Preparation of Ultrafiltration Membrane by Polyethylene Glycol Non-Covalent Functionalized Multi-Walled Carbon Nanotubes: Application for HA Removal and Fouling Control
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
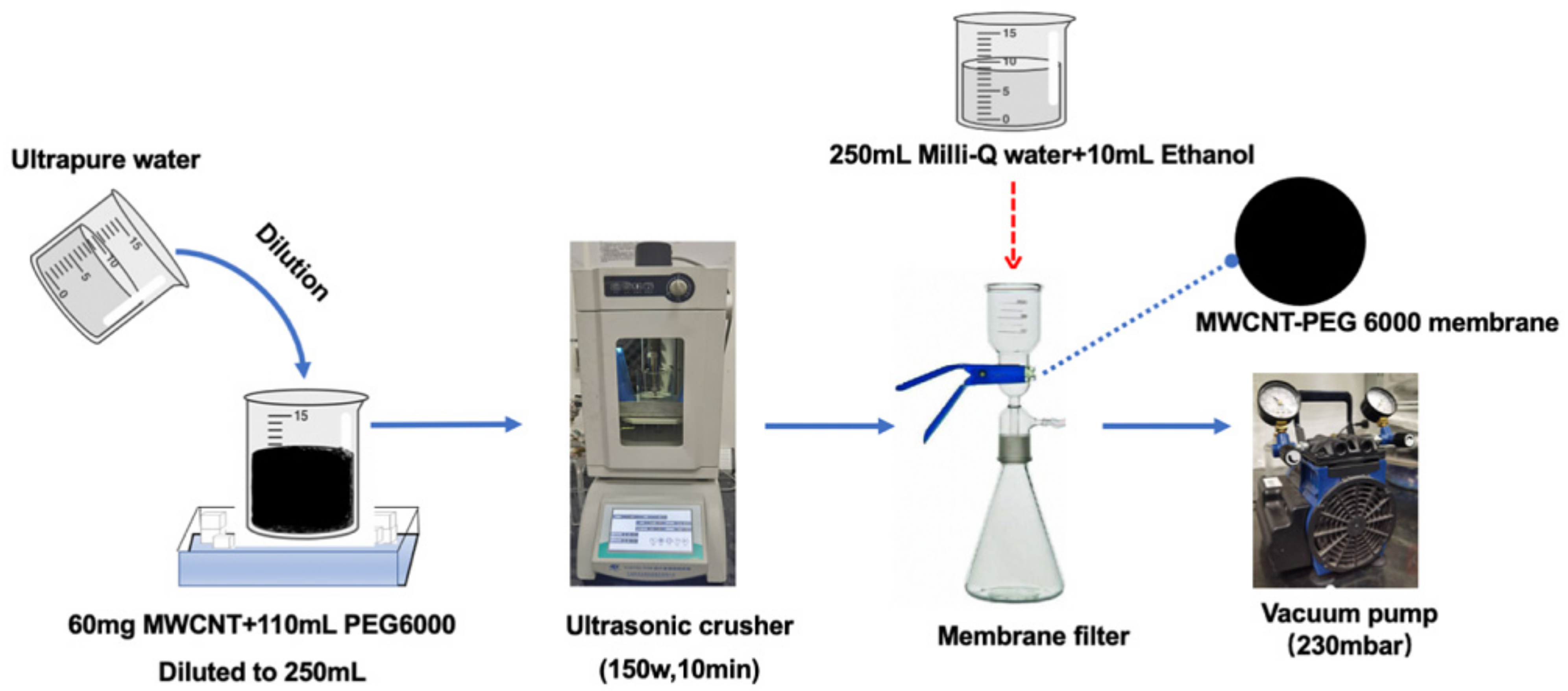
2.2. Non-Covalent Functionalization of MWCNT by PEG
2.3. Preparation of PEG Non-Covalent Functionalized MWCNT Membrane
2.4. Membrane Characterization
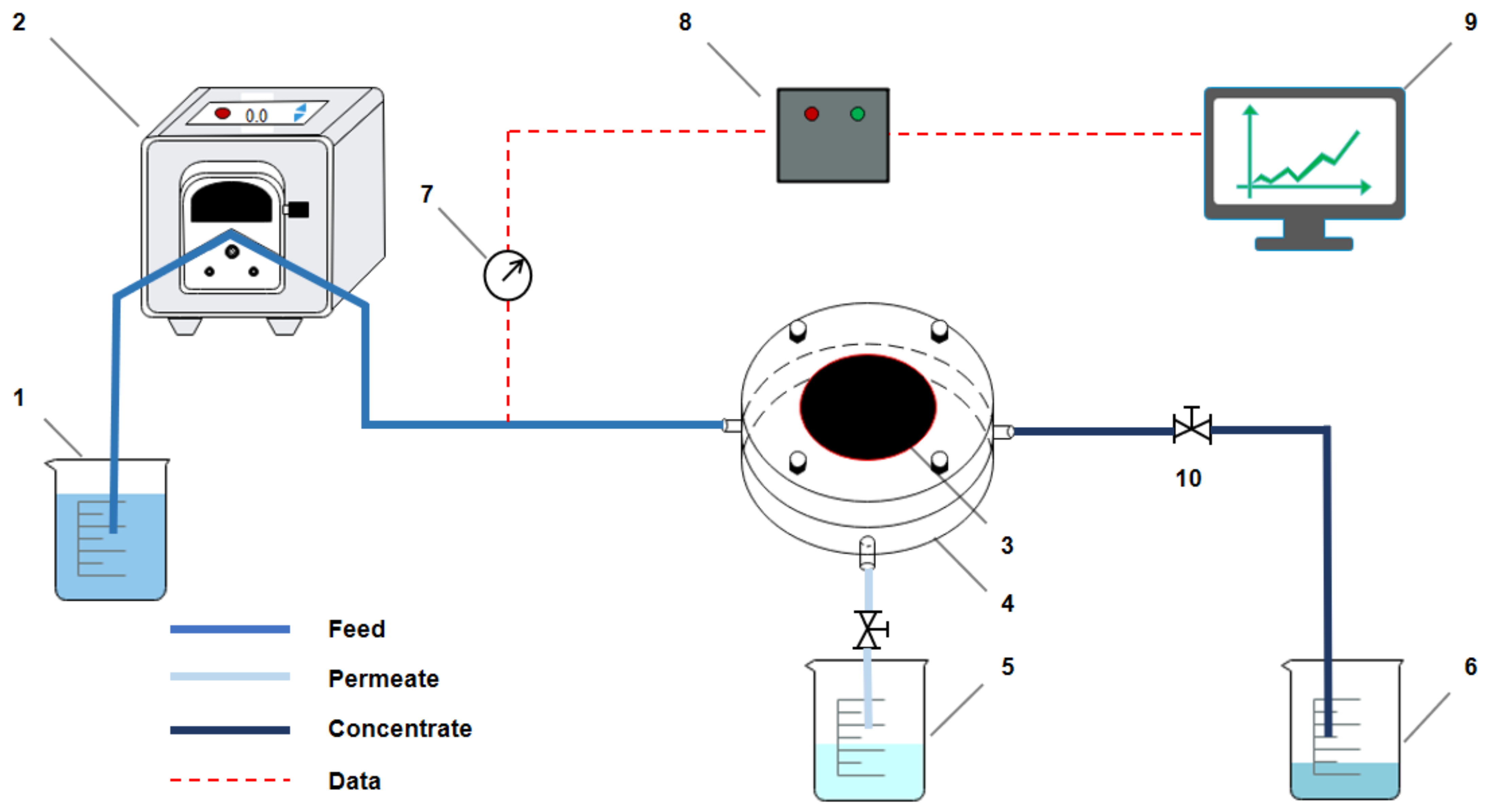
2.5. Permeability of PEG Non-Covalent-Functionalized MWCNT Membrane
