Performance of PVDF-TiO2 Membranes during Photo-Filtration in the Presence of Inorganic and Organic Components
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Membrane Preparation
2.3. Photo-Filtration Procedure
2.3.1. Apparatus
2.3.2. Filtration of Inorganic Contents
2.3.3. Filtration of Organic Contents
2.4. Analytical Tools
3. Results and Discussion
3.1. Influence of Inorganic Contents in Drinking Water on Photo-Filtration Performance
3.1.1. Photo-Filtration of Drinking Water
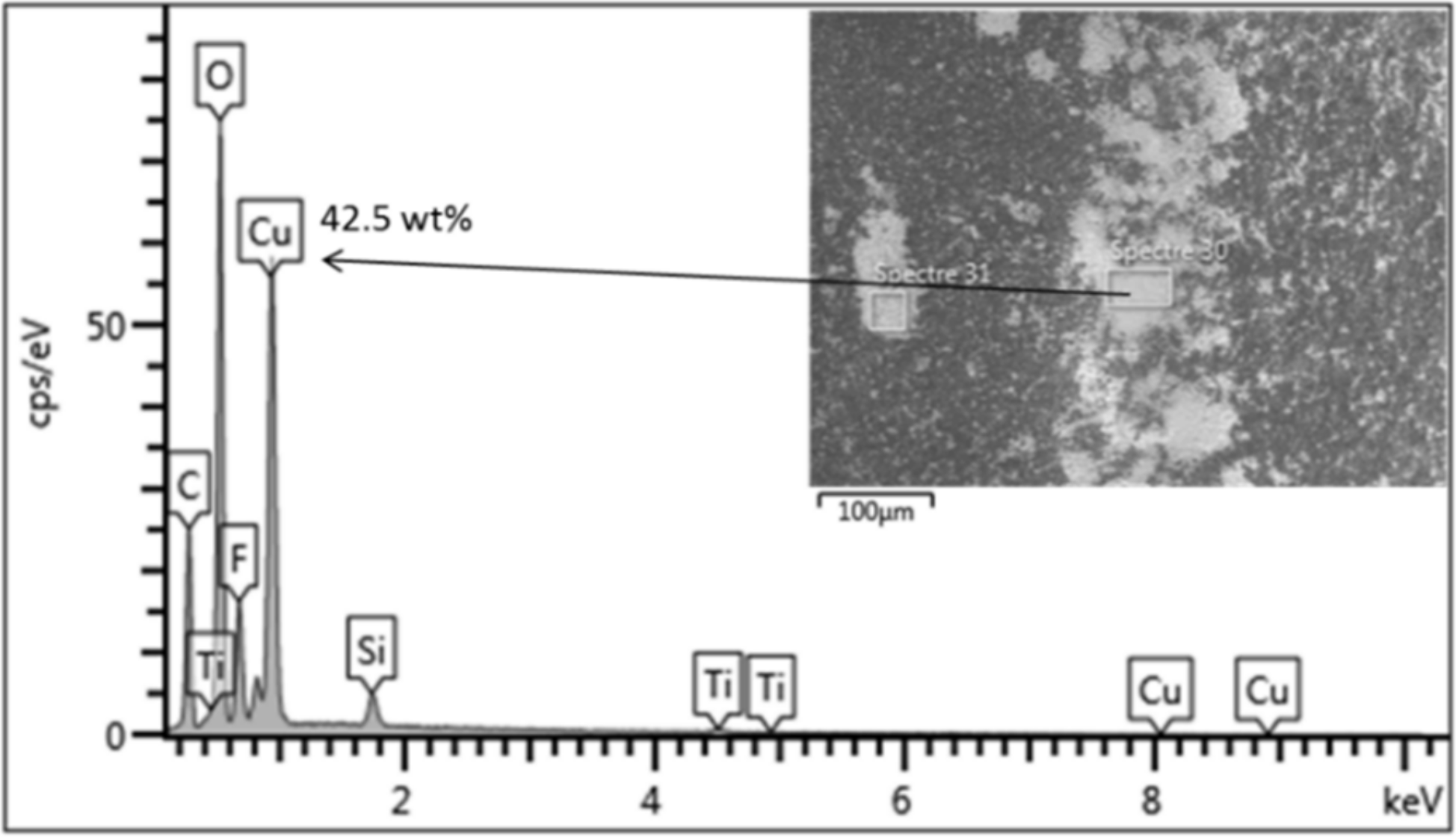
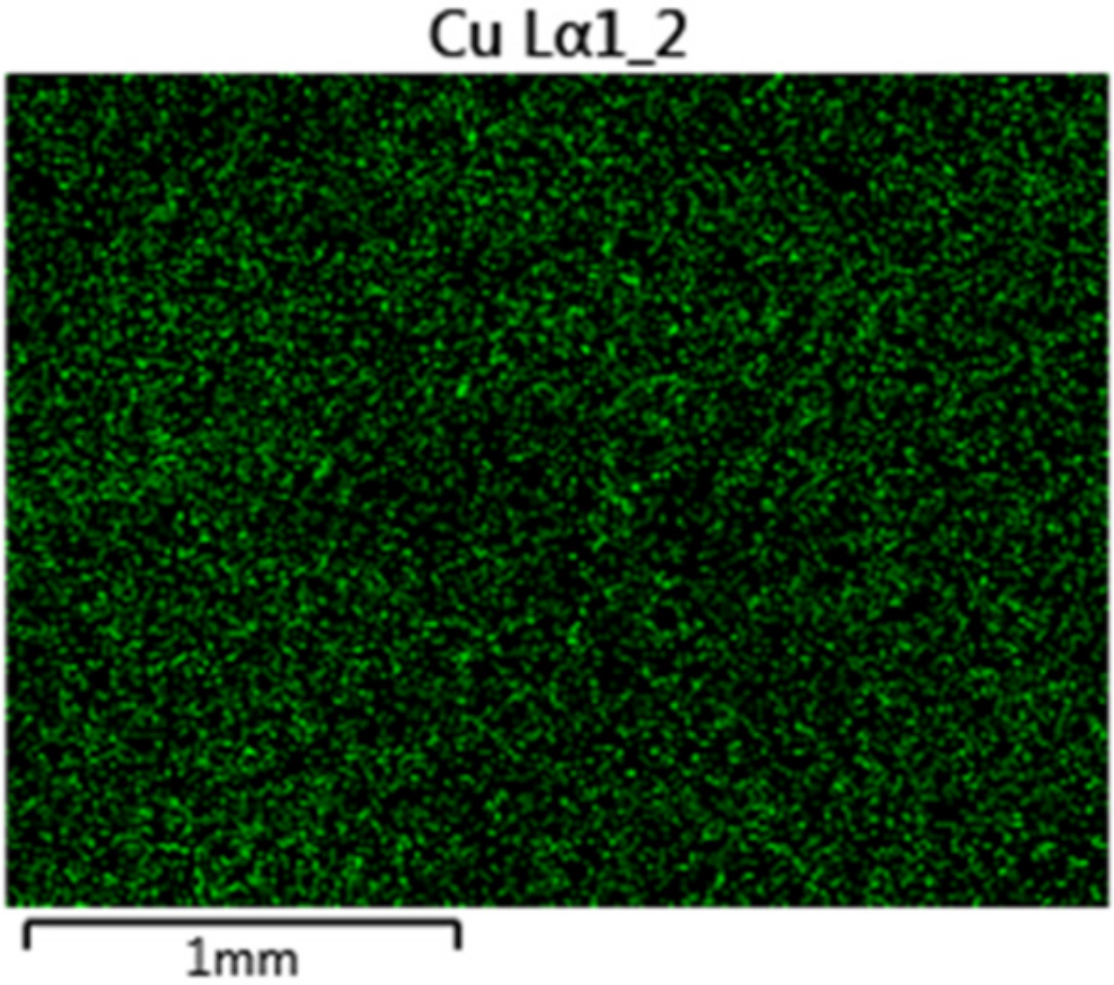
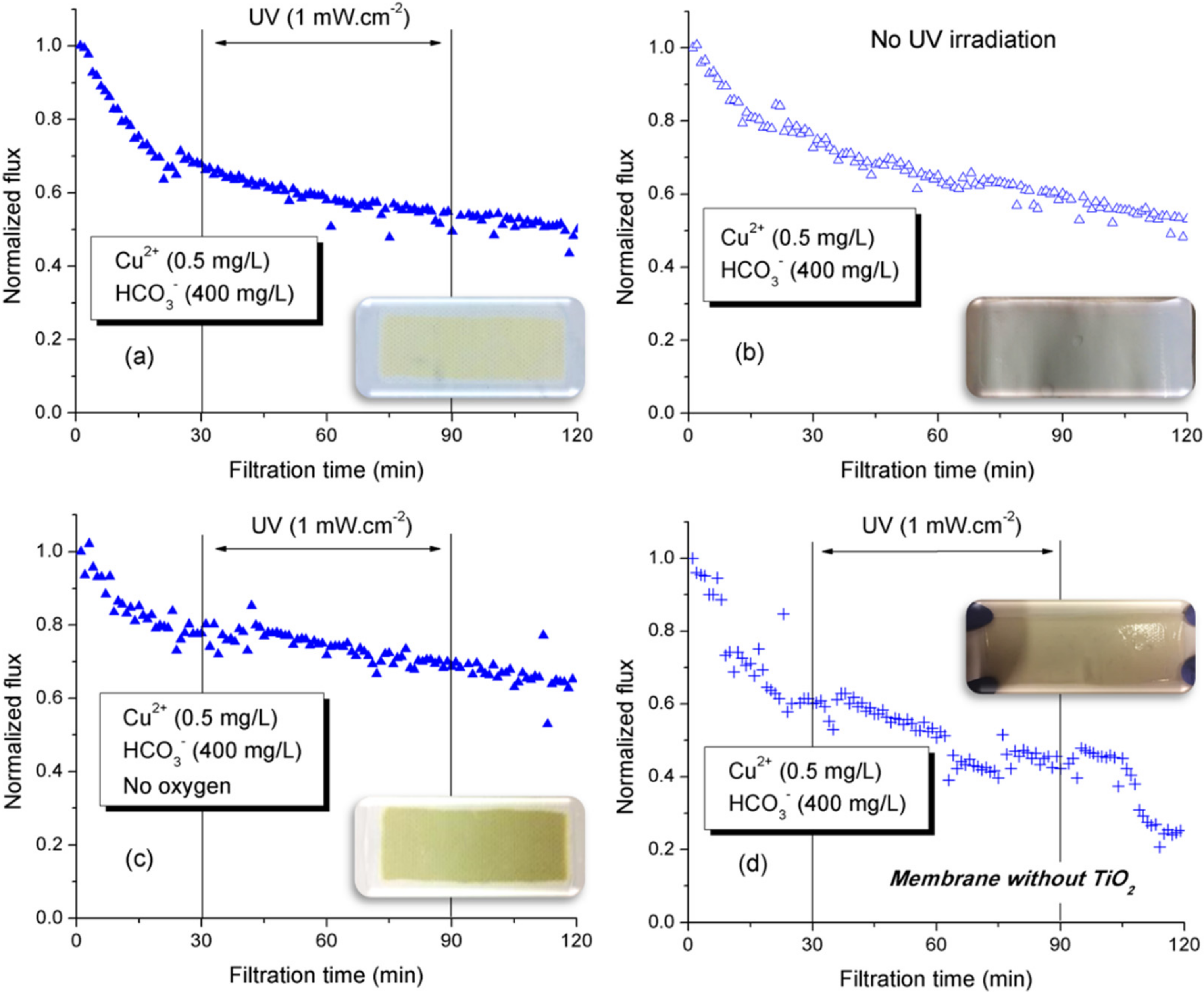
3.1.2. Influence of Metal Cations on Photo-Filtration Efficiency
3.1.3. Influence of Anions on Photo-Filtration Efficiency
3.1.4. Discussion
3.2. Influence of Organic Contents in Natural Water on Photo-Filtration Performance
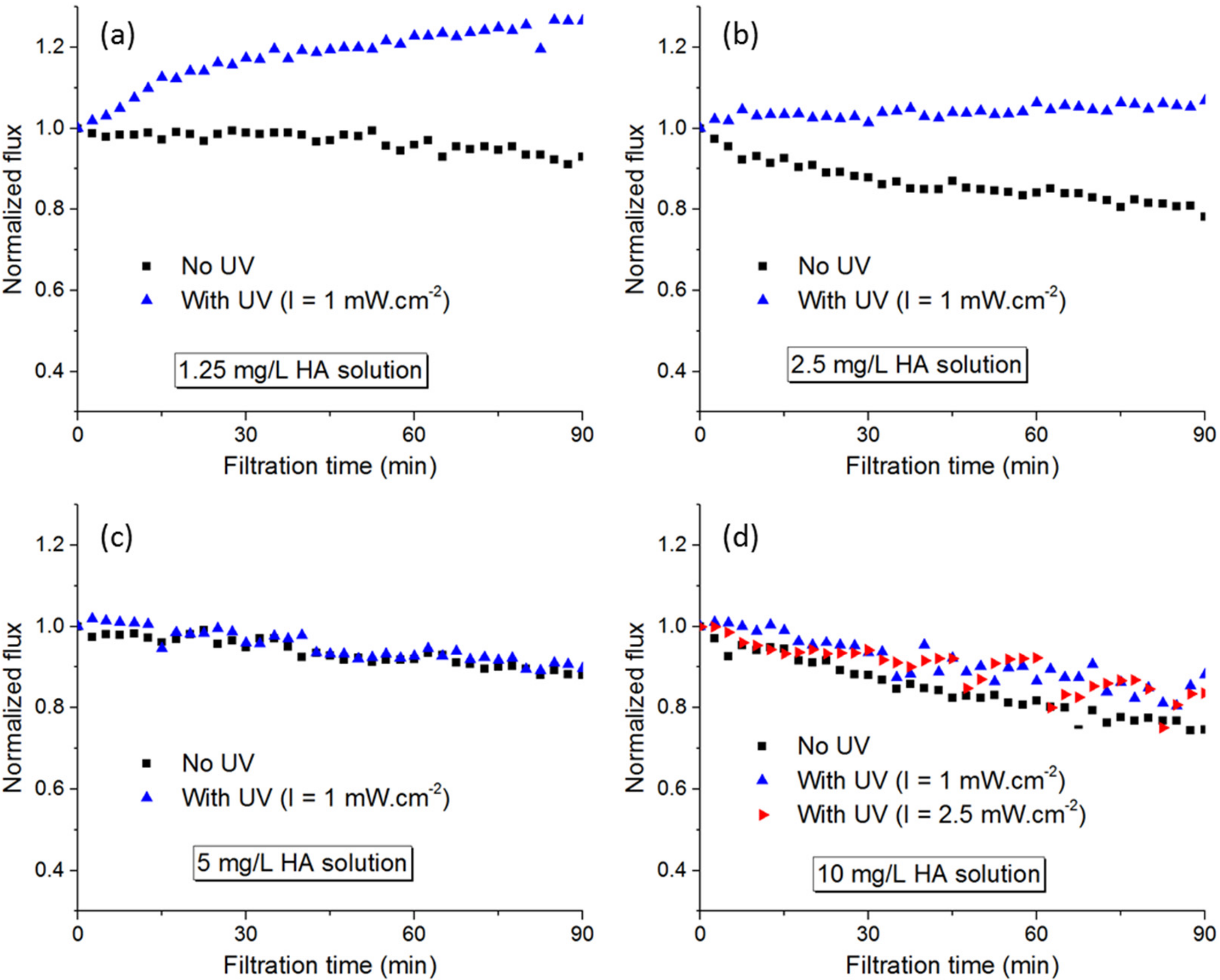
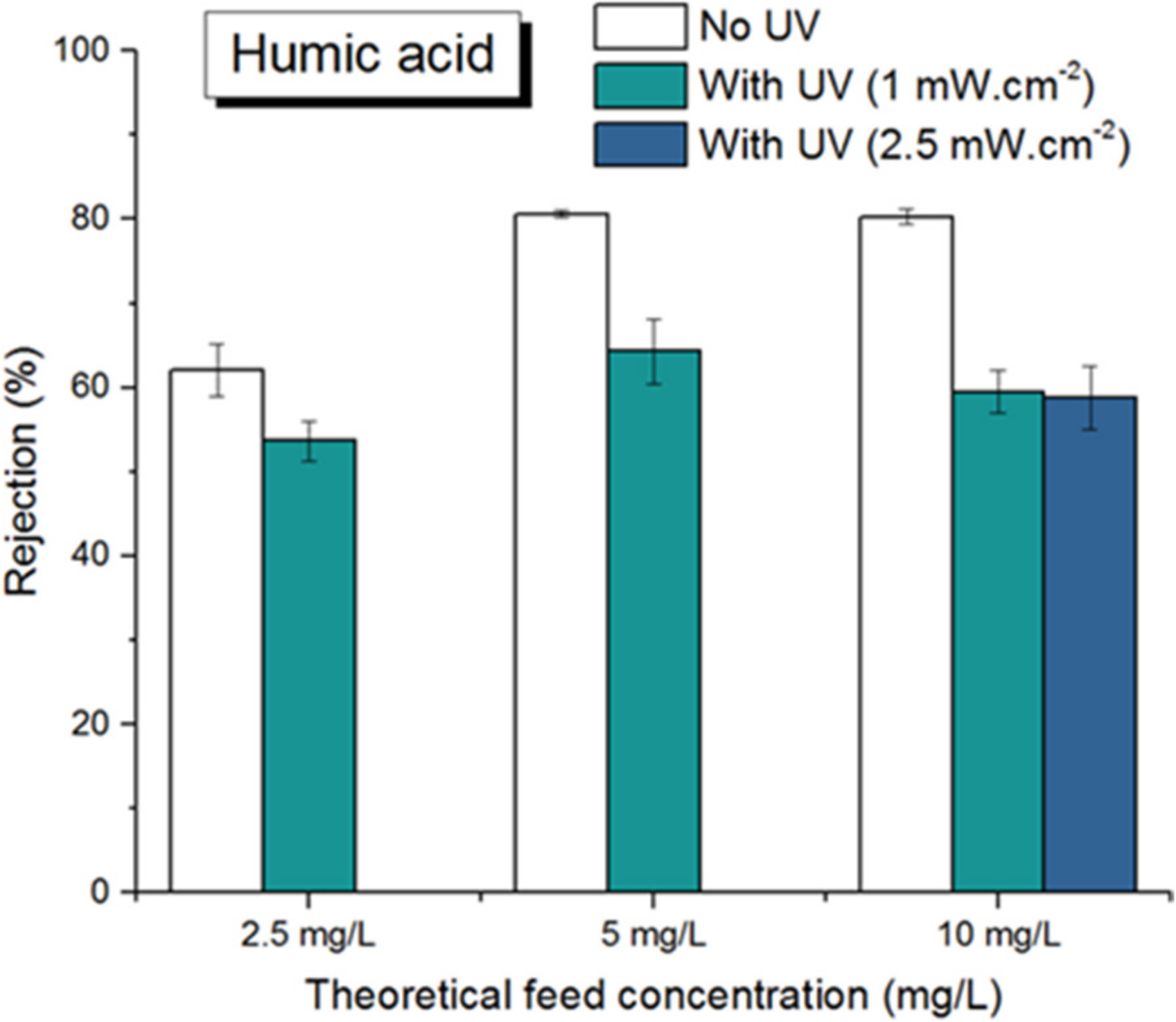
3.2.1. Humic Acids
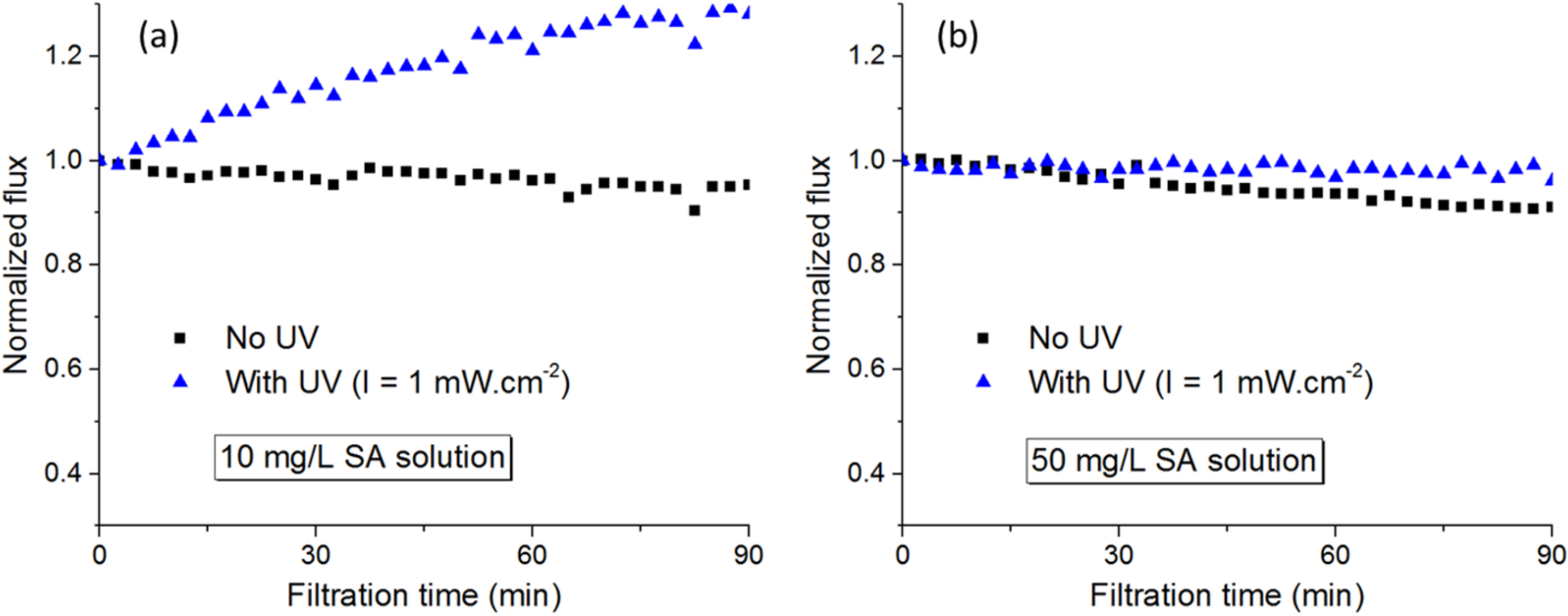
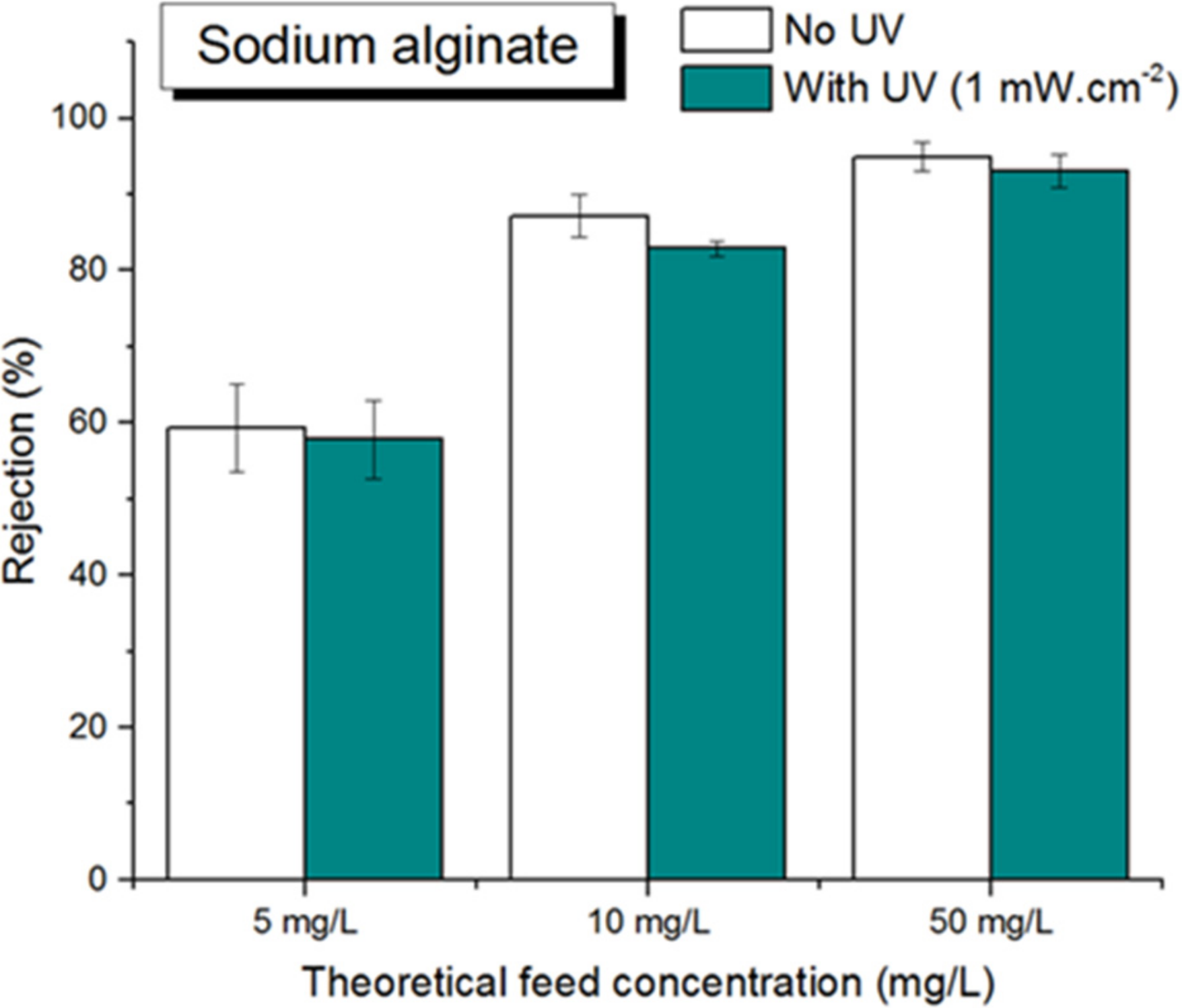
