Efficient Recovery of Noble Metal Ions (Pd2+, Ag+, Pt2+, and Au3+) from Aqueous Solutions Using N,N'-Bis(salicylidene)ethylenediamine (Salen) as an Extractant (Classic Solvent Extraction) and Carrier (Polymer Membranes)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Mass Spectrometry
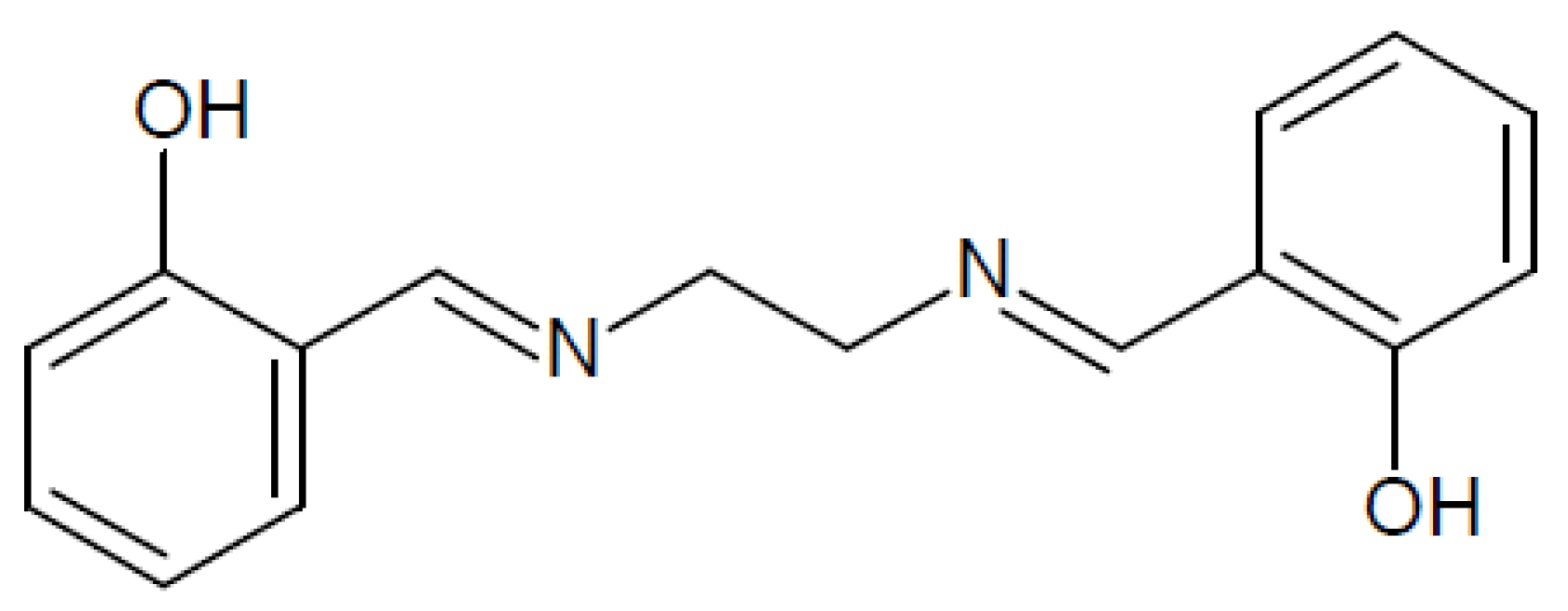
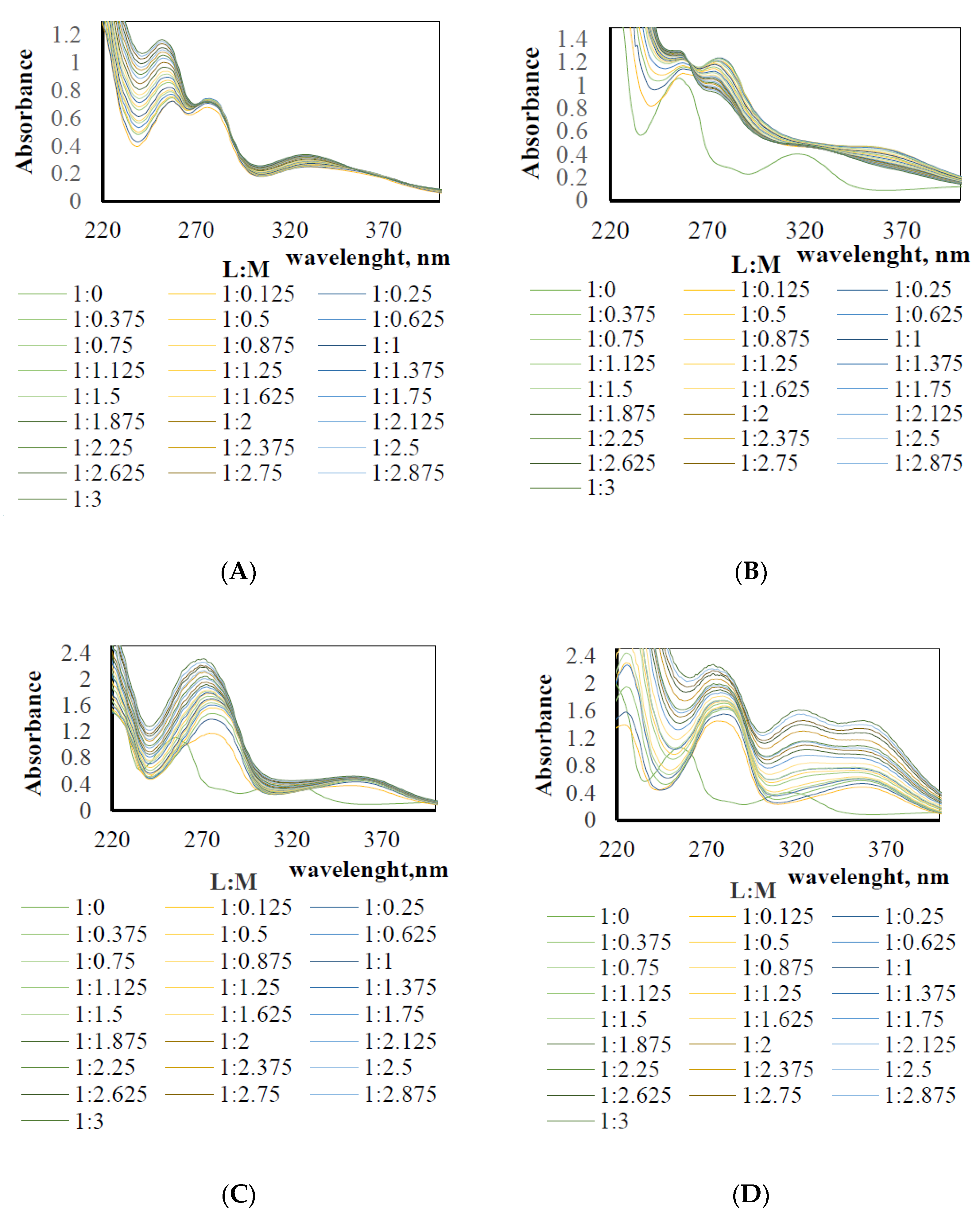
2.3. The Stability Constants
2.4. Solvent Extraction
2.5. The Preparation of Polymer Membranes
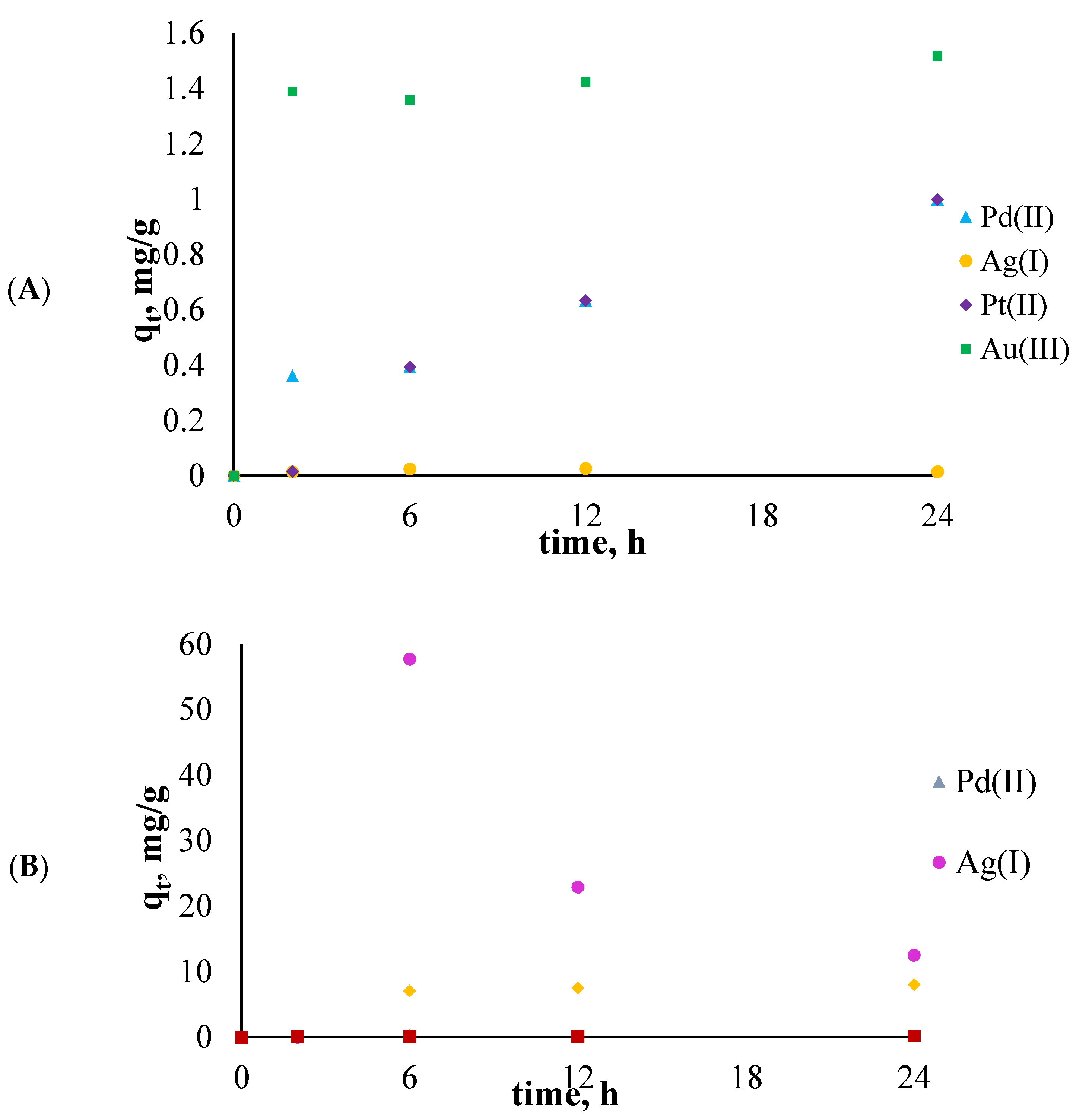
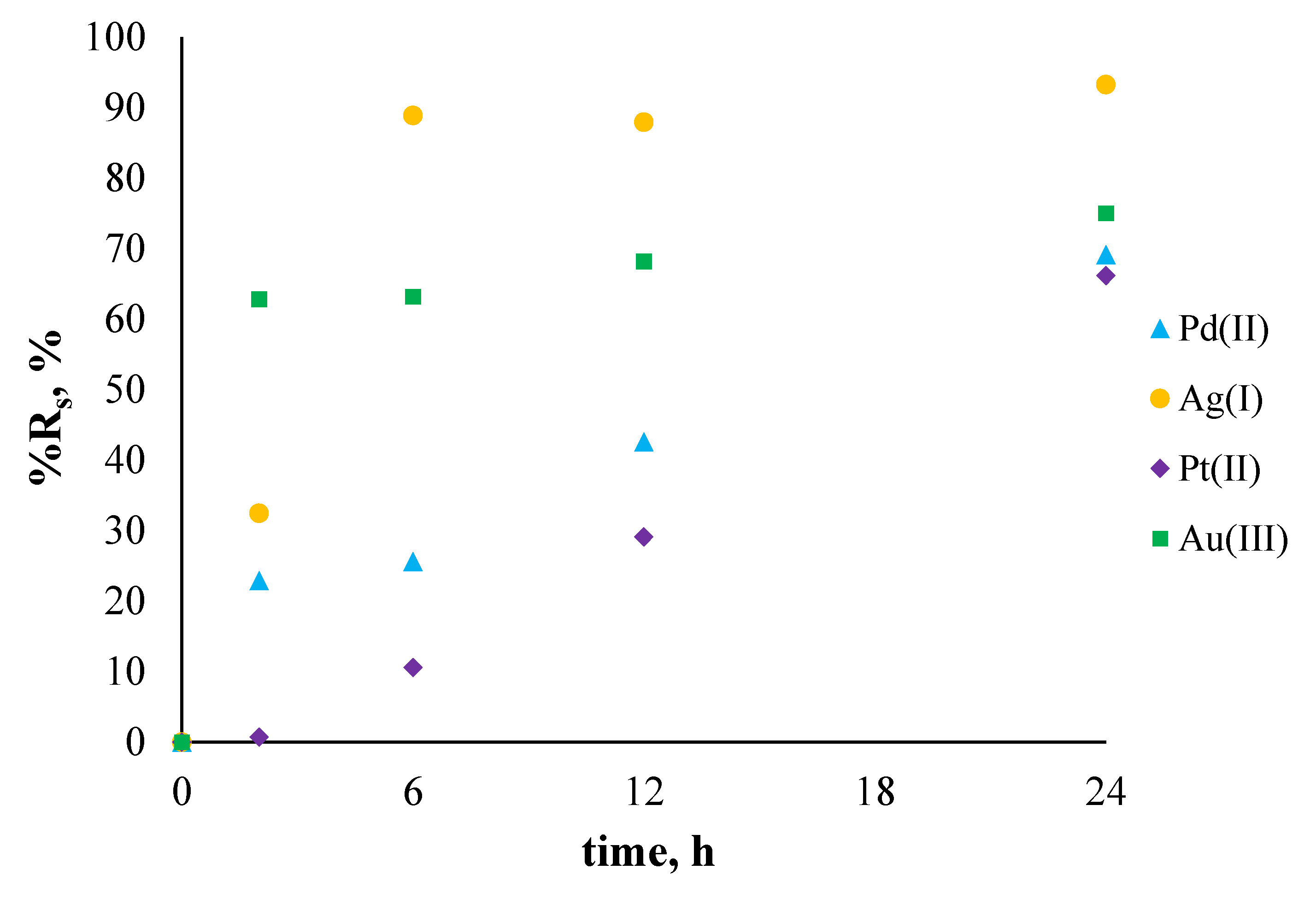
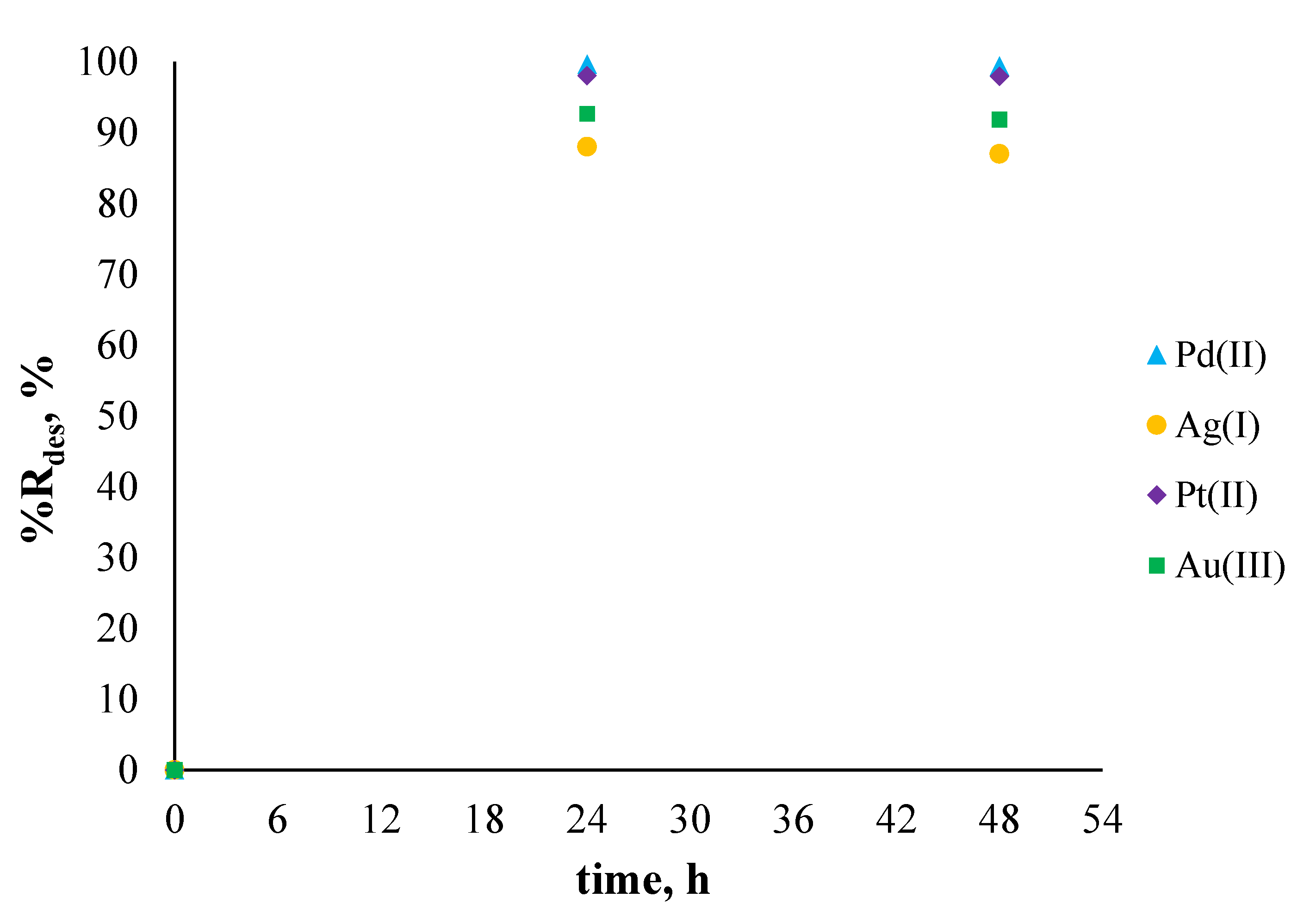
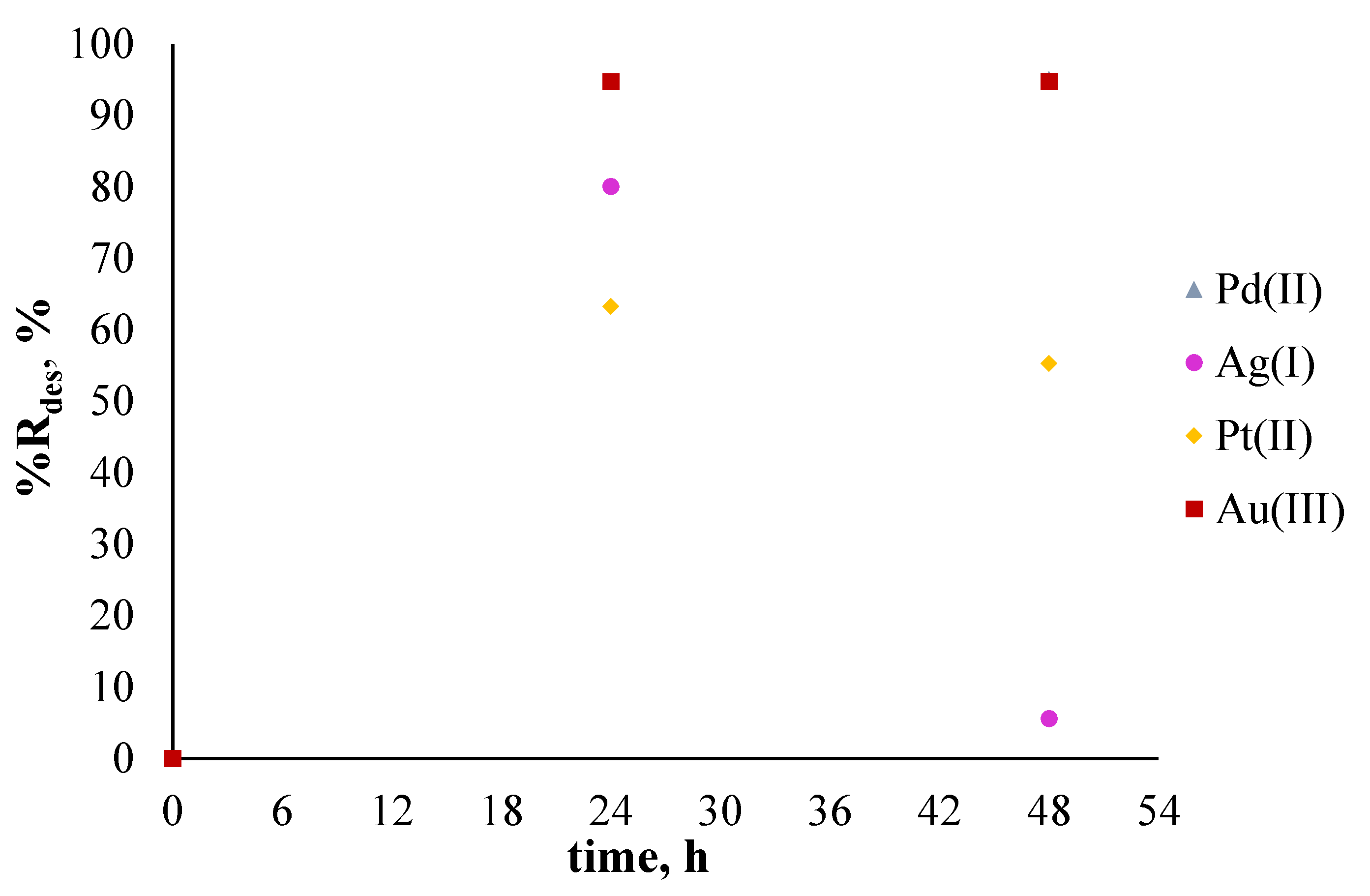
2.6. Sorption and Desorption Experiments
3. Results
3.1. The Stability Constant
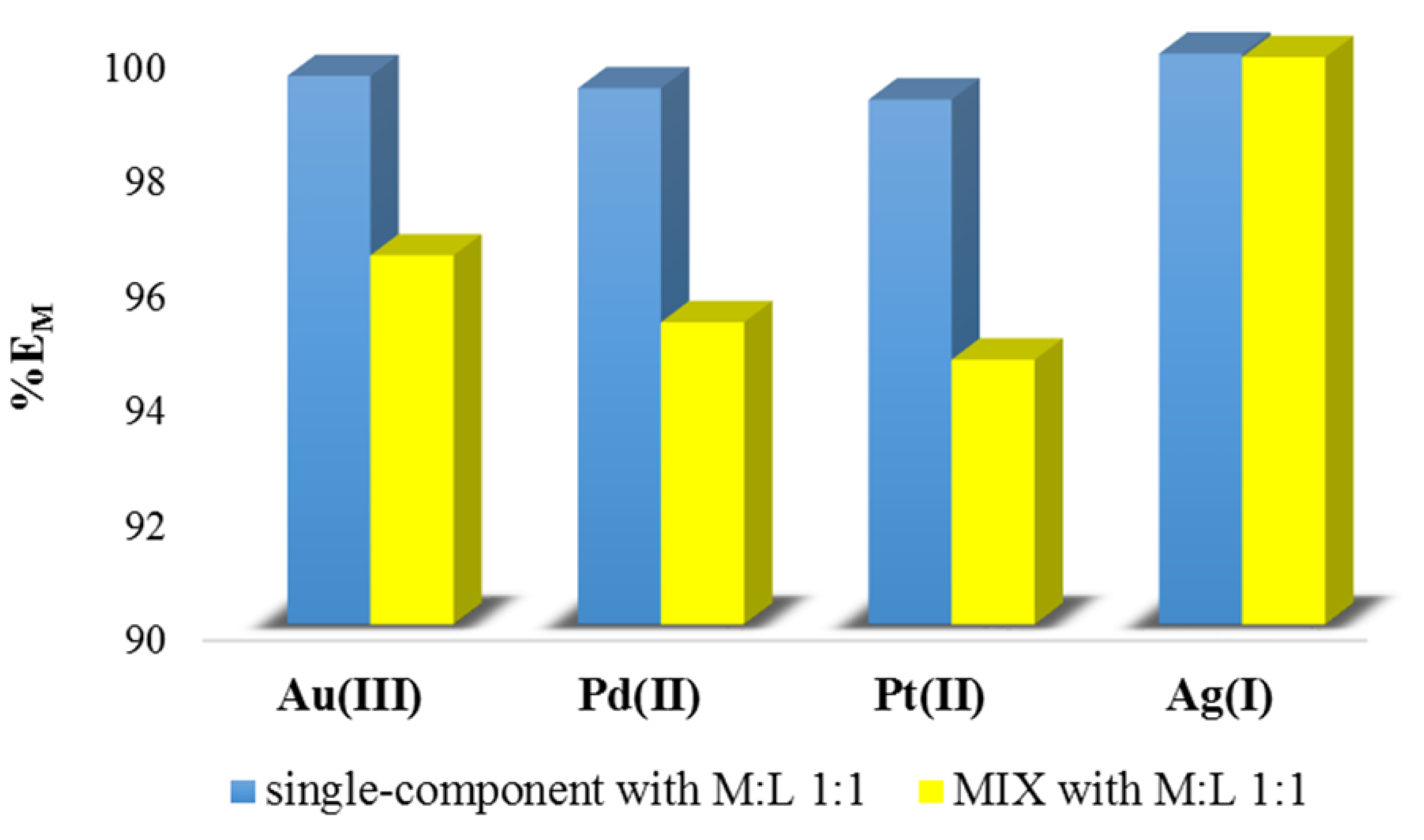
3.2. Solvent Extraction
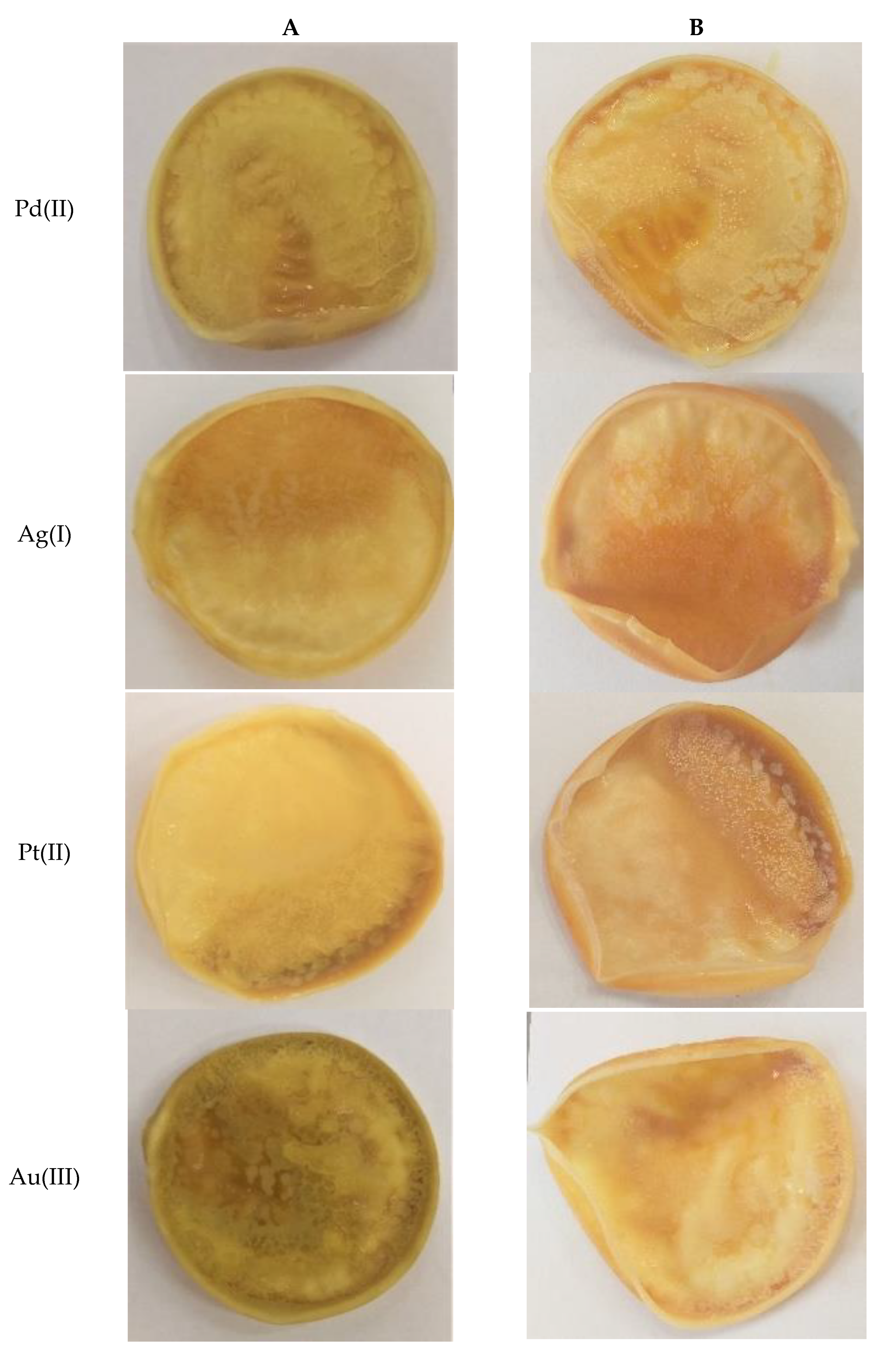
3.3. Sorption and Desorption Experiments
3.4. Mass Spectrometry
4. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Topal, G.; Tümerdem, R.; Basaran, I.; Gümüs, A.; Cakir, U. A Study of Complexation-ability of Neutral Schiff Bases to Some Metal Cations. Int. J. Mol. Sci. 2007, 8, 933–942. [Google Scholar] [CrossRef] [Green Version]
- Bader, N.R. Applications of schiff’s bases chelates in quantitative analysis: A review. Rasayan J. Chem. 2010, 3, 660–670. [Google Scholar]
- Elsherif, K.M.; Zubi, A.; Shawish, H.B.; Abajja, S.A.; Almelah, E.B.M. Complex Formation of Bis(salicylidene)ethyl,enediamine (Salen type ligand) with Cupper(II) Ions in Different Solvents: Spectrophotometric and Conductometric Study. Int. J. New Chem. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Hu, L.; Xie, Q.; Tang, J.; Pan, C.; Yu, G.; Tam, K.C. Co(III)-Salen immobilized cellulose nanocrystals for efficient catalytic CO2 fixation into cyclic carbonates under mild conditions. Carbohydr. Polym. 2021, 256, 117558. [Google Scholar] [CrossRef] [PubMed]
- Coxall, R.A.; Lindoy, L.F.; Miller, H.A.; Parkin, A.; Parsons, S.; Tasker, P.A.; White, D.J. Solvent extraction of metal sulfates by zwitterionic forms of ditopic ligands. Dalton Trans. 2003, 1, 55–64. [Google Scholar] [CrossRef]
- Forgan, R.S.; Davidson, J.E.; Fabbiani, F.P.A.; Galbraith, S.G.; Henderson, D.K.; Moggach, S.A.; Parsons, S.; Tasker, P.A.; White, F.J. Cation and anion selectivity of zwitterionic salicylaldoxime metal salt extractants. Dalton Trans. 2010, 39, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, S.G.; Wang, Q.; Li, L.; Blake, A.J.; Wilson, C.; Collinson, S.R.; Lindoy, L.F.; Plieger, P.G.; Schröder, M.; Tasker, P.A. Anion Selectivity in Zwitterionic Amide-Functionalised Metal Salt Extractants. Chem. A Eur. J. 2007, 13, 6091–6107. [Google Scholar] [CrossRef] [PubMed]
