Zwitterionic Polysulfone Copolymer/Polysulfone Blended Ultrafiltration Membranes with Excellent Thermostability and Antifouling Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of PSf-Co-SBPSf Copolymers
2.2.1. Synthesis of Bisphenol A with Pendant Tertiary Amine Groups
2.2.2. Synthesis of PSf-Co-Tertiary Amine-Modified PSf (PSf-Co-TAPSf) Copolymer
2.2.3. Synthesis of PSf-Co-SBPSf Copolymers
2.3. Characterization of PSf-Co-SBPSf Copolymers
2.4. Membrane Preparation
2.5. Characterization of the Membranes
2.6. Membrane Thermostability Tests
3. Results and Discussion
3.1. Synthesis and Characterization of PSf-Co-SBPSf Copolymers
3.2. Membrane Characterization
3.2.1. Membrane Morphology
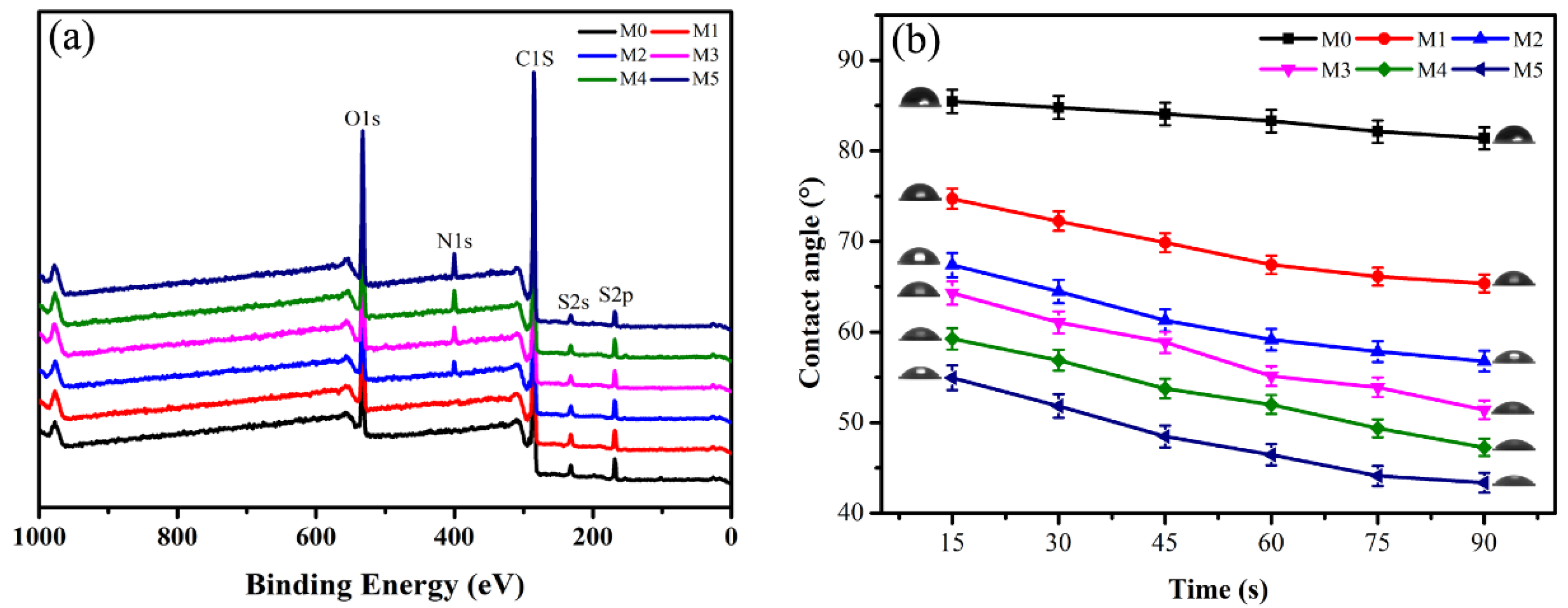
3.2.2. Membrane Surface Element Analysis and Hydrophilicity
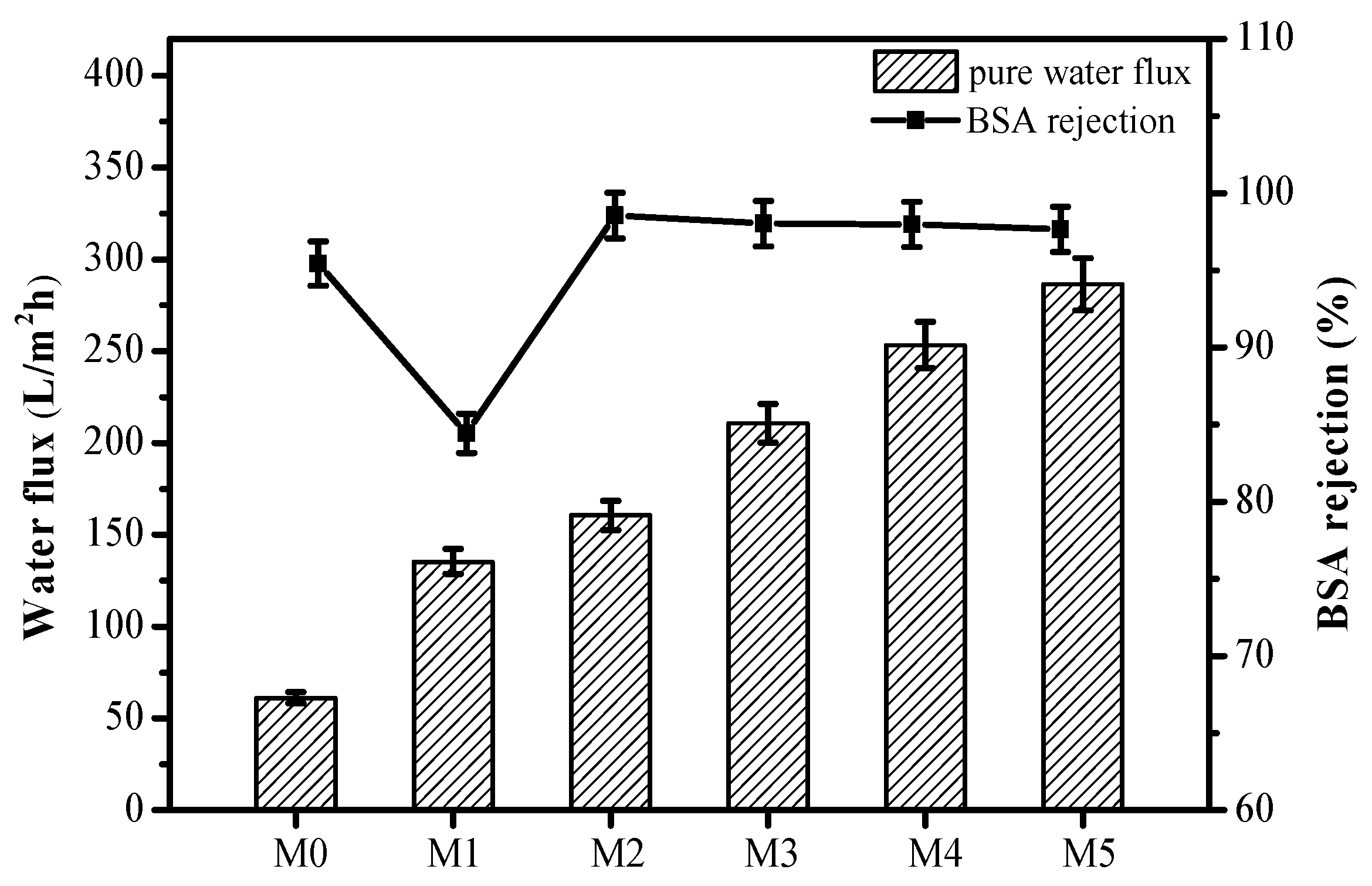
3.3. Permeation and Separation Properties of the Membranes
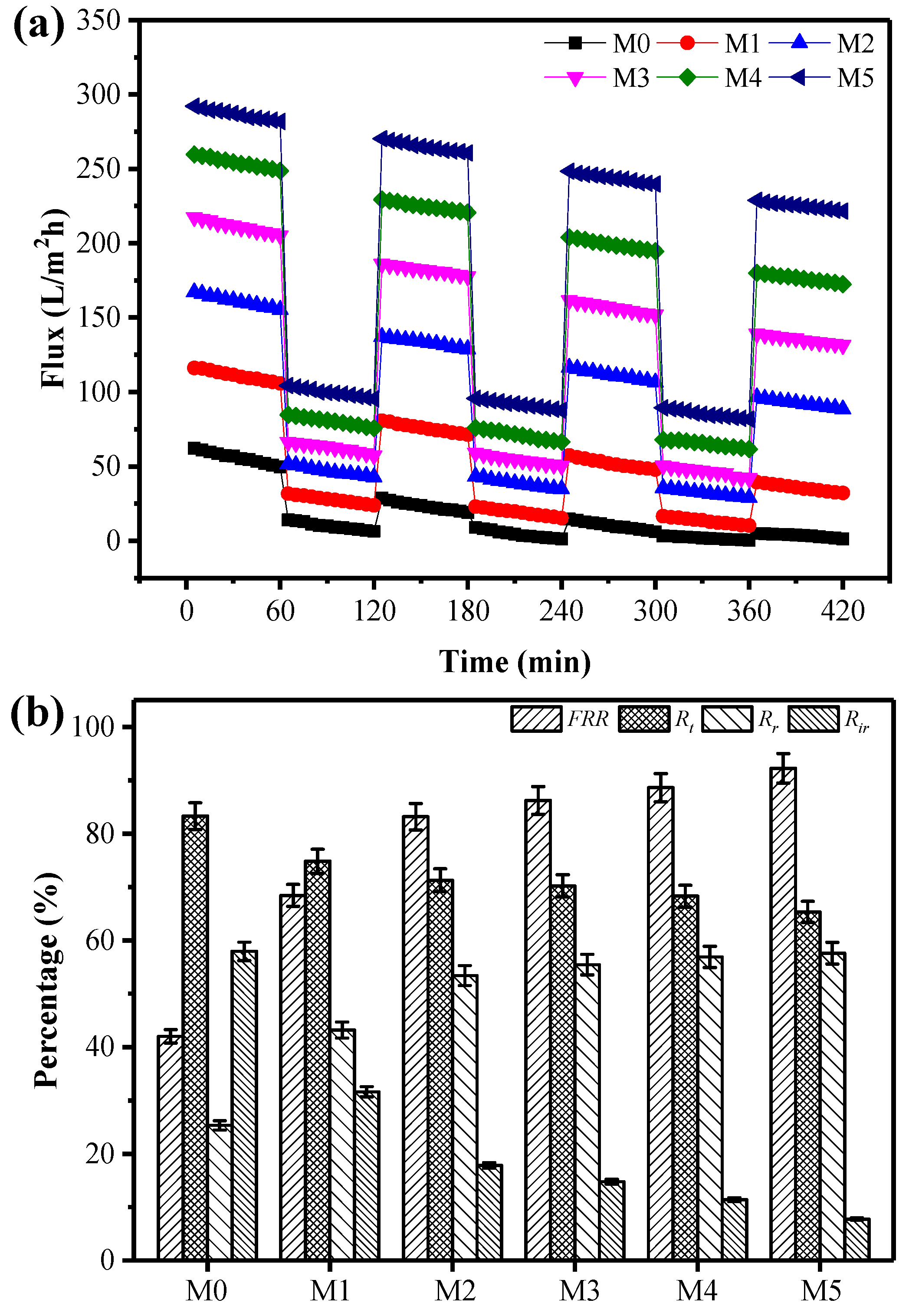
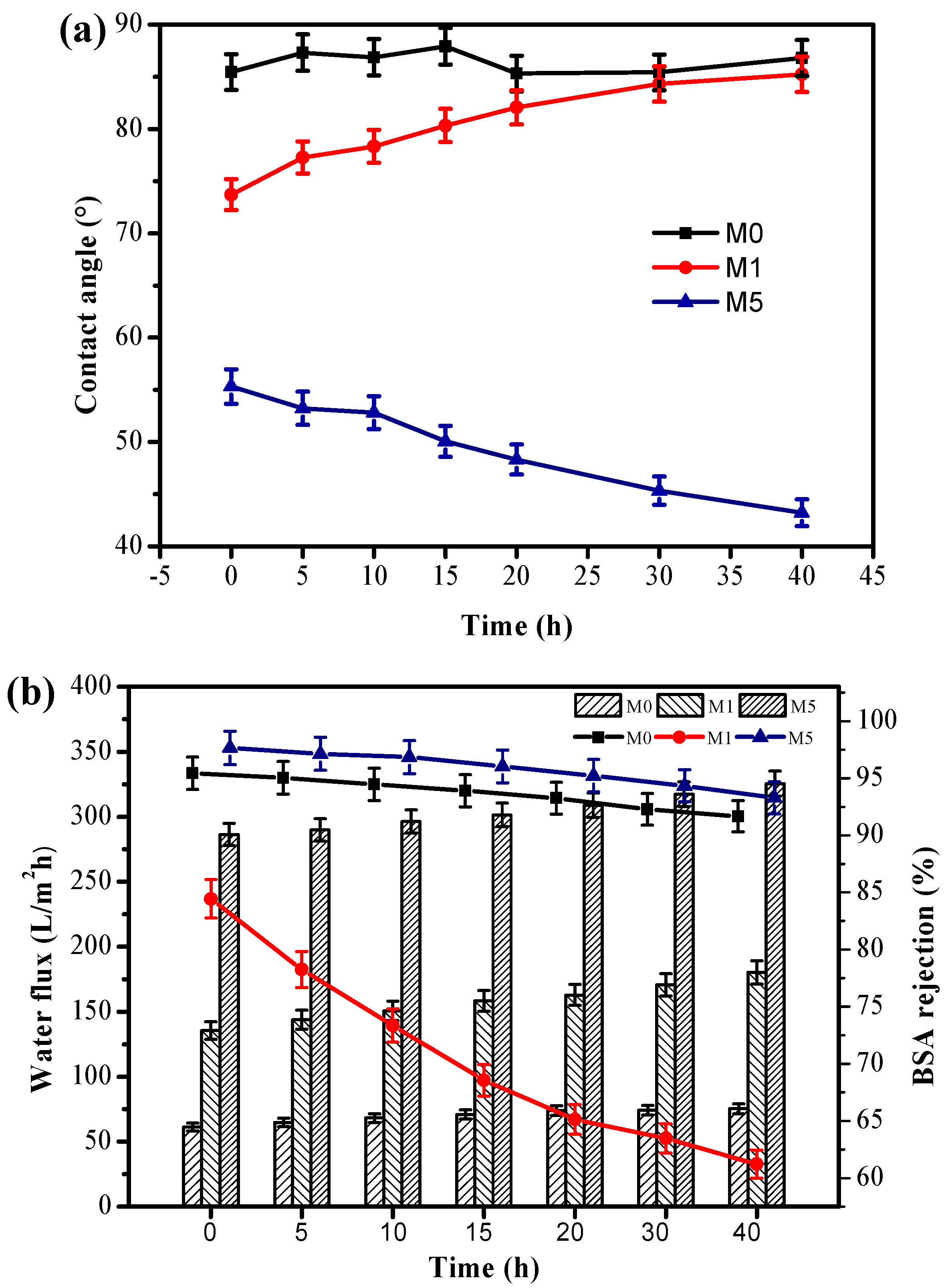
3.4. Antifouling Performance of the Membranes
3.5. Thermostability Performance of the Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Al Aani, S.; Mustafa, T.N.; Hilal, N. Ultrafiltration membranes for wastewater and water process engineering: A comprehensive statistical review over the past decade. J. Water Process. Eng. 2020, 35, 102141. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, H.W.; Nam, S.Y.; Park, H.B. Fouling-tolerant polysulfone-poly(ethylene oxide) random copolymer ultrafiltration membranes. J. Membr. Sci. 2011, 379, 296–306. [Google Scholar] [CrossRef]
- Sun, M.P.; Su, Y.L.; Mu, C.X.; Jiang, Z.Y. Improved antifouling property of PES ultrafiltration membranes using additive of silica-PVP nanocomposite. Ind. Eng. Chem. Res. 2010, 49, 790–796. [Google Scholar] [CrossRef]
