Lipids in Pathophysiology and Development of the Membrane Lipid Therapy: New Bioactive Lipids
Abstract
1. Introduction
2. Historical Perspective of Membrane Lipid Therapy
2.1. Recognition of the Role of Lipids and Lipid Structures in Molecular and Cellular Events
2.2. Relevance of Membrane Lipid Composition and Structure to Pathophysiology
2.3. Natural Bioactive Lipids and Rational Design of Lipid Bilayer-Targeted Therapies
3. Membrane Lipid Therapy in Oncology
3.1. Lipids in the Pathophysiology of Cancer
3.2. Relevant Lipid-Protein Interactions Involved in Cancer
3.2.1. Ras
3.2.2. EGFR
3.2.3. Signaling Pathways: WNT and Hedgehog
3.3. Lipid Therapies in Cancer
4. Membrane Lipid Therapy for Neurodegenerative Diseases
4.1. Lipids in the Pathophysiology of Neurodegenerative Diseases
4.1.1. Cholesterol and Sphingolipids
4.1.2. Phospholipids and Fatty Acids
4.2. Relevant Lipid-Protein Interactions in Neurodegenerative Diseases
4.2.1. APP
4.2.2. FABPs
4.2.3. α-Synuclein
4.3. Current and Lipid Therapies in Alzheimer’s Disease
5. Membrane Lipid Therapy for Infectious Diseases
5.1. Lipid-Dependent Steps in the Infectious Process as a Candidate for Lipid Therapy
5.1.1. Human Infections
5.1.2. Arthropod-Borne Pathogens
5.2. Lipid-Targeting Therapeutic Approaches for Infectious Disease
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Virchow, R. Physiological and Pathological Tissues. In Cellular Pathology; Pathological Institute of Berlin: Berlin, Germany, 1858; pp. 49–71. [Google Scholar]
- Escribá, P.V.; Ferrer-Montiel, A.V.; Ferragut, J.A.; Gonzalez-Ros, J.M. Role of Membrane Lipids in the Interaction of Daunomycin with Plasma Membranes from Tumor Cells: Implications in Drug-Resistance Phenomena. Biochemistry 1990, 29, 7275–7282. [Google Scholar] [CrossRef]
- Escribá, P.V. Membrane-Lipid Therapy: A New Approach in Molecular Medicine. Trends Mol. Med. 2006, 12, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. Lipid Replacement Therapy: A Nutraceutical Approach for Reducing Cancer-Associated Fatigue and the Adverse Effects of Cancer Therapy While Restoring Mitochondrial Function. Cancer Metastasis Rev. 2010, 29, 543–552. [Google Scholar] [CrossRef]
- Torres, M.; Rosselló, C.A.; Fernández-García, P.; Lladó, V.; Kakhlon, O.; Escribá, P.V. The Implications for Cells of the Lipid Switches Driven by Protein–Membrane Interactions and the Development of Membrane Lipid Therapy. Int. J. Mol. Sci. 2020, 21, 2322. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.; Zhan, Q.; Wang, Q.; Tan, Y.; Fang, C.; Wang, Y.; Zhou, J.; Yang, C.; Li, Y.; Kang, C. PTRF/Cavin-1 Remodels Phospholipid Metabolism to Promote Tumor Proliferation and Suppress Immune Responses in Glioblastoma by Stabilizing CPLA2. Neuro. Oncol. 2021, 23, 387–399. [Google Scholar] [CrossRef]
- Van Gijsel-Bonnello, M.; Acar, N.; Molino, Y.; Bretillon, L.; Khrestchatisky, M.; de Reggi, M.; Gharib, B. Pantethine Alters Lipid Composition and Cholesterol Content of Membrane Rafts, With Down-Regulation of CXCL12-Induced T Cell Migration. J. Cell. Physiol. 2015, 230, 2415–2425. [Google Scholar] [CrossRef]
- Emoto, K.; Kobayashi, T.; Yamaji, A.; Aizawa, H.; Yahara, I.; Inoue, K.; Umeda, M. Redistribution of Phosphatidylethanolamine at the Cleavage Furrow of Dividing Cells during Cytokinesis. Proc. Natl. Acad. Sci. USA 1996, 93, 12867–12872. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Nieuwenhuizen, W.F.; Dullens, H.F.J.; Catani, M.V.; Melino, G.; Veldink, G.A.; Vliegenthart, J.F.G.; AgrO, A.F. Membrane Modifications in Human Erythroleukemia K562 Cells During Induction of Programmed Cell Death by Transforming Growth Factor β1 or Cisplatin. Eur. J. Biochem. 1996, 241, 297–302. [Google Scholar] [CrossRef]
- Chellaiah, M.A.; Biswas, R.S.; Yuen, D.; Alvarez, U.M.; Hruska, K.A. Phosphatidylinositol 3,4,5-Trisphosphate Directs Association of Src Homology 2-Containing Signaling Proteins with Gelsolin. J. Biol. Chem. 2001, 276, 47434–47444. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the Diversity of Membrane Lipid Composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Robertson, J.D. The Structure of Biological Membranes: Current Status. Arch. Intern. Med. 1972, 129, 202–228. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; De Kruijff, B. Lipid Polymorphism and the Functional Roles of Lipids in Biological Membranes. Biochim. Biophys. Acta Rev. Biomembr. 1979, 559, 399–420. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Marcelja, S.; Horn, R.G.; Israelachvili, J.N. Physical Principles of Membrane Organization. Q. Rev. Biophys. 1980, 13, 121–200. [Google Scholar] [CrossRef] [PubMed]
- Escribá, P.V.; Ozaita, A.; Ribas, C.; Miralles, A.; Fodor, E.; Farkas, T.; García-Sevilla, J.A. Role of Lipid Polymorphism in G Protein-Membrane Interactions: Nonlamellar-Prone Phospholipids and Peripheral Protein Binding to Membranes. Proc. Natl. Acad. Sci. USA 1997, 94, 11375–11380. [Google Scholar] [CrossRef]
- Escribá, P.V. Membrane-Lipid Therapy: A Historical Perspective of Membrane-Targeted Therapies–From Lipid Bilayer Structure to the Pathophysiological Regulation of Cells. Biochim. Biophys. Acta 2017, 1859, 1493–1506. [Google Scholar] [CrossRef]
- Vögler, O.; Casas, J.; Capó, D.; Nagy, T.; Borchert, G.; Martorell, G.; Escribá, P.V. The Gβγ Dimer Drives the Interaction of Heterotrimeric Gi Proteins with Nonlamellar Membrane Structures. J. Biol. Chem. 2004, 279, 36540–36545. [Google Scholar] [CrossRef]
- Noguera-Salvà, M.A.; Guardiola-Serrano, F.; Martin, M.L.; Marcilla-Etxenike, A.; Bergo, M.O.; Busquets, X.; Escribá, P.V. Role of the C-Terminal Basic Amino Acids and the Lipid Anchor of the Gγ2 Protein in Membrane Interactions and Cell Localization. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1536–1547. [Google Scholar] [CrossRef]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane Lipid Composition: Effect on Membrane and Organelle Structure, Function and Compartmentalization and Therapeutic Avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef]
- Garofalo, T.; Manganelli, V.; Grasso, M.; Mattei, V.; Ferri, A.; Misasi, R.; Sorice, M. Role of Mitochondrial Raft-like Microdomains in the Regulation of Cell Apoptosis. Apoptosis 2015, 20, 621–634. [Google Scholar] [CrossRef]
- Cascianelli, G.; Villani, M.; Tosti, M.; Marini, F.; Bartoccini, E.; Viola Magni, M.; Albi, E. Lipid Microdomains in Cell Nucleus. Mol. Biol. Cell 2008, 19, 5289–5295. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Bharti, D.; Levental, I. Membrane Heterogeneity beyond the Plasma Membrane. Front. Cell Dev. Biol. 2020, 8, 1186. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Preta, G. Lipids in the Cell: Organisation Regulates Function. Cell. Mol. Life Sci. 2018, 75, 1909–1927. [Google Scholar] [CrossRef]
- Escribá, P.V.; Busquets, X.; Inokuchi, J.; Balogh, G.; Török, Z.; Horváth, I.; Harwood, J.L.; Vígh, L. Membrane Lipid Therapy: Modulation of the Cell Membrane Composition and Structure as a Molecular Base for Drug Discovery and New Disease Treatment. Prog. Lipid Res. 2015, 59, 38–53. [Google Scholar] [CrossRef]
- Bell, R.M.; Ballas, L.M.; Coleman, R.A. Lipid Topogenesis. J. Lipid Res. 1981, 22, 391–403. [Google Scholar] [CrossRef]
- Futerman, A.H.; Riezman, H. The Ins and Outs of Sphingolipid Synthesis. Trends Cell Biol. 2005, 15, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, G.; De Camilli, P. Phosphoinositides in Cell Regulation and Membrane Dynamics. Nature 2006, 443, 651–657. [Google Scholar] [CrossRef]
- Jain, M.; Ngoy, S.; Sheth, S.A.; Swanson, R.A.; Rhee, E.P.; Liao, R.; Clish, C.B.; Mootha, V.K.; Nilsson, R. A Systematic Survey of Lipids across Mouse Tissues. Am. J. Physiol. Endocrinol. Metab. 2014, 306, 854–868. [Google Scholar] [CrossRef]
- Pradas, I.; Huynh, K.; Cabré, R.; Ayala, V.; Meikle, P.J.; Jové, M.; Pamplona, R. Lipidomics Reveals a Tissue-Specific Fingerprint. Front. Physiol. 2018, 9, 1165. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Simons, K. Lipid Rafts as a Membrane-Organizing Principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional Rafts in Cell Membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Doan, J.E.S.; Windmiller, D.A.; Riches, D.W.H. Differential Regulation of TNF-R1 Signaling: Lipid Raft Dependency of P42mapk/Erk2 Activation, but Not NF-ΚB Activation. J. Immunol. 2004, 172, 7654–7660. [Google Scholar] [CrossRef]
- Chen, X.; Xun, K.; Chen, L.; Wang, Y. TNF-α, a Potent Lipid Metabolism Regulator. Cell Biochem. Funct. 2009, 27, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.; França, A.; Florentino, R.; Fonseca, R.; Lima Filho, A.; Vidigal, P.; Oliveira, A.; Dubuquoy, L.; Nathanson, M.; Leite, M. Cholesterol-Enriched Membrane Microdomains Are Needed for Insulin Signaling and Proliferation in Hepatic Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G80–G94. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, B.; Deng, Z.; Fan, Y.; Li, J.; Li, H. Lipid Rafts Promote Trans Fatty Acid-Induced Inflammation in Human Umbilical Vein Endothelial Cells. Lipids 2016, 52, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Resh, M.D. Cholesterol Depletion from the Plasma Membrane Triggers Ligand-Independent Activation of the Epidermal Growth Factor Receptor. J. Biol. Chem. 2002, 277, 49631–49637. [Google Scholar] [CrossRef]
- Roepstorff, K.; Thomsen, P.; Sandvig, K.; Van Deurs, B. Sequestration of Epidermal Growth Factor Receptors in Non-Caveolar Lipid Rafts Inhibits Ligand Binding. J. Biol. Chem. 2002, 277, 18954–18960. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.E.; Bohin, N.; Boerner, J.L. Src Family Kinases Mediate Epidermal Growth Factor Receptor Signaling from Lipid Rafts in Breast Cancer Cells. Cancer Biol. Ther. 2011, 12, 718. [Google Scholar] [CrossRef] [PubMed]
- Balbina, I.S.; Donatello, S.; Nabi, I.R.; Hopkins, A.M. Lipid Rafts as Master Regulators of Breast Cancer Cell Function. In Breast Cancer–Carcinogenesis, Cell Growth and Signalling Pathways; IntechOpen: London, UK, 2011; ISBN 978-953-307-714-7. [Google Scholar]
- Hama, K. The Fine Structure of Some Blood Vessels of the Earthworm, Eisenia Foetida. J. Biophys. Biochem. Cytol. 1960, 7, 717–724. [Google Scholar] [CrossRef]
- Thomsen, P.; Roepstorff, K.; Stahlhut, M.; Van Deurs, B. Caveolae Are Highly Immobile Plasma Membrane Microdomains, Which Are Not Involved in Constitutive Endocytic Trafficking. Mol. Biol. Cell 2002, 13, 238–250. [Google Scholar] [CrossRef]
- Shin, J.S.; Abraham, S.N. Co-Option of Endocytic Functions of Cellular Caveolae by Pathogens. Immunology 2001, 102, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo, M.A.; Lolo, F.N.; Echarri, A. Caveolae: Mechanosensing and Mechanotransduction Devices Linking Membrane Trafficking to Mechanoadaptation. Curr. Opin. Cell Biol. 2021, 68, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; Ash, M.E. Lipid Replacement Therapy: A Natural Medicine Approach to Replacing Damaged Lipids in Cellular Membranes and Organelles and Restoring Function. Biochim. Biophys. Acta 2014, 1838, 1657–1679. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Zendran, I.; Zalesińska, A.; Tarek, M.; Kulbacka, J. Lipid Composition of the Cancer Cell Membrane. J. Bioenerg. Biomembr. 2020, 52, 321–342. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Martin, M.L.; de Almeida, R.F.M.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; Guardiola-Serrano, F.; Lüth, A.; Kleuser, B.; Halver, J.E.; Escribá, P.V. Sphingomyelin and Sphingomyelin Synthase (SMS) in the Malignant Transformation of Glioma Cells and in 2-Hydroxyoleic Acid Therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 19569–19574. [Google Scholar] [CrossRef]
- Martin, M.L.; Barceló-Coblijn, G.; de Almeida, R.F.M.; Noguera-Salvà, M.A.; Terés, S.; Higuera, M.; Liebisch, G.; Schmitz, G.; Busquets, X.; Escribá, P.V. The Role of Membrane Fatty Acid Remodeling in the Antitumor Mechanism of Action of 2-Hydroxyoleic Acid. Biochim. Biophys. Acta 2013, 1828, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.L.; Liebisch, G.; Lehneis, S.; Schmitz, G.; Alonso-Sande, M.; Bestard-Escalas, J.; Lopez, D.H.; García-Verdugo, J.M.; Soriano-Navarro, M.; Busquets, X.; et al. Sustained Activation of Sphingomyelin Synthase by 2-Hydroxyoleic Acid Induces Sphingolipidosis in Tumor Cells. J. Lipid Res. 2013, 54, 1457–1465. [Google Scholar] [CrossRef]
- Marcilla-Etxenike, A.; Martín, M.L.; Noguera-Salvà, M.A.; García-Verdugo, J.M.; Soriano-Navarro, M.; Dey, I.; Escribá, P.V.; Busquets, X. 2-Hydroxyoleic Acid Induces ER Stress and Autophagy in Various Human Glioma Cell Lines. PLoS ONE 2012, 7, e48235. [Google Scholar] [CrossRef]
- Terés, S.; Lladó, V.; Higuera, M.; Barceló-Coblijn, G.; Martin, M.L.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; García-Verdugo, J.M.; Soriano-Navarro, M.; Saus, C.; et al. 2-Hydroxyoleate, a Nontoxic Membrane Binding Anticancer Drug, Induces Glioma Cell Differentiation and Autophagy. Proc. Natl. Acad. Sci. USA 2012, 109, 8489–8494. [Google Scholar] [CrossRef]
- Terés, S.; Lladó, V.; Higuera, M.; Barceló-Coblijn, G.; Martin, M.L.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; García-Verdugo, J.M.; Soriano-Navarro, M.; Saus, C.; et al. Normalization of Sphingomyelin Levels by 2-Hydroxyoleic Acid Induces Autophagic Cell Death of SF767 Cancer Cells. Autophagy 2012, 8, 1542–1544. [Google Scholar] [CrossRef]
- Mollinedo, F.; Gajate, C. Mitochondrial Targeting Involving Cholesterol-Rich Lipid Rafts in the Mechanism of Action of the Antitumor Ether Lipid and Alkylphospholipid Analog Edelfosine. Pharmaceutics 2021, 13, 763. [Google Scholar] [CrossRef]
- Vetica, F.; Sansone, A.; Meliota, C.; Batani, G.; Roberti, M.; Chatgilialoglu, C.; Ferreri, C. Free-Radical-Mediated Formation of Trans-Cardiolipin Isomers, Analytical Approaches for Lipidomics and Consequences of the Structural Organization of Membranes. Biomolecules 2020, 10, 1189. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Ash, M.E. Membrane Lipid Replacement for Chronic Illnesses, Aging and Cancer Using Oral Glycerolphospholipid Formulations with Fructooligosaccharides to Restore Phospholipid Function in Cellular Membranes, Organelles, Cells and Tissues. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1704–1724. [Google Scholar] [CrossRef]