2.6. Fouling Tests of PEG Non-Covalent-Functionalized MWCNT Membrane
3. Results and Discussion
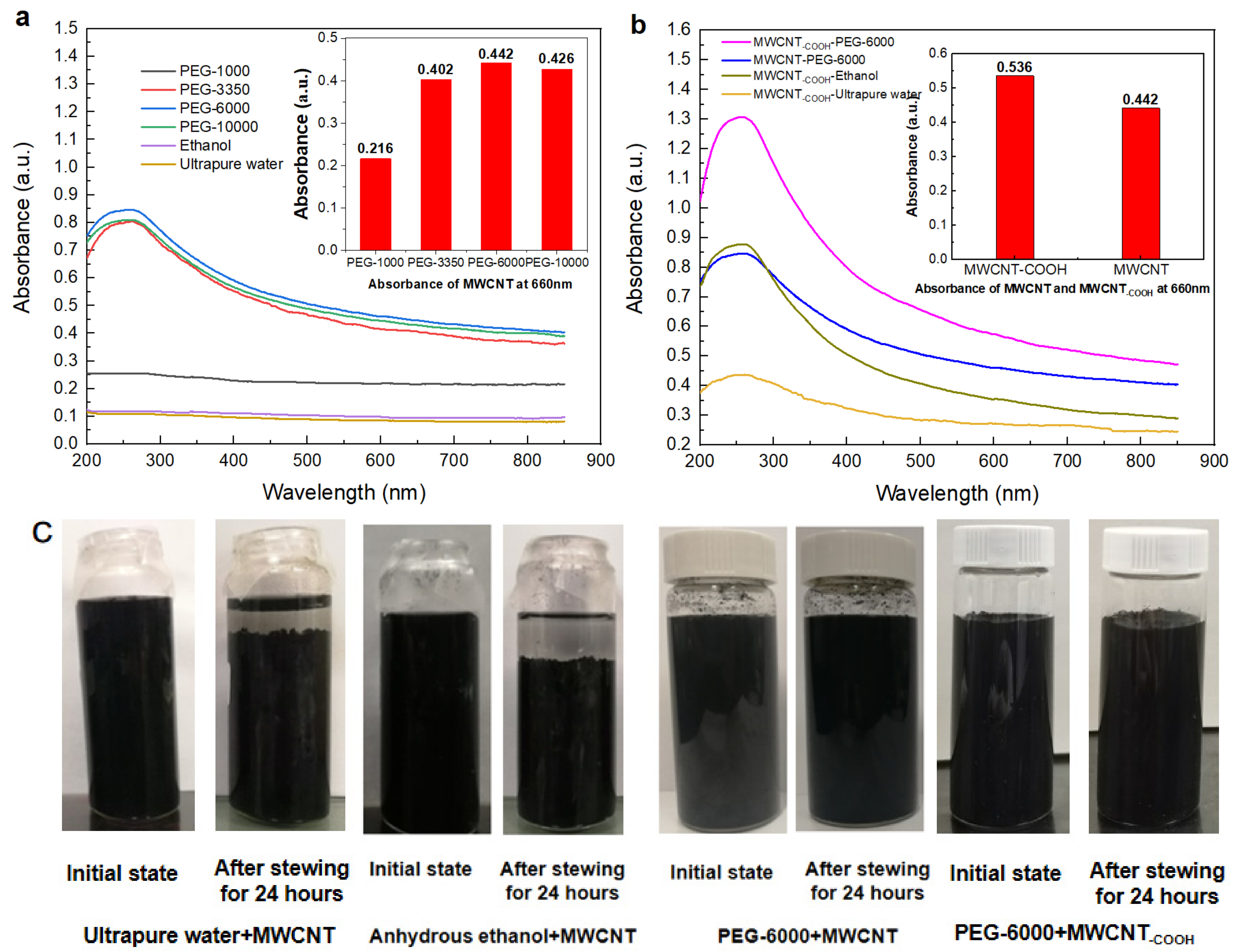
3.1. The Dispersion and Stability of PEG Non-Covalent-Functionalized MWCNT
3.2. Characterization of PEG Non-Covalent-Functionalized MWCNT Membranes
3.3. HA Removal by PEG Non-Covalent-Functionalized MWCNT Membranes
3.4. Antifouling Ability of PEG Non-Covalent-Functionalized MWCNT Membranes
4. Conclusions
- (1)
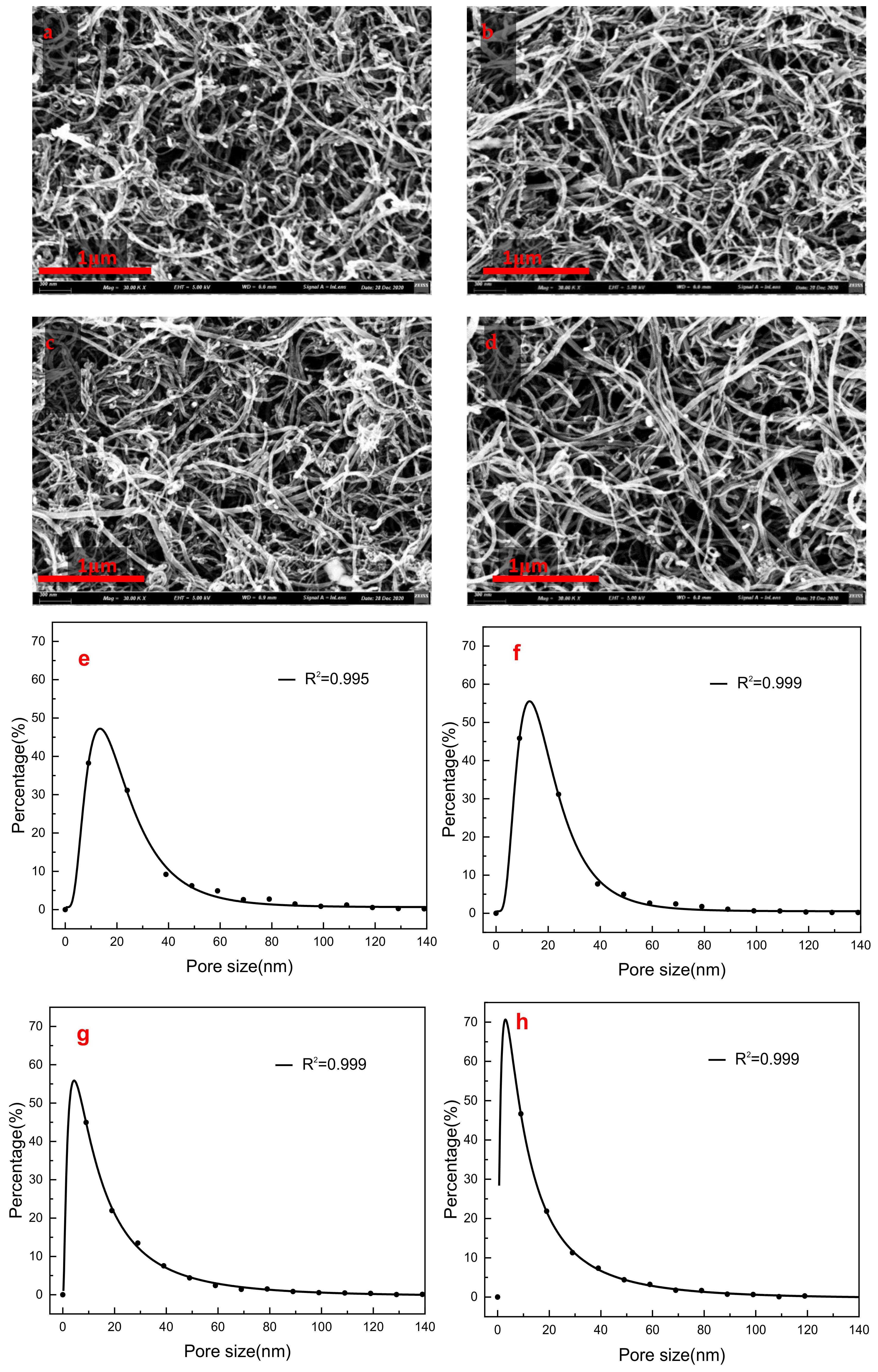
- MWCNT non-covalent functionalized with PEG–6000 had the best dispersion effect and the pore size of PEG-MWNT membrane distributed in a narrower range of diameter, which corresponded to a more concentrated membrane surface. Compared with MWCNT and MWCNT–COOH membrane, the oxygen content of PEG–MWCNT and PEG–MWCNT–COOH membrane was increased, which proved that PEG non-covalent functionalization of MWCNT was successful. PEG non-covalent functionalization greatly enhanced the hydrophilicity of the MWCNT membranes. The results of pure water flux showed that the PEG MWCNT membranes could be categorized into low pressure membranes.
