Influence of Preparation Temperature on the Properties and Performance of Composite PVDF-TiO2 Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
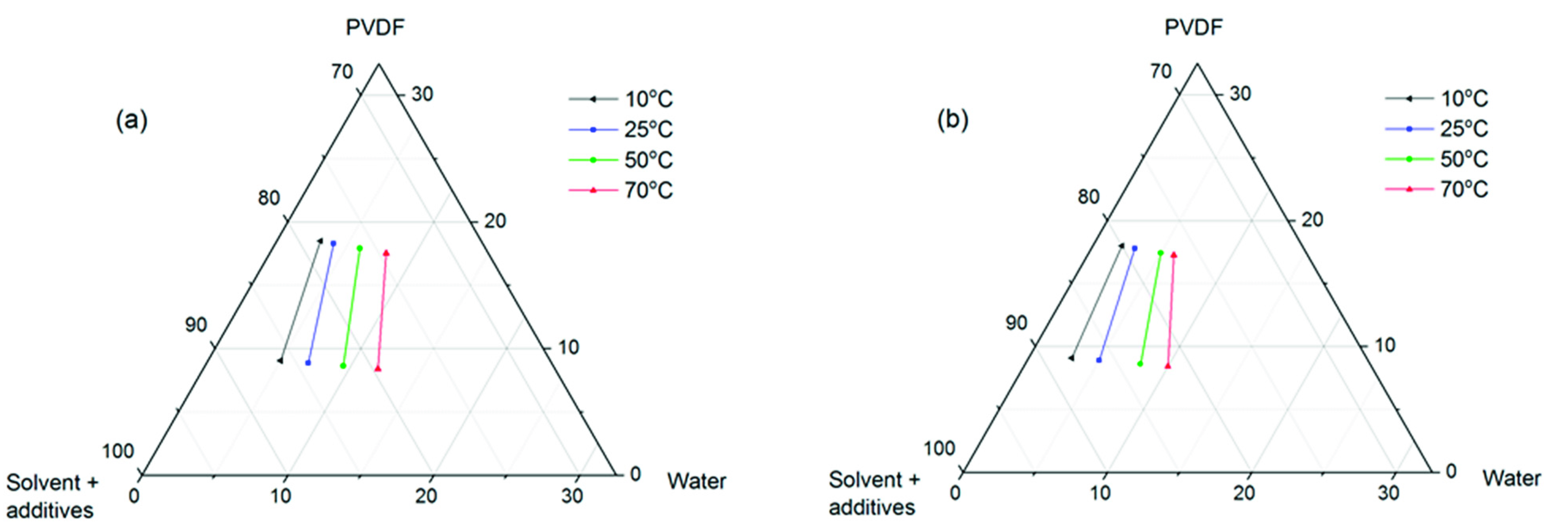
2.3. Study of Phase Separation Thermodynamics via Ternary Phase Diagrams
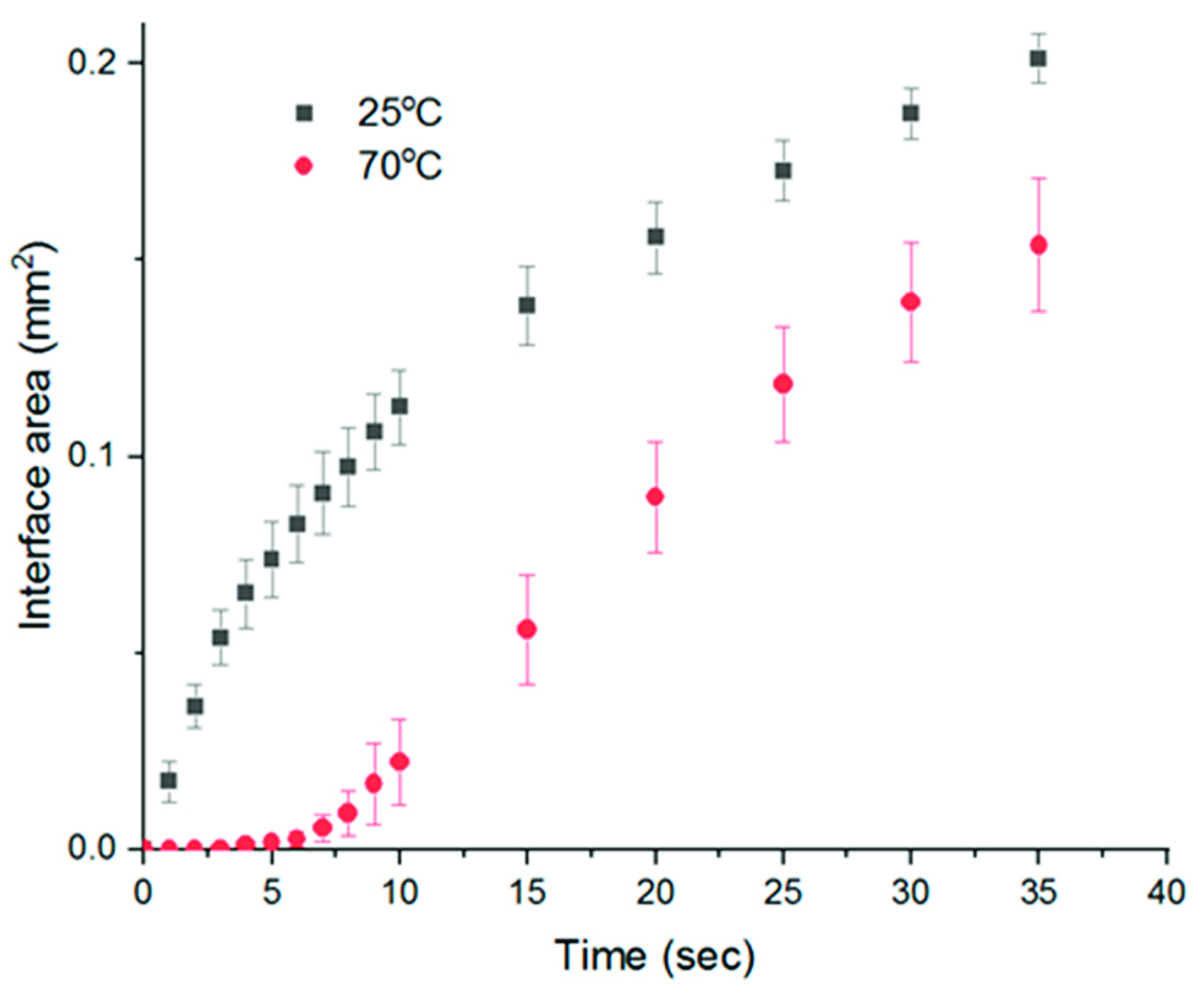
2.4. Study of Phase Separation Kinetics via Light Absorbance
2.5. Microscopic Observation of Water Entrance during NIPS
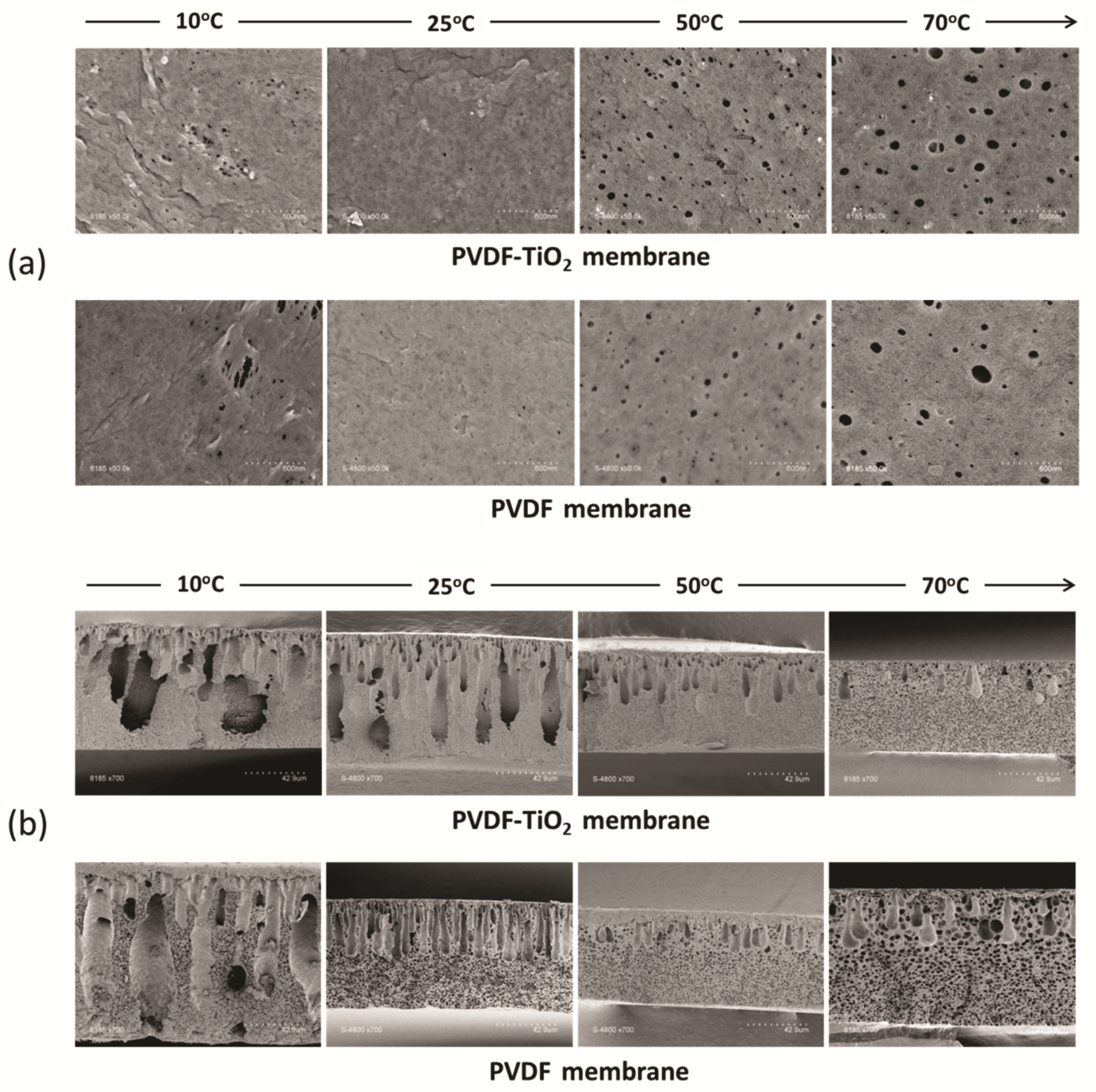
2.6. Membrane Structural Characterization
2.7. Thermal and Crystalline Properties
2.8. Membrane Permeability and Mechanical Properties
3. Results and Discussion
3.1. Phase Separation Thermodynamics
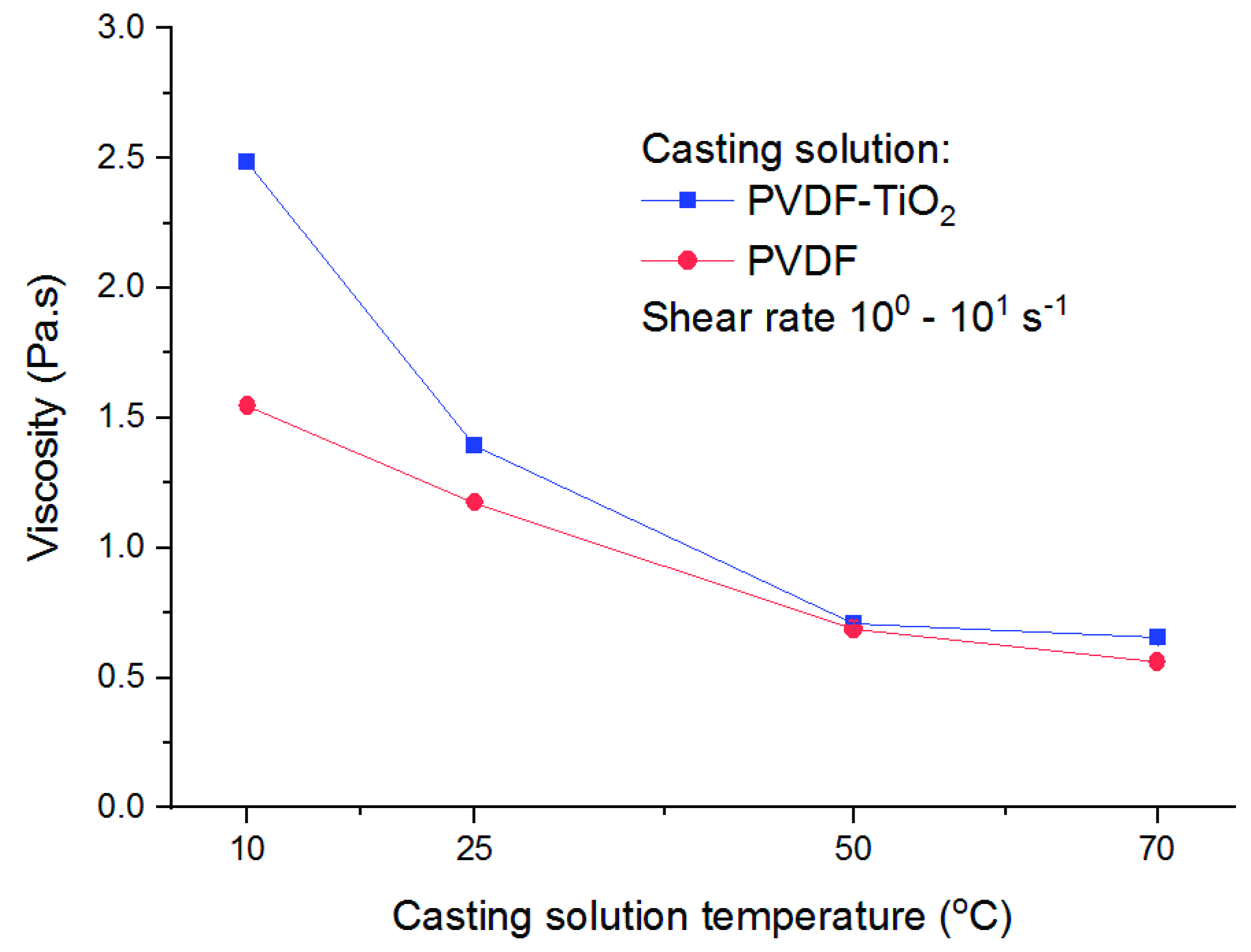
3.2. Phase Separation Kinetics
3.3. Microscopic Observation of Membrane Formation
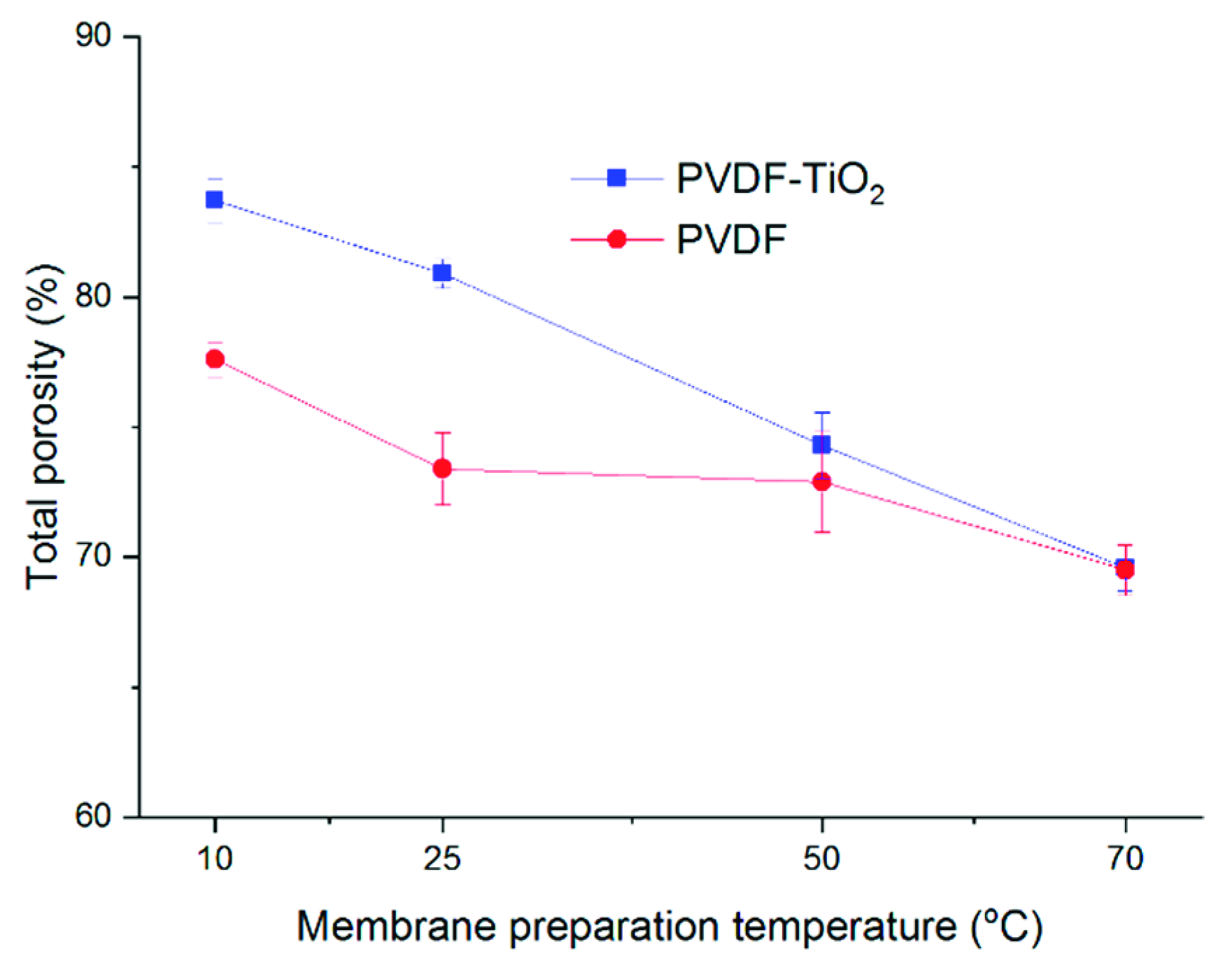
3.4. Membrane Structure and Properties
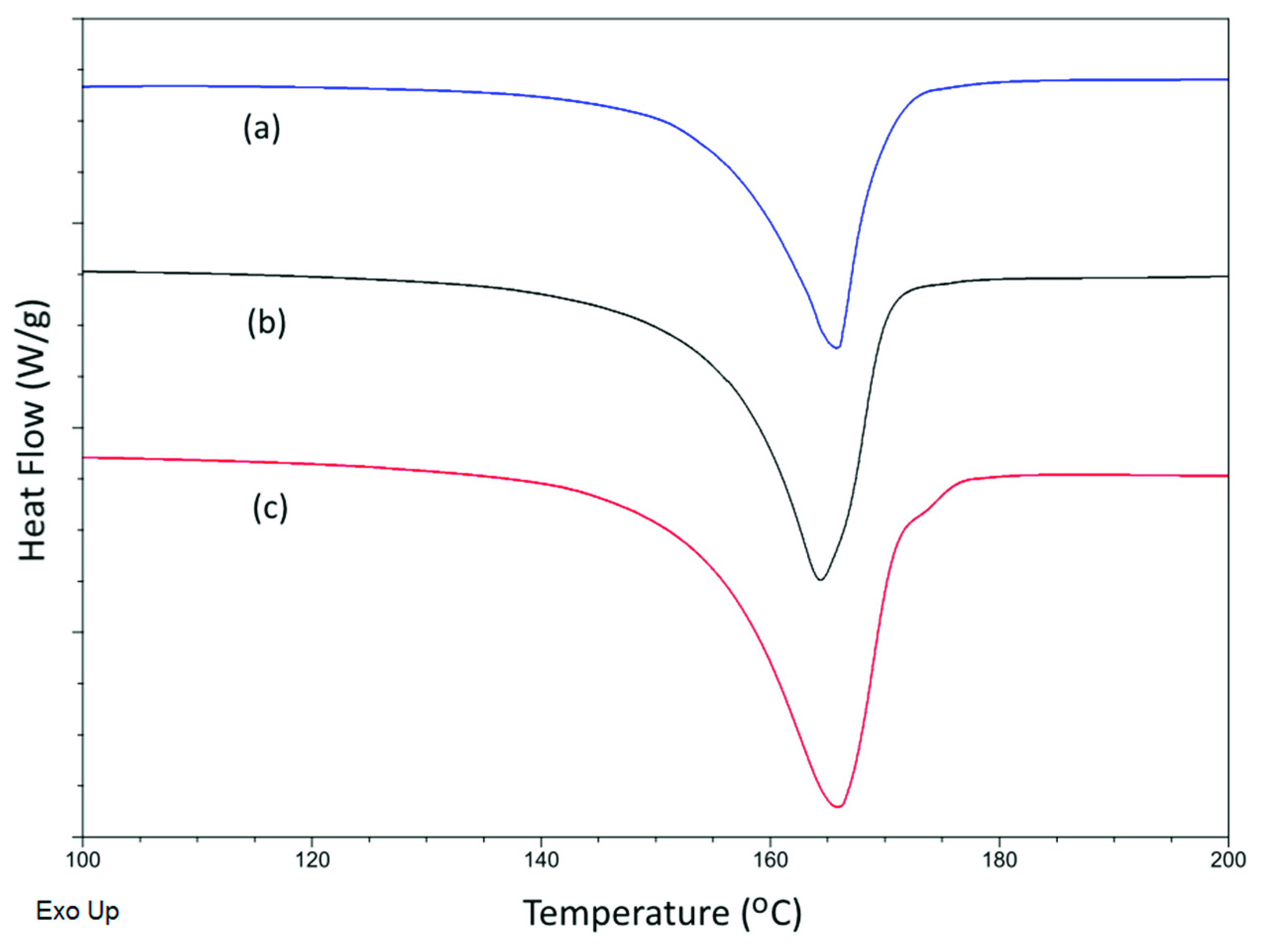
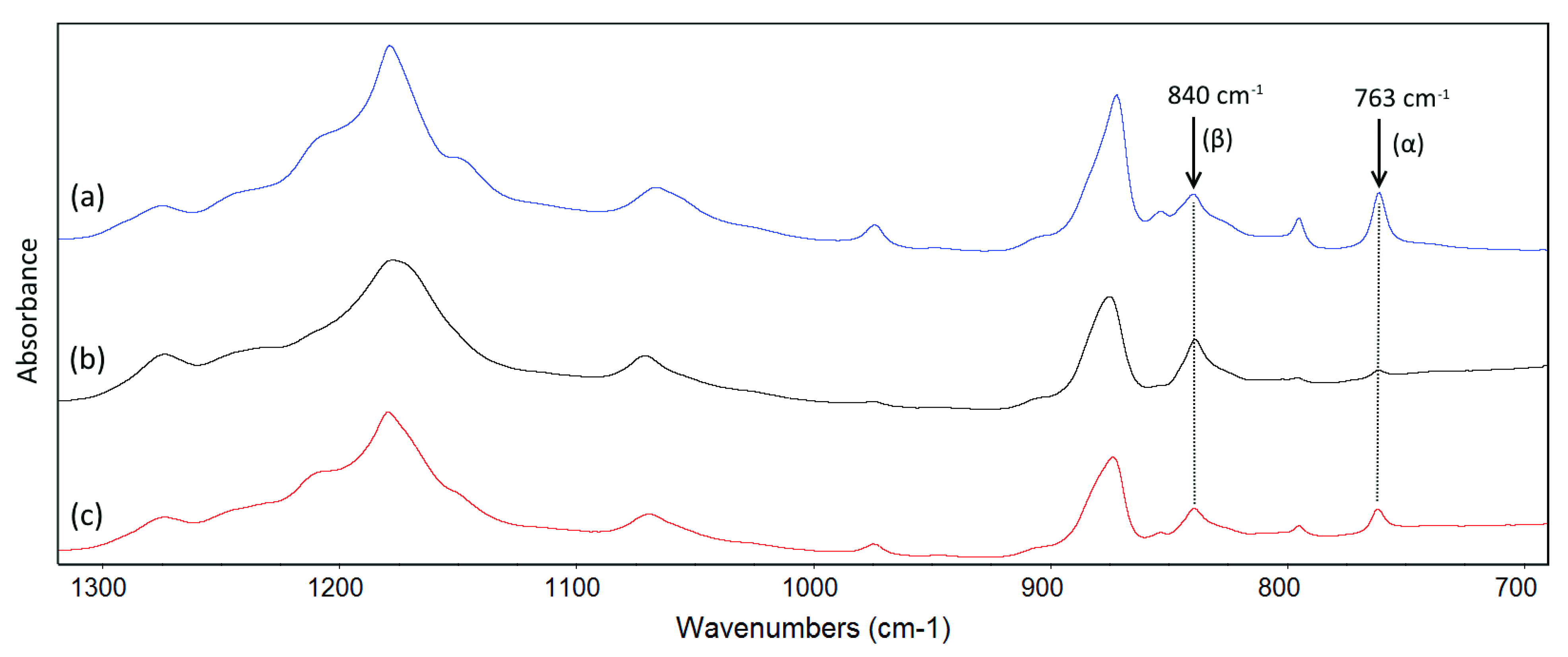
3.5. Thermal and Crystalline Properties
3.6. Membrane Permeability and Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goh, P.S.; Ng, B.C.; Lau, W.J.; Ismail, A.F. Inorganic Nanomaterials in Polymeric Ultrafiltration Membranes for Water Treatment. Sep. Purif. Rev. 2015, 44, 216–249. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Hoek, E.M.; Ghosh, A.K.; Huang, X.; Liong, M.; Zink, J.I. Physical–chemical properties, separation performance, and fouling resistance of mixed-matrix ultrafiltration membranes. Desalination 2011, 283, 89–99. [Google Scholar] [CrossRef]
- Li, J.-H.; Shao, X.-S.; Zhou, Q.; Li, M.-Z.; Zhang, Q.-Q. The double effects of silver nanoparticles on the PVDF membrane: Surface hydrophilicity and antifouling performance. Appl. Surf. Sci. 2013, 265, 663–670. [Google Scholar] [CrossRef]
- Wongchitphimon, S.; Wang, R.; Jiraratananon, R.; Shi, L.; Loh, C.H. Effect of polyethylene glycol (PEG) as an additive on the fabrication of polyvinylidene fluoride-co-hexafluropropylene (PVDF-HFP) asymmetric microporous hollow fiber membranes. J. Membr. Sci. 2011, 369, 329–338. [Google Scholar] [CrossRef]