- Maggio, B.; Fidelio, G.D.; Cumar, F.A.; Yu, R.K. Molecular Interactions and Thermotropic Behavior of Glycosphingolipids in Model Membrane Systems. Chem. Phys. Lipids 1986, 42, 49–63. [Google Scholar] [CrossRef]
- Ibarguren, M.; López, D.J.; Encinar, J.A.; González-Ros, J.M.; Busquets, X.; Escribá, P.V. Partitioning of Liquid-Ordered/Liquid-Disordered Membrane Microdomains Induced by the Fluidifying Effect of 2-Hydroxylated Fatty Acid Derivatives. Biochim. Biophys. Acta 2013, 1828, 2553–2563. [Google Scholar] [CrossRef]
- Khmelinskaia, A.; Ibarguren, M.; de Almeida, R.F.M.; López, D.J.; Paixão, V.A.; Ahyayauch, H.; Goñi, F.M.; Escribá, P.V. Changes in Membrane Organization upon Spontaneous Insertion of 2-Hydroxylated Unsaturated Fatty Acids in the Lipid Bilayer. Langmuir 2014, 30, 2117–2128. [Google Scholar] [CrossRef]
- Álvarez, R.; López, D.J.; Casas, J.; Lladó, V.; Higuera, M.; Nagy, T.; Barceló, M.; Busquets, X.; Escribá, P.V. G Protein–Membrane Interactions I: Gαi1 Myristoyl and Palmitoyl Modifications in Protein–Lipid Interactions and Its Implications in Membrane Microdomain Localization. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Virlogeux, A.; Scaramuzzino, C.; Lenoir, S.; Carpentier, R.; Louessard, M.; Genoux, A.; Lino, P.; Hinckelmann, M.-V.; Perrier, A.L.; Humbert, S.; et al. Increasing Brain Palmitoylation Rescues Behavior and Neuropathology in Huntington Disease Mice. Sci. Adv. 2021, 7, eabb0799. [Google Scholar] [CrossRef]
- Erickson, B.N.; Williams, H.H.; Avrin, I.; Lee, P. The lipid distribution of human platelets in health and disease 1. J. Clin. Investig. 1939, 18, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Member, S.; Bruger, M. Experimental Atherosclerosis; the Effect of Feeding Olive Oil on the Absorption and Deposition of Cholesterol. Arch Pathol. 1945, 40, 373–375. [Google Scholar]
- Stueck, G.H.; Rubin, S.H.; Clarke, D.H.; Graef, I.; Ralli, E.P. Studies on Patients with Cirrhosis of the Liver. Am. J. Med. 1948, 5, 188–201. [Google Scholar] [CrossRef]
- Field, B.C.; Gordillo, R.; Scherer, P.E. The Role of Ceramides in Diabetes and Cardiovascular Disease Regulation of Ceramides by Adipokines. Front. Endocrinol. 2020, 11, 569250. [Google Scholar] [CrossRef]
- Escribá, P.V.; Sanchez-Dominguez, J.M.; Alemany, R.; Perona, J.S.; Ruiz-Gutierrez, V. Alteration of Lipids, G Proteins, and PKC in Cell Membranes of Elderly Hypertensives. Hypertension 2003, 41, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E.; Chapman, D. Dynamics of Lipids in Membranes: Heterogeneity and the Role of Cholesterol. FEBS Lett. 1972, 23, 285–297. [Google Scholar] [CrossRef]
- Mabrey, S.; Mateo, P.L.; Sturtevant, J.M. High-Sensitivity Scanning Calorimetric Study of Mixtures of Cholesterol with Dimyristoyl- and Dipalmitoylphosphatidylcholines. Biochemistry 1978, 17, 2464–2468. [Google Scholar] [CrossRef]
- Harder, T.; Simons, K. Caveolae, DIGs, and the Dynamics of Sphingolipid—Cholesterol Microdomains. Curr. Opin. Cell Biol. 1997, 9, 534–542. [Google Scholar] [CrossRef]
- Shimshick, E.J.; McConnell, H.M. Lateral Phase Separation in Phospholipid Membranes. Biochemistry 1973, 12, 2351–2360. [Google Scholar] [CrossRef]
- Phillips, M.C.; Ladbrooke, B.D.; Chapman, D. Molecular Interactions in Mixed Lecithin Systems. Biochim. Biophys. Acta Biomembr. 1970, 196, 35–44. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Bloom, M. Mattress Model of Lipid-Protein Interactions in Membranes. Biophys. J. 1984, 46, 141–153. [Google Scholar] [CrossRef]
- Gomez, G.A.; Daniotti, J.L. Electrical Properties of Plasma Membrane Modulate Subcellular Distribution of K-Ras. FEBS J. 2007, 274, 2210–2228. [Google Scholar] [CrossRef]
- Barceló, F.; Prades, J.; Encinar, J.A.; Funari, S.S.; Vögler, O.; González-Ros, J.M.; Escribá, P.V. Interaction of the C-Terminal Region of the Gγ Protein with Model Membranes. Biophys. J. 2007, 93, 2530–2541. [Google Scholar] [CrossRef]
- Rodríguez-Alfaro, J.A.; Gomez-Fernandez, J.C.; Corbalan-Garcia, S. Role of the Lysine-Rich Cluster of the C2 Domain in the Phosphatidylserine-Dependent Activation of PKCα. J. Mol. Biol. 2004, 335, 1117–1129. [Google Scholar] [CrossRef]
- Pérez-Lara, Á.; Egea-Jiménez, A.L.; Ausili, A.; Corbalán-García, S.; Gómez-Fernández, J.C. The Membrane Binding Kinetics of Full-Length PKCα Is Determined by Membrane Lipid Composition. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2012, 1821, 1434–1442. [Google Scholar] [CrossRef]
- Corbalán-García, S.; Gómez-Fernández, J.C. Classical Protein Kinases C Are Regulated by Concerted Interaction with Lipids: The Importance of Phosphatidylinositol-4,5-Bisphosphate. Biophys. Rev. 2014, 6, 3–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Casas, J.; Ibarguren, M.; Álvarez, R.; Terés, S.; Lladó, V.; Piotto, S.P.; Concilio, S.; Busquets, X.; López, D.J.; Escribá, P.V. G Protein-Membrane Interactions II: Effect of G Protein-Linked Lipids on Membrane Structure and G Protein-Membrane Interactions. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1526–1535. [Google Scholar] [CrossRef]
- Cain, R.J.; Ridley, A.J. Phosphoinositide 3-Kinases in Cell Migration. Biol. Cell 2009, 101, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Norambuena, A.; Schwartz, M.A. Effects of Integrin-Mediated Cell Adhesion on Plasma Membrane Lipid Raft Components and Signaling. Mol. Biol. Cell 2011, 22, 3456–3464. [Google Scholar] [CrossRef]
- Mesa-Galloso, H.; Pedrera, L.; Ros, U. Pore-Forming Proteins: From Defense Factors to Endogenous Executors of Cell Death. Chem. Phys. Lipids 2021, 234, 105026. [Google Scholar] [CrossRef]
- Ros, U.; García-Sáez, A.J. More Than a Pore: The Interplay of Pore-Forming Proteins and Lipid Membranes. J. Membr. Biol. 2015, 248, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Kulma, M.; Anderluh, G. Beyond Pore Formation: Reorganization of the Plasma Membrane Induced by Pore-Forming Proteins. Cell. Mol. Life Sci. 2021, 78, 6229–6249. [Google Scholar] [CrossRef]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.-C.; Shao, F. Pore-Forming Activity and Structural Autoinhibition of the Gasdermin Family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef]
- Schön, P.; García-Sáez, A.J.; Malovrh, P.; Bacia, K.; Anderluh, G.; Schwille, P. Equinatoxin II Permeabilizing Activity Depends on the Presence of Sphingomyelin and Lipid Phase Coexistence. Biophys. J. 2008, 95, 691–698. [Google Scholar] [CrossRef]
- De Colibus, L.; Sonnen, A.F.-P.; Morris, K.J.; Siebert, C.A.; Abrusci, P.; Plitzko, J.; Hodnik, V.; Leippe, M.; Volpi, E.; Anderluh, G.; et al. Structures of Lysenin Reveal a Shared Evolutionary Origin for Pore-Forming Proteins and Its Mode of Sphingomyelin Recognition. Structure 2012, 20, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Kvetkina, A.; Malyarenko, O.; Pavlenko, A.; Dyshlovoy, S.; von Amsberg, G.; Ermakova, S.; Leychenko, E. Sea Anemone Heteractis Crispa Actinoporin Demonstrates In Vitro Anticancer Activities and Prevents HT-29 Colorectal Cancer Cell Migration. Molecules 2020, 25, 5979. [Google Scholar] [CrossRef]
- Ng, T.J.; Teo, M.Y.M.; Liew, D.S.; Effiong, P.E.; Hwang, J.S.; Lim, C.S.Y.; In, L.L.A. Cytotoxic and Apoptosis-Inducing Effects of Wildtype and Mutated Hydra Actinoporin-like Toxin 1 (HALT-1) on Various Cancer Cell Lines. PeerJ 2019, 7, e6639. [Google Scholar] [CrossRef] [PubMed]
- Schachter, D. Fluidity and Function of Hepatocyte Plasma Membranes. Hepatology 1984, 4, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Storck, E.M.; Özbalci, C.; Eggert, U.S. Lipid Cell Biology: A Focus on Lipids in Cell Division. Annu. Rev. Biochem. 2018, 87, 839–869. [Google Scholar] [CrossRef]
- Cauvin, C.; Echard, A. Phosphoinositides: Lipids with Informative Heads and Mastermind Functions in Cell Division. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 832–843. [Google Scholar] [CrossRef]
- Atilla-Gokcumen, G.E.; Muro, E.; Relat-Goberna, J.; Sasse, S.; Bedigian, A.; Coughlin, M.L.; Garcia-Manyes, S.; Eggert, U.S. Dividing Cells Regulate Their Lipid Composition and Localization. Cell 2014, 156, 428–439. [Google Scholar] [CrossRef]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581-594.e5. [Google Scholar] [CrossRef]
- Lewis, K.T.; Maddipati, K.R.; Taatjes, D.J.; Jena, B.P. Neuronal Porosome Lipidome. J. Cell. Mol. Med. 2014, 18, 1927–1937. [Google Scholar] [CrossRef]
- Lewis, K.T.; Maddipati, K.R.; Naik, A.R.; Jena, B.P. Unique Lipid Chemistry of Synaptic Vesicle and Synaptosome Membrane Revealed Using Mass Spectrometry. ACS Chem. Neurosci. 2017, 8, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D.; Astarita, G.; Rapaka, R. A Neuroscientist’s Guide to Lipidomics. Nat. Rev. Neurosci. 2007, 8, 743–754. [Google Scholar] [CrossRef]
- King, C.; Sengupta, P.; Seo, A.Y.; Lippincott-Schwartz, J. ER Membranes Exhibit Phase Behavior at Sites of Organelle Contact. Proc. Natl. Acad. Sci. USA 2020, 117, 7225–7235. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Surma, M.A.; Simons, K. Polarized Sorting and Trafficking in Epithelial Cells. Cell Res. 2012, 22, 793–805. [Google Scholar] [CrossRef]
- Sampaio, J.L.; Gerl, M.J.; Klose, C.; Ejsing, C.S.; Beug, H.; Simons, K.; Shevchenko, A. Membrane Lipidome of an Epithelial Cell Line. Proc. Natl. Acad. Sci. USA 2011, 108, 1903–1907. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in Saturated Fat Intake for Cardiovascular Disease. Cochrane Database Syst. Rev. 2020, 5, CD011737. [Google Scholar] [CrossRef]
- Terés, S.; Barceló-Coblijn, G.; Benet, M.; Alvarez, R.; Bressani, R.; Halver, J.E.; Escribá, P.V. Oleic Acid Content Is Responsible for the Reduction in Blood Pressure Induced by Olive Oil. Proc. Natl. Acad. Sci. USA 2008, 105, 13811–13816. [Google Scholar] [CrossRef]
- Delarue, J. Mediterranean Diet and Cardiovascular Health: An Historical Perspective. Br. J. Nutr. 2021, 1–14. [Google Scholar] [CrossRef]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 Fatty Acids for the Primary and Secondary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2020, 3, CD003177. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Marine Omega-3 (N-3) Fatty Acids for Cardiovascular Health: An Update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef]
- Pelucchi, C.; Bosetti, C.; Negri, E.; Lipworth, L.; La Vecchia, C. Olive Oil and Cancer Risk: An Update of Epidemiological Findings through 2010. Curr. Pharm. Des. 2011, 17, 805–812. [Google Scholar] [CrossRef]
- Xu, Z.-J.; Li, Q.; Ding, L.; Shi, H.-H.; Xue, C.-H.; Mao, X.-Z.; Wang, Y.-M.; Zhang, T.-T. A Comparative Study of the Effects of Phosphatidylserine Rich in DHA and EPA on Aβ-Induced Alzheimer’s Disease Using Cell Models. Food Funct. 2021, 12, 4411–4423. [Google Scholar] [CrossRef]
- Balakrishnan, J.; Kannan, S.; Govindasamy, A. Structured Form of DHA Prevents Neurodegenerative Disorders: A Better Insight into the Pathophysiology and the Mechanism of DHA Transport to the Brain. Nutr. Res. 2021, 85, 119–134. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Gao, X.; Guo, X.-F.; Li, K.-L.; Li, S.; Sinclair, A.J.; Li, D. Effects of Dietary Eicosapentaenoic Acid and Docosahexaenoic Acid Supplementation on Metabolic Syndrome: A Systematic Review and Meta-Analysis of Data from 33 Randomized Controlled Trials. Clin. Nutr. 2021, 40, 4538–4550. [Google Scholar] [CrossRef]
- Pawełczyk, T.; Grancow-Grabka, M.; Żurner, N.; Pawełczyk, A. Omega-3 Fatty Acids Reduce Cardiometabolic Risk in First-Episode Schizophrenia Patients Treated with Antipsychotics: Findings from the OFFER Randomized Controlled Study. Schizophr. Res. 2021, 230, 61–68. [Google Scholar] [CrossRef]
- Ngo Njembe, M.T.; Pachikian, B.; Lobysheva, I.; Van Overstraeten, N.; Dejonghe, L.; Verstraelen, E.; Buchet, M.; Rasse, C.; Gardin, C.; Mignolet, E.; et al. A Three-Month Consumption of Eggs Enriched with ω-3, ω-5 and ω-7 Polyunsaturated Fatty Acids Significantly Decreases the Waist Circumference of Subjects at Risk of Developing Metabolic Syndrome: A Double-Blind Randomized Controlled Trial. Nutrients 2021, 13, 663. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of Action of (n-3) Fatty Acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef] [PubMed]
- Colussi, G.; Catena, C.; Mos, L.; Sechi, L.A. The Metabolic Syndrome and the Membrane Content of Polyunsaturated Fatty Acids in Hypertensive Patients. Metab. Syndr. Relat. Disord. 2015, 13, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Qi, L. Clinical Lipidology Diet and Lifestyle Interventions on Lipids: Combination with Genomics and Metabolomics. Clin. Lipidol. 2014, 9, 417–427. [Google Scholar] [CrossRef]
- Fernández-García, P.; Rosselló, C.A.; Rodríguez-Lorca, R.; Beteta-Göbel, R.; Fernández-Díaz, J.; Lladó, V.; Busquets, X.; Escribá, P.V. The Opposing Contribution of SMS1 and SMS2 to Glioma Progression and Their Value in the Therapeutic Response to 2OHOA. Cancers 2019, 11, 88. [Google Scholar] [CrossRef]
- Torres, M.; Price, S.L.; Fiol-Deroque, M.A.; Marcilla-Etxenike, A.; Ahyayauch, H.; Barceló-Coblijn, G.; Terés, S.; Katsouri, L.; Ordinas, M.; López, D.J.; et al. Membrane Lipid Modifications and Therapeutic Effects Mediated by Hydroxydocosahexaenoic Acid on Alzheimer’s Disease. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Llado, V.; Lopez, D.J.; Ibarguren, M.; Alonso, M.; Soriano, J.B.; Escriba, P.V.; Busquets, X. Regulation of the Cancer Cell Membrane Lipid Composition by NaCHOleate: Effects on Cell Signaling and Therapeutical Relevance in Glioma. Biochim. Biophys Acta 2014, 1838, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Dick Katherine, J.; Eckhardt, M.; Paisán-Ruiz, C.; Alshehhi Aisha, A.; Proukakis, C.; Sibtain Naomi, A.; Maier, H.; Sharifi, R.; Patton Michael, A.; Bashir, W.; et al. Mutation of FA2H Underlies a Complicated Form of Hereditary Spastic Paraplegia (SPG35). Hum. Mutat. 2010, 31, E1251–E1260. [Google Scholar] [CrossRef]
