High Performance Mixed-Matrix Electrospun Membranes for Ammonium Removal from Wastewaters
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Mixed-Matrix Nanofibrous Membranes
2.3. Characterization
Membrane Morphology
2.4. Performance of Mixed-Matrix Membranes
3. Results and Discussion
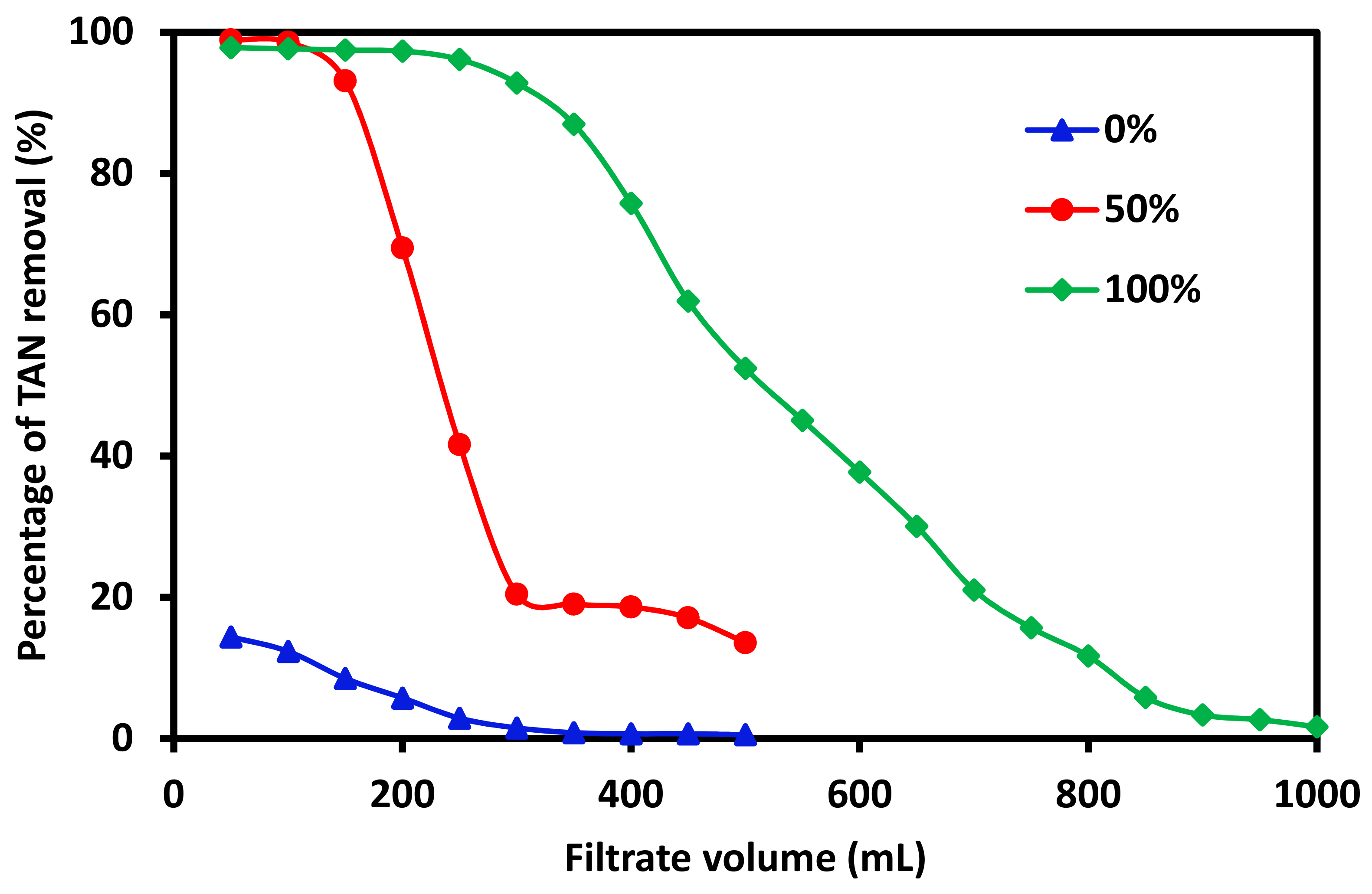
3.1. The Effects of Zeolite Loading on TAN Removal
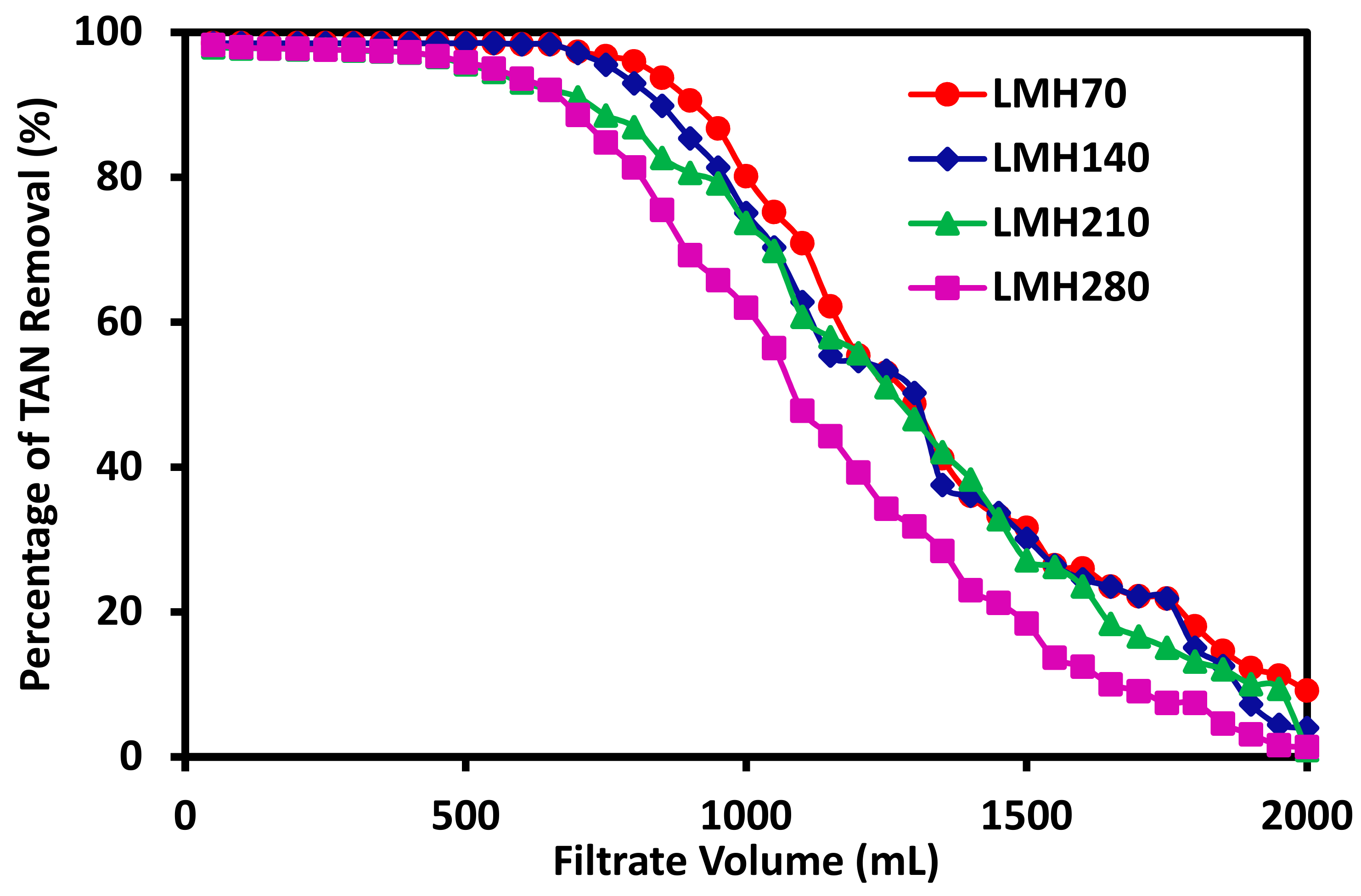
3.2. The Effects of Filtration Flux on Ammonium Exchange and Removal
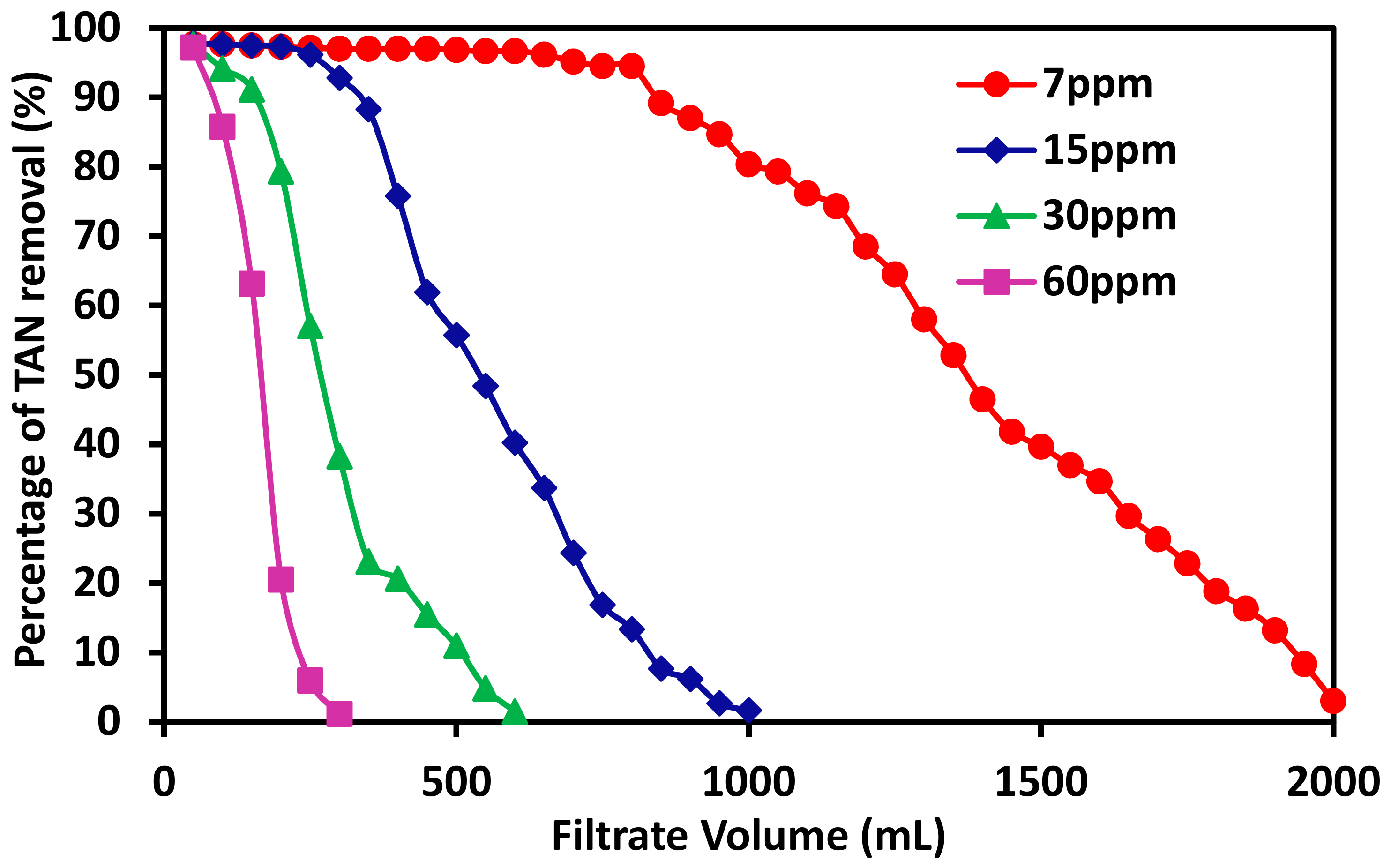
3.3. The Effects of TAN Concentration on Its Removal Capacity
3.4. The Effects of Membrane Regeneration on Its Performance
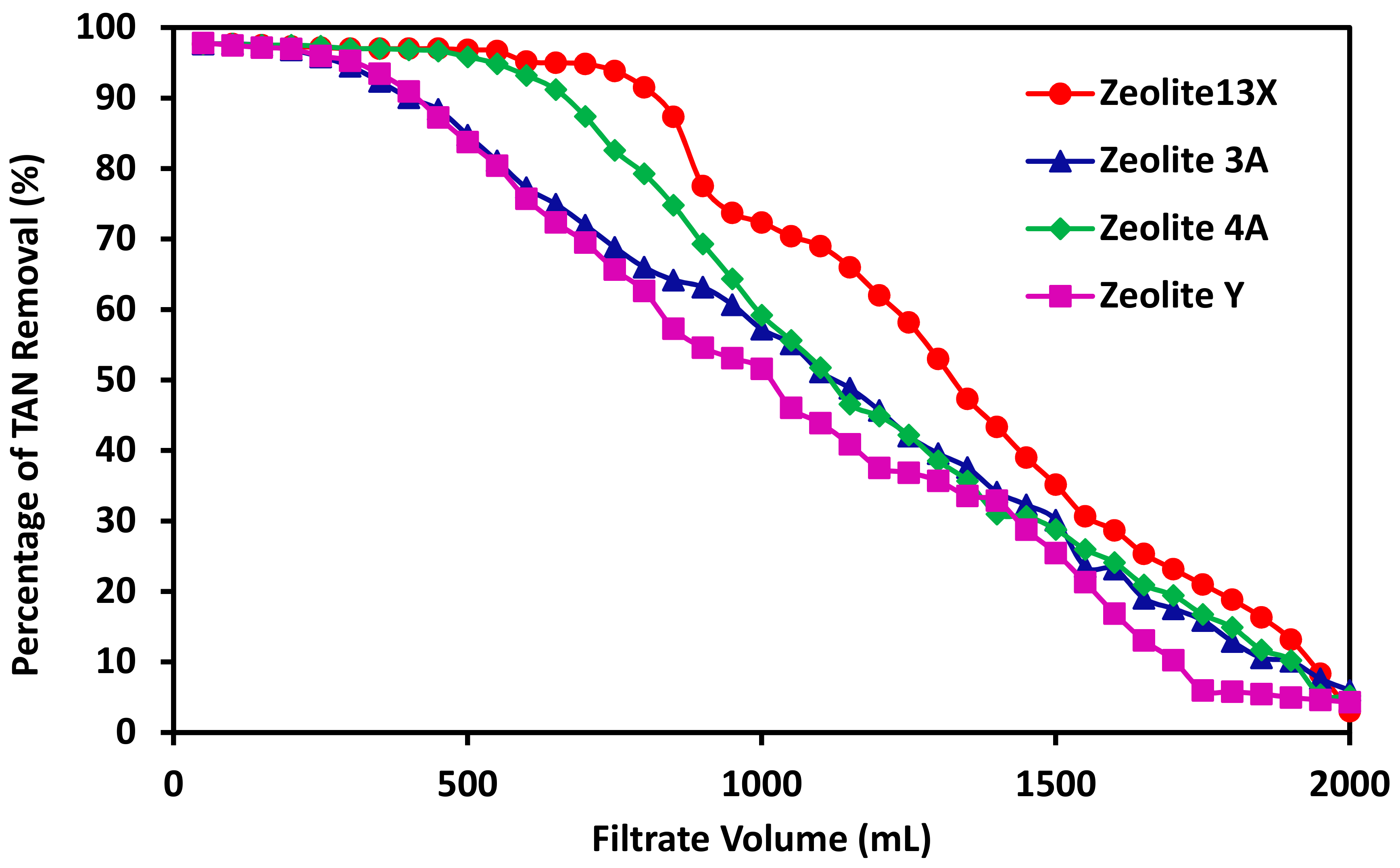
3.5. The Effects of Zeolite Type on the Performance of TAN Removal
3.6. Comparison with Other Zeolite Mixed-Matrix Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciambelli, P.; Corbo, P.; Porcelli, C.; Rimoli, A. Ammonia removal from wastewater by natural zeolites. I. Ammonium ion exchange properties of an Italian phillipsite tuff. Zeolites 1985, 5, 184–187. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Lin, F.; Pang, W.