Multicomponent Network Formation in Selective Layer of Composite Membrane for CO2 Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of Samples
2.2. Sample Characterization
3. Results and Discussion
3.1. Parameters of Individual Compounds
3.2. Network Formation within Thick Films
3.3. Network Formation in TFCM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pohlmann, J.; Bram, M.; Wilkner, K.; Brinkmann, T. Pilot scale separation of CO2 from power plant flue gases by membrane technology. Int. J. Greenh. Gas Control 2016, 53, 56–64. [Google Scholar] [CrossRef]
- Chauvy, R.; De Weireld, G. CO2 Utilization Technologies in Europe: A Short Review. Energy Technol. 2020, 8, 2000627. [Google Scholar] [CrossRef]
- Chen, X.; Liu, G.; Jin, W. Natural Gas Purification by Asymmetric Membranes: An Overview. Green Energy Environ. 2020. [Google Scholar] [CrossRef]
- Jansen, D.; Gazzani, M.; Manzolini, G.; van Dijk, E.; Carbo, M. Pre-combustion CO2 capture. Int. J. Greenh. Gas Control 2015, 40, 167–187. [Google Scholar] [CrossRef]
- Shirmohammadi, R.; Aslani, A.; Ghasempour, R.; Romeo, L.M. CO2 Utilization via Integration of an Industrial Post-Combustion Capture Process with a Urea Plant: Process Modelling and Sensitivity Analysis. Processes 2020, 8, 1144. [Google Scholar] [CrossRef]
- Zhao, L.; Primabudi, E.; Stolten, D. Investigation of a Hybrid System for Post-Combustion Capture. Energy Procedia 2014, 63, 1756–1772. [Google Scholar] [CrossRef]
- Karunakaran, M.; Kumar, M.; Shevate, R.; Akhtar, H.F.; Peinemann, K.-V. CO2-Philic Thin Film Composite Membranes: Synthesis and Characterization of PAN-r-PEGMA Copolymer. Polymers 2017, 9, 219. [Google Scholar] [CrossRef]
- Karunakaran, M.; Shevate, R.; Kumar, M.; Peinemann, K.V. CO2-selective PEO-PBT (PolyActive[trade mark sign])/graphene oxide composite membranes. Chem. Commun. 2015. [Google Scholar] [CrossRef]
