Mitigating Silica Fouling and Improving PPCP Removal by Modified NF90 Using In Situ Radical Graft Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane, Chemicals, and Reagents
2.2. Experimental Protocols
2.2.1. Filtration Experiments
2.2.2. Membrane Modification
2.2.3. Membrane Fouling Experiments
2.3. Analytical Methods
2.3.1. Membrane Characterization
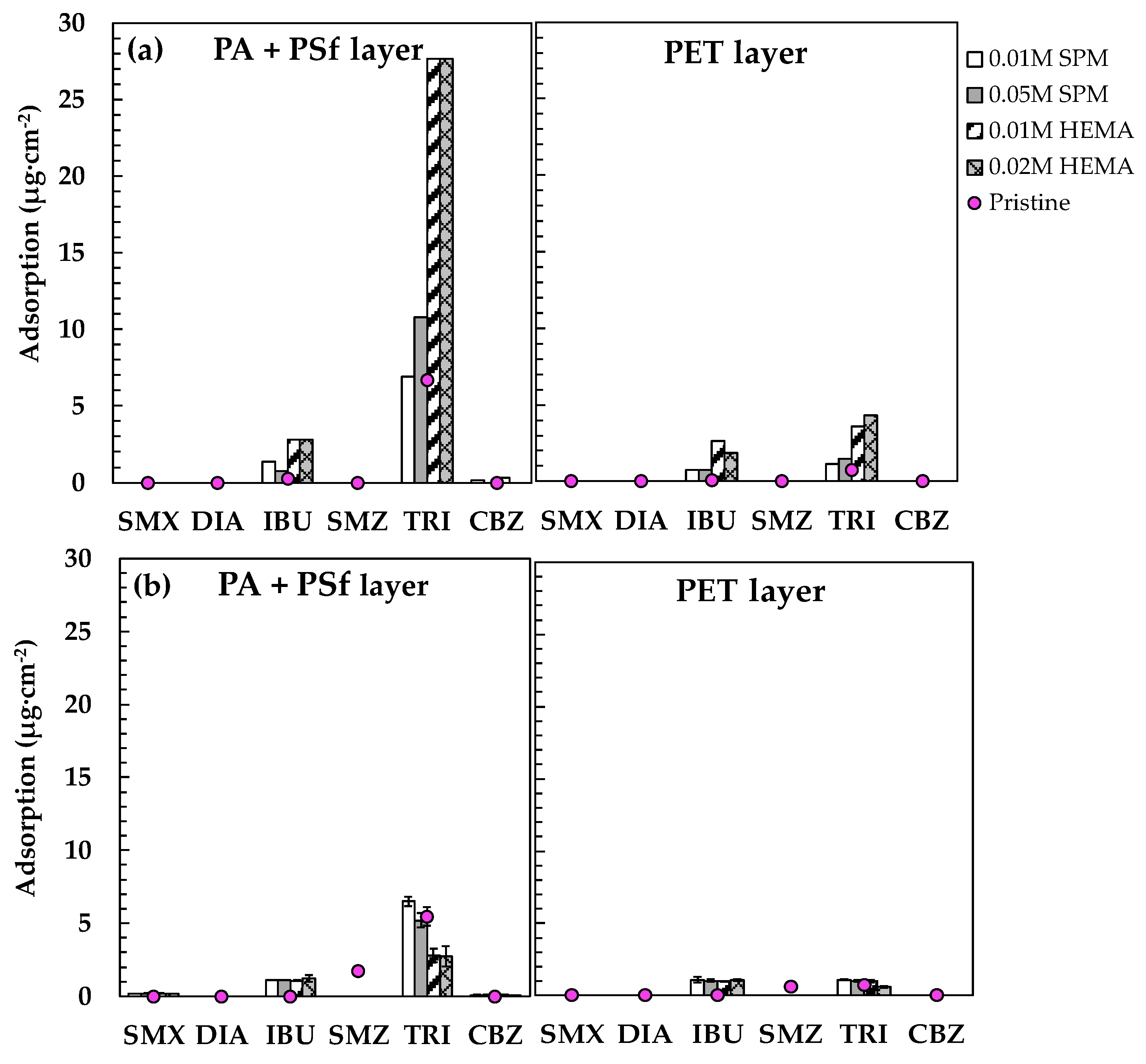
2.3.2. PPCP Extraction from Membranes
2.4. Confirmation of Fouling Mechanisms
3. Results and Discussion
3.1. Effect of Membrane Modification on Permeate Flux and Surface Characteristics
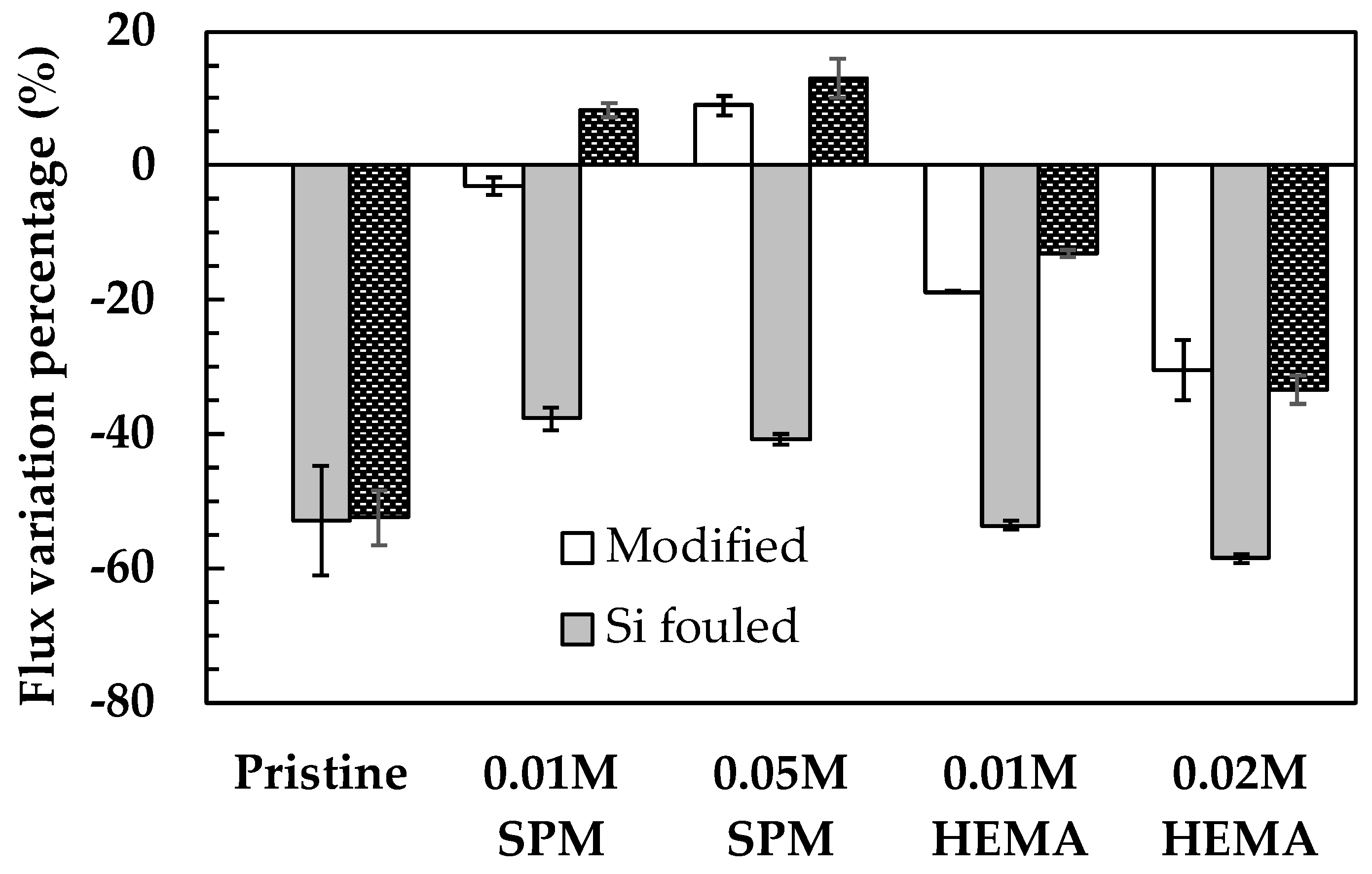
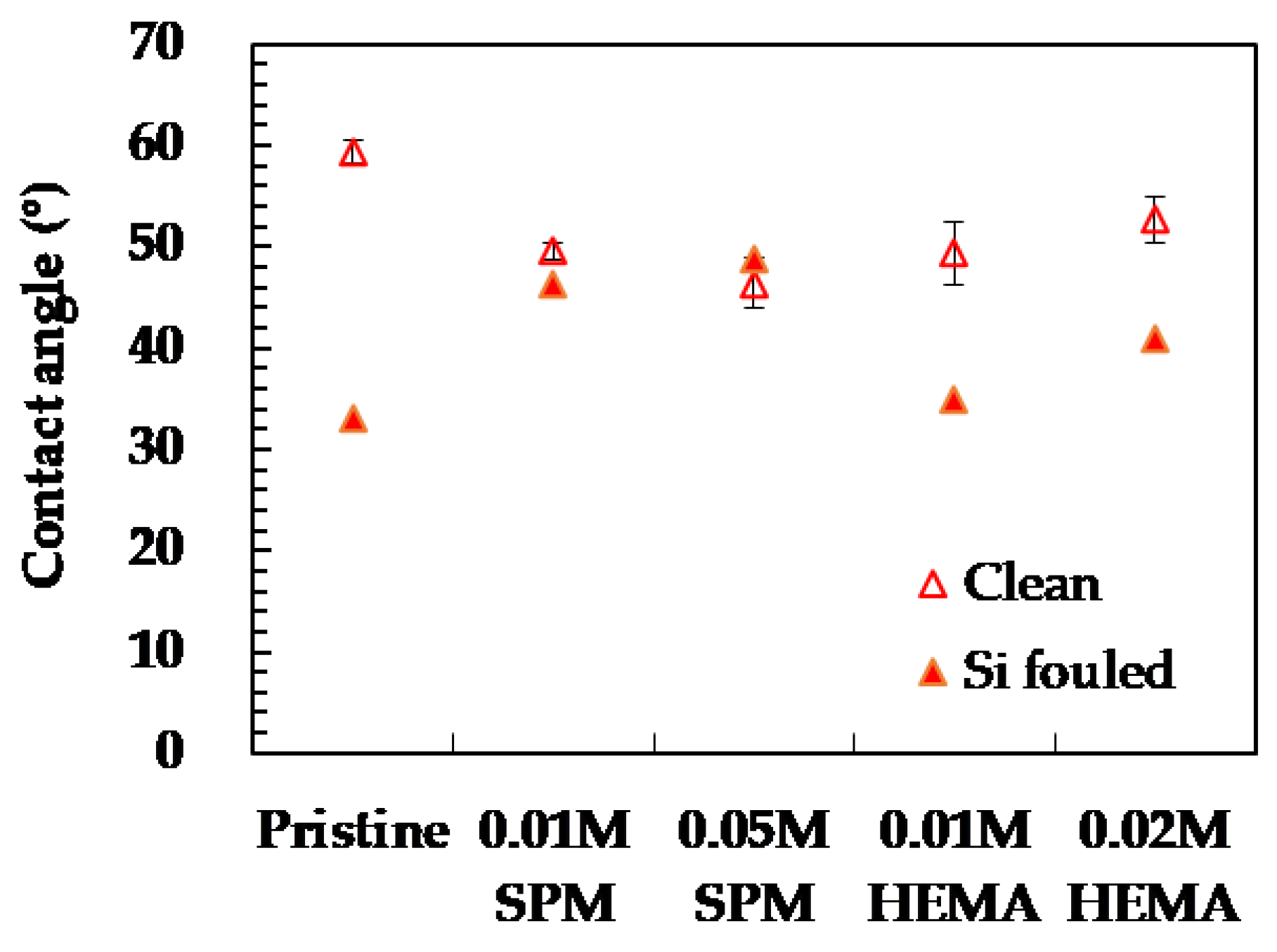
3.1.1. Before Silica Fouling
3.1.2. After Silica Fouling
3.2. Effect of Membrane Modification on Salt Rejection
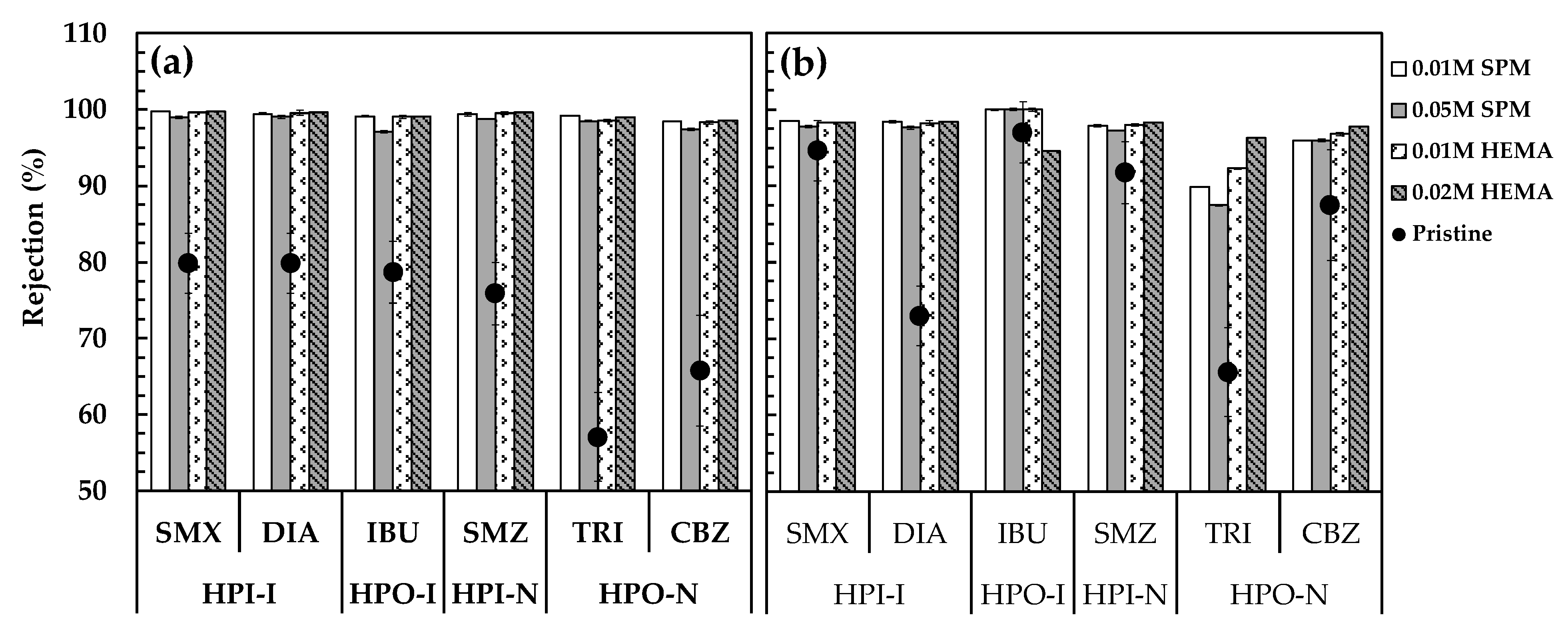
3.3. Effect of Membrane Modification on PPCP Rejection and Adsorption
3.3.1. Before Silica Fouling
3.3.2. After Silica Fouling
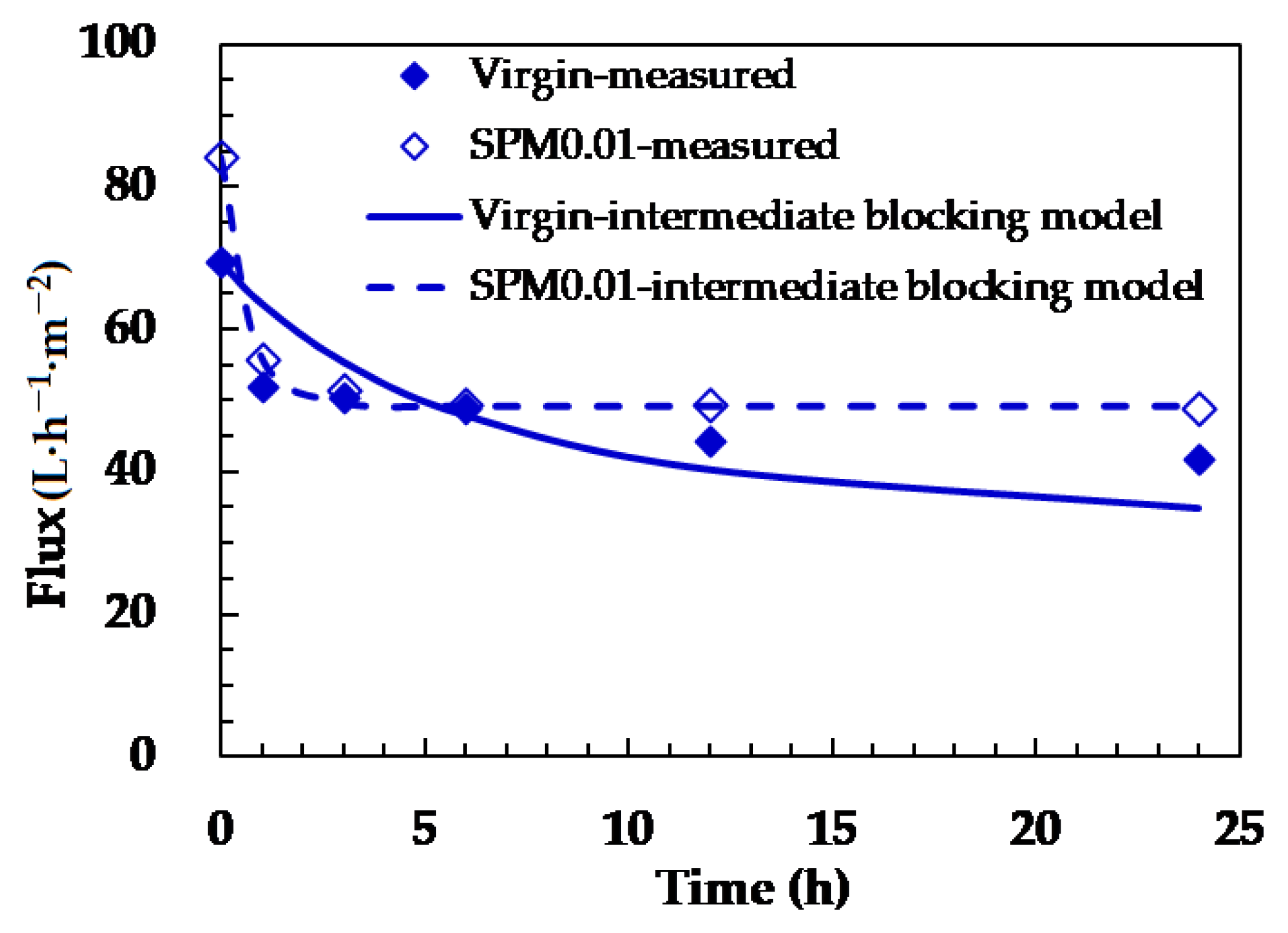
3.4. Confirmation of the Silica Fouling Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.; Chu, K.H.; Al-Hamadani, Y.A.J.; Park, C.M.; Jang, M.; Kim, D.-H.; Yu, M.; Heo, J.; Yoon, Y. Removal of contaminants of emerging concern by membranes in water and wastewater: A review. Chem. Eng. J. 2018, 335, 896–914. [Google Scholar] [CrossRef]
- Shamsuzzoha, M.; Rasheduzzaman, M.; Ghosh, R.C. Building Resilience for Drinking Water Shortages through Reverse Osmosis Technology in Coastal Areas of Bangladesh. Procedia Eng. 2018, 212, 559–566. [Google Scholar] [CrossRef]
- Huang, H.; Cho, H.; Schwab, K.; Jacangelo, J.G. Effects of feedwater pretreatment on the removal of organic microconstituents by a low fouling reverse osmosis membrane. Desalination 2011, 281, 446–454. [Google Scholar] [CrossRef]
