Fundamentals of Membrane Lipid Replacement: A Natural Medicine Approach to Repairing Cellular Membranes and Reducing Fatigue, Pain, and Other Symptoms While Restoring Function in Chronic Illnesses and Aging
Abstract
1. Introduction: Why Membrane Lipid Replacement (MLR)
2. Biological Membranes and GPL
3. Mitochondria and Their Membranes
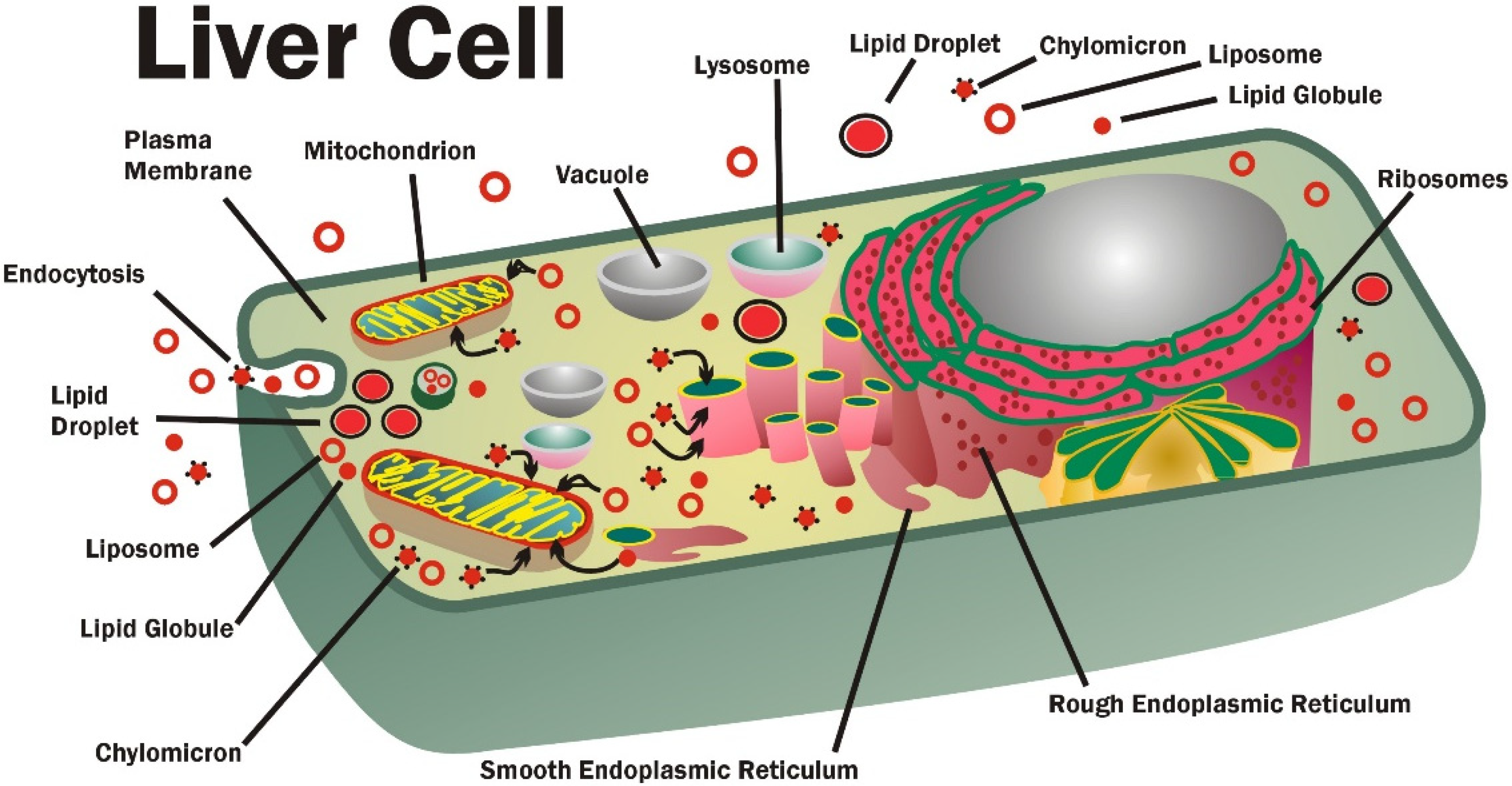
4. Liposomes, Chylomicrons, Lipid Globules, Lipid Micelles, and GPL Transport
5. MLR Formulations
6. Safety of MLR
7. MLR in Aging
8. MLR in Fatiguing Illnesses
9. MLR in Pain Control
10. MLR in Degenerative Diseases
11. MLR in Metabolic and Cardiovascular Diseases
12. MLR in Other Clinical Conditions
13. MLR: Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABR | auditory brainstem responses |
| AD | Alzheimers disease |
| AGEs | advanced glycation end products |
| CAPD | chronic ambulatory peritoneal dialysis |
| CFS | chronic fatigue syndrome |
| CL | cardiolipin |
| CoQ10 | coenzyme Q10 |
| CVD | cardiovascular disease |
| DAG | diacylglycerol |
| DAMPs | damage associated molecular patterns |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| EPL | essential phospholipids |
| ETC | electron transport chain |
| FA | fatty acid |
| FDA | US Federal Drug Administration |
| GPL | glycerolphospholipids |
| GRAS | generally recognized as safe |
| HDL | high density lipoproteins; HNE 4-hydroxynonenal |
| IL | interleukin |
| MDA | malondialdehyde |
| MetSyn | metabolic syndrome |
| MIM | mitochondrial inner membrane |
| MLR | membrane lipid replacement |
| mPTP | mitochondrial permeability transition pores |
| mtDNA | mitochondrial DNA |
| NCD | non-communicable diseases |
| NF-κB | nuclear factor kappa B |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PG | phosphatidylglycerol |
| PI | phosphatidylinositol |
| PS | phosphatidylserine |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| TNFα | tumor necrosis factor alpha |
| T2D | type 2 diabetes |
References
- Nicolson, G.L.; Ash, M.E. Membrane Lipid Replacement for chronic illnesses, aging and cancer using oral glycerolphospholipid formulations with fructooligosaccharides to restore phospholipid function in cellular membranes, organelles, cells and tissues. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1704–1724. [Google Scholar] [CrossRef]
- Nicolson, G.L. Membrane Lipid Replacement: Clinical studies using a natural medicine approach to restoring membrane function and improving health. Intern. J. Clin. Med. 2016, 7, 133–143. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Rosenblatt, S.; Ferreira de Mattos, G.; Settineri, R.; Breeding, P.C.; Ellithorpe, R.R.; Ash, M.E. Clinical uses of Membrane Lipid Replacement supplements in restoring membrane function and reducing fatigue in chronic illnesses and cancer. Discoveries 2016, 4, e54. [Google Scholar] [CrossRef]
- Escribá, P.V. Membrane-lipid therapy: A new approach in molecular medicine. Trends Mol. Med. 2006, 12, 34–43. [Google Scholar] [CrossRef]
- Ibarguren, M.; López, D.J.; Escribá, P.V. The effect of natural and synthetic fatty acids on membrane structure, cellular functions and human health. Biochim. Biophys. Acta 2014, 1838, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Gundermann, K.J. The “Essential” Phospholipids as a Membrane Therapeutic; European Society of Biochemical Pharmacology: Szcecin, Poland, 1993. [Google Scholar]
- Küllenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipids Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef]
- Edidin, M. Lipids on the frontier: A quarter century of cell-membrane bilayers. Nat. Rev. Mol. Cell Biol. 2003, 4, 414–418. [Google Scholar] [CrossRef]
- Tarnopolsky, M.; van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar]
- Ibarguaren, M.; López, D.J.; Escribá, P.V.; Tekpli, X.; Holme, J.A.; Sergent, O.; Lagadic-Gossmann, D. Role for membrane in cell death: Implication for health and disease. Toxicology 2013, 304, 141–157. [Google Scholar]
- Alvarez, R.; López, D.J.; Casas, J.; Liadó, V.; Higuera, M.; Barceló, M.; Busquets, X.; Escribá, P.V. G protein-membrane interactions: Gai1 myristoyl and palmitoyl modifications in protein-lipid interactions and its implications in membrane microdomain localization. Biochim. Biophys. Acta 2015, 1851, 1511–1520. [Google Scholar] [CrossRef] [PubMed]
- Escribá, P.V.; González-Ros, J.M.; Goñi, F.M.; Kinnunen, J.; Vigh, L.; Sánchez-Magraner, L.; Fernández, A.M.; Busquets, X.; Horváth, I.; Barceló-Coblijn, G. Membranes: A meeting point for lipids, proteins and therapies. J. Cell Mol. Med. 2008, 12, 829–875. [Google Scholar] [CrossRef]
- Knight, J.A. Diseases related to oxygen-derived free radicals. Ann. Clin. Lab. Sci. 1995, 25, 111–121. [Google Scholar] [PubMed]
- Gutteridge, J.M.C. Free radicals in disease processes: A compilation of cause and consequence. Free Rad. Res. Commun. 1993, 19, 141–158. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1953, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Kaur, H.; Halliwell, B. Oxygen free radicals and human diseases. J. Royal Soc. Health 1991, 111, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Catalá, A. Lipid peroxidation modifies the picture of membranes from the “Fluid Mosaic Model” to the “Lipid Whisker Model”. Biochimie 2012, 94, 101–109. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Hagen, T.M.; Ames, B.N. Oxidative damage and mitochondrial decay in aging. Proc. Nat. Acad. Sci. USA 1994, 91, 10771–10778. [Google Scholar] [CrossRef]
- Adam-Vizi, V.; Chinopoulos, C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol. Sci. 2006, 27, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.H.; Lee, H.C. Oxidative stress, mitochondrial DNA mutation and impairment of antioxidant enzymes in aging. Exp. Biol. Med. 2002, 227, 671–682. [Google Scholar] [CrossRef]
- Gems, D.; Partridge, L. Stress-response hormesis and aging: That which does not kill us makes us stronger. Cell Metabol. 2008, 7, 200–203. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Ruggiero, F.M. Mitochondrial dysfunction in brain aging: Role of oxidative stress and cardiolipin. Neurochem. Intern. 2011, 58, 447–457. [Google Scholar] [CrossRef]
- Arteel, G.E. Leveraging oxidative stress questions in vivo: Implications and limitations. Arch. Biochem. Biophys. 2016, 595, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Gilgun–Sherki, Y.; Melamed, E.; Offen, D. The role of oxidative stress in the pathogenesis of multiple sclerosis: The need for effective antioxidant therapy. J. Neurol. 2004, 251, 261–268. [Google Scholar]
- Adibhatla, R.M.; Hatcher, J.F. Lipid oxidation and peroxidation in CNS health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2010, 12, 125–169. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [CrossRef] [PubMed]
- Shibata, T.; Iio, K.; Kawai, Y.; Shibata, N.; Kawaguchi, M.; Toi, S.; Kobayashi, M.; Yamamoto, K.; Uchida, K. Identification of a lipid peroxidation product as a potential trigger of the p53 pathway. J. Biol. Chem. 2006, 281, 1196–1204. [Google Scholar] [CrossRef]
- Zierenberg, O.; Grundy, S.M. Intestinal absorption of polyenephosphatidylcholine in Man. J. Lipid Res. 1982, 23, 1136–1142. [Google Scholar] [CrossRef]
- Dobbins, W.O., III. Morphologic aspects of lipid absorption. Am. J. Clin. Nutr. 1969, 22, 257. [Google Scholar] [CrossRef]
- Iqbal, J.; Hussain, M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef]
- Carey, M.C.; Small, D.M.; Bliss, C.M. Lipid digestion and absorption. Annu. Rev. Physiol. 1983, 45, 651–677. [Google Scholar] [CrossRef]
- Mayor, S.; Presley, J.F.; Maxfield, F.R. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J. Cell Biol. 1993, 121, 1257–1269. [Google Scholar] [CrossRef]
- Ferreira, G.; Costa, C.; Bassaizteguy, V.; Santos, M.; Cardozo, R.; Montes, J.; Settineri, R.; Nicolson, G.L. Incubation of human sperm with micelles made from glycerophospholipid mixtures increases sperm motility and resistance to oxidative stress. PLoS ONE 2018, 13, e0197897. [Google Scholar] [CrossRef]
- Shevchenko, A.; Simons, K. Lipidomics: Coming to grips with lipid diversity. Nat. Rev. Mol. Cell Biol. 2010, 11, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Kinnunen, P.K.J. Biophysical properties of lipids and dynamic membranes. Trends Cell Biol. 2006, 16, 538–546. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Ferreira de Mattos, G. A brief introduction to some aspects of the Fluid—Mosaic Model of cell membrane structure and its importance in Membrane Lipid Replacement. Membranes 2021. accepted. [Google Scholar]
- Lagoutte-Renosi, J.; Allemand, F.; Ramseyer, C.; Rabani, V.; Davani, S. Influence of anti-platent agents on the composition of platelet plasma membrane: A lipidomics approach with ticagrelor and its active metabolite. Intern. J. Mol. Sci. 2021, 22, 1432. [Google Scholar] [CrossRef]
