Experimental and Theoretical Approaches to Describing Interactions in Natural Cell Membranes Occurring as a Result of Fatal Alcohol Poisoning
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
Microelectrophoretic Mobility Measurements
3. Results
4. Discussion
- -
- The equation for determining the membrane surface charge density:
- -
- Linear equations—simplifications of Equation (8)—for high (Equation (9)) and low (Equation (10)) concentrations of hydrogen ions:
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballard, H.S. The hematological complications of alcoholism. Alcohol. Clin. Exp. Res. 1989, 13, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Ballard, H.S. Alcohol, bone marrow, and blood. Alcohol. Health Res. World. 1993, 17, 310–315. [Google Scholar]
- Stouten, K.; Riedl, J.A.; Droogendijk, J.; Castel, R.; van Rosmalen, J.; van Houten, R.J.; Berendes, P.; Sonneveld, P.; Levin, M.D. Prevalence of potential underlying aetiology of macrocytic anaemia in Dutch general practice. BMC Fam. Pr. 2016, 17, 113. [Google Scholar] [CrossRef][Green Version]
- Thoma, E.; Bitri, S.; Mucaj, K.; Tahiri, A.; Pano, I. Changes of Some Blood Count Variables in Correlation with the Time of Alcohol Abuse. J. Addict. Res. 2015, 6, 2. [Google Scholar] [CrossRef]
- Marumo, M.; Wakabayashi, I. Sensitivity of thrombin-induced platelet aggregation to inhibition by ethanol. Clin. Chim. Acta 2009, 402, 156–159. [Google Scholar] [CrossRef]
- Ballard, H.S. Alcohol Abuse in Guide to Anemia, 2nd ed.; Garrison, C.D., Ed.; Cumberland House: Naperville, IL, USA, 2009; p. 165. [Google Scholar]
- De Pietri, L.; Bianchini, M.; Montalti, R.; De Maria, N.; Di Maira, T.; Begliomini, B.; Gerunda, G.E.; di Benedetto, F.; Garcia-Tsao, G.; Villa, E. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: A randomized, controlled trial. Hepatology 2016, 63, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Gurtovenko, A.A.; Anwar, J. Interaction of Ethanol with Biological Membranes: The Formation of Non-bilayer Structures within the Membrane Interior and their Significance. J. Phys. Chem. B 2009, 113, 1983–1992. [Google Scholar] [CrossRef]
- Sonmez, M.; Ince, H.Y.; Yalcin, O.; Ajdžanović, V.; Spasojević, I.; Meiselman, H.J.; Baskurt, O.K. The effect of alcohols on red blood cell mechanical properties and membrane fluidity depends on their molecular size. PLoS ONE 2013, 8, e76579. [Google Scholar] [CrossRef]