- Sun, C.; Zlotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; di Noto, V. [Nafion/(WO3)x] hybrid membranes for vanadium redox flow batteries. Solid State Ionics. 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Iglesias, O.; Rivero, M.; Urtiaga, A.M.; Ortiz, I. Membrane-based photocatalytic systems for process intensification. Chem. Eng. J. 2016, 305, 136–148. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.-J.; Chou, H.-H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, antifouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Madaeni, S.; Ghaemi, N.; Alizadeh, A.; Joshaghani, M. Influence of photo-induced superhydrophilicity of titanium dioxide nanoparticles on the anti-fouling performance of ultrafiltration membranes. Appl. Surf. Sci. 2011, 257, 6175–6180. [Google Scholar] [CrossRef]
- Ngang, H.P.; Ooi, B.S.; Ahmad, A.L.; Lai, S.O. Preparation of PVDF-TiO2 mixed-matrix membrane and its evaluation on dye adsorption and UV-cleaning properties. Chem. Eng. J. 2012, 197, 359–367. [Google Scholar] [CrossRef]
- Cui, Z.; Drioli, E.; Lee, Y.M. Recent progress in fluoropolymers for membranes. Prog. Polym. Sci. 2014, 39, 164–198. [Google Scholar] [CrossRef]
- Chin, S.S.; Chiang, K.; Fane, A.G. The stability of polymeric membranes in a TiO2 photocatalysis process. J. Membr. Sci. 2006, 275, 202–211. [Google Scholar] [CrossRef]
- Teow, Y.H.; Ahmad, A.L.; Lim, J.K.; Ooi, B. Studies on the surface properties of mixed-matrix membrane and its antifouling properties for humic acid removal. J. Appl. Polym. Sci. 2013, 128, 3184–3192. [Google Scholar] [CrossRef]
- Nor, N.; Jaafar, J.; Ismail, A.; Mohamed, M.A.; Rahman, M.; Othman, M.; Lau, W.; Yusof, N. Preparation and performance of PVDF-based nanocomposite membrane consisting of TiO2 nanofibers for organic pollutant decomposition in wastewater under UV irradiation. Desalination 2016, 391, 89–97. [Google Scholar] [CrossRef]
- Razmjou, A.; Arifin, E.; Dong, G.; Mansouri, J.; Chen, V. Superhydrophobic modification of TiO2 nanocomposite PVDF membranes for applications in membrane distillation. J. Membr. Sci. 2012, 415–416, 850–863. [Google Scholar] [CrossRef]