- Cantu, L.; Corti, M.; Brocca, P.; Del Favero, E. Structural aspects of ganglioside-containing membranes. Biochim. Biophys. Acta 2009, 1788, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Op den Kamp, J.A.F. Lipid asymmetry in membranes. Annu. Rev. Biochem. 1979, 48, 47–71. [Google Scholar] [CrossRef]
- Bagatolli, L.A.; Ipsen, J.H.; Simonsen, A.C.; Mouritsen, O.G. An outlook on organization of lipids in membranes: Searching for a realistic connection with the organization of biological membranes. Prog. Lipid Res. 2010, 49, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J.; Wolf, C. The liquid-ordered phase in membranes. Biochim. Biophys. Acta 2009, 1788, 33–46. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. The Fluid—Mosaic Model of membrane structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than forty years. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Simmons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Simmons, K.; Gerl, M.J. Revitalizing membrane rafts: New tools and insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Holte, L.L.; Peter, S.A.; Sinnwell, T.M.; Gawrisch, K. 2H Nuclear magnetic resonance order parameter profiles suggest a change of molecular shape for phosphatidylcholines containing a polyunsaturated acyl chain. Biophys. J. 1995, 68, 2396–2403. [Google Scholar] [CrossRef]
- Hille, B.; Armstrong, C.M.; MacKinnon, R. Ion channels: From idea to reality. Nat. Med. 1999, 5, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, B.; Cortassa, S.; Aon, M.A. Mitochondrial ion channels: Gatekeepers of life and death. Physiology 2005, 20, 303–315. [Google Scholar] [CrossRef]
- Stubbs, C.D.; Smith, A.D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim. Biophys. Acta 1984, 779, 89–137. [Google Scholar] [CrossRef]
- Chapman, D. Phase transitions and fluidity characteristics of lipids and cell membranes. Quart. Rev. Biophys. 1975, 8, 185–235. [Google Scholar] [CrossRef] [PubMed]
- Pelech, S.L.; Vance, D.E. Regulation of phosphatidylcholine biosynthesis. Biochim. Biophys. Acta 1984, 779, 217–251. [Google Scholar] [CrossRef]
- Vance, J.E.; Tasseva, G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta 2013, 1831, 543–554. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Bloom, M. Mattress model of lipid-protein interactions in membranes. Biophys. J. 1984, 46, 141–153. [Google Scholar] [CrossRef]
- Goni, F.M. The basic structure and dynamics of cell membranes: An update of the Singer-Nicolson model. Biochim. Biophys. Acta 2014, 1838, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, A.; Fujiwara, T.K.; Chadda, R.; Xie, M.; Tsunoyama, T.A.; Kalay, Z.; Kasai, R.S.; Suzuki, K.G. Dynamic organizing principals of the plasma membrane that regulate signal transduction: Commemorating the fortieth anniversary of Singer and Nicolson’s fluid-mosaic model. Annu. Rev. Cell Dev. Biol. 2012, 28, 215–250. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, S. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Bohovych, I.; Khalimonchuk, O. Sending out an SOS: Mitochondria as a signalling hub. Front. Cell Dev. Biol. 2016, 4, a109. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Monteiro, J.P.; Oliveira, P.J.; Jurado, A.S. Mitochondrial membrane lipid 30luminium30g in pathophysiology: A new target for diet and therapeutic interventions. Prog. Lipid Res. 2013, 52, 513–528. [Google Scholar] [CrossRef]
- Papa, S. Mitochondrial oxidative phosphorylation changes in the life span. Molecular aspects and physiopathological implications. Biochim. Biophys. Acta 1996, 1276, 87–105. [Google Scholar] [CrossRef]
- Xu, F.Y.; McBride, H.; Acehan, D.; Vaz, F.M.; Houtkooper, R.H.; Lee, R.M.; Mowat, M.A.; Hatch, G.M. The dynamics of cardiolipin synthesis post-mitochondrial fusion. Biochim. Biophys. Acta 2010, 1798, 1577–1585. [Google Scholar] [CrossRef][Green Version]
- Petrosillo, G.; Ruggiero, F.M.; Paradies, G. Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J. 2003, 17, 2202–2208. [Google Scholar] [CrossRef]
- Jiang, F.; Ryan, M.; Schlame, M.; Zhao, M.; Gu, Z.; Klingenberg, M.; Pfanner, N.; Greenberg, M.L. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J. Biol. Chem. 2000, 275, 22387–22394. [Google Scholar] [CrossRef]
- Lapuente-Brun, E.; Moreno-Loshuertos, R.; Acin-Pérez, R.; Latorre-Pellicer, A.; Colás, C.; Balsa, E.; Perales-Clemente, E.; Quirós, P.M.; Calvo, E.; Rodriguez-Hernández, M.A.; et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 2013, 340, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Chicco, A.J.; Sparagna, G.C. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am. J. Physiol. Cell Physiol. 2007, 292, C33–C44. [Google Scholar] [CrossRef]
- Rodriguez-Enriquez, S.; He, L.; Lemasters, J.J. Role of mitochondrial permeability transition pores in mitochondrial autophagy. Intern. J. Biochem. Cell Biol. 2004, 36, 2463–2472. [Google Scholar] [CrossRef]
- Kluck, R.M.; Bossy-Wetzel, E.; Green, D.R.; Newmeyer, D.D. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science 1997, 275, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.M.; Stotland, A.; Queliconi, B.B.; Gottlieb, R.A. A time to reap, a time to sow: Mitophagy and biogenesis in cardiac pathophysiology. J. Mol. Cell. Cardiol. 2015, 1, 62–72. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Bauernfeind, F.; Hornung, V. Of inflammasomes and apathogens—sensing of microbes by the inflammasome. EMBO Mol. Med. 2013, 5, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Bauemfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and 30luminium salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Alnemri, T.; Wu, J.; Yu, J.W.; Datta, P.; Mille, B.; Jankowski, W.; Rosenberg, S.; Zhang, J.; Alnemri, E.S. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007, 14, 1590–1604. [Google Scholar] [CrossRef] [PubMed]
- Faas, M.M.; de Vos, P. Mitochondrial function in immune cells in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165845. [Google Scholar] [CrossRef] [PubMed]
- Mignotte, B.; Vayssiere, J.L. Mitochondria and apoptosis. Eur. J. Biochem. 1998, 252, 1–15. [Google Scholar] [CrossRef]
- Al-Gubory, K.H. Mitochondria: Omega-3 in the route of mitochondrial reactive oxygen species. Intern. J. Biochem. Cell Biol. 2012, 44, 1569–1573. [Google Scholar] [CrossRef]
- Zeeshan, H.M.A.; Lee, G.H.; Kim, H.R.; Chae, H.J. Endoplasmic reticulum stress and associated ROS. Intern. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Jacobson, J.; Duchen, M.R. Mitochondrial oxidative stress and cell death in astrocytes—requirement for stored Ca2+ and susptained opening the permeability transition pore. J. Cell Sci. 2002, 115, 1175–1188. [Google Scholar] [CrossRef]
- Seidlmayer, L.K.; Juettner, V.V.; Kettlewell, S.; Pavlov, E.V.; Blatter, L.A.; Dedkova, E.N. Distinct mPTP activation mechanisms in ischaemia–reperfusion: Contribution of Ca2+, ROS, pH and inorganic polyphosphate. Cardiovas. Res. 2015, 106, 237–248. [Google Scholar] [CrossRef]
- Khairallah, R.J.; Kim, J.; O’Shea, K.M.; O’Connell, K.A.; Brow, B.H.; Galvao, T.; Daneault, C.; Des Rosiers, C.; Polster, B.M.; Hoppel, C.L.; et al. Improved mitochondrial function with diet-induced increase in either docosahexaenoic acid or arachidonic acid in membrane phospholipids. PLoS ONE 2012, 7, e34402. [Google Scholar] [CrossRef]
- Liao, T.H.; Hamosh, P.; Hamosh, M. Fat digestion by lingual lipase: Mechanism of lipolysis in the stomach and upper small intestine. Pediatric Res. 1984, 18, 402–409. [Google Scholar] [CrossRef]
- Zierenberg, G.; Odenthal, J.; Betzing, H. Incorporation of PPC into serum lipoproteins after oral or i.v. administration. Atherosclerosis 1979, 34, 259–276. [Google Scholar]
- Patton, J.S. Gastrointestinal lipid digestion in physiology of the gastrointestinal tract. In Physiology of the Gastrointestinal Tract; Johnson, L.R., Ed.; Raven Press: New York, NY, USA, 1981; pp. 1123–1146. [Google Scholar]
- Murota, K. Digestion and absorption of dietary glycerophospholipids in the small intestine: Their significance as carrier molecules of choline and n-3 polyunsaturated fatty acids. Biocatal. Agricult. Biotechnol. 2020, 26, 101633. [Google Scholar] [CrossRef]
- Wollaeger, E.E. Fat, faeces and the importance of the ileum. Proc. Mayo Clin. 1973, 48, 833–843. [Google Scholar]
- Hendry, G.A.F. Evolutionary origins and natural functions of fructans—A climatological, biogeographic and mechanistic appraisal. New Phytol. 1993, 123, 3–14. [Google Scholar] [CrossRef]
- Vereyken, I.J.; Chupin, V.; Demel, R.A.; Smeekens, S.C.M.; De Druijff, B. Fructans insert between the headgroups of phospholipids. Biochim. Biophys. Acta 2001, 1310, 307–320. [Google Scholar] [CrossRef]
