Physicochemical Properties of Membrane Adsorber from Palm Empty Fruit Bunch (PEFB) by Acid Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Materials
2.2. Preparation and Activation of Activated Carbon PEFB
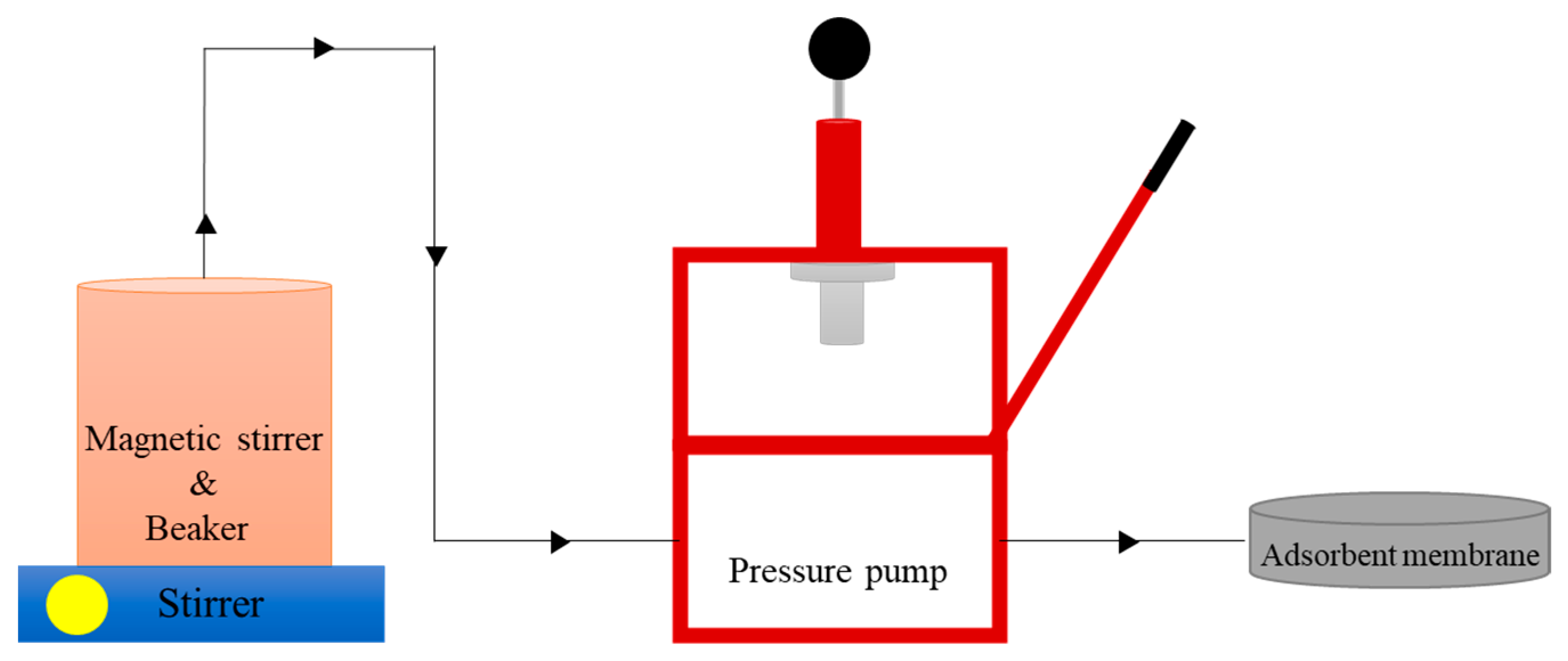
2.3. Preparation and Characterisation of Membrane Adsorber PEFB
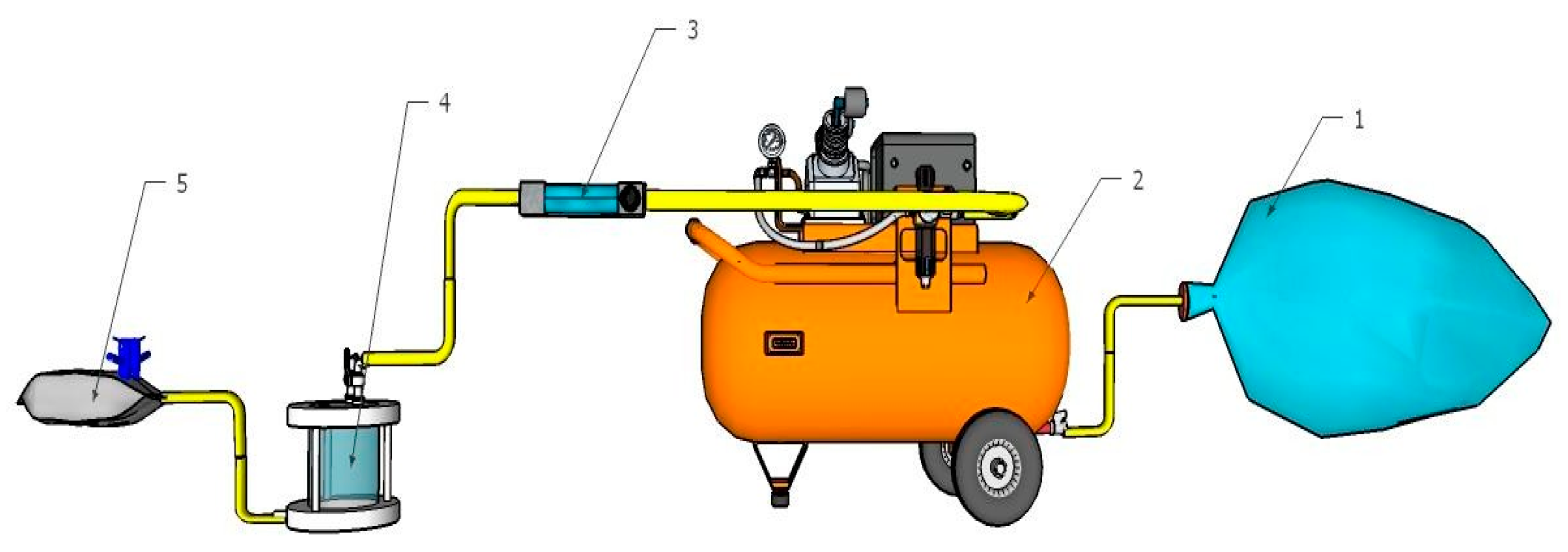
2.4. Biogas Purification Using Membrane Adsorber
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pertiwiningrum, A.; Dwi Koranto, C.A.; Wuri, M.A. Renewable Energy of Biogas Through Integrated Organic Cycle System in Tropical System. IntechOpen 2018, 74497, 99–117. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, R.; Meier, L.; Diaz, I.; Jeison, D. A review on the state-of-the-art of physical/chemical and biological technologies for biogas upgrading. Rev. Environ. Sci. Biotechnol. 2015, 14, 727–759. [Google Scholar] [CrossRef] [Green Version]
- Axelsson, L.; Franzén, M.; Ostwald, M.; Berndes, G.; Lakshmi, G.; Ravindranath, N.H. Perspective: Jatropha cultivation in southern India: Assessing farmers’ experiences. Biofuels Bioprod. Biorefining 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Petersson, A.; Holm-nielsen, J.B.; Baxter, D. Biogas upgrading technologies– developments and innovations. IEA Bioenergy 2009, 20, 1–19. [Google Scholar]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Susanto, H.; Wijaya, W.; Widiasa, I.N. Modifikasi Karbon Aktif Sebagai Adsorben Untuk Pemurnian Biogas. Teknik 2013, 34, 4–8. [Google Scholar] [CrossRef]
- McKinsey, S.Z. Removal of Hydrogen Sulfide from Biogas Using Cow—Manure Compost. Cornell Univ. 2003, 1–104. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.470.2484&rep=rep1&type=pdf (accessed on 18 November 2021).
