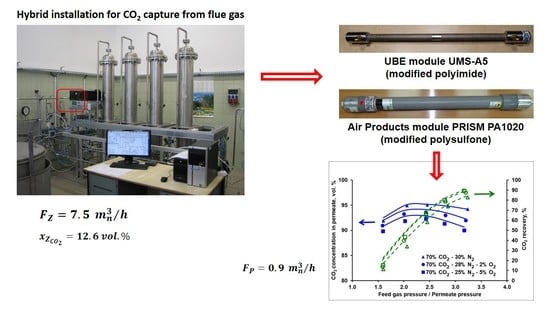
The Separative Performance of Modules with Polymeric Membranes for a Hybrid Adsorptive/Membrane Process of CO2 Capture from Flue Gas
Abstract
1. Introduction
2. Experimental Procedure and Mathematical Modeling
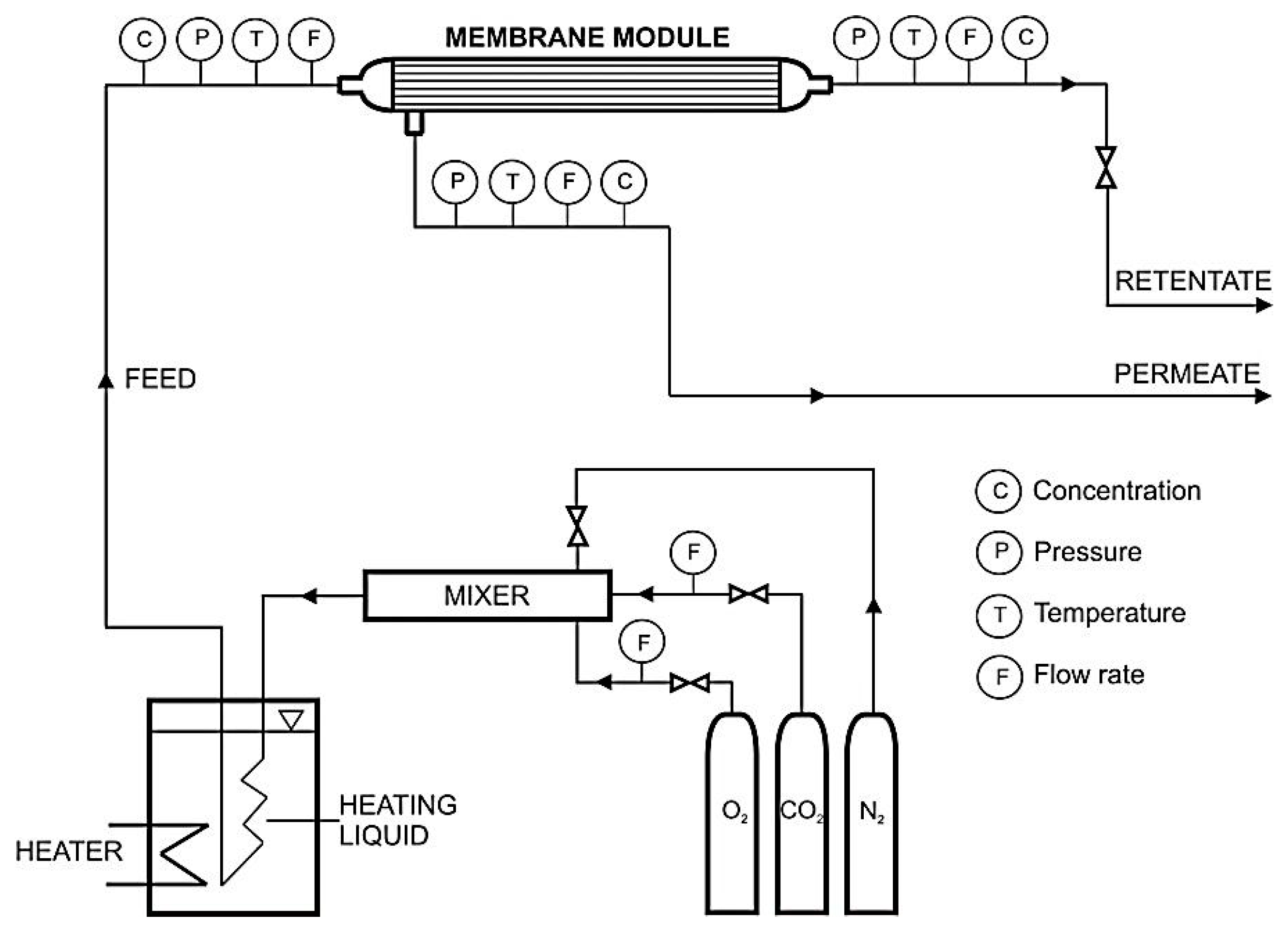
2.1. Experimental Setup
2.2. Membrane Modules
2.3. Gas Permeation Studies
2.4. Mathematical Model
3. Results and Discussion
3.1. Module Permeance and Ideal Selectivity
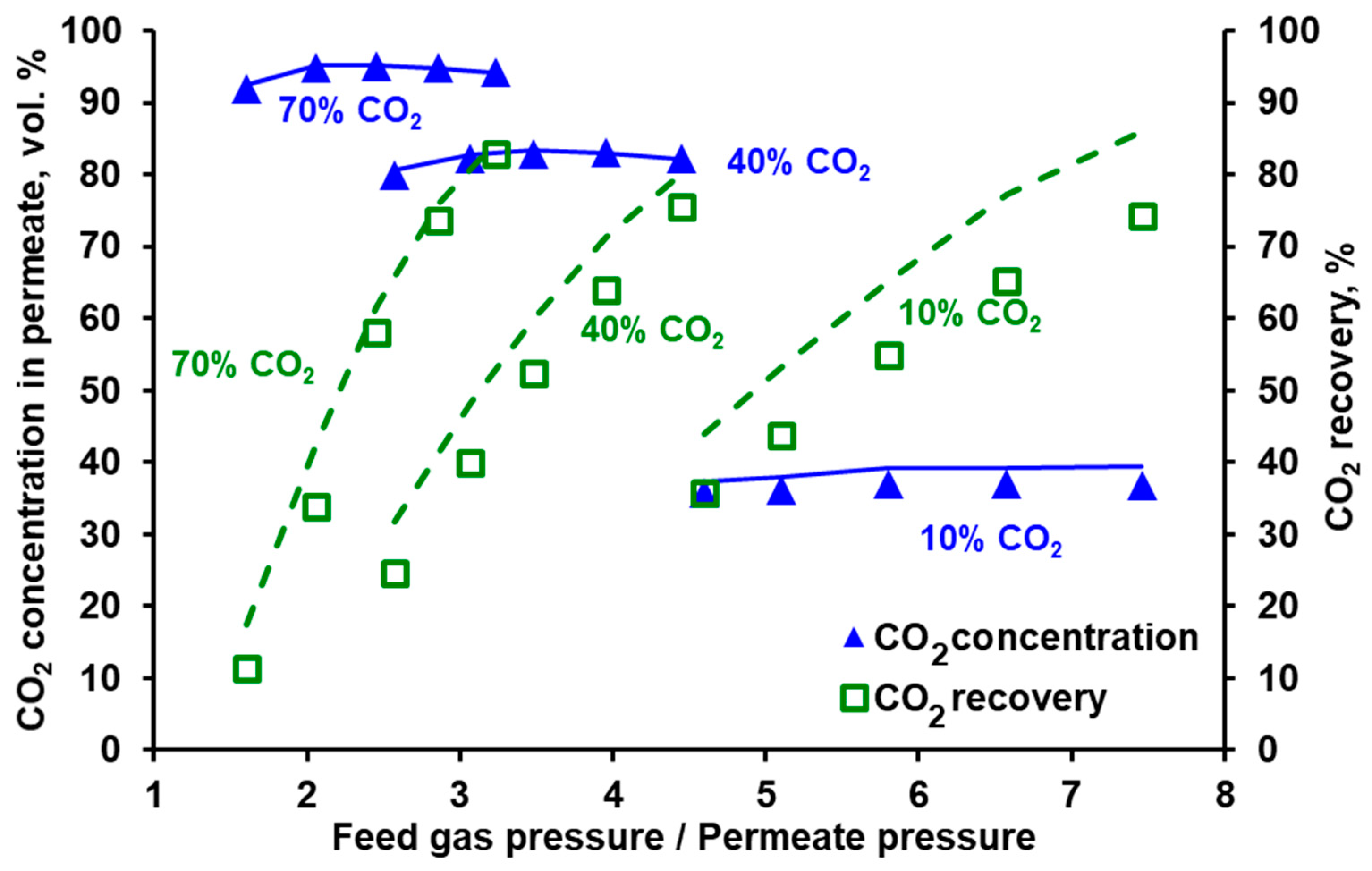
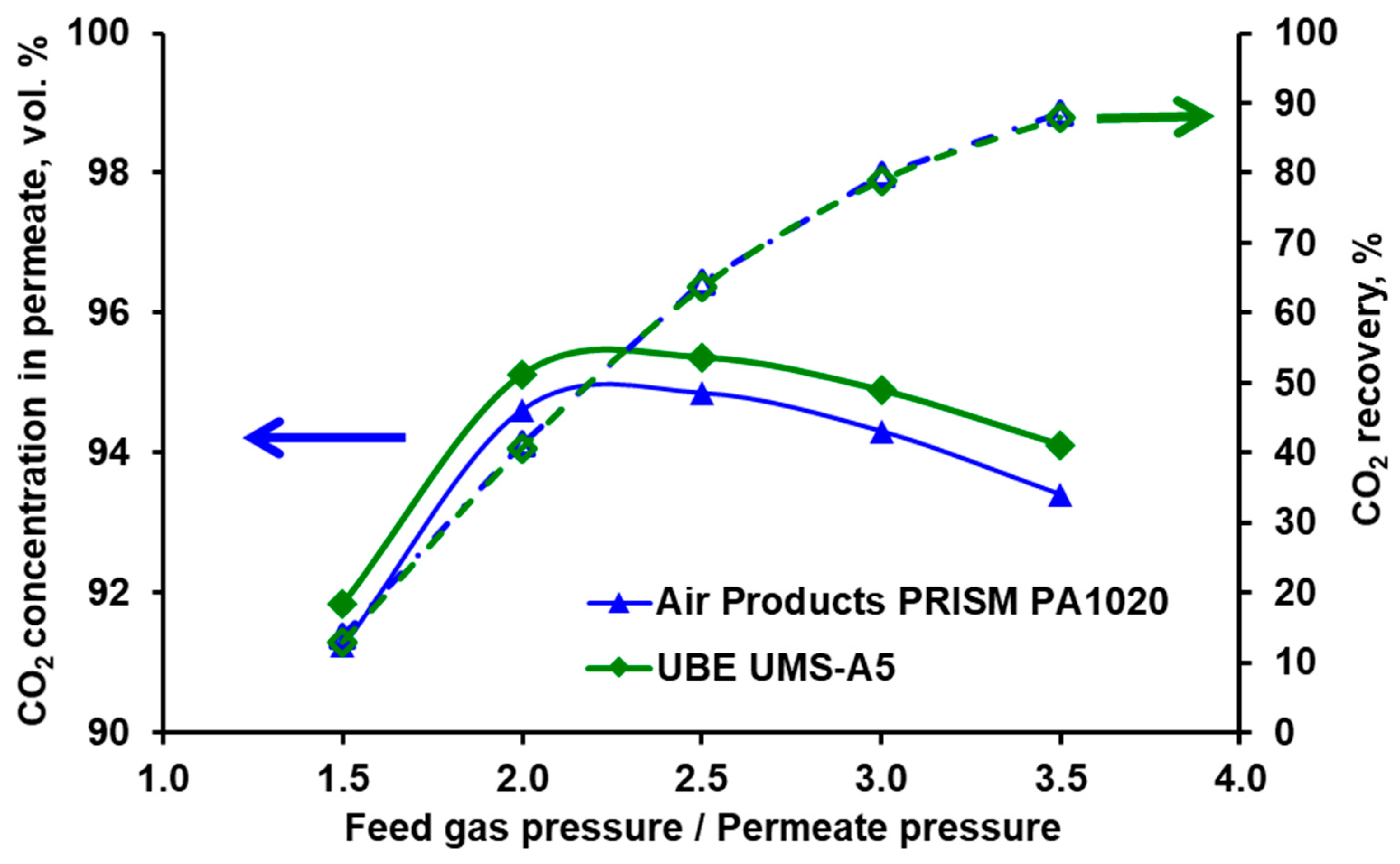
3.2. Separation of CO2/N2 and Model Validation
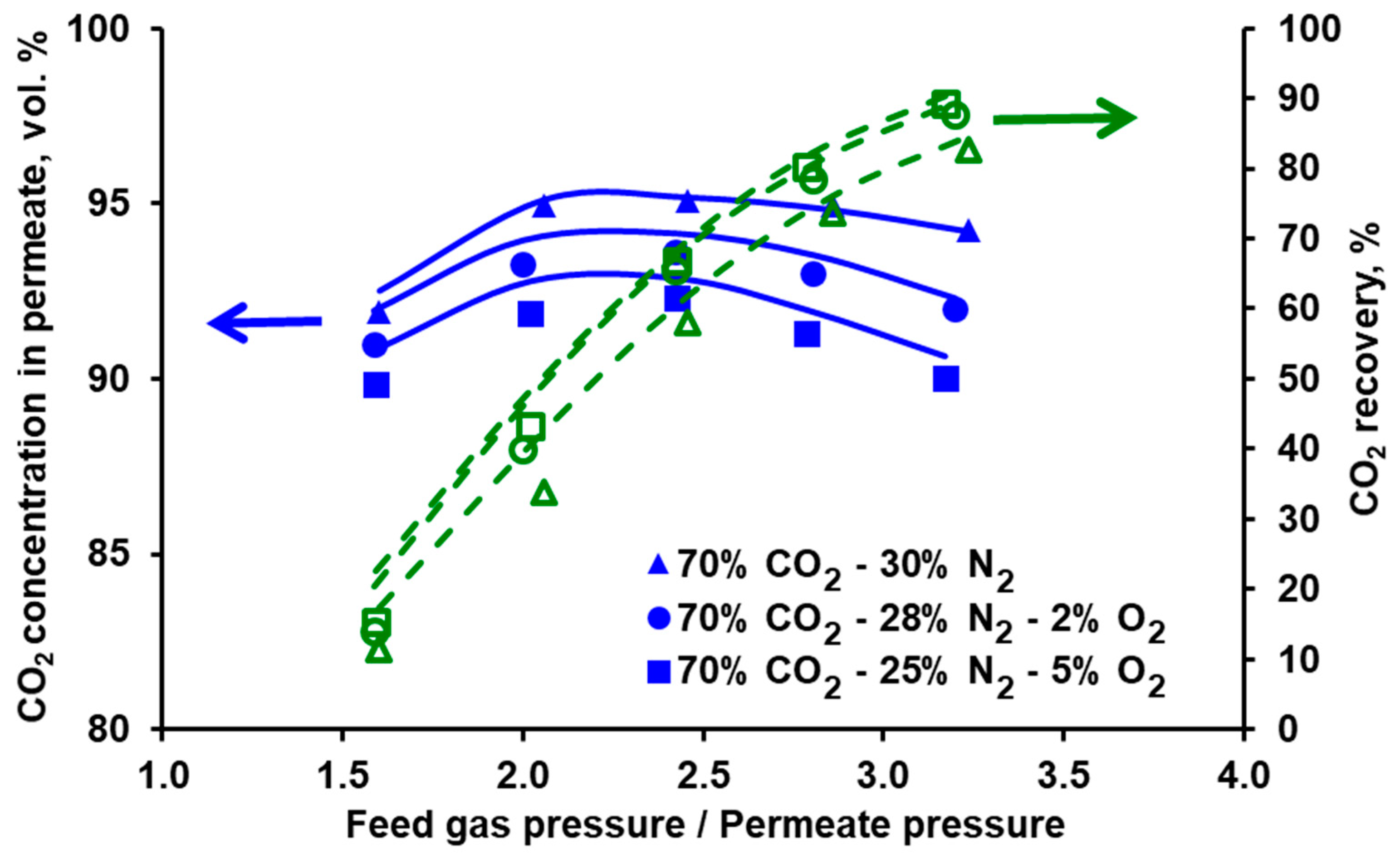
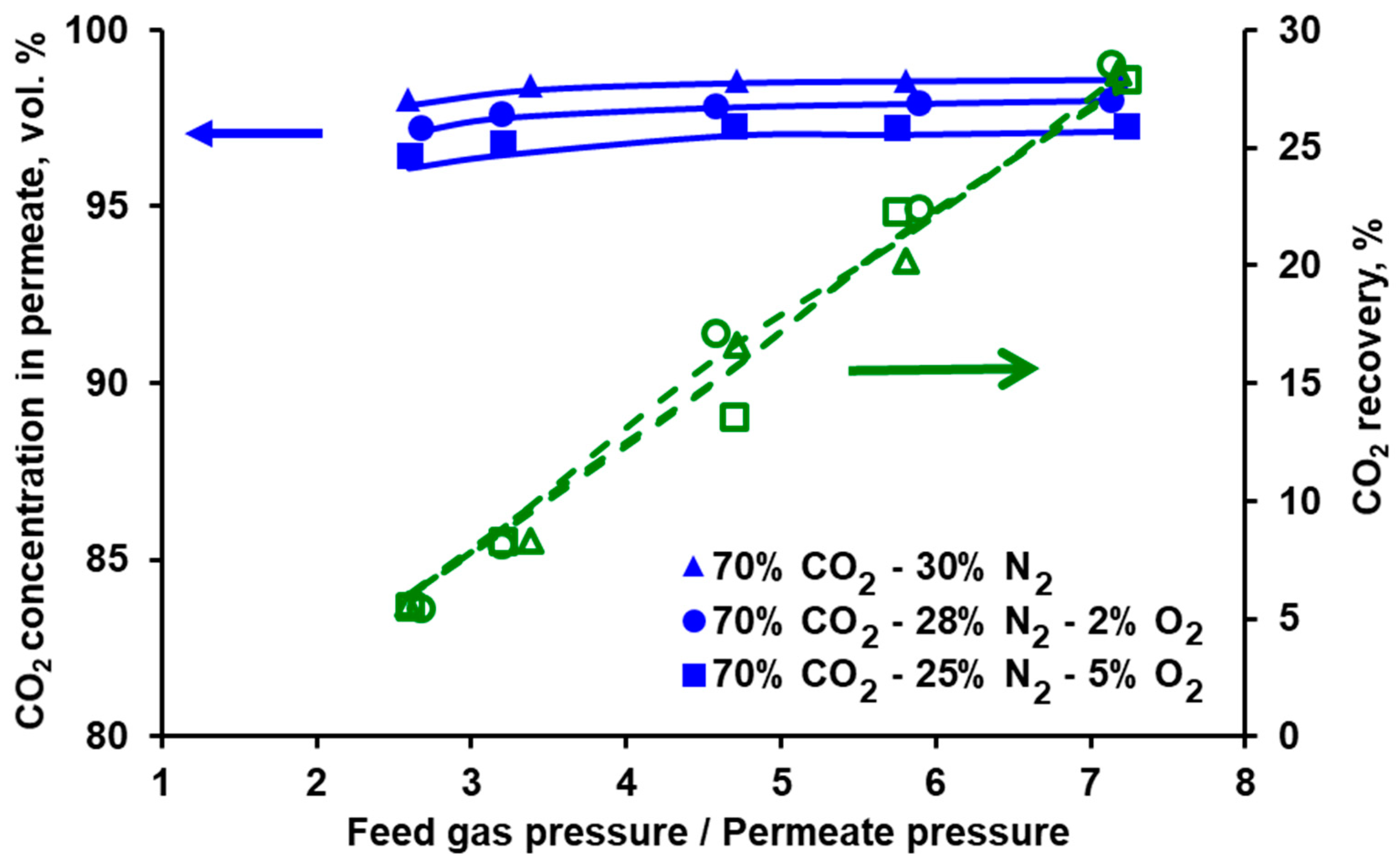
3.3. Impact of Oxygen on Separation Performance
3.4. Stability of Separation Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, C.; Sun, Y.; Fan, Z.; Liu, Q.; Ji, N.; Kitamura, Y. Parametric study of a novel cryogenic-membrane hybrid system for efficient CO2 separation. Int. J. Greenh. Gas Control. 2018, 72, 74–81. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- White, L.S.; Amo, K.D.; Wu, T.; Merkel, T.C. Extended field trials of Polaris sweep modules for carbon capture. J. Membr. Sci. 2017, 542, 217–225. [Google Scholar] [CrossRef]
- Majchrzak, A.; Nowak, W. Separation characteristics as a selection criteria of CO2 adsorbents. J. CO2 Util. 2017, 17, 69–79. [Google Scholar] [CrossRef]