- Alaoui, O.T.; Nguyen, Q.T.; Mbareck, C.; Rhlalou, T. Elaboration and study of poly(vinylidene fluoride)–anatase TiO2 composite membranes in photocatalytic degradation of dyes. Appl. Catal. A Gen. 2009, 358, 13–20. [Google Scholar] [CrossRef]
- Mericq, J.P.; Mendret, J.; Brosillon, S.; Faur, C. High performance PVDF-TiO2 membranes for water treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the non-solvent induced phase separation (NIPS) effect during the fabrication of microporous PVDF membranes via thermally induced phase separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Yeow, M.L.; Liu, Y.T.; Li, K. Isothermal phase diagrams and phase-inversion behavior of poly(vinylidene fluoride)/solvents/additives/water systems. J. Appl. Polym. Sci. 2003, 90, 2150–2155. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Sun, D.; An, Q.; Chen, H. Formation mechanism and crystallization of poly(vinylidene fluoride) membrane via immersion precipitation method. Desalination 2009, 236, 170–178. [Google Scholar] [CrossRef]
- Buonomenna, M.; Macchi, P.; Davoli, M.; Drioli, E. Poly(vinylidene fluoride) membranes by phase inversion: The role the casting and coagulation conditions play in their morphology, crystalline structure and properties. Eur. Polym. J. 2007, 43, 1557–1572. [Google Scholar] [CrossRef]
- Fadaei, A.; Salimi, A.; Mirzataheri, M. Structural elucidation of morphology and performance of the PVDF/PEG membrane. J. Polym. Res. 2014, 21, 1–8. [Google Scholar] [CrossRef]
- Bottino, A.; Camera-Roda, G.; Capannelli, G.; Munari, S. The formation of microporous polyvinylidene difluoride membranes by phase separation. J. Membr. Sci. 1991, 57, 1–20. [Google Scholar] [CrossRef]
- Guillen, G.R.; Pan, Y.; Li, M.; Hoek, E.M.V. Preparation and Characterization of Membranes Formed by Nonsolvent Induced Phase Separation: A Review. Ind. Eng. Chem. Res. 2011, 50, 3798–3817. [Google Scholar] [CrossRef]
- Lee, K.-W.; Seo, B.-K.; Nam, S.-T.; Han, M.-J. Trade-off between thermodynamic enhancement and kinetic hindrance during phase inversion in the preparation of polysulfone membranes. Desalination 2003, 159, 289–296. [Google Scholar] [CrossRef]
