Development of Pure Silica CHA Membranes for CO2 Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Si-CHA Crystals
2.2. Synthesis of Si-CHA Membranes
2.3. Characterization
3. Results and Discussion
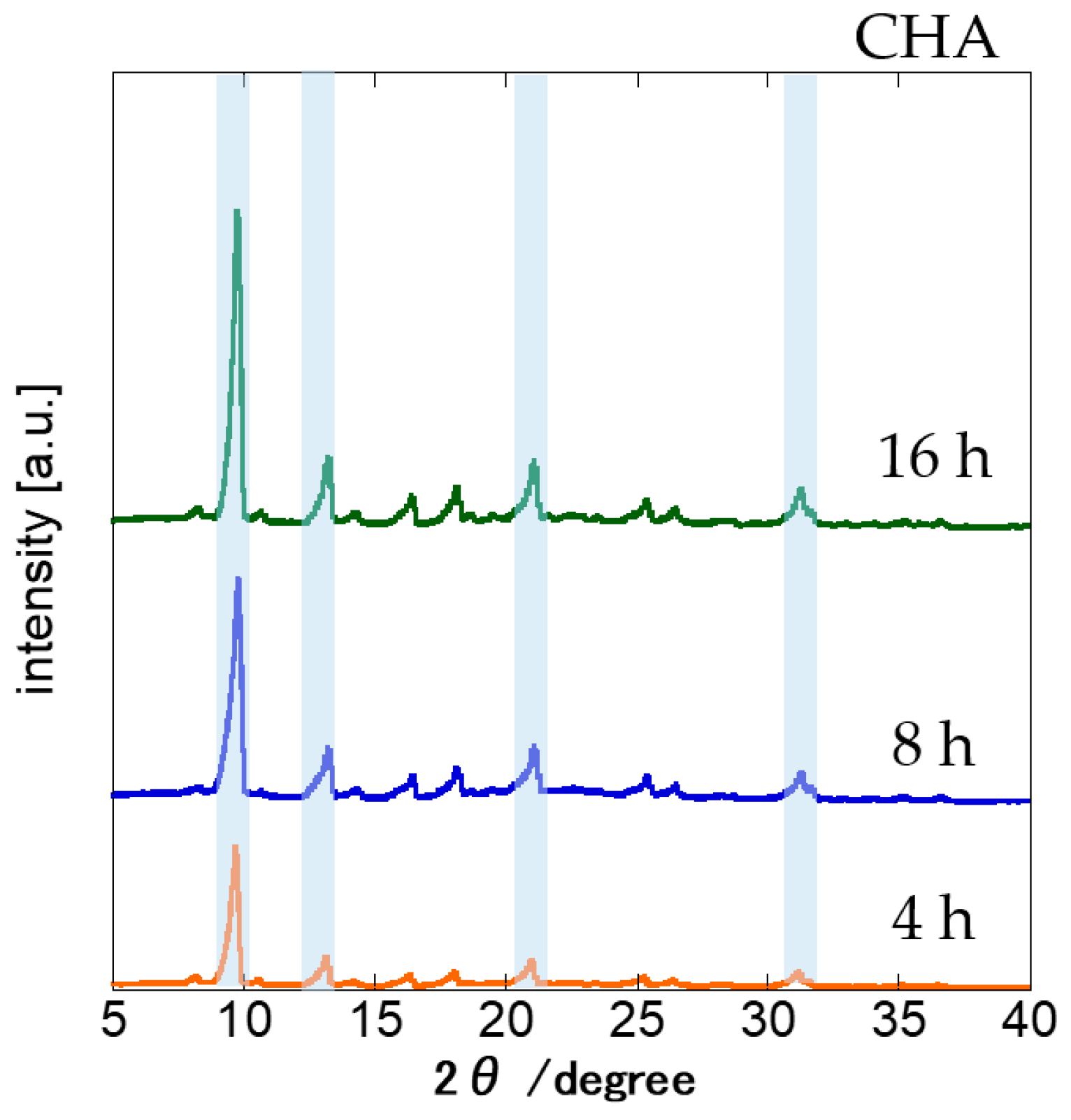
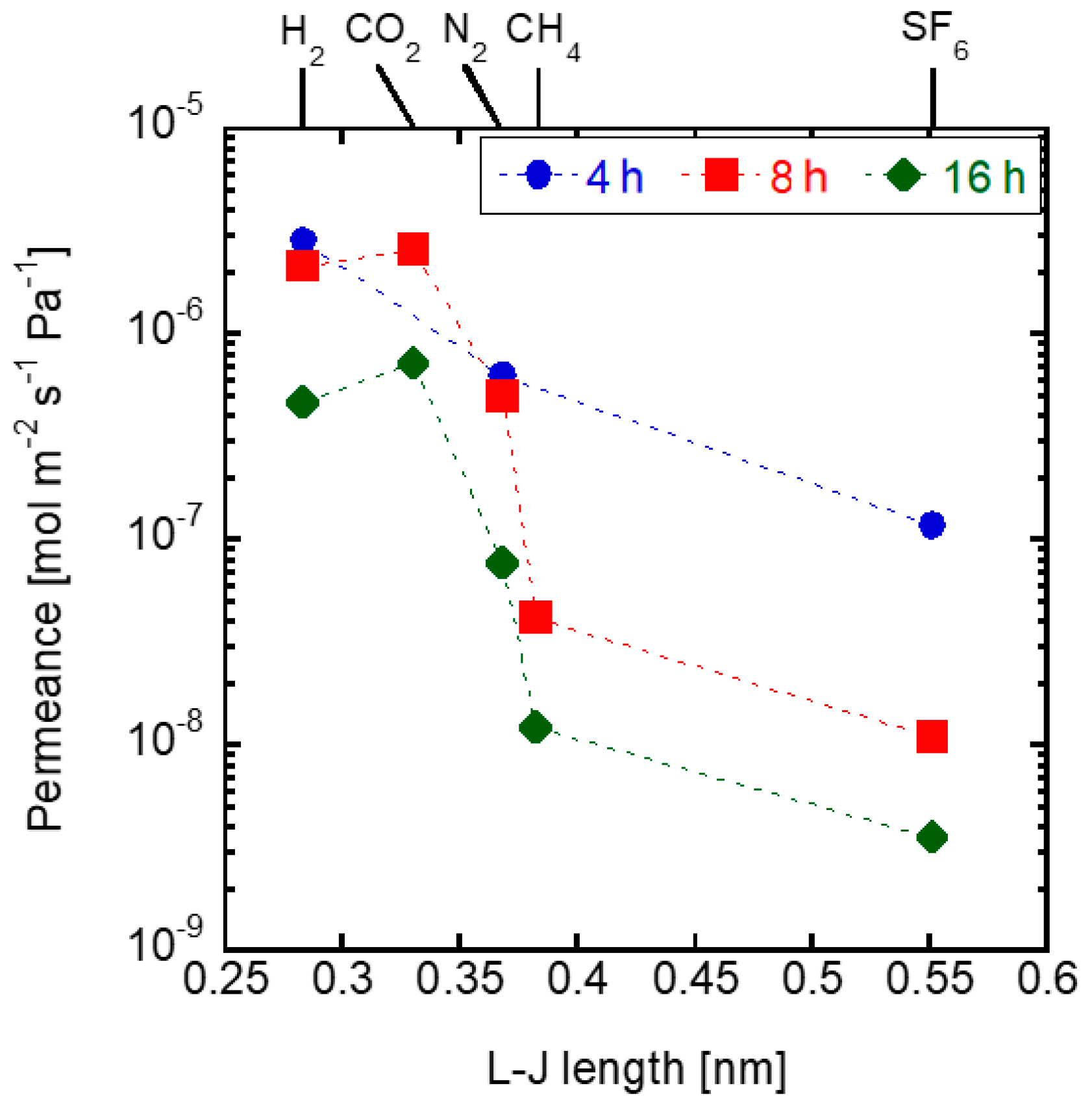
3.1. Effects of Synthesis Time
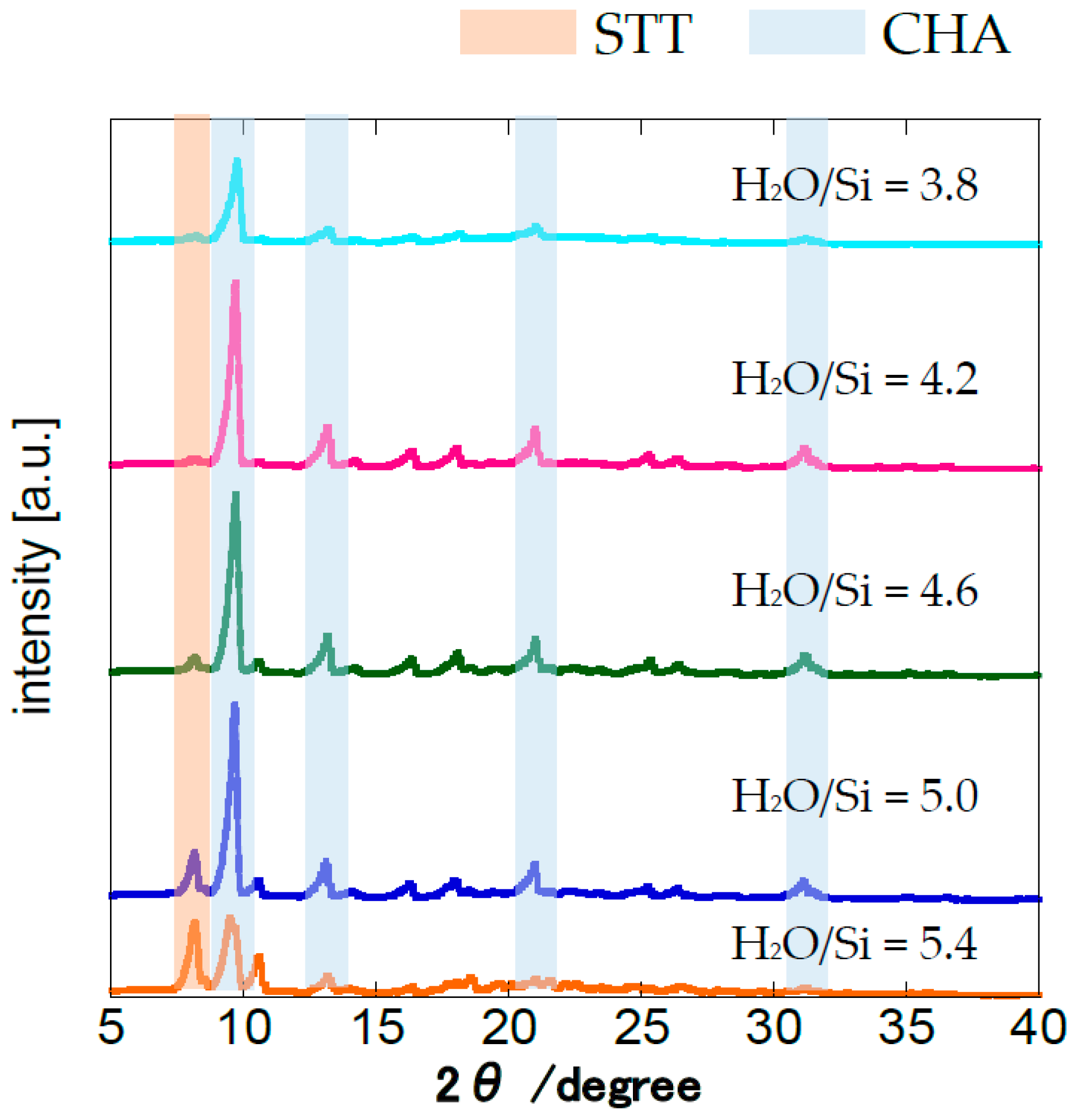
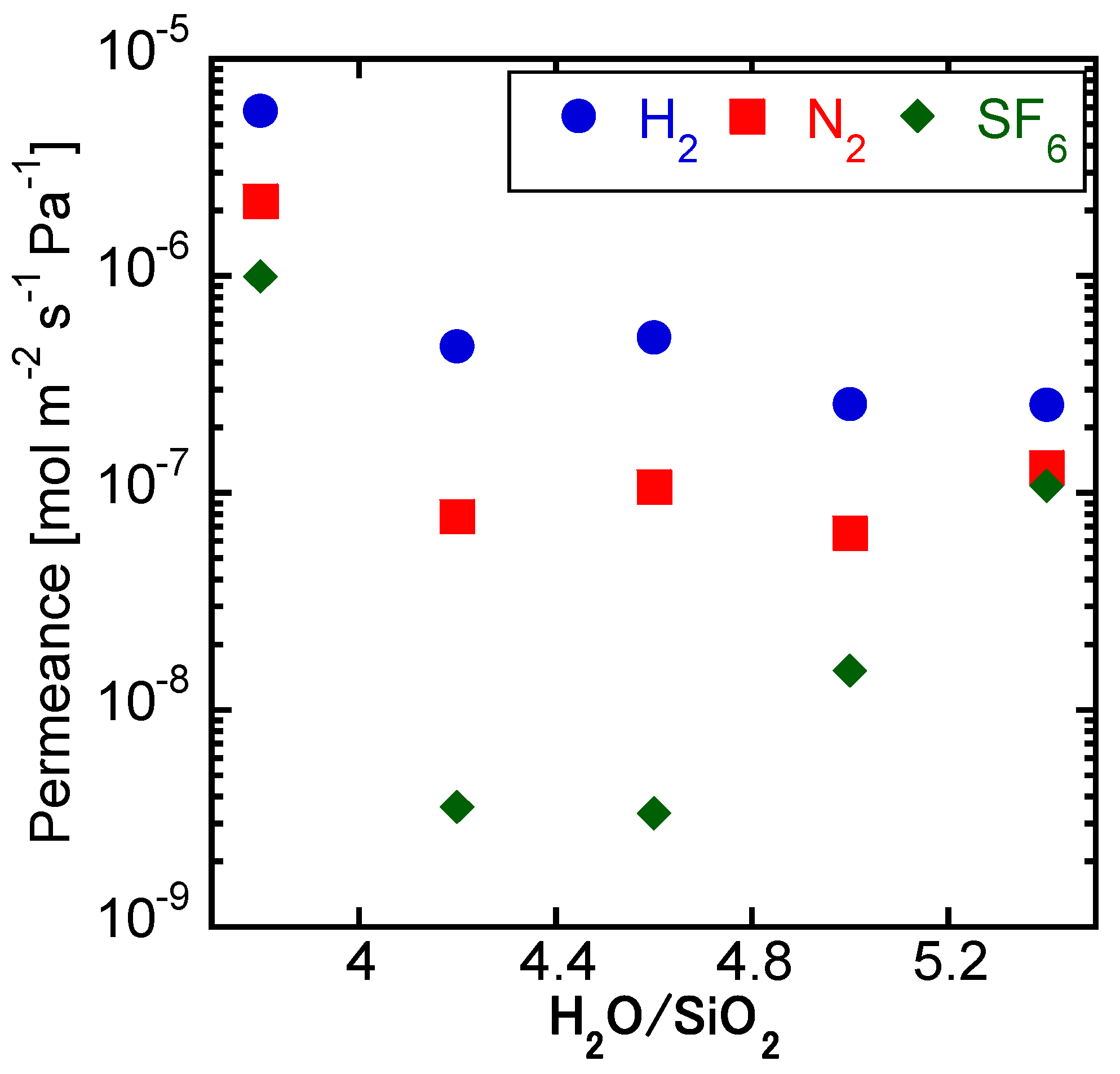
3.2. Effect of H2O/SiO2 Ratio of the Parent Gel
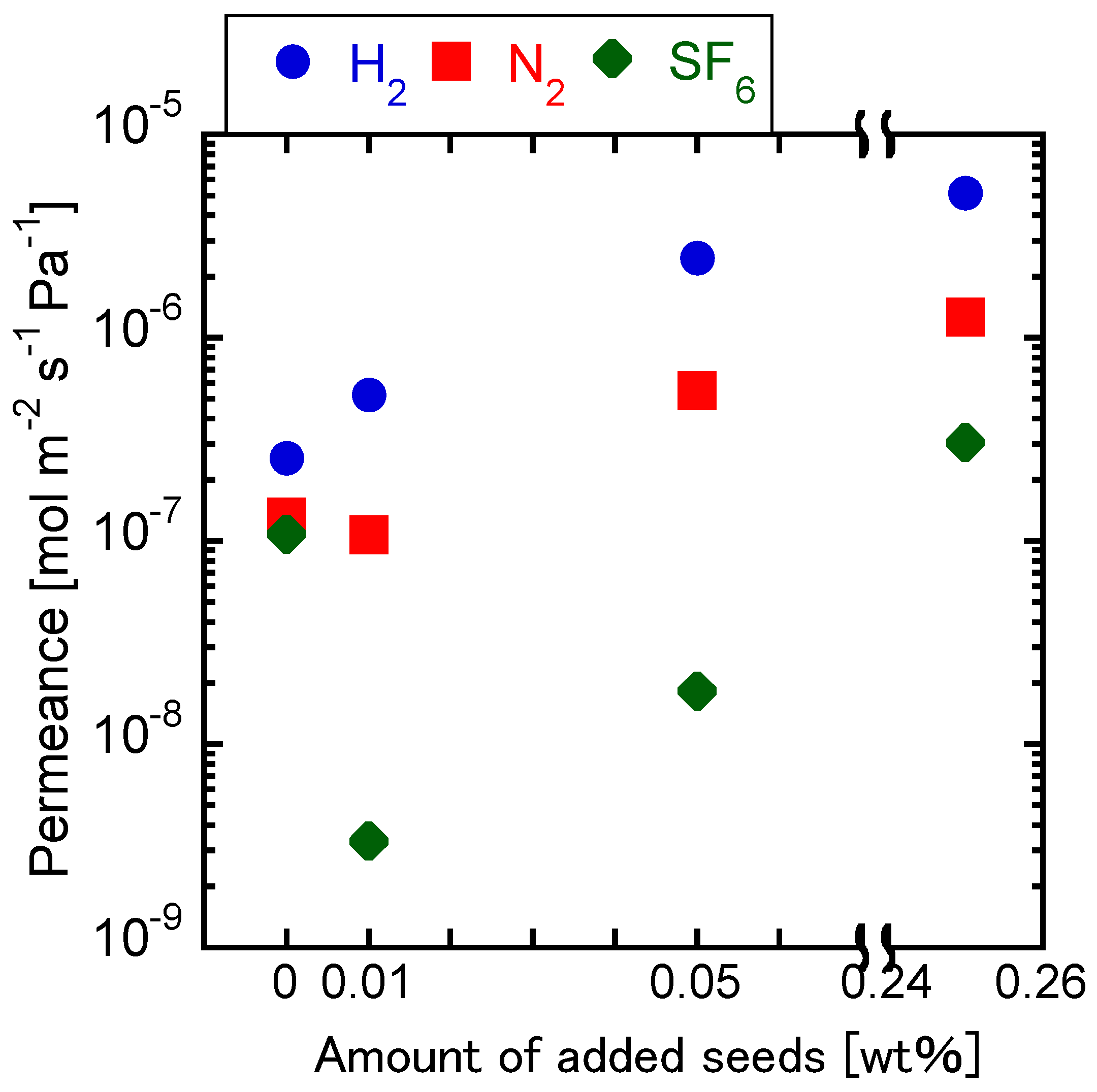
3.3. Effect of Adding Seed Crystals to the Synthesis Gel
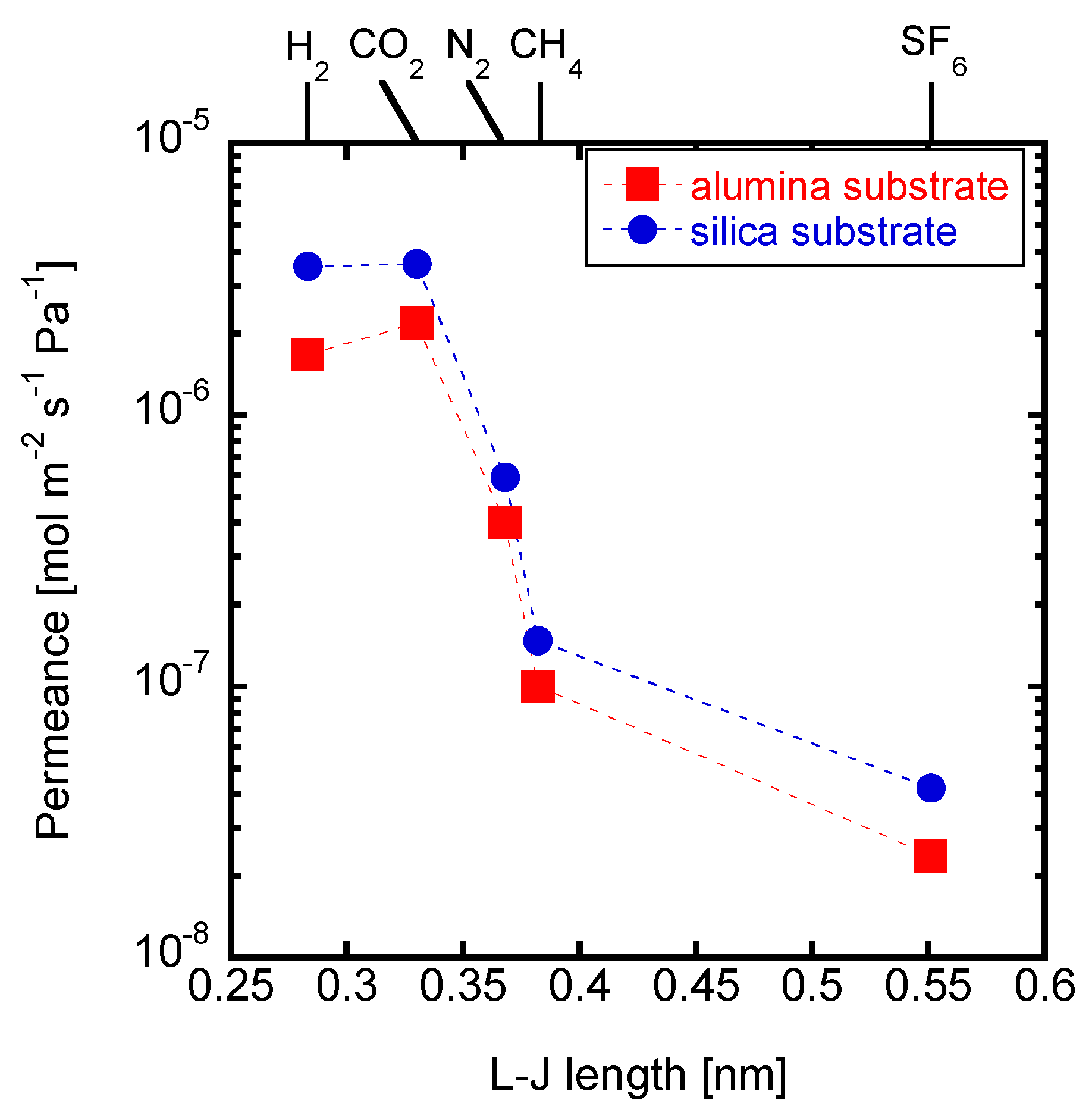
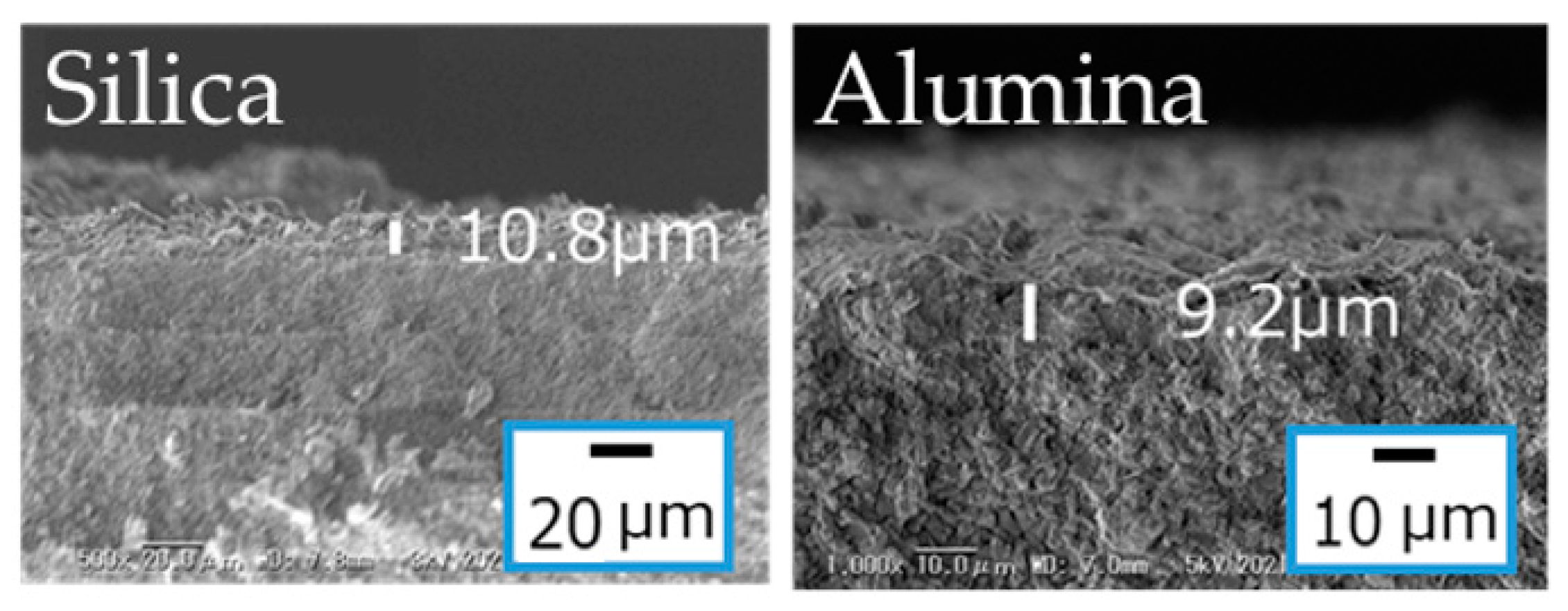
3.4. Effects of the Substrates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Whitfield, M. Accumulation of fossil CO2 in the atmosphere and in the sea. Nature 1974, 247, 523–525. [Google Scholar] [CrossRef]
- Rufford, T.E.; Smart, S.; Watson, G.C.Y.; Graham, B.F.; Boxall, J.; Diniz da Costa, J.C.; May, E.F. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Pet. Sci. Eng. 2012, 94–95, 123–154. [Google Scholar] [CrossRef]