- Dadfarnia, S.; Shabani, A.M.H.; Tamaddon, F.; Rezaei, M. Immobilized salen (N,N′-bis (salicylidene) ethylenediamine) as a complexing agent for on-line sorbent extraction/preconcentration and flow injection–flame atomic absorption spectrometry. Anal. Chim. Acta 2005, 539, 69–75. [Google Scholar] [CrossRef]
- Dadfarnia, S.; Shabani, A.M.; Jafari, A.A.; Saadat, Z.; Tamaddon, F.; Rezaei, M. Silver, zinc and copper determination in water and biological samples employing FI-FAAS and a microcolumn of immobilized 2, 2′-[3-aza-1,5- pentanediylebis(nitrilomethylidyne)]-bisphenol on surfactant coated alumina. Can. J. Anal. Sci. Spectrosc. 2006, 51, 302–311. [Google Scholar]
- Kim, Y.S.; In, G.; Choi, J.M. Solid Phase Extraction of Trace Cu(II), Mn(II), Pb(II) and Zn(II) in Water Samples with Pulverized Silica-salen(NEt2)2. Bull. Korean Chem. Soc. 2006, 27, 1557–1561. [Google Scholar]
- Kim, Y.-S.; In, G.; Han, C.-W.; Choi, J.-M. Studies on synthesis and application of XAD-4-salen chelate resin for separation and determination of trace elements by solid phase extraction. Microchem. J. 2005, 80, 151–157. [Google Scholar] [CrossRef]
- Attol, D.H.; Mihsen, H.H. Synthesis of Silica-Salen Derivative from Rice Husk Ash and its Use for Extraction of Divalent Metal Ions Co(II), Ni(II) and Cu(II), Indones. J. Chem. 2020, 20, 16–28. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Y.; Li, D.-S.; Bu, X.; Feng, P. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, e1705189. [Google Scholar] [CrossRef]
- Guo, X.-G.; Qiu, S.; Chen, X.; Gong, Y.; Sun, X. Postsynthesis Modification of a Metallosalen-Containing Metal–Organic Framework for Selective Th(IV)/Ln(III) Separation. Inorg. Chem. 2017, 56, 12357–12361. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Ochoa, G.; Campo-Cobo, L.F.; Gutiérrez-Valencia, T.M. Simultaneous extraction and reduction of gold using sodium tetraphenylborate in polymeric inclusion membranes. Sep. Purif. Technol. 2021, 276, 119334. [Google Scholar] [CrossRef]
- Campo-Cobo, L.F.; Pérez-Urbano, M.L.; Gutiérrez-Valencia, T.M.; Hoyos-Saavedra, O.L.; Cuervo-Ochoa, G. Selective Extraction of Gold with Polymeric Inclusion Membranes Based on Salen Ligands with Electron- Accepting Substituents. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2654–2664. [Google Scholar] [CrossRef]