- Moghadam, M.T.; Lesage, G.; Mohammadi, T.; Mericq, J.-P.; Mendret, J.; Heran, M.; Faur, C.; Brosillon, S.; Hemmati, M.; Naeimpoor, F. Improved antifouling properties of TiO2/PVDF nanocomposite membranes in UV-coupled ultrafiltration. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Tran, D.-T.; Mendret, J.; Méricq, J.-P.; Faur, C.; Brosillon, S. Study of permeate flux behavior during photo-filtration using photocatalytic composite membranes. Chem. Eng. Process. Process. Intensif. 2020, 148, 107781. [Google Scholar] [CrossRef]
- Guillen, G.R.; Ramon, G.Z.; Kavehpour, H.P.; Kaner, R.B.; Hoek, E.M. Direct microscopic observation of membrane formation by nonsolvent induced phase separation. J. Membr. Sci. 2013, 431, 212–220. [Google Scholar] [CrossRef]
- Zhang, G.; Lu, S.; Zhang, L.; Meng, Q.; Shen, C.; Zhang, J. Novel polysulfone hybrid ultrafiltration membrane prepared with TiO2-g-HEMA and its antifouling characteristics. J. Membr. Sci. 2013, 436, 163–173. [Google Scholar] [CrossRef]
- Shi, F.M.; Ma, Y.X.; Ma, J.; Wang, P.P.; Sun, W.X. Preparation and characterization of PVDF/TiO2 hybrid membranes with different dosage of nano-TiO2. J. Memb. Sci. 2012, 389, 522–531. [Google Scholar] [CrossRef]
- Lin, D.-J.; Chang, H.-H.; Chen, T.-C.; Lee, Y.-C.; Cheng, L.-P. Formation of porous poly(vinylidene fluoride) membranes with symmetric or asymmetric morphology by immersion precipitation in the water/TEP/PVDF system. Eur. Polym. J. 2006, 42, 1581–1594. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Bhattacharjee, S. Rational design of phase inversion membranes by tailoring thermodynamics and kinetics of casting solution using polymer additives. J. Membr. Sci. 2013, 441, 31–44. [Google Scholar] [CrossRef]
- Mohsenpour, S.; Safekordi, A.; Tavakolmoghadam, M.; Rekabdar, F.; Hemmati, M. Comparison of the membrane morphology based on the phase diagram using PVP as an organic additive and TiO2 as an inorganic additive. Polymer 2016, 97, 559–568. [Google Scholar] [CrossRef]