- Brinkmann, T.; Lillepärg, J.; Notzke, H.; Pohlmann, J.; Shishatskiy, S.; Wind, J.; Wolff, T. Development of CO2 Selective Poly(Ethylene Oxide)-Based Membranes: From Laboratory to Pilot Plant Scale. Engineering 2017, 3, 485–493. [Google Scholar] [CrossRef]
- Schuldt, K.; Pohlmann, J.; Shishatskiy, S.; Brinkmann, T. Applicability of PolyActive™ Thin Film Composite Membranes for CO2 Separation from C2H4 Containing Multi-Component Gas Mixtures at Pressures up to 30 Bar. Membranes 2018, 8, 27. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. Tailor-made Polymeric Membranes based on Segmented Block Copolymers for CO2 Separation. Adv. Funct. Mater. 2008, 18, 2815–2823. [Google Scholar] [CrossRef]
- Car, A.; Yave, W.; Peinemann, K.-V.; Stropnik, C. Tailoring Polymeric Membrane Based on Segmented Block Copolymers for CO2 Separation. In Membrane Gas Separation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; pp. 227–253. [Google Scholar] [CrossRef]
- Tena, A.; Marcos-Fernandez, A.; Palacio, L.; Pradanos, P.; Lozano, A.E.; de Abajo, J.; Hernandez, A. On the influence of the proportion of PEO in thermally controlled phase segregation of copoly(ether-imide)s for gas separation. J. Membr. Sci. 2013, 434, 26–34. [Google Scholar] [CrossRef]
- Lilleparg, J.; Georgopanos, P.; Shishatskiy, S. Stability of blended polymeric materials for CO2 separation. J. Membr. Sci. 2014, 467, 269–278. [Google Scholar] [CrossRef]
- Buerkle, L.E.; Rowan, S.J. Supramolecular gels formed from multi-component low molecular weight species. Chem. Soc. Rev. 2012, 41, 6089–6102. [Google Scholar] [CrossRef] [PubMed]
- Glass, S.; Kühnert, M.; Abel, B.; Schulze, A. Controlled Electron-Beam Synthesis of Transparent Hydrogels for Drug Delivery Applications. Polymers 2019, 11, 501. [Google Scholar] [CrossRef]