- Witt, K.; Bożejewicz, D.; Kaczorowska, M.A. N,N'-Bis(salicylidene)ethylenediamine (Salen) as an Active Compound for the Recovery of Ni(II), Cu(II), and Zn(II) Ions from Aqueous Solutions. Membranes 2020, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- O’Bryan, Y.; Truong, Y.B.; Cattrall, R.W.; Kyratzis, I.L.; Kolev, S.D. A new generation of highly stable and permeable polymer inclusion membranes (PIMs) with their carrier immobilized in a crosslinked semi-interpenetrating polymer network. Application to the transport of thiocyanate. J. Membr. Sci. 2017, 529, 55–62. [Google Scholar] [CrossRef]
- Carner, C.A.; Croft, C.F.; Kolev, S.D.; Almeida, M.I.G.S. Green solvents for the fabrication of polymer inclusion membranes (PIMs). Sep. Purif. Technol. 2020, 239, 116486. [Google Scholar] [CrossRef]
- Chen, L.; Wu, Y.; Dong, H.; Meng, M.; Li, C.; Yan, Y.; Chen, J. An overview on membrane strategies for rare earths extraction and separation. Sep. Purif. Technol. 2018, 197, 70–85. [Google Scholar] [CrossRef]
- Ismail, H.; Hanafiah, M.M. A review of sustainable e-waste generation and management: Present and future perspectives. J. Environ. Manag. 2020, 264. [Google Scholar] [CrossRef]
- Benfettouma, Z.; Youcef, M.H.; Benabdallah, T.; Reffas, H. Extraction study on recovery of nickel (II) from concentrated chloride medium with N,N′-bis(salicylaldeyde)ethylenediimine in the presence of CTAB and Brij® 35 surfactants. J. Dispers. Sci. Technol. 2020, 42, 1912–1925. [Google Scholar] [CrossRef]
- Ipeaiyeda, A.R.; Tesi, G.O. Sorption and desorption studies on toxic metals from brewery effluent using eggshell as adsorbent. Adv. Nat. Sci. 2014, 7, 15–24. [Google Scholar] [CrossRef]
- Ishtiaq, F.; Bhatti, H.N.; Khan, A.; Iqbal, M.; Kausar, A. Polypyrole, polyaniline and sodium alginate biocomposites and adsorption-desorption efficiency for imidacloprid insecticide. Int. J. Biol. Macromol. 2020, 147, 217–232. [Google Scholar] [CrossRef]
- Xian, F.; Hendrickson, C.L.; Marshall, A.G. High Resolution Mass Spectrometry. Anal. Chem. 2012, 84, 708–719. [Google Scholar] [CrossRef]
- Konermann, L.; Ahadi, E.; Rodriguez, A.D.; Vahidi, S. Unraveling the Mechanism of Electrospray Ionization. Anal. Chem. 2013, 85, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Bożejewicz, D.; Witt, K.; Kaczorowska, M.A.; Urbaniak, W.; Ośmiałowski, B. The Application of 2,6-Bis(4-Methoxybenzoyl)-Diaminopyridine in Solvent Extraction and Polymer Membrane Separation for the Recovery of Au(III), Ag(I), Pd(II) and Pt(II) Ions from Aqueous Solutions. Int. J. Mol. Sci. 2021, 22, 9123. [Google Scholar] [CrossRef]