- Zhang, L.; She, Q.; Wang, R.; Wongchitphimon, S.; Chen, Y.; Fane, A.G. Unique roles of aminosilane in developing anti-fouling thin film composite (TFC) membranes for pressure retarded osmosis (PRO). Desalination 2016, 389, 119–128. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Al-Amoudi, A.S. Factors affecting natural organic matter (NOM) and scaling fouling in NF membranes: A review. Desalination 2010, 259, 1–10. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Coleman, P.J. NF/RO filtration of the hydrophobic ionogenic compound triclosan: Transport mechanisms and the influence of membrane fouling. Sep. Purif. Technol. 2008, 62, 709–716. [Google Scholar] [CrossRef]
- Khan, I.A.; Lee, Y.S.; Kim, J.O. A comparison of variations in blocking mechanisms of membrane-fouling models for estimating flux during water treatment. Chemosphere 2020, 259, 127328. [Google Scholar] [CrossRef] [PubMed]
- Vrouwenvelder, H.S.; van Paassen, J.A.M.; Folmer, H.C.; Hofman, J.A.M.N.; Nederlof, M.M.; van der Kooij, D. Biofouling of membranes for drinking water production. Desalination 1998, 118, 157–166. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chong, T.H.; Fane, A.G. Colloidal interactions and fouling of NF and RO membranes: A review. Adv. Colloid Interface Sci. 2011, 164, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, M.; López-Serna, R.; Palacio, L.; Hernández, A.; Prádanos, P.; Peña, M. Study of the rejection of contaminants of emerging concern by a biomimetic aquaporin hollow fiber forward osmosis membrane. J. Water Process. Eng. 2021, 40, 101914. [Google Scholar] [CrossRef]
- Liu, F.M.; Zhu, B.K.; Xu, Y.Y. Improving the hydrophilicity of poly(vinylidene fluoride) porous membranes by electron beam initiated surface grafting of AA/SSS binary monomers. Appl. Surf. Sci. 2006, 253, 2096–2101. [Google Scholar] [CrossRef]