-Q. Ammonium exchange in aqueous solution using Chinese natural clinoptilolite and modified zeolite. J. Hazard. Mater. 2007, 142, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Han, L.; Ma, H.; Zheng, Y.; Zhang, H.; Liu, D.; Liang, S. Adsorption characteristics of ammonium ion by zeolite 13X. J. Hazard. Mater. 2008, 158, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xiao, X.; Yan, B.; Yang, L. Ammonium removal from aqueous solutions by using natural Chinese (Chende) zeolite as adsorbent. J. Hazard. Mater. 2010, 175, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Li, X.; Zhang, X. Ammonium removal from aqueous solutions using microwave-treated natural Chinese zeolite. Sep. Purif. Technol. 2008, 58, 359–366. [Google Scholar] [CrossRef]
- Arslan, A.; Veli, S. Zeolite 13X for adsorption of ammonium ions from aqueous solutions and hen slaughterhouse wastewaters. J. Taiwan Inst. Chem. Eng. 2012, 43, 393–398. [Google Scholar] [CrossRef]
- Ahmadiannamini, P.; Eswaranandam, S.; Wickramasinghe, R.; Qian, X. Mixed-matrix membranes for efficient ammonium removal from wastewaters. J. Membr. Sci. 2017, 526, 147–155. [Google Scholar] [CrossRef]
