Cytotoxicity of a Cell Culture Medium Treated with a High-Voltage Pulse Using Stainless Steel Electrodes and the Role of Iron Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Main Materials
2.2. Growth of Cells and Preparation of Cell Suspension
2.3. Electric Pulse Generator and Electrodes Used to Treat Medium or Cell Suspension
2.4. Treatment of the Culture Medium with an Electric Pulse
2.5. Treatment of the Cell Suspension with an Electric Pulse
2.6. Determination of the Fraction of Electroporated Cells
2.7. Determination of the Fraction of Cells Killed by the Electric Pulse
2.8. Influence of an Electric Pulse-Treated Medium, its pH, and Fe2+ or Fe3+ Ions on Cell Viability
2.9. Determination of Cell Viability
2.10. Determination of the Amount of Iron Ions (Fe2+ & Fe3+) Released into the Medium
2.11. Statistical Analysis
3. Results and Discussion
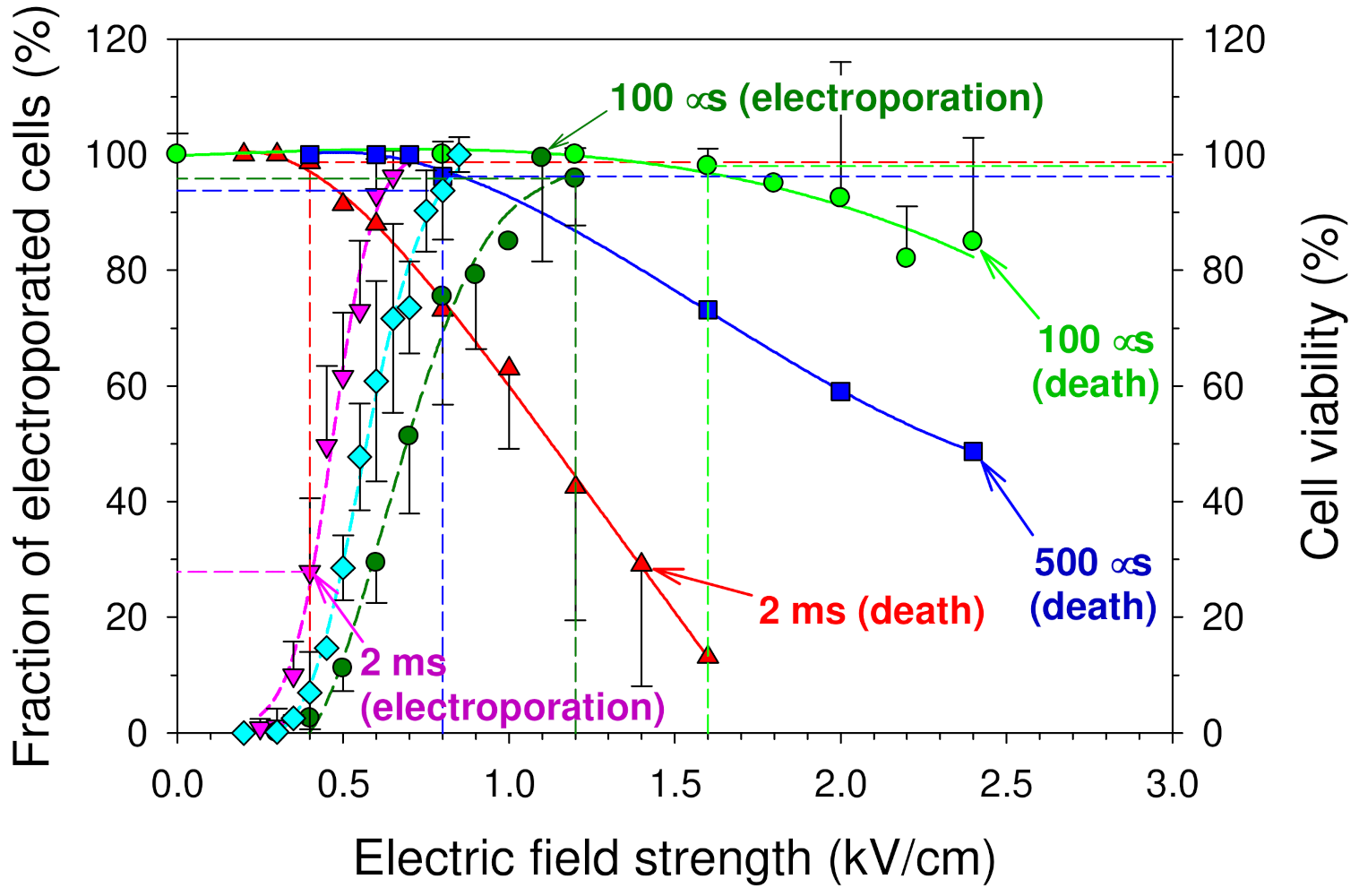
3.1. Influence of an Electric Pulse on the Integrity of the Cell Plasma Membrane (Electroporation) and Cell Viability
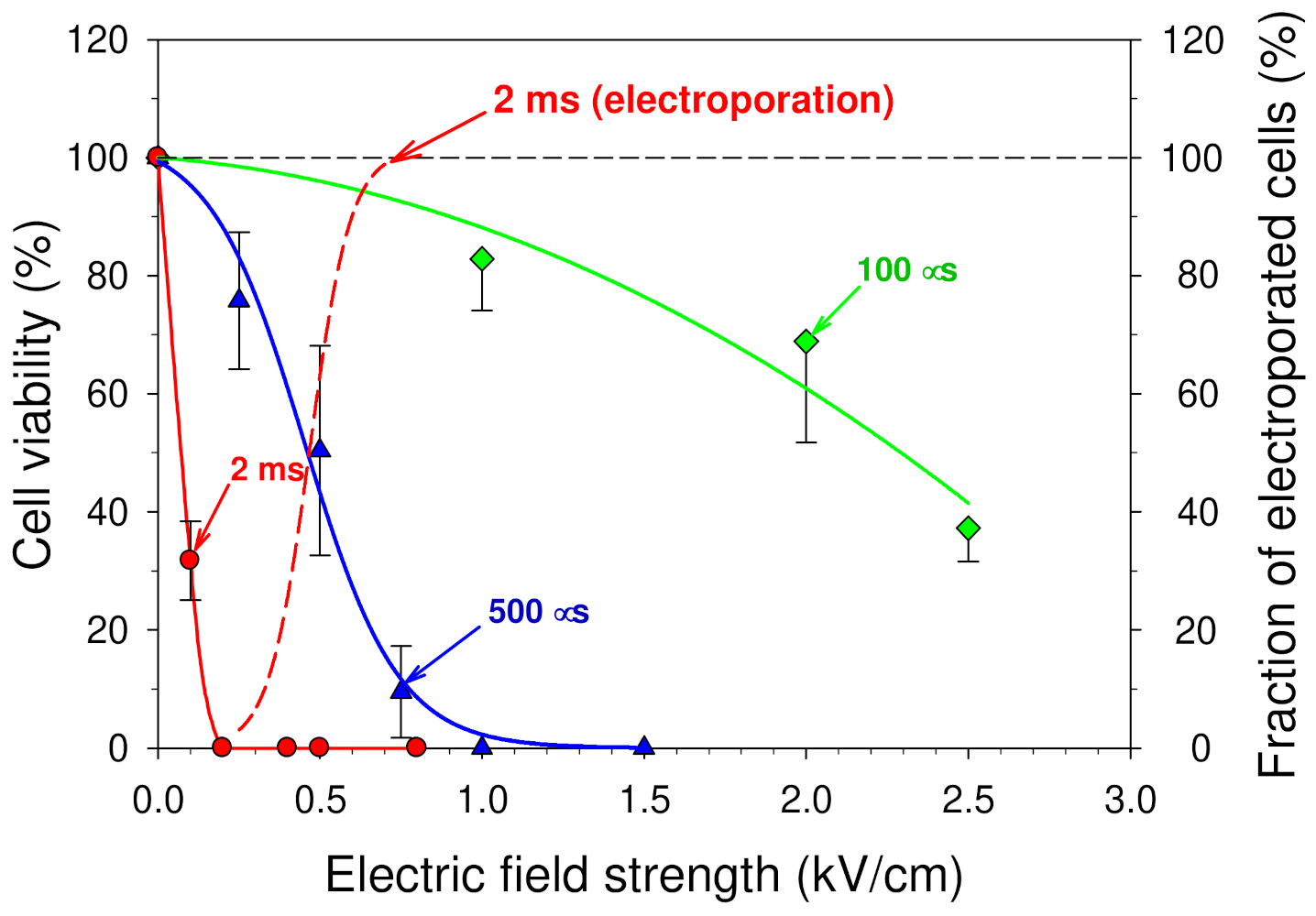
3.2. Influence of the Cell Culture Medium Treated by an Electric Pulse on the Cell Viability
3.3. Dependence of the Killing Ability of an Electric Field Pulse and the Medium Treated with the Pulse on the Electric Charge That has Flown through the Sample
3.4. Plausible Reasons of the Cytotoxicity of the Cell Culture Medium Treated with the Electric Pulse
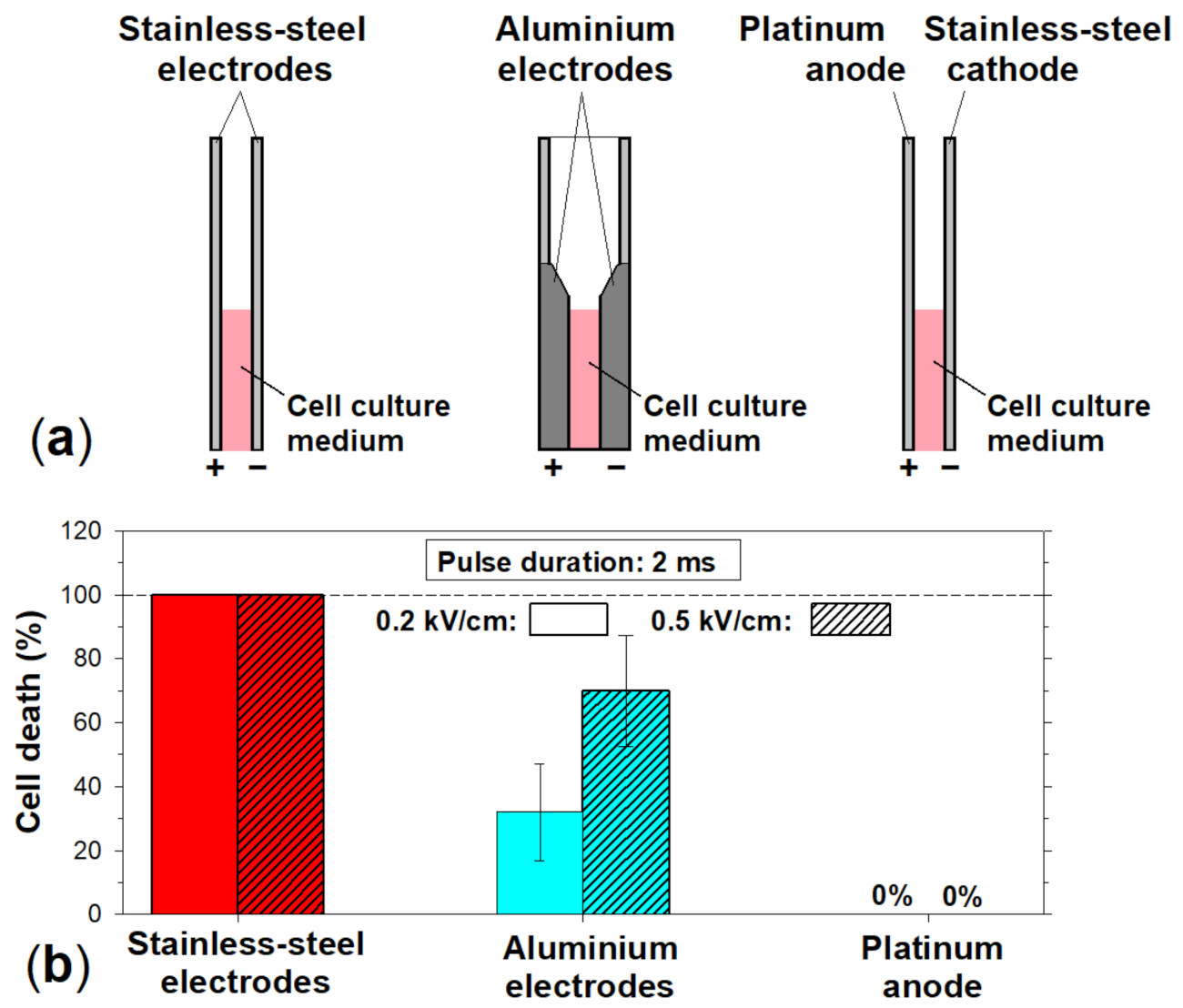
3.5. Influence of the Electrode Material on the Cytotoxicity of the Culture Medium Treated with an Electric Pulse
3.6. Release of Iron Ions from Stainless Steel Electrodes
