Scholar] [CrossRef]
- Sugimori, H.; Terao, T.; Nishino, Y.; Ito, Y.; Miyazawa, A.; Konosu, Y.; Koga, M.; Matsumoto, H.; Uemura, S.; Kameya, Y.; et al. Nanostructural Evolution during Catalyst Layer Formation Studied via Cryo-Electron Microscopy. ECS Trans 2017, 80, 253–258. [Google Scholar] [CrossRef]
- Takahashi, S.; Shimanuki, J.; Mashio, T.; Ohma, A.; Tohma, H.; Ishihara, A.; Ito, Y.; Nishino, Y.; Miyazawa, A. Observation of ionomer in catalyst ink of polymer electrolyte fuel cell using cryogenic transmission electron microscopy. Electrochim. Acta 2017, 224, 178–185. [Google Scholar] [CrossRef]
- Yang, F.; Xin, L.; Uzunoglu, A.; Qiu, Y.; Stanciu, L.; Ilavsky, J.; Li, W.; Xie, J. Investigation of the Interaction between Nafion Ionomer and Surface Functionalized Carbon Black Using Both Ultrasmall Angle X-ray Scattering and Cryo-TEM. ACS Appl. Mater. Interfaces 2017, 9, 6530–6538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, H.; Henning, S.; Herranz, J.; Eychmüller, A.; Uchida, M.; Schmidt, T.J. Tomographic Analysis and Modeling of Polymer Electrolyte Fuel Cell Unsupported Catalyst Layers. J. Electrochem. Soc. 2018, 165, F7–F16. [Google Scholar] [CrossRef] [Green Version]
- Terao, T.; Inoue, G.; Kawase, M.; Kubo, N.; Yamaguchi, M.; Yokoyama, K.; Tokunaga, T.; Shinohara, K.; Hara, Y.; Hara, T. Development of novel three-dimensional reconstruction method for porous media for polymer electrolyte fuel cells using focused ion beam-scanning electron microscope tomography. J. Power Sources 2017, 347, 108–113. [Google Scholar] [CrossRef]
- Morawietz, T.; Handl, M.; Oldani, C.; Friedrich, K.A.; Hiesgen, R. Quantitative in Situ Analysis of Ionomer Structure in Fuel Cell Catalytic Layers. ACS Appl. Mater. Interfaces 2016, 8, 27044–27054. [Google Scholar] [CrossRef] [PubMed]
- Cetinbas, F.C.; Ahluwalia, R.K.; Kariuki, N.; Andrade, V.D.; Fongalland, D.; Smith, L.; Sharman, J.; Ferreira, P.; Rasouli, S.; Myers, D.J. Hybrid approach combining multiple characterization techniques and simulations for microstructural analysis of proton exchange membrane fuel cell electrodes. J. Power Sources 2017, 344, 62–73. [Google Scholar] [CrossRef] [Green Version]
- Kusano, T.; Hiroi, T.; Amemiya, K.; Ando, M.; Takahashi, T.; Shibayama, M. Structural evolution of a catalyst ink for fuel cells during the drying process investigated by CV-SANS. Polym. J. 2015, 47, 546–555. [Google Scholar] [CrossRef]
- Haubold, H.G.; Wang, X.H.; Jungbluth, H.; Goerigk, G.; Schilling, W. In situ anomalous small-angle X-ray scattering and X-ray absorption near-edge structure investigation of catalyst structures and reactions. J. Mol. Struct. 1996, 383, 283–289. [Google Scholar] [CrossRef]
- Haubold, H.G.; Wang, X.H.; Goerigk, G.; Schilling, W. In Situ Anomalous Small-Angle X-ray Scattering Investigation of Carbon-Supported Electrocatalysts. J. Appl. Cryst. 1997, 30, 653–658. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Kariuki, N.N.; Subbaraman, R.; Kropf, A.J.; Smith, M.C.; Holby, E.F.; Morgan, D.; Myers, D.J. In Situ Anomalous Small-Angle X-ray Scattering Studies of Platinum Nanoparticle Fuel Cell Electrocatalyst Degradation. J. Am. Chem. Soc. 2012, 134, 14823–14833. [Google Scholar] [CrossRef]