- (2)
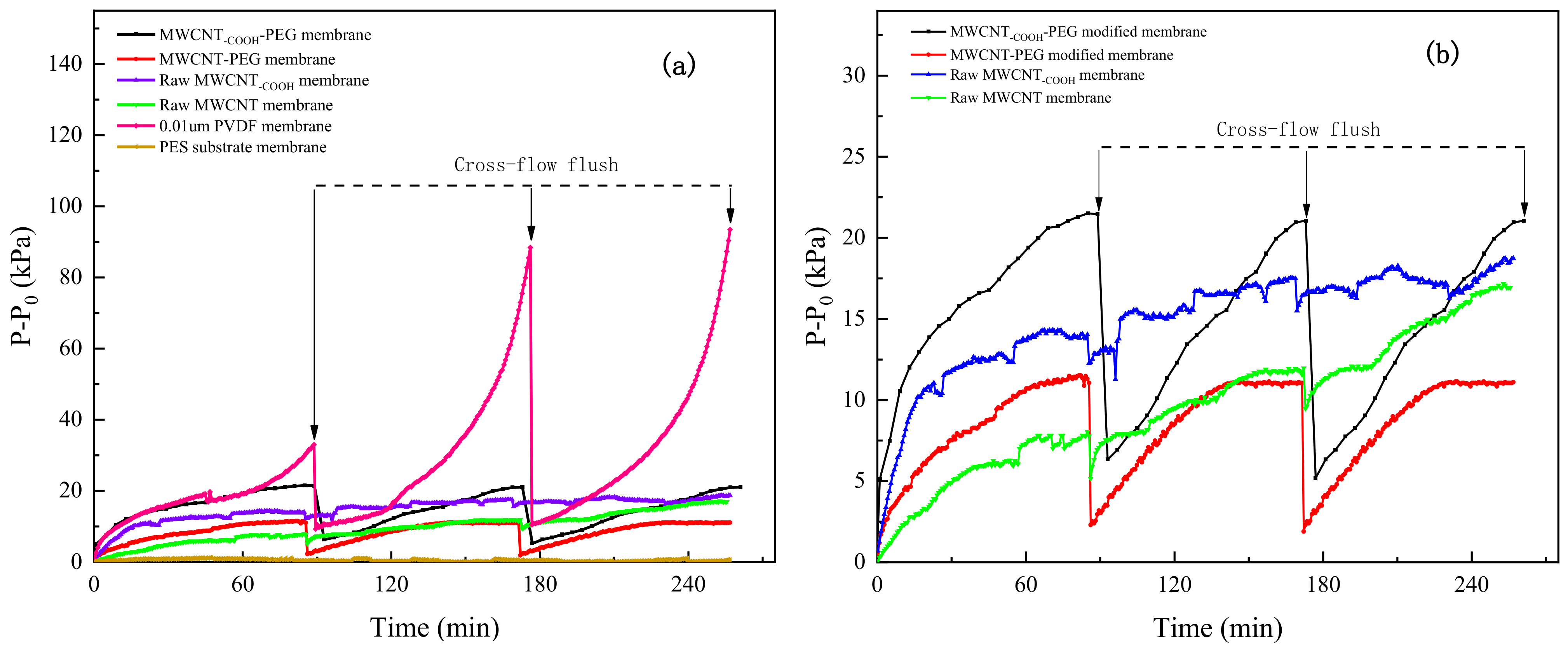
- All the MWCNT-modified membranes had lower TMP growth rates compared with the commercial 0.01 μm PVDF ultrafiltration membrane during the HA filtration. The PEG–MWCNT–COOH membrane had the best effectiveness on HA removal, while the PEG–MWCNT membrane had the best recoverability. According to the transformation of HA removal rate and TMP, we speculated that the adsorption of raw MWCNT–COOH/MWCNT membranes plays a major role in HA removal. Although PEG non-covalent functionalization occupied the adsorption site of MWCNT, the removal of HA would further rely on the interception effect of the PEG–MWCNT membranes. PEG non-covalent functionalization effectively prolonged the service life of PEG–MWCNT membrane.
- (3)
- Compared with the commercial 0.01-μm PVDF ultrafiltration membrane, the antifouling ability of the four MWCNT-modified membranes were improved during the filtration of BSA. The TMP recovery rate of PEG–MWCNT membrane after cross flushing was 79.4%, followed by the PEG–MWCNT-COOH membrane with a TMP recovery rate of 70%. The TMP recovery rates of raw MWCNT–COOH and MWCNT membrane were only 14.9% and 28.3%, respectively. PEG non-covalent functionalization improved the antifouling ability of the raw MWCNT/MWCNT–COOH membranes, and reduced the irreversible fouling of raw MWCNT/ MWCNT–COOH membranes.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chang, H.; Liang, H.; Qu, F.; Liu, B.; Yu, H.; Du, X.; Li, G.; Snyder, S.A. Hydraulic backwashing for low-pressure membranes in drinking water treatment: A review. J. Membr. Sci. 2017, 540, 362–380. [Google Scholar] [CrossRef]
- Huang, H.; Schwab, K.; Jacangelo, J.G. Pretreatment for Low Pressure Membranes in Water Treatment: A Review. Environ. Sci. Technol. 2009, 43, 3011–3019. [Google Scholar] [CrossRef]
- Li, K.; Wen, G.; Li, S.; Chang, H.; Shao, S.; Huang, T.; Li, G.; Liang, H. Effect of pre-oxidation on low pressure membrane (LPM) for water and wastewater treatment: A review. Chemosphere 2019, 231, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Amy, G. Fundamental understanding of organic matter fouling of membranes. Desalination 2008, 231, 44–51. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nat. Cell Biol. 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Villalonga, R.; Villalonga, M.L.; Díez, P.; Pingarrón, J. Decorating carbon nanotubes with polyethylene glycol-coated magnetic nanoparticles for implementing highly sensitive enzyme biosensors. J. Mater. Chem. 2011, 21, 12858–12864. [Google Scholar] [CrossRef]
- Eguílaz, M.; Venegas, C.J.; Gutiérrez, A.; Rivas, G.A.; Bollo, S. Carbon nanotubes non-covalently functionalized with cytochrome c: A new bioanalytical platform for building bienzymatic biosensors. Microchem. J. 2016, 128, 161–165. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Song, J.; Yang, W.; Wang, M.; Zhu, C.; Zhao, W.; Zheng, J.; Lin, Y. Self-supporting activated carbon/carbon nanotube/reduced graphene oxide flexible electrode for high performance supercapacitor. Carbon 2018, 129, 236–244. [Google Scholar] [CrossRef]