- Zhu, X.; Cui, W.; Li, X.; Jin, Y. Electrospun Fibrous Mats with High Porosity as Potential Scaffolds for Skin Tissue Engineering. Biomacromolecules 2008, 9, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Huo, P.; Ko, F.K. Fabrication of ultrafine fibrous polytetrafluoroethylene porous membranes by electrospinning. J. Mater. Res. 2009, 24, 2755–2761. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Wang, M.; Wang, X.; Yu, J.; Sun, G. Electrospun nanomaterials for ultrasensitive sensors. Mater. Today 2010, 13, 16–27. [Google Scholar] [CrossRef]
- Pilehvar-Soltanahmadi, Y.; Akbarzadeh, A.; Moazzez-Lalaklo, N.; Zarghami, N. An update on clinical applications of electrospun nanofibers for skin bioengineering. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1350–1364. [Google Scholar] [CrossRef] [PubMed]
- Thavasi, V.; Singh, G.; Ramakrishna, S. Electrospun nanofibers in energy and environmental applications. Energy Environ. Sci. 2008, 1, 205–221. [Google Scholar] [CrossRef]
- Mercante, L.A.; Scagion, V.P.; Migliorini, F.L.; Mattoso, L.H.; Correa, D.S. Electrospinning-based (bio)sensors for food and agricultural applications: A review. TrAC Trends Anal. Chem. 2017, 91, 91–103. [Google Scholar] [CrossRef]