- Garone, C.; Pippucci, T.; Cordelli, D.M.; Zuntini, R.; Castegnaro, G.; Marconi, C.; Graziano, C.; Marchiani, V.; Verrotti, A.; Seri, M.; et al. FA2H-Related Disorders: A Novel c.270+3A>T Splice-Site Mutation Leads to a Complex Neurodegenerative Phenotype. Dev. Med. Child Neurol. 2011, 53, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Camara-Lemarroy, C.R.; Gonzalez-Moreno, E.I.; Guzman-de la Garza, F.J.; Fernandez-Garza, N.E. Arachidonic Acid Derivatives and Their Role in Peripheral Nerve Degeneration and Regeneration. Sci. World J. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- He, Z.; Zhang, R.; Jiang, F.; Zhang, H.; Zhao, A.; Xu, B.; Jin, L.; Wang, T.; Jia, W.; Jia, W.; et al. FADS1-FADS2 Genetic Polymorphisms Are Associated with Fatty Acid Metabolism through Changes in DNA Methylation and Gene Expression. Clin. Epigenetics 2018, 10, 1–13. [Google Scholar] [CrossRef]
- Phillis, J.W.; Horrocks, L.A.; Farooqui, A.A. Cyclooxygenases, Lipoxygenases, and Epoxygenases in CNS: Their Role and Involvement in Neurological Disorders. Brain Res. Rev. 2006, 52, 201–243. [Google Scholar] [CrossRef]
- Das, U.N. “Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules 2021, 11, 241. [Google Scholar] [CrossRef]
- Lopez, D.H.; Fiol-Deroque, M.A.; Noguera-Salvà, M.A.; Terés, S.; Campana, F.; Piotto, S.; Castro, J.A.; Mohaibes, R.J.; Escribá, P.V.; Busquets, X. 2-Hydroxy Arachidonic Acid: A New Non-Steroidal Anti-Inflammatory Drug. PLoS ONE 2013, 8, e72052. [Google Scholar] [CrossRef]
- Avila-Martin, G.; Mata-Roig, M.; Galán-Arriero, I.; Taylor, J.S.; Busquets, X.; Escribá, P.V. Treatment with Albumin-Hydroxyoleic Acid Complex Restores Sensorimotor Function in Rats with Spinal Cord Injury: Efficacy and Gene Expression Regulation. PLoS ONE 2017, 12, e0189151. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.J. Obesity and Type 2 Diabetes: Which Patients Are at Risk? Diabetes Obes. Metab. 2012, 14, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Wang, B.; Palladino, E.N.D.; de Aguiar Vallim, T.Q.; Ford, D.A.; Tontonoz, P. ER Phospholipid Composition Modulates Lipogenesis during Feeding and in Obesity. J. Clin. Investig. 2017, 127, 3640–3651. [Google Scholar] [CrossRef]
- Perona, J.S. Membrane Lipid Alterations in the Metabolic Syndrome and the Role of Dietary Oils. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1690–1703. [Google Scholar] [CrossRef] [PubMed]
- Schuller, A.; Solis-Herruzo, J.A.; Moscat, J.; Fernandez-Checa, J.C.; Municio, A.M. The Fluidity of Liver Plasma Membranes from Patients with Different Types of Liver Injury. Hepatology 1986, 6, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.S.; Bruckdorfer, K.R.; Day, R.C.; McIntyre, N. Decreased Erythrocyte Membrane Fluidity and Altered Lipid Composition in Human Liver Disease. J. Lipid Res. 1982, 23, 124–132. [Google Scholar] [CrossRef]
- Pfisterer, S.G.; Peränen, J.; Ikonen, E. LDL-Cholesterol Transport to the Endoplasmic Reticulum: Current Concepts. Curr. Opin. Lipidol. 2016, 27, 282–287. [Google Scholar] [CrossRef]
- Imamura, T.; Doi, Y.; Arima, H.; Yonemoto, K.; Hata, J.; Kubo, M.; Tanizaki, Y.; Ibayashi, S.; Iida, M.; Kiyohara, Y. LDL Cholesterol and the Development of Stroke Subtypes and Coronary Heart Disease in a General Japanese Population the Hisayama Study. Stroke 2009, 40, 382–388. [Google Scholar] [CrossRef]
- Itabe, H.; Obama, T.; Kato, R. The Dynamics of Oxidized LDL during Atherogenesis. J. Lipids 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Liu, W.; Yin, Y.; Zhou, Z.; He, M.; Dai, Y. OxLDL-Induced IL-1β Secretion Promoting Foam Cells Formation Was Mainly via CD36 Mediated ROS Production Leading to NLRP3 Inflammasome Activation. Inflamm. Res. 2014, 63, 33–43. [Google Scholar] [CrossRef]
- Nie, J.; Yang, J.; Wei, Y.; Wei, X. The Role of Oxidized Phospholipids in the Development of Disease. Mol. Aspects Med. 2020, 76, 100909. [Google Scholar] [CrossRef] [PubMed]
- Vogl, F.; Humpolícková, J.; Amaro, M.; Koller, D.; Köfeler, H.; Zenzmaier, E.; Hof, M.; Hermetter, A. Role of Protein Kinase C δ in Apoptotic Signaling of Oxidized Phospholipids in RAW 264.7 Macrophages. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Juliano, R.A.; Mason, R.P. Eicosapentaenoic Acid (EPA) Has Optimal Chain Length and Degree of Unsaturation to Inhibit Oxidation of Small Dense LDL and Membrane Cholesterol Domains as Compared to Related Fatty Acids in Vitro. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183254. [Google Scholar] [CrossRef]
- Murphy, D.J.; Vance, J. Mechanisms of Lipid-Body Formation. Trends Biochem. Sci. 1999, 24, 109–115. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, P. The Lipid Droplet: A Conserved Cellular Organelle. Protein Cell 2017, 8, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Carvalho, P. Dynamics and Functions of Lipid Droplets. Nat. Rev. Mol. Cell Biol. 2018, 20, 137–155. [Google Scholar] [CrossRef]
- Funari, S.S.; Barceló, F.; Escribá, P.V. Effects of Oleic Acid and Its Congeners, Elaidic and Stearic Acids, on the Structural Properties of Phosphatidylethanolamine Membranes. J. Lipid Res. 2003, 44, 567–575. [Google Scholar] [CrossRef]
- Yang, Q.; Alemany, R.; Casas, J.; Kitajka, K.; Lanier, S.M.; Escriba, P.V. Influence of the Membrane Lipid Structure on Signal Processing via G Protein-Coupled Receptors. Mol Pharmacol 2005, 68, 210–217. [Google Scholar] [CrossRef]
- Serhan, C.N.; Gotlinger, K.; Hong, S.; Arita, M. Resolvins, Docosatrienes, and Neuroprotectins, Novel Omega-3-Derived Mediators, and Their Aspirin-Triggered Endogenous Epimers: An Overview of Their Protective Roles in Catabasis. Prostaglandins Other Lipid Mediat. 2004, 73, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.L.; Bazan, H.E.P. Docosanoid Signaling Modulates Corneal Nerve Regeneration: Effect on Tear Secretion, Wound Healing, and Neuropathic Pain. J. Lipid Res. 2021, 62, 100033. [Google Scholar] [CrossRef]
- Muñoz-Guardiola, P.; Casas, J.; Megías-Roda, E.; Solé, S.; Perez-Montoyo, H.; Yeste-Velasco, M.; Erazo, T.; Diéguez-Martínez, N.; Espinosa-Gil, S.; Muñoz-Pinedo, C.; et al. The Anti-Cancer Drug ABTL0812 Induces ER Stress-Mediated Cytotoxic Autophagy by Increasing Dihydroceramide Levels in Cancer Cells. Autophagy 2021, 17, 1349–1366. [Google Scholar] [CrossRef]
- París-Coderch, L.; Soriano, A.; Jiménez, C.; Erazo, T.; Muñoz-Guardiola, P.; Masanas, M.; Antonelli, R.; Boloix, A.; Alfón, J.; Pérez-Montoyo, H.; et al. The Antitumour Drug ABTL0812 Impairs Neuroblastoma Growth through Endoplasmic Reticulum Stress-Mediated Autophagy and Apoptosis. Cell Death Dis. 2020, 11, 773. [Google Scholar] [CrossRef]
- Hernando, S.; Requejo, C.; Herran, E.; Ruiz-Ortega, J.A.; Morera-Herreras, T.; Lafuente, J.V.; Gainza, E.; Pedraz, J.L.; Igartua, M.; Hernandez, R.M. Beneficial Effects of N-3 Polyunsaturated Fatty Acids Administration in a Partial Lesion Model of Parkinson’s Disease: The Role of Glia and NRf2 Regulation. Neurobiol. Dis. 2019, 121, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Vögler, O.; López-Bellan, A.; Alemany, R.; Tofé, S.; González, M.; Quevedo, J.; Pereg, V.; Barceló, F.; Escriba, P.V. Structure–Effect Relation of C18 Long-Chain Fatty Acids in the Reduction of Body Weight in Rats. Int. J. Obes. 2008, 32, 464–473. [Google Scholar] [CrossRef]
- Lossos, A.; Barash, V.; Soffer, D.; Argov, Z.; Gomori, M.; Ben-Nariah, Z.; Abramsky, O.; Steiner, I. Hereditary Branching Enzyme Dysfunction in Adult Polyglucosan Body Disease: A Possible Metabolic Cause in Two Patients. Ann. Neurol. 1991, 30, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Lossos, A.; Meiner, Z.; Barash, V.; Soffer, D.; Schlesinger, I.; Abramsky, O.; Argov, Z.; Shpitzen, S.; Meiner, V. Adult Polyglucosan Body Disease in Ashkenazi Jewish Patients Carrying the Tyr329 Ser Mutation in the Glycogen-Branching Enzyme Gene. Ann. Neurol. 1998, 44, 867–872. [Google Scholar] [CrossRef]
- Orhan Akman, H.; Emmanuele, V.; Kurt, Y.G.; Kurt, B.; Sheiko, T.; DiMauro, S.; Craigen, W.J. A Novel Mouse Model That Recapitulates Adult-Onset Glycogenosis Type 4. Hum. Mol. Genet. 2015, 24, 6801–6810. [Google Scholar] [CrossRef] [PubMed]
- Wierzba-Bobrowicz, T.; Lewandowska, E.; Stepien, T.; Modzelewska, J. Immunohistochemical and Ultrastructural Changes in the Brain in Probable Adult Glycogenosis Type IV: Adult Polyglucosan Body Disease. Folia Neuropathol 2008, 46, 165–175. [Google Scholar]
- Alvarez, R.; Casas, J.; López, D.J.; Ibarguren, M.; Suari-Rivera, A.; Terés, S.; Guardiola-Serrano, F.; Lossos, A.; Busquets, X.; Kakhlon, O.; et al. Triacylglycerol Mimetics Regulate Membrane Interactions of Glycogen Branching Enzyme: Implications for Therapy. J. Lipid Res. 2017, 58, 1598–1612. [Google Scholar] [CrossRef] [PubMed]
- Kakhlon, O.; Ferreira, I.; Solmesky, L.J.; Khazanov, N.; Lossos, A.; Alvarez, R.; Yetil, D.; Pampou, S.; Weil, M.; Senderowitz, H.; et al. Guaiacol as a Drug Candidate for Treating Adult Polyglucosan Body Disease. JCI Insight 2018, 3, e99694. [Google Scholar] [CrossRef]
- Gombos, I.; Crul, T.; Piotto, S.; Güngör, B.; Török, Z.; Balogh, G.; Péter, M.; Slotte, J.P.; Campana, F.; Pilbat, A.-M.; et al. Membrane-Lipid Therapy in Operation: The HSP Co-Inducer BGP-15 Activates Stress Signal Transduction Pathways by Remodeling Plasma Membrane Rafts. PLoS ONE 2011, 6, e28818. [Google Scholar] [CrossRef]
- Wachal, Z.; Szilágyi, A.; Takács, B.; Szabó, A.M.; Priksz, D.; Bombicz, M.; Szilvássy, J.; Juhász, B.; Szilvássy, Z.; Varga, B. Improved Survival and Retinal Function of Aging ZDF Rats in Long-Term, Uncontrolled Diabetes by BGP-15 Treatment. Front. Pharmacol. 2021, 12, 650207. [Google Scholar] [CrossRef]
- Sintov, A.C.; Berkovich, L.; Ben-Shabat, S. Inhibition of Cancer Growth and Induction of Apoptosis by BGP-13 and BGP-15, New Calcipotriene-Derived Vitamin D3 Analogs, in-Vitro and in-Vivo Studies. Investig. New Drugs 2013, 31, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Covic, L.; Misra, M.; Badar, J.; Singh, C.; Kuliopulos, A. Pepducin-Based Intervention of Thrombin-Receptor Signaling and Systemic Platelet Activation. Nat. Med. 2002, 8, 1161–1165. [Google Scholar] [CrossRef]
- Yang, E.; Boire, A.; Agarwal, A.; Nguyen, N.; O’Callaghan, K.; Tu, P.; Kuliopulos, A.; Covic, L. Blockade of PAR1 Signaling with Cell-Penetrating Pepducins Inhibits Akt Survival Pathways in Breast Cancer Cells and Suppresses Tumor Survival and Metastasis. Cancer Res. 2009, 69, 6223–6231. [Google Scholar] [CrossRef]
- Gurbel, P.A.; Bliden, K.P.; Turner, S.E.; Tantry, U.S.; Gesheff, M.G.; Barr, T.P.; Covic, L.; Kuliopulos, A. Cell-Penetrating Pepducin Therapy Targeting PAR1 in Subjects with Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 189–197. [Google Scholar] [CrossRef]
- Panettieri, R.A.; Pera, T.; Liggett, S.B.; Benovic, J.L.; Penn, R.B. Pepducins as a Potential Treatment Strategy for Asthma and COPD. Curr. Opin. Pharmacol. 2018, 40, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Dorlo, T.P.C.; Balasegaram, M.; Beijnen, J.H.; de Vries, P.J. Miltefosine: A Review of Its Pharmacology and Therapeutic Efficacy in the Treatment of Leishmaniasis. J. Antimicrob. Chemother. 2012, 67, 2576–2597. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.A.; Mendanha, S.A.; Hansen, D.; Alonso, A. Interaction of Miltefosine with the Lipid and Protein Components of the Erythrocyte Membrane. J. Pharm. Sci. 2013, 102, 1661–1669. [Google Scholar] [CrossRef]
- Zulueta Díaz, Y.d.l.M.; Ambroggio, E.E.; Fanani, M.L. Miltefosine Inhibits the Membrane Remodeling Caused by Phospholipase Action by Changing Membrane Physical Properties. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183407. [Google Scholar] [CrossRef]
- Castro, B.M.; Fedorov, A.; Hornillos, V.; Delgado, J.; Acuña, A.U.; Mollinedo, F.; Prieto, M. Edelfosine and Miltefosine Effects on Lipid Raft Properties: Membrane Biophysics in Cell Death by Antitumor Lipids. J. Phys. Chem. B 2013, 117, 7929–7940. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Reprogramming of Fatty Acid Metabolism in Cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Cantley, L.C.; Neel, B.G. New Insights into Tumor Suppression: PTEN Suppresses Tumor Formation by Restraining the Phosphoinositide 3-Kinase/AKT Pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 4240–4245. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.A.; Iliopoulos, D.; Joshi, A.; Zhang, Y.; Jaeger, S.A.; Bulyk, M.; Tsichlis, P.N.; Shirley Liu, X.; Struhl, K. A Transcriptional Signature and Common Gene Networks Link Cancer with Lipid Metabolism and Diverse Human Diseases. Cancer Cell 2010, 17, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Denkert, C.; Lehtinen, L.; Müller, B.; Brockmöller, S.; Seppänen-Laakso, T.; Budczies, J.; Bucher, E.; Yetukuri, L.; Castillo, S.; et al. Novel Theranostic Opportunities Offered by Characterization of Altered Membrane Lipid Metabolism in Breast Cancer Progression. Cancer Res. 2011, 71, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid Metabolic Reprogramming in Cancer Cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef]
- Sara Woodman; Kyoungtae Kim Membrane Lipids: Implication for Diseases and Membrane Trafficking. SM J. Biol. 2017, 3, 1016.
- Björkholm, P.; Ernst, A.M.; Hacke, M.; Wieland, F.; Brügger, B.; von Heijne, G. Identification of Novel Sphingolipid-Binding Motifs in Mammalian Membrane Proteins. Biochim. Biophys. Acta Biomembr. 2014, 1838, 2066–2070. [Google Scholar] [CrossRef]
- Weiser, B.P.; Salari, R.; Eckenhoff, R.G.; Brannigan, G. Computational Investigation of Cholesterol Binding Sites on Mitochondrial VDAC. J. Phys. Chem. B 2014, 118, 9852–9860. [Google Scholar] [CrossRef]
- Stafford, J.H.; Thorpe, P.E. Increased Exposure of Phosphatidylethanolamine on the Surface of Tumor Vascular Endothelium. Neoplasia 2011, 13, 299–308. [Google Scholar] [CrossRef]
