Giant Unilamellar Vesicle Electroformation: What to Use, What to Avoid, and How to Quantify the Results
Abstract
:1. Introduction
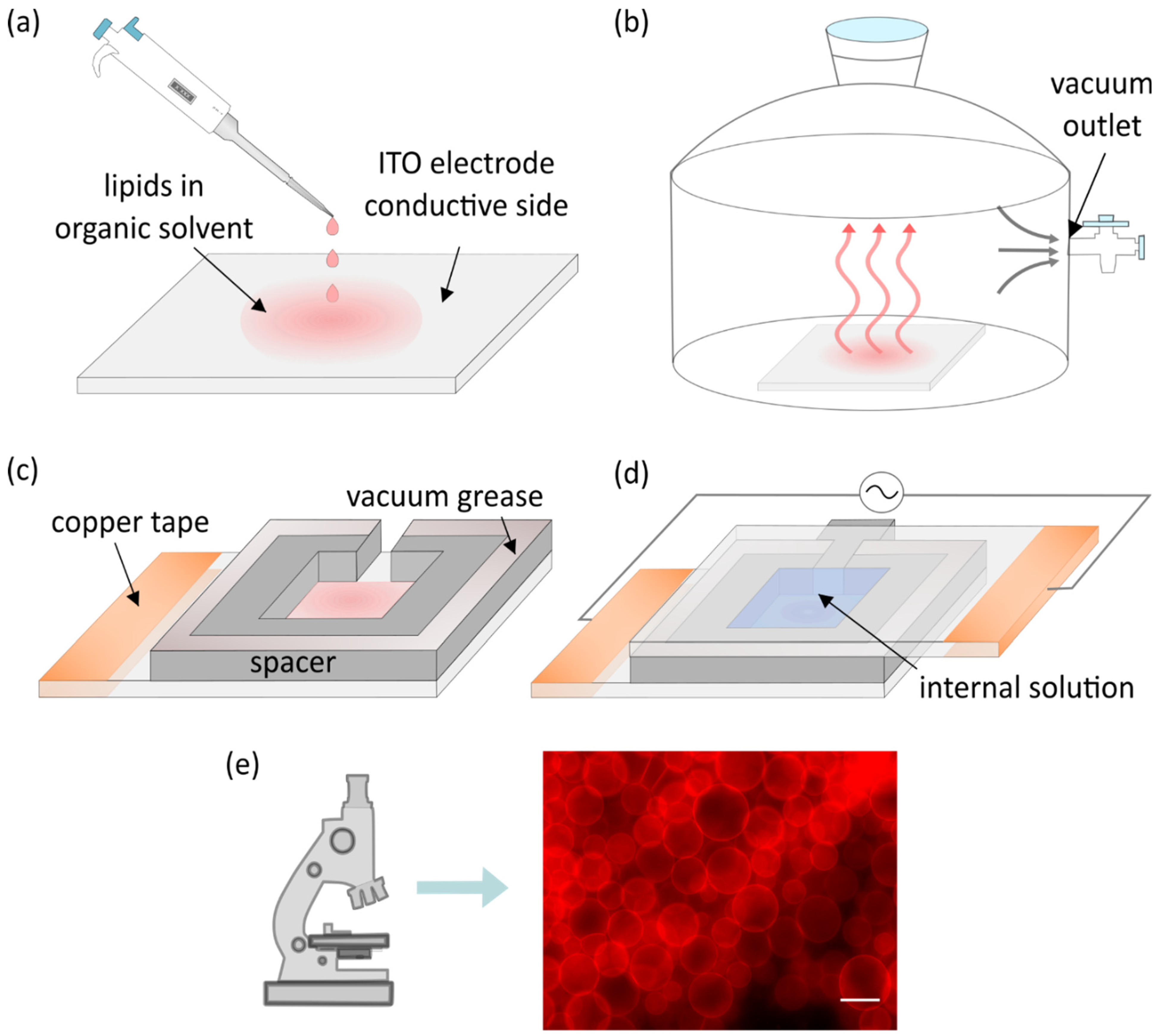
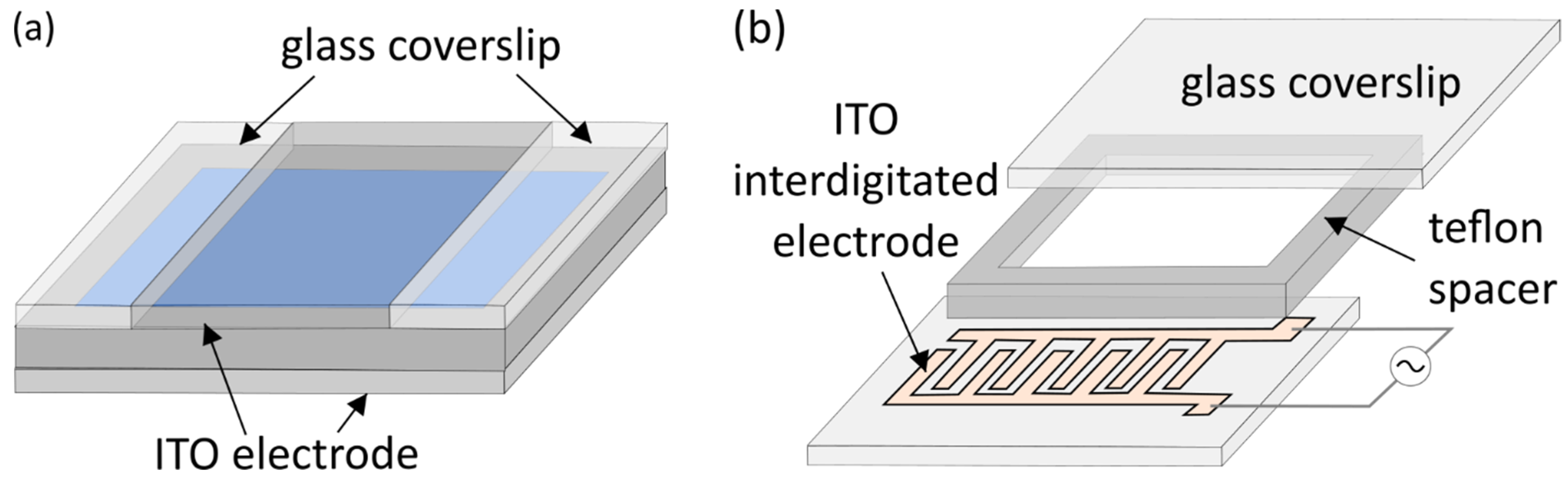
2. Classic Electroformation Protocol
3. Electrode Materials and Cleaning
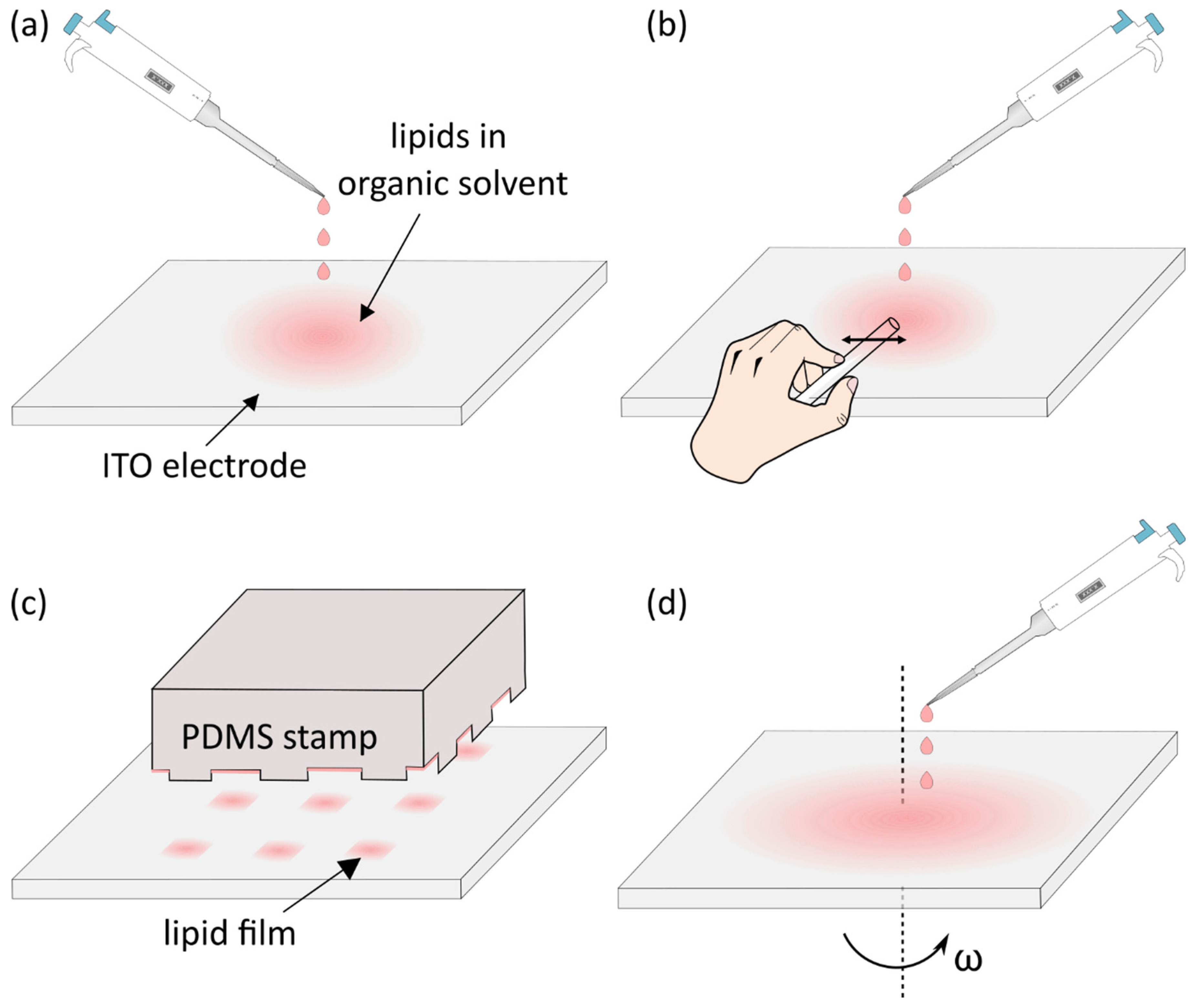
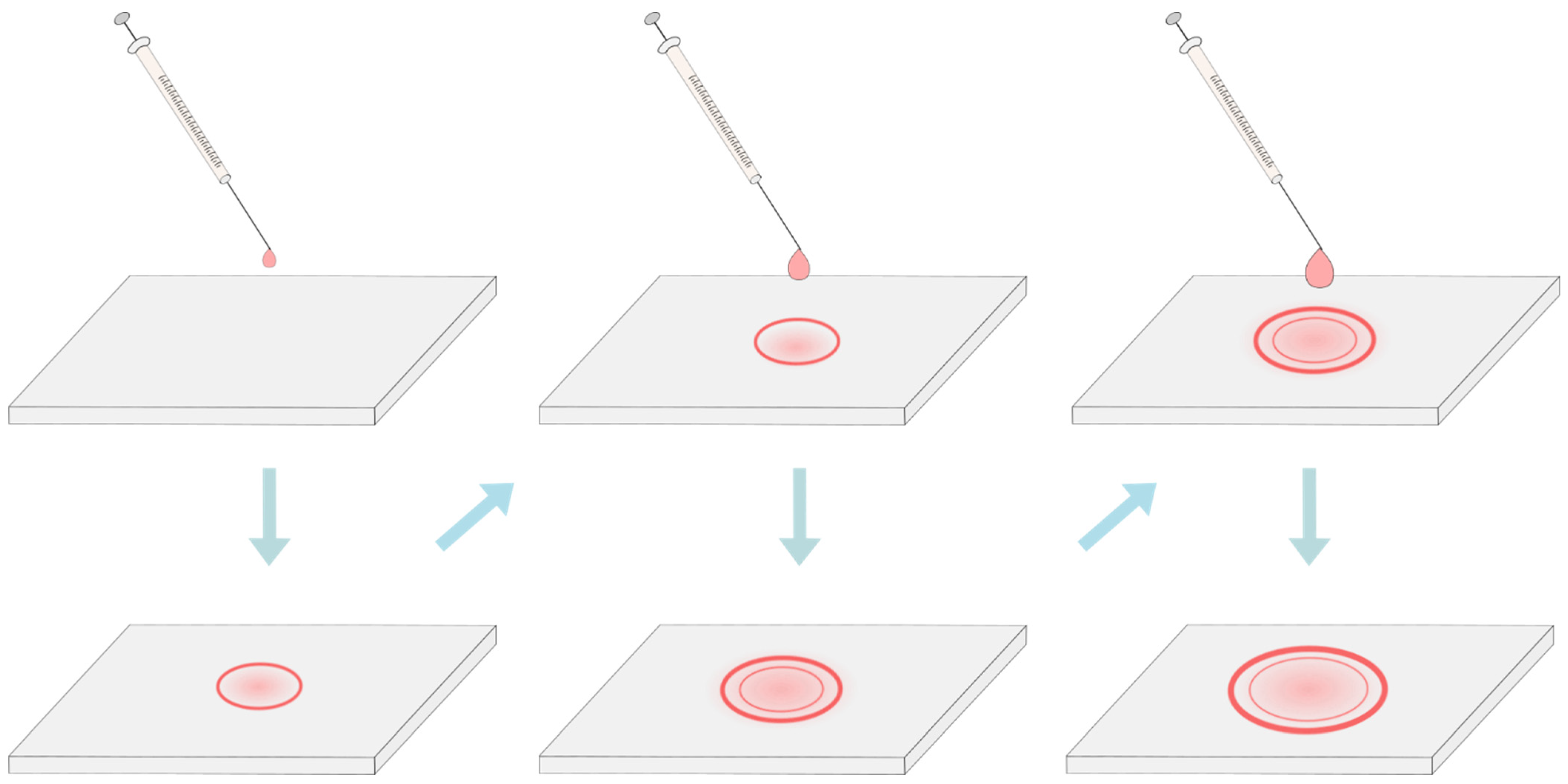
4. Deposition of the Lipid Solution and Removal of the Solvent
5. Lipid Compositions and Internal Solutions
6. Electrical Parameters
7. Temperature and Electroformation Duration
8. Vesicles Population Count and Size
8.1. Manual Analysis
8.2. Automated Microscopy Analysis
8.3. Light Scattering Methods
8.4. Electrical Impedance-Based Analysis
9. Final Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Menger, F.M.; Angelova, M.I. Giant vesicles: Imitating the cytological processes of cell membranes. Acc. Chem. Res. 1998, 31, 789–797. [Google Scholar] [CrossRef]
- Veatch, S.L.; Keller, S.L. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 2002, 89, 268101. [Google Scholar] [CrossRef] [Green Version]
- Reeves, J.P.; Dowben, R.M. Formation and properties of thin-walled phospholipid vesicles. J. Cell. Physiol. 1969, 73, 49–60. [Google Scholar] [CrossRef]
- Pott, T.; Bouvrais, H.; Méléard, P. Giant unilamellar vesicle formation under physiologically relevant conditions. Chem. Phys. Lipids 2008, 154, 115–119. [Google Scholar] [CrossRef]
- Valkenier, H.; López Mora, N.; Kros, A.; Davis, A.P. Visualization and quantification of transmembrane ion transport into giant unilamellar vesicles. Angew. Chemie Int. Ed. 2015, 54, 2137–2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subczynski, W.; Raguz, M.; Widomska, J. Studying lipid organization in biological membranes using liposomes and EPR spin labeling. Methods Mol. Biol. 2010, 606, 247–269. [Google Scholar] [CrossRef]
- Zylberberg, C.; Matosevic, S. Pharmaceutical liposomal drug delivery: A review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef] [Green Version]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veatch, S.L. Electro-formation and fluorescence microscopy of giant vesicles with coexisting liquid phases. Methods Mol. Biol. 2007, 398, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Morales-Penningston, N.F.; Wu, J.; Farkas, E.R.; Goh, S.L.; Konyakhina, T.M.; Zheng, J.Y.; Webb, W.W.; Feigenson, G.W. GUV preparation and imaging: Minimizing artifacts. Biochim. Biophys. Acta-Biomembr. 2010, 1798, 1324–1332. [Google Scholar] [CrossRef] [Green Version]
- Stein, H.; Spindler, S.; Bonakdar, N.; Wang, C. Production of Isolated Giant Unilamellar Vesicles under High Salt Concentrations. Front. Physiol. 2017, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2019, 0, 1–30. [Google Scholar] [CrossRef]