- Woo, K.T.; Dong, G.; Lee, J.; Kim, J.S.; Do, Y.S.; Lee, W.H.; Lee, H.S.; Lee, Y.M. Ternary mixed-gas separation for flue gas CO2 capture using high performance thermally rearranged (TR) hollow fiber membranes. J. Membr. Sci. 2016, 510, 472–480. [Google Scholar] [CrossRef]
- Rios, R.B.; Correia, L.S.; Bastos-Neto, M.; Torres, A.E.B.; Hatimondi, S.A.; Ribeiro, A.M.; Li, P.; Cavalcante, C.L.; De Azevedo, D.C.S. Evaluation of carbon dioxide–nitrogen separation through fixed bed measurements and simulations. Adsorption 2014, 20, 945–957. [Google Scholar] [CrossRef]
- Pires, J.; Martins, F.; Alvim-Ferraz, M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Fout, T.; I Plasynski, S.; McIlvried, H.G.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control. 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Report of DOE NETL. Office of Program. Performance and Benefits. CO2 Impurity Desingn Parameters. Quality Guidelines for Energy Systems Studies. DOE NETL 2012. DOE/NETL-341/011212. Draft Report. August 2013. Available online: https://www.osti.gov/biblio/1566771-quality-guidelines-energy-system-studies-co2-impurity-design-parameters (accessed on 27 October 2020).
- Abanades, J.; Arias, B.; Lyngfelt, A.; Mattisson, T.; Wiley, D.; Li, H.; Ho, M.; Mangano, E.; Brandani, S. Emerging CO2 capture systems. Int. J. Greenh. Gas Control. 2015, 40, 126–166. [Google Scholar] [CrossRef]