- Radzyminska-Lenarcik, E.; Pyszka, I.; Kosciuszko, A. Separation and Recovery of Gold(III), Palladium(II) and Platinum(IV) by Solvent Extraction Using a New β-Diketone Derivative from Acidic Solutions. Materials 2021, 14, 4436. [Google Scholar] [CrossRef]
- Sathuluri, R.R.; Kurniawan, Y.S.; Kim, I.Y.; Maeki, M.; Iwasaki, W.; Morisada, S.; Kawakita, H.; Miyazaki, M.; Ohto, K. Droplet-based microreactor system for stepwise recovery of precious metal ions from real metal waste with calix[4]arene derivatives. Sep. Sci. Technol. 2018, 53, 1261–1272. [Google Scholar] [CrossRef]
- Yamada, M.; Gandhi, M.R.; Shibayama, A. Rapid and selective recovery of palladium from platinum group metals and base metals using a thioamide-modified calix[4]arene extractant in environmentally friendly hydrocarbon fluids. Sci. Rep. 2018, 8, 16909. [Google Scholar] [CrossRef]
- Akin, I.; Erdemir, S.; Yilmaz, M.; Ersoz, M. Calix[4]arene derivative bearing imidazole groups as carrier for the transport of palladium by using bulk liquid membrane. J. Hazard. Mater. 2012, 223–224, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Nowik-Zajac, A.; Zawierucha, I.; Kozlowski, C. Selective Transport of Ag(I) through a Polymer Inclusion Membrane Containing a Calix[4]pyrrole Derivative from Nitrate Aqueous Solutions. Int. J. Mol. Sci. 2020, 21, 5348. [Google Scholar] [CrossRef]
- Nowik-Zajac, A.; Zawierucha, I.; Kozlowski, C. Selective removal of silver(i) using polymer inclusion membranes containing calixpyrroles. RSC Adv. 2019, 9, 31122–31132. [Google Scholar] [CrossRef] [Green Version]
- Kubota, F.; Kono, R.; Yoshida, W.; Sharaf, M.; Kolev, S.D.; Goto, M. Recovery of gold ions from discarded mobile phone leachate by solvent extraction and polymer inclusion membrane (PIM) based separation using an amic acid extractant. Sep. Purif. Technol. 2019, 214, 156–161. [Google Scholar] [CrossRef]
- Yoshida, W.; Baba, Y.; Kubota, F.; Kamiya, N.; Goto, M. Extraction and Stripping Behavior of Platinum Group Metals Using an Amic-Acid-Type Extractant. J. Chem. Eng. Jpn. 2017, 50, 521–526. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Tsai, T.-H. Solvent extraction of palladium(II) from acidic chloride solutions using tri-octyl/decyl ammonium chloride (Aliquat 336). Desalin. Water Treat. 2014, 52, 1101–1121. [Google Scholar] [CrossRef]