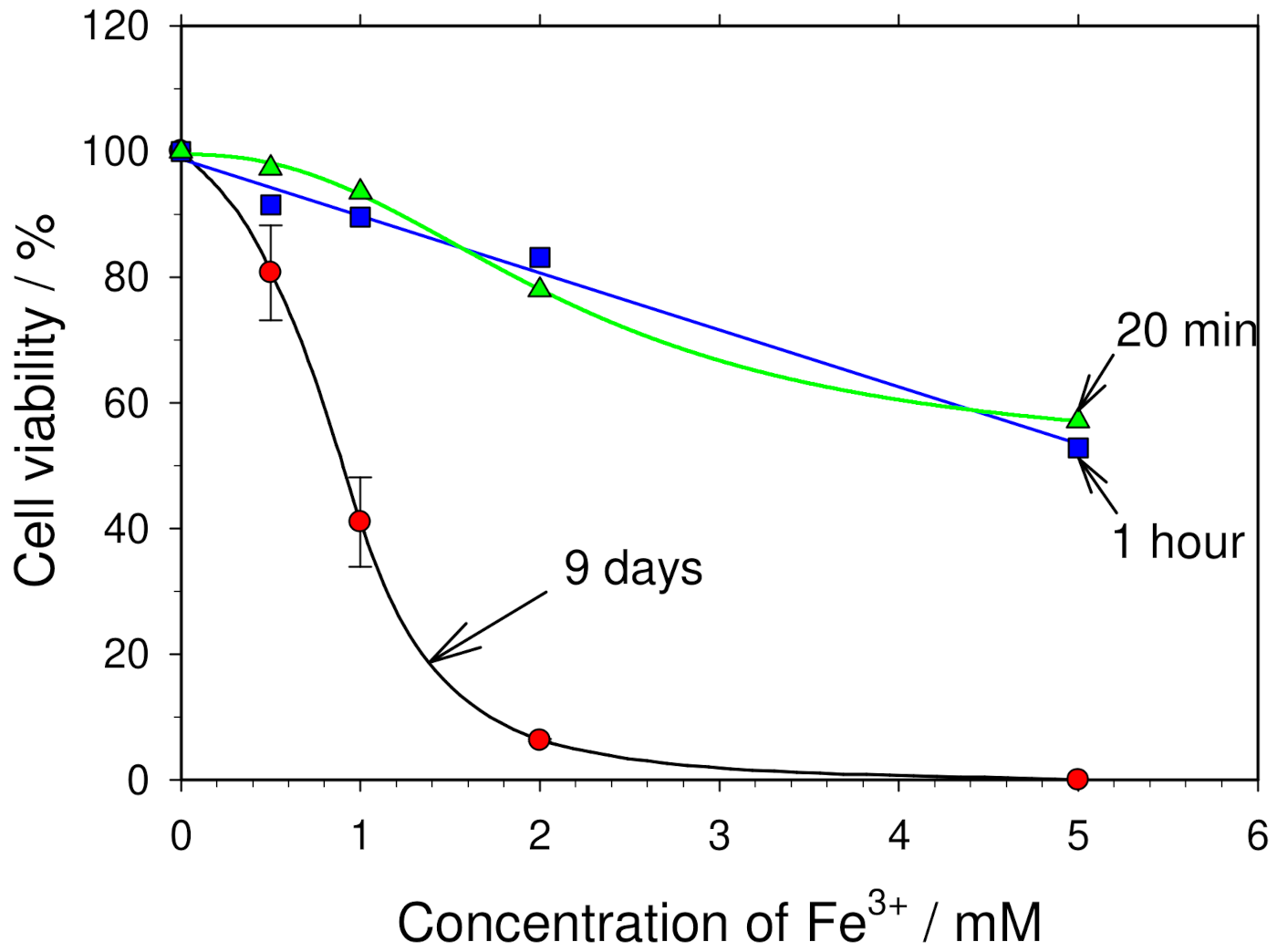
3.7. Influence of Iron Ions on Cell Viability
3.8. Plausible Reasons of the Electric Pulse-Treated Cell Culture Medium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Golberg, A.; Sack, M.; Teissie, J.; Pataro, G.; Pliquett, U.; Saulis, G.; Stefan, T.; Miklavcic, D.; Vorobiev, E.; Frey, W. Energy-efficient biomass processing with pulsed electric fields for bioeconomy and sustainable development. Biotechnol. Biofuels 2016, 9, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haberl, S.; Miklavcic, D.; Sersa, G.; Frey, W.; Rubinsky, B. Cell membrane electroporation-Part 2: The applications. IEEE Electr. Insul. Mag. 2013, 29, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Gowrishankar, T.R.; Esser, A.T.; Vasilkoski, Z.; Smith, K.C.; Weaver, J.C. Microdosimetry for conventional and supraelectroporation in cells with organelles. Biochem. Biophys. Res. Commun. 2006, 341, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, T.; Kramar, P.; Pucihar, G.; Miklavcic, D.; Tarek, M. Cell membrane electroporation- Part 1: The phenomenon. IEEE Electr. Insul. Mag. 2012, 28, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Abidor, I.G.; Arakelyan, L.V.; Chernomordik, L.V.; Chizmadzhev, Y.; Pastushenko, V.F.; Tarasevich, M.R. Electric breakdown of bilayer lipid membranes. I. The main experimental facts and their qualitative discussion. Bioelectrochem. Bioenerg. 1979, 6, 37–52. [Google Scholar] [CrossRef]
- Krassowska, W.; Filev, P.D. Modeling electroporation in a single cell. Biophys. J. 2007, 92, 404–417. [Google Scholar] [CrossRef] [Green Version]