- Zhu, L.J.; Zhu, L.P.; Zhao, Y.F.; Zhu, B.K.; Xu, Y.Y. Anti-fouling and anti-bacterial polyethersulfone membranes quaternized from the additive of poly(2-dimethylamino ethyl methacrylate) grafted SiO2 nanoparticles. J. Mater. Chem. A 2014, 2, 15566–15574. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Zhu, L.P.; Yi, Z.; Zhu, B.K.; Xu, Y.Y. Improving the hydrophilicity and fouling-resistance of polysulfone ultrafiltration membranes via surface zwitterionicalization mediated by polysulfone-based triblock copolymer additive. J. Membr. Sci. 2013, 440, 40–47. [Google Scholar] [CrossRef]
- Gao, B.J.; Zhang, D.D.; Li, Y.B. Preparation of POSS-grafted polysulfone microfiltration membrane and its rejection and removal properties towards heavy metal ions. Polym. Adv. Technol. 2019, 30, 1096–1105. [Google Scholar] [CrossRef]
- Adams, F.V.; Nxumalo, E.N.; Krause, R.W.M.; Hoek, E.M.V.; Mamba, B.B. Preparation and characterization of polysulfone/beta-cyclodextrin polyurethane composite nanofiltration membranes. J. Membr. Sci. 2012, 405, 291–299. [Google Scholar] [CrossRef]
- Park, H.M.; Jee, K.Y.; Lee, Y.T. Preparation and characterization of a thin-film composite reverse osmosis membrane using a polysulfone membrane including metal-organic frameworks. J. Membr. Sci. 2017, 541, 510–518. [Google Scholar] [CrossRef]
- Yuan, X.S.; Guo, Z.Y.; Geng, H.Z.; Rhen, D.S.; Wang, L.D.; Yuan, X.T.; Li, J. Enhanced performance of conductive polysulfone/MWCNT/PANI ultrafiltration membrane in an online fouling monitoring application. J. Membr. Sci. 2019, 575, 160–169. [Google Scholar] [CrossRef]