- Raeburn, J.; Adams, D.J. Multicomponent low molecular weight gelators. Chem. Commun. 2015, 51, 5170–5180. [Google Scholar] [CrossRef]
- Lilleparg, J.; Georgopanos, P.; Emmler, T.; Shishatskiy, S. Effect of the reactive amino and glycidyl ether terminated polyethylene oxide additives on the gas transport properties of Pebax[registered sign] bulk and thin film composite membranes. RSC Adv. 2016, 6, 11763–11772. [Google Scholar] [CrossRef]
- Jiang, X.; He, S.; Li, S.; Bai, Y.; Shao, L. Penetrating chains mimicking plant root branching to build mechanically robust, ultra-stable CO2-philic membranes for superior carbon capture. J. Mater. Chem. A 2019, 7, 704–711. [Google Scholar] [CrossRef]
- Deng, J.; Dai, Z.; Yan, J.; Sandru, M.; Sandru, E.; Spontak, R.J.; Deng, L. Facile and solvent-free fabrication of PEG-based membranes with interpenetrating networks for CO2 separation. J. Membr. Sci. 2019, 570–571, 455–463. [Google Scholar] [CrossRef]
- Sharifi, M.; Jang, C.W.; Abrams, C.F.; Palmese, G.R. Toughened epoxy polymers via rearrangement of network topology. J. Mater. Chem. A 2014, 2, 16071–16082. [Google Scholar] [CrossRef]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F.R.; Frey, H. Polymerization of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef]
- Lillepärg, J.; Breitenkamp, S.; Shishatskiy, S.; Pohlmann, J.; Wind, J.; Scholles, C.; Brinkmann, T. Characteristics of Gas Permeation Behaviour in Multilayer Thin Film Composite Membranes for CO2 Separation. Membranes 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Van Krevelen, D.W.; Te Nijenhuis, K. Chapter 7—Cohesive Properties and Solubility. In Properties of Polymers, 4th ed.; Nijenhuis, K.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 189–227. [Google Scholar] [CrossRef]