- Ben-David, A.; Bernstein, R.; Oren, Y.; Belfer, S.; Dosoretz, C.; Freger, V. Facile surface modification of nanofiltration membranes to target the removal of endocrine-disrupting compounds. J. Membr. Sci. 2010, 357, 152–159. [Google Scholar] [CrossRef]
- Feng, X.; Imran, Q.; Zhang, Y.; Sixdenier, L.; Lu, X.; Kaufman, G.; Gabinet, U.; Kawabata, K.; Elimelech, M.; Osuji, C.O. Precise nanofiltration in a fouling-resistant self-assembled membrane with water-continuous transport pathways. Sci. Adv. 2019, 5, eaav9308. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.-R.; Wang, J.-N.; Li, C.-J. Strategies for improving the performance of the polyamide thin film composite (PA-TFC) reverse osmosis (RO) membranes: Surface modifications and nanoparticles incorporations. Desalination 2013, 328, 83–100. [Google Scholar] [CrossRef]
- Lin, Y.-L. In situ concentration-polarization-enhanced radical graft polymerization of NF270 for mitigating silica fouling and improving pharmaceutical and personal care product rejection. J. Membr. Sci. 2018, 552, 387–395. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Tsai, J.-Z.; Hung, C.-H. Using in situ modification to enhance organic fouling resistance and rejection of pharmaceutical and personal care products in a thin-film composite nanofiltration membrane. Environ. Sci. Pollut. Res. 2019, 26, 34073–34084. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.-F.; Boo, C.; Lu, X.; Zhou, X.; Yu, H.-Q.; Elimelech, M. Surface functionalization of reverse osmosis membranes with sulfonic groups for simultaneous mitigation of silica scaling and organic fouling. Water Res. 2020, 185, 116203. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-L.; Chiou, J.-H.; Lee, C.-H. Effect of silica fouling on the removal of pharmaceuticals and personal care products by nanofiltration and reverse osmosis membranes. J. Hazard. Mater. 2014, 277, 102–109. [Google Scholar] [CrossRef]