- Saini, B.; Khuntia, S.; Sinha, M.K. Incorporation of cross-linked poly(AA-co-ACMO) copolymer with pH responsive and hydrophilic properties to polysulfone ultrafiltration membrane for the mitigation of fouling behavior. J. Membr. Sci. 2019, 572, 184–197. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Hliavitskaya, T.A.; Penkova, A.V.; Ermakov, S.S.; Ulbricht, M. Modification of Polysulfone Ultrafiltration Membranes via Addition of Anionic Polyelectrolyte Based on Acrylamide and Sodium Acrylate to the Coagulation Bath to Improve Antifouling Performance in Water Treatment. Membranes 2020, 10, 264. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef]
- Wang, Z.X.; Lin, S.H. Membrane fouling and wetting in membrane distillation and their mitigation by novel membranes with special wettability. Water Res. 2017, 112, 38–47. [Google Scholar] [CrossRef]
- She, Q.H.; Wang, R.; Fane, A.G.; Tang, C.Y.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Hernandez, M.; Melian-Martel, N.; Ruiz-Garcia, A.; Del Rio-Gamero, B. Fouling characterization during initial stage of cross-flow ultrafiltration. J. Water Process. Eng. 2020, 38, 101611–101621. [Google Scholar] [CrossRef]
- Olimattel, K.; Church, J.; Lee, W.H.; Chumbimuni-Torres, K.Y.; Zhai, L.; Sadmani, A.H.M.A. Enhanced fouling resistance and antimicrobial property of ultrafiltration membranes via polyelectrolyte-assisted silver phosphate nanoparticle immobilization. Membranes 2020, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface modification of water purification membranes. Angew. Chem. Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bano, S.; Mahmood, A.; Kim, S.J.; Lee, K.H. Graphene oxide modified polyamide nanofiltration membrane with improved flux and antifouling properties. J. Mater. Chem. A 2015, 3, 2065–2071. [Google Scholar] [CrossRef]