- Mohsenpour, S.; Esmaeilzadeh, F.; Safekordi, A.; Tavakolmoghadam, M.; Rekabdar, F.; Hemmati, M. The role of thermodynamic parameter on membrane morphology based on phase diagram. J. Mol. Liq. 2016, 224, 776–785. [Google Scholar] [CrossRef]
- Feng, Y.; Han, G.; Zhang, L.; Chen, S.-B.; Chung, T.-S.; Weber, M.; Staudt, C.; Maletzko, C. Rheology and phase inversion behavior of polyphenylenesulfone (PPSU) and sulfonated PPSU for membrane formation. Polymer 2016, 99, 72–82. [Google Scholar] [CrossRef]
- Chang, J.; Zuo, J.; Zhang, L.; O’Brien, G.S.; Chung, T.S. Using green solvent, triethyl phosphate (TEP), to fabricate highly porous PVDF hollow fiber membranes for membrane distillation. J. Memb. Sci. 2017, 539, 295–304. [Google Scholar] [CrossRef]
- Matz, R. The structure of cellulose acetate membranes 1. The development of porous structures in anisotropic membranes. Desalination 1972, 10, 1–15. [Google Scholar] [CrossRef]
- Strathmann, H.; Kock, K.; Amar, P.; Baker, R. The formation mechanism of asymmetric membranes. Desalination 1975, 16, 179–203. [Google Scholar] [CrossRef]
- Strathmann, H.; Kock, K. The formation mechanism of phase inversion membranes. Desalination 1977, 21, 241–255. [Google Scholar] [CrossRef]
- Paulsen, F.G.; Shojaie, S.S.; Krantz, W. Effect of evaporation step on macrovoid formation in wet-cast polymeric membranes. J. Membr. Sci. 1994, 91, 265–282. [Google Scholar] [CrossRef]
- Frommer, M.A.; Messalem, R.M. Mechanism of Membrane Formation. VI. Convective Flows and Large Void Formation during Membrane Precipitation. Prod. Res. Dev. 1973, 12, 328–333. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Lin, F.-C.; Wu, T.-T.; Wang, D.-M. On the formation of macrovoids in PMMA membranes. J. Membr. Sci. 1999, 155, 31–43. [Google Scholar] [CrossRef]
- Khare, V.P.; Greenberg, A.R.; Zartman, J.; Krantz, W.B.; Todd, P. Macrovoid growth during polymer membrane casting. Desalination 2002, 145, 17–23. [Google Scholar] [CrossRef]
- Smolders, C.; Reuvers, A.; Boom, R.; Wienk, I. Microstructures in phase-inversion membranes. Part 1. Formation of macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef] [Green Version]