- Hidalgo, D.; Sanz-Bedate, S.; Martín-Marroquín, J.M.; Castro, J.; Antolín, G. Selective separation of CH4 and CO2 using membrane contactors. Renew. Energy 2020, 150, 935–942. [Google Scholar] [CrossRef]
- Angelidaki, I.; Xie, L.; Luo, G.; Zhang, Y.; Oechsner, H.; Lemmer, A.; Munoz, R.; Kougias, P.G. Biogas Upgrading: Current and Emerging Technologies, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128168561. [Google Scholar]
- Baena-Moreno, F.M.; Rodríguez-Galán, M.; Vega, F.; Vilches, L.F.; Navarrete, B. Review: Recent advances in biogas purifying technologies. Int. J. Green Energy 2019, 16, 401–412. [Google Scholar] [CrossRef]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane gas separation technologies for biogas upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Zhou, K.; Chaemchuen, S.; Verpoort, F. Alternative materials in technologies for Biogas upgrading via CO2 capture. Renew. Sustain. Energy Rev. 2017, 79, 1414–1441. [Google Scholar] [CrossRef]
- Mutia, E. Proses Pemisahan Menggunakan Teknologi Membran; Lambung Mangkurat University Press: Kalimantan Selatan, Indonesia, 2019; Volume 53, ISBN 978-857-811-079-6. [Google Scholar]
- Li, Y.; Wang, L.; Hu, X.; Jin, P.; Song, X. Surface modification to produce superhydrophobic hollow fiber membrane contactor to avoid membrane wetting for biogas purification under pressurized conditions. Sep. Purif. Technol. 2018, 194, 222–230. [Google Scholar] [CrossRef]
- Jin, P.; Huang, C.; Li, J.; Shen, Y.; Wang, L. Surface modification of poly(Vinylidene fluoride) hollow fibre membranes for biogas purification in a gas–liquid membrane contactor system. R. Soc. Open Sci. 2017, 4, 171321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Žák, M.; Bendová, H.; Friess, K.; Bara, J.E.; Izák, P. Single-step purification of raw biogas to biomethane quality by hollow fiber membranes without any pretreatment—An innovation in biogas upgrading. Sep. Purif. Technol. 2018, 203, 36–40. [Google Scholar] [CrossRef]
- He, Q.; Yu, G.; Yan, S.; Dumée, L.F.; Zhang, Y.; Strezov, V.; Zhao, S. Renewable CO2 absorbent for carbon capture and biogas upgrading by membrane contactor. Sep. Purif. Technol. 2018, 194, 207–215. [Google Scholar] [CrossRef]
- Harasimowicz, M.; Orluk, P.; Zakrzewska-Trznadel, G.; Chmielewski, A.G. Application of polyimide membranes for biogas purification and enrichment. J. Hazard. Mater. 2007, 144, 698–702. [Google Scholar] [CrossRef]
- Saiful, S. Performance of Mixed Matrix Membrane Adsorbers for Lysozyme Separation. J. Rekayasa Kim. Lingkung. 2011, 8, 29–34. [Google Scholar]
- Boi, C.; Malavasi, A.; Carbonell, R.G.; Gilleskie, G. A direct comparison between membrane adsorber and packed column chromatography performance. J. Chromatogr. A 2020, 1612, 460629. [Google Scholar] [CrossRef]
- Indonesia B.P.S. Indonesian Oil Palm Statistic 2019; Perkebunan, S.S.T., Ed.; Badan Pusat Statistik: Jakarta, Indonesia, 2019; ISBN 1978-9947. [Google Scholar]
- Ayuni, S.T.; Larasaty, P.; Anam, C.; Riyadi, A.; Hastuti, A.; Kurniasih, A.; Saputri, V.G.; Pratiwi, A.I.; Meilaningsih, T. Laporan Perekonomian Global. ©BPS RI/BPS-Statistics Indonesia, Jakarta. 2020. Available online: www.bi.go.id (accessed on 18 November 2021).