- Olney, T.N.; Cann, N.M.; Cooper, G.; Brion, C.E. Absolute scale determination for photoabsorption spectra and the calculation of molecular properties using dipole sum-rules. Chem. Phys. 1997, 223, 59–98. [Google Scholar] [CrossRef]
- Marcus, Y. Solubility Parameter of Carbon Dioxide—An Enigma. ACS Omega 2018, 3, 524–528. [Google Scholar] [CrossRef]
- Mazinani, S.; Ramezani, R.; Darvishmanesh, S.; Molelekwa, G.F.; Di Felice, R.; Van der Bruggen, B. A ground breaking polymer blend for CO2/N2 separation. J. CO2 Util. 2018, 27, 536–546. [Google Scholar] [CrossRef]
- D’Angelico, S.; Urban, M. Method for Determining and/or Monitoring Viscosity and Corresponding Apparatus. U.S. Patant 9,709,475 B2, 18 July 2017. [Google Scholar]
- Khan, M.M.; Halder, K.; Shishatskiy, S.; Filiz, V. Synthesis and Crosslinking of Polyether-Based Main Chain Benzoxazine Polymers and Their Gas Separation Performance. Polymers 2018, 10, 221. [Google Scholar] [CrossRef]
- Jan Roman, P.; Detlev, F.; Thomas, K.; Klaus-Viktor, P. Gas permeation measurement under defined humidity via constant volume/variable pressure method. J. Membr. Sci. 2012, 389, 343–348. [Google Scholar] [CrossRef]
- Sebastian, H.M.; Lin, H.-M.; Chao, K.-C. Correlation of the solubility of carbon dioxide in hydrocarbon solvents. Ind. Eng. Chem. Process Des. Dev. 1981, 20, 508–511. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Jain, L.; Khanna, A. Validation and prediction of solubility parameters of ionic liquids for CO2 capture. Sep. Purif. Technol. 2012, 97, 51–64. [Google Scholar] [CrossRef]
- Marcus, Y. Solubility Parameters of Permanent Gases. J. Chem. 2016, 2016, 1–18. [Google Scholar] [CrossRef]
- Williams, L.L.; Rubin, J.B.; Edwards, H.W. Calculation of Hansen Solubility Parameter Values for a Range of Pressure and Temperature Conditions, Including the Supercritical Fluid Region. Ind. Eng. Chem. Res. 2004, 43, 4967–4972. [Google Scholar] [CrossRef]