- Behrooz, H.A.; Hoseini, M.; Mahamadzade, M.; Ranjbaran, N. Economic Comparison between Membrane and Adsorption Processes for Separation of CO2 and CH4 Mixture. In Proceedings of the 5th National Conference on New Researches in Chemistry and Chemical Engineering, Tehran, Iran, 4 February 2019. [Google Scholar]
- Belaissaoui, B.; Moullec, Y.L.; Willson, D.; Favre, E. Hybrid membrane cryogenic process for post-combustion CO2 capture. J. Memb. Sci. 2012, 415–416, 424–434. [Google Scholar] [CrossRef]
- Brunetti, A.; Macedonio, F.; Barbieri, G.; Drioli, E. Membrane engineering for environmental protection and sustainable industrial growth: Options for water and gas treatment. Environ. Eng. Res. 2015, 20, 307–328. [Google Scholar] [CrossRef]
- Fard, A.K.; McKay, G.; Buekenhoudt, A.; Sulaiti, H.A.; Motmans, F.; Khraisheh, M.; Atieh, M. Inorganic Membranes: Preparation and Application for Water Treatment and Desalination. Materials 2018, 11, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benfer, S.; Popp, U.; Richter, H.; Siewert, C.; Tomandl, G. Development and characterization of ceramic nanofiltration membranes. Sep. Purif. Technol. 