- Sreenivasulu, B.; Gayatri, D.; Sreedhar, I.; Raghavan, K. A journey into the process and engineering aspects of carbon capture technologies. Renew. Sustain. Energy Rev. 2015, 41, 1324–1350. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Heck, R.; Qahtani, M.S.; Yahaya, G.O.; Tanis, I.; Brown, D.; Bahamdan, A.A.; Ameen, A.W.; Vaidya, M.; Ballaguet, J.-P.; Alhajry, R.; et al. Block copolyimide membranes for pure- and mixed-gas separation. Sep. Purif. Technol. 2017, 173, 183–192. [Google Scholar] [CrossRef]
- Roussanaly, S.; Anantharaman, R.; Lindqvist, K.; Hagen, B. A new approach to the identification of high-potential materials for cost-efficient membrane-based post-combustion CO2 capture. Sustain. Energy Fuels 2018, 2, 1225–1243. [Google Scholar] [CrossRef]
- Hossain, I.; Al Munsur, A.Z.; Choi, O.; Kim, T.-H. Bisimidazolium PEG-mediated crosslinked 6FDA-durene polyimide membranes for CO2 separation. Sep. Purif. Technol. 2019, 224, 180–188. [Google Scholar] [CrossRef]
- White, L.S.; Wei, X.; Pande, S.; Wu, T.; Merkel, T.C. Extended flue gas trials with a membrane-based pilot plant at a one-ton-per-day carbon capture rate. J. Membr. Sci. 2015, 496, 48–57. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Freeman, B.; Hao, P.; Baker, R.; Kniep, J.; Chen, E.; Ding, J.; Zhang, Y.; Rochelle, G.T. Hybrid Membrane-absorption CO2 Capture Process. Energy Procedia 2014, 63, 605–613. [Google Scholar] [CrossRef]