- Zwaal, R.F.A.; Comfurius, P.; Bevers, E.M. Surface Exposure of Phosphatidylserine in Pathological Cells. Cell. Mol. Life Sci. 2005, 62, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.; ten Hagen, T.L.M. Cell Membrane Modulation as Adjuvant in Cancer Therapy. Cancer Treat. Rev. 2017, 52, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, N.; Fialho, A. Perturbing the Dynamics and Organization of Cell Membrane Components: A New Paradigm for Cancer-Targeted Therapies. Int. J. Mol. Sci. 2018, 19, 3871. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, F.; Rosa, C.; Cicalini, I.; Sacchetta, P.; Del Boccio, P.; Genovesi, D.; Pieragostino, D. Advances in Lipidomics for Cancer Biomarkers Discovery. Int. J. Mol. Sci. 2016, 17, 1992. [Google Scholar] [CrossRef] [PubMed]
- Burgert, A.; Schlegel, J.; Bécam, J.; Doose, S.; Bieberich, E.; Schubert-Unkmeir, A.; Sauer, M. Characterization of Plasma Membrane Ceramides by Super-Resolution Microscopy. Angew. Chemie Int. Ed. 2017, 56, 6131–6135. [Google Scholar] [CrossRef]
- Head, B.P.; Patel, H.H.; Insel, P.A. Interaction of Membrane/Lipid Rafts with the Cytoskeleton: Impact on Signaling and Function. Biochim. Biophys. Acta Biomembr. 2014, 1838, 532–545. [Google Scholar] [CrossRef]
- Monaco, M.E. Fatty Acid Metabolism in Breast Cancer Subtypes. Oncotarget 2017, 8, 29487–29500. [Google Scholar] [CrossRef]
- Qu, L.; Pan, C.; He, S.-M.; Lang, B.; Gao, G.-D.; Wang, X.-L.; Wang, Y. The Ras Superfamily of Small GTPases in Non-Neoplastic Cerebral Diseases. Front. Mol. Neurosci. 2019, 12, 121. [Google Scholar] [CrossRef]
- Muñoz-Maldonado, C.; Zimmer, Y.; Medová, M. A Comparative Analysis of Individual RAS Mutations in Cancer Biology. Front. Oncol. 2019, 9, 1088. [Google Scholar] [CrossRef]
- Prior, I.A.; Lewis, P.D.; Mattos, C. A Comprehensive Survey of Ras Mutations in Cancer. Cancer Res. 2012, 72, 2457–2467. [Google Scholar] [CrossRef]
- Campbell, S.L.; Philips, M.R. Post-Translational Modification of RAS Proteins. Curr. Opin. Struct. Biol. 2021, 71, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Osaka, N.; Hirota, Y.; Ito, D.; Ikeda, Y.; Kamata, R.; Fujii, Y.; Chirasani, V.R.; Campbell, S.L.; Takeuchi, K.; Senda, T.; et al. Divergent Mechanisms Activating RAS and Small GTPases through Post-Translational Modification. Front. Mol. Biosci. 2021, 8, 642. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Bai, W.; Bepler, G.; Zhang, X. Activation of Ras by Post-Translational Modifications. In Conquering RAS; Academic Press: Cambridge, MA, USA, 2017; pp. 97–118. [Google Scholar]
- Busquets-Hernández, C.; Triola, G. Palmitoylation as a Key Regulator of Ras Localization and Function. Front. Mol. Biosci. 2021, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Niv, H.; Gutman, O.; Kloog, Y.; Henis, Y.I. Activated K-Ras and H-Ras Display Different Interactions with Saturable Nonraft Sites at the Surface of Live Cells. J. Cell Biol. 2002, 157, 865–872. [Google Scholar] [CrossRef]
- Vogel, A.; Nikolaus, J.; Weise, K.; Triola, G.; Waldmann, H.; Winter, R.; Herrmann, A.; Huster, D. Interaction of the Human N-Ras Protein with Lipid Raft Model Membranes of Varying Degrees of Complexity. Biol. Chem. 2014, 395, 779–789. [Google Scholar] [CrossRef]
- Lin, D.T.S.; Davis, N.G.; Conibear, E. Targeting the Ras Palmitoylation/Depalmitoylation Cycle in Cancer. Biochem. Soc. Trans. 2017, 45, 913–921. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal Growth Factor Receptor (EGFR) Signaling in Cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Kim, D.H.; Triet, H.M.; Ryu, S.H. Regulation of EGFR Activation and Signaling by Lipids on the Plasma Membrane. Prog. Lipid Res. 2021, 83, 101115. [Google Scholar] [CrossRef]
- Li, X.; Ortiz, M.A.; Kotula, L. The Physiological Role of Wnt Pathway in Normal Development and Cancer. Exp. Biol. Med. 2020, 245, 411–426. [Google Scholar] [CrossRef]
- Sezgin, E.; Azbazdar, Y.; Ng, X.W.; Teh, C.; Simons, K.; Weidinger, G.; Wohland, T.; Eggeling, C.; Ozhan, G. Binding of Canonical Wnt Ligands to Their Receptor Complexes Occurs in Ordered Plasma Membrane Environments. FEBS J. 2017, 284, 2513–2526. [Google Scholar] [CrossRef]
- Nusse, R. Disarming Wnt. Nat. Cell Biol. 2015, 519, 163–164. [Google Scholar] [CrossRef]
- Riitano, G.; Manganelli, V.; Capozzi, A.; Mattei, V.; Recalchi, S.; Martellucci, S.; Longo, A.; Misasi, R.; Garofalo, T.; Sorice, M. LRP6 Mediated Signal Transduction Pathway Triggered by Tissue Plasminogen Activator Acts through Lipid Rafts in Neuroblastoma Cells. J. Cell Commun. Signal. 2020, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Kurayoshi, M.; Yamamoto, H.; Izumi, S.; Kikuchi, A. Post-Translational Palmitoylation and Glycosylation of Wnt-5a Are Necessary for Its Signalling. Biochem. J. 2007, 402, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Komekado, H.; Yamamoto, H.; Chiba, T.; Kikuchi, A. Glycosylation and Palmitoylation of Wnt-3a Are Coupled to Produce an Active Form of Wnt-3a. Genes Cells 2007, 12, 521–534. [Google Scholar] [CrossRef]
- Montagnani, V.; Stecca, B. Role of Protein Kinases in Hedgehog Pathway Control and Implications for Cancer Therapy. Cancers 2019, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Resh, M.D. Palmitoylation of Hedgehog Proteins by Hedgehog Acyltransferase: Roles in Signalling and Disease. Open Biol. 2021, 11, 200414. [Google Scholar] [CrossRef] [PubMed]
- Blassberg, R.; Jacob, J. Lipid Metabolism Fattens up Hedgehog Signaling. BMC Biol. 2017, 15, 1–14. [Google Scholar] [CrossRef]
- Pietrobono, S.; Stecca, B. Targeting the Oncoprotein Smoothened by Small Molecules: Focus on Novel Acylguanidine Derivatives as Potent Smoothened Inhibitors. Cells 2018, 7, 272. [Google Scholar] [CrossRef]
- Long, J.; Zhang, C.-J.; Zhu, N.; Du, K.; Yin, Y.-F.; Tan, X.; Liao, D.-F.; Qin, L. Lipid Metabolism and Carcinogenesis, Cancer Development. Am. J. Cancer Res. 2018, 8, 778–791. [Google Scholar]
- Srivatsav, A.T.; Mishra, M.; Kapoor, S. Small-Molecule Modulation of Lipid-Dependent Cellular Processes against Cancer: Fats on the Gunpoint. BioMed Res. Int. 2018, 2018, 1–17. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Chan, K.-G.; Pusparajah, P.; Lee, W.-L.; Chuah, L.-H.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Targeting Membrane Lipid a Potential Cancer Cure? Front. Pharmacol. 2017, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- van der Hoeven, D.; Cho, K.; Zhou, Y.; Ma, X.; Chen, W.; Naji, A.; Montufar-Solis, D.; Zuo, Y.; Kovar, S.E.; Levental, K.R.; et al. Sphingomyelin Metabolism Is a Regulator of K-Ras Function. Mol. Cell. Biol. 2018, 38, e00373-17. [Google Scholar] [CrossRef]
- Xie, G.; Wang, Z.; Chen, Y.; Zhang, S.; Feng, L.; Meng, F.; Yu, Z. Dual Blocking of PI3K and MTOR Signaling by NVP-BEZ235 Inhibits Proliferation in Cervical Carcinoma Cells and Enhances Therapeutic Response. Cancer Lett. 2017, 388, 12–20. [Google Scholar] [CrossRef]
- Soler, A.; Figueiredo, A.M.; Castel, P.; Martin, L.; Monelli, E.; Angulo-Urarte, A.; Milà-Guasch, M.; Viñals, F.; Baselga, J.; Casanovas, O.; et al. Therapeutic Benefit of Selective Inhibition of P110α PI3-Kinase in Pancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2016, 22, 5805–5817. [Google Scholar] [CrossRef]
- Manara, M.C.; Nicoletti, G.; Zambelli, D.; Ventura, S.; Guerzoni, C.; Landuzzi, L.; Lollini, P.-L.; Maira, S.-M.; García-Echeverría, C.; Mercuri, M.; et al. NVP-BEZ235 as a New Therapeutic Option for Sarcomas. Clin. Cancer Res. 2010, 16, 530–540. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Ghosh, S.; Sen, T.; Shadab, M.; Banerjee, I.; Basu, S.; Ali, N. A Novel Therapeutic Strategy for Cancer Using Phosphatidylserine Targeting Stearylamine-Bearing Cationic Liposomes. Mol. Ther. Nucleic Acids 2018, 10, 9–27. [Google Scholar] [CrossRef]
- Desai, T.J.; Udugamasooriya, D.G. A Comprehensive Lipid Binding and Activity Validation of a Cancer-Specific Peptide-Peptoid Hybrid PPS1. Biochem. Biophys. Res. Commun. 2017, 486, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; He, G.; Tang, D.; Xiong, L.; Wen, Y.; Miao, X.; Hong, Z.; Yao, H.; Chen, C.; Yan, S.; et al. Lovastatin Inhibits Cancer Stem Cells and Sensitizes to Chemo- and Photodynamic Therapy in Nasopharyngeal Carcinoma. J. Cancer 2017, 8, 1655–1664. [Google Scholar] [CrossRef]
- Zhao, Y.; He, L.; Wang, T.; Zhu, L.; Yan, N. 2-Hydroxypropyl-β-Cyclodextrin Regulates the Epithelial to Mesenchymal Transition in Breast Cancer Cells by Modulating Cholesterol Homeostasis and Endoplasmic Reticulum Stress. Metabolites 2021, 11, 562. [Google Scholar] [CrossRef]
- Borgquist, S.; Bjarnadottir, O.; Kimbung, S.; Ahern, T.P. Statins: A Role in Breast Cancer Therapy? J. Intern. Med. 2018, 284, 346–357. [Google Scholar] [CrossRef]
- Smorenburg, C.H.; Seynaeve, C.; Bontenbal, M.; Planting, A.S.; Sindermann, H.; Verweij, J. Phase II Study of Miltefosine 6% Solution as Topical Treatment of Skin Metastases in Breast Cancer Patients. Anticancer Drugs 2000, 11, 825–828. [Google Scholar] [CrossRef]
- Teixeira, S.F.; Rodrigues, C.P.; Costa, C.J.S.; Pettinati, T.N.; de Azevedo, R.A.; Mambelli, L.I.; Jorge, S.D.; Ramos, R.N.; Ferro, E.S.; Barbuto, J.A.M.; et al. Edelfosine: An Antitumor Drug Prototype. Anticancer Agents Med. Chem. 2018, 18, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Garizo, A.R.; Coelho, L.F.; Pinto, S.; Dias, T.P.; Fernandes, F.; Bernardes, N.; Fialho, A.M. The Azurin-Derived Peptide CT-P19LC Exhibits Membrane-Active Properties and Induces Cancer Cell Death. Biomedicines 2021, 9, 1194. [Google Scholar] [CrossRef] [PubMed]
- Guardiola-Serrano, F.; Beteta-Göbel, R.; Rodríguez-Lorca, R.; Ibarguren, M.; López, D.J.; Terés, S.; Alvarez, R.; Alonso-Sande, M.; Busquets, X.; Escribá, P.V. The Novel Anticancer Drug Hydroxytriolein Inhibits Lung Cancer Cell Proliferation via a Protein Kinase C α–and Extracellular Signal-Regulated Kinase 1/2–Dependent Mechanism. J. Pharmacol. Exp. Ther. 2015, 354, 213–224. [Google Scholar] [CrossRef]
- Guardiola-Serrano, F.; Beteta-Göbel, R.; Rodríguez-Lorca, R.; Ibarguren, M.; López, D.J.; Terés, S.; Alonso-Sande, M.; Higuera, M.; Torres, M.; Busquets, X.; et al. The Triacylglycerol, Hydroxytriolein, Inhibits Triple Negative Mammary Breast Cancer Cell Proliferation through a Mechanism Dependent on Dihydroceramide and Akt. Oncotarget 2019, 10, 2486–2507. [Google Scholar] [CrossRef]
- Beteta-Göbel, R.; Fernández-Díaz, J.; Arbona-González, L.; Rodríguez-Lorca, R.; Torres, M.; Busquets, X.; Fernández-García, P.; Escribá, P.V.; Lladó, V. The Novel Antitumor Compound HCA Promotes Glioma Cell Death by Inducing Endoplasmic Reticulum Stress and Autophagy. Cancers 2021, 13, 4290. [Google Scholar] [CrossRef] [PubMed]
- Grilley-Olson, J.E.; Weiss, J.; Ivanova, A.; Villaruz, L.C.; Moore, D.T.; Stinchcombe, T.E.; Lee, C.; Shan, J.S.; Socinski, M.A. Phase Ib Study of Bavituximab with Carboplatin and Pemetrexed in Chemotherapy-Naive Advanced Nonsquamous Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2018, 19, e481–e487. [Google Scholar] [CrossRef]
- Menendez, J.A.; Vellon, L.; Lupu, R. Antitumoral Actions of the Anti-Obesity Drug Orlistat (XenicalTM) in Breast Cancer Cells: Blockade of Cell Cycle Progression, Promotion of Apoptotic Cell Death and PEA3-Mediated Transcriptional Repression of Her2/Neu (ErbB-2) Oncogene. Ann. Oncol. 2005, 16, 1253–1267. [Google Scholar] [CrossRef]
- Di Vizio, D.; Adam, R.M.; Kim, J.; Kim, R.; Sotgia, F.; Williams, T.; Demichelis, F.; Solomon, K.R.; Loda, M.; Rubin, M.A.; et al. Caveolin-1 Interacts with a Lipid Raft-Associated Population of Fatty Acid Synthase. Cell Cycle 2008, 7, 2257–2267. [Google Scholar] [CrossRef]
- Matsushita, Y.; Nakagawa, H.; Koike, K. Lipid Metabolism in Oncology: Why It Matters, How to Research, and How to Treat. Cancers 2021, 13, 474. [Google Scholar] [CrossRef]
- Guais, A.; Baronzio, G.; Sanders, E.; Campion, F.; Mainini, C.; Fiorentini, G.; Montagnani, F.; Behzadi, M.; Schwartz, L.; Abolhassani, M. Adding a Combination of Hydroxycitrate and Lipoic Acid (METABLOCTM) to Chemotherapy Improves Effectiveness against Tumor Development: Experimental Results and Case Report. Investig. New Drugs 2012, 30, 200–211. [Google Scholar] [CrossRef]
- Li, E.-Q.; Zhao, W.; Zhang, C.; Qin, L.-Z.; Liu, S.-J.; Feng, Z.-Q.; Wen, X.; Chen, C.-P. Synthesis and Anti-Cancer Activity of ND-646 and Its Derivatives as Acetyl-CoA Carboxylase 1 Inhibitors. Eur. J. Pharm. Sci. 2019, 137, 105010. [Google Scholar] [CrossRef] [PubMed]
- Monleon Comparative Metabolic Profiling of Paediatric Ependymoma, Medulloblastoma and Pilocytic Astrocytoma. Int. J. Mol. Med. 2010, 26, 941–948. [CrossRef]
- Clark, A.R.; Calligaris, D.; Regan, M.S.; Pomeranz Krummel, D.; Agar, J.N.; Kallay, L.; MacDonald, T.; Schniederjan, M.; Santagata, S.; Pomeroy, S.L.; et al. Rapid Discrimination of Pediatric Brain Tumors by Mass Spectrometry Imaging. J. Neurooncol. 2018, 140, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Cummins, C.L.; MacPherson, L.; Sun, Y.; Natarajan, K.; Grundy, R.G.; Arvanitis, T.N.; Kauppinen, R.A.; Peet, A.C. Magnetic Resonance Spectroscopy Metabolite Profiles Predict Survival in Paediatric Brain Tumours. Eur. J. Cancer 2013, 49, 457–464. [Google Scholar] [CrossRef]
- Wang, L.; Habib, A.A.; Mintz, A.; Li, K.C.; Zhao, D. Phosphatidylserine-Targeted Nanotheranostics for Brain Tumor Imaging and Therapeutic Potential. Mol. Imaging 2017, 16, 1536012117708722. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase III Trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Zhou, H.; Stafford, J.H.; Hallac, R.R.; Zhang, L.; Huang, G.; Mason, R.P.; Gao, J.; Thorpe, P.E.; Zhao, D. Phosphatidylserine-Targeted Molecular Imaging of Tumor Vasculature by Magnetic Resonance Imaging. J. Biomed. Nanotechnol. 2014, 10, 846–855. [Google Scholar] [CrossRef]
- Torres, M.; Busquets, X.; Escribá, P.V. Brain Lipids in the Pathophysiology and Treatment of Alzheimer’s Disease. In Update on Dementia; Moretti, D.V., Ed.; InTech: Rijeka, Croatia, 2016; pp. 127–167. [Google Scholar]
- Sastry, P.S. Lipids of Nervous Tissue: Composition and Metabolism. Prog. Lipid Res. 1985, 24, 69–176. [Google Scholar] [CrossRef]
- Willis, L.M.; Shukitt-Hale, B.; Joseph, J.A. Dietary Polyunsaturated Fatty Acids Improve Cholinergic Transmission in the Aged Brain. Genes Nutr. 2009, 4, 309–314. [Google Scholar] [CrossRef]