- Pavlin, M.; Miklavcic, D. Theoretical and experimental analysis of conductivity, ion diffusion and molecular transport during cell electroporation--relation between short-lived and long-lived pores. Bioelectrochemistry 2008, 74, 38–46. [Google Scholar] [CrossRef]
- Saulis, G. Pore disappearance in a cell after electroporation: Theoretical simulation and comparison with experiments. Biophys. J. 1997, 73, 1299–1309. [Google Scholar] [CrossRef] [Green Version]
- Pakhomov, A.G.; Bowman, A.M.; Ibey, B.L.; Andre, F.M.; Pakhomova, O.N.; Schoenbach, K.H. Lipid nanopores can form a stable, ion channel-like conduction pathway in cell membrane. Biochem. Biophys. Res. Commun. 2009, 385, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Saulis, G. Electrochemical Processes Occuring during Cell Electromanipulation Procedures. A Short Review. In Electroporation in Laboratory and Clinical Investigations; Spugnini, E.P., Baldi, A., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2012; pp. 99–113. [Google Scholar]
- Saulis, G.; Rodaite-Riseviciene, R.; Dainauskaite, V.S.; Saule, R. Electrochemical Processes Occurring during High-Voltage Electric Pulses and their Importance for the Technology of Food Processing by Pulsed Electric Fields; Ravishankar, R.V., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 575–591. [Google Scholar]
- Milazzo, G. Electrochemistry: Theoretical Principles and Practical Applications; Elsevier: Amsterdam, The Netherlands, 1963. [Google Scholar]
- Loomis-Husselbee, J.W.; Cullen, P.J.; Irvine, R.F.; Dawson, A.P. Electroporation can cause artefacts due to solubilization of cations from the electrode plates. Aluminum ions enhance conversion of inositol 1,3,4,5-tetrakisphosphate into inositol 1,4,5-trisphosphate in electroporated L1210 cells. Biochem. J. 1991, 277, 883–885. [Google Scholar] [CrossRef] [Green Version]