- McKelvey, S.A.; Koros, W.J. Phase separation, vitrification, and the manifestation of macrovoids in polymeric asymmetric membranes. J. Memb. Sci. 1996, 112, 29–39. [Google Scholar] [CrossRef]
- Ren, J.; Li, Z.; Wong, F.S. Membrane structure control of BTDA-TDI/MDI (P84) co-polyimide asymmetric membranes by wet-phase inversion process. J. Memb. Sci. 2004, 241, 305–314. [Google Scholar] [CrossRef]
- Wang, B.; Lai, Z. Finger-like voids induced by viscous fingering during phase inversion of alumina/PES/NMP suspensions. J. Membr. Sci. 2012, 405–406, 275–283. [Google Scholar] [CrossRef]
- Kosma, V.A.; Beltsios, K.G. Macrovoids in solution-cast membranes: Direct probing of systems exhibiting horizontal macrovoid growth. J. Membr. Sci. 2012, 407–408, 93–107. [Google Scholar] [CrossRef]
- Yu, L.; Yang, F.; Xiang, M. Phase separation in a PSf/DMF/water system: A proposed mechanism for macrovoid formation. RSC Adv. 2014, 4, 42391–42402. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Z.; Zhao, S.; Li, S.; Wang, J.; Wang, S. The influence of the nonsolvent intrusion through the casting film bottom surface on the macrovoid formation. J. Membr. Sci. 2014, 464, 8–19. [Google Scholar] [CrossRef]
- Hung, W.-L.; Wang, D.-M.; Lai, J.-Y.; Chou, S.-C. On the initiation of macrovoids in polymeric membranes—Effect of polymer chain entanglement. J. Membr. Sci. 2016, 505, 70–81. [Google Scholar] [CrossRef]
- Lin, K.-Y.; Wang, D.-M.; Lai, J.-Y. Nonsolvent-Induced Gelation and Its Effect on Membrane Morphology. Macromolecules 2002, 35, 6697–6706. [Google Scholar] [CrossRef]
- Su, Y.; Kuo, C.; Wang, D.; Lai, J.; Deratani, A.; Pochat, C.; Bouyer, D. Interplay of mass transfer, phase separation, and membrane morphology in vapor-induced phase separation. J. Membr. Sci. 2009, 338, 17–28. [Google Scholar] [CrossRef]
- Benz, M.; Euler, W.B. Determination of the crystalline phases of poly(vinylidene fluoride) under different preparation conditions using differential scanning calorimetry and infrared spectroscopy. J. Appl. Polym. Sci. 2003, 89, 1093–1100. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, A.-Q.; Zhu, B.-K.; Du, C.-H.; Xu, Y.-Y. Polymorphism in porous poly(vinylidene fluoride) membranes formed via immersion precipitation process. J. Membr. Sci. 2008, 319, 169–175. [Google Scholar] [CrossRef]