3.2.2. Sodium Alginate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bet-Moushoul, E.; Mansourpanah, Y.; Farhadi, K.; Tabatabaei, M. TiO2 nanocomposite based polymeric membranes: A review on performance improvement for various applications in chemical engineering processes. Chem. Eng. J. 2016, 283, 29–46. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Mendret, J.; Hatat-Fraile, M.; Rivallin, M.; Brosillon, S. Influence of solution pH on the performance of photocatalytic membranes during dead-end filtration. Sep. Purif. Technol. 2013, 118, 406–414. [Google Scholar] [CrossRef]
- Iglesias, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Membrane-based photocatalytic systems for process intensification. Chem. Eng. J. 2016, 305, 136–148. [Google Scholar] [CrossRef]
- Leong, S.; Razmjou, A.; Wang, K.; Hapgood, K.; Zhang, X.; Wang, H. TiO2 based photocatalytic membranes: A review. J. Membr. Sci. 2014, 472, 167–184. [Google Scholar] [CrossRef]
- Li, J.-H.; Xu, Y.-Y.; Zhu, L.-P.; Wang, J.-H.; Du, C.-H. Fabrication and characterization of a novel TiO2 nanoparticle self-assembly membrane with improved fouling resistance. J. Membr. Sci. 2009, 326, 659–666. [Google Scholar] [CrossRef]