- Zhang, W.B.; Zhu, Y.Z.; Liu, X.; Wang, D.; Li, J.Y.; Jiang, L.; Jin, J. Salt-induced fabrication of superhydrophilic and underwater superoleophobic PAA-g-PVDF membranes for effective separation of oil-in-water emulsions. Angew. Chem. Int. Ed. 2014, 53, 856–860. [Google Scholar] [CrossRef]
- Teow, Y.H.; Ooi, B.S.; Ahmad, A.L.; Lim, J.K. Investigation of anti-fouling and UV-cleaning properties of PVDF/TiO2 mixed-matrix membrane for humic acid removal. Membranes 2020, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Loh, C.H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S.D. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; Bilad, M.R.; Giwa, A.; Hasan, S.W.; Arafat, H.A. Leaching of PVP from PVDF/PVP blend membranes: Impacts on membrane structure and fouling in membrane bioreactors. J. Mater. Sci. 2016, 51, 4328–4341. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, K.H. Effect of PEG additive on membrane formation by phase inversion. J. Membr. Sci. 1998, 138, 153–163. [Google Scholar] [CrossRef]
- Miyano, T.; Matsuura, T.; Carlsson, D.J.; Sourirajan, S. Retention of polyvinylpyrrolidone swelling agent in the Poly(ether para-phenylenesulfone) ultrafiltration membrane. J. Appl. Polym. Sci. 1990, 41, 407–417. [Google Scholar] [CrossRef]
- Ozcan, S.; Kaner, P.; Thomas, D.; Cebe, P.; Asatekin, A. Hydrophobic antifouling electrospun mats from zwitterionic amphiphilic copolymers. ACS Appl. Mater. Inter. 2018, 10, 18300–18309. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.F.; Jeon, S.; Kakihana, Y.; Kakehi, J.; Zhu, B.K.; Matsuyama, H.; Zhao, S.F. Improved antifouling properties of polyvinyl chloride blend membranes by novel phosphate based-zwitterionic polymer additive. J. Membr. Sci. 2017, 528, 326–335. [Google Scholar] [CrossRef]
- Manawi, Y.; Kochkodan, V.; Mohammad, A.W.; Atieh, M.A. Arabic gum as a novel pore-forming and hydrophilic agent in polysulfone membranes. J. Membr. Sci. 2017, 529, 95–104. [Google Scholar] [CrossRef]
