Separation of H2O/CO2 Mixtures by MFI Membranes: Experiment and Monte Carlo Study
Abstract
1. Introduction
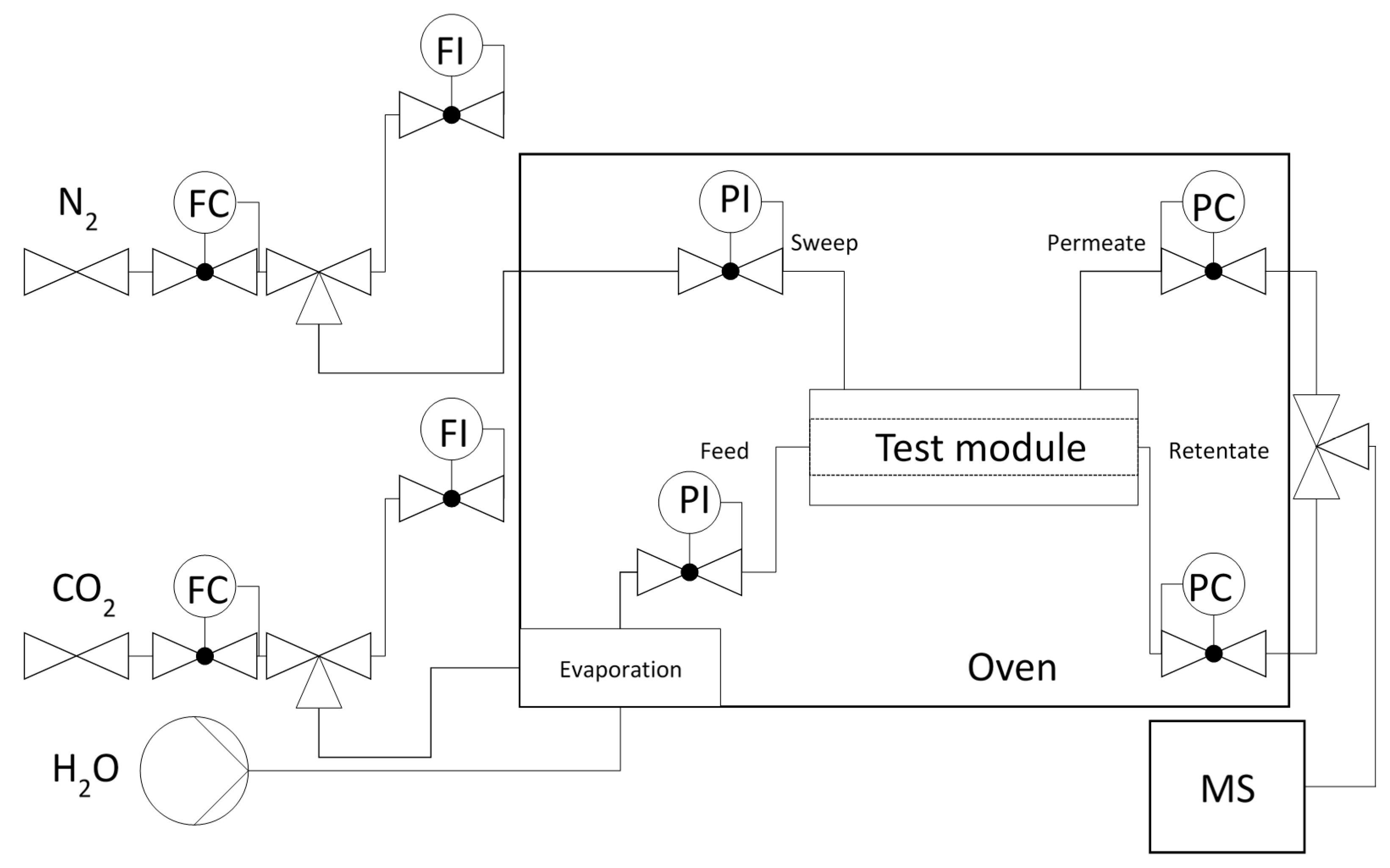
2. Materials and Methods
3. Results
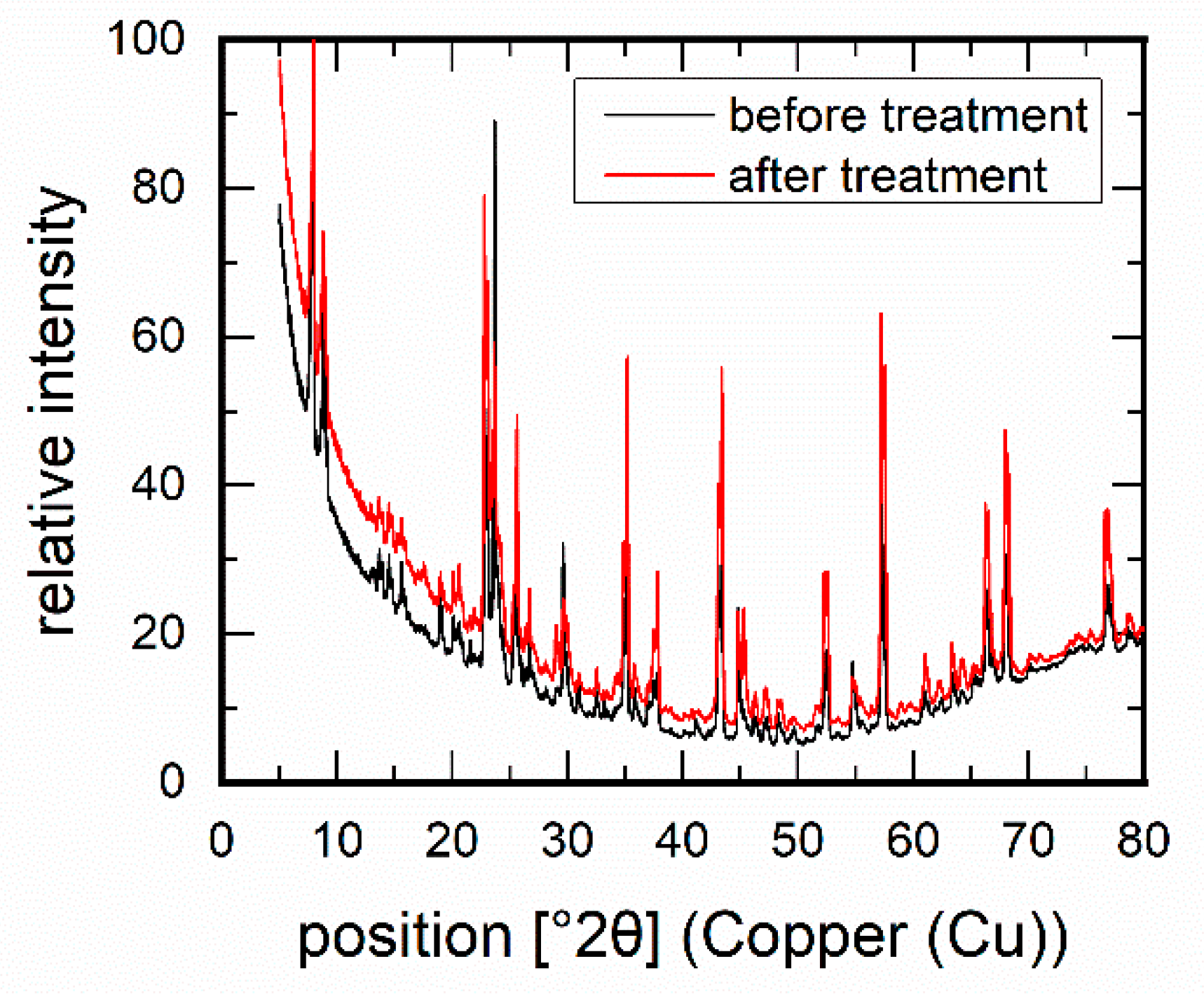
3.1. Membrane Stability in the Presence of Amines
3.2. Determination of Possible Separation Conditions via Monte Carlo Simulation
3.3. Separation of CO2/H2O Gas Streams at MFI Membranes
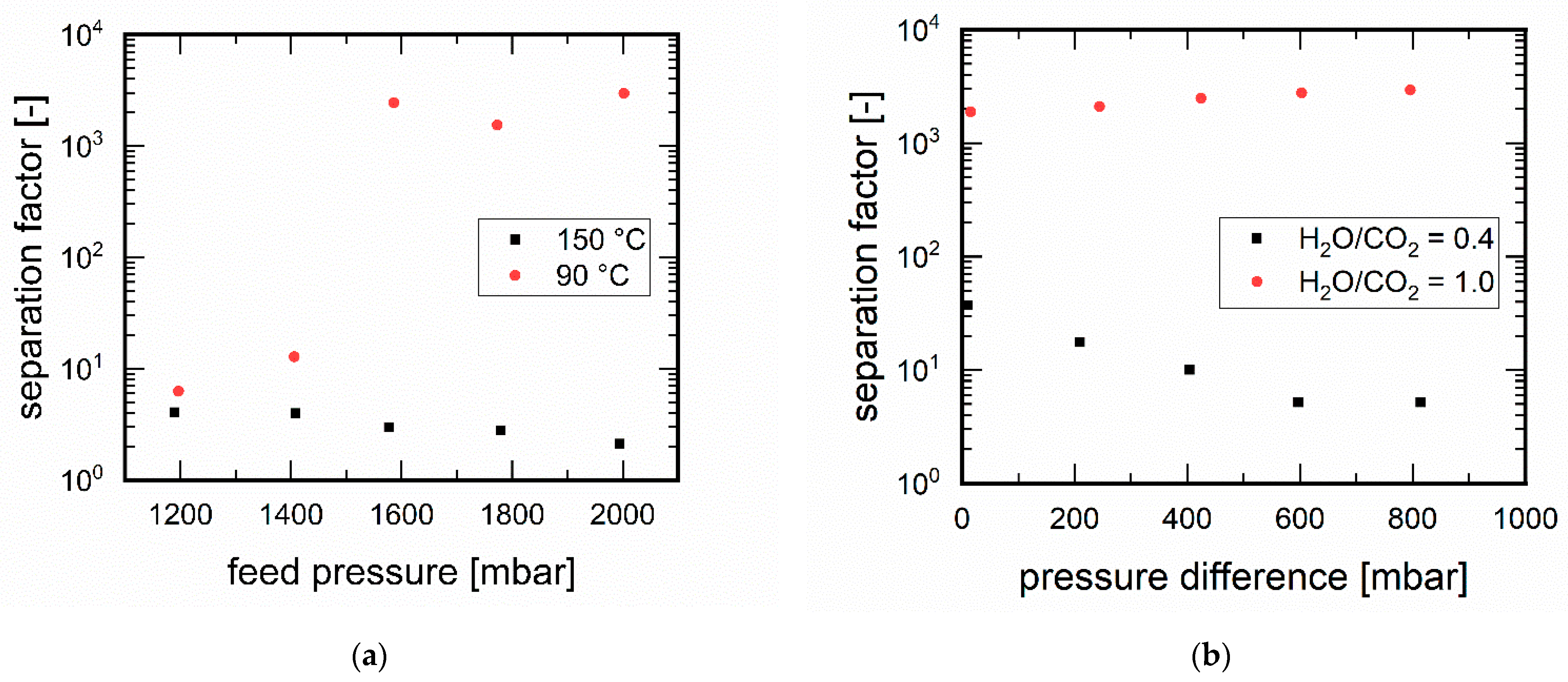
3.3.1. Influence of Temperature
3.3.2. Influence of Pressure
3.3.3. Influence of Feed Flux
3.3.4. Separation Performance of Amine-Treated MFI Membranes
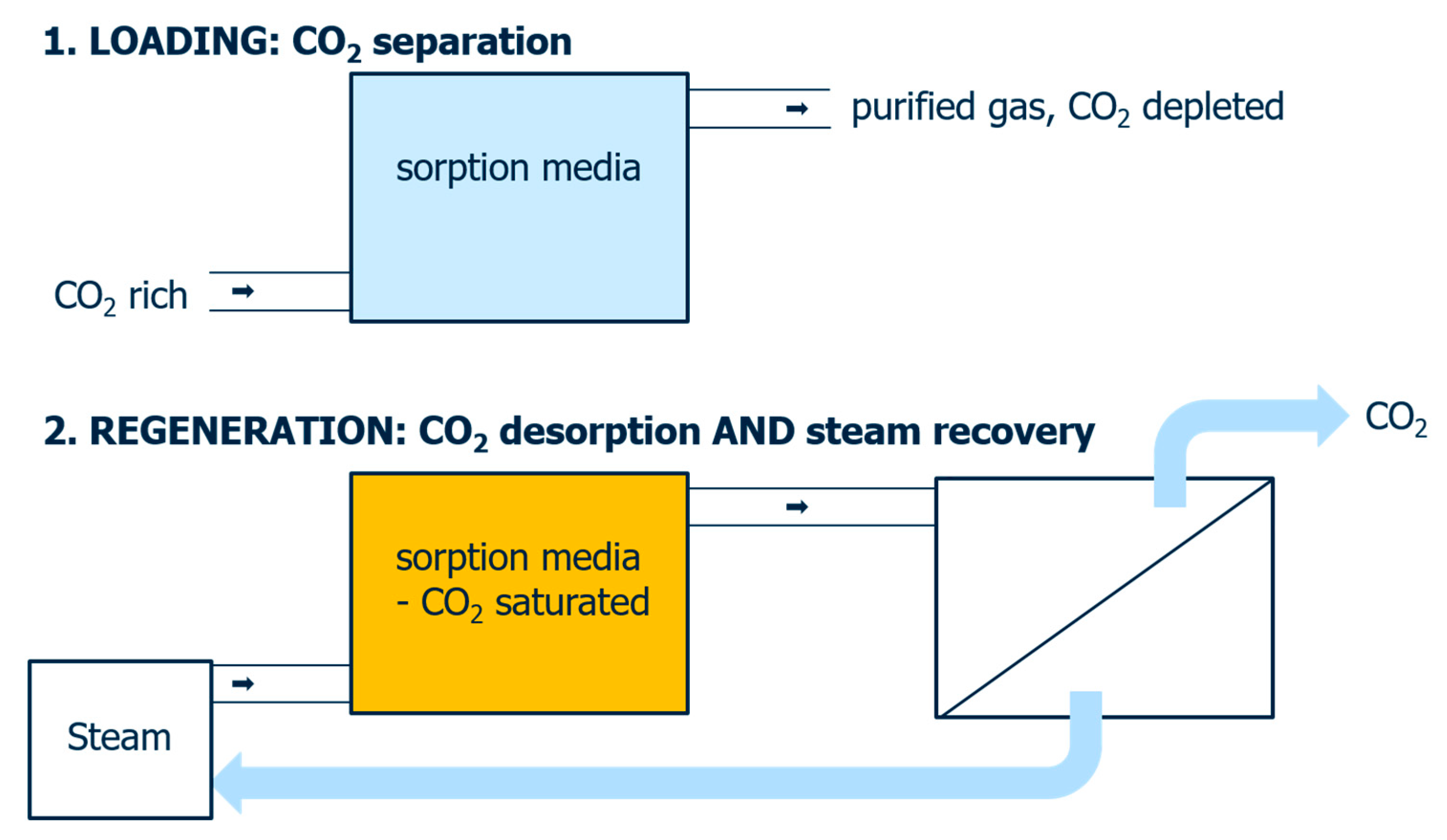
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chowdhury, F.A.; Yamada, H.; Higashii, T.; Goto, K.; Onoda, M. CO2 Capture by Tertiary Amine Absorbents: A Performance Comparison Study. Ind. Eng. Chem. Res. 2013, 52, 8323–8331. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Russell, A.G. Amine-Based CO2 Capture Technology Development from the Beginning of 2013—A Review. ACS Appl. Mater. Interf. 2015, 7, 2137–2148. [Google Scholar] [CrossRef] [PubMed]
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon dioxide capture using liquid absorption methods: A review. Environ. Chem. Lett. 2021, 19, 77–109. [Google Scholar] [CrossRef]
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Rochelle, G.T. 3–Conventional Amine Scrubbing for CO2 Capture. In Absorption-Based Post-Combustion Capture of Carbon Dioxide; Feron, P.H.M., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 35–67. [Google Scholar]