- Walde, P.; Cosentino, K.; Engel, H.; Stano, P. Giant vesicles: Preparations and applications. ChemBioChem 2010, 11, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Méléard, P.; Bagatolli, L.; Pott, T. Giant unilamellar vesicle electroformation from lipid mixtures to native membranes under physiological conditions. Methods Enzymol. 2009, 465, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Mason, R.P.; Tulenko, T.N.; Jacob, R.F. Direct evidence for cholesterol crystalline domains in biological membranes: Role in human pathobiology. Biochim. Biophys. Acta-Biomembr. 2003, 1610, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Subczynski, W.K.; Pasenkiewicz-Gierula, M. Hypothetical Pathway for Formation of Cholesterol Microcrystals Initiating the Atherosclerotic Process. Cell Biochem. Biophys. 2020, 78, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of membranes derived from the total lipids extracted from clear and cataractous lenses of 61–70-year-old human donors. Eur. Biophys. J. 2014, 44, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Raguz, M.; Kumar, S.N.; Zareba, M.; Ilic, N.; Mainali, L.; Subczynski, W.K. Confocal Microscopy Confirmed that in Phosphatidylcholine Giant Unilamellar Vesicles with very High Cholesterol Content Pure Cholesterol Bilayer Domains Form. Cell Biochem. Biophys. 2019, 77, 309–317. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Raguz, M.; Widomska, J.; Mainali, L.; Konovalov, A. Functions of cholesterol and the cholesterol bilayer domain specific to the fiber-cell plasma membrane of the eye lens. J. Membr. Biol. 2012, 245, 51–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mainali, L.; Raguz, M.; Subczynski, W.K. Formation of cholesterol bilayer domains precedes formation of cholesterol crystals in cholesterol/dimyristoylphosphatidylcholine membranes: EPR and DSC studies. J. Phys. Chem. B 2013, 117, 8994–9003. [Google Scholar] [CrossRef] [Green Version]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of membranes derived from the total lipids extracted from the human lens cortex and nucleus. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 1432–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subczynski, W.K.; Mainali, L.; Raguz, M.; O’Brien, W.J. Organization of lipids in fiber-cell plasma membranes of the eye lens. Exp. Eye Res. 2017, 156, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widomska, J.; Subczynski, W.K.; Mainali, L.; Raguz, M. Cholesterol Bilayer Domains in the Eye Lens Health: A Review. Cell Biochem. Biophys. 2017, 75, 387–398. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Pasenkiewicz-Gierula, M.; Widomska, J.; Mainali, L.; Raguz, M. High Cholesterol/Low Cholesterol: Effects in Biological Membranes Review. Cell Biochem. Biophys. 2017, 75, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Buboltz, J.T.; Feigenson, G.W. Maximum solubility of cholesterol in phosphatidylcholine and phosphatidylethanolamine bilayers. Biochim. Biophys. Acta-Biomembr. 