- Metz, S.J.; Mulder, M.H.V.; Wessling, M. Gas-Permeation Properties of Poly(ethylene oxide) Poly(butylene terephthalate) Block Copolymers. Macromolecules 2004, 37, 4590–4597. [Google Scholar] [CrossRef]
- Castillejos, S.; Cerna, J.; Meléndez, F.; Castro, M.E.; Aguilar, R.; Márquez-Beltrán, C.; González, M. Bulk Modification of Poly(lactide) (PLA) via Copolymerization with Poly(propylene glycol) Diglycidylether (PPGDGE). Polymers 2018, 10, 1184. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Georgopanos, P.; Khan, M.M.; Neumann, S.; Abetz, V. Influence of Poly(ethylene glycol) Segment Length on CO Permeation and Stability of PolyActive Membranes and Their Nanocomposites with PEG POSS. ACS Appl. Mater. Interfaces 2014, 7, 12289–12298. [Google Scholar] [CrossRef]
- Maus, A.; Saalwächter, K. Crystallization Kinetics of Poly(dimethylsiloxane) Molecular-Weight Blends—Correlation with Local Chain Order in the Melt? Macromol. Chem. Phys. 2007, 208, 2066–2075. [Google Scholar] [CrossRef]
- Rahman, M.M.; Abetz, C.; Shishatskiy, S.; Martin, J.; Müller, A.J.; Abetz, V. CO2 Selective PolyActive Membrane: Thermal Transitions and Gas Permeance as a Function of Thickness. ACS Appl. Mater. Interfaces 2018, 10, 26733–26744. [Google Scholar] [CrossRef]
- Lillepärg, J.; Shishatskiy, S.; Wind, J. Stability of Pebax® or PolyActive® blended with low molecular weight PEG as materials for CO2 selective membranes. In Proceedings of the Euromembrane Conference 2012, London, UK, 23–27 September 2012. [Google Scholar]
- Derjaguin, B.V.; Muller, V.M.; Toporov, Y.P. Effect of contact deformations on the adhesion of particles. J. Colloid Interface Sci. 1975, 53, 314–326. [Google Scholar] [CrossRef]