- Mi, L.; Jiang, S.Y. Integrated antimicrobial and nonfouling zwitterionic polymers. Angew. Chem. Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef]
- Wang, S.Y.; Fang, L.F.; Cheng, L.; Jeon, S.; Kato, N.; Matsuyama, H. Improved antifouling properties of membranes by simple introduction of zwitterionic copolymers via electrostatic adsorption. J. Membr. Sci. 2018, 564, 672–681. [Google Scholar] [CrossRef]
- Weinman, S.T.; Bass, M.; Pandit, S.; Herzberg, M.; Freger, V.; Husson, S.M. A switchable zwitterionic membrane surface chemistry for biofouling control. J. Membr. Sci. 2018, 548, 490–501. [Google Scholar] [CrossRef]
- Liu, C.H.; Lee, J.; Ma, J.; Elimelech, M. Antifouling thin-film composite membranes by controlled architecture of zwitterionic polymer brush layer. Environ. Sci. Technol. 2017, 51, 2161–2169. [Google Scholar] [CrossRef]
- Bengani-Lutz, P.; Converse, E.; Cebe, P.; Asatekin, A. Self-assembling zwitterionic copolymers as membrane selective layers with excellent fouling resistance: Effect of zwitterion chemistry. ACS Appl. Mater. Interfaces 2017, 9, 20859–20872. [Google Scholar] [CrossRef]
- Koberle, P.; Laschewsky, A. Hydrophobically-modified zwitterionic polymers—synthesis, bulk properties, and miscibility with inorganic salts. Macromolecules 1994, 27, 2165–2173. [Google Scholar] [CrossRef]
- Lewis, A.L. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids Surf. B 2000, 18, 261–275. [Google Scholar] [CrossRef]