- Kusuma, V.A.; Li, Z.; Hopkinson, D.; Luebke, D.R.; Chen, S. Evaluating the Energy Performance of a Hybrid Membrane-Solvent Process for Flue Gas Carbon Dioxide Capture. Ind. Eng. Chem. Res. 2016, 55, 11329–11337. [Google Scholar] [CrossRef]
- Frimpong, R.A.; Irvin, B.D.; Nikolic, H.; Liu, K.; Figueroa, J. Integrated hybrid process for solvent-based CO2 capture using a pre-concentrating membrane: A pilot scale study. Int. J. Greenh. Gas Control. 2019, 82, 204–209. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Le Moullec, Y.; Willson, D.; Favre, E. Hybrid membrane cryogenic process for post-combustion CO2 capture. J. Membr. Sci. 2012, 10, 424–434. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Ji, N.; Deng, S.; Zhao, J.; Li, Y.; Kitamura, Y. Reducing the energy consumption of membrane-cryogenic hybrid CO2 capture by process optimization. Energy 2017, 124, 29–39. [Google Scholar] [CrossRef]
- Mat, N.C.; Lipscomb, G. Global sensitivity analysis for hybrid membrane-cryogenic post combustion carbon capture process. Int. J. Greenh. Gas Control. 2019, 81, 157–169. [Google Scholar] [CrossRef]
- Jeon, J.-K.; Ihm, S.-K.; Park, Y.-K.; Kim, J.S.; Dong, J.I.; Kim, S.; Kim, J.; Kim, S.-S.; Yoo, K.-S. Membrane/PSA Hybrid Process for Carbon Dioxide Recovery at Low Concentration. Adv. Pharmacol. 2004, 153, 543–546. [Google Scholar] [CrossRef]