2001, 22–23, 231–237. [Google Scholar] [CrossRef]
- Nomura, M.; Yamaguchi, T.; Nakao, S. Silicalite Membranes Modified by Counter diffusion CVD Technique. Ind. Eng. Chem. Res. 1997, 36, 4217–4223. [Google Scholar] [CrossRef]
- van Veen, H.M.; van Delft, Y.C.; Engelen, C.W.R.; Pex, P.P.A.C. Dewatering of organics by pervaporation with silica membranes. Sep. Purif. Technol. 2001, 22–23, 361–366. [Google Scholar] [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Ravanchi, M.T.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 2014, 828131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, M.; Fujioka, Y.; Yogo, K. Pure silica CHA type zeolite for CO2 separation using pressure swing adsorption at high pressure. J. Mater. Chem. 2012, 22, 20186. [Google Scholar] [CrossRef]
- Kida, K.; Maeta, Y.; Yogo, K. Preparation and gas permeation properties on pure silica CHA-type zeolite membranes. J. Memb. Sci. 2017, 522, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Kean, W. The ‘ideal selectivity’ vs. ‘true selectivity’ for permeation of gas mixtures in nanoporous membranes. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Kida, K.; Maeta, Y.; Yogo, K. Pure silica CHA-type zeolite membranes for dry and humidified CO2/CH4 mixtures separation. Sep. Purif. Technol. 2018, 197, 116–121. [Google Scholar] [CrossRef]
- Yu, L.; Nobandegani, M.S.; Hedlund, J. Industrially relevant CHA membranes for CO2/CH4 separation. J. Memb. Sci. 2022, 641, 119888. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Abe, C.; Natsui, M.; Ikeda, A. Gas Permeation Properties of High-Silica CHA-Type Membrane. Membranes 2021, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Qiu, H.; Zhang, Y.; Tang, X.; Meng, D.; Yang, S.; Guo, W.; Xu, N.; Kong, L.; Zhang, Y.; et al. Seeded synthesis of all-silica CHA zeolites in diluted mother liquor. Micro. Meso. Mater. 2021, 316, 110914. [Google Scholar] [CrossRef]