- Sulihatimarsyila, N.U.R.; Wafti, A.B.D.; Lik, H.; Lau, N.; Loh, S.O.H.K.; Aziz, A.A.; Rahman, Z.A.B.; May, C.Y. Activated Carbon From Oil Palm Biomass as Potential Adsorbent for Palm Oil Mill Effluent Treatment. J. Oil Palm Res. 2017, 29, 278–290. [Google Scholar]
- Wang, Y.; Pan, J.; Li, Y.; Zhang, P.; Zhang, X.; Li, M.; Zheng, H.; Sun, Y.; Wang, H.; Du, Q. Preparation and Characterization of Activated Carbon from Oil-palm Fiber and Its Evaluation for Methylene Blue Adsorption. Mater. Tehnol. 2021, 55, 449–457. [Google Scholar] [CrossRef]
- Ooi, C.; Lee, T.; Pung, S.; Yeoh, F. Activated Carbon Fiber Derived From Single Step Carbonization-Activation Process. ASEAN Eng. J. 2013, 4, 40–50. [Google Scholar]
- Gaol, M.R.L.L.; Sitorus, R.; Yanthi, S.; Surya, I.; Manurung, R. Pembuatan Selulosa Asetat Dari α -Selulosa Tandan Kosong Kelapa Sawit. J. Tek. Kim. USU 2013, 2, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Rani, N.H.A.; Mohamad, N.F.; Matali, S.; Sharifah Aishah, S.A.S. Preparation and characterization of activated carbon made from oil palm empty fruit bunch. Key Eng. Mater. 2014, 594–595, 44–48. [Google Scholar] [CrossRef]
- Hidayu, A.R.; Mohamad, N.F.; Matali, S.; Sharifah, A.S.A.K. Characterization of activated carbon prepared from oil palm empty fruit bunch using BET and FT-IR techniques. Procedia Eng. 2013, 68, 379–384. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Gui, B.; Xiang, S.X.; Bao, X.T.; Zhang, H.J.; Lua, A.C. Preparation of activated carbons by utilizing solid wastes from palm oil processing mills. J. Porous Mater. 2008, 15, 535–540. [Google Scholar] [CrossRef]