- Ouyang, Z.; Huang, Z.; Tang, X.; Xiong, C.; Tang, M.; Lu, Y. A dually charged nanofiltration membrane by pH-responsive polydopamine for pharmaceuticals and personal care products removal. Sep. Purif. Technol. 2019, 211, 90–97. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kashif, A.; Fuwad, A.; Choi, Y. Current advances in treatment technologies for removal of emerging contaminants from water—A critical review. Co-ord. Chem. Rev. 2021, 442, 213993. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Farid, M.U.; Kharraz, J.A.; Choi, J.; Tang, C.Y.; Nghiem, L.D.; Jang, A.; An, A.K. Removal of organic micropollutants using advanced membrane-based water and wastewater treatment: A review. J. Membr. Sci. 2020, 598, 117672. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C.; Yoon, J. Removal of endocrine disrupting compounds and pharmaceuticals by nanofiltration and ultrafiltration membranes. Desalination 2007, 202, 16–23. [Google Scholar] [CrossRef]
- Qi, Y.; Tong, T.; Zhao, S.; Zhang, W.; Wang, Z.; Wang, J. Reverse osmosis membrane with simultaneous fouling- and scaling-resistance based on multilayered metal-phytic acid assembly. J. Membr. Sci. 2020, 601, 117888. [Google Scholar] [CrossRef]
- Lin, Y.-L. Effects of organic, biological and colloidal fouling on the removal of pharmaceuticals and personal care products by nanofiltration and reverse osmosis membranes. J. Membr. Sci. 2017, 542, 342–351. [Google Scholar] [CrossRef]