- Vetrivel, K.S.; Thinakaran, G. Membrane Rafts in Alzheimer’s Disease β-Amyloid Production. Biochim. Biophys. Acta 2010, 1801, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhong, C. Membrane Aging as the Real Culprit of Alzheimer’s Disease: Modification of a Hypothesis. Neurosci. Bull. 2018, 34, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of Lipids in Alzheimer’s Disease Pathology and Potential Therapies. Front. Physiol. 2020, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Escribá, P.V.; González-Ros, J.M.; Goñi, F.M.; Kinnunen, P.K.J.; Vigh, L.; Sánchez-Magraner, L.; Fernández, A.M.; Busquets, X.; Horváth, I.; Barceló-Coblijn, G. Membranes: A Meeting Point for Lipids, Proteins and Therapies. J. Cell. Mol. Med. 2008, 12, 829–875. [Google Scholar] [CrossRef]
- Martins, I.J.; Hone, E.; Foster, J.K.; Sünram-Lea, S.I.; Gnjec, A.; Fuller, S.J.; Nolan, D.; Gandy, S.E.; Martins, R.N. Apolipoprotein E, Cholesterol Metabolism, Diabetes, and the Convergence of Risk Factors for Alzheimer’s Disease and Cardiovascular Disease. Mol. Psychiatry 2006, 11, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Ashford, J.W. APOE Genotype Effects on Alzheimer’s Disease Onset and Epidemiology. J. Mol. Neurosci. 2004, 23, 157–165. [Google Scholar] [CrossRef]
- Poirier, J. Apolipoprotein E, Cholesterol Transport and Synthesis in Sporadic Alzheimer’s Disease. Neurobiol. Aging 2005, 26, 355–361. [Google Scholar] [CrossRef]
- Rapp, A.; Gmeiner, B.; Hüttinger, M. Implication of ApoE Isoforms in Cholesterol Metabolism by Primary Rat Hippocampal Neurons and Astrocytes. Biochimie 2006, 88, 473–483. [Google Scholar] [CrossRef]
- Thimiri Govinda Raj, D.B.; Ghesquière, B.; Tharkeshwar, A.K.; Coen, K.; Derua, R.; Vanderschaeghe, D.; Rysman, E.; Bagadi, M.; Baatsen, P.; De Strooper, B.; et al. A Novel Strategy for the Comprehensive Analysis of the Biomolecular Composition of Isolated Plasma Membranes. Mol Syst Biol 2011, 7, 541. [Google Scholar] [CrossRef]
- Tamboli, I.Y.; Prager, K.; Thal, D.R.; Thelen, K.M.; Dewachter, I.; Pietrzik, C.U.; St George-Hyslop, P.; Sisodia, S.S.; De Strooper, B.; Heneka, M.T.; et al. Loss of Gamma-Secretase Function Impairs Endocytosis of Lipoprotein Particles and Membrane Cholesterol Homeostasis. J. Neurosci. 2008, 28, 12097–12106. [Google Scholar] [CrossRef]
- Kuo, Y.M.; Emmerling, M.R.; Bisgaier, C.L.; Essenburg, A.D.; Lampert, H.C.; Drumm, D.; Roher, A.E. Elevated Low-Density Lipoprotein in Alzheimer’s Disease Correlates with Brain Aβ 1-42 Levels. Biochem. Biophys. Res. Commun. 1998, 252, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Hottman, D.A.; Chernick, D.; Cheng, S.; Wang, Z.; Li, L. HDL and Cognition in Neurodegenerative Disorders. Neurobiol. Dis. 2014, 72, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Haughey, N.J. Could Plasma Sphingolipids Be Diagnostic or Prognostic Biomarkers for Alzheimer’s Disease? Clin. Lipidol. 2012, 7, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Wattenberg, B.W. Intra- and Intercellular Trafficking in Sphingolipid Metabolism in Myelination. Adv. Biol. Regul. 2019, 71, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. In Sphingolipids as Signaling and Regulatory Molecules. Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2010; Volume 688, pp. 1–23. [Google Scholar]
- Varma, V.R.; Oommen, A.M.; Varma, S.; Casanova, R.; An, Y.; Andrews, R.M.; O’Brien, R.; Pletnikova, O.; Troncoso, J.C.; Toledo, J.; et al. Brain and Blood Metabolite Signatures of Pathology and Progression in Alzheimer Disease: A Targeted Metabolomics Study. PLoS Med. 2018, 15, e1002482. [Google Scholar] [CrossRef]
- He, X.; Huang, Y.; Li, B.; Gong, C.-X.; Schuchman, E.H. Deregulation of Sphingolipid Metabolism in Alzheimer’s Disease. Neurobiol. Aging 2010, 31, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-T.; Xu, J.; Lee, J.-M.; Ku, G.; Han, X.; Yang, D.-I.; Chen, S.; Hsu, C.Y. Amyloid-β Peptide Induces Oligodendrocyte Death by Activating the Neutral Sphingomyelinase–Ceramide Pathway. J. Cell Biol. 2004, 164, 123–131. [Google Scholar] [CrossRef]
- Filippov, V.; Song, M.A.; Zhang, K.; Vinters, H.V.; Tung, S.; Kirsch, W.M.; Yang, J.; Duerksen-Hughes, P.J. Increased Ceramide in Brains with Alzheimer’s and Other Neurodegenerative Diseases. J. Alzheimer’s Dis. 2012, 29, 537–547. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Hof, P.R.; Šimić, G. Ceramides in Alzheimer’s Disease: Key Mediators of Neuronal Apoptosis Induced by Oxidative Stress and A β Accumulation. Oxid. Med. Cell. Longev. 2015, 2015, 1–17. [Google Scholar] [CrossRef]
- Satoi, H.; Tomimoto, H.; Ohtani, R.; Kitano, T.; Kondo, T.; Watanabe, M.; Oka, N.; Akiguchi, I.; Furuya, S.; Hirabayashi, Y.; et al. Astroglial Expression of Ceramide in Alzheimer’s Disease Brains: A Role during Neuronal Apoptosis. Neuroscience 2005, 130, 657–666. [Google Scholar] [CrossRef]
- Han, X.; David, M.H.; Daniel, W.M.; Kelley, J.; Morris John, C. Substantial Sulfatide Deficiency and Ceramide Elevation in Very Early Alzheimer’s Disease: Potential Role in Disease Pathogenesis. J. Neurochem. 2002, 82, 809–818. [Google Scholar] [CrossRef]
- Han, X.; Fagan, A.M.; Cheng, H.; Morris, J.C.; Xiong, C.; Holtzman, D.M. Cerebrospinal Fluid Sulfatide Is Decreased in Subjects with Incipient Dementia. Ann. Neurol. 2003, 54, 115–119. [Google Scholar] [CrossRef]
- Riboldi, G.M.; Fonzo, A.B. Di GBA, Gaucher Disease, and Parkinson’s Disease: From Genetic to Clinic to New Therapeutic Approaches. Cells 2019, 8, 364. [Google Scholar] [CrossRef]
- Mazzulli, J.; Xu, Y.; Sun, Y.; Knight, A.; McLean, P.; Caldwell, G.; Sidransky, E.; Grabowski, G.; Krainc, D. Gaucher Disease Glucocerebrosidase and α-Synuclein Form a Bidirectional Pathogenic Loop in Synucleinopathies. Cell 2011, 146, 37–52. [Google Scholar] [CrossRef]
- Wilson, M.W.; Shu, L.; Hinkovska-Galcheva, V.; Jin, Y.; Rajeswaran, W.; Abe, A.; Zhao, T.; Luo, R.; Wang, L.; Wen, B.; et al. Optimization of Eliglustat-Based Glucosylceramide Synthase Inhibitors as Substrate Reduction Therapy for Gaucher Disease Type 3. ACS Chem. Neurosci. 2020, 11, 3464–3473. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Fedier, A.; Kohler, R.S.; Jacob, F. Glucosylceramide Synthase Inhibitors Differentially Affect Expression of Glycosphingolipids. Glycobiology 2015, 25, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Sardi, S.P.; Viel, C.; Clarke, J.; Treleaven, C.M.; Richards, A.M.; Park, H.; Olszewski, M.A.; Dodge, J.C.; Marshall, J.; Makino, E.; et al. Glucosylceramide Synthase Inhibition Alleviates Aberrations in Synucleinopathy Models. Proc. Natl. Acad. Sci. USA 2017, 114, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Podbielska, M.; Szulc, Z.M.; Ariga, T.; Pokryszko-Dragan, A.; Fortuna, W.; Bilinska, M.; Podemski, R.; Jaskiewicz, E.; Kurowska, E.; Yu, R.K.; et al. Distinctive Sphingolipid Patterns in Chronic Multiple Sclerosis Lesions. J. Lipid Res. 2020, 61, 1464–1479. [Google Scholar] [CrossRef]
- Giussani, P.; Prinetti, A.; Tringali, C. The Role of Sphingolipids in Myelination and Myelin Stability and Their Involvement in Childhood and Adult Demyelinating Disorders. J. Neurochem. 2021, 156, 403–414. [Google Scholar] [CrossRef]
- Penke, B.; Paragi, G.; Gera, J.; Berkecz, R.; Kovács, Z.; Crul, T.; VÍgh, L. The Role of Lipids and Membranes in the Pathogenesis of Alzheimer’s Disease: A Comprehensive View. Curr. Alzheimer Res. 2018, 15, 1191–1212. [Google Scholar] [CrossRef]
- Niu, Z.; Zhang, Z.; Zhao, W.; Yang, J. Interactions between Amyloid β Peptide and Lipid Membranes. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1663–1669. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A. Plasmalogen-Selective Phospholipase A2 and Its Involvement in Alzheimer’s Disease. Biochem. Soc. Trans. 1998, 26, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.R.; Lovell, M.A.; Yatin, M.; Dhillon, H.; Markesbery, W.R. Regional Membrane Phospholipid Alterations in Alzheimer’s Disease. Neurochem. Res. 1998, 23, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, R.M.; Blusztajn, J.K.; Pittas, A.G.; Slack, B.E.; Growdon, J.H.; Wurtman, R.J. Evidence for a Membrane Defect in Alzheimer Disease Brain. Proc. Natl. Acad. Sci. USA 1992, 89, 1671–1675. [Google Scholar] [CrossRef]
- Wood, P.L. Lipidomics of Alzheimer’s Disease: Current Status. Alzheimers. Res. Ther. 2012, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Ma, K.; Gao, F.; Kim, H.-W.; Rapoport, S.I.; Rao, J.S. Disturbed Choline Plasmalogen and Phospholipid Fatty Acid Concentrations in Alzheimer’s Disease Prefrontal Cortex. J. Alzheimer’s Dis. 2011, 24, 507–517. [Google Scholar] [CrossRef]
- Haughey, N.J.; Bandaru, V.V.R.; Bae, M.; Mattson, M.P. Roles for Dysfunctional Sphingolipid Metabolism in Alzheimer’s Disease Neuropathogenesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 878–886. [Google Scholar] [CrossRef]
- Kao, Y.C.; Ho, P.C.; Tu, Y.K.; Jou, I.M.; Tsai, K.J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef]
- Hazel, J. The Role of Alterations in Membrane Lipid Composition in Enabling Physiological Adaptation of Organisms to Their Physical Environment. Prog. Lipid Res. 1990, 29, 167–227. [Google Scholar] [CrossRef]
- Yehuda, S.; Rabinovitz, S.; Carasso, R.L.; Mostofsky, D.I. The Role of Polyunsaturated Fatty Acids in Restoring the Aging Neuronal Membrane. Neurobiol. Aging 2002, 23, 843–853. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C. Dietary Fat Composition and Dementia Risk. Neurobiol. Aging 2014, 35, S59–S64. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Schneider, J.; Wilson, R.S. Dietary Fats and the Risk of Incident Alzheimer Disease. Arch. Neurol. 2003, 60, 194–200. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Cipolla, M.; Chiang, J.; Arakaki, X.; Harrington, M.G. Human Cerebrospinal Fluid Fatty Acid Levels Differ between Supernatant Fluid and Brain-Derived Nanoparticle Fractions, and Are Altered in Alzheimer’s Disease. PLoS ONE 2014, 9, e100519. [Google Scholar] [CrossRef]
- Rosselló, C.A.; Torres, M.; Busquets, X.; Escribá, P.V. Polyunsaturated Fatty Acids. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2008; pp. 3665–3671. ISBN 978-3-662-46875-3. [Google Scholar]
- Naudí, A.; Cabré, R.; Dominguez-Gonzalez, M.; Ayala, V.; Jové, M.; Mota-Martorell, N.; Piñol-Ripoll, G.; Gil-Villar, M.P.; Rué, M.; Portero-Otín, M.; et al. Region-Specific Vulnerability to Lipid Peroxidation and Evidence of Neuronal Mechanisms for Polyunsaturated Fatty Acid Biosynthesis in the Healthy Adult Human Central Nervous System. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 485–495. [Google Scholar] [CrossRef]
- Söderberg, M.; Edlund, C.; Kristensson, K.; Dallner, G. Fatty Acid Composition of Brain Phospholipids in Aging and in Alzheimer’s Disease. Lipids 1991, 26, 421–425. [Google Scholar] [CrossRef]
- Han, X.; Holtzman, D.M.; McKeel, D.W. Plasmalogen Deficiency in Early Alzheimer’s Disease Subjects and in Animal Models: Molecular Characterization Using Electrospray Ionization Mass Spectrometry. J Neurochem 2001, 77, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Cui, J.-G.; Marcheselli, V.L.; Bodker, M.; Botkjaer, A.; Gotlinger, K.; Serhan, C.N.; Bazan, N.G. A Role for Docosahexaenoic Acid-Derived Neuroprotectin D1 in Neural Cell Survival and Alzheimer Disease. J Clin. Investig. 2005, 115, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Astarita, G.; Jung, K.-M.; Berchtold, N.C.; Nguyen, V.Q.; Gillen, D.L.; Head, E.; Cotman, C.W.; Piomelli, D. Deficient Liver Biosynthesis of Docosahexaenoic Acid Correlates with Cognitive Impairment in Alzheimer’s Disease. PLoS ONE 2010, 5, e12538. [Google Scholar] [CrossRef]
- Belkouch, M.; Hachem, M.; Elgot, A.; Van, A.L.; Picq, M.; Guichardant, M.; Lagarde, M.; Bernoud-Hubac, N. The Pleiotropic Effects of Omega-3 Docosahexaenoic Acid on the Hallmarks of Alzheimer’s Disease. J. Nutr. Biochem. 2016, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Poljak, A.; Braidy, N.; Crawford, J.; Sachdev, P. Blood Fatty Acids in Alzheimer’s Disease and Mild Cognitive Impairment: A Meta-Analysis and Systematic Review. Ageing Res. Rev. 2020, 60, 101043. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Cipolla, M.; Chiang, A.J.; Edminster, S.P.; Arakaki, X.; Harrington, M.G. Polyunsaturated Fatty Acid Composition of Cerebrospinal Fluid Fractions Shows Their Contribution to Cognitive Resilience of a Pre-Symptomatic Alzheimer’s Disease Cohort. Front. Physiol. 2020, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; An, Y.; Pletnikova, O.; O’Brien, R.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Association between Fatty Acid Metabolism in the Brain and Alzheimer Disease Neuropathology and Cognitive Performance: A Nontargeted Metabolomic Study. PLoS Med. 2017, 14, e1002266. [Google Scholar] [CrossRef] [PubMed]
- Iuliano, L.; Pacelli, A.; Ciacciarelli, M.; Zerbinati, C.; Fagioli, S.; Piras, F.; Orfei, M.D.; Bossù, P.; Pazzelli, F.; Serviddio, G.; et al. Plasma Fatty Acid Lipidomics in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimer’s Dis. 2013, 36, 545–553. [Google Scholar] [CrossRef]
- Thomas, M.H.; Pelleieux, S.; Vitale, N.; Olivier, J.L. Dietary Arachidonic Acid as a Risk Factor for Age-Associated Neurodegenerative Diseases: Potential Mechanisms. Biochimie 2016, 130, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Amtul, Z.; Uhrig, M.; Beyreuther, K. Additive Effects of Fatty Acid Mixtures on the Levels and Ratio of Amyloid Β40/42 Peptides Differ from the Effects of Individual Fatty Acids. J. Neurosci. Res. 2011, 89, 1795–1801. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.; McEvoy, M.; Magennis, E.; Schofield, P.; Patterson, A.; Zacharia, K. Dietary Long-Chain Fatty Acids and Cognitive Performance in Older Australian Adults. Nutrients 2019, 11, 711. [Google Scholar] [CrossRef]
- Doyle, R.; Sadlier, D.M.; Godson, C. Pro-Resolving Lipid Mediators: Agents of Anti-Ageing? Semin. Immunol. 2018, 40, 36–48. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized Pro-Resolving Mediators: Endogenous Regulators of Infection and Inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Whittington, R.A.; Planel, E.; Terrando, N. Impaired Resolution of Inflammation in Alzheimer’s Disease: A Review. Front. Immunol. 2017, 8, 1464. [Google Scholar] [CrossRef]