- Zamani, S.A.; Yunus, R.; Samsuri, A.W.; Salleh, M.A.M.; Asady, B. Removal of Zinc from Aqueous Solution by Optimized Oil Palm Empty Fruit Bunches Biochar as Low Cost Adsorbent. Bioinorg. Chem. Appl. 2017, 2017, 7914714. [Google Scholar] [CrossRef] [Green Version]
- Septevani, A.A.; Rifathin, A.; Sari, A.A.; Sampora, Y.; Ariani, G.N.; Sondari, D. Oil palm empty fruit bunch-based nanocellulose as a super-adsorbent for water remediation. Carbohydr. Polym. 2020, 229, 115433. [Google Scholar] [CrossRef]
- Elias, M.A.; Hadibarata, T.; Sathishkumar, P. Modified oil palm industry solid waste as a potential adsorbent for lead removal. Environ. Chem. Ecotoxicol. 2021, 3, 1–7. [Google Scholar] [CrossRef]
- Hendrawan, Y.; Nurseta, D.Y.; Utami, R.; Trilaksana, M.I.A.; Sumarlan, S.H.; Wibisono, Y. Effect of carbonisation temperature and activating agents on the characteristics of activated carbon produced from oil palm empty fruit bunch. IOP Conf. Ser. Earth Environ. Sci. 2021, 733, 012004. [Google Scholar] [CrossRef]
- Ooi, C.H.; Cheah, W.K.; Sim, Y.L.; Pung, S.Y.; Yeoh, F.Y. Conversion and characterization of activated carbon fiber derived from palm empty fruit bunch waste and its kinetic study on urea adsorption. J. Environ. Manag. 2017, 197, 199–205. [Google Scholar] [CrossRef]