- Saulis, G.; Lape, R.; Praneviciute, R.; Mickevicius, D. Changes of the solution pH due to exposure by high-voltage electric pulses. Bioelectrochemistry 2005, 67, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, T.; Miklavcic, D.; Mir, L.M. Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses. Part II. Reduced electrolytic contamination. Bioelectrochemistry 2001, 54, 91–95. [Google Scholar] [CrossRef]
- Saulis, G.; Mickevicius, D. Cell Electromanipulation Procedures Change the pH of a solution. In Electricity and Magnetism in Biology and Medicine; Bersani, F., Ed.; Plenum Publishing Corporation: New York, NY, USA, 1999; pp. 263–266. [Google Scholar] [CrossRef]
- Stapulionis, R. Electric pulse-induced precipitation of biological macromolecules in electroporation. Bioelectrochem. Bioenerg. 1999, 48, 249–254. [Google Scholar] [CrossRef]
- Roodenburg, B.; Morren, J.; Berg, H.E.I.; de Haan, S.W.H. Metal release in a stainless steel pulsed electric field (PEF) system. Part I. Effect of different pulse shapes; theory and experimental method. Innov. Food Sci. Emerg. Technol. 2005, 6, 327–336. [Google Scholar] [CrossRef]
- Pataro, G.; Falcone, M.; Donsi, G.; Ferrari, G. Metal release from stainless steel electrodes of a PEF treatment chamber: Effects of electrical parameters and food composition. Innov. Food Sc. Emerg. Technol. 2014, 21, 58–65. [Google Scholar] [CrossRef]
- Tomov, T.; Tsoneva, I. Are the stainless steel electrodes inert? Bioelectrochemistry 2000, 51, 207–209. [Google Scholar] [CrossRef]