- Wei, W.; Cho, C.-W.; Kim, S.; Song, M.-H.; Bediako, J.K.; Yun, Y.-S. Selective recovery of Au(III), Pt(IV), and Pd(II) from aqueous solutions by liquid–liquid extraction using ionic liquid Aliquat-336. J. Mol. Liq. 2016, 216, 18–24. [Google Scholar] [CrossRef]
- Kumar, J.R.; Choi, I.-H.; Lee, J.-Y. Precious metals extraction processing in chloride media by using liquids as novel extractant system. Korean Chem. Eng. Res. 2017, 55, 503–509. [Google Scholar] [CrossRef]
- Vereycken, W.; van Stee, J.; Riaño, S.; van Gerven, T.; Binnemans, K. Non-equilibrium solvent extraction in milliflow reactors: Precious and base metal separations with undiluted ionic liquids. Sep. Purif. Technol. 2021, 265, 118490. [Google Scholar] [CrossRef]
- Herce-Sesa, B.; López-López, J.A.; Moreno, C. Multi-elemental ionic liquid-based solvent bar micro-extraction of priority and emerging trace metallic pollutants (Cd, Ag, Pd) in natural waters. J. Hazard. Mater. 2019, 370, 63–69. [Google Scholar] [CrossRef]
- He, K.; Tang, J.; Weng, H.; Chen, G.; Wu, Z.; Lin, M. Efficient extraction of precious metal ions by a membrane emulsification circulation extractor. Sep. Purif. Technol. 2019, 213, 93–100. [Google Scholar] [CrossRef]
- Mahandra, H.; Faraji, F.; Ghahreman, A. Novel Extraction Process for Gold Recovery from Thiosulfate Solution Using Phosphonium Ionic Liquids. ACS Sustain. Chem. Eng. 2021, 9, 8179–8185. [Google Scholar] [CrossRef]
- Regel-Rosocka, M.; Rzelewska, M.; Baczynska, M.; Janus, M.; Wisniewski, M. Removal of palladium(II) from aqueous chloride solution with cyphos phosphonium ionic liquids as metal ion carriers for liquid-liquid extraction and transport across polymer inclusion membrane. Physicochem. Probl. Miner. Process. 2015, 51, 621–631. [Google Scholar] [CrossRef]
- Fajar, A.T.N.; Kubota, F.; Firmansyah, M.L.; Goto, M. Separation of Palladium(II) and Rhodium(III) Using a Polymer Inclusion Membrane Containing a Phosphonium-Based Ionic Liquid Carrier. Ind. Eng. Chem. Res. 2019, 58, 22334–22342. [Google Scholar] [CrossRef]
- Boudesocque, S.; Mohamadou, A.; Conreux, A.; Marin, B.; Dupont, L. The recovery and selective extraction of gold and platinum by novel ionic liquids. Sep. Purif. Technol. 2019, 210, 824–834. [Google Scholar] [CrossRef]
- Vojoudi, H.; Badiei, A.; Banaei, A.; Bahar, S.; Karimi, S.; Ziarani, G.M.; Ganjali, M.R. Extraction of gold, palladium and silver ions using organically modified silica-coated magnetic nanoparticles and silica gel as a sorbent. Microchim. Acta 2017, 184, 3859–3866. [Google Scholar] [CrossRef]