1999, 1417, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Boban, Z.; Puljas, A.; Kovač, D.; Subczynski, W.K.; Raguz, M. Effect of Electrical Parameters and Cholesterol Concentration on Giant Unilamellar Vesicles Electroformation. Cell Biochem. Biophys. 2020, 78, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Baykal-Caglar, E.; Hassan-Zadeh, E.; Saremi, B.; Huang, J. Preparation of giant unilamellar vesicles from damp lipid film for better lipid compositional uniformity. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 2598–2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, N.; Pincet, F.; Cribier, S. Giant vesicles formed by gentle hydration and electroformation: A comparison by fluorescence microscopy. Colloids Surf. B Biointerfaces 2005, 42, 125–130. [Google Scholar] [CrossRef]
- Angelova, M.I.; Dimitrov, D.S. Liposome electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303–311. [Google Scholar] [CrossRef]
- Dimitrov, D.S.; Angelova, M.I. Lipid swelling and liposome formation on solid surfaces in external electric fields. In New Trends in Colloid Science; Springer: Berlin/Heidelberg, Germany, 1987; pp. 48–56. [Google Scholar]
- Has, C.; Pan, S. Vesicle formation mechanisms: An overview. J. Liposome Res. 2020, 31, 90–111. [Google Scholar] [CrossRef]
- Li, W.; Wang, Q.; Yang, Z.; Wang, W.; Cao, Y.; Hu, N.; Luo, H.; Liao, Y.; Yang, J. Impacts of electrical parameters on the electroformation of giant vesicles on ITO glass chips. Colloids Surf. B Biointerfaces 2016, 140, 560–566. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Ma, S.; Zhang, Y.; Han, X. Electroformation of giant unilamellar vesicles in saline solution. Colloids Surf. B Biointerfaces 2016, 147, 368–375. [Google Scholar] [CrossRef]
- Angelova, M.; Dimitrov, D.S. A mechanism of liposome electroformation. In Trends in Colloid and Interface Science II; Springer: Berlin/Heidelberg, Germany, 1988; pp. 59–67. [Google Scholar]
- Herold, C.; Chwastek, G.; Schwille, P.; Petrov, E.P. Efficient electroformation of supergiant unilamellar vesicles containing cationic lipids on ITO-coated electrodes. Langmuir 2012, 28, 5518–5521. [Google Scholar] [CrossRef]
- Drabik, D.; Doskocz, J.; Przybyło, M. Effects of electroformation protocol parameters on quality of homogeneous GUV populations. Chem. Phys. Lipids 2018, 212, 88–95. [Google Scholar] [CrossRef]
- Pereno, V.; Carugo, D.; Bau, L.; Sezgin, E.; Bernardino De La Serna, J.; Eggeling, C.; Stride, E. Electroformation of Giant Unilamellar Vesicles on Stainless Steel Electrodes. ACS Omega 2017, 2, 994–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behuria, H.G.; Biswal, B.K.; Sahu, S.K. Electroformation of liposomes and phytosomes using copper electrode. J. Liposome Res. 2021, 31, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Ayuyan, A.G.; Cohen, F.S. Lipid peroxides promote large rafts: Effects of excitation of probes in fluorescence microscopy and electrochemical reactions during vesicle formation. Biophys. J. 2006, 91, 2172–2183. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Berry, C.K.; Storer, P.A.; Raphael, R.M. Peroxidation of polyunsaturated phosphatidyl-choline lipids during electroformation. Biomaterials 2007, 28, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Farkas, E.R.; Webb, W.W. Multiphoton polarization imaging of steady-state molecular order in ternary lipid vesicles for the purpose of lipid phase assignment. J. Phys. Chem. B 2010, 114, 15512–15522. [Google Scholar] [CrossRef]
- Okumura, Y.; Sugiyama, T. Electroformation of Giant Vesicles on a Polymer Mesh. Membranes 2011, 1, 184–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, Y.; Zhang, H.; Sugiyama, T.; Iwata, Y. Electroformation of giant vesicles on a non-electroconductive substrate. J. Am. Chem. Soc. 2007, 129, 1490–1491. [Google Scholar] [CrossRef] [PubMed]
- Lefrançois, P.; Goudeau, B.; Arbault, S. Electroformation of phospholipid giant unilamellar vesicles in physiological phosphate buffer. Integr. Biol. 2018, 10, 429–434. [Google Scholar] [CrossRef]
- Bi, H.; Yang, B.; Wang, L.; Cao, W.; Han, X. Electroformation of giant unilamellar vesicles using interdigitated ITO electrodes. J. Mater. Chem. A 2013, 1, 7125–7130. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, J.; Shao, H.; Kong, F.; Jain, N.; Hunt, G.; Feigenson, G. Phase studies of model biomembranes: Macroscopic coexistence of Lα + Lβ, with light-induced coexistence of Lα + Lo Phases. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 2777–2786. [Google Scholar] [CrossRef] [Green Version]
- Witkowska, A.; Jablonski, L.; Jahn, R. A convenient protocol for generating giant unilamellar vesicles containing SNARE proteins using electroformation. Sci. Rep. 2018, 8, 9422. [Google Scholar] [CrossRef]
- Estes, D.J.; Mayer, M. Electroformation of giant liposomes from spin-coated films of lipids. Colloids Surf. B Biointerfaces 2005, 42, 115–123. [Google Scholar] [CrossRef]
- Politano, T.J.; Froude, V.E.; Jing, B.; Zhu, Y. AC-electric field dependent electroformation of giant lipid vesicles. Colloids Surf. B Biointerfaces 2010, 79, 75–82. [Google Scholar] [CrossRef]
- Angelova, M.I.; Soléau, S.; Méléard, P.; Faucon, F.; Bothorel, P. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. In Trends in Colloid and Interface Science VI; Springer: Berlin/Heidelberg, Germany, 1992; pp. 127–131. [Google Scholar]
- Ghellab, S.E.; Mu, W.; Li, Q.; Han, X. Prediction of the size of electroformed giant unilamellar vesicle using response surface methodology. Biophys. Chem. 2019, 253, 106217. [Google Scholar] [CrossRef] [PubMed]
- Gracià, R.S.; Bezlyepkina, N.; Knorr, R.L.; Lipowsky, R.; Dimova, R. Effect of cholesterol on the rigidity of saturated and unsaturated membranes: Fluctuation and electrodeformation analysis of giant vesicles. Soft Matter 2010, 6, 1472–1482. [Google Scholar] [CrossRef]