- Yue, W.W.; Li, H.J.; Xiang, T.; Qin, H.; Sun, S.D.; Zhao, C.S. Grafting of zwitterion from polysulfone membrane via surface-initiated ATRP with enhanced antifouling property and biocompatibility. J. Membr. Sci. 2013, 446, 79–91. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, S.L.; Luan, J.S.; Mu, Y.F.; Du, Y.L.; Wang, G.B. Fabrication of ultrafiltration membranes with enhanced antifouling capability and stable mechanical properties via the strategies of blending and crosslinking. J. Membr. Sci. 2017, 539, 116–127. [Google Scholar] [CrossRef]
- Dennis, J.M.; Fahs, G.B.; Moore, R.B.; Turner, S.R.; Long, T.E. Synthesis and characterization of polysulfone-containing poly(butylene terephthalate) segmented block copolymers. Macromolecules 2014, 47, 8171–8177. [Google Scholar] [CrossRef]
- Li, Y.; Huang, S.B.; Zhou, S.F.; Fane, A.G.; Zhang, Y.Q.; Zhao, S.F. Enhancing water permeability and fouling resistance of polyvinylidene fluoride membranes with carboxylated nanodiamonds. J. Membr. Sci. 2018, 556, 154–163. [Google Scholar] [CrossRef]
- Wang, Z.X.; Lau, C.H.; Zhang, N.Q.; Bai, Y.P.; Shao, L. Mussel-inspired tailoring of membrane wettability for harsh water treatment. J. Mater. Chem. A 2015, 3, 2650–2657. [Google Scholar] [CrossRef]
- Zhang, G.L.; Lu, S.F.; Zhang, L.; Meng, Q.; Shen, C.; Zhang, J.W. Novel polysulfone hybrid ultrafiltration membrane prepared with TiO2-g-HEMA and its antifouling characteristics. J. Membr. Sci. 2013, 436, 163–173. [Google Scholar] [CrossRef]
- Basu, S.; Khan, A.L.; Cano-Odena, A.; Liu, C.Q.; Vankelecom, I.F.J. Membrane-based technologies for biogas separations. Chem. Soc. Rev. 2010, 39, 750–768. [Google Scholar] [CrossRef]
- Kobayashi, M.; Terayama, Y.; Yamaguchi, H.; Terada, M.; Murakami, D.; Ishihara, K.; Takahara, A. Wettability and antifouling behavior on the surfaces of superhydrophilic polymer brushes. Langmuir 2012, 28, 7212–7222. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, C.; Bridge, A.T.; Hunley, M.T.; Long, T.E.; Green, M.D. Segmented imidazolium ionenes: Solution rheology, thermomechanical properties, and electrospinning. Polymer 2017, 114, 257–265. [Google Scholar] [CrossRef]