- Tahmasebi, E.; Yamini, Y. Polythiophene-coated Fe3O4 nanoparticles as a selective adsorbent for magnetic solid-phase extraction of silver(I), gold(III), copper(II) and palladium(II). Microchim. Acta 2014, 181, 543–551. [Google Scholar] [CrossRef]
- Wei, W.; Desireddy, H.K.R.; Bediako, J.K.; Yun, Y.-S. Aliquat-336-impregnated alginate capsule as a green sorbent for selective recovery of gold from metal mixtures. Chem. Eng. J. 2016, 289, 413–422. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, M.; Wang, S.; Dai, L.; Zhang, L.; Wang, C. Selective recovery of gold ions in aqueous solutions by a novel trithiocyanuric-Zr based MOFs adsorbent. J. Mol. Liq. 2020, 298, 112090. [Google Scholar] [CrossRef]




| Type of Solution | Metal Ions | Sample | M:L | pH | CM Metal Ion in the Aqueous Phase, [mol/L] | CM Ligand in the Organic Phase, [mol/L] |
|---|---|---|---|---|---|---|
| single component solution | Pd2+ | 1 | 1:1 | 8.997 | 0.009 | 0.009 |
| 2 | 1:5 | 9.015 | 0.002 | 0.010 | ||
| 3 | 1:10 | 9.111 | 0.001 | 0.010 | ||
| Ag+ | 4 | 1:1 | 10.247 | 0.009 | 0.009 | |
| 5 | 1:5 | 10.354 | 0.002 | 0.010 | ||
| 6 | 1:10 | 10.119 | 0.001 | 0.010 | ||
| Pt2+ | 7 | 1:1 | 8.997 | 0.005 | 0.005 | |
| 8 | 1:5 | 9.214 | 0.002 | 0.010 | ||
| 9 | 1:10 | 9.059 | 0.001 | 0.010 | ||
| Au3+ | 10 | 1:1 | 9.132 | 0.005 | 0.005 | |
| 11 | 1:5 | 9.325 | 0.002 | 0.010 | ||
| 12 | 1:10 | 9.456 | 0.001 | 0.010 | ||
| polymetallic solution | Pd2+ | MIX | 1:1 (for the sum of all precious metal ions) 1:4 (for single metal ion) | 9.285 | 0.00062 | 0.0025 |
| Ag+ | ||||||
| Pt2+ | ||||||
| Au3+ | ||||||
| Pd2+ | 1:4 (for the sum of all precious metal ions) 1:16 (for single metal ion) | 9.197 | 0.00062 | 0.01 | ||
| Ag+ | ||||||
| Pt2+ | ||||||
| Au3+ |
| L:M Complex Type | Stability Constants | M | |||
|---|---|---|---|---|---|
| Pd2+ | Ag+ | Pt2+ | Au3+ | ||
| log K | |||||
| 1:1 | log K1 | 5.54 | 2.30 | 5.48 | 0.52 |
| 1:2 | log K2 | 4.60 | 4.40 | 4.60 | 4.63 |
| 1:3 | log K3 | 4.00 | 4.30 | 4.30 | 4.00 |
| Type of Solution | Metal Ions | M:L | The Extraction Percentage, %EM [%] |
|---|---|---|---|
| single component solution | Pd2+ | 1:1 | 99.35 |
| 1:5 | 97.39 | ||
| 1:10 | 92.98 | ||
| Ag+ | 1:1 | 99.95 | |
| 1:5 | 99.86 | ||
| 1:10 | 99.83 | ||
| Pt2+ | 1:1 | 99.15 | |
| 1:5 | 97.80 | ||
| 1:10 | 94.98 | ||
| Au3+ | 1:1 | 99.56 | |
| 1:5 | 97.77 | ||
| 1:10 | 94.89 | ||
| polymetallic solution (MIX) | Pd2+ | 1:1 (for the sum of all precious metal ions) 1:4 (for single metal ion) | 96.43 |
| Ag+ | 95.27 | ||
| Pt2+ | 94.62 | ||
| Au3+ | 99.89 | ||