- Teow, Y.H.; Ooi, B.S.; Ahmad, A.L. Fouling behaviours of PVDF-TiO2 mixed -matrix membrane applied to humic acid treatment. J. Water Process Eng. 2017, 15, 89–98. [Google Scholar] [CrossRef]
- Damodar, R.A.; You, S.-J.; Chou, H.-H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, antifouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Nor, N.A.M.; Jaafar, J.; Ismail, A.F.; Mohamed, M.A.; Rahman, M.A.; Othman, M.H.D.; Lau, W.J.; Yusof, N. Preparation and performance of PVDF-based nanocomposite membrane consisting of TiO2 nanofibers for organic pollutant decomposition in wastewater under UV irradiation. Desalination 2016, 391, 89–97. [Google Scholar] [CrossRef]
- Bian, X.K.; Sin, L.Q.; Yang, X.X.; Lu, X.F.; Shi, L.; Yang, X.X.; Lu, X.F. Effect of nano-TiO2 particles on the performance of PVDF, PVDF-g-(maleic anhydride), and PVDF- g-poly(acryl amide) membranes. Ind. Eng. Chem. Res. 2011, 50, 12113–12123. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Mollahosseini, A.; Rajaeian, B. Structural and performance properties of UV-assisted TiO2 deposited nano-composite PVDF/SPES membranes. Desalination 2012, 285, 31–38. [Google Scholar] [CrossRef]
- Mericq, J.P.; Mendret, J.; Brosillon, S.; Faur, C. High performance PVDF-TiO2 membranes for water treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Yang, F.; Yang, X. Excellent antimicrobial properties of mesoporous anatase TiO2 and Ag/TiO2 composite films. Microporous Mesoporous Mater. 2008, 114, 431–439. [Google Scholar] [CrossRef]
- Razmjou, A.; Resosudarmo, A.; Holmes, R.L.; Li, H.; Mansouri, J.; Chen, V. The effect of modified TiO2 nanoparticles on the polyethersulfone ultrafiltration hollow fiber membranes. Desalination 2012, 287, 271–280. [Google Scholar] [CrossRef]
- Tran, D.-T.; Mendret, J.; Méricq, J.-P.; Faur, C.; Brosillon, S. Study of permeate flux behavior during photo-filtration using photocatalytic composite membranes. Chem. Eng. Process. Process Intensif. 2020, 148, 107781. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Ma, W.; Zhao, J.; Hidaka, H.; Serpone, N. Effect of Transition Metal Ions on the TiO2-Assisted Photodegradation of Dyes under Visible Irradiation: A Probe for the Interfacial Electron Transfer Process and Reaction Mechanism. J. Phys. Chem. B 2002, 106, 318–324. [Google Scholar] [CrossRef]
- Guillard, C.; Lachheb, H.; Houas, A.; Ksibi, M.; Elaloui, E.; Herrmann, J.-M. Influence of chemical structure of dyes, of pH and of inorganic salts on their photocatalytic degradation by TiO2 comparison of the efficiency of powder and supported TiO2. J. Photochem. Photobiol. A Chem. 2003, 158, 27–36. [Google Scholar] [CrossRef]
- Guillard, C.; Puzenat, E.; Lachheb, H.; Houas, A.; Herrmann, J.M. Why inorganic salts decrease the TiO2 photocatalytic efficiency. Int. J. Photoenergy. 2005, 7, 641208. [Google Scholar] [CrossRef] [Green Version]
- Budarz, J.F.; Turolla, A.; Piasecki, A.F.; Bottero, J.-Y.; Antonelli, M.; Wiesner, M.R. Influence of Aqueous Inorganic Anions on the Reactivity of Nanoparticles in TiO2 Photocatalysis. Langmuir 2017, 33, 2770–2779. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Méricq, J.P.; Mendret, J.; Brosillon, S.; Faur, C. Influence of preparation temperature on the properties and performance of composite pvdf-tio2 membranes. Membranes 2021, 11, 876. [Google Scholar] [CrossRef]
- Rodrigues, A.; Brito, A.; Janknecht, P.; Proença, M.F.; Nogueira, R. Quantification of humic acids in surface water: Effects of divalent cations, pH, and filtration. J. Environ. Monit. 2009, 11, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brink, P.V.D.; Zwijnenburg, A.; Smith, G.; Temmink, H.; van Loosdrecht, M. Effect of free calcium concentration and ionic strength on alginate fouling in cross-flow membrane filtration. J. Membr. Sci. 2009, 345, 207–216. [Google Scholar] [CrossRef]
- Guo, X.; Chen, X.; Hu, W. Studies on cleaning the polyvinylchloride ultrafiltration membrane fouled by sodium alginate. Environ. Technol. 2009, 30, 431–435. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Bekbolet, M. Evaluation of humic acid photocatalytic degradation by UV—vis and fluorescence spectroscopy. Catal. Today 2005, 101, 267–274. [Google Scholar] [CrossRef]
- Dziedzic, J.; Wodka, D.; Nowak, P.; Warszyński, P.; Simon, C.; Kumakiri, I. Photocatalytic degradation of the humic species as a method of their removal from water—comparison of UV and artificial sunlight irradiation. Physicochem. Probl. Miner. Process. 2010, 45, 15–28. [Google Scholar]
- Kaspar, P.; Sobola, D.; Částková, K.; Knápek, A.; Burda, D.; Orudzhev, F.; Dallaev, R.; Tofel, P.; Trčka, T.; Grmela, L.; et al. Characterization of polyvinylidene fluoride (Pvdf) electrospun fibers doped by carbon flakes. Polymers 2020, 12, 2766. [Google Scholar] [CrossRef]
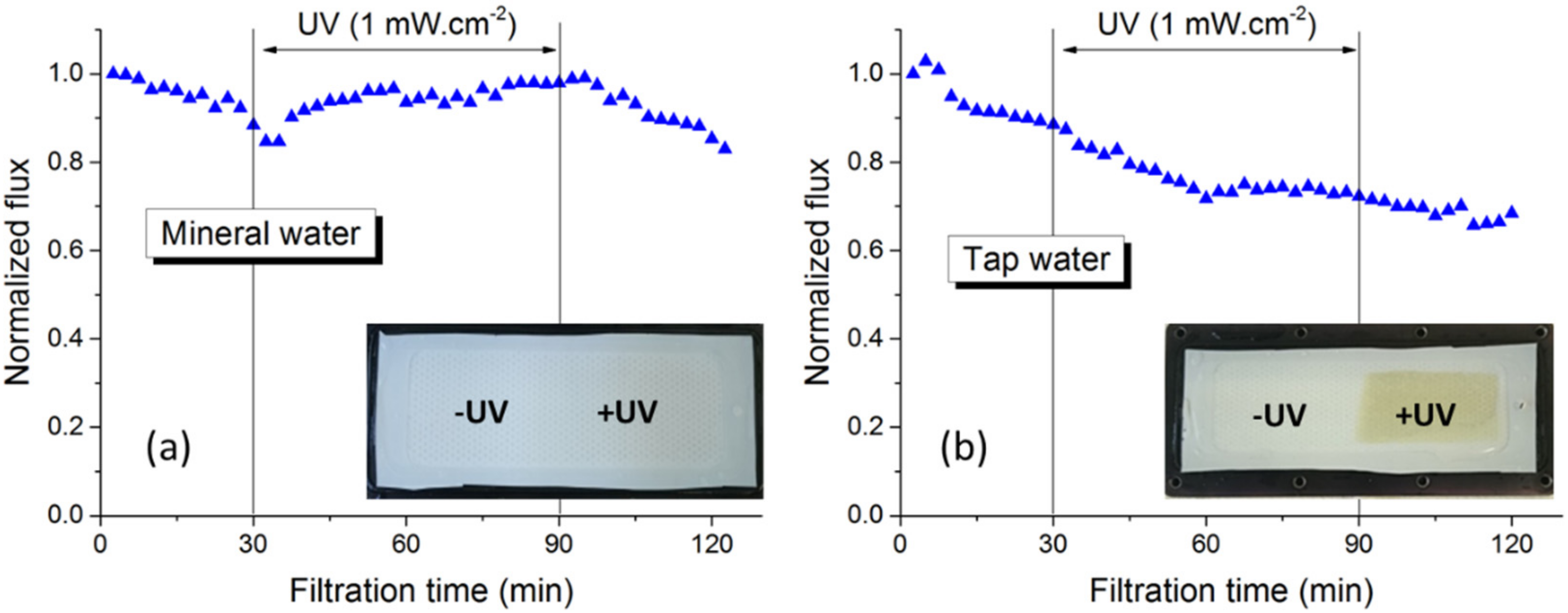



| Species (mg/L) | Pure Water | Tap Water | Mineral Water | Note |
|---|---|---|---|---|
| HCO3− | n/d | 441.1 | 383.7 | Analyzed by ionic chromatography and chemical titration |
| Cl− | n/d | 36.8 | 18.8 | |
| NO3− | n/d | 3.8 | 4.3 | |
| SO42− | n/d | 24.4 | 1530.0 | |
| Na+ | n/d | 21.1 | 14.2 | |
| K+ | n/d | 1.4 | 4.1 | |
| Mg2+ | n/d | 8.6 | 119.0 | |
| Ca2+ | 0.05 | 117.1 | 549.0 | |
| Cu | n/d | 0.47 | n/d | Analyzed by ICP-MS |