- Biringer, R.G. The Role of Eicosanoids in Alzheimer’s Disease. Int. J. Environ. Res. Public Health 2019, 16, 2560. [Google Scholar] [CrossRef]
- Van der Kant, R.; Goldstein, L.S.B. Cellular Functions of the Amyloid Precursor Protein from Development to Dementia. Dev. Cell 2015, 32, 502–515. [Google Scholar] [CrossRef] [PubMed]
- Sáez-Orellana, F.; Octave, J.-N.; Pierrot, N. Alzheimer’s Disease, a Lipid Story: Involvement of Peroxisome Proliferator-Activated Receptor α. Cells 2020, 9, 1215. [Google Scholar] [CrossRef]
- Area-Gomez, E.; Schon, E.A. On the Pathogenesis of Alzheimer’s Disease: The MAM Hypothesis. FASEB J. 2017, 31, 864–867. [Google Scholar] [CrossRef]
- Pera, M.; Larrea, D.; Guardia-Laguarta, C.; Montesinos, J.; Velasco, K.R.; Agrawal, R.R.; Xu, Y.; Chan, R.B.; Di Paolo, G.; Mehler, M.F.; et al. Increased Localization of APP-C99 in Mitochondria-Associated ER Membranes Causes Mitochondrial Dysfunction in Alzheimer Disease. EMBO J. 2017, 36, 3356–3371. [Google Scholar] [CrossRef]
- Jimenez, S.; Torres, M.; Vizuete, M.; Sanchez-Varo, R.; Sanchez-Mejias, E.; Trujillo-Estrada, L.; Carmona-Cuenca, I.; Caballero, C.; Ruano, D.; Gutierrez, A.; et al. Age-Dependent Accumulation of Soluble Amyloid β (Aβ) Oligomers Reverses the Neuroprotective Effect of Soluble Amyloid Precursor Protein-α (SAPP(α)) by Modulating Phosphatidylinositol 3-Kinase (PI3K)/Akt-GSK-3β Pathway in Alzheimer Mouse Model. J. Biol. Chem. 2011, 286, 18414–18425. [Google Scholar] [CrossRef] [PubMed]
- Parets, S.; Irigoyen, Á.; Ordinas, M.; Cabot, J.; Miralles, M.; Arbona, L.; Péter, M.; Balogh, G.; Fernández-García, P.; Busquets, X.; et al. 2-Hydroxy-Docosahexaenoic Acid Is Converted into Heneicosapentaenoic Acid via α-Oxidation: Implications for Alzheimer’s Disease Therapy. Front. Cell Dev. Biol. 2020, 8, 164. [Google Scholar] [CrossRef]
- McKillop, I.H.; Girardi, C.A.; Thompson, K.J. Role of Fatty Acid Binding Proteins (FABPs) in Cancer Development and Progression. Cell. Signal. 2019, 62, 109336. [Google Scholar] [CrossRef]
- Amiri, M.; Yousefnia, S.; Seyed Forootan, F.; Peymani, M.; Ghaedi, K.; Nasr Esfahani, M.H. Diverse Roles of Fatty Acid Binding Proteins (FABPs) in Development and Pathogenesis of Cancers. Gene 2018, 676, 171–183. [Google Scholar] [CrossRef]
- Su, X.; Tan, Q.S.W.; Parikh, B.H.; Tan, A.; Mehta, M.N.; Wey, Y.S.; Tun, S.B.B.; Li, L.-J.; Han, X.-Y.; Wong, T.Y.; et al. Characterization of Fatty Acid Binding Protein 7 (FABP7) in the Murine Retina. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3397–3408. [Google Scholar] [CrossRef][Green Version]
- Mita, R.; Beaulieu, M.J.; Field, C.; Godbout, R. Brain Fatty Acid-Binding Protein and ω-3/ω-6 Fatty Acids: Mechanistic Insight into Malignant Glioma Cell Migration. J. Biol. Chem. 2010, 285, 37005–37015. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Owada, Y. Possible Involvement of Fatty Acid Binding Proteins in Psychiatric Disorders. Anat. Sci. Int. 2021, 96, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Uusitalo, M.; Klenow, M.B.; Laulumaa, S.; Blakeley, M.P.; Simonsen, A.C.; Ruskamo, S.; Kursula, P. Human Myelin Protein P2: From Crystallography to Time-Lapse Membrane Imaging and Neuropathy-Associated Variants. FEBS J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Laulumaa, S.; Nieminen, T.; Raasakka, A.; Krokengen, O.C.; Safaryan, A.; Hallin, E.I.; Brysbaert, G.; Lensink, M.F.; Ruskamo, S.; Vattulainen, I.; et al. Structure and Dynamics of a Human Myelin Protein P2 Portal Region Mutant Indicate Opening of the β Barrel in Fatty Acid Binding Proteins. BMC Struct. Biol. 2018, 18, 8. [Google Scholar] [CrossRef]
- Galvagnion, C. The Role of Lipids Interacting with α-Synuclein in the Pathogenesis of Parkinson’s Disease. J. Parkinsons. Dis. 2017, 7, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Broersen, K.; Van Den Brink, D.; Fraser, G.; Goedert, M.; Davletov, B. α-Synuclein Adopts an α-Helical Conformation in the Presence of Polyunsaturated Fatty Acids to Hinder Micelle Formation. Biochemistry 2006, 45, 15610–15616. [Google Scholar] [CrossRef]
- Perry, E.E.; Perry, R.H. The Cholinergic System in Alzheimer’s Disease. Trends Neurosci. 1982, 5, 261–262. [Google Scholar] [CrossRef]
- Texidó, L.; Martín-Satué, M.; Alberdi, E.; Solsona, C.; Matute, C. Amyloid β Peptide Oligomers Directly Activate NMDA Receptors. Cell Calcium 2011, 49, 184–190. [Google Scholar] [CrossRef]
- Rogawski, M.A.; Wenk, G.L. The Neuropharmacological Basis for the Use of Memantine in the Treatment of Alzheimer’s Disease. CNS Drug Rev. 2003, 9, 275–308. [Google Scholar] [CrossRef]
- Raina, P.; Santaguida, P.; Ismaila, A.; Patterson, C.; Cowan, D.; Levine, M.; Booker, L.; Oremus, M. Effectiveness of Cholinesterase Inhibitors and Memantine for Treating Dementia: Evidence Review for a Clinical Practice Guideline. Ann. Intern. Med. 2008, 148, 379–397. [Google Scholar] [CrossRef]
- Kaduszkiewicz, H.; Zimmermann, T.; Beck-Bornholdt, H.-P.; van den Bussche, H. Cholinesterase Inhibitors for Patients with Alzheimer’s Disease: Systematic Review of Randomised Clinical Trials. BMJ 2005, 331, 321–327. [Google Scholar] [CrossRef]
- Karlawish, J. Aducanumab and the Business of Alzheimer Disease—Some Choice. JAMA Neurol. 2021, 78, 1303. [Google Scholar] [CrossRef]
- Knopman, D.S.; Jones, D.T.; Greicius, M.D. Failure to Demonstrate Efficacy of Aducanumab: An Analysis of the EMERGE and ENGAGE Trials as Reported by Biogen, December 2019. Alzheimer’s Dement. 2021, 17, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Amiri, S.; Pecic, S.; Machaj, F.; Rosik, J.; Łos, M.J.; Alizadeh, J.; Mahdian, R.; da Silva Rosa, S.C.; Schaafsma, D.; et al. Pleiotropic Effects of Statins: A Focus on Cancer. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165968. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, D.; Kim, H.; Lester, R.; Fukuchi, K.-I. Simvastatin Enhances Learning and Memory Independent of Amyloid Load in Mice. Ann. Neurol. 2006, 60, 729–739. [Google Scholar] [CrossRef]
- Fassbender, K.; Simons, M.; Bergmann, C.; Stroick, M.; Lutjohann, D.; Keller, P.; Runz, H.; Kuhl, S.; Bertsch, T.; von Bergmann, K.; et al. Simvastatin Strongly Reduces Levels of Alzheimer’s Disease β-Amyloid Peptides Aβ 42 and Aβ 40 in Vitro and in Vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 5856–5861. [Google Scholar] [CrossRef]
- Geifman, N.; Brinton, R.D.; Kennedy, R.E.; Schneider, L.S.; Butte, A.J. Evidence for Benefit of Statins to Modify Cognitive Decline and Risk in Alzheimer’s Disease. Alzheimers. Res. Ther. 2017, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, M.; Gustafsson, K.; Syversen, S.; Olsson, A.; Edman, Å.; Davidsson, P.; Wallin, A.; Blennow, K. Treatment with Simvastatin in Patients with Alzheimer’s Disease Lowers Both α- and β-Cleaved Amyloid Precursor Protein. Dement. Geriatr. Cogn. Disord. 2003, 16, 25–30. [Google Scholar] [CrossRef]
- Lin, F.-C.; Chuang, Y.-S.; Hsieh, H.-M.; Lee, T.-C.; Chiu, K.-F.; Liu, C.-K.; Wu, M.-T. Early Statin Use and the Progression of Alzheimer Disease. Medicine 2015, 94, e2143. [Google Scholar] [CrossRef]
- Hoglund, K.; Thelen, K.M.; Syversen, S.; Sjogren, M.; von Bergmann, K.; Wallin, A.; Vanmechelen, E.; Vanderstichele, H.; Lutjohann, D.; Blennow, K. The Effect of Simvastatin Treatment on the Amyloid Precursor Protein and Brain Cholesterol Metabolism in Patients with Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 2005, 19, 256–265. [Google Scholar] [CrossRef]
- Sano, M.; Bell, K.L.; Galasko, D.; Galvin, J.E.; Thomas, R.G.; van Dyck, C.H.; Aisen, P.S. A Randomized, Double-Blind, Placebo-Controlled Trial of Simvastatin to Treat Alzheimer Disease. Neurology 2011, 77, 556–563. [Google Scholar] [CrossRef]
- Feldman, H.H.; Doody, R.S.; Kivipelto, M.; Sparks, D.L.; Waters, D.D.; Jones, R.W.; Schwam, E.; Schindler, R.; Hey-Hadavi, J.; DeMicco, D.A.; et al. Randomized Controlled Trial of Atorvastatin in Mild to Moderate Alzheimer Disease: LEADe. Neurology 2010, 74, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.W.; Kivipelto, M.; Feldman, H.; Sparks, L.; Doody, R.; Waters, D.D.; Hey-Hadavi, J.; Breazna, A.; Schindler, R.J.; Ramos, H.; et al. The Atorvastatin/Donepezil in Alzheimer’s Disease Study (LEADe): Design and Baseline Characteristics. Alzheimers Dement. 2008, 4, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.; Blauw, G.J.; Murphy, M.B.; Bollen, E.L.E.M.; Buckley, B.M.; Cobbe, S.M.; Ford, I.; Gaw, A.; Hyland, M.; Jukema, J.W.; et al. Pravastatin in Elderly Individuals at Risk of Vascular Disease (PROSPER): A Randomised Controlled Trial. Lancet 2002, 360, 1623–1630. [Google Scholar] [CrossRef]
- Raederstorff, D.; Wyss, A.; Calder, P.C.; Weber, P.; Eggersdorfer, M. Vitamin E Function and Requirements in Relation to PUFA. Br. J. Nutr. 2015, 114, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Labazi, M.; McNeil, A.K.; Kurtz, T.; Lee, T.C.; Pegg, R.B.; Angeli, J.P.F.; Conrad, M.; McNeil, P.L. The Antioxidant Requirement for Plasma Membrane Repair in Skeletal Muscle. Free Radic. Biol. Med. 2015, 84, 246–253. [Google Scholar] [CrossRef]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma Antioxidants Are Similarly Depleted in Mild Cognitive Impairment and in Alzheimer’s Disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef]
- Mullan, K.; Cardwell, C.R.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Plasma Antioxidant Status in Patients with Alzheimer’s Disease and Cognitively Intact Elderly: A Meta-Analysis of Case-Control Studies. J. Alzheimer’s Dis. 2018, 62, 305–317. [Google Scholar] [CrossRef]
- Ding, Y.; Qiao, A.; Wang, Z.; Goodwin, J.S.; Lee, E.-S.; Block, M.L.; Allsbrook, M.; McDonald, M.P.; Fan, G.-H. Retinoic Acid Attenuates β-Amyloid Deposition and Rescues Memory Deficits in an Alzheimer’s Disease Transgenic Mouse Model. J. Neurosci. 2008, 28, 11622–11634. [Google Scholar] [CrossRef]
- Wang, S.; Yang, S.; Liu, W.; Zhang, Y.; Xu, P.; Wang, T.; Ling, T.; Liu, R. α-Tocopherol Quinine Ameliorates Spatial Memory Deficits by Reducing β-Amyloid Oligomers, Neuroinflammation and Oxidative Stress in Transgenic Mice with Alzheimer’s Disease. Behav. Brain Res. 2016, 296, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Farina, N.; Llewellyn, D.; Isaac, M.G.E.K.N.; Tabet, N. Vitamin E for Alzheimer’s Dementia and Mild Cognitive Impairment. Cochrane Database Syst. Rev. 2017, 4, CD002854. [Google Scholar] [CrossRef]
- Kryscio, R.J.; Abner, E.L.; Caban-Holt, A.; Lovell, M.; Goodman, P.; Darke, A.K.; Yee, M.; Crowley, J.; Schmitt, F.A. Association of Antioxidant Supplement Use and Dementia in the Prevention of Alzheimer’s Disease by Vitamin E and Selenium Trial (PREADViSE). JAMA Neurol. 2017, 74, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Shinto, L.; Quinn, J.; Montine, T.; Dodge, H.H.; Woodward, W.; Baldauf-Wagner, S.; Waichunas, D.; Bumgarner, L.; Bourdette, D.; Silbert, L.; et al. A Randomized Placebo-Controlled Pilot Trial of Omega-3 Fatty Acids and α Lipoic Acid in Alzheimer’s Disease. J. Alzheimers Dis. 2014, 38, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Raman, R.; Thomas, R.G.; Yurko-Mauro, K.; Nelson, E.B.; Van Dyck, C.; Galvin, J.E.; Emond, J.; Jack, C.R.; Weiner, M.; et al. Docosahexaenoic Acid Supplementation and Cognitive Decline in Alzheimer Disease: A Randomized Trial. J. Am. Med. Assoc. 2010, 304, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Freund-Levi, Y.; Eriksdotter-Jönhagen, M.; Cederholm, T.; Basun, H.; Faxén-Irving, G.; Garlind, A.; Vedin, I.; Vessby, B.; Wahlund, L.O.; Palmblad, J. ω-3 Fatty Acid Treatment in 174 Patients with Mild to Moderate Alzheimer Disease: OmegAD Study–A Randomized Double-Blind Trial. Arch. Neurol. 2006, 63, 1402–1408. [Google Scholar] [CrossRef]
- Janssen, C.I.F.; Kiliaan, A.J. Long-Chain Polyunsaturated Fatty Acids (LCPUFA) from Genesis to Senescence: The Influence of LCPUFA on Neural Development, Aging, and Neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; McCarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N.; Stedman, M.; Investigators, M. Beneficial Effects of Docosahexaenoic Acid on Cognition in Age-Related Cognitive Decline. Alzheimers Dement. 2010, 6, 456–464. [Google Scholar] [CrossRef]
- Su, H.-M. Mechanisms of N-3 Fatty Acid-Mediated Development and Maintenance of Learning Memory Performance. J. Nutr. Biochem. 2010, 21, 364–373. [Google Scholar] [CrossRef]
- Martín, V.; Fabelo, N.; Santpere, G.; Puig, B.; Marín, R.; Ferrer, I.; Díaz, M. Lipid Alterations in Lipid Rafts from Alzheimer’s Disease Human Brain Cortex. J. Alzheimers Dis. 2010, 19, 489–502. [Google Scholar] [CrossRef]
- Fiol-Deroque, M.A.; Gutierrez-Lanza, R.; Terés, S.; Torres, M.; Barceló, P.; Rial, R.V.; Verkhratsky, A.; Escribá, P.V.; Busquets, X.; Rodríguez, J.J. Cognitive Recovery and Restoration of Cell Proliferation in the Dentate Gyrus in the 5XFAD Transgenic Mice Model of Alzheimer’s Disease Following 2-Hydroxy-DHA Treatment. Biogerontology 2013, 14, 763–775. [Google Scholar] [CrossRef]
- Torres, M.; Marcilla-Etxenike, A.; Fiol-deRoque, M.A.; Escribá, P.V.; Busquets, X. The Unfolded Protein Response in the Therapeutic Effect of Hydroxy-DHA against Alzheimer’s Disease. Apoptosis 2015, 20, 712–724. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane Lipids: Where They Are and How They Behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Mazzon, M.; Mercer, J. Lipid Interactions during Virus Entry and Infection. Cell. Microbiol. 2014, 16, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Bukrinsky, M.I.; Mukhamedova, N.; Sviridov, D. Lipid Rafts and Pathogens: The Art of Deception and Exploitation. J. Lipid Res. 2020, 61, 601–610. [Google Scholar] [CrossRef]
- Bagam, P.; Singh, D.P.; Inda, M.E.; Batra, S. Unraveling the Role of Membrane Microdomains during Microbial Infections. Cell Biol. Toxicol. 2017, 33, 429–455. [Google Scholar] [CrossRef]
- Dumas, F.; Haanappel, E. Lipids in Infectious Diseases–The Case of AIDS and Tuberculosis. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1636–1647. [Google Scholar] [CrossRef]