- Rodaite-Riseviciene, R.; Saule, R.; Snitka, V.; Saulis, G. Release of iron ions from the stainless-steel anode occurring during high-voltage pulses and its consequences for cell electroporation technology. IEEE Trans. Plasma Sci. 2014, 42, 249–254. [Google Scholar] [CrossRef]
- Suehiro, J.; Hatano, T.; Shutou, M.; Hara, M. Improvement of electric pulse shape for electropermeabilization-assisted die-lectrophoretic impedance measurement for high sensitive bacteria detection. Sens. Actuators B 2005, 109, 209–215. [Google Scholar] [CrossRef]
- Saulis, G.; Rodaite-Riseviciene, R.; Snitka, V. Increase of the roughness of the stainless-steel anode surface due to the exposure to high-voltage electric pulses as revealed by atomic force microscopy. Bioelectrochemistry 2007, 70, 519–523. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Stremersch, S.; Braeckmans, K.; de Smedt, S.C.; Hendrix, A.; Wood, M.J.A.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release 2013, 172, 229–238. [Google Scholar] [CrossRef]
- Pliquett, U.F.; Gusbeth, C.A. Overcoming electrically induced artifacts in penetration studies with fluorescent tracers. Bioelectrochemistry 2000, 51, 75–79. [Google Scholar] [CrossRef]
- Kinosita, K.; Tsong, T.Y. Formation and resealing of pores of controlled sizes in human erythrocyte membrane. Nature 1977, 268, 438–441. [Google Scholar] [CrossRef]
- Neumann, E.; Toensing, K.; Kakorin, S.; Budde, P.; Frey, J. Mechanism of electroporative dye uptake by mouse B cells. Biophys. J. 1998, 74, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Saulis, G. Cell electroporation: Estimation of the number of pores and their sizes. Biomed. Sci. Instrum. 1999, 35, 291–296. [Google Scholar]