- Gao, H.W.; Sun, X.H.; Gao, C.L. Antifouling polysulfone ultrafiltration membranes with sulfobetaine polyimides as novel additive for the enhancement of both water flux and protein rejection. J. Membr. Sci. 2017, 542, 81–90. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Zhang, S.B.; Dai, L.; Chen, X.S. Novel zwitterionic poly(arylene ether sulfone)s as antifouling membrane material. J. Membr. Sci. 2010, 349, 217–224. [Google Scholar] [CrossRef]
- Susanto, H.; Ulbricht, M. Characteristics, performance and stability of polyethersulfone ultrafiltration membranes prepared by phase separation method using different macromolecular additives. J. Membr. Sci. 2009, 327, 125–135. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.S.; Xiang, C.B.; Xianda, S. Effect of nano-sized Al2O3-particle addition on PVDF ultrafiltration membrane performance. J. Membr. Sci. 2006, 276, 162–167. [Google Scholar] [CrossRef]
- Shi, X.F.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and cleaning of ultrafiltration membranes: A review. J. Water Process. Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.L.; Han, Z.S.; Li, G.B. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Sun, H.G.; Yang, X.B.; Zhang, Y.Q.; Cheng, X.Q.; Xu, Y.C.; Bai, Y.P.; Shao, L. Segregation-induced in situ hydrophilic modification of poly (vinylidene fluoride) ultrafiltration membranes via sticky poly (ethylene glycol) blending. J. Membr. Sci. 2018, 563, 22–30. [Google Scholar] [CrossRef]


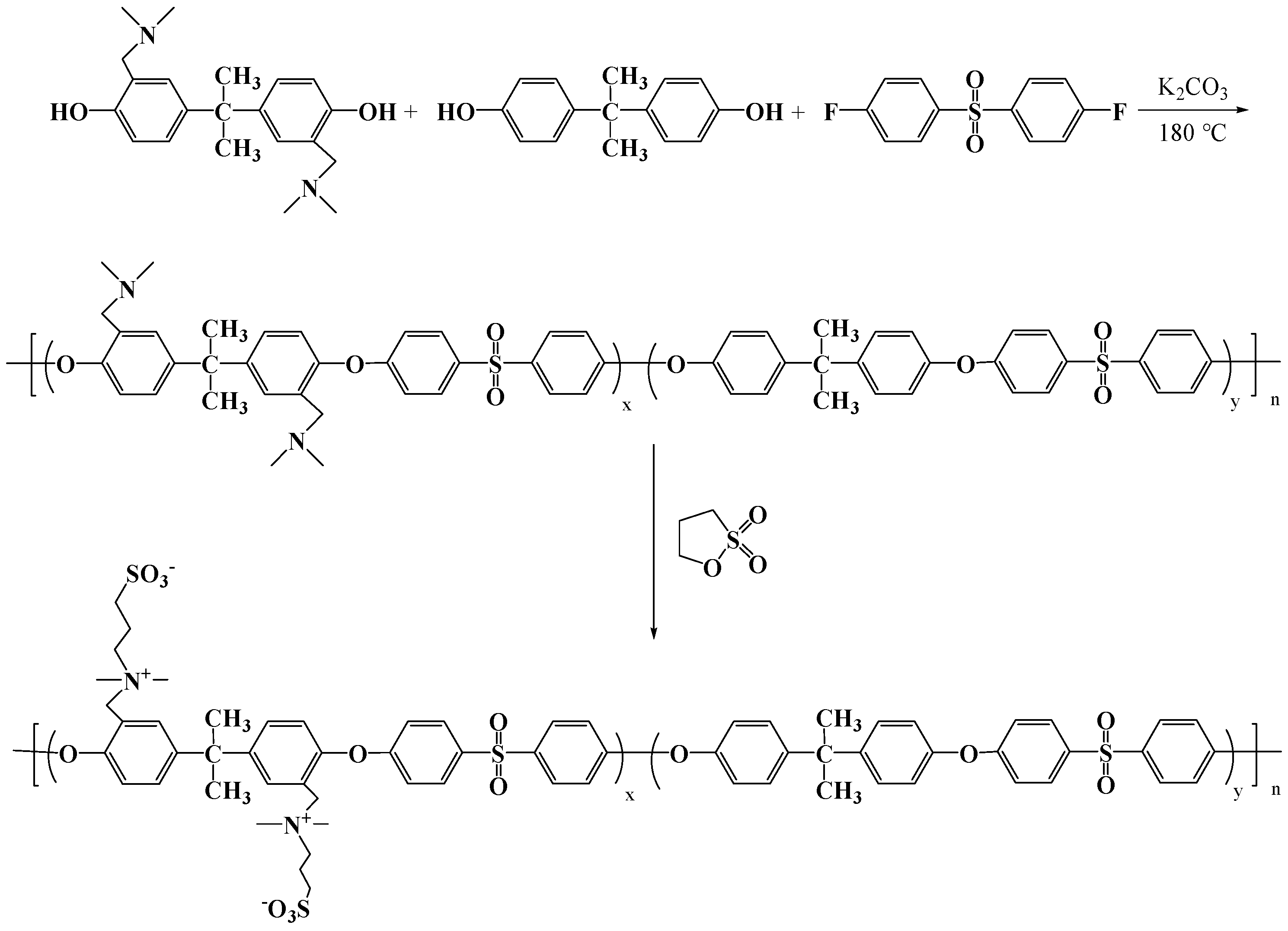


| Membrane | Casting Solution (wt%) | |||
|---|---|---|---|---|
| PSf | PSf-co-SBPSf | PEG | NMP | |