- Puxty, G.; Rowland, R.; Allport, A.; Yang, Q.; Bown, M.; Burns, R.; Maeder, M.; Attalla, M. Carbon Dioxide Postcombustion Capture: A Novel Screening Study of the Carbon Dioxide Absorption Performance of 76 Amines. Environ. Sci. Technol. 2009, 43, 6427–6433. [Google Scholar] [CrossRef]
- Mukesh, C.; Khokarale, S.G.; Virtanen, P.; Mikkola, J.-P. Rapid desorption of CO2 from deep eutectic solvents based on polyamines at lower temperatures: An alternative technology with industrial potential. Sustain. Energy Fuels 2019, 3, 2125–2134. [Google Scholar] [CrossRef]
- de Vos, R.M.; Maier, W.F.; Verweij, H. Hydrophobic silica membranes for gas separation. J. Membr. Sci. 1999, 158, 277–288. [Google Scholar] [CrossRef]
- Pakizeh, M.; Omidkhah, M.R.; Zarringhalam, A. Synthesis and characterization of new silica membranes using template–sol–gel technology. Int. J. Hydrogen Energy 2007, 32, 1825–1836. [Google Scholar] [CrossRef]
- Assa, F. Synthesis and Performance of Nanostructure Templated Silica Membranes Surface-modified by Two Different Procedures. Chem. Biochem. Eng. Quart. 2015, 29, 417–427. [Google Scholar] [CrossRef]
- Fan, S.; Liu, J.; Zhang, F.; Zhou, S.; Sun, F. Fabrication of zeolite MFI membranes supported by α-Al2O3 hollow ceramic fibers for CO2 separation. J. Mater. Res. 2013, 28, 1870–1876. [Google Scholar] [CrossRef]
- Sublet, J.; Pera-Titus, M.; Guilhaume, N.; Farrusseng, D.; Schrive, L.; Chanaud, P.; Siret, B.; Durécu, S. Technico-economical assessment of MFI-type zeolite membranes for CO2 capture from postcombustion flue gases. AIChE J. 2012, 58, 3183–3194. [Google Scholar] [CrossRef]
- Yang, S.; Cao, Z.; Arvanitis, A.; Sun, X.; Xu, Z.; Dong, J. DDR-type zeolite membrane synthesis, modification and gas permeation studies. J. Membr. Sci. 2016, 505, 194–204. [Google Scholar] [CrossRef]