- Rigler, M.W.; Honkanen, R.E.; Patton, J.M. Visualization by freeze fracture, in vitro and in vivo of the products of fat digestion. J. Lipid Res. 1986, 27, 836–857. [Google Scholar] [CrossRef]
- Rostgaard, J.; Barrnett, R.J. Fine structural observations of the absorption of lipid particles in the small intestine of the rat. Anat. Record 1965, 152, 325–349. [Google Scholar] [CrossRef] [PubMed]
- Dermer, G.B. Ultrastructural changes in the microvillous plasma membrane during lipid absorption and the form of absorbed lipid: An in vitro study at 37 °C. J. Ultrastruct. Res. 1967, 20, 311–320. [Google Scholar] [CrossRef]
- Ehehalt, R.; Braun, A.; Karner, M.; Füllekrug, J.; Stremmel, W. Phosphatidylcholine as a constituent in the colonic mucosal barrier—physiological and clinical relevance. Biochim. Biophys. Acta 2010, 1801, 983–993. [Google Scholar] [CrossRef]
- Contreras, F.-X.; Sánchez-Magraner, L.; Alonso, A.; Goñi, F.M. Transbilayer (flip-flop) lipid motion and lipid scrambling in membranes. FEBS Lett. 2010, 584, 1779–1786. [Google Scholar] [CrossRef]
- Tanaka, K.; Fujimura-Kamada, K.; Yamamoto, T. Functions of phospholipid flippases. J. Biochem. 2011, 149, 131–143. [Google Scholar] [CrossRef]
- Lev, S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat. Rev. Mol. Cell. Biol. 2010, 11, 739–750. [Google Scholar] [CrossRef]
- Penno, A.; Hackenbroich, G.; Thiele, C. Phospholipids and lipid droplets. Biochim. Biophys. Acta 2013, 1831, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Toulmay, A.; Prinz, W.A. Membrane contact sites, gateways for lipid homeostasis. Curr. Opin. Cell Biol. 2015, 33, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Toulmay, A.; Prinz, W.A. Lipid transfer and signaling at organelle contact sites: The tip of the iceberg. Curr. Opin. Cell Biol. 2011, 23, 458–463. [Google Scholar] [CrossRef]
- Prinz, W.A. Lipid trafficking sans vesicles: Where, why, how? Cell 2010, 143, 870–874. [Google Scholar] [CrossRef]
- Poloncová, K.; Griac, P. Phospholipid transport and remodeling in health and disease. Gen. Physiol. Biophys. 2011, 30, S25–S35. [Google Scholar] [CrossRef]
- Osman, C.; Voelker, D.R.; Langer, T. Making heads or tails of phospholipids in mitochondria. J. Cell Biol. 2011, 192, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Schlattner, U.; Tokarska-Schlattner, M.; Rousseau, D.; Boissan, M.; Mannella, C.; Epand, R.; Lacombe, M.-L. Mitochondrial cardiolipin/phospholipid trafficking: The role of membrane contact site complexes and lipid transfer proteins. Chem. Phys. Lipids 2014, 179, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Samuel, A.Z.; Miyaoka, R.; Ando, M.; Gaebler, A.; Thiele, C.; Takeyama, H. Molecular profiling of lipid droplets inside HuH7 cells with Raman micro-spectroscopy. Commun. Biol. 2020, 3, 372. [Google Scholar] [CrossRef]
- Leonov, A.; Titorenko, V.I. A network of interorganellar communications underlies cellular aging. IUBMB Life 2013, 65, 665–674. [Google Scholar] [CrossRef]
- Helle, S.C.J.; Kanfer, G.; Kolar, K.; Lang, A.; Michel, A.H.; Kornmann, B. Organization and function of membrane contact sites. Biochim. Biophys. Acta 2013, 1833, 2526–2541. [Google Scholar] [CrossRef]
- Pol, A.; Gross, S.F.; Parton, R.G. Biogenesis of the multifunctional lipid droplet: Lipids, proteins and sites. J. Cell Biol. 2014, 204, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Goodman, J.M. The lipid droplet—a well-connected organelle. Front. Cell Devel. Biol. 2015, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Krahmer, M.; Farese, R.V.; Walther, T.C. Balancing the fat: Lipid droplets and hman disease. EMBO Mol. Med. 2013, 5, 905–915. [Google Scholar] [CrossRef]
- Bhagat, U.; Das, U.N. Potential role of dietary lipids in the prophylaxis of some clinical conditions. Arch. Med. Sci. 2015, 11, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Zilversmit, D.B. The composition and structure of lymph chylomicrons in dog, rat and man. J. Clin. Investig. 1965, 44, 1610–1622. [Google Scholar] [CrossRef]
- Barrnett, R.J. Brush border membranes. Yale J. Biol. Med. 1983, 56, 347–348. [Google Scholar]
- Child, P.; Myher, J.J.; Kuypers, F.A.; Op den Kamp, J.A.; van Deenen, L.L. Acyl selectivity in the transfer of molecular species of phosphatidylcholines from human erythrocytes. Biochim. Biophys. Acta 1985, 812, 321–322. [Google Scholar] [CrossRef]
- Liu, P.S.; Barts, R.; Li, W.H.; Venables, B.; Zehmer, J.K.; Roth, M.R.; Welti, R.; Anderson, R.G.W.; Chapman, K.D. Lipodomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 2007, 48, 837–847. [Google Scholar]
- Welti, R.; Helmkamp, G.M., Jr. Acyl chain specificity of phosphatidylcholine transfer protein from bovine liver. J. Biol. Chem. 1984, 259, 6937–6941. [Google Scholar] [CrossRef]
- Child, P.; Op den Kamp, J.A.; Roelofsen, B.; van Deenen, L.L. Molecular species compositon of membrane phosphatidylcholine influences the rate of cholesterol efflux from human erythrocytes and vesicles of erythrocyte lipid. Biochim. Biophys. Acta 1985, 814, 237–246. [Google Scholar] [CrossRef]
- Borodin, E.A.; Lanio, M.E.; Khalilov, E.M.; Markin, S.S.; Thorkhovskaya, T.I.; Rozkin, M.M.; Sapelkina, I.M.; Kulakova, S.N.; Levechev, M.M.; Archakov, A.I.; et al. Cholesterol removal from biological membranes by positively charged phosphatidylcholine micelles. Bull. Exp. Biol. Med. 1985, 2, 164–166. [Google Scholar]
- Nierle, W.; el Wahab el Baya, A. Examination and composition of some legume seeds. Z. lebensm. Unters. Forsch. 1977, 164, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E. Importance of n-3 fatty acids in health and disease. Am. J. Clin. Nutr. 2000, 71, S171–S178. [Google Scholar] [CrossRef]
- Schmidt, J.; Skou, E.B.; Christensen, H.A.; Dyerberg, J.H. N-3 fatty acids from fish and coronary artery disease: Implications for public health. Public Health Nutr. 2000, 3, 91–98. [Google Scholar] [CrossRef]
- Sparagna, G.C.; Lesnefsky, E.J. Cardiolipin remodeling in the heart. J. Cardiovasc. Pharmacol. 2009, 53, 290–301. [Google Scholar] [CrossRef]
- O’Shea, K.M.; Khairallah, R.J.; Sparagna, G.C.; Xu, W.; Hecker, P.A.; Robillard-Frayne, I.; Des Rosiers, C.; Kristian, T.; Murphy, R.C.; Fiskum, G.; et al. Dietary omega-3 fatty acids alter cardiac mitochondrial phospholipids composition and delay Ca2+-induced mitochondrial permeability transition. J. Mol. Cell Cardiol. 2009, 47, 819–827. [Google Scholar] [CrossRef]
- Cernacchi, T.; Bertoldin, T.; Farina, C.; Flori, M.G.; Crepaldi, G. Cognitive decline in the elderly: A double-blind, placebo-controlled multicenter study on the efficacy of phosphatidylserine administration. Aging 1993, 5, 123–133. [Google Scholar]
- Jorissen, B.L.; Brouns, F.; van Boxtel, M.P.; Ponds, R.W.; Verhey, F.R.; Jolles, J.; Riedel, W.J. The influence of soy-derived phosphatidylserine on cognition in age-associated memory impairment. Nutr. Neurosci. 2001, 4, 121–134. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Settineri, R.; Ellithorpe, R. Lipid Replacement Therapy with a glycophospholipid formulation with NADH and CoQ10 significantly reduces fatigue in intractable chronic fatiguing illnesses and chronic Lyme disease. Intern. J. Clin. Med. 2012, 3, 163–170. [Google Scholar] [CrossRef]
- Nicolson, G.L. Mitochondrial dysfunction and chronic disease: Treatment with natural supplements. Alt. Ther. Health Med. 2014, 20, 18–25. [Google Scholar]
- Polinsky, A.J.; Ebert, M.; Cain, E.D.; Ludlow, C.; Bassich, C.J. Cholinergic treatment in Tourette syndrome. N. Engl. J. Med. 1980, 302, 1310–1311. [Google Scholar]
- Petera, V.; Prokop, V. The compensated cirrhosis of the liver. Therapeutic experience with Essentiale® forte. Therapiewoche 1986, 36, 540–544. [Google Scholar]
- Seidman, M.D.; Khan, M.J.; Tang, W.X.; Quirk, W.S. Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol. Head Neck Surg. 2002, 127, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Ellithorpe, R.R.; Settineri, R.; Ellithorpe, T.; Nicolson, G.L. Blood homocysteine and fasting insulin levels are reduced and erythrocyte sedimentation rates are increased with a glycophospholipid-vitamin formulation: A retrospective study in older subjects. Funct. Food Health Dis. 2015, 5, 126–135. [Google Scholar] [CrossRef]
- Cohn, J.S.; Wat, E.; Kamili, A.; Tandy, S. Dietary phospholipids, hepatic metabolism and cardiovascular disease. Curr. Opin. Lipidol. 2008, 19, 257–262. [Google Scholar] [CrossRef]