- Pliquett, U.; Elez, R.; Piiper, A.; Neumann, E. Electroporation of subcutaneous mouse tumors by rectangular and trapezium high voltage pulses. Bioelectrochemistry 2004, 62, 83–93. [Google Scholar] [CrossRef]
- Mir, L.M.; Orlowski, S.; Belehradek, J.; Paoletti, C. Electrochemotherapy potentiation of antitumor effect of bleomycin by local electric pulses. Eur. J. Cancer 1991, 27, 68–72. [Google Scholar] [CrossRef]
- Davalos, R.V.; Mir, I.L.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231. [Google Scholar] [CrossRef]
- Lv, Y.; Yao, C.; Rubinsky, B. A conceivable mechanism responsible for the synergy of high and low voltage irreversible elec-troporation pulses. Ann. Biomed. Eng. 2019, 47, 1552–1563. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Bose, V.G.; Langer, R.; Weaver, J.C. Electroporation of mammalian skin: A new mechanism to enhance transdermal drug delivery. Proc. Natl. Acad. Sci. USA 1993, 90, 10504–10508. [Google Scholar] [CrossRef] [Green Version]
- Titomirov, A.V.; Sukharev, S.; Kistanova, E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim. Biophys. Acta 1991, 1088, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Golberg, A.; Sheviryov, J.; Solomon, O.; Anavy, L.; Yakhini, Z. Molecular harvesting with electroporation for tissue profiling. Sci. Rep. 2019, 9, 15750. [Google Scholar] [CrossRef]
- Pataro, G.; Ferrari, G. Limitations of Pulsed Electric Field Utilization in Food Industry. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Barba, F.J., Parniakov, O., Wiktor, A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 283–310. [Google Scholar] [CrossRef]