- Patel, A.C.; Li, S.; Wang, C.; Zhang, W.; Wei, Y. Electrospinning of Porous Silica Nanofibers Containing Silver Nanoparticles for Catalytic Applications. Chem. Mater. 2007, 19, 1231–1238. [Google Scholar] [CrossRef]
- Filimon, A.; Olaru, N.; Doroftei, F.; Coroaba, A.; Dunca, S. Processing of quaternized polysulfones solutions as tool in design of electrospun nanofibers: Microstructural characteristics and antimicrobial activity. J. Mol. Liq. 2021, 330, 115664. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-T.; Wickramasinghe, S.R.; Qian, X. Electrospun Weak Anion-Exchange Fibrous Membranes for Protein Purification. Membranes 2020, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]



| Membrane Composition | Membrane Weight (g) | Flux (LMH) | Total TAN Removed (mg) | TAN Removal Capacity (mg/gzeolite) |
|---|---|---|---|---|
| 20%PES-100%Zeolite13X | 1.18 ± 0.06 | 70 | 31.7 ± 2.26 | 53.7 ± 3.83 |
| 20%PES-100%Zeolite13X | 1.18 ± 0.09 | 140 | 30.8 ± 1.02 | 52.2 ± 1.73 |
| 20%PES-100%Zeolite13X | 1.10 ± 0.09 | 210 | 28.8 ± 1.89 | 52.4 ± 3.44 |