- Federal Drug Administration. Scientific literature reviews on generally recognized as safe (GRAS) food ingredients: Lecithins. GRAS Rep. 1970, PB-241, 970. [Google Scholar]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Goldberg, J.; Currais, A.; Ates, G.; Huang, L.; Shokhirev, M.; Maher, P.; Schubert, D. Targeting of intracellular Ca2+ stores as a therapeutic strategy against age-related neurotoxicilties. NPI Aging Mech. Dis. 2020, 6, 10. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; del Idelchik, P.S.M.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Palikaras, K.; Tavernarakis, N. Mitophagy in neurodegeneration and aging. Front. Genet. 2012, 3, 297. [Google Scholar] [CrossRef]
- Franceschi, P.; Garangnani, G.; Vitale, G.; Capri, M.; Salvioli, S. Inflamminging and ‘Garb-aging’. Trends Endrocrinol. Metabol. 2016, 28, P199–P212. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; Van Remmen, H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef]
- Kirkwood, T.B.; Kowald, A. The free-radical theory of ageing—older, wiser and still alive: Modelling positional effects of the primary targets of ROS reveals new support. Bioessays 2012, 34, 692–700. [Google Scholar] [CrossRef]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Wu, S.B.; Wu, Y.T.; Wei, Y.H. Oxidative stress response elicited by mitochondrial dysfunction: Implication in the pathophysiology of aging. Exp. Biol. Med. 2013, 238, 450–460. [Google Scholar] [CrossRef]
- Ames, B.N.; Shigenaga, M.K.; Hagen, T.M. Mitochondrial decay in aging. Biochim. Biophys. Acta 1995, 1271, 154–170. [Google Scholar] [CrossRef]
- Gelino, S.; Hansen, M. Autophagy—An Emerging Anti-Aging Mechanism. J. Clin. Exp. Pathol. 2012, 4, 6. [Google Scholar] [CrossRef]
- Petrosillo, G.; Matera, M.; Moro, N.; Ruggiero, F.M.; Paradies, G. Mitochondrial complex I dysfunction in rat heart with aging: Critical role of reactive oxygen species and cardiolipin. Free Radic. Biol. Med. 2009, 46, 88–94. [Google Scholar] [CrossRef]
- Morrison, J.D. Fatigue as a presenting complaint in family practice. J. Family Pract. 1980, 10, 795–801. [Google Scholar]
- Agadjanyan, M.; Vasilevko, V.A.; Ghochikyan, A.; Berns, P.; Kesslak, P.; Settineri, R.; Nicolson, G.L. Nutritional supplement (NTFactor) restores mitochondrial function and reduces moderately severe fatigue in aged subjects. J. Chronic Fatigue Syndr. 2003, 11, 23–36. [Google Scholar] [CrossRef]
- Pamplona, R.; Barja, G.; Portero-Otín, M. Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: A homeoviscous-longevity adaptation? Ann. N. Y. Acad. Sci. 2002, 959, 475–490. [Google Scholar] [CrossRef]
- Spiekerkoetter, U.; Bastin, J.; Gillingham, M.; Morris, A.; Wijburg, F.; Wilcken, B. Current issues regarding treatment of mitochondrial fatty acid oxidation disorders. J. Inherit. Metab. Dis. 2010, 33, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Changes in the mitochondrial permeability transition pore in aging and age-associated diseases. Mech. Ageing Dev. 2013, 134, 1–9. [Google Scholar] [CrossRef]
- Kagan, V.E.; Chu, C.T.; Yyurina, Y.Y.; Cheikhi, A.; Bayir, H. Cardiolipin asymmetry, oxidation and signalling. Chem. Phys. Lipids 2014, 179, 64–69. [Google Scholar] [CrossRef]
- Claypool, S.M.; Oktay, Y.; Boontheung, P.; Loo, J.A.; Koehler, C.M. Cardiolipin defines the interactome of the major ADT/ATP carrier protein of the mitochondrial inner membrane. J. Cell Biol. 2008, 182, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Ostan, R.; Borelli, V.; Castellani, G.; Franceschi, C. Inflammaging and human longevity in the omics era. Mech. Ageing Dev. 2017, 165, 129–138. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Krysko, D.V.; Agostinis, P.; Krysko, O.; Garg, A.D.; Bachert, C.; Lambrecht, B.N.; Vandenabeele, P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 2010, 32, 157–164. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Vandenabeele, P.; Krysko, D.V. Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity 2013, 38, 209–223. [Google Scholar] [CrossRef]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Pelletier, M.; Lepow, T.S.; Billingham, L.K.; Murphy, M.P.; Siegel, R.M. New tricks from an old dog: Mitochondrial redox signaling in cellular inflammation. Semin. Immunol. 2012, 24, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef] [PubMed]
- Grivennikova, V.G.; Kareyeva, A.V.; Vinogradov, A.D. What are the sources of hydrogen peroxide production by heart mitochondria? Biochim. Biophys. Acta 2010, 1797, 939–944. [Google Scholar] [CrossRef]
- Pereira, C.A.; Carlos, D.; Ferreira, N.S.; Silva, J.F.; Zanotto, C.Z.; Zamboni, D.S.; Garcia, V.D.; Ventura, D.F.; Silva, J.S.; Tostes, R.C. Mitochondrial DNA promotes NLRP3 inflammasome activation and contributes to endothelial dysfunction and inflammation in Type 1 Diabetes. Front. Physiol. 2020, 10, 1557. [Google Scholar] [CrossRef]
- Chen, G.; Shaw, M.H.; Kim, Y.G.; Nunez, G. NOD-like receptors: Role in innate immunity and inflammatory disease. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 365–398. [Google Scholar] [CrossRef]
- Marty-Roix, R.; Lien, E. (De-)oiling inflammasomes. Immunity 2013, 38, 1088–1090. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Williams-Bey, Y.; Boularan, C.; Vural, A.; Huang, N.-N.; Hwang, I.-Y.; Shan-Shi, C.; Kehrl, J.H. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-κB activation and enhancing autophagy. PLoS ONE 2014, 9, e97957. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.K. Lipids, inflammasomes, metabolism, and disease. Immunol. Rev. 2020, 297, 108–122. [Google Scholar] [CrossRef]
- Karbowski, M. Mitochondria on guard: Role of mitochondrial fusion and fission in the regulation of apoptosis. Adv. Exp. Med. Biol. 2010, 687, 131–142. [Google Scholar] [PubMed]
- Bertholet, A.M.; Deierue, T.; Millet, A.M.; Moulis, M.F.; David, C.; Daloyau, C.M.; Arnauné-Pelloguin, L.; Davezac, N.; Mils, V.; Miguel, M.C.; et al. Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol. Dis. 2016, 90, 3–19. [Google Scholar] [CrossRef]
- Chan, D.C. Fusion and fission: Interlinked processes critical for mitochondrial health. Annu. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef]
- Nakada, K.; Inoue, K.; Ono, T.; Isobe, K.; Ogura, K.A.; Goto, Y.-I.; Nonaka, I.; Hayashi, J.-I. Inter-mitochondrial complementation: Mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nat. Med. 2001, 7, 934–940. [Google Scholar] [CrossRef]
- Arduíno, D.M.; Esteves, A.R.; Cardoso, S.M. Mitochondria drive autophagy pathology via microtubule disassembly: A new hypothesis for Parkinson disease. Autophagy 2013, 9, 112–114. [Google Scholar] [CrossRef]
- Westrate, L.M.; Drocco, J.A.; Martin, K.R.; Hlavacek, W.S.; MacKeigan, J.P. Mitochondrial morphological features are associated with fission and fusion events. PLoS ONE 2014, 9, e95265. [Google Scholar]
- Gazaryan, I.G.; Brown, A.M. Intersection between mitochondrial permeability pores and mitochondrial fusion/fission. Neurochem. Res. 2007, 32, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Frohman, M.A. Lipid signaling on the mitochondrial surface. Biochim. Biophys. Acta 2009, 1791, 839–844. [Google Scholar] [CrossRef]
- Hirose, A.; Terauchi, M.; Osaka, Y.; Akiyoshi, M.; Kato, K.; Miyasaka, N. Effect of soy lecithin on fatigue and menopausal symptoms in middle-aged women: A randomized, double-blind, placebo-controlled study. Nutrit. J. 2018, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L.; Settineri, R.; Ellithorpe, R. Neurodegenerative and fatiguing Illnesses, infections and mitochondrial dysfunction: Use of natural supplements to restore mitochondrial function. Funct. Foods Health Dis. 2014, 4, 23–65. [Google Scholar] [CrossRef]
- Orsucci, D.; Mancuso, M.; Ienco, E.C.; LoGerfo, A.; Siciliano, G. Targeting mitochondrial dysfunction and neurodegeneration by means of coenzyme Q10 and its analogues. Curr. Med. Chem. 2011, 18, 4053–4064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tamura, Y.; Roy, M.; Adachi, Y.; Iijima, M.; Sesaki, H. Biosynthesis and roles of phospholipids in mitochondrial fusion, division and mitophagy. Cell Mol. Life Sci. 2014, 71, 3767–3778. [Google Scholar] [CrossRef]
- Tapia, P.C. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “Mitohormesis” for health and vitality. Med. Hypotheses 2006, 66, 832–843. [Google Scholar]
- Brandhorst, S.; Longo, V.D. Protein quantity and source, fasting-mimicking diets, and longevity. Adv Nutr. 2019, 10, S340–S350. [Google Scholar] [CrossRef]
- Kroenke, K.; Wood, D.R.; Mangelsdorff, A.D.; Meier, N.J.; Powell, J.B. Chronic fatigue in primary care. Prevalence, patient characteristics, and outcome. JAMA 1998, 260, 929–934. [Google Scholar] [CrossRef]
- Krupp, L.B.; Pollina, D.A. Mechanisms and management of fatigue in progressive neurological disorders. Curr. Opin. Neurol. 1996, 9, 456–460. [Google Scholar] [CrossRef]
- Myhill, S.; Booth, N.E.; McLaren-Howard, J. Chronic fatigue syndrome and mitochondrial dysfunction. Intern. J. Clin. Exp. Med. 2009, 2, 1–16. [Google Scholar]