- Hidayat, A.; Sutrisno, B. Comparison on pore development of activated carbon produced by chemical and physical activation from palm empty fruit bunch. IOP Conf. Ser. Mater. Sci. Eng. 2016, 162, 012008. [Google Scholar] [CrossRef] [Green Version]
- Wibisono, Y.; Amanah, A.; Sukoyo, A.; Anugroho, F.; Kurniati, E. Activated Carbon Loaded Mixed Matrix Membranes Extracted from Oil Palm Empty Fruit Bunches for Vehicle Exhaust Gas Adsorbers Extracted from Oil Palm Empty Fruit Bunches for Vehicle Exhaust Gas Adsorbers. Evergr. Jt. J. Nov. Carbon Resour. Sci. Green Asia Strateg. 2021, 8, 593–600. [Google Scholar]
- Said, S.M.; Subriyer, N.; Priadi, D.P. The Effect of Addition of Activated Carbon Made from Oil Palm Empty Fruit Bunch and Iron Powder on Ceramic Membrane Characteristics. In Proceedings of the 7th Annual Basic Science International Conference, Malang, Indonesia, 7–8 March 2017; Volume 2, p. 278. [Google Scholar]
- Elma, M.; Sumardi, A.; Paramita, A.; Rahma, A.; Lestari, A.E.; Yanto, D.H.Y.; Hadi, S.; Assyaifi, Z.L.; Raharjo, Y. Physicochemical properties of mesoporous organo-silica xerogels fabricated through organo catalyst. Membranes 2021, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.A.W.; Abdullah, M.O.; Lim, L.L.P.; Yeo, T.H.C. Surface Modification and Characterization of Coconut Shell-Based Activated Carbon Subjected to Acidic and Alkaline Treatments. J. Appl. Sci. Process Eng. 2017, 4, 186–194. [Google Scholar]