- Sviridov, D.; Bukrinsky, M. Interaction of Pathogens with Host Cholesterol Metabolism. Curr. Opin. Lipidol. 2014, 25, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.A.; Chou, T. Mechanisms of Receptor/Coreceptor-Mediated Entry of Enveloped Viruses. Biophys. J. 2009, 96, 2624–2636. [Google Scholar] [CrossRef]
- Zaas, D.W.; Duncan, M.; Rae Wright, J.; Abraham, S.N. The Role of Lipid Rafts in the Pathogenesis of Bacterial Infections. Biochim. Biophys. Acta Mol. Cell Res. 2005, 1746, 305–313. [Google Scholar] [CrossRef]
- Ewers, H.; Helenius, A. Lipid-Mediated Endocytosis. Cold Spring Harb. Perspect. Biol. 2011, 3, a004721. [Google Scholar] [CrossRef]
- Sviridov, D.; Miller, Y.I.; Ballout, R.A.; Remaley, A.T.; Bukrinsky, M. Targeting Lipid Rafts—A Potential Therapy for COVID-19. Front. Immunol. 2020, 11, 2361. [Google Scholar] [CrossRef]
- Baorto, D.M.; Gao, Z.; Malaviya, R.; Dustin, M.L.; Van Der Merwe, A.; Lublin, D.M.; Abraham, S.N. Survival of FimH-Expressing Enterobacteria in Macrophages Relies on Glycolipid Traffic. Nature 1997, 389, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.S.; Corrêa, G.; Einicker-Lamas, M.; Coutinho-Silva, R. Host-Cell Lipid Rafts: A Safe Door for Micro-Organisms? Biol. Cell 2010, 102, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Braun, E.; Sauter, D. Furin-Mediated Protein Processing in Infectious Diseases and Cancer. Clin. Transl. Immunol. 2019, 8, e1073. [Google Scholar] [CrossRef]
- Van der Meer-Janssen, Y.P.; van Galen, J.; Batenburg, J.J.; Helms, J.B. Lipids in Host-Pathogen Interactions: Pathogens Exploit the Complexity of the Host Cell Lipidome. Prog. Lipid Res. 2010, 49, 1–26. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, D.; Hu, Y.; Wu, T.; Chong, H.; He, Y. SARS-CoV-2-Derived Fusion Inhibitor Lipopeptides Exhibit Highly Potent and Broad-Spectrum Activity against Divergent Human Coronaviruses. Signal Transduct. Target. Ther. 2021, 6, 294. [Google Scholar] [CrossRef]
- Martín-Acebes, M.A.; Vazquez-Calvo, A.; Caridi, F.; Saiz, J.-C.; Sobrino, F. Lipid Involvement in Viral Infections: Present and Future Perspectives for the Design of Antiviral Strategies. In Lipid Metabolism; InTech Open: London, UK, 2013. [Google Scholar]
- Soares, M.M.; King, S.W.; Thorpe, P.E. Targeting Inside-out Phosphatidylserine as a Therapeutic Strategy for Viral Diseases. Nat. Med. 2008, 14, 1357–1362. [Google Scholar] [CrossRef]
- Gerber, D.E.; Stopeck, A.T.; Wong, L.; Rosen, L.S.; Thorpe, P.E.; Shan, J.S.; Ibrahim, N.K. Phase I Safety and Pharmacokinetic Study of Bavituximab, a Chimeric Phosphatidylserine-Targeting Monoclonal Antibody, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2011, 17, 6888–6896. [Google Scholar] [CrossRef] [PubMed]
- Meester, I.; Rosas-Taraco, A.G.; Solís-Soto, J.M.; Salinas-Carmona, M.C. The Roles of Lipid Droplets in Human Infectious Disease. Med. Univ. 2011, 13, 207–216. [Google Scholar]
- Monson, E.A.; Crosse, K.M.; Duan, M.; Chen, W.; O’Shea, R.D.; Wakim, L.M.; Carr, J.M.; Whelan, D.R.; Helbig, K.J. Intracellular Lipid Droplet Accumulation Occurs Early Following Viral Infection and Is Required for an Efficient Interferon Response. Nat. Commun. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Zhang, J.; Lan, Y.; Sanyal, S. Modulation of Lipid Droplet Metabolism—A Potential Target for Therapeutic Intervention in Flaviviridae Infections. Front. Microbiol. 2017, 8, 2286. [Google Scholar] [CrossRef]
- O’Neal, A.J.; Butler, L.R.; Rolandelli, A.; Gilk, S.D.; Pedra, J.H.F. Lipid Hijacking: A Unifying Theme in Vector-Borne Diseases. eLife 2020, 9, e61675. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, C.L.; Green, R.S.; Damle, S.R.; Martin, R.K.; Ghahrai, N.N.; Colonne, P.M.; Fullerton, M.S.; Conrad, D.H.; Chalfant, C.E.; Voth, D.E.; et al. Functional Inhibition of Acid Sphingomyelinase Disrupts Infection by Intracellular Bacterial Pathogens. Life Sci. Alliance 2019, 2, e201800292. [Google Scholar] [CrossRef] [PubMed]
- Dunning Hotopp, J.C.; Lin, M.; Madupu, R.; Crabtree, J.; Angiuoli, S.V.; Eisen, J.; Seshadri, R.; Ren, Q.; Wu, M.; Utterback, T.R.; et al. Comparative Genomics of Emerging Human Ehrlichiosis Agents. PLoS Genet. 2006, 2, 208–223. [Google Scholar] [CrossRef]
- Toledo, A.; Benach, J.L. Hijacking and Use of Host Lipids by Intracellular Pathogens. Microbiol. Spectr. 2015, 3, 637–666. [Google Scholar] [CrossRef]
- Hossain, H.; Wellensiek, H.J.; Geyer, R.; Lochnit, G. Structural Analysis of Glycolipids from Borrelia Burgdorferi. Biochimie 2001, 83, 683–692. [Google Scholar] [CrossRef]
- Lin, M.; Grandinetti, G.; Hartnell, L.M.; Bliss, D.; Subramaniam, S.; Rikihisa, Y. Host Membrane Lipids Are Trafficked to Membranes of Intravacuolar Bacterium Ehrlichia Chaffeensis. Proc. Natl. Acad. Sci. USA 2020, 117, 8032–8043. [Google Scholar] [CrossRef]
- Semini, G.; Paape, D.; Paterou, A.; Schroeder, J.; Barrios-Llerena, M.; Aebischer, T. Changes to Cholesterol Trafficking in Macrophages by Leishmania Parasites Infection. Microbiologyopen 2017, 6, e00469. [Google Scholar] [CrossRef]
- Zhang, K.; Beverley, S.M. Phospholipid and Sphingolipid Metabolism in Leishmania. Mol. Biochem. Parasitol. 2010, 170, 55–64. [Google Scholar] [CrossRef]
- Vaughan, A.M.; O’neill, M.T.; Tarun, A.S.; Camargo, N.; Phuong, T.M.; Aly, A.S.I.; Cowman, A.F.; Kappe, S.H.I. Type II Fatty Acid Synthesis Is Essential Only for Malaria Parasite Late Liver Stage Development. Cell. Microbiol. 2009, 11, 506–520. [Google Scholar] [CrossRef]
- Itoe, M.A.; Sampaio, J.L.; Cabal, G.G.; Real, E.; Zuzarte-Luis, V.; March, S.; Bhatia, S.N.; Frischknecht, F.; Thiele, C.; Shevchenko, A.; et al. Host Cell Phosphatidylcholine Is a Key Mediator of Malaria Parasite Survival during Liver Stage Infection. Cell Host Microbe 2014, 16, 778–786. [Google Scholar] [CrossRef]
- Gazos-Lopes, F.; Martin, J.L.; Dumoulin, P.C.; Burleigh, B.A. Host Triacylglycerols Shape the Lipidome of Intracellular Trypanosomes and Modulate Their Growth. PLoS Pathog. 2017, 13, e1006800. [Google Scholar] [CrossRef]
- Soto-Acosta, R.; Mosso, C.; Cervantes-Salazar, M.; Puerta-Guardo, H.; Medina, F.; Favari, L.; Ludert, J.E.; Del Angel, R.M. The Increase in Cholesterol Levels at Early Stages after Dengue Virus Infection Correlates with an Augment in LDL Particle Uptake and HMG-CoA Reductase Activity. Virology 2013, 442, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Heaton, N.S.; Perera, R.; Berger, K.L.; Khadka, S.; LaCount, D.J.; Kuhn, R.J.; Randall, G. Dengue Virus Nonstructural Protein 3 Redistributes Fatty Acid Synthase to Sites of Viral Replication and Increases Cellular Fatty Acid Synthesis. Proc. Natl. Acad. Sci. USA 2010, 107, 17345–17350. [Google Scholar] [CrossRef]
- Vial, T.; Tan, W.L.; Xiang, B.W.W.; Missé, D.; Deharo, E.; Marti, G.; Pompon, J. Dengue Virus Reduces AGPAT1 Expression to Alter Phospholipids and Enhance Infection in Aedes Aegypti. PLoS Pathog. 2019, 15, e1008199. [Google Scholar] [CrossRef] [PubMed]
- Leier, H.C.; Weinstein, J.B.; Kyle, J.E.; Lee, J.Y.; Bramer, L.M.; Stratton, K.G.; Kempthorne, D.; Navratil, A.R.; Tafesse, E.G.; Hornemann, T.; et al. A Global Lipid Map Defines a Network Essential for Zika Virus Replication. Nat. Commun. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Merino-Ramos, T.; Vázquez-Calvo, Á.; Casas, J.; Sobrino, F.; Saiz, J.C.; Martín-Acebes, M.A. Modification of the Host Cell Lipid Metabolism Induced by Hypolipidemic Drugs Targeting the Acetyl Coenzyme A Carboxylase Impairs West Nile Virus Replication. Antimicrob. Agents Chemother. 2016, 60, 307–315. [Google Scholar] [CrossRef]
- Jiménez de Oya, N.; Esler, W.P.; Huard, K.; El-Kattan, A.F.; Karamanlidis, G.; Blázquez, A.B.; Ramos-Ibeas, P.; Escribano-Romero, E.; Louloudes-Lázaro, A.; Casas, J.; et al. Targeting Host Metabolism by Inhibition of Acetyl-Coenzyme A Carboxylase Reduces Flavivirus Infection in Mouse Models. Emerg. Microbes Infect. 2019, 8, 624–636. [Google Scholar] [CrossRef] [PubMed]
- York, A.G.; Williams, K.J.; Argus, J.P.; Zhou, Q.D.; Brar, G.; Vergnes, L.; Gray, E.E.; Zhen, A.; Wu, N.C.; Yamada, D.H.; et al. Limiting Cholesterol Biosynthetic Flux Spontaneously Engages Type i IFN Signaling. Cell 2015, 163, 1716–1729. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Naim, H.Y. Miglustat-Induced Intestinal Carbohydrate Malabsorption Is Due to the Inhibition of α-Glucosidases, but Not β-Galactosidases. J. Inherit. Metab. Dis. 2012, 35, 949–954. [Google Scholar] [CrossRef]
- Chao, C.C.; Gutiérrez-Vázquez, C.; Rothhammer, V.; Mayo, L.; Wheeler, M.A.; Tjon, E.C.; Zandee, S.E.J.; Blain, M.; de Lima, K.A.; Takenaka, M.C.; et al. Metabolic Control of Astrocyte Pathogenic Activity via CPLA2-MAVS. Cell 2019, 179, 1483–1498.e22. [Google Scholar] [CrossRef]
- Falagas, M.E.; Makris, G.C.; Matthaiou, D.K.; Rafailidis, P.I. Statins for Infection and Sepsis: A Systematic Review of the Clinical Evidence. J. Antimicrob. Chemother. 2008, 61, 774–785. [Google Scholar] [CrossRef]
- Mills, E.J.; Wu, P.; Chong, G.; Ghement, I.; Singh, S.; Akl, E.A.; Eyawo, O.; Guyatt, G.; Berwanger, O.; Briel, M. Efficacy and Safety of Statin Treatment for Cardiovascular Disease: A Network Meta-Analysis of 170 255 Patients from 76 Randomized Trials. QJM Int. J. Med. 2011, 104, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Parihar, S.P.; Guler, R.; Brombacher, F. Statins: A Viable Candidate for Host-Directed Therapy against Infectious Diseases. Nat. Rev. Immunol. 2019, 19, 104–117. [Google Scholar] [CrossRef] [PubMed]
- Longo, J.; van Leeuwen, J.E.; Elbaz, M.; Branchard, E.; Penn, L.Z. Statins as Anticancer Agents in the Era of Precision Medicine. Clin. Cancer Res. 2020, 26, 5791–5800. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, D.L.; McCune, S.A. Inhibition of Fatty Acid Synthesis in Isolated Adipocytes by 5-(Tetradecyloxy)-2-Furoic Acid. Lipids 1984, 19, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Munger, J.; Bennett, B.D.; Parikh, A.; Feng, X.J.; McArdle, J.; Rabitz, H.A.; Shenk, T.; Rabinowitz, J.D. Systems-Level Metabolic Flux Profiling Identifies Fatty Acid Synthesis as a Target for Antiviral Therapy. Nat. Biotechnol. 2008, 26, 1179–1186. [Google Scholar] [CrossRef]
- Yao, J.; Rock, C.O. How Bacterial Pathogens Eat Host Lipids: Implications for the Development of Fatty Acid Synthesis Therapeutics. J. Biol. Chem. 2015, 290, 5940–5946. [Google Scholar] [CrossRef]
- Fernández-Oliva, A.; Ortega-González, P.; Risco, C. Targeting Host Lipid Flows: Exploring New Antiviral and Antibiotic Strategies. Cell. Microbiol. 2019, 21, e12996. [Google Scholar] [CrossRef]
- Rendina, A.R.; Cheng, D. Characterization of the Inactivation of Rat Fatty Acid Synthase by C75: Inhibition of Partial Reactions and Protection by Substrates. Biochem. J. 2005, 388, 895–903. [Google Scholar] [CrossRef]
- Gullberg, R.C.; Steel, J.J.; Pujari, V.; Rovnak, J.; Crick, D.C.; Perera, R. Stearoly-CoA Desaturase 1 Differentiates Early and Advanced Dengue Virus Infections and Determines Virus Particle Infectivity. PLoS Pathog. 2018, 14, e1007261. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Lim, Y.-S.; Pham, L.V.; Shin, H.-Y.; Kim, Y.-S.; Hwang, S.B. Stearoyl Coenzyme A Desaturase 1 Is Associated with Hepatitis C Virus Replication Complex and Regulates Viral Replication. J. Virol. 2014, 88, 12311–12325. [Google Scholar] [CrossRef] [PubMed]
- Uto, Y. Recent Progress in the Discovery and Development of Stearoyl CoA Desaturase Inhibitors. Chem. Phys. Lipids 2016, 197, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Beck, Z.; Balogh, A.; Kis, A.; Izsépi, E.; Cervenak, L.; László, G.; Bíró, A.; Liliom, K.; Mocsár, G.; Vámosi, G.; et al. New Cholesterol-Specific Antibodies Remodel HIV-1 Target Cells’ Surface and Inhibit Their in Vitro Virus Production. J. Lipid Res. 2010, 51, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Roy, S. TAM Receptors: A Phosphatidylserine Receptor Family and Its Implications in Viral Infections. Int. Rev. Cell Mol. Biol. 2020, 357, 81–122. [Google Scholar] [CrossRef] [PubMed]
- Pompei, R.; Flore, O.; Marccialis, M.A.; Pani, A.; Loddo, B. Glycyrrhizic Acid Inhibits Virus Growth and Inactivates Virus Particles. Nature 1979, 281, 689–690. [Google Scholar] [CrossRef]
- Harada, S. The Broad Anti-Viral Agent Glycyrrhizin Directly Modulates the Fluidity of Plasma Membrane and HIV-1 Envelope. Biochem. J. 2005, 392, 191–199. [Google Scholar] [CrossRef]
- Selyutina, O.Y.; Polyakov, N.E.; Korneev, D.V.; Zaitsev, B.N. Influence of Glycyrrhizin on Permeability and Elasticity of Cell Membrane: Perspectives for Drugs Delivery. Drug Deliv. 2016, 23, 858–865. [Google Scholar] [CrossRef]
- Harada, S.; Yokomizo, K.; Monde, K.; Maeda, Y.; Yusa, K. A Broad Antiviral Neutral Glycolipid, Fattiviracin FV-8, Is a Membrane Fluidity Modulator. Cell. Microbiol. 2007, 9, 196–203. [Google Scholar] [CrossRef]
- Matsuda, K.; Hattori, S.; Komizu, Y.; Kariya, R.; Ueoka, R.; Okada, S. Cepharanthine Inhibited HIV-1 Cell-Cell Transmission and Cell-Free Infection via Modification of Cell Membrane Fluidity. Bioorganic Med. Chem. Lett. 2014, 24, 2115–2117. [Google Scholar] [CrossRef]
- Matsuda, K.; Hattori, S.; Kariya, R.; Komizu, Y.; Kudo, E.; Goto, H.; Taura, M.; Ueoka, R.; Kimura, S.; Okada, S. Inhibition of HIV-1 Entry by the Tricyclic Coumarin GUT-70 through the Modification of Membrane Fluidity. Biochem. Biophys. Res. Commun. 2015, 457, 288–294. [Google Scholar] [CrossRef]
- Bajimaya, S.; Frankl, T.; Hayashi, T.; Takimoto, T. Cholesterol Is Required for Stability and Infectivity of Influenza A and Respiratory Syncytial Viruses. Virology 2017, 510, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Bryan-Marrugo, O.L.; Arellanos-Soto, D.; Rojas-Martinez, A.; Barrera-Saldaña, H.; Ramos-Jimenez, J.; Vidaltamayo, R.; Rivas-Estilla, A.M. The Anti-Dengue Virus Properties of Statins May Be Associated with Alterations in the Cellular Antiviral Profile Expression. Mol. Med. Rep. 2016, 14, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, C.M.; Rawat, S.S.; Cho, E.H.; Guiffre, D.L.; Lockett, S.; Merrill, A.H.; Blumenthal, R. Sphingomyelinase Restricts the Lateral Diffusion of CD4 and Inhibits Human Immunodeficiency Virus Fusion. J. Virol. 2007, 81, 5294–5304. [Google Scholar] [CrossRef]