- Warmuziński, K.; Tańczyk, M.; Jaschik, M. Experimental study on the capture of CO2 from flue gas using adsorption combined with membrane separation. Int. J. Greenh. Gas Control. 2015, 37, 182–190. [Google Scholar] [CrossRef]
- Jaschik, M.; Tanczyk, M.; Jaschik, J.; Janusz-Cygan, A. The performance of a hybrid VSA-membrane process for the capture of CO2 from flue gas. Int. J. Greenh. Gas Control. 2020, 97, 103037. [Google Scholar] [CrossRef]
- Warmuziński, K.; Tańczyk, M.; Jaschik, M.; Janusz-Cygan, A. A Hybrid Separation Process for the Recovery of Carbon Dioxide From Flue Gases. Energy Procedia 2013, 37, 2154–2163. [Google Scholar] [CrossRef]
- Fong, J.C.L.Y.; Anderson, C.J.; Xiao, G.; Webley, P.A.; Hoadley, A. Multi-objective optimisation of a hybrid vacuum swing adsorption and low-temperature post-combustion CO2 capture. J. Clean. Prod. 2016, 111, 193–203. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Sengupta, A.; Sirkar, K. Multicomponent gas separation by an asymmetric permeator containing two different membranes. J. Membr. Sci. 1984, 21, 73–109. [Google Scholar] [CrossRef]
- Sengupta, A.; Sirkar, K.K. Ternary gas mixture separation in two-membrane permeators. AIChE J. 1987, 33, 529–539. [Google Scholar] [CrossRef]
- Ettouney, H. Permeability functions for pure and mixture gases in silicone rubber and polysulfone membranes: Dependence on pressure and composition. J. Membr. Sci. 1997, 135, 251–261. [Google Scholar] [CrossRef]