- Booth, N.E.; Myhill, S.; McLaren-Howard, J. Mitochondrial dysfunction and the pathophysiology of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Intern. J. Clin. Exp. Med. 2012, 5, 208–220. [Google Scholar]
- Logan, A.C.; Wong, C. Chronic fatigue syndrome: Oxidative stress and dietary modifications. Altern. Med. Rev. 2001, 6, 450–459. [Google Scholar] [PubMed]
- Manuel y Keenoy, B.; Moorkens, G.; Vertommen, J.; De Leeuw, I. Antioxidant status and lipoprotein peroxidation in chronic fatigue syndrome. Life Sci. 2001, 68, 2037–2049. [Google Scholar] [CrossRef]
- Fulle, S.; Mecocci, P.; Fano, G.; Vecchiet, I.; Racciotti, D.; Cherubini, A.; Pizzigallo, E.; Vecchiet, L.; Senin, U.; Beal, M.F. Specific oxidative alterations in vastus lateralis muscle of patients with the diagnosis of chronic fatigue syndrome. Free Radic. Biol. Med. 2000, 29, 1252–1259. [Google Scholar] [CrossRef]
- Richards, R.S.; Roberts, T.K.; McGregor, N.R.; Dunstan, R.H.; Butt, H.L. Blood parameters indicative of oxidative stress are associated with symptom expression in chronic fatigue syndrome. Redox Rep. 2000, 5, 35–41. [Google Scholar] [CrossRef]
- Pall, M.L. Elevated, sustained peroxynitrite levels as the cause of chronic fatigue syndrome. Med. Hypoth. 2000, 54, 115–125. [Google Scholar] [CrossRef]
- Nicolson, G.L. Lipid replacement therapy: A nutraceutical approach for reducing cancer-associated fatigue and the adverse effects of cancer therapy while restoring mitochondrial function. Cancer Metastasis Rev. 2010, 29, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Prue, G.; Rankin, J.; Allen, J.; Gracey, J.; Cramp, F. Cancer-related fatigue: A critical appraisal. Eur. J. Cancer 2006, 42, 846–863. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Marler, M.R.; Parker, B.A.; Jones, V.; Johnson, S.; Cohen-Zion, M.; Firoentino, L.; Sadler, G.R.; Ancoli-Israel, S. The relationship between fatigue and light exposure during chemotherapy. Supp. Care Cancer 2005, 13, 1010–1017. [Google Scholar] [CrossRef]
- Hofman, M.; Ryan, J.L.; Figueroa-Moseley, C.D.; Jean-Pierre, P.; Morrow, G.R. Cancer-related fatigue: The scale of the problem. Oncologist 2007, 12, 4–10. [Google Scholar] [CrossRef]
- Brown, L.F.; Kroenke, K. Cancer-related fatigue and its association with depression and anxiety: A systematic review. Psychosomatic 2009, 50, 440–447. [Google Scholar] [CrossRef]
- Bender, C.M.; Engberg, S.J.; Donovan, H.S. Symptom clusters in adults with chronic health problems and cancer as a comorbidity. Oncol. Nurs. Forum 2008, 35, E1–E11. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Settineri, R. Lipid Replacement Therapy: A functional food approach with new formulations for reducing cellular oxidative damage, cancer-associated fatigue and the adverse effects of cancer therapy. Funct. Food Health Dis. 2011, 1, 135–160. [Google Scholar] [CrossRef]
- Manzullo, E.F.; Escalante, C.P. Research into fatigue. Hematol. Oncol. Clin. N. Amer. 2002, 16, 619–628. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Conklin, K.A. Reversing mitochondrial dysfunction, fatigue and the adverse effects of chemotherapy of metastatic disease by Molecular Replacement Therapy. Clin. Expl. Metastasis 2008, 25, 161–169. [Google Scholar] [CrossRef]
- Nicolson, G.L. Lipid Replacement/Antioxidant Therapy as an adjunct supplement to reduce the adverse effects of cancer therapy and restore mitochondrial function. Pathol. Oncol. Res. 2005, 11, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Colodny, L.; Lynch, K.; Farber, C.; Papish, R.; Phillips, K.; Sanchez, M.; Cooper, K.; Pickus, O.; Palmer, D.; Percy, T.B.; et al. Results of a study to evaluate the use of Propax to reduce adverse effects of chemotherapy. J. Am. Nutraceutical Assoc. 2001, 3, 17–25. [Google Scholar]
- Martínez, J.; Vögler, O.; Casas, J.; Barceló, F.; Alemany, R.; Prades, J.; Nagy, T.; Baamonde, C.; Kasprzyk, P.G.; Terés, S.; et al. Membrane structure modulation, protein kinase Cα activation and anticancer activity of minerval. Mol. Pharmacol. 2005, 67, 531–540. [Google Scholar] [CrossRef]
- Terés, S.; Lladó, V.; Higuera, M.; Barceló-Coblijn, G.; Martin, M.L.; Noguera-Salvà, M.A.; Marcilla-Etxenike, A.; García-Verdugo, J.M.; Soriano-Navarro, M.; Saus, C.; et al. 2-Hydroxyoleate, a nontoxic membrane binding anticancer drug, induces glioma cell differentiation and autophagy. Proc. Natl. Acad. Sci. USA 2012, 109, 8489–8494. [Google Scholar] [CrossRef]
- Vidal, L.; Victoria, I.; Martín, M.G.; Brunet, M.; Colom, H.; Cortal, M.; Gómez-Ferreira, M.; Yeste-Velasco, M.; Pérez, A.; Rodon, J.; et al. A first-in-human phase I/Ib dose-escalation clinical trial of the autophagy inducer ABTL0812 in patients with advanced solid tumours. Eur. J. Cancer 2021, 146, 87–94. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Ellithrope, R. Lipid replacement and antioxidant nutritional therapy for restoring mitochondrial function and reducing fatigue in chronic fatigue syndrome and other fatiguing illnesses. J. Chronic Fatigue Syndr. 2006, 13, 57–68. [Google Scholar] [CrossRef]
- Conklin, K.A.; Nicolson, G.L. Molecular replacement in cancer therapy: Reversing cancer metabolic and mitochondrial dysfunction, fatigue and the adverse effects of therapy. Curr. Cancer Therapy Rev. 2008, 4, 66–76. [Google Scholar]
- Ellithorpe, R.R.; Settineri, R.; Nicolson, G.L. Reduction of fatigue by use of a dietary supplement containing glycophospholipids. J. Am. Nutraceut. Assoc. 2003, 6, 23–28. [Google Scholar]
- Hamilton, D.; Jensen, G.S. Nutraceutical support of mitochondrial function associated with reduction of long-term fatigue and inflammation. Altern. Ther. 2021, 27, 8–18. [Google Scholar]
- Nicolson, G.L.; Settineri, R.; Ferreira, G.; Breeding, P. Reduction of pain, fatigue, gastrointestinal and other symptoms and improvement in quality of life indicators in fibromyalgia patients with Membrane Lipid Replacement glycerolphospholipids and controlled-release caffeine. Intern. J. Clin. Med. 2018, 9, 560–579. [Google Scholar] [CrossRef]
- Scott, J.B.; Hassett, A.L.; Brummett, C.M.; Clauw, D.J.; Harte, S.E. Caffeine as an opioid analgesic adjuvant in fibromyalgia. J. Pain Res. 2017, 10, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Tarnopolsky, M.A. The mitochondrial cocktail: Rationale for combined nutraceutical therapy in mitochondrial cytopathies. Adv. Drug Deliv. Rev. 2008, 60, 1561–1567. [Google Scholar] [CrossRef]
- Gonzalez, M.J.; Seyfrie, T.; Nicolson, G.L.; Barclay, B.J.; Matta, J.; Vasquez, A.; Agostino, D.D.; Olalde, J.; Duconge, J.; Hunninghake, R.; et al. Mitochondrial correction: A new therapeutic paradigm for cancer and degenerative diseases. J. Orthomol. Med. 2018, 33, 1–20. [Google Scholar]
- Campbell, J.N.; Meyer, R.A. Mechanisms of neuropathic pain. Neuron 2006, 52, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Staud, R. Peripheral pain mechanisms in chronic widespread pain. Best Pract. Res. Clin. Rheumatol. 2011, 25, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Hall, W.C.; LaMantia, A.S.; Mooney, R.D.; Platt, M.L.; White, L.E. Neuroscience; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Nicolson, G.L.; Breeding, P.C. Membrane Lipid Replacement for reduction of pain, fatigue, gastrointestinal and other symptoms in patients with peripheral pain: Case reports. Case Rep. Rev. 2020, 1, 1–3. [Google Scholar] [CrossRef]
- Nicolson, G.L.; Breeding, P.C.; Settineri, R.; Ferreira de Mattos, G. Aging and chronic illnesses: Membrane Lipid Replacement for restoring mitochondrial function and reducing fatigue, pain, and other symptoms in aged individuals. Bioact. Comp. Health Dis. 2020, 3, 194–203. [Google Scholar] [CrossRef]
- Taberner, F.J.; Fernandez-Ballester, G.; Fernandez-Carvajal, A.; Ferrer-Montiel, A. TRP channels interaction with lipids and its implications in disease. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1818–1827. [Google Scholar] [CrossRef]
- Giniatullin, R. Ion channels of nociception. Intern. J. Mol. Sci. 2020, 21, 3553. [Google Scholar] [CrossRef]
- Grimm, A.; Eckert, A. Increased reactive oxygen species (ROS) formation and mitochondrial inner membrane lipid and protein oxidative damage with aging. Brain aging and neurodegeneration: From a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. [Google Scholar] [CrossRef]
- Parikh, S.; Goldstein, A.; Koenig, M.K.; Scaglia, F.; Enns, G.M.; Saneto, R.; Anselm, I.; Cohen, B.H.; Falk, M.J.; Greene, C.; et al. Diagnosis and management of mitochondrial disease: A consensus statement from the Mitochonrial Medicine Society. Genet. Med. 2015, 17, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Lenoir-Wijnkoop, I.; Jones, P.F.; Uauy, R.; Segal, L.; Milner, J. Nutrition economics—food as an ally of public health. Br. J. Nutr. 2013, 109, 777–784. [Google Scholar] [CrossRef]