- Dora, M.D.; Goldstein, B. Effect of alcohol on cellular membranes. Ann. Emerg. Med. 1986, 15, 1013–1018. [Google Scholar]
- Chin, J.H.; Goldstein, B.D. Effects of low concentrations of ethanol on the fluidity of spin-labeled erythrocyte and brain membranes. Mol. Pharm. 1977, 13, 435–441. [Google Scholar]
- Wood, W.G.; Lahiri, S.; Gorka, C.; Armbrecht, H.J.; Strong, R. In vitro effects of ethanol on erythrocyte membrane fluidity of alcoholic patients: An electron spin resonance study. Alcohol. Clin. Exp. Res. 1987, 11, 332–335. [Google Scholar] [CrossRef]
- Beaug, F.; Gallay, J.; Stibler, H.; Borg, S. Alcohol abuse increases the lipid structural order in human erythrocyte membranes: A steady-state and time-resolved anisotropy study. Biochem. Pharmacol. 1988, 37, 3823–3828. [Google Scholar] [CrossRef]
- Stibler, H.; Beauge, F.; Leguicher, A.; Borg, S. Biophysical and biochemical alterations in erythrocyte membranes from chronic alcoholics. Scand. J. Clin. Lab. Investig. 1991, 51, 309–319. [Google Scholar] [CrossRef]
- Hrelia, S.; Lercker, G.; Biagi, P.; Bordoni, A.; Stefanini, F.; Zunarelli, P.; Rossi, C.A. Effect of ethanol intake on human erythrocyte membrane fluidity and lipid composition. Biochem. Int. 1986, 12, 741–750. [Google Scholar]
- Dobrzynska, I.; Szachowicz-Petelska, B.; Skrzydlewska, E.; Figaszewski, Z.A. Changes in electric charge and phospholipids composition in erythrocyte membrane of ethanol-poisoned rats after administration of teas. Acta Pol. Pharm. 2004, 61, 483–487. [Google Scholar] [PubMed]
- Szachowicz-Petelska, B.; Dobrzynska, I.; Skrzydlewska, E.; Figaszewski, Z. Influence of green tea on surface charge density and phospholipids composition of erythrocytes membrane in ethanol intoxicated rats. Cell Biol. Toxicol. 2005, 21, 61–70. [Google Scholar] [CrossRef]
- Szachowicz-Petelska, B.; Dobrzynska, I.; Skrzydlewska, E.; Figaszewski, Z. Protective effect of blackcurrant on liver cell membrane of rats intoxicated with ethanol. J. Membr. Biol. 2012, 245, 191–200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chachaj-Brekiesz, A.; Kobierski, J.; Wnętrzak, A.; Dynarowicz-Latka, P. Electrical Properties of Membrane Phospholipids in Langmuir Monolayers. Membranes 2021, 11, 53. [Google Scholar] [CrossRef]