- Taylor, P.; Xu, C.; Fletcher, P.D.I.; Paunov, V.N. A novel technique for preparation of monodisperse giant liposomes. Chem. Commun. 2003, 44, 1732–1733. [Google Scholar] [CrossRef]
- Berre, L.; Chen, Y.; Baigl, D. From Convective Assembly to Landau—Levich Deposition of Multilayered Phospholipid Films of Controlled Thickness. Langmuir 2009, 2554–2557. [Google Scholar] [CrossRef]
- Bagatolli, L.A.; Parasassi, T.; Gratton, E. Giant phospholipid vesicles: Comparison among the whole lipid sample characteristics using different preparation methods: A two photon fluorescence microscopy study. Chem. Phys. Lipids 2000, 105, 135–147. [Google Scholar] [CrossRef]
- Collins, M.D.; Gordon, S.E. Giant liposome preparation for imaging and patch-clamp electrophysiology. J. Vis. Exp. 2013, 76, e50227. [Google Scholar] [CrossRef] [PubMed]
- Oropeza-Guzman, E.; Riós-Ramírez, M.; Ruiz-Suárez, J.C. Leveraging the Coffee Ring Effect for a Defect-Free Electroformation of Giant Unilamellar Vesicles. Langmuir 2019, 35, 16528–16535. [Google Scholar] [CrossRef]
- Bhatia, T.; Husen, P.; Brewer, J.; Bagatolli, L.A.; Hansen, P.L.; Ipsen, J.H.; Mouritsen, O.G. Preparing giant unilamellar vesicles (GUVs) of complex lipid mixtures on demand: Mixing small unilamellar vesicles of compositionally heterogeneous mixtures. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 3175–3180. [Google Scholar] [CrossRef] [Green Version]
- Girard, P.; Pécréaux, J.; Lenoir, G.; Falson, P.; Rigaud, J.L.; Bassereau, P. A new method for the reconstitution of membrane proteins into giant unilamellar vesicles. Biophys. J. 2004, 87, 419–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miele, Y.; Mingotaud, A.F.; Caruso, E.; Malacarne, M.C.; Izzo, L.; Lonetti, B.; Rossi, F. Hybrid giant lipid vesicles incorporating a PMMA-based copolymer. Biochim. Biophys. Acta-Gen. Subj. 2021, 1865, 129611. [Google Scholar] [CrossRef]
- Magnani, C.; Mpntis, C.; Mangiapia, G.; Mingotaud, A.F.; Mingotaud, C.; Roux, C.; Joseph, P.; Berti, D.; Lonetti, B. Hybrid vesicles from lipids and block copolymers: Phase behavior from the micro- to the nano-scale. Colloids Surf. B Biointerfaces 2018, 168, 18–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, J.; Beales, P.A.; Vanderlick, T.K. Giant Phospholipid/Block Copolymer Hybrid Vesicles: Mixing Behavior and Domain Formation. Langmuir 2010, 27, 1–6. [Google Scholar] [CrossRef]
- Le Meins, J.F.; Schatz, C.; Lecommandoux, S.; Sandre, O. Hybrid polymer/lipid vesicles: State of the art and future perspectives. Mater. Today 2013, 16, 397–402. [Google Scholar] [CrossRef]