- Junaidi, M.U.M.; Leo, C.P.; Ahmad, A.L.; Ahmad, N.A. Fluorocarbon functionalized SAPO-34 zeolite incorporated in asymmetric mixed matrix membranes for carbon dioxide separation in wet gases. Microporous Mesoporous Mat. 2015, 206, 23–33. [Google Scholar] [CrossRef]
- Wang, J.; Hao, Z.; Wohlrab, S. Continuous CO2 esterification to diethyl carbonate (DEC) at atmospheric pressure: Application of porous membranes for in situ H2O removal. Green Chem. 2017, 19, 3595–3600. [Google Scholar] [CrossRef]
- Wohlrab, S.; Meyer, T.; Stöhr, M.; Hecker, C.; Lubenau, U.; Oßmann, A. On the performance of customized MFI membranes for the separation of n-butane from methane. J. Membr. Sci. 2011, 369, 96–104. [Google Scholar] [CrossRef]
- Bolto, B.; Hoang, M.; Xie, Z. A review of water recovery by vapour permeation through membranes. Water Res. 2012, 46, 259–266. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M. Zeolite membranes–Recent developments and progress. Microporous Mesoporous Mat. 2008, 115, 215–233. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Su, X.; Cao, S.; Liu, Y.; Gao, D.; An, L. Experimental study of water recovery from flue gas using hollow micro–nano porous ceramic composite membranes. J. Ind. Eng. Chem. 2018, 57, 349–355. [Google Scholar] [CrossRef]
- Kim, J.F.; Park, A.; Kim, S.-J.; Lee, P.; Cho, Y.; Park, H.; Nam, S.; Park, Y. Harnessing Clean Water from Power Plant Emissions Using Membrane Condenser Technology. ACS Sustain. Chem. Eng. 2018, 6, 6425–6433. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Zhang, H.; Zhang, J.; Chen, H.; Fu, H. Moisture recovery from gas-fired boiler exhaust using membrane module array. J. Clean. Prod. 2019, 231, 1110–1121. [Google Scholar] [CrossRef]