- Lota, K.; Acznik, I.; Sierczynska, A.; Lota, G. Enhancing the performance of polypyrrole composites as electrode materials for supercapacitors by carbon nanotubes additives. J. Appl. Polym. Sci. 2019, 137. [Google Scholar] [CrossRef]
- Qin, P.; Huang, C.; Gao, B.; Pi, C.; Fu, J.; Zhang, X.; Huo, K.; Chu, P.K. Ultrathin carbon layer-encapsulated TiN nanotubes array with enhanced capacitance and electrochemical stability for supercapacitors. Appl. Surf. Sci. 2020, 503, 144293. [Google Scholar] [CrossRef]
- Jiang, R.; Zeng, B.; Liu, J.; Chai, X.; Chen, T.; Wu, Z.; Zhang, X.; Yang, K. Enhanced field electron emission of single-walled carbon nanotubes prepared by imprinting technique. J. Alloy. Compd. 2020, 816, 152669. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, Z.; Cai, Z.; Yan, N.; Lu, X.; Zhu, X.; Chen, L. Enhanced hydrogen storage properties of MgH2 by the synergetic catalysis of Zr0.4Ti0.6Co nanosheets and carbon nanotubes. Appl. Surf. Sci. 2020, 504, 144465. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-F.; Fan, J.-L.; Wang, S.-G.; Ma, G.-H. Adsorption of natural organic matter analogues by multi-walled carbon nanotubes: Comparison with powdered activated carbon. Chem. Eng. J. 2013, 219, 450–458. [Google Scholar] [CrossRef]
- Barrejón, M.; Syrgiannis, Z.; Burian, M.; Bosi, S.; Montini, T.; Fornasiero, P.; Amenitsch, H.; Prato, M. Cross-Linked Carbon Nanotube Adsorbents for Water Treatment: Tuning the Sorption Capacity through Chemical Functionalization. ACS Appl. Mater. Interfaces 2019, 11, 12920–12930. [Google Scholar] [CrossRef]
- Brady-Estévez, A.S.; Kang, S.; Elimelech, M. A Single-Walled-Carbon-Nanotube Filter for Removal of Viral and Bacterial Pathogens. Small 2008, 4, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Brady-Estévez, A.S.; Schnoor, M.H.; Vecitis, C.D.; Saleh, N.B.; Elimelech, M. Multiwalled Carbon Nanotube Filter: Improving Viral Removal at Low Pressure. Langmuir 2010, 26, 14975–14982. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon 2012, 50, 3–33. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, Y.; Li, Y.; Zhang, P.; Cao, Z.; Yang, H.; Liu, C.; Tao, G.; Gong, F.; Matsuyama, H. Synthesis of hydrophilic carbon nanotubes by grafting poly(methyl methacrylate) via click reaction and its effect on poly(vinylidene fluoride)-carbon nanotube composite membrane properties. Appl. Surf. Sci. 2018, 435, 79–90. [Google Scholar] [CrossRef]
- Madaeni, S.; Zinadini, S.; Vatanpour, V. Convective flow adsorption of nickel ions in PVDF membrane embedded with multi-walled carbon nanotubes and PAA coating. Sep. Purif. Technol. 2011, 80, 155–162. [Google Scholar] [CrossRef]