- Naumowicz, M.; Zając, M.; Kusaczuk, M.; Gál, M.; Kotyńska, J. Electrophoretic Light Scattering and Electrochemical Impedance Spectroscopy Studies of Lipid Bilayers Modified by Cinnamic Acid and Its Hydroxyl Derivatives. Membranes 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Dziubak, D.; Strzelak, K.; Sek, S. Electrochemical Properties of Lipid Membranes Self-Assembled from Bicelles. Membranes 2021, 11, 11. [Google Scholar] [CrossRef]
- Fernandes, H.P.; Cesar, C.L.; de Lourdes Barjas-Castro, M. Electrical properties of the red blood cell membrane and immunohematological investigation. Rev. Bras. Hematol. Hemoter. 2011, 33, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiang, Y.; Alvarez, O.; Du, E. Electrical Impedance Characterization of Erythrocyte Response to Cyclic Hypoxia in Sickle Cell Disease. ACS Sens. 2019, 4, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Kotyńska, J.; Petelska, A.D.; Szeremeta, M.; Niemcunowicz-Janica, A.; Figaszewski, Z.A. Changes in surface charge density of blood cells after sudden unexpected death. J. Membr. Biol. 2012, 245, 185–190. [Google Scholar] [CrossRef][Green Version]
- Szeremeta, M.; Petelska, A.D.; Kotyńska, J.; Niemcunowicz-Janica, A.; Figaszewski, Z.A. The effect of fatal carbon monoxide poisoning on the surface charge of blood cells. J. Membr. Biol. 2013, 246, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Petelska, A.D.; Kotyńska, J.; Figaszewski, Z.A. The effect of fatal carbon monoxide poisoning on the equilibria between cell membranes and the electrolyte solution. J. Membr. Biol. 2015, 248, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Szeremeta, M.; Petelska, A.D.; Kotyńska, J.; Pepiński, W.; Naumowicz, M.; Figaszewski, Z.A.; Niemcunowicz-Janica, A. Changes in surface charge density of blood cells in fatal accidental hypothermia. J. Membr. Biol. 2015, 248, 1175–1180. [Google Scholar] [CrossRef]
- Petelska, A.D.; Kotyńska, J.; Naumowicz, M.; Figaszewski, Z.A. Equilibria between cell membranes and electrolyte solution: Effect of fatal accidental hypothermia. J. Membr. Biol. 2016, 249, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Salonen, E.; Terama, E.; Vattulainen, I.; Faller, R.; Lee, B.W.; Holopainen, J.; Karttunen, M. Under the Influence of Alcohol: The Effect of Ethanol and Methanol on Lipid Bilayers. Biophys. J. 2006, 90, 1121–1135. [Google Scholar] [CrossRef]