| Pd2+ | 1:4 (for the sum of all precious metal ions) 1:16 (for single metal ion) | 96.50 | |
| Ag+ | 96.46 | ||
| Pt2+ | 94.86 | ||
| Au3+ | 99.90 |
| Type of Solution | Metal Ions | The Percentage of Sorption, %Rs [%] | The Percentage of Desorption, %Rdes [%] |
|---|---|---|---|
| single component solution | Pd2+ | 69.11 | 99.68 |
| Ag+ | 93.23 | 88.04 | |
| Pt2+ | 66.13 | 98.07 | |
| Au3+ | 74.99 | 92.72 | |
| polymetallic solution (MIX) | Pd2+ | 92.96 | 94.81 |
| Ag+ | 80.94 | 80.06 | |
| Pt2+ | 48.36 | 63.25 | |
| Au3+ | 84.26 | 94.72 |
| Au(NO3)3 and L (C16H16N2O2) | |||
|---|---|---|---|
| m/zmeas | m/zcalc | Assignment | Mass Error [ppm] |
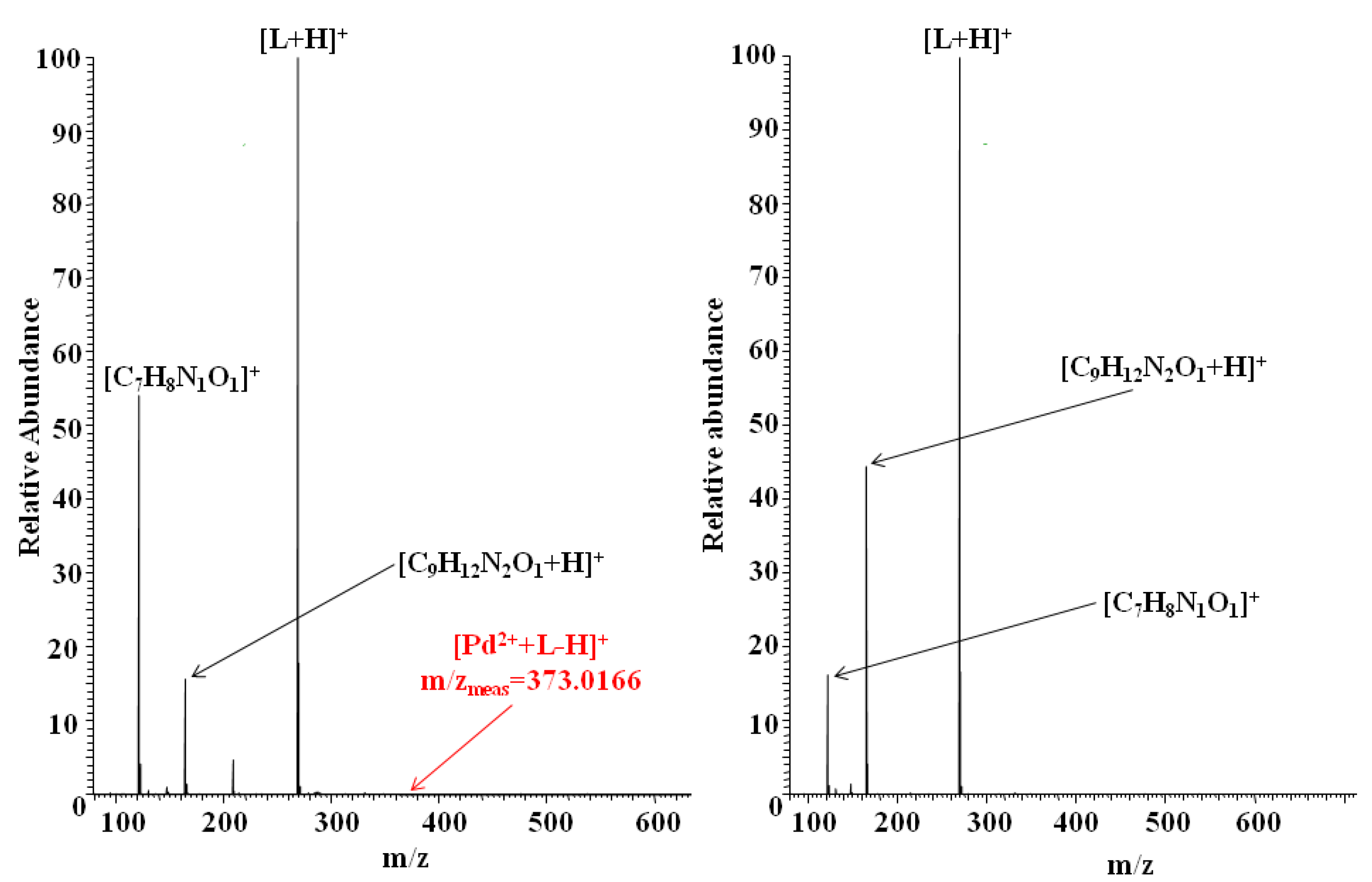
| 122.0603 | 122.0606 | [C7H8N1O1]+ | 2.46 |
| 165.1023 | 165.1028 | [C9H12N2O1 + H]+ | 3.03 |
| 269.1283 | 269.1290 | [L + H]+, (C16H17N2O2)+ | 2.60 |
| 463.0715 | 463.0720 | [Au3+ + L-2H]+, (AuC16H14N2O2)+ | 1.08 |
| Pd(NO3)2 and L(C16H16N2O2) | |||
| m/zmeas | m/zcalc | Assignment | Mass Error [ppm] |
| 122.0604 | 122.0606 | [C7H8N1O1]+ | 1.64 |
| 165.1025 | 165.1028 | [C9H12N2O1 + H]+ | 1.82 |
| 269.1287 | 269.1290 | [L + H]+, (C16H17N2O2)+ | 1.11 |
| 373.0166 | 373.0168 | [Pd2+ + L-H]+, (PdC16H15N2O2)+ | 0.53 |
| Pt(NO3)2 and L(C16H16N2O2) | |||
| m/zmeas | m/zcalc | Assignment | Mass Error [ppm] |
| 122.0604 | 122.0606 | [C7H8N1O1]+ | 1.64 |
| 165.1025 | 165.1028 | [C9H12N2O1 + H]+ | 1.82 |
| 269.1288 | 269.1290 | [L + H]+, (C16H17N2O2)+ | 0.74 |
| N,N’-bis(salicylidene)ethylenediamine) | |||||
|---|---|---|---|---|---|
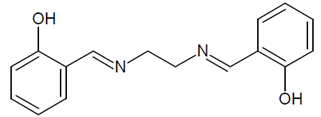 | |||||
| [%] | Pd(II) | Ag(I) | Pt(II) | Au(III) | Ref. |
| %EM | 92.98–99.35% | 99.83–99.95% | 94.98–99.15% | 94.89–99.56% | [This work] |
| %Rs | 69.11% | 93.23% | 66.12% | 74.99% | [This work] |
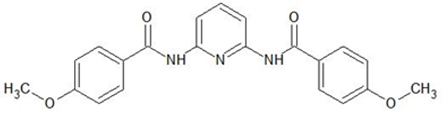
| 2,6-bis(4-methoxybenzoyl)-diaminopyridine | |||||
 | |||||
| [%] | Pd(II) | Ag(I) | Pt(II) | Au(III) | Ref. |
| %EM | ~99% | ~99% | ~99% | ~99% | [27] |
| %Rs | 23.82% | 94.89% | 38.99% | 63.46% | [27] |
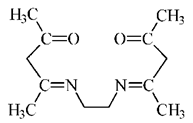
| Ethylenodiamino-bis-acetylacetone | |||||
 | |||||
| [%] | Pd(II) | Ag(I) | Pt(II) | Au(III) | Ref. |
| %EM | 87–93% | - | - | 56–65% | [28] |
| %Rs | - | - | - | - | - |
| Calix[4]arene derivatives | |||||
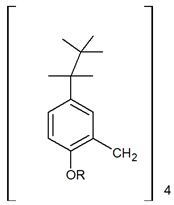 | |||||
| [%] | Pd(II) | Ag(I) | Pt(II) | Au(III) | Ref. |
| %EM | 70.9% | 13.3% | 64.4% | - | [29] |
| 99% | - | - | - | [30] | |
| 95% | - | - | - | [31] | |
| %Rs | - | - | - | - | - |