- Akiba, I.; Takechi, A.; Sakou, M.; Handa, M.; Shinohara, Y.; Amemiya, Y.; Yagi, N.; Sakurai, K. Anomalous Small-Angle X-ray Scattering Study of Structure of Polymer Micelles Having Bromines in Hydrophobic Core. Macromolecules 2012, 45, 6150–6157. [Google Scholar] [CrossRef]
- Beaucage, G. Approximations Leading to a Unified Exponential/Power-Law Approach to Small-Angle Scattering. J. Appl. Cryst. 1995, 28, 717–728. [Google Scholar] [CrossRef]
- Koga, T.; Hashimoto, T.; Takenaka, M.; Aizawa, K.; Amino, N.; Nakamura, M.; Yamaguchi, D.; Koizumi, S. New Insight into Hierarchical Structures of Carbon Black Dispersed in Polymer Matrices: A Combined Small-Angle Scattering Study. Macromolecules 2008, 41, 453–464. [Google Scholar] [CrossRef]
- Hashimoto, T.; Amino, N.; Nishitsuji, S.; Takenaka, M. Hierarchically self-organized filler particles in polymers: Cascade evolution of dissipative structures to ordered structures. Polym. J. 2019, 51, 109–130. [Google Scholar] [CrossRef]
- Khaneft, M.; Holderer, O.; Ivanova, O.; Lüke, W.; Kentzinger, E.; Appavou, M.S.; Zorn, R.; Lehnert, W. Structure and Proton Dynamics in Catalytic Layer of HT-PEFC. Fuel Cells 2016, 16, 406–413. [Google Scholar] [CrossRef]
- Domhoff, A.; Balwani, A.; Martin, T.B.; Davis, E.M. Leveraging Nanoparticle Dispersion State To Tune Vanadium Ion Selectivity of Nanophase-Segregated Ionomer Nanocomposites for Redox Flow Batteries. ACS Appl. Energy Mater. 2019, 2, 8535–8549. [Google Scholar] [CrossRef]
- Wang, D.; Cao, G. (Eds.) Nanomaterials for Energy Conversion and Storage; World Scientific: London, UK, 2018; pp. 757–809. [Google Scholar] [CrossRef] [Green Version]

| Component | Pt | CB | Nafion | H2O | NPA | EtOH |
|---|---|---|---|---|---|---|
| Content (wt%) | 1.8 | 4.4 | 3.3 | 50.6 | 5.6 | 34.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koga, M.; Matsumoto, H.; Kunishima, M.; Tokita, M.; Masunaga, H.; Ohta, N.; Takeuchi, A.; Mizukado, J.; Sugimori, H.; Shinohara, K.; et al. Microstructure Investigation of Polymer Electrolyte Fuel Cell Catalyst Layers Containing Perfluorosulfonated Ionomer. Membranes 2021, 11, 466. https://doi.org/10.3390/membranes11070466
Koga M, Matsumoto H, Kunishima M, Tokita M, Masunaga H, Ohta N, Takeuchi A, Mizukado J, Sugimori H, Shinohara K, et al. Microstructure Investigation of Polymer Electrolyte Fuel Cell Catalyst Layers Containing Perfluorosulfonated Ionomer. Membranes. 2021; 11(7):466. https://doi.org/10.3390/membranes11070466
Chicago/Turabian StyleKoga, Maito, Hidetoshi Matsumoto, Mitsunori Kunishima, Masatoshi Tokita, Hiroyasu Masunaga, Noboru Ohta, Akihisa Takeuchi, Junji Mizukado, Hidekazu Sugimori, Kazuhiko Shinohara, and et al. 2021. "Microstructure Investigation of Polymer Electrolyte Fuel Cell Catalyst Layers Containing Perfluorosulfonated Ionomer" Membranes 11, no. 7: 466. https://doi.org/10.3390/membranes11070466
APA StyleKoga, M., Matsumoto, H., Kunishima, M., Tokita, M., Masunaga, H., Ohta, N., Takeuchi, A., Mizukado, J., Sugimori, H., Shinohara, K., Uemura, S., Yoshida, T., & Hirai, S. (2021). Microstructure Investigation of Polymer Electrolyte Fuel Cell Catalyst Layers Containing Perfluorosulfonated Ionomer. Membranes, 11(7), 466. https://doi.org/10.3390/membranes11070466