- Tani, H.; Shiokawa, M.; Kaname, Y.; Kambara, H.; Mori, Y.; Abe, T.; Moriishi, K.; Matsuura, Y. Involvement of Ceramide in the Propagation of Japanese Encephalitis Virus. J. Virol. 2010, 84, 2798–2807. [Google Scholar] [CrossRef]
- Voisset, C.; Lavie, M.; Helle, F.; Op De Beeck, A.; Bilheu, A.; Bertrand-Michel, J.; Tercé, F.; Cocquerel, L.; Wychowski, C.; Vu-Dac, N.; et al. Ceramide Enrichment of the Plasma Membrane Induces CD81 Internalization and Inhibits Hepatitis C Virus Entry. Cell. Microbiol. 2008, 10, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Ismaili, A.; Meddings, J.B.; Ratnam, S.; Sherman, P.M. Modulation of Host Cell Membrane Fluidity: A Novel Mechanism for Preventing Bacterial Adhesion. Am. J. Physiol. Gastrointest. Liver Physiol. 1999, 277, G201–G208. [Google Scholar] [CrossRef]
- Jerwood, S.; Cohen, J. Unexpected Antimicrobial Effect of Statins. J. Antimicrob. Chemother. 2008, 61, 362–364. [Google Scholar] [CrossRef]
- Welsh, A.M.; Kruger, P.; Faoagali, J. Antimicrobial Action of Atorvastatin and Rosuvastatin. Pathology 2009, 41, 689–691. [Google Scholar] [CrossRef]
- Liao, W.C.; Huang, M.Z.; Wang, M.L.; Lin, C.J.; Lu, T.L.; Lo, H.R.; Pan, Y.J.; Sun, Y.C.; Kao, M.C.; Lim, H.J.; et al. Statin Decreases Helicobacter Pylori Burden in Macrophages by Promoting Autophagy. Front. Cell. Infect. Microbiol. 2017, 6, 203. [Google Scholar] [CrossRef]
- Varela-M, R.E.; Villa-Pulgarin, J.A.; Yepes, E.; Müller, I.; Modolell, M.; Muñoz, D.L.; Robledo, S.M.; Muskus, C.E.; López-Abán, J.; Muro, A.; et al. In Vitro and in Vivo Efficacy of Ether Lipid Edelfosine against Leishmania Spp. and SbV-Resistant Parasites. PLoS Negl. Trop. Dis. 2012, 6, e1612. [Google Scholar] [CrossRef]
- Dinesh, N.; Neelagiri, S.; Kumar, V.; Singh, S. Glycyrrhizic Acid Attenuates Growth of Leishmania Donovani by Depleting Ergosterol Levels. Exp. Parasitol. 2017, 176, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Maria-Neto, S.; De Almeida, K.C.; Macedo, M.L.R.; Franco, O.L. Understanding Bacterial Resistance to Antimicrobial Peptides: From the Surface to Deep Inside. Biochim. Biophys. Acta Biomembr. 2015, 1848, 3078–3088. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, A.; Neundorf, I. Design and Application of Antimicrobial Peptide Conjugates. Int. J. Mol. Sci. 2016, 17, 701. [Google Scholar] [CrossRef]
- Bayramov, D.F.; Neff, J.A. Beyond Conventional Antibiotics—New Directions for Combination Products to Combat Biofilm. Adv. Drug Deliv. Rev. 2017, 112, 48–60. [Google Scholar] [CrossRef]
- Palmieri, G.; Tatè, R.; Gogliettino, M.; Balestrieri, M.; Rea, I.; Terracciano, M.; Proroga, Y.T.; Capuano, F.; Anastasio, A.; De Stefano, L. Small Synthetic Peptides Bioconjugated to Hybrid Gold Nanoparticles Destroy Potentially Deadly Bacteria at Submicromolar Concentrations. Bioconjug. Chem. 2018, 29, 3877–3885. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Epand, R.F. Bacterial Membrane Lipids in the Action of Antimicrobial Agents. J. Pept. Sci. 2011, 17, 298–305. [Google Scholar] [CrossRef]
- Carter, G.C.; Bernstone, L.; Sangani, D.; Bee, J.W.; Harder, T.; James, W. HIV Entry in Macrophages Is Dependent on Intact Lipid Rafts. Virology 2009, 386, 192–202. [Google Scholar] [CrossRef]
- Fallah, Z.; Isfahani, H.N.; Tajbakhsh, M.; Mohseni, M.; Zabihi, E.; Abedian, Z. Antibacterial and Cytotoxic Effects of Cyclodextrin-Triazole-Titanium Based Nanocomposite. Brazilian Arch. Biol. Technol. 2021, 64, 1–13. [Google Scholar] [CrossRef]
- Sun, P.; Lu, X.; Xu, C.; Wang, Y.; Sun, W.; Xi, J. CD-SACE2 Inclusion Compounds: An Effective Treatment for Coronavirus Disease 2019 (COVID-19). J. Med. Virol. 2020, 92, 1721–1723. [Google Scholar] [CrossRef]
- Karginov, V.A. Cyclodextrin Derivatives as Anti-Infectives. Curr. Opin. Pharmacol. 2013, 13, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Miller, Y.I. Regulation of Lipid Rafts, Angiogenesis and Inflammation by AIBP. Curr. Opin. Lipidol. 2019, 30, 218. [Google Scholar] [CrossRef] [PubMed]
- Woller, S.A.; Choi, S.-H.; An, E.J.; Low, H.; Schneider, D.A.; Ramachandran, R.; Kim, J.; Bae, Y.S.; Sviridov, D.; Corr, M.; et al. Inhibition of Neuroinflammation by AIBP: Spinal Effects upon Facilitated Pain States. Cell Rep. 2018, 23, 2667. [Google Scholar] [CrossRef] [PubMed]
- Lasso, G.; Mayer, S.V.; Winkelmann, E.R.; Chu, T.; Elliot, O.; Patino-Galindo, J.A.; Park, K.; Rabadan, R.; Honig, B.; Shapira, S.D. A Structure-Informed Atlas of Human-Virus Interactions. Cell 2019, 178, 1526–1541. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, S.; Maillard, P.; Yamamoto, M.; Gondeau, C.; Douam, F.; Lebreton, S.; Lagaye, S.; Pol, S.; Helle, F.; Plengpanich, W.; et al. Up-Regulation of the ATP-Binding Cassette Transporter A1 Inhibits Hepatitis C Virus Infection. PLoS ONE 2014, 9, e92140. [Google Scholar] [CrossRef]
- Girard, E.; Paul, J.L.; Fournier, N.; Beaune, P.; Johannes, L.; Lamaze, C.; Védie, B. The Dynamin Chemical Inhibitor Dynasore Impairs Cholesterol Trafficking and Sterol-Sensitive Genes Transcription in Human HeLa Cells and Macrophages. PLoS ONE 2011, 6, e29042. [Google Scholar] [CrossRef]
- Preta, G.; Lotti, V.; Cronin, J.G.; Sheldon, I.M. Protective Role of the Dynamin Inhibitor Dynasore against the Cholesterol-Dependent Cytolysin of Trueperella Pyogenes. FASEB J. 2015, 29, 1516–1528. [Google Scholar] [CrossRef]
- Abban, C.Y.; Bradbury, N.A.; Meneses, P.I. HPV16 and BPV1 Infection Can Be Blocked by the Dynamin Inhibitor Dynasore. Am. J. Ther. 2008, 15, 304–311. [Google Scholar] [CrossRef]
- Miyauchi, K.; Kim, Y.; Latinovic, O.; Morozov, V.; Melikyan, G.B. HIV Enters Cells via Endocytosis and Dynamin-Dependent Fusion with Endosomes. Cell 2009, 137, 433–444. [Google Scholar] [CrossRef]
- Mues, M.B.; Cheshenko, N.; Wilson, D.W.; Gunther-Cummins, L.; Herold, B.C.; Longnecker, R.M. Dynasore Disrupts Trafficking of Herpes Simplex Virus Proteins. J. Virol. 2015, 89, 6673–6684. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin Promotes Atherosclerosis Regression via Macrophage Reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef]
- Reyes, A.Z.; Hu, K.A.; Teperman, J.; Wampler Muskardin, T.L.; Tardif, J.C.; Shah, B.; Pillinger, M.H. Anti-Inflammatory Therapy for COVID-19 Infection: The Case for Colchicine. Ann. Rheum. Dis. 2021, 80, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Sartorius, R.; D’Apice, L.; Barba, P.; Cipria, D.; Grauso, L.; Cutignano, A.; De Berardinis, P. Vectorized Delivery of α-Galactosylceramide and Tumor Antigen on Filamentous Bacteriophage Fd Induces Protective Immunity by Enhancing Tumor-Specific T Cell Response. Front. Immunol. 2018, 9, 1496. [Google Scholar] [CrossRef] [PubMed]
- Dubrovsky, L.; Ward, A.; Choi, S.H.; Pushkarsky, T.; Brichacek, B.; Vanpouille, C.; Adzhubei, A.A.; Mukhamedova, N.; Sviridov, D.; Margolis, L.; et al. Inhibition of HIV Replication by Apolipoprotein A-I Binding Protein Targeting the Lipid Rafts. mBio 2020, 11, e02956-19. [Google Scholar] [CrossRef] [PubMed]
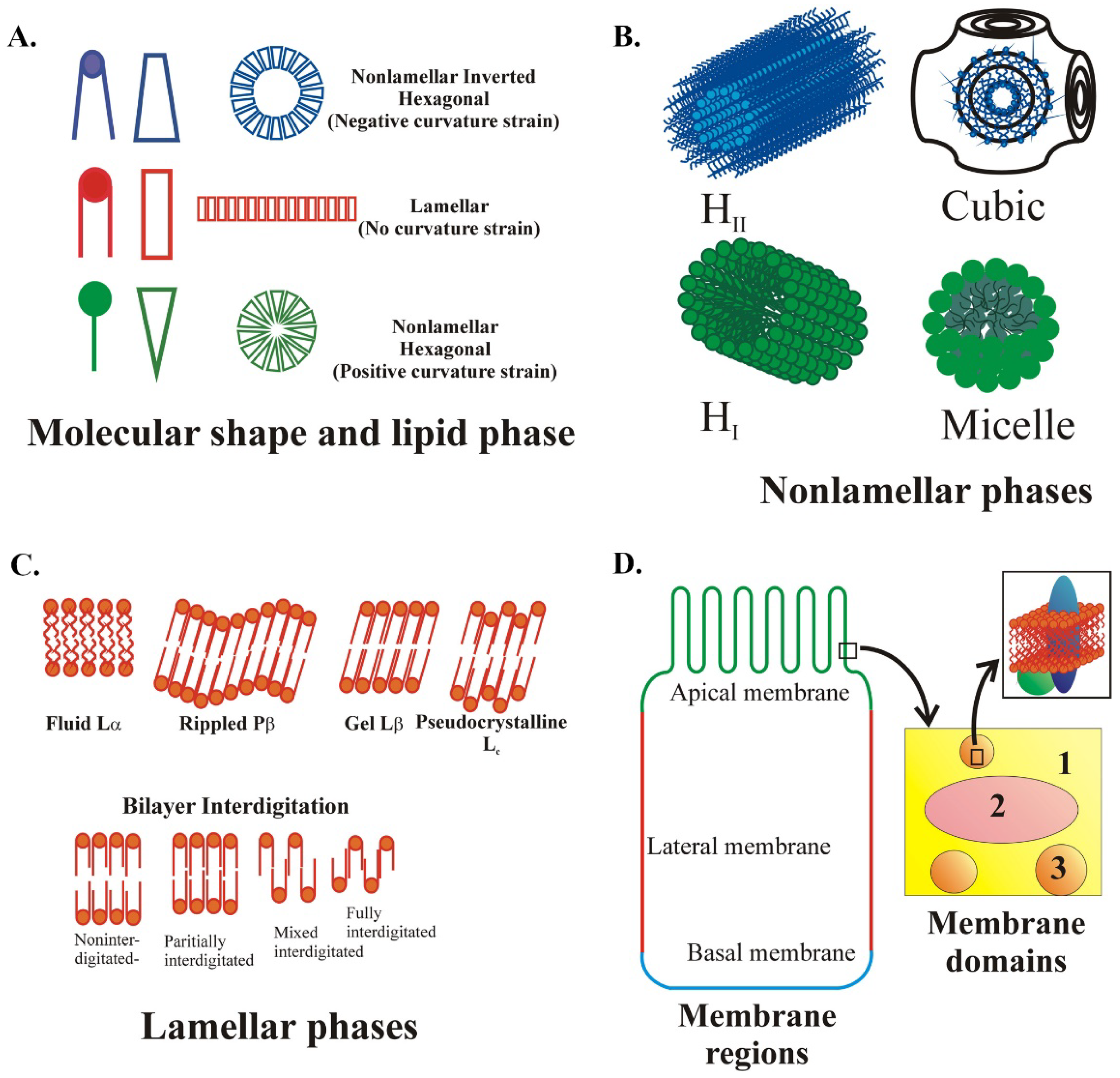
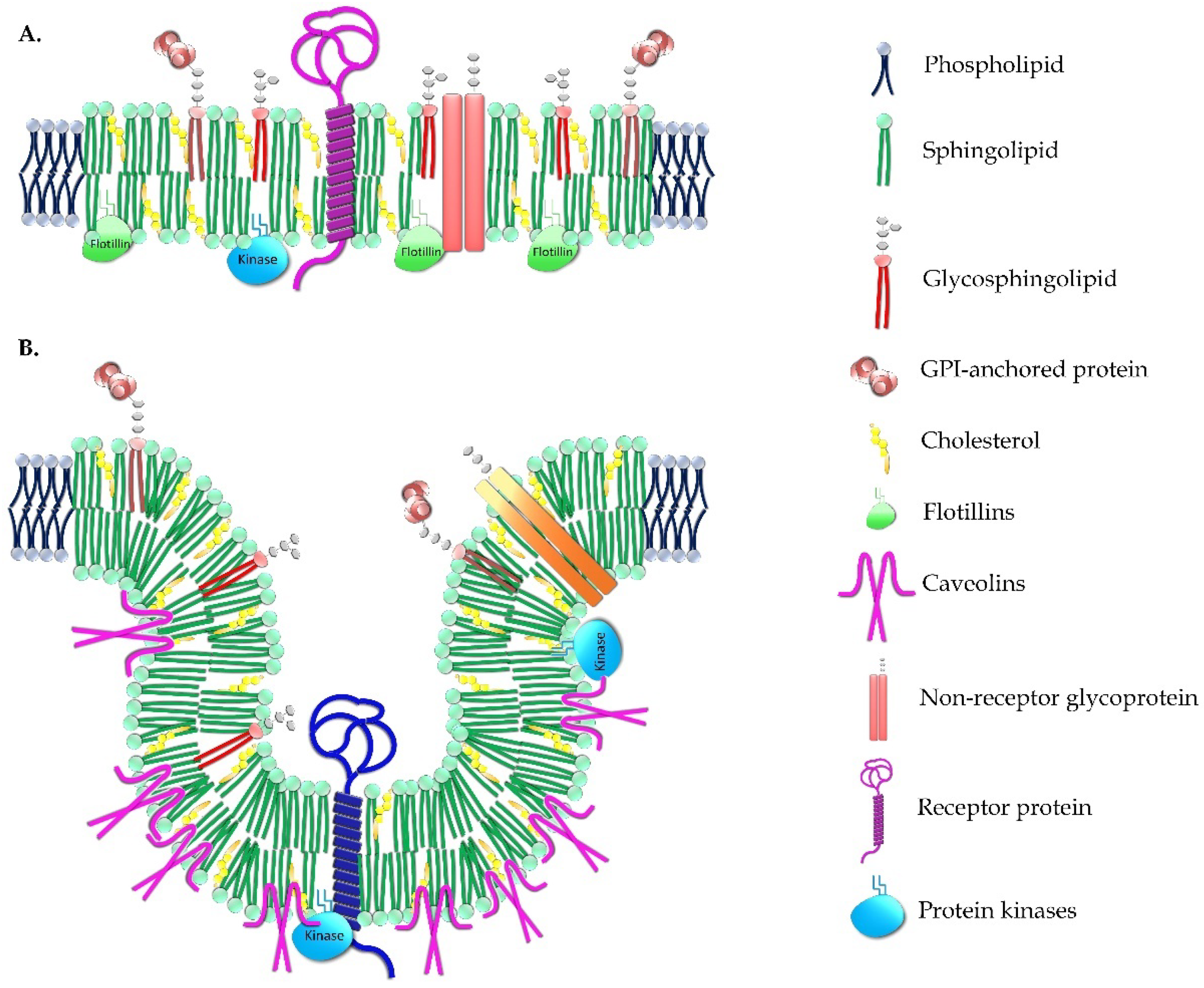
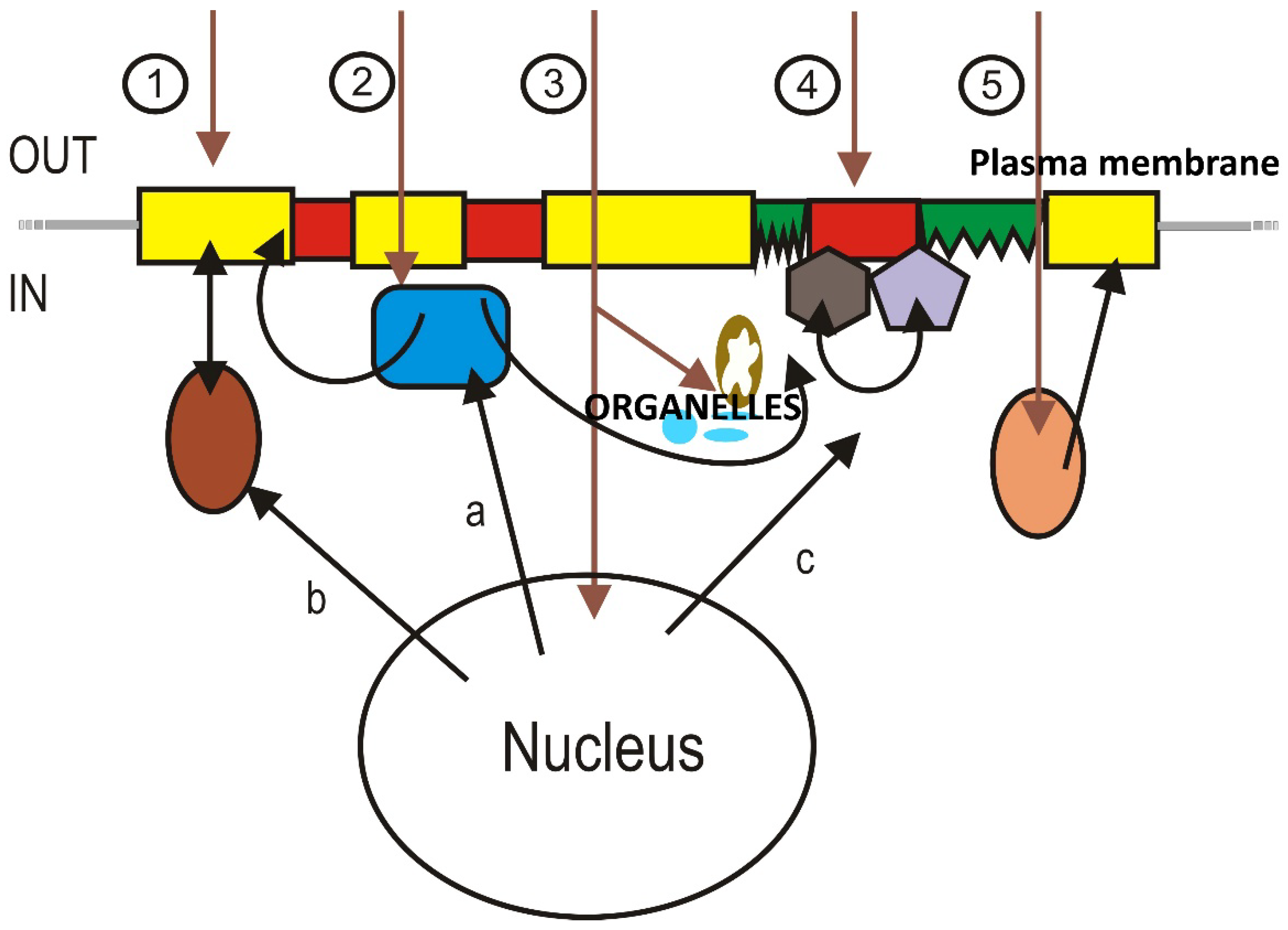
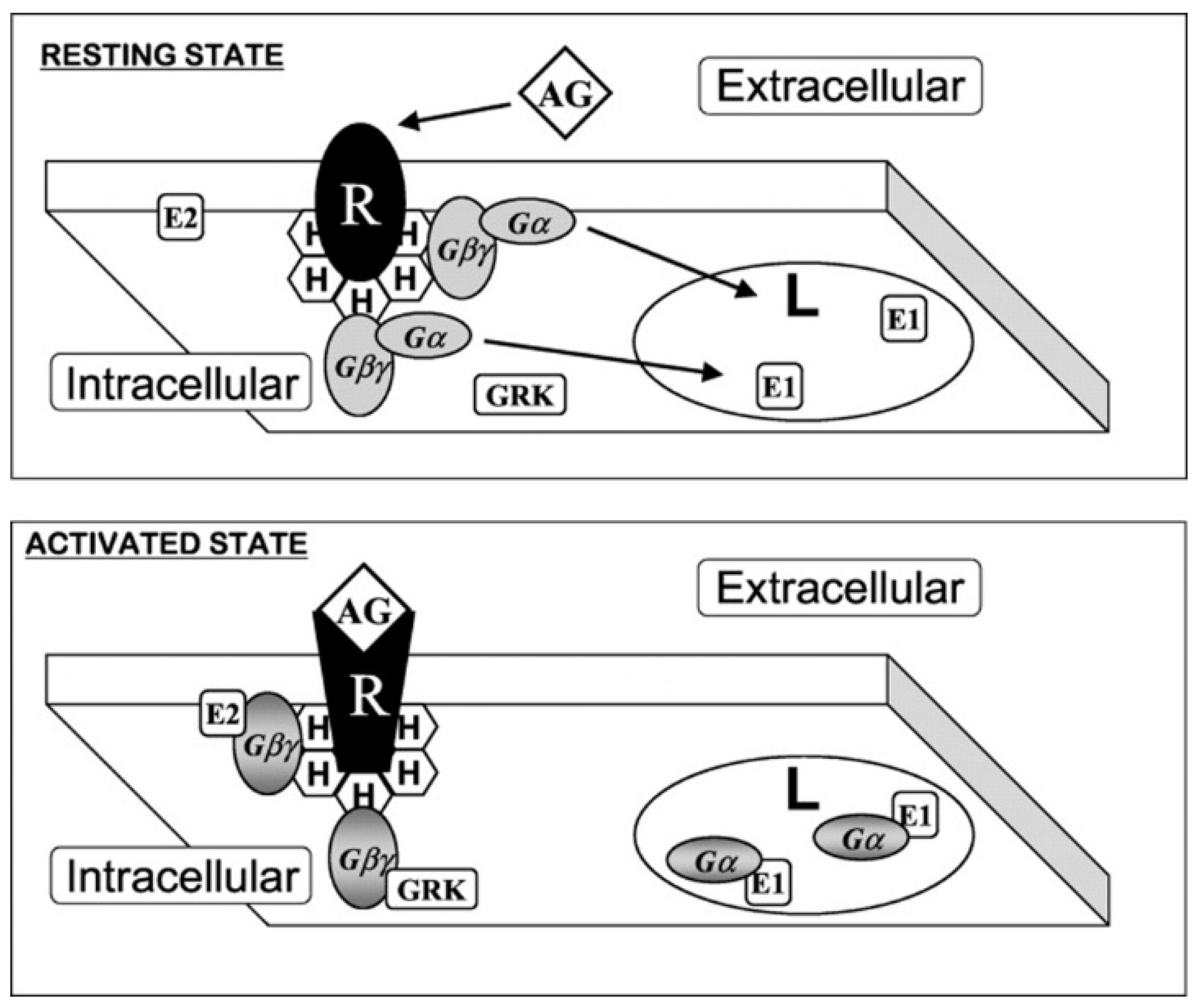

|
Target Element |
Therapeutic Molecule | Indication | Mechanism of Action | Status | Reference |
| Free Cho | Statins | Inhibition of pathogen replication | Inhibition of 3-hydroxy-3-methyl-glutaryl-CoaA reductase | IV/M for other indications | [388,389,390,391] NCT03971019 |
| Fatty acid biosynthesis and lipid droplets | 5-tetradecyloxy-2-furoic acid (TOFA) | Blocking replication of HCMV and influenza A virus | Inhibition of ACC | IV | [392,393,394,395] |
| CeruleninC75 | DENV, WNV, USUV and FHV viruses | Specific inhibition of different FASN activities | IV | [396] | |
| A939572 (piperidine–aryl urea-based inhibitor) | HCV and DENV infection | Specific inhibition of SCD1 | IV | [397,398,399] | |
| Specific lipids on the lipid envelope of the host or the pathogen | Cho-specific antibodies | Viral and bacterial infection | Membrane remodeling induced by Cho-specific antibodies on the target cells | IV/M for other indications | [400] |
| Phospahtidylserine specific antibodies | Arenavirus and CMV infection | Targeting of a pre-apoptotic event in cells infected by a variety of viruses | CT | [363,364,401] | |
| Membrane fluidity | Glycyrrhizin | A 5% decrease in fluidity reduces HIV infectivity by 56% | Saponin, structurally similar to Cho, promotes changes in the mobility of the lipids and modulates fusion processes | IV | [402,403,404] |
| Fattiviracin FV | Broad antiviral | Neutral glycolipid isolated from Streptomycetes that promotes changes in lipid mobility | IV | [405] | |
| Cepharantine | Inhibition of HIV infection and transmission | Natural plant alkaloid promoting changes in lipid mobility | IV/M for other indications | [406] | |
| Trimeric coumarin GUT-70 | Inhibition of HIV entry | Natural product derived from the stem bark of Chlophyllum Brasiliense promoting changes in lipid mobility | IV | [407] | |
| Gemfibrocil, lovastatin, fluvastatin, atorvastatin, pravastatin, simvastatin HMGCR-RNAi | Dengue, parainfluenza, Sendia virus | Cho lowering agents affecting Cho metabolism and lipid rafts, inhibiting the viral cell cycle | IV/M for other indications | [408,409] | |
| Treatment with sphingomyelinase (SMase), or by exogenous addition of long-chain Cer | Japanese encephalitis virus, HIV-1, HCV, Sindbis virus, rhinovirus | Modulating the fusion processes for viral entry and/or the exit of new virions | IV | [410,411,412] | |
| Hexanol benzyl alcohol and A2C | Inhibition of bacterial (e.g., Helicobacter pylori) and non-virus pathogen (e.g., Leishmania spp) infection | Promotes changes in lipid mobility and prevents bacterial adhesion | IV | [413,414,415,416,417,418] | |
| AMPs most studied groups are cationic α-helical polypeptides | Effective agents against a variety of Gram-positive and -negative bacteria, fungi, and protozoans | Most AMPs belong to the class of membrane-active peptides. AMPs penetrate bacterial membranes, causing membrane destabilization and bacterial death while reducing possible bacterial drug resistance. Current strategies to improve the design of AMPs as human medicines is their local delivery combining device coatings and nanomaterials Cationic α-helical polypeptides interact with negatively charged cell membranes through electrostatic interactions resulting in membrane adsorption and conformational changes | M | [419,420,421,422,423] | |
| Distribution of receptors and co-receptors | Increase in Cer content | Blocking HIV fusion | Induction of CD4 receptor clustering and the prevention of co-receptors engagement | IV | [410] |
| Lipid rafts | ACHAs (IgG type monoclonal) | HIV-1 | Sequestration of Cho or sphingomyelin preventing selective budding from glycolipid-enriched membrane lipid rafts | IV/M for other indications | [400] |
| Cyclodextrin and derivatives | HIV-1, SARS-CoV-2, Helicobacter pylori, and other bacteria | Sequestration of Cho or sphingomyelin, reduction in lipid raft stability, and protection against pore-forming activities | IV/M for other indications | [424,425,426,427] | |
| Statins | Broad inhibition of bacterial (Helicobacter pylori, Pneumonia, etc.) and viral (SARS-CoV-2) infection | Reduction in Cho or sphingomyelin biosynthesis and reduction in lipid raft stability | IV/M for other indications | [390] | |
| AIBP | SARS-CoV-2 | Stimulation of Cho efflux in cells that are Cho-loaded or infected and a reduction in lipid raft abundance to the “healthy level” but not reducing it beyond that or affecting healthy cells | IV | [428,429] | |
| Clomiphene and toremifene | Ebola virus, Zika virus | Selective estrogen modulators altering lipid rafts | IV/M for other indications | [430] | |
| GW3965 (liver X receptor agonist) | HCV | Stimulation of ABCA1 expression, regulation of Cho or sphingolipids, and alteration of lipid rafts | IV/M for other indications | [431] | |
| Dynasore | BPV1, HIV, HPV16, HSV, Trueperella pyogenes | Impairment of Cho trafficking and disruption of lipid raft organization | IV | [432,433,434,435,436] | |
| Lipid-based defense strategies in human hosts (immune system and host cell) | Cyclodextrin and derivatives | Virus and bacteria | Anti-inflammatory properties | IV/M for other indications | [437] |
| Colchicine | SARS-CoV-2 | Anti-inflammatory properties for symptomatic treatment | CT | [438] | |
| Filamentous bacteriophages | Stimulation of immune response | Carriers of immunologically active lipids and antigenic peptides | IV/PCS | [439] | |
| AIBP | HIV | Anti- inflammatory properties | IV/PCS | [440] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, M.; Parets, S.; Fernández-Díaz, J.; Beteta-Göbel, R.; Rodríguez-Lorca, R.; Román, R.; Lladó, V.; Rosselló, C.A.; Fernández-García, P.; Escribá, P.V. Lipids in Pathophysiology and Development of the Membrane Lipid Therapy: New Bioactive Lipids. Membranes 2021, 11, 919. https://doi.org/10.3390/membranes11120919
Torres M, Parets S, Fernández-Díaz J, Beteta-Göbel R, Rodríguez-Lorca R, Román R, Lladó V, Rosselló CA, Fernández-García P, Escribá PV. Lipids in Pathophysiology and Development of the Membrane Lipid Therapy: New Bioactive Lipids. Membranes. 2021; 11(12):919. https://doi.org/10.3390/membranes11120919
Chicago/Turabian StyleTorres, Manuel, Sebastià Parets, Javier Fernández-Díaz, Roberto Beteta-Göbel, Raquel Rodríguez-Lorca, Ramón Román, Victoria Lladó, Catalina A. Rosselló, Paula Fernández-García, and Pablo V. Escribá. 2021. "Lipids in Pathophysiology and Development of the Membrane Lipid Therapy: New Bioactive Lipids" Membranes 11, no. 12: 919. https://doi.org/10.3390/membranes11120919
APA StyleTorres, M., Parets, S., Fernández-Díaz, J., Beteta-Göbel, R., Rodríguez-Lorca, R., Román, R., Lladó, V., Rosselló, C. A., Fernández-García, P., & Escribá, P. V. (2021). Lipids in Pathophysiology and Development of the Membrane Lipid Therapy: New Bioactive Lipids. Membranes, 11(12), 919. https://doi.org/10.3390/membranes11120919