- González González, M.; Cabanelas Valcárcel, J.C.; Baselga Llidó, J. Applications of FTIR on Epoxy Resins—Identification, Monitoring the Curing Process, Phase Separation and Water Uptake; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
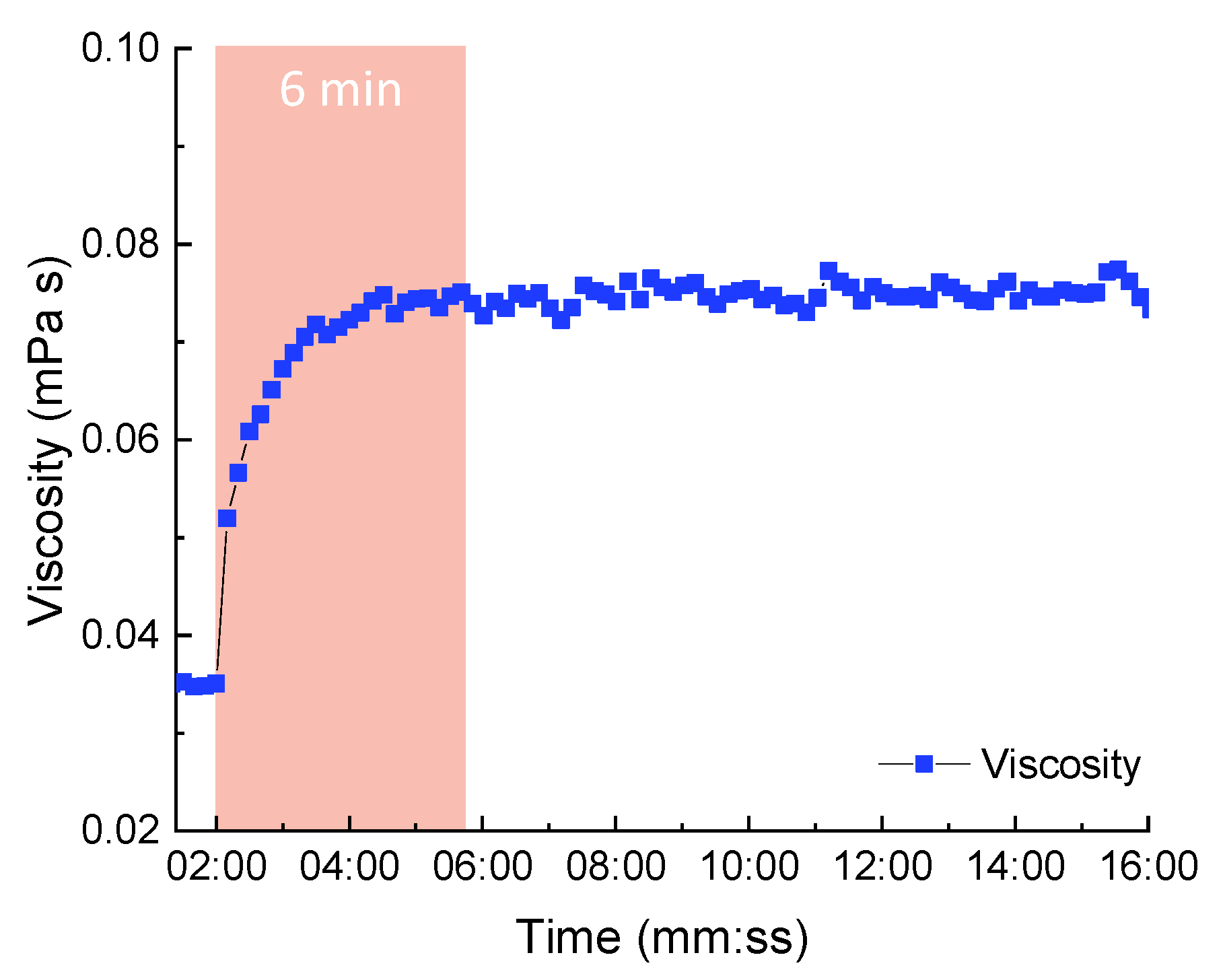

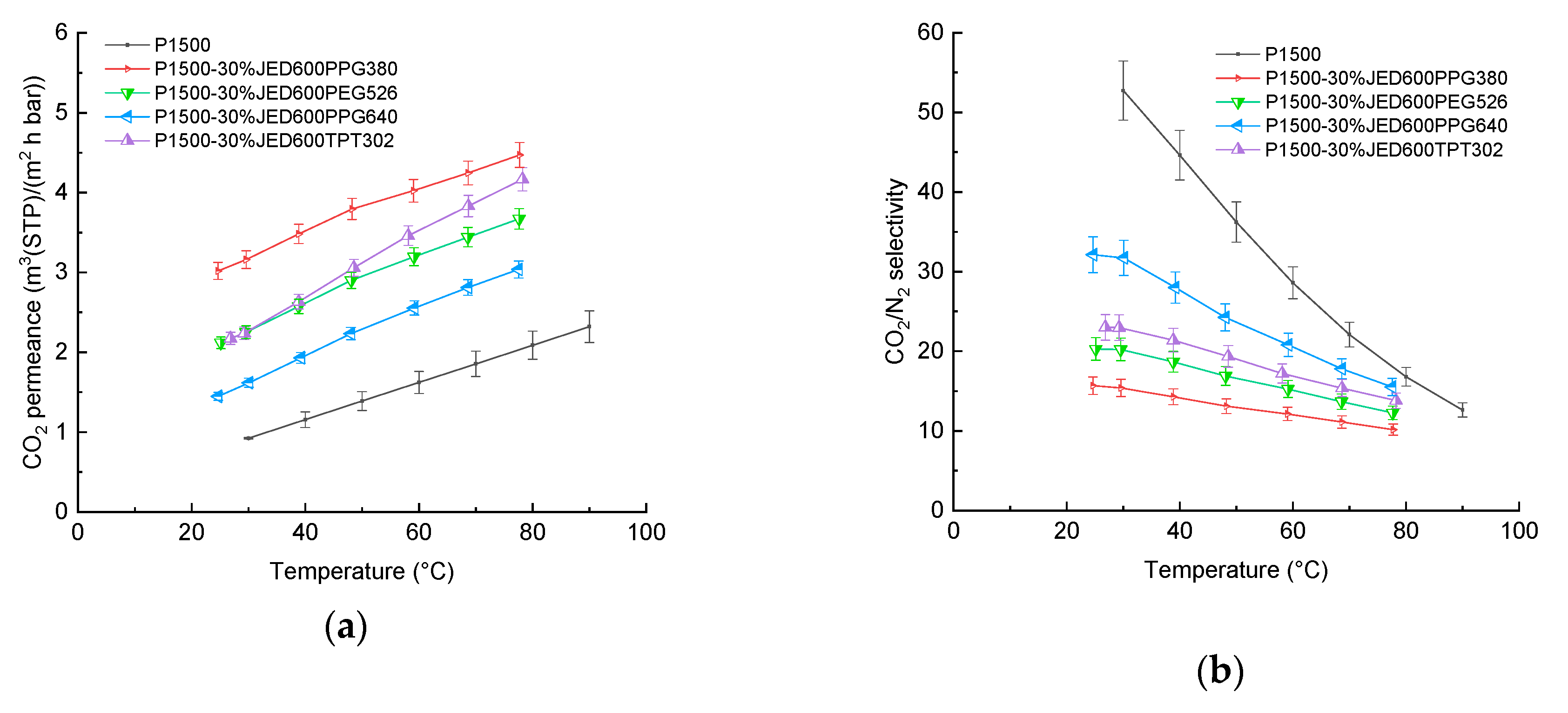

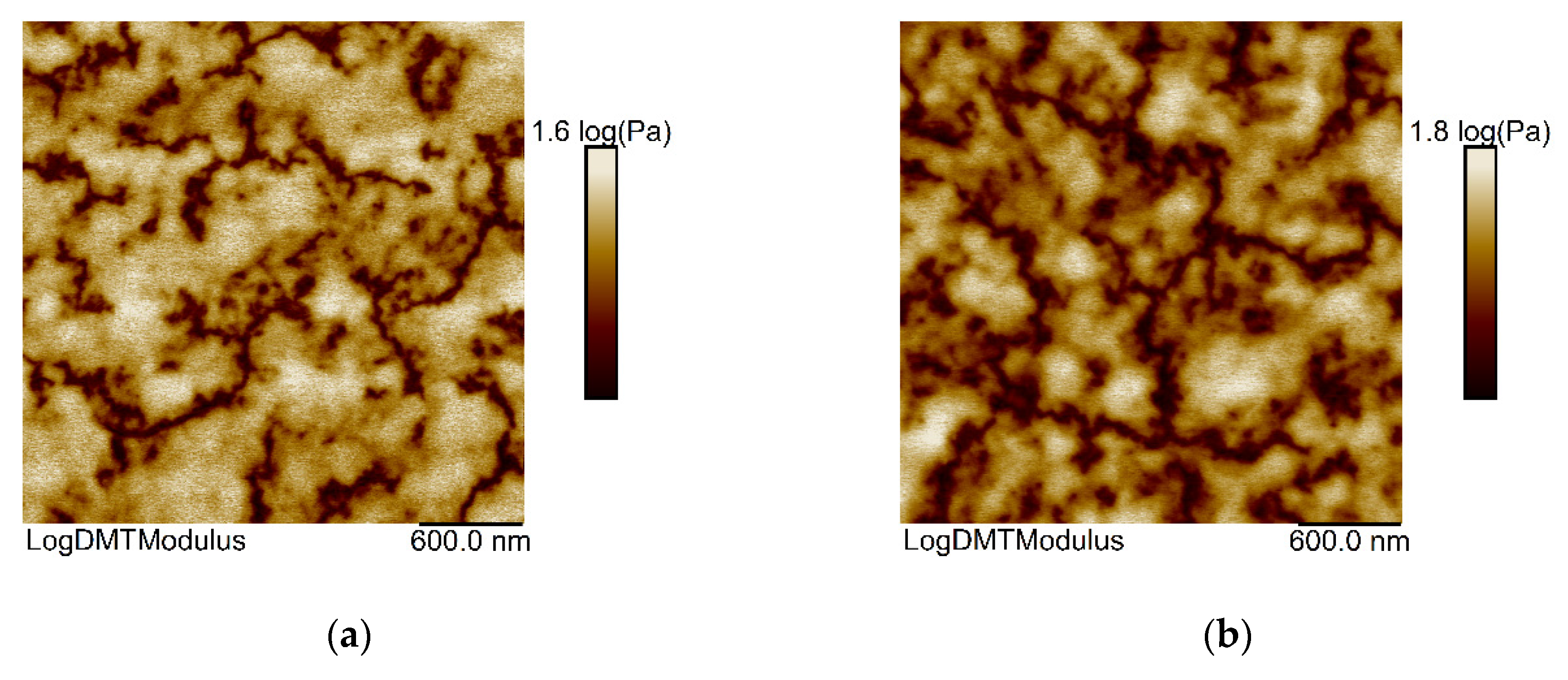


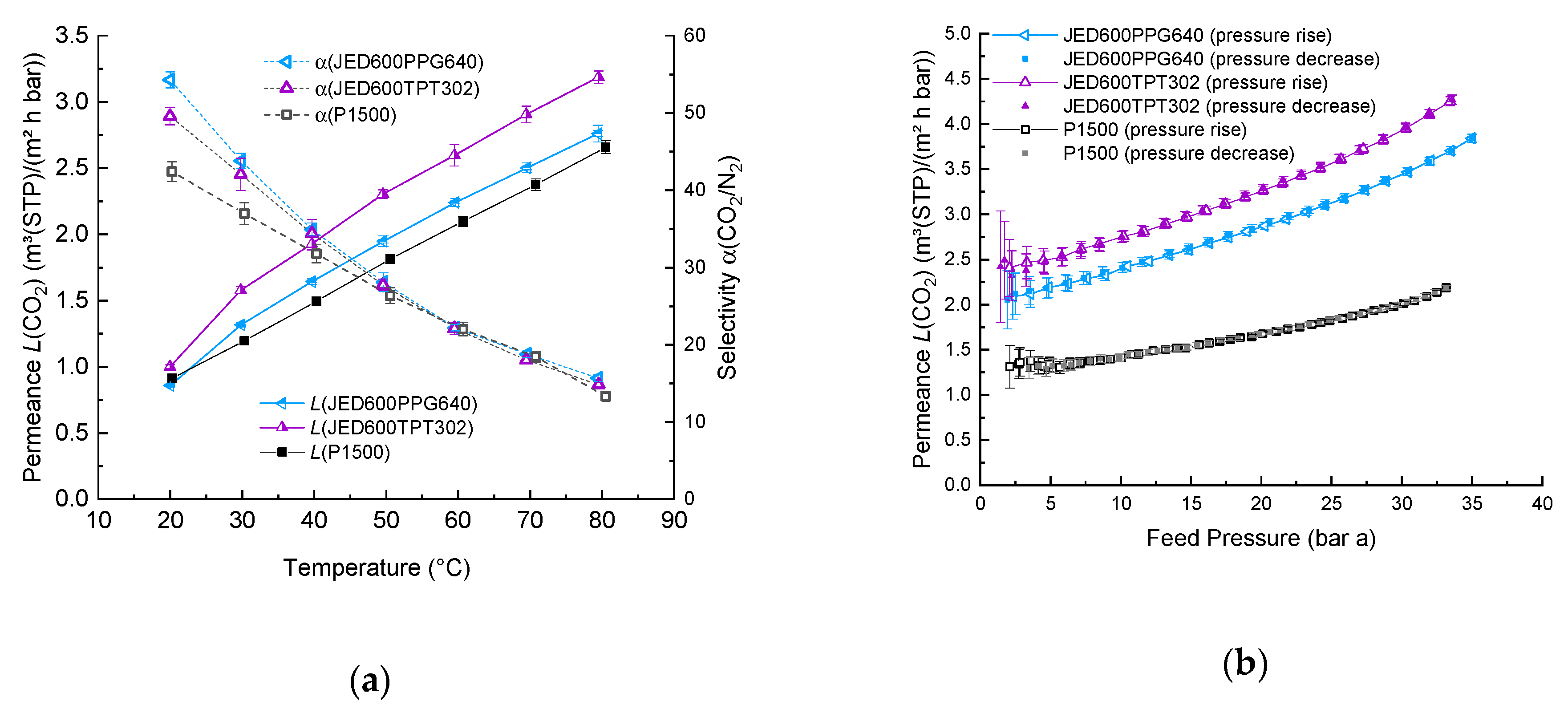
| Important Morphological Feature | Commercial Name | Code | Molecular Mass MW, (g/mol) | Density (at 25 °C) 1, (g/cm3) | Flash Point 1, (°C) |
|---|---|---|---|---|---|
| Matrix forming polymer | PolyActiveTM 1500PEGT77PBT23 | P1500 | 1500 2 | 1.188 3 | - |
| Two reactive glycidyl groups | Poly(propylene glycol) diglycidyl ether | PPG380 | 380 | 1.14 | 113 |
| PPG640 | 640 | 1.06 | 113 | ||
| Poly(ethylene glycol) diglycidyl ether | PEG526 | 526 | 1.14 | 197 | |
| Three reactive glycidyl groups | Trimethylolpropane triglycidyl ether | TPT302 | 302 | 1.157 | n.d. |
| Two reactive amino groups | Jeffamine® ED600 | JED600 | 600 | 1.035 | 113 |
| Jeffamine® ED900 | JED900 | 900 | 1.065 | 113 | |
| Jeffamine® ED2003 | JED2003 | 2000 | 1.068 | 113 | |
| Three reactive amino groups | Jeffamine® T403 | JT403 | 482 | 0.978 | 113 |
| Component | Molar Volume, (cm3/mol) | (MPa1/2) | (MPa1/2) | (MPa1/2) | (MPa1/2) | |
|---|---|---|---|---|---|---|
| Soft segment PEGT1500 1 | 1249.4 | 18.8 | 11.9 | 9.4 | 24.2 | 9.94 |
| Hard segment PBT 1 | 113.4 | 28.8 | 21.6 | 9.4 | 37.2 | 31.29 |
| P1500 2 | n.a. | 21.1 | 14.2 | 9.4 | 27.1 | 14.66 |
| PPG380 | 370.4 | 13.9 | 7.8 | 7.6 | 17.7 | 4.6 |
| PPG640 | 704.9 | 15.0 | 7.7 | 7.9 | 18.4 | 3.3 |
| PEG526 | 281.9 | 16.0 | 10.0 | 8.7 | 20.8 | 5.65 |
| TPT302 | 238.6 | 13.8 | 8.5 | 8.0 | 18.1 | 5.33 |
| JED600 | 693.1 | 16.9 | 8.3 | 9.4 | 21.0 | 5.39 |
| JED900 | 970.9 | 16.5 | 8.4 | 9.0 | 20.6 | 4.91 |
| JED2003 | 1906.9 | 15.9 | 9.1 | 8.7 | 20.3 | 4.89 |