- Huang, S.; McDonald, J.A.; Kuchel, R.P.; Khan, S.J.; Leslie, G.; Tang, C.Y.; Mansouri, J.; Fane, A.G. Surface modification of nanofiltration membranes to improve the removal of organic micropollutants: Linking membrane characteristics to solute transmission. Water Res. 2021, 203, 117520. [Google Scholar] [CrossRef] [PubMed]
- Arsuaga, J.M.; López-Muñoz, M.-J.; Sotto, A. Correlation between retention and adsorption of phenolic compounds in nanofiltration membranes. Desalination 2010, 250, 829–832. [Google Scholar] [CrossRef]
- Wilke, C.R.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Vincent-Vela, M.C.; Álvarez-Blanco, S.; García, J.L.; Bergantiños, E. Analysis of membrane pore blocking models adapted to crossflow ultrafiltration in the ultrafiltration of PEG. Chem. Eng. J. 2009, 149, 232–241. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, P.-K.; Lee, C.-H.; Kwon, H.-H. Surface modification of nanofiltration membranes to improve the removal of organic micro-pollutants (EDCs and PhACs) in drinking water treatment: Graft polymerization and cross-linking followed by functional group substitution. J. Membr. Sci. 2008, 321, 190–198. [Google Scholar] [CrossRef]
- Eskandari, P.; Abousalman-Rezvani, Z.; Roghani-Mamaqani, H.; Salami-Kalajahi, M.; Mardani, H. Polymer grafting on graphene layers by controlled radical polymerization. Adv. Colloid Interface Sci. 2019, 273, 102021. [Google Scholar] [CrossRef]
- Rahimpour, A.; Madaeni, S.S.; Mansourpanah, Y. Nano-porous polyethersulfone (PES) membranes modified by acrylic acid (AA) and 2-hydroxyethylmethacrylate (HEMA) as additives in the gelation media. J. Membr. Sci. 2010, 364, 380–388. [Google Scholar] [CrossRef]