- Kim, J.F.; Drioli, E. Transport Membrane Condenser Heat Exchangers to Break the Water-Energy Nexus—A Critical Review. Membranes 2021, 11, 12. [Google Scholar] [CrossRef]
- Lin, H.; Thompson, S.M.; Serbanescu-Martin, A.; Wijmans, J.G.; Amo, K.D.; Lokhandwala, K.A.; Merkel, T.C. Dehydration of natural gas using membranes. Part I: Composite membranes. J. Membr. Sci. 2012, 413–414, 70–81. [Google Scholar] [CrossRef]
- Li, G.M.; Feng, C.; Li, J.F.; Liu, J.Z.; Wu, Y.L. Water vapor permeation and compressed air dehydration performances of modified polyimide membrane. Sep. Purif. Technol. 2008, 60, 330–334. [Google Scholar] [CrossRef]
- Zagho, M.M.; Hassan, M.K.; Khraisheh, M.; Al-Maadeed, M.A.A.; Nazarenko, S. A review on recent advances in CO2 separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chem. Eng. J. Adv. 2021, 6, 100091. [Google Scholar] [CrossRef]
- Dubbeldam, D.; Calero, S.; Ellis, D.E.; Snurr, R.Q. RASPA: Molecular simulation software for adsorption and diffusion in flexible nanoporous materials. Mol. Simul. 2016, 42, 81–101. [Google Scholar] [CrossRef]
- Vlugt, T.J.H.; Martin, M.G.; Smit, B.; Siepmann, J.I.; Krishna, R. Improving the efficiency of the configurational-bias Monte Carlo algorithm. Mol. Phys. 1998, 94, 727–733. [Google Scholar] [CrossRef]
- Ewald, P.P. Die Berechnung optischer und elektrostatischer Gitterpotentiale. Ann. Phys. 1921, 369, 253–287. [Google Scholar] [CrossRef]
- Harris, J.G.; Yung, K.H. Carbon Dioxide’s Liquid-Vapor Coexistence Curve And Critical Properties as Predicted by a Simple Molecular Model. J. Phys. Chem. 1995, 99, 12021–12024. [Google Scholar] [CrossRef]