- Kase, Y. Gas Separation by Polyimide Membranes. In Advanced Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 581–598. [Google Scholar]
- Ho, W.; Sirkar, K. Membrane Handbook; Van Nostrand Reinhold: New York, NY, USA, 1992; pp. 17–101. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 301–353. [Google Scholar]
- Nunes, S.; Peinemann, K.-V. Membrane Technology in the Chemical Industry, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 53–90. [Google Scholar]
- Carreon, M.; Dahe, G.; Feng, J.; Venna, S.R.; Carreon, M.A. Mixed Matrix Membranes for Gas Separation Applications. In Membranes for Gas Separations; World Scientific Pub Co Pte Ltd.: Hackensack, NJ, USA, 2017; pp. 1–57. [Google Scholar]
- Jaschik, M.; Tańczyk, M.; Janusz-Cygan, A.; Wojdyła, A.; Warmuziński, K. The separation of carbon dioxide from CO2/N2/O2 mixtures using polyimide and polysulphone membranes. Chem. Proc. Eng. 2018, 39, 449–456. [Google Scholar] [CrossRef]
- Matteucci, S.; Yampolskii, Y.; Freeman, B.D.; Pinnau, I. Transport of Gases and Vapors in Glassy and Rubbery Polymers. In Materials Science of Membranes for Gas and Vapor Separation; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 1–47. [Google Scholar]
- Duthie, X.; Kentish, S.E.; Powell, C.; Nagai, K.; Qiao, G.; Stevens, G. Operating temperature effects on the plasticization of polyimide gas separation membranes. J. Membr. Sci. 2007, 294, 40–49. [Google Scholar] [CrossRef]