- Goushki, M.N.; Mousavi, S.A.; Abdekhodaie, M.J.; Sadeghi, M. Free radical graft polymerization of 2-hydroxyethyl methacrylate and acrylic acid on the polysulfone membrane surface through circulation of reaction media to improve its performance and hemocompatibility properties. J. Membr. Sci. 2018, 564, 762–772. [Google Scholar] [CrossRef]
- Gorzalski, A.; Donley, C.; Coronell, O. Elemental composition of membrane foulant layers using EDS, XPS, and RBS. J. Membr. Sci. 2017, 522, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes: I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Suo, Y.; Ren, Y.S. Research on the mechanism of nanofiltration membrane fouling in zero discharge process of high salty wastewater from coal chemical industry. Chem. Eng. Sci. 2021, 245, 116810. [Google Scholar] [CrossRef]
- Zhao, G.; Chen, W.N. Design of poly(vinylidene fluoride)-g-p(hydroxyethyl methacrylate-co-N-isopropylacrylamide) membrane via surface modification for enhanced fouling resistance and release property. Appl. Surf. Sci. 2017, 398, 103–115. [Google Scholar] [CrossRef]
- Lay, H.T.; Wang, R.; Chew, J.W. Membrane fouling by mixtures of oppositely charged particles. J. Membr. Sci. 2021, 625, 119093. [Google Scholar] [CrossRef]
- Sabir, A.; Islam, A.; Shafiq, M.; Shafeeq, A.; Butt, M.T.Z.; Ahmad, N.; Sanaullah, K.; Jamil, T. Novel polymer matrix composite membrane doped with fumed silica particles for reverse osmosis desalination. Desalination 2015, 368, 159–170. [Google Scholar] [CrossRef]
- Ren, T.; Han, L.; Liu, R.; Ma, C.; Chen, X.; Zhao, S.; Zhang, Y. Influence of inorganic salt on retention of ibuprofen by nanofiltration. Sep. Purif. Technol. 2017, 189, 382–388. [Google Scholar] [CrossRef]