- Miyamoto, M.; Nakatani, T.; Fujioka, Y.; Yogo, K. Verified synthesis of pure silica CHA-type zeolite in fluoride media. Micro. Meso. Mater. 2015, 206, 67–74. [Google Scholar] [CrossRef]
- Kim, E.; Cai, W.; Baik, H.; Choi, J. Uniform Si-CHA Zeolite Layers Formed by a Selective Sonication-Assisted Deposition Method. Angew. Chem. Int. Ed. 2013, 52, 5280–5284. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, F.; Sun, K.; Jin, X.; Zhang, Y.; Liu, B.; Zhou, R.; Benfer, S.; Popp, U.; Richter, H.; et al. Green Synthesis of highly CO2-selective CHA zeolite membranes in all-silica and fluoride-free solution for CO2/CH4 separations. Energy Fuels 2020, 34, 11307–11314. [Google Scholar] [CrossRef]
- Zarubin, D.P.; Nemkina, N.V. The solubility of amorphous silica in an alkaline aqueous medium at a constant ionic strength. Russ. J. Inorg. Chem. 1990, 35, 31–38. [Google Scholar]
- Franke, M.D.; Ernst, W.R.; Myerson, A.S. Kinetics of dissolution of alumina in acidic solution. Am. Inst. Chem. Eng. 1987, 33, 267–273. [Google Scholar] [CrossRef]