- Lu, G.Q.; Diniz da Costa, J.C.; Duke, M.; Giessler, S.; Socolow, R.; Williams, R.H.; Kreutz, T. Inorganic membranes for hydrogen production and purification: A critical review and perspective. J. Colloid Interface Sci. 2007, 314, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Yavari, S.; Malakahmad, A.; Sapari, N.B.; Yavari, S. Synthesis optimization of oil palm empty fruit bunch and rice husk biochars for removal of imazapic and imazapyr herbicides. J. Environ. Manage. 2017, 193, 201–210. [Google Scholar] [CrossRef]
- Chen, J.P.; Wu, S. Acid/Base-Treated Activated Carbons: Characterization of Functional Groups and Metal Adsorptive Properties. Langmuir 2004, 20, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Prahas, D.; Kartika, Y.; Indraswati, N.; Ismadji, S. Activated carbon from jackfruit peel waste by H3PO4 chemical activation: Pore structure and surface chemistry characterization. Chem. Eng. J. 2008, 140, 32–42. [Google Scholar] [CrossRef]
- Tang, Y.B.; Liu, Q.; Chen, F.Y. Preparation and characterization of activated carbon from waste ramulus mori. Chem. Eng. J. 2012, 203, 19–24. [Google Scholar] [CrossRef]
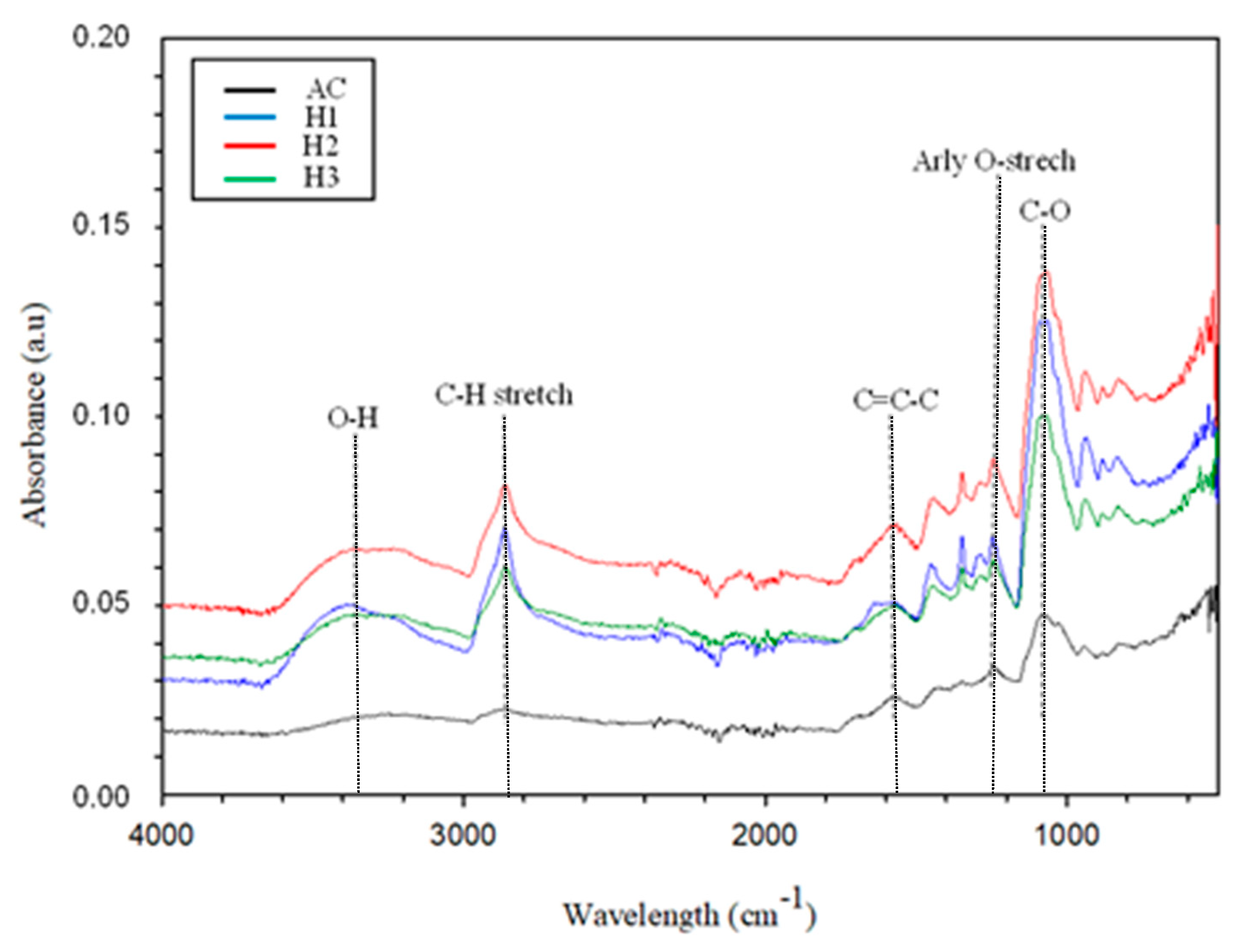
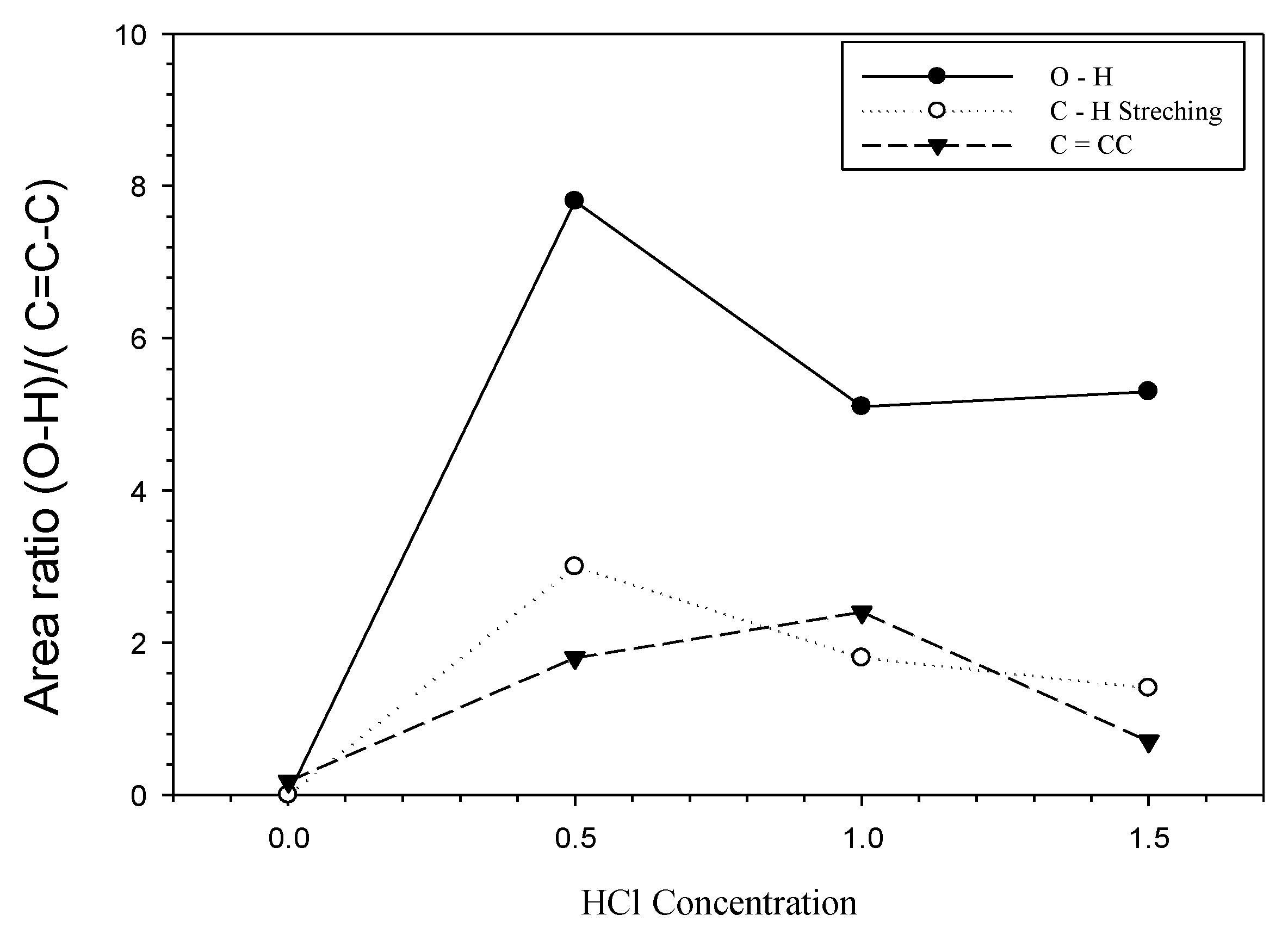


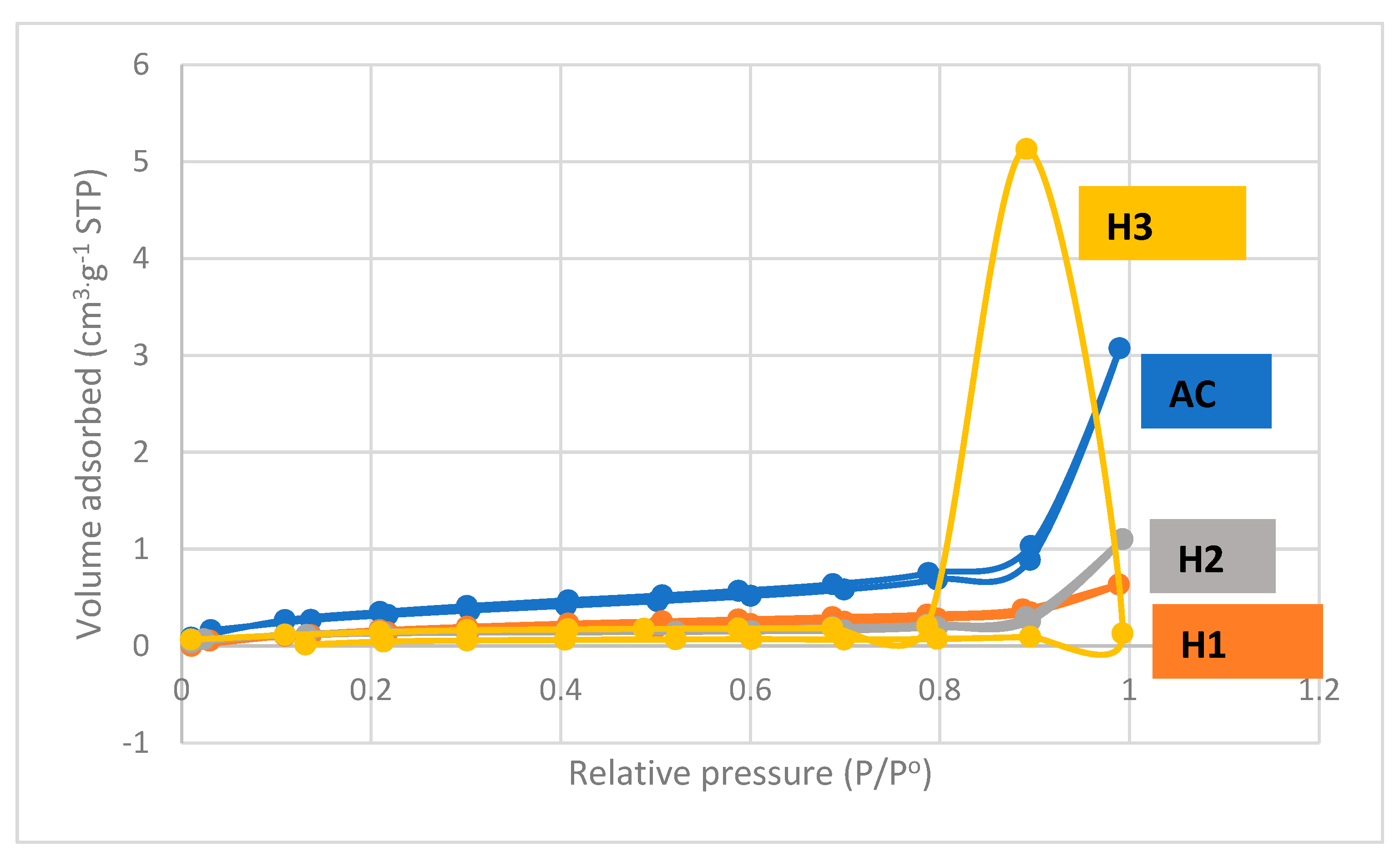
| HCl Concentration | Area (Qn) | Area Ratio | |
|---|---|---|---|
| O-H | C=C-C | H/C=C-C | |
| 0 | 0.01 | 0.18 | 0.00 |
| 0.5 | 7.8 | 1.8 | 4.33 |
| 1 | 5.1 | 2.4 | 2.13 |
| 1.5 | 5.3 | 0.7 | 7.57 |