- Rick, S.W. A reoptimization of the five-site water potential (TIP5P) for use with Ewald sums. J. Chem. Phys. 2004, 120, 6085–6093. [Google Scholar] [CrossRef]
- Mahoney, M.W.; Jorgensen, W.L. A five-site model for liquid water and the reproduction of the density anomaly by rigid, nonpolarizable potential functions. J. Chem. Phys. 2000, 112, 8910–8922. [Google Scholar] [CrossRef]
- Jorabchi, M.N.; Ludwig, R.; Paschek, D. Quasi-Universal Solubility Behavior of Light Gases in Imidazolium-Based Ionic Liquids with Varying Anions: A Molecular Dynamics Simulation Study. J. Phys. Chem. B 2021, 125, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Koros, W.J.; Ma, Y.H.; Shimidzu, T. Terminology for Membranes and membrane processes (IUPAC recommendations 1996). Pure Appl. Chem. 1996, 68, 1479–1489. [Google Scholar] [CrossRef]
- Caro, J.; Noack, M.; Kölsch, P. Zeolite Membranes: From the Laboratory Scale to Technical Applications. Adsorption 2005, 11, 215–227. [Google Scholar] [CrossRef]
- Hedlund, J.; Korelskiy, D.; Sandström, L.; Lindmark, J. Permporometry analysis of zeolite membranes. J. Membr. Sci. 2009, 345, 276–287. [Google Scholar] [CrossRef]
- van Koningsveld, H.; Jansen, J.C. Single crystal structure analysis of zeolite H-ZSM-5 loaded with naphthalene. Microporous Mater. 1996, 6, 159–167. [Google Scholar] [CrossRef]
- Dragomirova, R.; Stohr, M.; Hecker, C.; Lubenau, U.; Paschek, D.; Wohlrab, S. Desorption-controlled separation of natural gas alkanes by zeolite membranes. RSC Adv. 2014, 4, 59831–59834. [Google Scholar] [CrossRef]