| 20%PES-100%Zeolite13X | 1.05 ± 0.10 | 280 | 25.6 ± 2.53 | 48.8 ± 4.87 |
| Membrane Composition | Membrane Weight (g) | Flux (LMH) | Feed Conc. (ppm) | Total TAN Removed (mg) | TAN Removal Capacity (mg/gzeolite) |
|---|---|---|---|---|---|
| 20%PES-100%Zeolite13X | 1.15 ± 0.09 | 70 | 7 | 31.7 ± 1.38 | 55.1 ± 2.38 |
| 20%PES-100%Zeolite13X | 1.16 ± 0.07 | 70 | 15 | 31.5 ± 1.52 | 54.4 ± 2.62 |
| 20%PES-100%Zeolite13X | 1.20 ± 0.13 | 70 | 30 | 31.1 ± 2.17 | 51.8 ± 3.62 |
| 20%PES-100%Zeolite13X | 1.17 ± 0.11 | 70 | 60 | 31.4 ± 2.01 | 53.7 ± 3.44 |
| Membrane Composition | Membrane Weight (g) | Flux (LMH) | Permeate Volume with >90% TAN Removal (mL) | Total TAN Removed (mg) | TAN Removal Capacity (mg/gzeolite) |
|---|---|---|---|---|---|
| 20%PES-100%Zeolite 13X | 1.15 ± 0.07 | 70 | 800 | 30.2 ± 0.75 | 52.5 ± 1.30 |
| 20%PES-100%Zeolite Y | 1.08 ± 0.11 | 70 | 400 | 23.6 ± 1.87 | 43.6 ± 3.46 |
| 20%PES-100%Zeolite 3A | 1.12 ± 0.09 | 70 | 400 | 24.2 ± 1.79 | 43.1 ± 3.19 |
| 20%PES-100%Zeolite 4A | 1.13 ± 0.08 | 70 | 650 | 27.2 ± 1.59 | 48.1 ± 2.81 |
| Composite Membrane | TAN Removal Capacity (mg/gzeolite) | TAN Removal Volume Capacity * (L/m2) |