- Alexander, A.E.; Johnson, P. Colloid Science; Clarendon Press: Oxford, UK, 1949. [Google Scholar]
- Barrow, G.M. Physical Chemistry; McGraw-Hill Inc: New York, NY, USA, 1996. [Google Scholar]
- Dobrzyńska, I.; Skrzydlewska, E.; Figaszewski, Z. Parameters characterizing acid-base equilibria between cell membrane and solution and their application to monitoring the effect of various factors on the membrane. Bioelectrochemistry 2006, 69, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Scheidt, H.A.; Huster, D. The interaction of small molecules with phospholipid membranes studied by 1 H NOESY NMR under magic-angle spinning. Acta Pharmacol. Sin. 2008, 29, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Rowe, E.S. Effects of ethanol on membrane lipids. In Alcohol and Neurobiology: Receptors, Membranes, and Channels, 1st ed.; Watson, R.R., Ed.; CRC Press: Boca Raton, FL, USA, 1992; pp. 239–268. [Google Scholar]
- Henderson, C.M.; Block, D.E. Examining the Role of Membrane Lipid Composition in Determining the Ethanol Tolerance of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2014, 80, 2966–2972. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, H.J.; Best-Popescu, C.; Jang, S.; Park, Y.K. The Effects of Ethanol on the Morphological and Biochemical Properties of Individual Human Red Blood Cells. PLoS ONE 2015, 10, e0145327. [Google Scholar] [CrossRef] [PubMed]
- Killian, J.A. Hydrophobic mismatch between proteins and lipids in membranes. Biochim. Biophys. Acta 1998, 1376, 401–416. [Google Scholar] [CrossRef]
- Zeng, J.; Smith, K.E.; Chong, P.L. Effects of alcohol-induced lipid interdigitation on proton permeability in L-α-dipalmitoylphosphatidylcholine vesicles. Biophys. J. 1993, 65, 1404–1414. [Google Scholar] [CrossRef]
- Lee, A.G. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta 2004, 1666, 62–87. [Google Scholar] [CrossRef]
- Bulle, S.; Reddy, V.D.; Padmavathi, P.; Maturu, P.; Puvvada, P.K.; Nallanchakravarthula, V. Association between alcohol-induced erythrocyte membrane alterations and hemolysis in chronic alcoholic. J. Clin. Biochem. Nutr. 2017, 60, 63–69. [Google Scholar] [CrossRef] [PubMed]
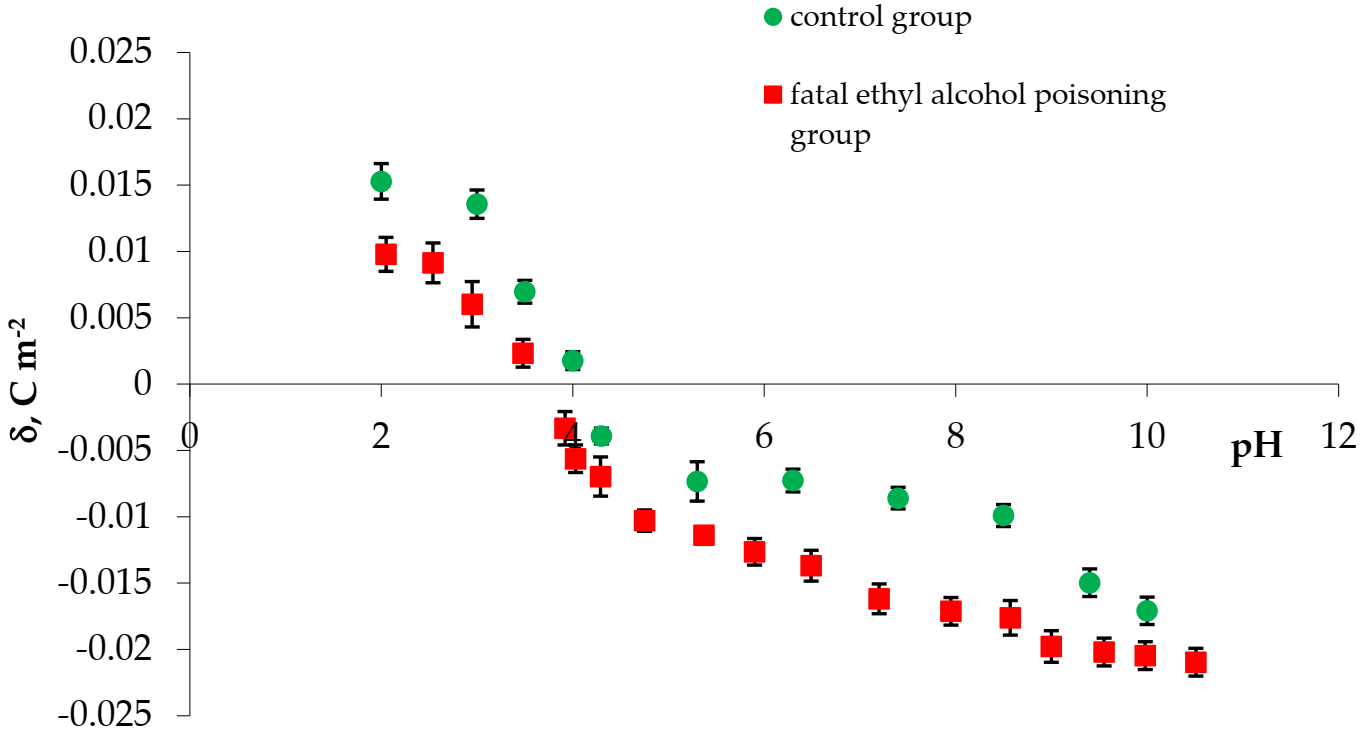