- Yang, X.; Lee, J.; Yuan, L.; Chae, S.-R.; Peterson, V.K.; Minett, A.I.; Yin, Y.; Harris, A.T. Removal of natural organic matter in water using functionalised carbon nanotube buckypaper. Carbon 2013, 59, 160–166. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, Y.; Ramasamy, R.P. Non-Covalent Functionalization of Carbon Nanotubes for Electrochemical Biosensor Development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef] [PubMed]
- Sweetman, L.J.; Nghiem, L.; Chironi, I.; Triani, G.; Panhuis, M.I.H.; Ralph, S.F. Synthesis, properties and water permeability of SWNT buckypapers. J. Mater. Chem. 2012, 22, 13800–13810. [Google Scholar] [CrossRef]
- Rashid, H.-O.; Triani, G.; Scales, N.; Panhuis, M.I.H.; Nghiem, L.D.; Ralph, S.F. Nanofiltration applications of tough MWNT buckypaper membranes containing biopolymers. J. Membr. Sci. 2017, 529, 23–34. [Google Scholar] [CrossRef]
- El-Hammadi, M.M.; Delgado, Á.V.; Melguizo, C.; Prados, J.C.; Arias, J.L. Folic acid-decorated and PEGylated PLGA nanoparticles for improving the antitumour activity of 5-fluorouracil. Int. J. Pharm. 2017, 516, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-H.; Zhu, B.-K.; Kong, L.; Xu, Y.-Y. Improving Hydrophilicity and Protein Resistance of Poly(vinylidene fluoride) Membranes by Blending with Amphiphilic Hyperbranched-Star Polymer. Langmuir 2007, 23, 5779–5786. [Google Scholar] [CrossRef]
- Chinpa, W.; Quémener, D.; Beche, E.; Jiraratananon, R.; Deratani, A. Preparation of poly(etherimide) based ultrafiltration membrane with low fouling property by surface modification with poly(ethylene glycol). J. Membr. Sci. 2010, 365, 89–97. [Google Scholar] [CrossRef]
- Chen, X.; Su, Y.; Shen, F.; Wan, Y. Antifouling ultrafiltration membranes made from PAN-b-PEG copolymers: Effect of copolymer composition and PEG chain length. J. Membr. Sci. 2011, 384, 44–51. [Google Scholar] [CrossRef]
- Phomdum, P.; Gassara, S.; Deratani, A.; Chinpa, W. Enhancement of Resistance to Protein Fouling of Poly(ether imide) Membrane by Surface Grafting with PEG under Organic Solvent-free Condition. Chin. J. Polym. Sci. 2018, 36, 1157–1167. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Shi, J.-Y.; Wang, J.-L.; Li, Y.-L.; Gao, N.-N.; Liu, Z.-X.; Lian, W.-T. Preparation and characterization of PEG-g-MWCNTs/PSf nano-hybrid membranes with hydrophilicity and antifouling properties. RSC Adv. 2015, 5, 84746–84753. [Google Scholar] [CrossRef]
- Bai, L.; Liang, H.; Crittenden, J.; Qu, F.; Ding, A.; Ma, J.; Du, X.; Guo, S.; Li, G. Surface modification of UF membranes with functionalized MWCNTs to control membrane fouling by NOM fractions. J. Membr. Sci. 2015, 492, 400–411. [Google Scholar] [CrossRef]
- Sharmeen, S.; Rahman, A.M.; Lubna, M.M.; Salem, K.S.; Islam, R.; Khan, M.A. Polyethylene glycol functionalized carbon nanotubes/gelatin-chitosan nanocomposite: An approach for significant drug release. Bioact. Mater. 2018, 3, 236–244. [Google Scholar] [CrossRef]