| Concentration (M) | SBET (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| 0 | 0.2699 | 0.00878 | 130.12732 |
| 0.5 | 0.5345 | 0.000983 | 7.34468 |
| 1 | 0.4387 | 0.001705 | 15.54718 |
| 1.5 | 0.4001 | 0.001484 | 14.83716 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidayah, N.; Elma, M.; Darsono, P.V.; Syauqiah, I.; Amenia, A.; Laksana Putra, D.G.; Akbar, H.R.; Huda, N.; Rahma, A. Physicochemical Properties of Membrane Adsorber from Palm Empty Fruit Bunch (PEFB) by Acid Activation. Membranes 2021, 11, 917. https://doi.org/10.3390/membranes11120917
Hidayah N, Elma M, Darsono PV, Syauqiah I, Amenia A, Laksana Putra DG, Akbar HR, Huda N, Rahma A. Physicochemical Properties of Membrane Adsorber from Palm Empty Fruit Bunch (PEFB) by Acid Activation. Membranes. 2021; 11(12):917. https://doi.org/10.3390/membranes11120917
Chicago/Turabian StyleHidayah, Nur, Muthia Elma, Putri Vidiasari Darsono, Isna Syauqiah, Angelica Amenia, Daniel Guntur Laksana Putra, Heru Renaldi Akbar, Nurul Huda, and Aulia Rahma. 2021. "Physicochemical Properties of Membrane Adsorber from Palm Empty Fruit Bunch (PEFB) by Acid Activation" Membranes 11, no. 12: 917. https://doi.org/10.3390/membranes11120917
APA StyleHidayah, N., Elma, M., Darsono, P. V., Syauqiah, I., Amenia, A., Laksana Putra, D. G., Akbar, H. R., Huda, N., & Rahma, A. (2021). Physicochemical Properties of Membrane Adsorber from Palm Empty Fruit Bunch (PEFB) by Acid Activation. Membranes, 11(12), 917. https://doi.org/10.3390/membranes11120917