- Cai, X.M.; Lei, T.P.; Sun, D.H.; Lin, L.W. A critical analysis of the alpha, beta and gamma phases in poly(vinylidene fluoride) using FTIR. Rsc. Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Gregorio, R. Determination of the alpha, beta, and gamma crystalline phases of poly(vinylidene fluoride) films prepared at different conditions. J. Appl. Polym. Sci. 2006, 100, 3272–3279. [Google Scholar] [CrossRef]
- Li, C.-L.; Wang, D.-M.; Deratani, A.; Quémener, D.; Bouyer, D.; Lai, J.-Y. Insight into the preparation of poly(vinylidene fluoride) membranes by vapor-induced phase separation. J. Membr. Sci. 2010, 361, 154–166. [Google Scholar] [CrossRef]
- Liu, J.; Lu, X.; Wu, C. Effect of Preparation Methods on Crystallization Behavior and Tensile Strength of Poly(vinylidene fluoride) Membranes. Membranes 2013, 3, 389–405. [Google Scholar] [CrossRef] [Green Version]
- Rahimpour, A.; Jahanshahi, M.; Mollahosseini, A.; Rajaeian, B. Structural and performance properties of UV-assisted TiO2 deposited nanocomposite PVDF/SPES membranes. Desalination 2012, 285, 31–38. [Google Scholar] [CrossRef]




| Mechanism | References |
|---|---|
| Shrinkage of polymer matrix Initiation of macrovoids is induced by fractures in skin layer of the polymer solution, then the shrinkage of polymer matrix drains new precipitates to the finger side, enlarging the macrovoids.
| [42,43] |
| Surface tension gradient-induced convective flow Interfacial tension of the polymer solution/water interface becomes zero at one point, leading to water intrusion in the polymer solution and the initiation of macrovoids. Afterwards, solvent diffusion to the intrusions cause the macrovoids to grow in size.
| [41,44,45,46,47] |
| Instantaneous phase demixing Initiation of macrovoids is induced by the expansion of nuclei droplets of the polymer-lean phase to very large dimensions, then the combined effect of diffusional flows of the polymer lean phase, relative to the polymer-rich phase and liquid–liquid demixing, leads to the growth of macrovoids.