| Zn | n/d | 0.16 | n/d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, D.-T.; Mendret, J.; Méricq, J.-P.; Faur, C.; Brosillon, S. Performance of PVDF-TiO2 Membranes during Photo-Filtration in the Presence of Inorganic and Organic Components. Membranes 2022, 12, 245. https://doi.org/10.3390/membranes12020245
Tran D-T, Mendret J, Méricq J-P, Faur C, Brosillon S. Performance of PVDF-TiO2 Membranes during Photo-Filtration in the Presence of Inorganic and Organic Components. Membranes. 2022; 12(2):245. https://doi.org/10.3390/membranes12020245
Chicago/Turabian StyleTran, Duc-Trung, Julie Mendret, Jean-Pierre Méricq, Catherine Faur, and Stephan Brosillon. 2022. "Performance of PVDF-TiO2 Membranes during Photo-Filtration in the Presence of Inorganic and Organic Components" Membranes 12, no. 2: 245. https://doi.org/10.3390/membranes12020245
APA StyleTran, D.-T., Mendret, J., Méricq, J.-P., Faur, C., & Brosillon, S. (2022). Performance of PVDF-TiO2 Membranes during Photo-Filtration in the Presence of Inorganic and Organic Components. Membranes, 12(2), 245. https://doi.org/10.3390/membranes12020245