| M0 | 15 | 0 | 0 | 85 |
| M1 | 15 | 0 | 5 | 85 |
| M2 | 13 | 2 | 0 | 85 |
| M3 | 12 | 3 | 0 | 85 |
| M4 | 11 | 4 | 0 | 85 |
| M5 | 10 | 5 | 0 | 85 |
| Membrane | Porosity (%) | Mean Pore Size (nm) | Over Membrane Thickness (μm) | Skin Layer Thickness (μm) |
|---|---|---|---|---|
| M0 | 46.3 ± 0.6 | 3.9 | 110 ± 3 | 1.46 ± 0.4 |
| M1 | 62.2 ± 0.3 | 13.4 | 106 ± 11 | 1.23 ± 0.4 |
| M2 | 76.7 ± 0.4 | 5.6 | 109 ± 8 | 1.06 ± 0.7 |
| M3 | 79.6 ± 0.2 | 6.1 | 105 ± 2 | 0.98 ± 0.2 |
| M4 | 81.1 ± 0.3 | 5.8 | 106 ± 6 | 0.91 ± 0.6 |
| M5 | 85.8 ± 0.5 | 6.5 | 103 ± 4 | 0.83 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Gao, C.; Wang, X.; Wu, G.; Yin, J.; Huang, Y.; Sun, X. Zwitterionic Polysulfone Copolymer/Polysulfone Blended Ultrafiltration Membranes with Excellent Thermostability and Antifouling Properties. Membranes 2021, 11, 932. https://doi.org/10.3390/membranes11120932
Li D, Gao C, Wang X, Wu G, Yin J, Huang Y, Sun X. Zwitterionic Polysulfone Copolymer/Polysulfone Blended Ultrafiltration Membranes with Excellent Thermostability and Antifouling Properties. Membranes. 2021; 11(12):932. https://doi.org/10.3390/membranes11120932
Chicago/Turabian StyleLi, Dalong, Changlu Gao, Xinyue Wang, Gang Wu, Jinghua Yin, Yudong Huang, and Xiuhua Sun. 2021. "Zwitterionic Polysulfone Copolymer/Polysulfone Blended Ultrafiltration Membranes with Excellent Thermostability and Antifouling Properties" Membranes 11, no. 12: 932. https://doi.org/10.3390/membranes11120932
APA StyleLi, D., Gao, C., Wang, X., Wu, G., Yin, J., Huang, Y., & Sun, X. (2021). Zwitterionic Polysulfone Copolymer/Polysulfone Blended Ultrafiltration Membranes with Excellent Thermostability and Antifouling Properties. Membranes, 11(12), 932. https://doi.org/10.3390/membranes11120932