| JT403 | 537.7 | 15.6 | 5.0 | 9.5 | 19.0 | 3.7 |
| Component | Tg, (°C) | Tc, (°C) | Tm, (°C) |
|---|---|---|---|
| P1500 | −49 | 11 1 | 28 1 |
| PPG380 | −78 | n.a. | n.a. |
| PPG640 | −76 | n.a. | n.a. |
| PEG526 | −72 | −45 | −14 |
| TPT302 | −66 | n.a. | n.a. |
| JED600 | −49 | −26 | −12 (−10) 2 |
| JED900 | −64 | 2 | 21 (22) 2 |
| JED2003 | −56 | −6 | 36 (43) 2 |
| JT403 | −69 | n.a. | n.a. |
| Sample | P(O2), (Barrer) | P(N2), (Barrer) | P(CH4), (Barrer) | P(H2), (Barrer) | P(CO2), (Barrer) | S(CO2), (cm3(STP) cm−3 cmHg−1) | D(CO2), (10−6 cm2 s−1) |
|---|---|---|---|---|---|---|---|
| P1500 1 | 8.7 | 3.4 | 10.5 | 17.6 | 181 (9.26) | 0.019 (0.001) | 0.91 (0.06) |
| Thick P1500 films blended with one component | |||||||
| P1500-40%PPG380 | 20.0 (1.35) | 7.9 (0.53) | 27.3 (1.84) | 34.4 (2.33) | 368 (24.8) | 0.025 (0.002) | 1.504 (0.13) |
| P1500-40%PPG640 | 22.3 (1.9) | 8.6 (0.73) | 30.5 (2.59) | 37.3 (3.17) | 357 (30.3) | 0.026 (0.002) | 1.351 (0.16) |
| P1500-40%PEG526 | 15.5 (1.97) | 6.4 (0.81) | 21.1 (2.67) | 26.7 (3.39) | 326 (41.3) | 0.024 (0.003) | 1.349 (0.24) |
| P1500-40%TPT302 | 45.1 (4.49) | 43.6 (4.20) | 68.5 (6.34) | n.a. | 246 (22.9) | 0.026 (0.003) | 0.963 (0.13) |
| P1500-40%JT403 2 | 20.1 (1.2) | n.a. | n.a. | n.a. | 3.5 (0.09) | 0.581 (1.04) | 0.0006 (0.011) |
| Thick P1500 films blended with two reactive components | |||||||
| 50%JED600PPG380 | n.a | n.a. | n.a. | n.a. | 208.9 (16.3) | 0.034 0.004) | 0.621 (0.008) |
| 50%JED900PPG380 2 | n.a. | n.a. | 7.9 (0.45) | n.a. | 116.0 (6.70) | 0.026 (0.001) | 0.444 (0.036) |
| 50%JED2003PPG380 | 11.2 (0.59) | 4.1 (0.22) | 14.3 (0.75) | 22.3 (1.17) | 212.8 (11.1) | 0.024 (0.001) | 0.003 (0.06) |
| 50%JED600PEG526 | 7.2 (0.47) | 4.9 (0.32) | 8.6 (0.56) | 32.0 (2.10) | 96.7 (6.35) | 0.136 (0.049) | 0.072 (0.025) |