- Scholes, C.A.; Chen, G.Q.; Stevens, G.W.; Kentish, S.E. Plasticization of ultra-thin polysulfone membranes by carbon dioxide. J. Membr. Sci. 2010, 346, 208–214. [Google Scholar] [CrossRef]
- Adewole, J.; Ahmad, A.; Ismail, S.; Leo, C. Current challenges in membrane separation of CO2 from natural gas: A review. Int. J. Greenh. Gas Control. 2013, 17, 46–65. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Gas-Separation: Applications. In Membrane Operations; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 167–194. [Google Scholar]
- Zhao, L.; Riensche, E.; Blum, L.; Stolten, D. Multi-stage gas separation membrane processes used in post-combustion capture: Energetic and economic analyses. J. Membr. Sci. 2010, 359, 160–172. [Google Scholar] [CrossRef]






| AQ, GPU m2 | Feed Pressure, bar (a *) | 288 K | 293 K | 298 K |
|---|---|---|---|---|
| CO2 | 1.8−7.5 | 19.6 ± 2.2 | 20.4 ± 2.0 | 21.7 ± 1.6 |
| O2 | 2.0−7.5 | 3.5 ± 0.1 | 3.8 ± 0.1 | 4.5 ± 0.1 |
| N2 | 3.0−7.5 | 0.39 ± 0.01 | 0.44 ± 0.02 | 0.48 ± 0.01 |
| AQ, GPU m2 | Feed Pressure, bar (a *) | 288 K | 293 K | 298 K |
|---|---|---|---|---|
| CO2 | 1.8−3.0 | 321.0 ± 3.9 | 342.2 ± 5.2 | 361.4 ± 6.1 |
| O2 | 4.7−7.5 | 54.0 ± 1.0 | 61.6 ± 0.6 | 68.0 ± 1.1 |
| N2 | 1.5−7.5 | 7.1 ± 0.3 | 8.4 ± 0.6 | 9.8 ± 0.5 |