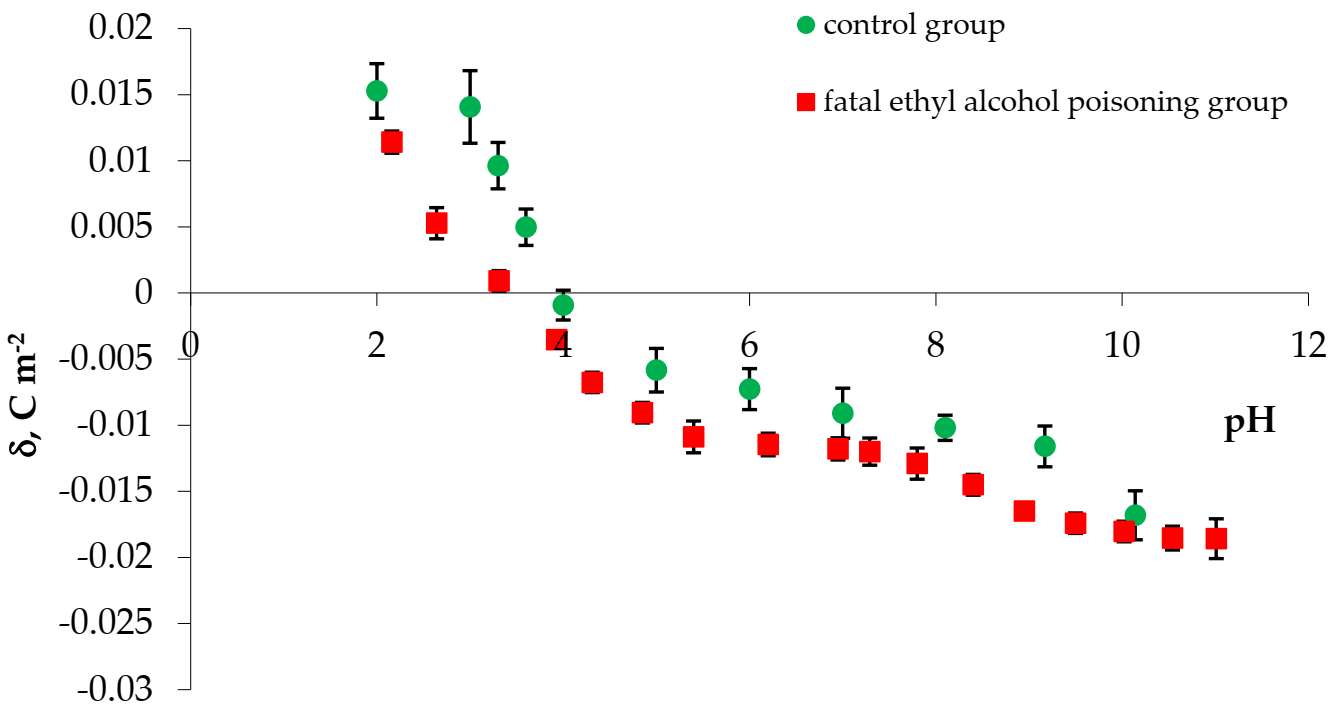


| Examined Groups | Isoelectric Point | Surface Charge Density [10−2 C m−2] | |
|---|---|---|---|
| pH ~ 3 | pH ~ 10 | ||
| control | 4.05 | 1.357 ± 0.113 | −1.710 ± 0.109 |
| fatal ethyl alcohol poisoning | 3.92 | 0.978 ± 0.068 | −2.300 ± 0.106 |
| Examined Groups | Isoelectric Point | Surface Charge Density [10−2 C m−2] | |
|---|---|---|---|
| pH ~ 3 | pH ~ 10 | ||
| control | 4.20 | 1.407 ± 0.212 | −1.681 ± 0.178 |
| fatal ethyl alcohol poisoning | 3.31 | 1.141 ± 0.138 | −1.858 ± 0.155 |
| Groups | Parameters | |||
|---|---|---|---|---|
| cA [10−6 mol/m2] | cB [10−6 mol/m2] | KAH [102 m3/mol] | KBOH [107 m3/mol] | |
| control | 7.11 ± 0.44 | 1.61 ± 0.51 | 3.44 ± 1.11 | 3.56 ± 0.81 |
| fatal ethyl alcohol poisoning | 1.16 ± 0.08 | 3.22 ± 0.91 | 0.38 ± 0.04 | 2.12 ± 0.26 |
| Groups | Parameters | |||
|---|---|---|---|---|
| cA [10−6 mol/m2] | cB [10−6 mol/m2] | KAH [102 m3/mol] | KBOH [107 m3/mol] | |
| control | 3.76 ± 0.81 | 1.21 ± 0.23 | 2.88 ± 1.58 | 2.11 ± 0.79 |
| fatal ethyl alcohol poisoning | 5.56 ± 0.33 | 0.17 ± 0.03 | 3.47 ± 0.65 | 5.21 ± 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petelska, A.D.; Szeremeta, M.; Kotyńska, J.; Niemcunowicz-Janica, A. Experimental and Theoretical Approaches to Describing Interactions in Natural Cell Membranes Occurring as a Result of Fatal Alcohol Poisoning. Membranes 2021, 11, 189. https://doi.org/10.3390/membranes11030189
Petelska AD, Szeremeta M, Kotyńska J, Niemcunowicz-Janica A. Experimental and Theoretical Approaches to Describing Interactions in Natural Cell Membranes Occurring as a Result of Fatal Alcohol Poisoning. Membranes. 2021; 11(3):189. https://doi.org/10.3390/membranes11030189
Chicago/Turabian StylePetelska, Aneta D., Michał Szeremeta, Joanna Kotyńska, and Anna Niemcunowicz-Janica. 2021. "Experimental and Theoretical Approaches to Describing Interactions in Natural Cell Membranes Occurring as a Result of Fatal Alcohol Poisoning" Membranes 11, no. 3: 189. https://doi.org/10.3390/membranes11030189
APA StylePetelska, A. D., Szeremeta, M., Kotyńska, J., & Niemcunowicz-Janica, A. (2021). Experimental and Theoretical Approaches to Describing Interactions in Natural Cell Membranes Occurring as a Result of Fatal Alcohol Poisoning. Membranes, 11(3), 189. https://doi.org/10.3390/membranes11030189