- Desbiens, N.; Boutin, A.; Demachy, I. Water Condensation in Hydrophobic Silicalite-1 Zeolite: A Molecular Simulation Study. J. Phys. Chem. B 2005, 109, 24071–24076. [Google Scholar] [CrossRef]
- Ektefa, F.; Javadian, S.; Rahmati, M. Computational comparison of the efficiency of nanoporous zeolite frameworks for separation of phenol from water. J. Taiwan Inst. Chem. Eng. 2018, 88, 104–113. [Google Scholar] [CrossRef]
- Lu, L.; Shao, Q.; Huang, L.; Lu, X. Simulation of adsorption and separation of ethanol–water mixture with zeolite and carbon nanotube. Fluid Phase Equilib. 2007, 261, 191–198. [Google Scholar] [CrossRef]
- van den Broeke, L.J.P.; Bakker, W.J.W.; Kapteijn, F.; Moulijn, J.A. Transport and separation properties of a silicalite-1 membrane—I. Operating conditions. Chem. Eng. Sci. 1999, 54, 245–258. [Google Scholar] [CrossRef]
- Arruebo, M.; Coronas, J.; Menéndez, M.; Santamaría, J. Separation of hydrocarbons from natural gas using silicalite membranes. Sep. Purif. Technol. 2001, 25, 275–286. [Google Scholar] [CrossRef]
- Gump, C.J.; Lin, X.; Falconer, J.L.; Noble, R.D. Experimental configuration and adsorption effects on the permeation of C4 isomers through ZSM-5 zeolite membranes. J. Membr. Sci. 2000, 173, 35–52. [Google Scholar] [CrossRef]
- van de Graaf, J.M.; Kapteijn, F.; Moulijn, J.A. Methodological and operational aspects of permeation measurements on silicalite-1 membranes. J. Membr. Sci. 1998, 144, 87–104. [Google Scholar] [CrossRef]