| 50%JT403PPG380 | 3.0 (0.34) | n.a | 2.4 (0.27) | 12.0 (1.34) | 22.8 (2.56) | 0.180 (0.029) | 0.018 (0.003) |
| Polymer Systems | (MPa1/2) | (MPa1/2) | (MPa1/2) | δt, (MPa1/2) | |
|---|---|---|---|---|---|
| P1500-40%PPG380 | 18.2 | 11.6 | 8.7 | 23.3 | 8.80 |
| P1500-40%PPG640 | 18.6 | 11.6 | 8.7 | 23.6 | 9.28 |
| P1500-40%PEG526 | 19.1 | 12.5 | 9.1 | 24.6 | 10.60 |
| P1500-40%TPT302 | 18.2 | 11.9 | 8.8 | 23.5 | 9.03 |
| P1500-40%JT403 | 18.9 | 10.5 | 9.4 | 23.6 | 9.24 |
| 50%JED600PPG380 | 18.2 | 11.1 | 8.9 | 23.2 | 8.55 |
| 50%JED900PPG380 | 18.2 | 11.1 | 8.9 | 23.1 | 8.42 |
| 50%JED2003PPG380 | 18.0 | 11.3 | 8.8 | 23.0 | 8.32 |
| 50%JED600PEG526 | 18.8 | 11.7 | 9.2 | 23.9 | 9.68 |
| 50%JT403PPG380 | 17.9 | 10.4 | 8.9 | 22.5 | 7.56 |
| 50%JT403TPT302 | 17.9 | 10.5 | 9.1 | 22.7 | 7.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lillepärg, J.; Sperling, E.; Blanke, M.; Held, M.; Shishatskiy, S. Multicomponent Network Formation in Selective Layer of Composite Membrane for CO2 Separation. Membranes 2021, 11, 174. https://doi.org/10.3390/membranes11030174
Lillepärg J, Sperling E, Blanke M, Held M, Shishatskiy S. Multicomponent Network Formation in Selective Layer of Composite Membrane for CO2 Separation. Membranes. 2021; 11(3):174. https://doi.org/10.3390/membranes11030174
Chicago/Turabian StyleLillepärg, Jelena, Evgeni Sperling, Marit Blanke, Martin Held, and Sergey Shishatskiy. 2021. "Multicomponent Network Formation in Selective Layer of Composite Membrane for CO2 Separation" Membranes 11, no. 3: 174. https://doi.org/10.3390/membranes11030174
APA StyleLillepärg, J., Sperling, E., Blanke, M., Held, M., & Shishatskiy, S. (2021). Multicomponent Network Formation in Selective Layer of Composite Membrane for CO2 Separation. Membranes, 11(3), 174. https://doi.org/10.3390/membranes11030174