- Takeuchi, K.; Takizawa, Y.; Kitazawa, H.; Fujii, M.; Hosaka, K.; Ortiz-Medina, J.; Morelos-Gomez, A.; Cruz-Silva, R.; Fujishige, M.; Akuzawa, N.; et al. Salt rejection behavior of carbon nanotube-polyamide nanocomposite reverse osmosis membranes in several salt solutions. Desalination 2018, 443, 165–171. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Tsai, C.-C.; Zheng, N.-Y. Improving the organic and biological fouling resistance and removal of pharmaceutical and personal care products through nanofiltration by using in situ radical graft polymerization. Sci. Total Environ. 2018, 635, 543–550. [Google Scholar] [CrossRef]
- Dang, H.Q.; Price, W.E.; Nghiem, L.D. The effects of feed solution temperature on pore size and trace organic contaminant rejection by the nanofiltration membrane NF270. Sep. Purif. Technol. 2014, 125, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Lv, G.; Li, Z.; Hoeppner, N.; Wu, L.; Liao, L. Interactions between sulfa drug sulfadiazine and hydrophobic talc surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2014, 446, 172–178. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, B.; Yang, F. Removal of Sulfadiazine by Polyamide Nanofiltration Membranes: Measurement, Modeling, and Mechanisms. Membranes 2021, 11, 104. [Google Scholar] [CrossRef]
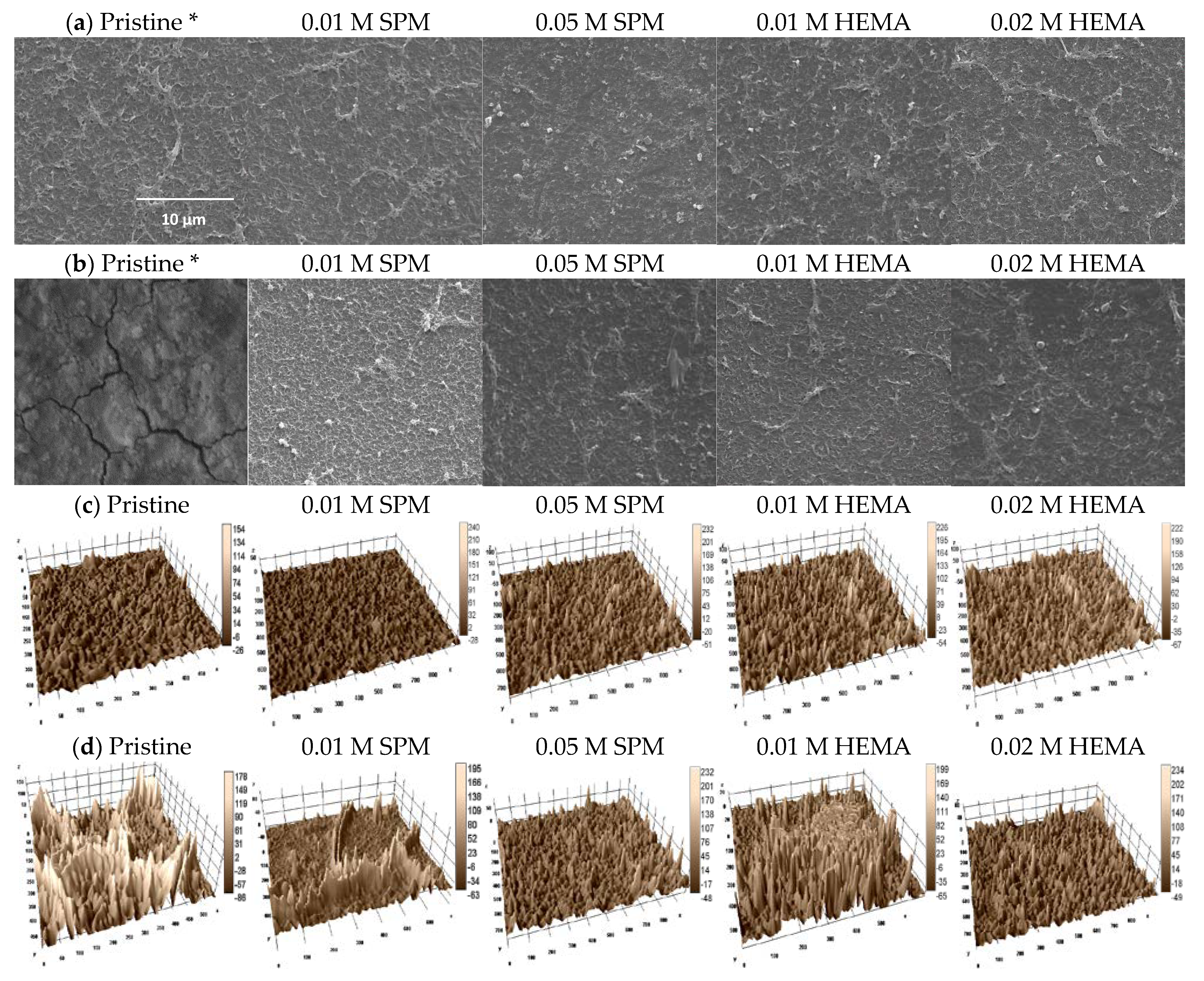
| Membrane | Silica Fouling | Surface Morphology | Rq a (μm) | Ra b (μm) |
|---|---|---|---|---|
| Pristine | No | Smooth valley | 8.0 | 6.0 |
| 0.01 M SPM | No | Ridge valley | 8.3 | 6.1 |
| 0.05 M SPM | No | Ridge valley | 18.2 | 12.9 |
| 0.01 M HEMA | No | Ridge valley | 20.7 | 15.0 |
| 0.02 M HEMA | No | Ridg valley | 22.9 | 17.0 |
| Pristine | Yes | Ridge valley | 40.8 | 33.0 |
| 0.01 M SPM | Yes | Ridge valley | 18.0 | 12.1 |
| 0.05 M SPM | Yes | Ridge valley | 16.5 | 11.8 |
| 0.01 M HEMA | Yes | Ridge valley | 19.1 | 13.8 |
| 0.02 M HEMA | Yes | Ridge valley | 16.6 | 11.6 |
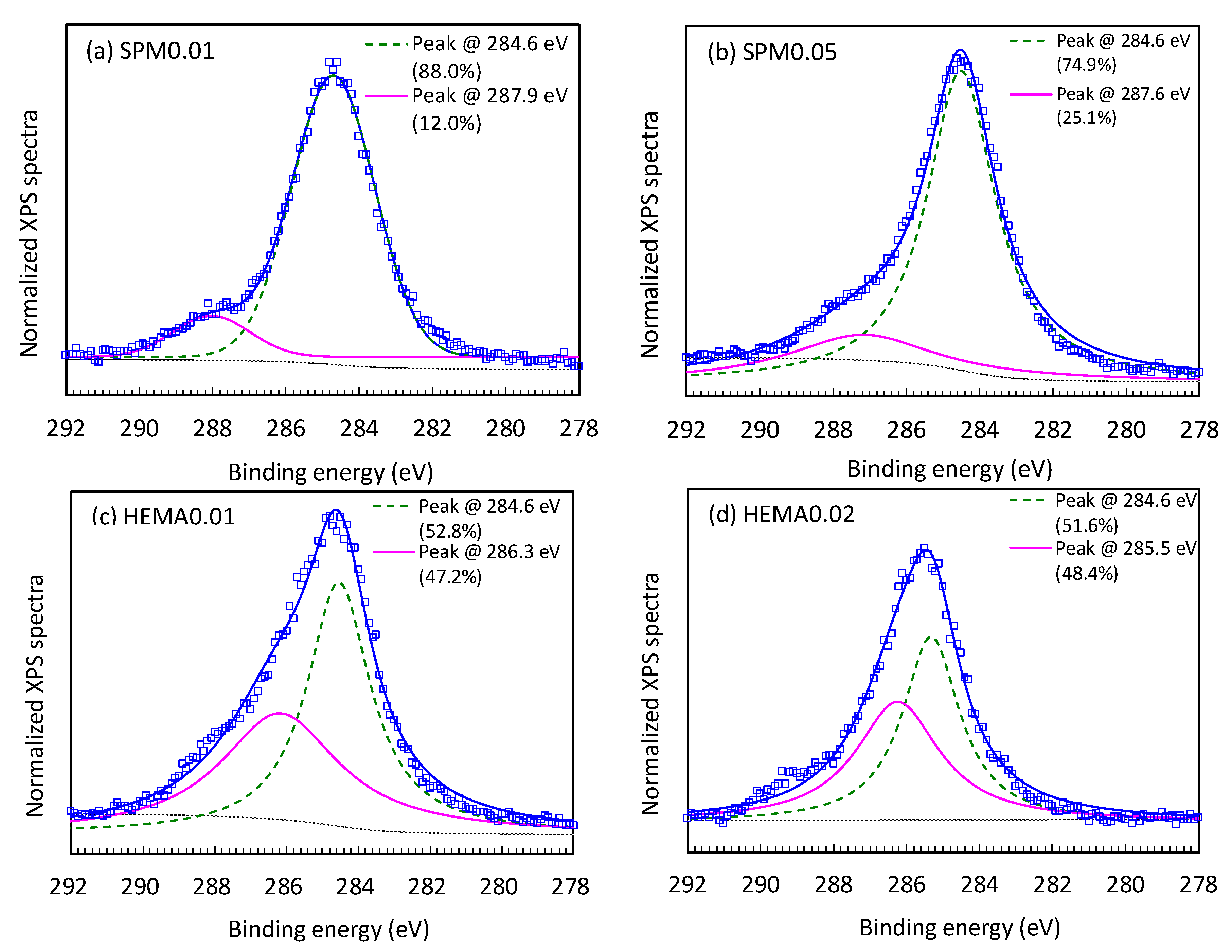