| Air Products, PRISM PA1020 AQ, GPU m2 (at 295 K) | UBE, UMS-A5 AQ, GPU m2 (at 296 K) | |||
|---|---|---|---|---|
| Year 0 | Year 8 | Year 0 | Year 7 | |
| CO2 | 353 | 322 | 21.7 | 17.9 |
| N2 | 9.33 | 8.97 | 0.457 | 0.404 |
| O2 | 61.7 | 54.3 | 4.13 | 3.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janusz-Cygan, A.; Jaschik, J.; Wojdyła, A.; Tańczyk, M. The Separative Performance of Modules with Polymeric Membranes for a Hybrid Adsorptive/Membrane Process of CO2 Capture from Flue Gas. Membranes 2020, 10, 309. https://doi.org/10.3390/membranes10110309
Janusz-Cygan A, Jaschik J, Wojdyła A, Tańczyk M. The Separative Performance of Modules with Polymeric Membranes for a Hybrid Adsorptive/Membrane Process of CO2 Capture from Flue Gas. Membranes. 2020; 10(11):309. https://doi.org/10.3390/membranes10110309
Chicago/Turabian StyleJanusz-Cygan, Aleksandra, Jolanta Jaschik, Artur Wojdyła, and Marek Tańczyk. 2020. "The Separative Performance of Modules with Polymeric Membranes for a Hybrid Adsorptive/Membrane Process of CO2 Capture from Flue Gas" Membranes 10, no. 11: 309. https://doi.org/10.3390/membranes10110309
APA StyleJanusz-Cygan, A., Jaschik, J., Wojdyła, A., & Tańczyk, M. (2020). The Separative Performance of Modules with Polymeric Membranes for a Hybrid Adsorptive/Membrane Process of CO2 Capture from Flue Gas. Membranes, 10(11), 309. https://doi.org/10.3390/membranes10110309