- Neustadt, J.; Pieczenik, S.R. Medication-induced mitochondrial damage and disease. Mol. Nutr. Food Res. 2008, 52, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aguilera, A.; Rull, A.; Rodrigueq-Gallego, E.; Riera-Borrull, M.; Luciano-Mateo, F.; Camps, J.; Menéndez, J.A.; Joven, J. Mitochondrial dysfunction: A basic mechanism in inflammation-related non-communicable diseases and their therapeutic opportunities. Mediat. Inflammat. 2013, 2013, 135698. [Google Scholar] [CrossRef]
- Amati, F.; Dubé, J.J.; Coen, P.M.; Stefanovic-Racic, J.; Toledo, F.G.S.; Goodpaster, B.H. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care 2009, 32, 1547–1549. [Google Scholar] [CrossRef]
- Dubé, J.J.; Coen, P.M.; DiStefano, G.; Chacon, A.C.; Helbling, N.L.; Desimone, M.E.; Stafanovic-Racic, M.; Hames, K.C.; Despines, A.A.; Toledo, F.G.; et al. The effects of acute lipid overload on skeletal muscle insulin resistance, metabolic flexibility and mitochondrial performance. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E1117–E1124. [Google Scholar] [CrossRef]
- Pintus, F.; Floris, G.; Rufini, A. Nutrient availability links mitochondria, apoptosis and obesity. Aging 2012, 4, 1–8. [Google Scholar] [CrossRef]
- Kramer, P.; Bressan, P. Mitochondria inspire a lifestyle. Cell. Mol. Basis Mitochondrial Inherit. 2019, 231, 105–126. [Google Scholar]
- Silva, B.S.C.; DiGiovanni, L.; Kumar, R.; Carmichael, R.E.; Kim, P.K.; Schrader, M. Maintaining social contacts: The physiological relevance of organelle interactions. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118800. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I. Anti-ageing strategies: Prevention or therapy? Showing ageing from within. EMBO Rep. 2005, 6, S25–S29. [Google Scholar] [CrossRef]
- Menendez, J.A.; Cufi, S.; Oliveras-Ferraros, C.; Vellon, L.; Joven, J.; Vazquez-Martin, A. Gerosuppressant metformin: Less is more. Aging 2011, 3, 348–362. [Google Scholar] [CrossRef]
- Coen, P.M.; Menshikova, E.V.; Distefano, G.; Zheng, D.; Tanner, C.J.; Standley, R.A.; Helbling, N.L.; Dubis, G.S.; Ritov, V.B.; Xie, H.; et al. Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning and insulin sensitivity after gastric bypass surgery. Diabetes 2015, 64, 3737–3750. [Google Scholar] [CrossRef]
- Logan, A.; Cochemé, H.M.; Trnka, J.; Prime, T.A.; Abakumova, I.; Jones, B.A.; Filipovska, A.; Murphy, M.P. Mitochondria-targeted antioxidants in the treatment of disease. Ann. N. Y. Acad. Sci. 2008, 1147, 105–111. [Google Scholar]
- Ross, M.F.; Kelso, G.F.; Blaikie, F.H.; James, A.M.; Cocheme, H.M.; Filipovska, A.; Da Ros, T.; Hurd, T.R.; Smith, R.A.; Murphy, M.P. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry 2005, 70, 222–230. [Google Scholar] [CrossRef]
- Anton, S.; Leeuwenburgh, C. Fasting or caloric restriction for Healthy Aging. Exp. Gerontol. 2013, 48, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Lettieri-Barbato, D.; Cannata, S.M.; Casagrande, V.; Ciriolo, M.R.; Aquilano, K. Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle. PLoS ONE 2018, 13, e0195912. [Google Scholar] [CrossRef]
- Mehrabani, S.; Bagherniya, M.; Askari, G.; Read, M.I.; Sahebkar, A. The effect of fasting or calorie restriction on mitophagy induction: A literature review. J. Cachexia Sarcopenia Muscle 2020, 11, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.A.; Adlam, V.J.; Blaikie, F.H.; Manas, A.R.; Porteous, C.M.; James, A.M.; Ross, M.F.; Bach, D.; Naon, D.; Pich, S.; et al. Expression of Mfn2, the Charcot-Marie-Tooth neuropathy type 2A gene, in human skeletal muscle: Effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes 2005, 54, 2685–2693. [Google Scholar]
- Kim, S.-J.; Cheresh, P.; Jabionski, R.P.; Williams, D.B.; Kamp, D.W. The role of mitochondrial DNA in mediating alveolar epithelial cell apoptosis and pulmonary fibrosis. Int. J. Mol. Sci. 2015, 16, 21486–21519. [Google Scholar] [CrossRef] [PubMed]
- Coppotelli, G.; Ross, J.M. Mitochondria in ageing and diseases: The super trouper of the cell. Int. J. Mol. Sci. 2016, 17, 711. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Isobe, K.; Nakada, K.; Hayashi, J.I. Human cells are protected from mitochondrial dysfunction by complementation of DNA products in fused mitochondria. Nat. Genet. 2001, 28, 272–275. [Google Scholar] [CrossRef]
- Gough, N.R. Focus issue: TOR signaling, a tale of two complexes. Sci. Signal. 2012, 5, 212–217. [Google Scholar] [CrossRef]
- Takahara, T.; Maeda, T. Evolutionarily conserved regulation of TOR signalling. Biochemistry 2013, 154, 1–10. [Google Scholar] [CrossRef]
- Wu, J.J.; Quijano, C.; Chen, E.; Liu, H.; Cao, L.; Fergusson, M.M.; Rovira, I.I.; Gutkind, S.; Daniels, M.P.; Komats, M.; et al. Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy. Aging 2009, 1, 425–437. [Google Scholar] [CrossRef]
- McIver, C.M.; Wycherley, T.P.; Clifton, P.M. mTOR signaling and ubiquitin-proteosome gene expression in the preservation of fat free mass following high protein, calorie restricted weight loss. Nutr. Metab. 2012, 9, 83. [Google Scholar] [CrossRef]
- Cunningham, J.T.; Rodgers, J.T.; Arlow, D.H.; Vazquez, F.; Mootha, V.K.; Puigserver, P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1 transcriptional complex. Nature 2007, 450, 736–740. [Google Scholar] [CrossRef]
- Ellithorpe, R.A.; Settineri, R.; Jacques, B.; Nicolson, G.L. Lipid Replacement Therapy functional food with NT Factor for reducing weight, girth, body mass, appetite, cravings for foods and fatigue while improving blood lipid profiles. Funct. Food Health Dis. 2012, 2, 11–24. [Google Scholar] [CrossRef]
- Vögler, O.; López-Bellan, A.; Alemany, R.; Tofé, S.; González, M.; Quevedo, J.; Pereg, V.; Barceló, F.; Escribá, P.V. Structure-effect relation of C18 long-chain fatty acids in the reduction of body weight in rats. Int. J. Obes. 2008, 32, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Richter, Y.; Herzog, Y.; Lifshitz, Y.; Hayun, R.; Zchut, S. The effect of soybean phosphatidylserine on cognitive performance in elderly with subjective memory complaints: A pilot study. Clin. Intervent. Aging 2013, 8, 557–563. [Google Scholar]
- Kato-Kataoka, A.; Sukai, M.; Ebina, R.; Nonaka, C.; Asano, T.; Miyamori, T. Soybean-derived phosphatidylserine improves memory function of elderly Japanese subjects with memory complaints. J. Clin. Biochem. Nutr. 2010, 47, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Busquets, X.; Escribá, P.V. Brain lipids in the pathophysiology and treatment of alzheimer’s disease. In Update on Dementia; Moretti, D.V., Ed.; InTech: Rijeka, Croatia, 2016; pp. 127–167. [Google Scholar]
- Xiang, Y.; Lam, S.M.; Shui, G. What can lipidomics tell us about the pathogenesis of Alzheimer disease? J. Biol. Chem. 2015, 396, 1281–1291. [Google Scholar] [CrossRef]
- Torres, M.; Perice, S.L.; Fiol-deRoque, M.A.; Marcilla-Etxekike, A.; Ahyayauch, H.; Barceló-Coblijn, G.; Terés, S.; Katsouri, L.; Ordinas, M.; López, D.J.; et al. Membrane lipid modifications and therapeutic effects mediated by hydroxydocosahexaenoic acid on Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1838, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Escribá, P.V.; Busquets, X.; Inokuchi, J.I.; Balogh, G.; Törok, Z.; Horvath, I.; Hrwood, J.L.; Vígh, L. Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug disscovery and new disease treatment. Prog. Lipid Res. 2015, 59, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Fiol-deRoque, M.; Gutierrez-Lanza, R.; Terés, S.; Torres, M.; Barceló, P.; Rial, R.V.; Verkhratsky, A.; Escribá, P.V.; Busquets, X.; Rodríguez, J.J. Cognitive recovery and restoration of cell proliferation in the dentate gyrus in the 5XFAD transgenic mice model of Alzheimer’s disease following 2-hydroxy-DHA treatment. Biogerontology 2013, 14, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Arenaza-Urquijo, E.M.; Sánchez-Benavides, G.; Suárez-Calvet, M.; Milà-Alomà, M.; Grau-Rivera, O.; González-de-Echávarri, J.M.; Crous-Bou, M.; Minguillón, C.; Fauria, K.; et al. DHA intake relates to better cerebrovascular and neurodegeneration neuroimaging phenotypes in middle-age adults at increased genetic risk of Alzheimer disease. Am. J. Clin. Nutr. 2021, 113, 1627–1635. [Google Scholar] [CrossRef]
- Hernando, S.; Requejo, C.; Herran, E.; Ruiz-Ortega, J.A.; Morera-Herreras, T.; Lafuente, J.V.; Ugedo, L.; Gainza, E.; Pedraz, J.L.; Igartua, M.; et al. Beneficial effects of n-3 polyunsaturated fatty acids administration in a partial lesion model of Parkinson’s disease: The role of glia and NRf2 regulation. Neurobiol. Dis. 2019, 121, 252–262. [Google Scholar] [CrossRef]