- Sano, T.; Yanagishita, H.; Kiyozumi, Y.; Mizukami, F.; Haraya, K. Separation of ethanol/water mixture by silicalite membrane on pervaporation. J. Memb. Sci. 1994, 95, 221–228. [Google Scholar] [CrossRef]
- Eilertsen, E.A.; Milsen, M.H.; Wendelbo, R.; Olsbye, U.; Lillerud, K.P. Synthesis of high silica CHA zeolites with controlled Si/Al ratio. In Proceedings of the 4th International FEZA Conference, Paris, France, 2–6 September 2008; pp. 265–268. [Google Scholar]
- Imasaka, S.; Nakai, A.; Araki, S.; Yamamoto, H. Synthesis and Gas Permeation of STT-type Zeolite Membranes. J. Jpn. Pet. Inst. 2018, 61, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, Y.; Ikarugi, S.; Oura, K.; Ikeda, A.; Matsuyama, E.; Ono, R.; Tawarayama, H.; Saito, T.; Kuwahara, K.; Nomura, M. MFI zeolite membranes prepared on novel silica substrates. J. Chem. Eng. Jpn. 2015, 48, 891–896. [Google Scholar] [CrossRef]
- Tanizume, S.; Maehara, S.; Ishii, K.; Onoki, T.; Okuno, T.; Tawarayama, H.; Ishikawa, S.; Nomura, M. Reaction of methanol to olefin using a membrane contactor on a silica substrate. Sep. Purif. Tech. 2020, 254, 117647. [Google Scholar] [CrossRef]