- Van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Stevens, M.M.; Honerkamp-Smith, A.R.; Keller, S.L. Solubility limits of cholesterol, lanosterol, ergosterol, stigmasterol, and β-sitosterol in electroformed lipid vesicles. Soft Matter 2010, 6, 5882–5890. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-K.; So, L.; Spector, A. Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. J. Lipid Res. 1985, 26, 600–609. [Google Scholar] [CrossRef]
- Li, L.K.; So, L. Age dependent lipid and protein changes in individual bovine lenses. Curr. Eye Res. 1987, 6, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Mainali, L.; Pasenkiewicz-Gierula, M.; Subczynski, W.K. Formation of cholesterol Bilayer Domains Precedes Formation of Cholesterol Crystals in Membranes Made of the Major Phospholipids of Human Eye Lens Fiber Cell Plasma Membranes. Curr. Eye Res. 2020, 45, 162–172. [Google Scholar] [CrossRef]
- Kusumi, A.; Fujiwara, T.K.; Tsunoyama, T.A.; Kasai, R.S.; Koichiro, A.L.; Masanao, M.H.; Matsumori, N.; Komura, N.; Ando, H.; Suzuki, K.G.N. Defining raft domains in the plasma membrane. Traffic 2020, 21, 106–137. [Google Scholar] [CrossRef]
- Dimova, R.; Aranda, S.; Bezlyepkina, N.; Nikolov, V.; Riske, K.A.; Lipowsky, R. A practical guide to giant vesicles. Probing the membrane nanoregime via optical microscopy. J. Phys. Condens. Matter 2006, 18, 1151–1176. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Li, W.; Hu, N.; Chen, X.; Fan, T.; Wang, Z.; Yang, Z.; Cheney, M.A.; Yang, J. Biointerfaces Ion concentration effect ( Na+ and Cl−) on lipid vesicle formation. Colloids Surf. B Biointerfaces 2017, 155, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Q.; Lei, J.; Tao, K.; Huang, J.; Zhao, S.; Hu, N.; Yang, J. Mechanism study of how lipid vesicle electroformation is suppressed by the presence of sodium chloride. Colloids Surf. B Biointerfaces 2021, 206, 111951. [Google Scholar] [CrossRef]
- Estes, D.J.; Mayer, M. Giant liposomes in physiological buffer using electroformation in a flow chamber. Biochim. Biophys. Acta-Biomembr. 2005, 1712, 152–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montes, L.-R.; Alonso, A.; Goni, F.M.; Bagatolli, L.A. Giant unilamellar vesicles electroformed from native membranes and organic lipid mixtures under physiological conditions. Biophys. J. 2007, 93, 3548–3554. [Google Scholar] [CrossRef] [Green Version]
- Hristova, N.I.; Angelova, M.I.; Tsoneva, I. An experimental approach for direct observation of the interaction of polyanions with sphingosine-containing giant vesicles. Bioelectrochemistry 2002, 58, 65–73. [Google Scholar] [CrossRef]
- Bucher, P.; Fischer, A.; Luisi, P.L.; Oberholzer, T.; Walde, P. Giant vesicles as biochemical compartments: The use of microinjection techniques. Langmuir 1998, 14, 2712–2721. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, N.; Lei, J.; Qing, Q.; Huang, J.; Tao, K.; Zhao, S.; Sun, K.; Yang, J. Formation of Giant Lipid Vesicles in the Presence of Nonelectrolytes—Glucose, Sucrose, Sorbitol and Ethanol. Processes 2021, 9, 945. [Google Scholar] [CrossRef]