- Parets, S.; Irigoyen, A.; Ordinas, M.; Cabot, J.; Miralles, M.; Arbona, L.; Péter, M.; Balogh, G.; Fernández-García, P.; Busquets, X.; et al. 2-Hydroxy-docosahexaenoic acid is converted into heneicosapentaenoic acid via α-oxidation: Implications for Alzheimer’s disease therapy. Frot. Cell Dev. Biol. 2020, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Brewer, H.B.; Cleeman, J.I.; Smith, S.C.; Lenfant, C. Definition of metabolic syndrome. Report of the National Heart, Lung and Blood Institute/American Heart Association Conference on Scientific Issues Related to Definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, V.A. The metabolic syndrome, hyperlipidemia and insulin resistance. Clin. Cornerstone 2005, 7, 61–72. [Google Scholar] [CrossRef]
- Reaven, G.M. Role of insulin resistance in human Samochowiec disease (syndrome X). Annu. Rev. Med. 1993, 44, 121–131. [Google Scholar] [CrossRef]
- Park, Y.W.; Zhu, S.; Palaniappan, L.; Heska, S.; Carenthon, M.R.; Heymsfield, S.B. The metabolic syndrome. Prevalence and associated risk factor findings in the U.S. population form the Third National Health and Nutrition Examination Survey, 1988-1994. Arch. Intern. Med. 2003, 163, 427–436. [Google Scholar] [CrossRef]
- Cifkova, R.; Erdine, S.; Fagard, R.; Farsand, C.; Heagerty, A.M.; Kiolski, W.; Kjeldsen, S.; Luscher, T.; Mallion, J.M.; Mancia, G.; et al. Practice guidelines for primary care physicians: 2003 ESH/ESC hypertension guidelines. J. Hypertens. 2003, 21, 1779–1786. [Google Scholar]
- Whitworth, J.A. World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J. Hypertens. 2003, 21, 1983–1992. [Google Scholar]
- Reaven, G.M. The metabolic syndrome: Is this diagnosis necessary? Am. J. Clin. Nutr. 2006, 83, 1237–1247. [Google Scholar] [CrossRef]
- Dandona, P.; Aljada, A.; Chaudhuri, A.; Mohanty, P.; Garg, R. Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes and inflammation. Circulation 2005, 111, 1448–1454. [Google Scholar] [CrossRef]
- Einhorn, D.; Reaven, G.M.; Cobin, R.H.; Ford, E.; Ganda, O.P.; Handelsman, Y.; Hellman, R.; Jellinger, P.S.; Kendall, D.; Krauss, R.M.; et al. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr. Pract. 2003, 9, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C. Biochemical and molecular basis of insulin resistance. Curr. Protein Peptide Sci. 2006, 7, 113–131. [Google Scholar] [CrossRef]
- Houston, M.C.; Egan, B.M. The Metabolic Syndrome. Pathophysiology, diagnosis, clinical aspects, prevention and nonpharmacologic treatment: Emphasis on lifestyle modifications, nutrition, nutritional supplements, vitamins, minerals, antioxidants, weight management and exercise. J. Am. Nutraceutical Assoc. 2005, 8, 3–83. [Google Scholar]
- Aguilar-Salinas, C.A.; Viveros-Ruiz, T. Recent advances in managing/understanding the metabolic syndrome. F1000 Res. 2019, 8, 370. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Halle, V.; Hornung, V.; Petzold, G.C.; Stewart, C.R.; Monks, B.G.; Reinheckel, T.; Fitzgerald, K.A.; Latz, E.; Moore, K.J.; Golenbock, D.T. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 2008, 9, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Gris, D.; Lei, Y.; Jia, S.; Zhang, L.; Huang, M.T.; Brickey, W.J.; Ting, J.P. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signalling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Gora, I.M.; Ciechanowska, A.; Ladyzynski, P. NLRP3 Inflammasome at the Interface of inflammation, endothelial dysfunction, and type 2 diabetes. Cells 2021, 10, 314. [Google Scholar] [CrossRef]
- Benetti, E.; Chiazza, F.; Patel, N.S.; Collino, M. The NLRP3 inflammasome as a novel player of the intercellular crosstalk in metabolic disorders. Mediat. Inflamm. 2013, 2013, 678627. [Google Scholar] [CrossRef]
- Wu, J.; Wang, K.; Wang, X.; Pang, Y.; Jiang, C. The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 2021, 12, 360–373. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef]
- Schrauwen, P.; Hesselink, M.K.C. Oxidative capacity, lipotoxicity and mitochondrial damage in type 2 diabetes. Diabetes 2004, 53, 1412–1417. [Google Scholar] [CrossRef]
- Itani, S.I.; Ruderman, N.B.; Schmieder, F.; Boden, G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 2002, 51, 2005–2011. [Google Scholar] [CrossRef]
- Sonnenberg, G.E.; Krakower, G.R.; Kissebah, A.H. A novel pathway to the manifestations of metabolic syndrome. Obes. Res. 2004, 12, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.E.; He, J.; Menshikova, E.V.; Ritov, V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 2002, 51, 2944–2950. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Schiffrin, E.L. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem. Cell Biol. 2004, 122, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, P.; Schrauwen-Hinderling, V.; Hoeks, J.; Hesselink, M.K. Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta 2010, 1801, 266–271. [Google Scholar] [CrossRef]
- Green, K.; Brand, M.D.; Murphy, M.P. Prevention of mitochondrial oxidative damage as a therpeutic strategy in diabetes. Diabetes 2004, 53, S110–S118. [Google Scholar] [CrossRef]
- Rosen, P.; Nawroth, P.P.; King, G.; Moller, W.; Tritschler, H.J.; Packer, L. The role of oxidative stress in the onset and progression of diabetes and its complications: A summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab. Res. Rev. 2001, 17, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A. New insights on oxidative stress and diabetic complications may lead to a “causal” antioxidant therapy. Diabetes Care 2003, 26, 1589–1596. [Google Scholar] [CrossRef]
- Opara, E.C. Oxidative stress, micronutrients, diabetes mellitus and its complications. J. Royal Soc. Health 2002, 122, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with in type type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Opara, E.C. Role of oxidative stress in the etiology of type 2 diabetes and the effect of antioxidant supplementation on glycemic control. J. Investig. Med. 2004, 52, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Sheu, S.-S.; Nauduri, D.; Anders, M.W. Targeting antioxidants to mitochondria: A new therapeutic direction. Biochim. Biophys. Acta 2006, 1762, 256–265. [Google Scholar] [CrossRef]
- Nicolson, G.L. Metabolic syndrome and mitochondrial function: Molecular replacement and antioxidant supplements to prevent membrane oxidation and restore mitochondrial function. J. Cell. Biochem. 2007, 100, 1352–1369. [Google Scholar] [CrossRef]
- Zierenberg, O.; Assmann, G.; Schmitz, G.; Rosseneu, M. Effect of polyenephosphatidylcholine on cholesterol uptake by human high density lipoprotein. Atherosclerosis 1981, 39, 527–542. [Google Scholar] [CrossRef]
- Shimizu, N.; Sakajiri, S. Effects of EPL capsules on lipid in diabetic (part II). Jap. J. New Rem. Clin. 1973, 22, 2277–2283. [Google Scholar]
- Serkova, V.K. Dynamics of blood lipids, parameters of lipid peroxidation and energy metabolism in patients with ischemic heart disease treated with Essentiale. Klin. Med. 1986, 64, 91–95. [Google Scholar]
- Martines, G.; Restori, G.; Caffé, C.; Cortesi, R. Relationship between glycide tolerance and polyunsaturated phosphatidylcholine (EPL). Ter. Mod. 1990, 4, 155–157. [Google Scholar]
- Strain, J.J. Disturbances of micronutrient and antioxidant status in diabetes. Proc. Nutr. Soc. 1991, 50, 591–604. [Google Scholar] [CrossRef]
- Preuss, H.G. The insulin system: Influence of antioxidants. J. Am. Coll. Nutr. 1998, 17, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Yasunari, K. What we learned from randomized clinical trials and cohort studies of antioxidant vitamins. Focus on vitamin E and cardiovascular disease. Curr. Pharm. Biotechnol. 2006, 7, 69–72. [Google Scholar] [CrossRef]
- Sowers, J.R.; Frohlich, E.D. Insulin and insulin resistance: Impact on blood pressure and cardiovascular disease. Med. Clin. N. Am. 2004, 88, 63–82. [Google Scholar] [CrossRef]
- Irani, K. Oxidant signaling in vascular cell growth, death and survival: A review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signalling. Circ. Res. 2000, 87, 179–183. [Google Scholar] [CrossRef]
- Zambon, A.; Pauletto, P.; Crepaldi, G. The metabolic syndrome—a chronic cardiovascular inflammatory condition. Aliment. Pharmacol. Ther. 2005, 22, 20–23. [Google Scholar] [CrossRef]
- Stancu, C.S.; Toma, L.; Sima, A.V. Dual role of lipoproteins in endothelial cell dysfunction in atherosclerosis. Cell Tissue Res. 2012, 349, 433–446. [Google Scholar] [CrossRef]
- Bremer, A.A.; Jialal, I. Adipose tissue dysfunction in nascent metabolic syndrome. J. Obes. 2013, 2013, 393192. [Google Scholar] [CrossRef]
- Hsueh, W.A.; Quiñones, M.J. Role of endothelial dysfunction in insulin resistance. Am. J. Cardiol. 2003, 92 (Suppl. 4A), 10J–17J. [Google Scholar] [CrossRef]