|---|---|---|
| Phase Inversion Mixed-Matrix Membrane (15% PSU-50% Zeolite 13x) | 19.8 | 35 |
| Pored-Filled | 10.4 | 140 |
| Electrospun Mixed-Matrix Membrane (20% PES-100% Zeolite 13x) | 55.1 | 750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-T.; Wickramasinghe, S.R.; Qian, X. High Performance Mixed-Matrix Electrospun Membranes for Ammonium Removal from Wastewaters. Membranes 2021, 11, 440. https://doi.org/10.3390/membranes11060440
Chen S-T, Wickramasinghe SR, Qian X. High Performance Mixed-Matrix Electrospun Membranes for Ammonium Removal from Wastewaters. Membranes. 2021; 11(6):440. https://doi.org/10.3390/membranes11060440
Chicago/Turabian StyleChen, Shu-Ting, Sumith Ranil Wickramasinghe, and Xianghong Qian. 2021. "High Performance Mixed-Matrix Electrospun Membranes for Ammonium Removal from Wastewaters" Membranes 11, no. 6: 440. https://doi.org/10.3390/membranes11060440
APA StyleChen, S.-T., Wickramasinghe, S. R., & Qian, X. (2021). High Performance Mixed-Matrix Electrospun Membranes for Ammonium Removal from Wastewaters. Membranes, 11(6), 440. https://doi.org/10.3390/membranes11060440