- Grit, M.; Zuidam, N.J.; Underberg, W.J.M.; Crommelin, D.J.A. Hydrolysis of Partially Saturated Egg Phosphatidylcholine in Aqueous Liposome Dispersions and the Effect of Cholesterol Incorporation on Hydrolysis Kinetics. J. Pharm. Pharmacol. 1993, 45, 490–495. [Google Scholar] [CrossRef]
- Veatch, S.L.; Keller, S.L. Separation of Liquid Phases in Giant Vesicles of Ternary Mixtures of Phospholipids and Cholesterol. Biophys. J. 2003, 85, 3074–3083. [Google Scholar] [CrossRef] [Green Version]
- Juhasz, J.; Davis, J.H.; Sharom, F.J. Biochimica et Biophysica Acta Fluorescent probe partitioning in GUVs of binary phospholipid mixtures: Implications for interpreting phase behavior. Biochim. Biophys. Acta-Biomembr. 2012, 1818, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Betaneli, V.; Worch, R.; Schwille, P. Effect of temperature on the formation of liquid phase-separating giant unilamellar vesicles (GUV). Chem. Phys. Lipids 2012, 165, 630–637. [Google Scholar] [CrossRef]
- Hossain, R.; Adamiak, K. Dynamic properties of the electric double layer in electrolytes. J. Electrostat. 2013, 71, 829–838. [Google Scholar] [CrossRef]
- Breton, M.; Amirkavei, M.; Mir, L.M. Optimization of the Electroformation of Giant Unilamellar Vesicles (GUVs) with Unsaturated Phospholipids. J. Membr. Biol. 2015, 248, 827–835. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Umakoshi, H.; Kuboi, R. Kinetic Study on Giant Vesicle Formation with Electroformation Method. Langmuir 2009, 25, 4835–4840. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.; Mouritsen, O.G. Phase separation dynamics and lateral organization of two-component lipid membranes. Biophys. J. 1995, 69, 942–954. [Google Scholar] [CrossRef] [Green Version]
- De Almeida, R.F.M.; Loura, L.M.S.; Fedorov, A.; Prieto, M. Nonequilibrium Phenomena in the Phase Separation of a Two-Component Lipid Bilayer. Biophys. J. 2002, 82, 823–834. [Google Scholar] [CrossRef] [Green Version]
- Cuevas, E.; Zaldivar, D.; Pérez-Cisneros, M.; Ramírez-Ortegón, M. Circle detection using discrete differential evolution optimization. Pattern Anal. Appl. 2011, 14, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Zupanc, J.; Drobne, D.; Ster, B. Markov random field model for segmenting large populations of lipid vesicles from micrographs. J. Liposome Res. 2011, 21, 315–323. [Google Scholar] [CrossRef]
- Hermann, E.; Bleicken, S.; Subburaj, Y.; García-Sáez, A.J. Automated analysis of giant unilamellar vesicles using circular Hough transformation. Bioinformatics 2014, 30, 1747–1754. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Witkowska, A. Guv Membrane Linearization Macro. Available online: https://zenodo.org/record/376618 (accessed on 14 January 2021).
- Witkowska, A. Macro for Quantification of GUV Diameter, Membrane Fluorescence Intensity, and FRET. Available online: https://zenodo.org/record/1249321 (accessed on 14 January 2021).