- Wang, D.; Bao, A.; Kunc, W.; Liss, W. Coal power plant flue gas waste heat and water recovery. Appl. Energy 2012, 91, 341–348. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Li, W.; Choi, S.; Drese, J.H.; Hornbostel, M.; Krishnan, G.; Eisenberger, P.M.; Jones, C.W. Steam-Stripping for Regeneration of Supported Amine-Based CO2 Adsorbents. ChemSusChem 2010, 3, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Gebald, C.; Wurzbacher, J.A.; Tingaut, P.; Zimmermann, T.; Steinfeld, A. Amine-Based Nanofibrillated Cellulose As Adsorbent for CO2 Capture from Air. Environ. Sci. Technol. 2011, 45, 9101–9108. [Google Scholar] [CrossRef]
- Sujan, A.R.; Pang, S.H.; Zhu, G.; Jones, C.W.; Lively, R.P. Direct CO2 Capture from Air using Poly(ethylenimine)-Loaded Polymer/Silica Fiber Sorbents. ACS Sustain. Chem. Eng. 2019, 7, 5264–5273. [Google Scholar] [CrossRef]
- Sehaqui, H.; Gálvez, M.E.; Becatinni, V.; cheng Ng, Y.; Steinfeld, A.; Zimmermann, T.; Tingaut, P. Fast and Reversible Direct CO2 Capture from Air onto All-Polymer Nanofibrillated Cellulose—Polyethylenimine Foams. Environ. Sci. Technol. 2015, 49, 3167–3174. [Google Scholar] [CrossRef]
- Deng, X.; Yang, W.; Li, S.; Liang, H.; Shi, Z.; Qiao, Z. Large-Scale Screening and Machine Learning to Predict the Computation-Ready, Experimental Metal-Organic Frameworks for CO2 Capture from Air. Appl. Sci. 2020, 10, 569. [Google Scholar] [CrossRef]
- Didas, S.A.; Choi, S.; Chaikittisilp, W.; Jones, C.W. Amine–Oxide Hybrid Materials for CO2 Capture from Ambient Air. Acc. Chem. Res. 2015, 48, 2680–2687. [Google Scholar] [CrossRef] [PubMed]
- Wijesiri, R.P.; Knowles, G.P.; Yeasmin, H.; Hoadley, A.F.A.; Chaffee, A.L. CO2 Capture from Air Using Pelletized Polyethylenimine Impregnated MCF Silica. Ind. Eng. Chem. Res. 2019, 58, 3293–3303. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Eisenberger, P.M.; Jones, C.W. Application of Amine-Tethered Solid Sorbents for Direct CO2 Capture from the Ambient Air. Environ. Sci. Technol. 2011, 45, 2420–2427. [Google Scholar] [CrossRef] [PubMed]