- Blum, C.B.; Levy, R.I.; Eisenberg, S.; Hall, M.; Goebel, R.H.; Berman, M. High density lipoprotein metabolism in man. J. Clin. Investig. 1977, 60, 795–807. [Google Scholar] [CrossRef]
- Kudinov, V.A.; Alekseeva, O.Y.; Torkhovskaya, T.I.; Baskaev, K.K.; Artyushev, R.I.; Saburina, I.N.; Markin, S.S. High-density lipoproteins as homeostatic nanoparticles of blood plasma. Int. J. Mol. Sci. 2020, 21, 8737. [Google Scholar] [CrossRef]
- Wong, E.K.; Nicolosi, R.J.; Low, P.A.; Herd, J.A.; Hayes, K.C. Lecithin influenceon hyperlipemia in rhesus monkeys. Lipids 1980, 15, 428–433. [Google Scholar] [CrossRef]
- Samochowiec, L.; Kadlubowska, D.; Rozewicka, M.; Kuzna, W.; Szyszka, K. Investigations in experimental atherosclerosis. Part 2. The effect of phosphatidyicholine (EPL) on experimental atherosclerotic changes in miniature pigs. Atherosclerosis 1976, 23, 319–331. [Google Scholar] [CrossRef]
- Escribá, P.V.; Sánchez-Domínguez, J.M.; Alemany, R.; Perona, J.S.; Ruiz-Gutierrez, V. Alteration of lipids, G proteins, and PKC in cell membranes of elderly hypertensives. Hypertension 2003, 41, 176–182. [Google Scholar] [CrossRef]
- Terés, S.; Barceló-Coblijn, G.; Benet, M.; Álvarez, R.; Bressani, R.; Halver, J.E.; Escribá, P.V. Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Proc. Natl. Acad. Sci. USA 2008, 105, 13811–13816. [Google Scholar] [CrossRef]
- Cynshi, O.; Stocker, R. Inhibition of lipoprotein lipid oxidation. Handb. Exp. Pharmacol. 2005, 170, 563–590. [Google Scholar]
- Kirsten, R.; Heintz, B.; Nelson, K.; Oremek, G. Reduction of hyperlipidemia with 3-sn-polyenylphosphatidylcholine in dialysis patients. Intern. J. Clin. Pharmacol. Ther. Toxicol. 1989, 27, 129–134. [Google Scholar]
- Noseda, G.; Suva, F.; Fragiacomo, C. Modification of serum lipids, lipoproteins and apoproteins A1 and B in patients with hyperlipidemia type Iia and Iib using polyenylphosphatidylcholine. Schweiz. Med. Wochenschr. 1985, 115, 1064–1070. [Google Scholar]
- Sykes, D.L.; Holdsworth, L.; Jawad, N.; Gunasekera, P.; Morica, A.H.; Crooks, M.G. Post-COVID-19 symptom burden: What is Long-COVID and how should we manage it? Lung 2021, 199, 113–119. [Google Scholar] [CrossRef]
- Wallnöfer, H.; Hanusch, M. “Essential” phospholipids in the treatment of hepatic disease. Med. Wschr. 1973, 27, 331–336. [Google Scholar]
- Kordac, V.; Brodanová, M.; Marecek, Z.; Jirásek, A. Essentiale forte in the treatment of chronic active hepatitis. Prakt. Lék. 1985, 65, 834–837. [Google Scholar]
- Pogromov, A.P.; Otbinskaya, L.I.; Antonenko, N.I.; Gitel, N.P.; Smolyanitsky, A.; Verkhovskaya, P.B. Use of Essentiale in the treatment of liver diseases. Klin. Med. 1978, 10, 97–101. [Google Scholar]
- Fassati, P.; Horejsi, J.; Fassati, M.; Jezkova, Z.; Spizek, J. The effect of essential choline phospholipids on HBsAg and on certain biochemical tests in cirrhosis of the liver. Cas. Lek. Cesk. 1981, 120, 56–60. [Google Scholar]
- di Paolo, N.; Buoncristiani, U.; Capotondo, L.; Gaggiotti, E.; de Mia, M.; Rossi, P.; Sansoni, E.; Bernini, M. Phosphatidylcholine and peritoneal transport during peritoneal dialysis. Nephron 1986, 44, 365–370. [Google Scholar] [CrossRef]
- Graeff, H.; von Hugo, R.; Schröck, R. Recent aspects of hemostatis, hematology and hemorheology in preeclampsia-eclampsis. Eur. J. Obstet. Gynecol. Reprod. Biol. 1984, 17, 91–102. [Google Scholar] [CrossRef]
- Shalina, R.I.; Kusch, I.B.; Oreshkina, V.P.; Azizova, O.A.; Kozlov, A.V.; Panasenko, O.M. Antioxidants as a part of combined treatment of patients with late gestosis. Obstet. Gynecol. 1989, 65, 37–41. [Google Scholar]
- Bottiglioni, F.; Tirelli, R. “Essentielle” Phospholipide in der Therapie der Spätgestosen. Ärztl. Praxis 1968, 20, 2656–2657. [Google Scholar]
- Nicolson, G.L.; Settineri, R.; Ellithorpe, R. Glycophospholipid formulation with NADH and CoQ10 significantly reduces intractable fatigue in chronic Lyme disease patients: Preliminary report. Funct. Food Health Dis. 2012, 2, 35–47. [Google Scholar] [CrossRef]
- Medica, A.J.; Aitkin, R.J.; Nicolson, G.L.; Sheridan, A.R.; Swegan, A.; De Luliis, A.; Gib, Z. Glycerolphospholipids protect stallion spermatozoa from oxidative damage in vitro. Reproduct. Fertil. 2021, 2, 199–209. [Google Scholar] [CrossRef]
- Hille, B.; Dickson, E.J.; Kruse, M.; Vivas, O.; Suh, B.C. Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 2015, 1851, 844–856. [Google Scholar] [CrossRef]
- Stieger, B.; Steiger, J.; Locher, K.P. Membrane lipids and transporter function. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166079. [Google Scholar] [CrossRef]
- Jafurulla, M.; Chattopadhyay, A. Membrane lipids in the function of serotonin and adrenergic receptors. Curr. Med. Chem. 2013, 20, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Sunshine, H.; Iruela-Arispe, M.L. Membrane lipids and cell signaling. Curr. Opin. Lipidol. 2017, 28, 408–413. [Google Scholar] [CrossRef]
- Suh, B.C.; Leal, K.; Hille, B. Modulation of high-voltage activated Ca(2+) channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron 2010, 67, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Hagar, R.E.; Ehrlich, B.E. Regulation of the type III InsP3 receptor by InsP3 and ATP. Biophys. J. 2000, 79, 271–278. [Google Scholar] [CrossRef][Green Version]
| Use | Subjects/Patients | Age | MLR Lipid | NTFL Dose b | NTFL Dose c | Example |
|---|---|---|---|---|---|---|
| Group | Supplement | Range (g/day) | Range (g/day) | Reference | ||
| (Original) | (Revised) | |||||
| General health | Aged | senior | NTFactor/L d | 2 | 2–3 | Nicolson et al. [3] |
| Fatigue | Aged | senior | NTFactor/L | 3 | 4 | Agadjanyan et al. [145] |
| Fatigue | CFS/ME | adult/teen | NTFactor/L | 2–4 | 4 | Nicolson & Ellithorpe [203] |
| Fatigue | CFS/ME | adult | ATP Fuel | 4 | 4 | Nicolson et al. [123] |
| Inflammation | Chronic fatigue | adult | ATP360 | 0.4 | N/A e | Hamilton & Jensen [206] |
| Fatigue | Fibromyalgia | adult | NTFactor/L | 3–4 | 4 | Nicolson et al. [215] |
| Fatigue | Menopause | adult | NTFactor/L | 1.2 | 3 | Hirose et al. [174] |
| Weight loss | Obesity, fatigue d | adult | NTFactor | 2 | 3–4 | Ellithrope et al. [245] |
| Brain health | Neurodegen. dis. | adult | NTFactor/L | 3–4 | 4 | Nicolson et al. [175] |
| CD health | CD risk/CD dis. | adult | NTFactor/L | 2–4 | 4 | Ellithorpe et al. [128] |
| Metabolic health | MetSyn/diabetes | adult | NTFactor/L | 2–4 | 4 | Nicolson [289] |
| Metabolic health | Diabetes | adult | ATP Fuel | 4 | 4 | Nicolson et al. [123] |
| Neurobehavior | Autism Spectrum dis. | child | NTFactor/L | 1–2 | 1–3 | Nicolson et al. [175] |
| Infections | Lyme/mycoplasma | adult | ATP Fuel | 4 | 4 | Nicolson et al. [321] |
| Fertility | Fertility Diseases | adult | NTFactor/L | 2–3 | 4 | Ferreira et al. [33] |
| Fatigue | Cancer | adult | NTFactor/L | 2–3 | 4 | Nicolson & Conklin [197] |
| Anemia | Anemia | adult | NTFactor/L | 1–2 | 4 | Nicolson et al. [123] |
| Injury | Spinal injury | adult | NTFactor/L | 1–2 | 4 | Ellithorpe et al. [123] |
| Autoimmune | Rheumatoid arthritis | adult | ATP Fuel | 3 | 4 | Nicolson et al. [123] |
| General health | Pregnancy | adult | NTFactor/L | 1–2 | 2–3 | Ellithorpe et al. [205] |
| Chemical detox | GW Illnesses | adult | NTFactor/L | >4 | >6 | Nicolson & Breeding [214] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolson, G.L.; Ferreira de Mattos, G.; Ash, M.; Settineri, R.; Escribá, P.V. Fundamentals of Membrane Lipid Replacement: A Natural Medicine Approach to Repairing Cellular Membranes and Reducing Fatigue, Pain, and Other Symptoms While Restoring Function in Chronic Illnesses and Aging. Membranes 2021, 11, 944. https://doi.org/10.3390/membranes11120944
Nicolson GL, Ferreira de Mattos G, Ash M, Settineri R, Escribá PV. Fundamentals of Membrane Lipid Replacement: A Natural Medicine Approach to Repairing Cellular Membranes and Reducing Fatigue, Pain, and Other Symptoms While Restoring Function in Chronic Illnesses and Aging. Membranes. 2021; 11(12):944. https://doi.org/10.3390/membranes11120944
Chicago/Turabian StyleNicolson, Garth L., Gonzalo Ferreira de Mattos, Michael Ash, Robert Settineri, and Pablo V. Escribá. 2021. "Fundamentals of Membrane Lipid Replacement: A Natural Medicine Approach to Repairing Cellular Membranes and Reducing Fatigue, Pain, and Other Symptoms While Restoring Function in Chronic Illnesses and Aging" Membranes 11, no. 12: 944. https://doi.org/10.3390/membranes11120944
APA StyleNicolson, G. L., Ferreira de Mattos, G., Ash, M., Settineri, R., & Escribá, P. V. (2021). Fundamentals of Membrane Lipid Replacement: A Natural Medicine Approach to Repairing Cellular Membranes and Reducing Fatigue, Pain, and Other Symptoms While Restoring Function in Chronic Illnesses and Aging. Membranes, 11(12), 944. https://doi.org/10.3390/membranes11120944