- Sych, T.; Schubert, T.; Vauchelles, R.; Madl, J.; Omidvar, R.; Thuenauer, R.; Richert, L.; Mély, Y.; Römer, W. GUV-AP: Multifunctional Fiji-based tool for quantitative image analysis of Giant Unilamellar Vesicles. Bioinformatics 2019, 35, 2340–2342. [Google Scholar] [CrossRef]
- Carvalho, K.; Ramos, L.; Roy, C.; Picart, C. Giant Unilamellar Vesicles Containing Phosphatidylinositol(4,5)bisphosphate: Characterization and Functionality. Biophys. J. 2008, 95, 4348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husen, P.; Arriaga, L.R.; Monroy, F.; Ipsen, J.H.; Bagatolli, L.A. Morphometric image analysis of giant vesicles: A new tool for quantitative thermodynamics studies of phase separation in lipid membranes. Biophys. J. 2012, 103, 2304–2310. [Google Scholar] [CrossRef] [Green Version]
- Sendra, G.H.; Hoerth, C.H.; Wunder, C.; Lorenz, H. 2D map projections for visualization and quantitative analysis of 3D fluorescence micrographs. Sci. Rep. 2015, 5, 12457. [Google Scholar] [CrossRef] [Green Version]
- Vorauer-Uhl, K.; Wagner, A.; Borth, N.; Katinger, H. Determination of liposome size distribution by flow cytometry. Cytometry 2000, 39, 166–171. [Google Scholar] [CrossRef]
- Simonsen, J.B. A liposome-based size calibration method for measuring microvesicles by flow cytometry. J. Thromb. Haemost. 2016, 14, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Sato, K.; Obinata, K.; Sugawara, T.; Urabe, I.; Yomo, T. Quantification of structural properties of cell-sized individual liposomes by flow cytometry. J. Biosci. Bioeng. 2006, 3, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Hema Sagar, G.; Tiwari, M.D.; Bellare, J.R. Flow cytometry as a novel tool to evaluate and separate vesicles using characteristic scatter signatures. J. Phys. Chem. B 2010, 114, 10010–10016. [Google Scholar] [CrossRef] [PubMed]
- Matsushita-Ishiodori, Y.; Hanczyc, M.M.; Wang, A.; Szostak, J.W.; Yomo, T. Using imaging flow cytometry to quantify and optimize giant vesicle production by water-in-oil emulsion transfer methods. Langmuir 2019, 35, 2375–2382. [Google Scholar] [CrossRef]
- Nishimura, K.; Hosoi, T.; Sunami, T.; Toyota, T.; Fujinami, M.; Oguma, K.; Matsuura, T.; Suzuki, H.; Yomo, T. Population analysis of structural properties of giant liposomes by flow cytometry. Langmuir 2009, 25, 10439–10443. [Google Scholar] [CrossRef]
- van der Pol, E.; Coumans, F.A.W.; Grootemaat, A.E.; Gardiner, C.; Sargent, I.L.; Harrison, P.; Sturk, A.; van Leeuwen, T.G.; Nieuwland, R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 2014, 12, 1182–1192. [Google Scholar] [CrossRef]
- Hoffman, R.A. Pulse width for particle sizing. Curr. Protoc. Cytom. 2009, 1, 1–17. [Google Scholar] [CrossRef]
- Kang, K.; Lee, S.B.; Yoo, J.H.; Nho, C.W. Flow cytometric fluorescence pulse width analysis of etoposide-induced nuclear enlargement in HCT116 cells. Biotechnol. Lett. 2010, 32, 1045–1052. [Google Scholar] [CrossRef] [Green Version]
- Vembadi, A.; Menachery, A.; Qasaimeh, M.A. Cell Cytometry: Review and Perspective on Biotechnological Advances. Front. Bioeng. Biotechnol. 2019, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Parigoris, E.; Dunkelmann, D.L.; Murphy, A.; Wili, N.; Kaech, A.; Dumrese, C.; Jimenez-Rojo, N.; Silvan, U. Facile generation of giant unilamellar vesicles using polyacrylamide gels. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Buboltz, J.T.; Feigenson, G.W. A novel strategy for the preparation of liposomes: Rapid solvent exchange. Biochim. Biophys. Acta-Biomembr. 1999, 1417, 232–245. [Google Scholar] [CrossRef] [Green Version]
- Buboltz, J.T. A more efficient device for preparing model-membrane liposomes by the rapid solvent exchange method. Rev. Sci. Instrum. 2009, 80, 124301. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boban, Z.; Mardešić, I.; Subczynski, W.K.; Raguz, M. Giant Unilamellar Vesicle Electroformation: What to Use, What to Avoid, and How to Quantify the Results. Membranes 2021, 11, 860. https://doi.org/10.3390/membranes11110860
Boban Z, Mardešić I, Subczynski WK, Raguz M. Giant Unilamellar Vesicle Electroformation: What to Use, What to Avoid, and How to Quantify the Results. Membranes. 2021; 11(11):860. https://doi.org/10.3390/membranes11110860
Chicago/Turabian StyleBoban, Zvonimir, Ivan Mardešić, Witold Karol Subczynski, and Marija Raguz. 2021. "Giant Unilamellar Vesicle Electroformation: What to Use, What to Avoid, and How to Quantify the Results" Membranes 11, no. 11: 860. https://doi.org/10.3390/membranes11110860
APA StyleBoban, Z., Mardešić, I., Subczynski, W. K., & Raguz, M. (2021). Giant Unilamellar Vesicle Electroformation: What to Use, What to Avoid, and How to Quantify the Results. Membranes, 11(11), 860. https://doi.org/10.3390/membranes11110860