| Membrane | C (%) | O (%) | N (%) | O/C | N/C | O/N | |
|---|---|---|---|---|---|---|---|
| Pristine a | 75.99 | 13.22 | 5.52 | 0.17 | 0.07 | 2.39 | |
| Modified | 0.01 M SPM | 71.22 | 19.65 | 9.14 | 0.28 | 0.13 | 2.15 |
| 0.05 M SPM | 71.72 | 19.64 | 8.63 | 0.27 | 0.12 | 2.28 | |
| 0.01 M HEMA | 70.32 | 21.89 | 7.79 | 0.31 | 0.11 | 2.81 | |
| 0.02 M HEMA | 70.38 | 21.06 | 8.56 | 0.30 | 0.12 | 2.46 | |
| Membrane | Kc b (1·m−1) | R2 | Ki c (1·m−1) | R2 | Ks d (10−3·s−0.5·m−0.5) | R2 | Kgl e (10−5·s·m−2) | R2 | |
|---|---|---|---|---|---|---|---|---|---|
| Pristine a | 1.95 | 0.80 | 13.30 | 0.91 | 6.27 | 0.63 | 3.36 | 0.82 | |
| Modified | SPM0.01 | 0.02 | 1.00 | 0.02 | 1.00 | 0.01 | 0.41 | NA | NA |
| SPM0.05 | 0.01 | 0.90 | 0.02 | 0.94 | 0.01 | 0.63 | NA | NA | |
| HEMA0.01 | 0.02 | 0.94 | 0.03 | 0.96 | 0.01 | 0.53 | NA | NA | |
| HEMA0.02 | 0.02 | 0.98 | 0.03 | 0.99 | 0.01 | 0.57 | NA | NA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-L.; Zheng, N.-Y.; Gan, H.-Y.; Chang, A.-X.; Luo, H.-X.; Mao, Y.-J. Mitigating Silica Fouling and Improving PPCP Removal by Modified NF90 Using In Situ Radical Graft Polymerization. Membranes 2021, 11, 904. https://doi.org/10.3390/membranes11110904
Lin Y-L, Zheng N-Y, Gan H-Y, Chang A-X, Luo H-X, Mao Y-J. Mitigating Silica Fouling and Improving PPCP Removal by Modified NF90 Using In Situ Radical Graft Polymerization. Membranes. 2021; 11(11):904. https://doi.org/10.3390/membranes11110904
Chicago/Turabian StyleLin, Yi-Li, Nai-Yun Zheng, Hao-Yu Gan, An-Xian Chang, Huai-Xuan Luo, and Yao-Jie Mao. 2021. "Mitigating Silica Fouling and Improving PPCP Removal by Modified NF90 Using In Situ Radical Graft Polymerization" Membranes 11, no. 11: 904. https://doi.org/10.3390/membranes11110904