- Adeli, M.; Soleyman, R.; Beiranvand, Z.; Madani, F. Carbon nanotubes in cancer therapy: A more precise look at the role of carbon nanotube–polymer interactions. Chem. Soc. Rev. 2013, 42, 5231–5256. [Google Scholar] [CrossRef]
- Lee, H. Adsorption of plasma proteins onto PEGylated single-walled carbon nanotubes: The effects of protein shape, PEG size and grafting density. J. Mol. Graph. Model. 2017, 75, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Ge, C.; Liu, Y.; Bai, R.; Li, D.; Yang, Y.; Liao, L.; Chen, C. The interaction of serum proteins with carbon nanotubes depend on the physicochemical properties of nanotubes. J. Nanosci. Nanotechnol. 2011, 11, 10102–10110. [Google Scholar] [CrossRef] [PubMed]
- Canapè, C.; Foillard, S.; Bonafè, R.; Maiocchi, A.; Doris, E. Comparative assessment of the in vitro toxicity of some functionalized carbon nanotubes and fullerenes. RSC Adv. 2015, 5, 68446–68453. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, K.; Zhou, T.; Jia, X.; Li, J.; Liu, P. PEGylated multi-walled carbon nanotubes as versatile vector for tumor-specific intracellular triggered release with enhanced anti-cancer efficiency: Optimization of length and PEGylation degree. Colloids Surf. B Biointerfaces 2018, 168, 43–49. [Google Scholar] [CrossRef]
- Boge, J.; Sweetman, L.J.; Panhuis, M.I.H.; Ralph, S.F. The effect of preparation conditions and biopolymer dispersants on the properties of SWNT buckypapers. J. Mater. Chem. 2009, 19, 9131–9140. [Google Scholar] [CrossRef]
- Lee, J.U.; Huh, J.; Kim, K.H.; Park, C.; Jo, W.H. Aqueous suspension of carbon nanotubes via non-covalent functionalization with oligothiophene-terminated poly(ethylene glycol). Carbon 2007, 45, 1051–1057. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Li, C.; Hou, L.-A. Fabrication of GO modified PVDF membrane for dissolved organic matter removal: Removal mechanism and antifouling property. Sep. Purif. Technol. 2019, 209, 482–490. [Google Scholar] [CrossRef]
- Kundu, S.; Wang, Y.; Xia, W.; Muhler, M. Thermal Stability and Reducibility of Oxygen-Containing Functional Groups on Multiwalled Carbon Nanotube Surfaces: A Quantitative High-Resolution XPS and TPD/TPR Study. J. Phys. Chem. C 2008, 112, 16869–16878. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Shan, M.; Zhou, B.; Li, Y.; Li, B.; Niu, J.; Qian, X. Synergetic effects of oxidized carbon nanotubes and graphene oxide on fouling control and anti-fouling mechanism of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2013, 448, 81–92. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Ke, B.; He, Z.; Cui, Y.; Pan, Z.; Li, D.; Huang, S.; Lai, C.; Su, J. Magnetic multi-walled carbon nanotubes modified with polyaluminium chloride for removal of humic acid from aqueous solution. J. Mol. Liq. 2019, 279, 241–250. [Google Scholar] [CrossRef]