- Oleksiak, M.D.; Rimer, J.D. Synthesis of zeolites in the absence of organic structure-directing agents: Factors governing crystal selection and polymorphism. Rev. Chem. Eng. 2014, 30, 1–49. [Google Scholar] [CrossRef]
- Chew, T.L.; Ahmad, A.L. Gas Permeation Properties of Modified SAPO-34 Zeolite Membranes. Procedia Eng. 2016, 148, 1225–1231. [Google Scholar] [CrossRef] [Green Version]
- Grand, J.; Awala, H.; Mintova, S. Mechanism of zeolites crystal growth: New findings and open questions. Cryst. Eng. Comm. 2016, 18, 650–664. [Google Scholar] [CrossRef]
- Nanev, C.N. Relationship between number and sizes of crystals growing in batchcrystallization: Nuclei number density, nucleation kinetics and crystal polydispersity. J. Cryst. Growth 2020, 546, 125786. [Google Scholar] [CrossRef]
- Drummond, D.; De Jonge, A.; Rees, L.V.C. Ion exchange kinetics in zeolite A. J. Phys. Chem. 1983, 87, 1967–1971. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, G.G.d.S.; Takayama, D.; Ishii, K.; Nomura, M.; Onoki, T.; Okuno, T.; Tawarayama, H.; Ishikawa, S. Development of Pure Silica CHA Membranes for CO2 Separation. Membranes 2021, 11, 926. https://doi.org/10.3390/membranes11120926
Figueiredo GGdS, Takayama D, Ishii K, Nomura M, Onoki T, Okuno T, Tawarayama H, Ishikawa S. Development of Pure Silica CHA Membranes for CO2 Separation. Membranes. 2021; 11(12):926. https://doi.org/10.3390/membranes11120926
Chicago/Turabian StyleFigueiredo, Gabriel Gama da Silva, Daishi Takayama, Katsunori Ishii, Mikihiro Nomura, Takamasa Onoki, Takuya Okuno, Hiromasa Tawarayama, and Shinji Ishikawa. 2021. "Development of Pure Silica CHA Membranes for CO2 Separation" Membranes 11, no. 12: 926. https://doi.org/10.3390/membranes11120926
APA StyleFigueiredo, G. G. d. S., Takayama, D., Ishii, K., Nomura, M., Onoki, T., Okuno, T., Tawarayama, H., & Ishikawa, S. (2021). Development of Pure Silica CHA Membranes for CO2 Separation. Membranes, 11(12), 926. https://doi.org/10.3390/membranes11120926