| [46,48,49] |
| Viscous fingering Osmotic pressure leads to water diffusion into polymer-lean phase and causes the initiation of macrovoids. Then, viscous fingering causes the forming of complex finger-like patterns of water inside the polymer solution, leading to macrovoids growth. In addition, delayed demixing also occurs at the walls of the fingers, leading to the propagation of the phase separation front.
| [50,51] |
| Membrane | Tm (°C) | Xc (%) | Fα (%) | Membrane | Tm (°C) | Xc (%) | Fα (%) |
|---|---|---|---|---|---|---|---|
| PVDF-10 | 165.9 ± 0.0 | 61.6 ± 1.0 | 38.9% | PVDF-TiO2-10 | 164.5 ± 0.2 | 61.2 ± 1.3 | 36.4% |
| PVDF-25 | 166.7 ± 0.2 | 58.0 ± 1.2 | 56.6% | PVDF-TiO2-25 | 164.7 ± 0.1 | 60.3 ± 1.2 | 42.7% |
| PVDF-50 | 167.4 ± 0.1 | 59.0 ± 0.7 | 64.2% | PVDF-TiO2-50 | 165.4 ± 0.1 | 58.4 ± 0.8 | 55.9% |
| PVDF-70 | 167.5 ± 0.2 | 54.8 ± 0.7 | 68.8% | PVDF-TiO2-70 | 165.9 ± 0.0 | 55.8 ± 2.1 | 60.3% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, D.-T.; Méricq, J.-P.; Mendret, J.; Brosillon, S.; Faur, C. Influence of Preparation Temperature on the Properties and Performance of Composite PVDF-TiO2 Membranes. Membranes 2021, 11, 876. https://doi.org/10.3390/membranes11110876
Tran D-T, Méricq J-P, Mendret J, Brosillon S, Faur C. Influence of Preparation Temperature on the Properties and Performance of Composite PVDF-TiO2 Membranes. Membranes. 2021; 11(11):876. https://doi.org/10.3390/membranes11110876
Chicago/Turabian StyleTran, Duc-Trung, Jean-Pierre Méricq, Julie Mendret, Stephan Brosillon, and Catherine Faur. 2021. "Influence of Preparation Temperature on the Properties and Performance of Composite PVDF-TiO2 Membranes" Membranes 11, no. 11: 876. https://doi.org/10.3390/membranes11110876
APA StyleTran, D.-T., Méricq, J.-P., Mendret, J., Brosillon, S., & Faur, C. (2021). Influence of Preparation Temperature on the Properties and Performance of Composite PVDF-TiO2 Membranes. Membranes, 11(11), 876. https://doi.org/10.3390/membranes11110876