- Zghal, S.; Jedidi, I.; Cretin, M.; Cerneaux, S.; Abdelmouleh, M. One-step synthesis of highly porous carbon graphite/carbon nanotubes composite by in-situ growth of carbon nanotubes for the removal of humic acid and copper (II) from wastewater. Diam. Relat. Mater. 2020, 101, 107557. [Google Scholar] [CrossRef]

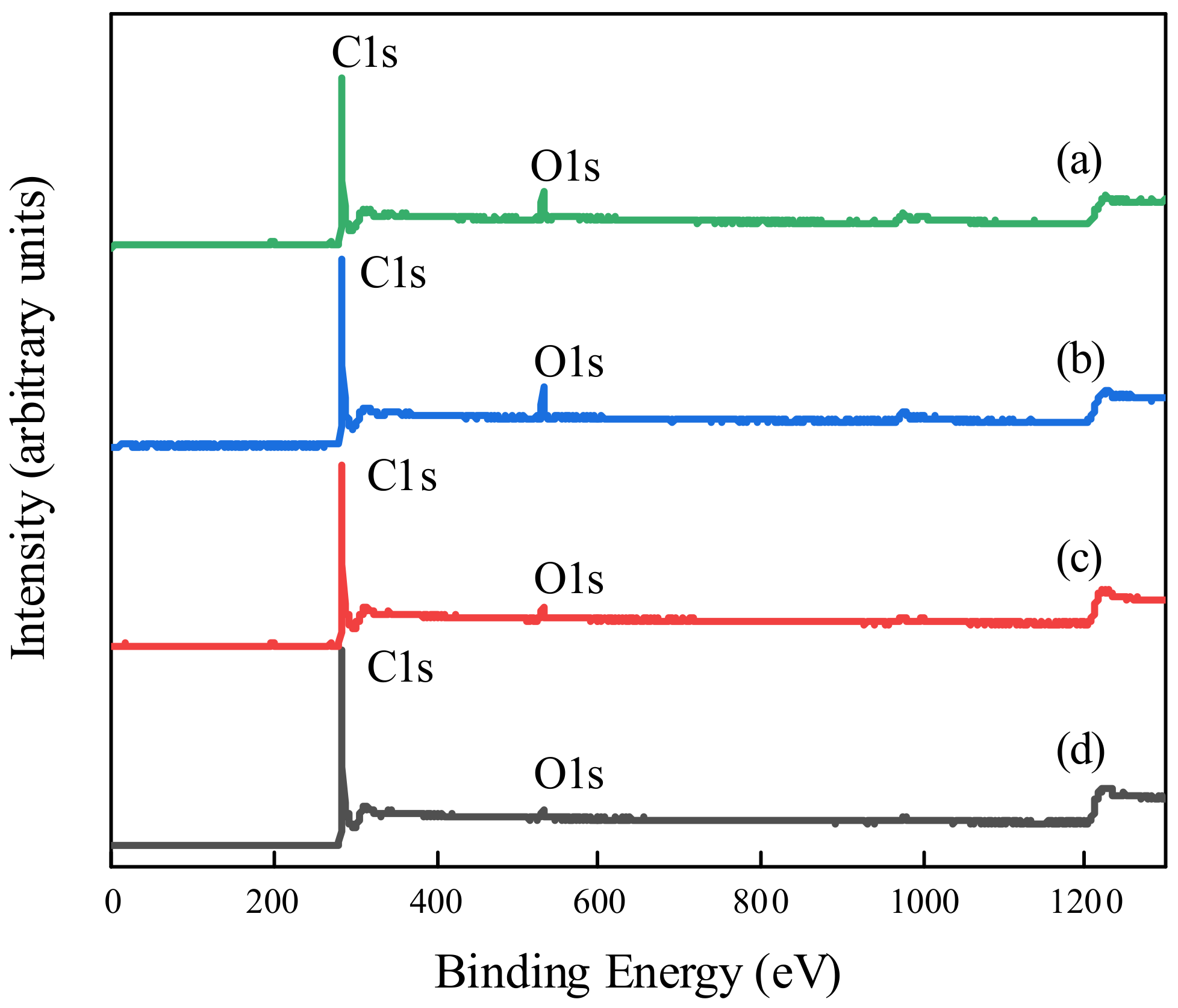
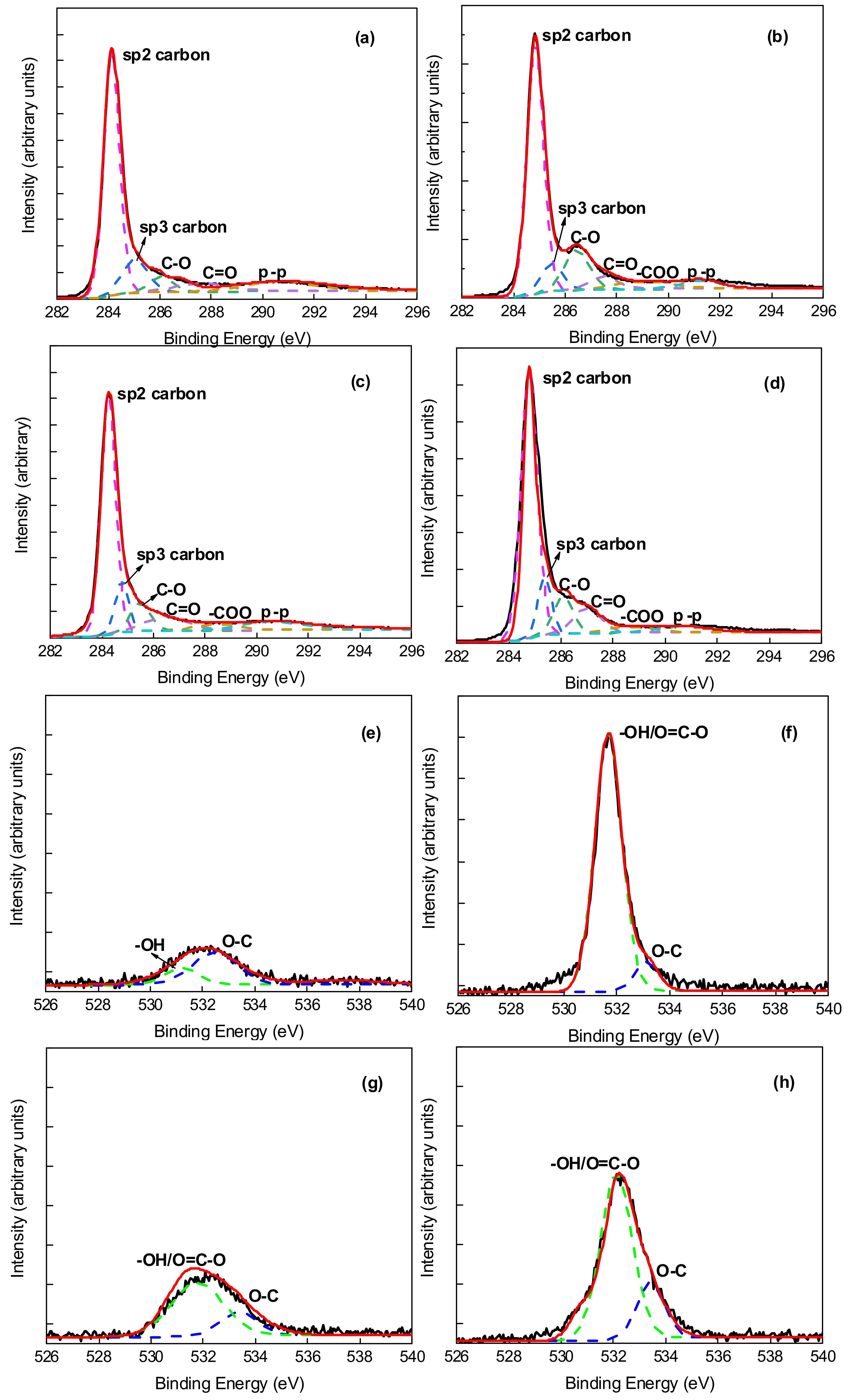
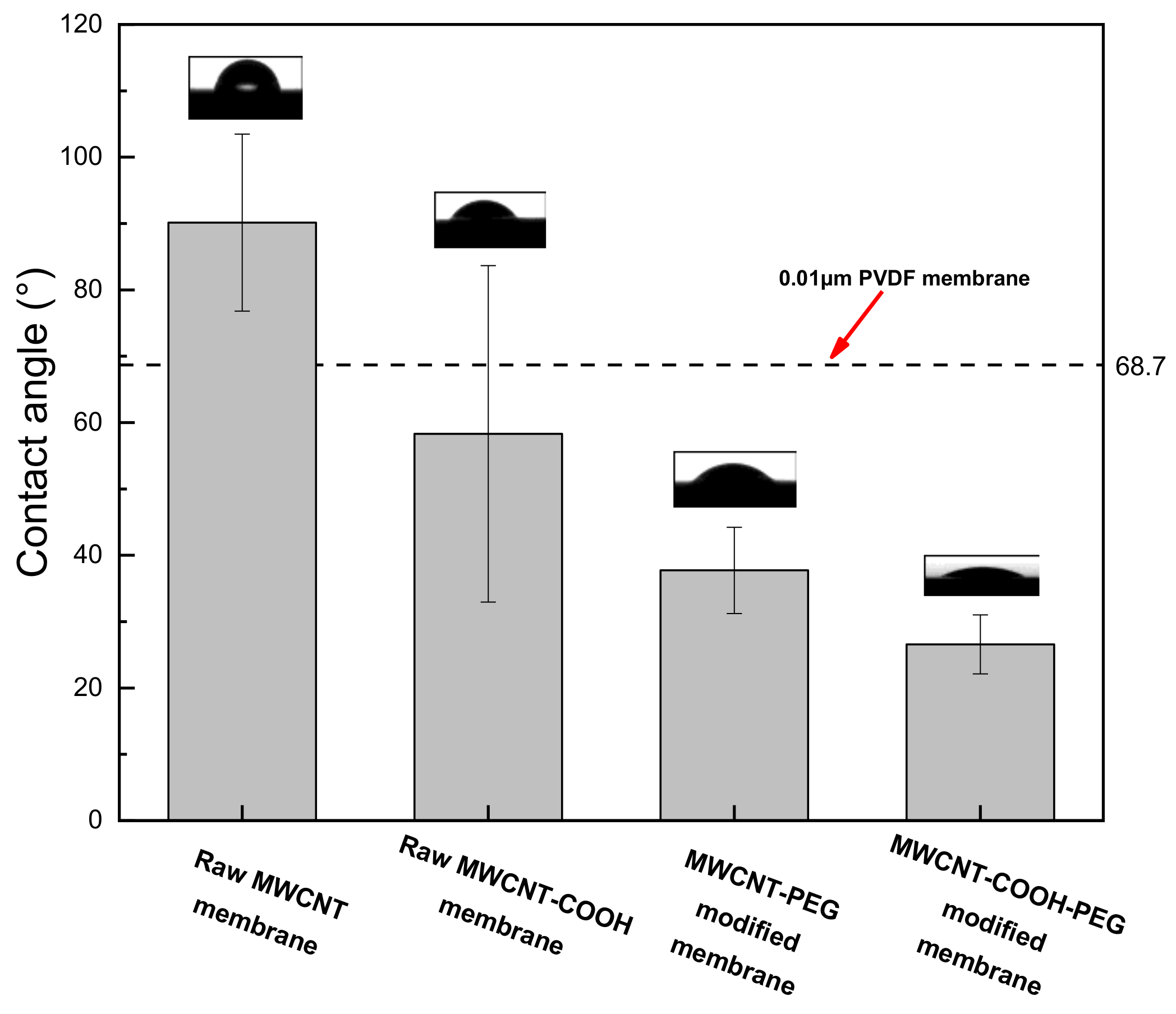


| Membrane Type | PEG–MWCNT–COOH Membrane | PEG–MWCNT Membrane | Raw MWCNT–COOH Membrane | Raw MWCNT Membrane |
|---|---|---|---|---|
| C content (%) | 92.65 | 94.09 | 96.21 | 97.40 |
| O content (%) | 7.35 | 5.91 | 3.79 | 2.60 |
| –OH/O–C=O content (%) | 2.47 | 2.25 | 0.96 | 0.32 |
| O–C content (%) | 0.41 | 0.13 | 0.32 | 0.42 |
| Type | Pore Size (nm) | Porosity (%) | ||
|---|---|---|---|---|
| Mean Size | SD | Ratio | SD | |
| Raw–MWCNT | 29.0035 | 27.0679 | 18.46 | 0.0518 |
| Raw–MWCNT–COOH | 23.0691 | 24.3588 | 17.44 | 0.0017 |
| PEG–MWCNT | 24.0633 | 23.9308 | 19.56 | 0.0184 |
| PEG–MWCNT–COOH | 21.9863 | 20.4326 | 7.81 | 0.0910 |
| Membrane Type | Cycle 1 (%) | Cycle 2 (%) | Average (%) |
|---|---|---|---|
| 0.01 µm PVDF membrane | 71.9 | 88.1 | 80.0 |
| PEG–MWCNT–COOH-modified membrane | 71.4 | 76.2 | 73.8 |
| PEG–MWCNT-modified membrane | 79.8 | 79.1 | 79.4 |
| Raw MWCNT–COOH membrane | 14.7 | 15.1 | 14.9 |
| Raw MWCNT membrane | 35.6 | 20.9 | 28.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Dong, M.; Xiong, X.; Gai, X.; Zeng, J.; Luan, G.; Wang, Y.; Wu, Y.; Guo, J. Preparation of Ultrafiltration Membrane by Polyethylene Glycol Non-Covalent Functionalized Multi-Walled Carbon Nanotubes: Application for HA Removal and Fouling Control. Membranes 2021, 11, 362. https://doi.org/10.3390/membranes11050362
Wang Y, Dong M, Xiong X, Gai X, Zeng J, Luan G, Wang Y, Wu Y, Guo J. Preparation of Ultrafiltration Membrane by Polyethylene Glycol Non-Covalent Functionalized Multi-Walled Carbon Nanotubes: Application for HA Removal and Fouling Control. Membranes. 2021; 11(5):362. https://doi.org/10.3390/membranes11050362
Chicago/Turabian StyleWang, Yu, Mengchan Dong, Xinya Xiong, Xiaoli Gai, Jia Zeng, Guirong Luan, Yufei Wang, Yaochen Wu, and Jin Guo. 2021. "Preparation of Ultrafiltration Membrane by Polyethylene Glycol Non-Covalent Functionalized Multi-Walled Carbon Nanotubes: Application for HA Removal and Fouling Control" Membranes 11, no. 5: 362. https://doi.org/10.3390/membranes11050362
APA StyleWang, Y., Dong, M., Xiong, X., Gai, X., Zeng, J., Luan, G., Wang, Y., Wu, Y., & Guo, J. (2021). Preparation of Ultrafiltration Membrane by Polyethylene Glycol Non-Covalent Functionalized Multi-Walled Carbon Nanotubes: Application for HA Removal and Fouling Control. Membranes, 11(5), 362. https://doi.org/10.3390/membranes11050362