| Calix[4]pyrrole derivatives | |||||
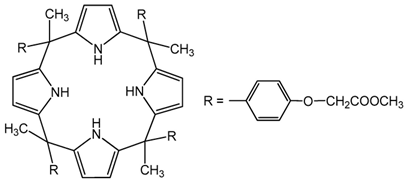 | |||||
| [%] | Pd(II) | Ag(I) | Pt(II) | Au(III) | Ref. |
| %EM | - | - | - | - | - |
| %Rs | - | 92.77% | - | - | [32] |
| - | 80.1%, | - | - | [33] | |
| D2EHAG | |||||
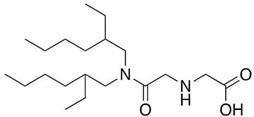 | |||||
| [%] | Pd(II) | Ag(I) | Pt(II) | Au(III) | Ref. |
| %EM | - | - | - | 69% | [34] |
| 98% | - | - | - | [35] | |
| %Rs | - | - | - | 96% | [34] |
| Aliquat 336 | |||||
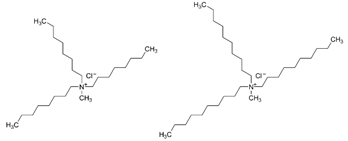 | |||||
| [%] | Pd(II) | Ag(I) | Pt(II) | Au(III) | Ref. |
| %EM | 99% | - | - | - | [36] |
| 99% | - | - | 99% | [37] | |
| - | - | ~100% | [38] | ||
| %Rs | 80% | - | - | - | [39] |
| Cyphos IL 101 | |||||
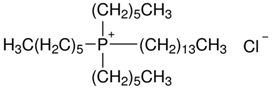 | |||||
| [%] | Pd(II) | Ag(I) | Pt(II) | Au(III) | Ref. |
| %EM | - | - | ~100% | - | [38] |
| >90% | 93–95% | - | - | [40] | |
| 99.9% | - | - | 99.9% | [41] | |
| - | - | - | 98.4% | [42] | |
| 84–90% | - | - | - | [43] | |
| %Rs | ~45% | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witt, K.; Kaczorowska, M.A.; Bożejewicz, D.; Urbaniak, W. Efficient Recovery of Noble Metal Ions (Pd2+, Ag+, Pt2+, and Au3+) from Aqueous Solutions Using N,N'-Bis(salicylidene)ethylenediamine (Salen) as an Extractant (Classic Solvent Extraction) and Carrier (Polymer Membranes). Membranes 2021, 11, 863. https://doi.org/10.3390/membranes11110863
Witt K, Kaczorowska MA, Bożejewicz D, Urbaniak W. Efficient Recovery of Noble Metal Ions (Pd2+, Ag+, Pt2+, and Au3+) from Aqueous Solutions Using N,N'-Bis(salicylidene)ethylenediamine (Salen) as an Extractant (Classic Solvent Extraction) and Carrier (Polymer Membranes). Membranes. 2021; 11(11):863. https://doi.org/10.3390/membranes11110863
Chicago/Turabian StyleWitt, Katarzyna, Małgorzata A. Kaczorowska, Daria Bożejewicz, and Włodzimierz Urbaniak. 2021. "Efficient Recovery of Noble Metal Ions (Pd2+, Ag+, Pt2+, and Au3+) from Aqueous Solutions Using N,N'-Bis(salicylidene)ethylenediamine (Salen) as an Extractant (Classic Solvent Extraction) and Carrier (Polymer Membranes)" Membranes 11, no. 11: 863. https://doi.org/10.3390/membranes11110863
APA StyleWitt, K., Kaczorowska, M. A., Bożejewicz, D., & Urbaniak, W. (2021). Efficient Recovery of Noble Metal Ions (Pd2+, Ag+, Pt2+, and Au3+) from Aqueous Solutions Using N,N'-Bis(salicylidene)ethylenediamine (Salen) as an Extractant (Classic Solvent Extraction) and Carrier (Polymer Membranes). Membranes, 11(11), 863. https://doi.org/10.3390/membranes11110863