| m/u | q/e | 𝞼/Å | ||
|---|---|---|---|---|
| O_CO2 | 15.9994 | −0.3256 | 80.507 | 3.033 |
| C_CO2 | 12.0 | 0.6512 | 28.129 | 2.76 |
| O_H2O | 15.9994 | 0.0 | 89.633 | 3.097 |
| H_H2O | 1.008 | 0.241 | - | - |
| V_H2O | 0.0 | −0.241 | - | - |
| H2O/CO2 Ratio | Change of the Separation Factor (–) | CO2 Permeance (L⋅m−2⋅h−1⋅bar−1) | H2O Permeance (m−2⋅h−1⋅bar−1) |
|---|---|---|---|
| 0.4 | 2.37 | 6936 | 12893 |
| ↓ | ↓ | ↓ | |
| 3.43 | 2630 | 7981 | |
| 0.6 | 217 | 26.5 | 6604 |
| ↓ | ↓ | ↓ | |
| 401 | 16.4 | 7417 | |
| 1.0 | 726 | 9.0 | 7429 |
| ↓ | ↓ | ↓ | |
| 505 | 14.8 | 8584 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wotzka, A.; Jorabchi, M.N.; Wohlrab, S. Separation of H2O/CO2 Mixtures by MFI Membranes: Experiment and Monte Carlo Study. Membranes 2021, 11, 439. https://doi.org/10.3390/membranes11060439
Wotzka A, Jorabchi MN, Wohlrab S. Separation of H2O/CO2 Mixtures by MFI Membranes: Experiment and Monte Carlo Study. Membranes. 2021; 11(6):439. https://doi.org/10.3390/membranes11060439
Chicago/Turabian StyleWotzka, Alexander, Majid Namayandeh Jorabchi, and Sebastian Wohlrab. 2021. "Separation of H2O/CO2 Mixtures by MFI Membranes: Experiment and Monte Carlo Study" Membranes 11, no. 6: 439. https://doi.org/10.3390/membranes11060439
APA StyleWotzka, A., Jorabchi, M. N., & Wohlrab, S. (2021). Separation of H2O/CO2 Mixtures by MFI Membranes: Experiment and Monte Carlo Study. Membranes, 11(6), 439. https://doi.org/10.3390/membranes11060439