- Phillips, M.; Rubinsky, L.; Meir, A.; Raju, N.; Rubinsky, B. Combining electrolysis and electroporation for tissue ablation. Technol. Cancer Res. Treat. 2015, 14, 395–410. [Google Scholar] [CrossRef]
- Reyns, K.M.F.A.; Diels, A.M.J.; Michiels, C.W. Generation of bactericidal and mutagenic components by pulsed electric field treatment. Int. J. Food Microbiol. 2004, 93, 165–173. [Google Scholar] [CrossRef]
- Krassowska, W.; Nanda, G.S.; Austin, M.B.; Dev, S.B.; Rabussay, D.P. Viability of cancer cells exposed to pulsed electric fields: The role of pulse charge. Ann. Biomed. Eng. 2003, 31, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Hulsheger, H.; Niemann, E.G. Lethal effect of high-voltage pulses on E. coli K12. Radiat. Environ. Biophys. 1980, 18, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.; Mercadal, B.; Stehling, M.; Ivorra, A. In vitro study on the mechanisms of action of electrolytic electroporation (E2). Bioelectrochemistry 2020, 133, 107482. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Sakamoto, M.; Fujimura, T. Efficient gene introduction into rice by electroporation and analysis of transgenic plants: Use of electroporation buffer lacking chloride ions. Theor. Appl. Genet. 1990, 80, 475–480. [Google Scholar] [CrossRef]
- Rodaite-Riseviciene, R.; Saule, R.; Saulis, G. Cytotoxicity of cell culture medium treated by high-voltage pulses. In Proceedings of the International Conference Bio & Food Electrotechnologies—BFE 2012, Fisciano, Salerno, Italy, 26–28 September 2012; pp. 83–87. [Google Scholar]
- Puc, M.; Corovic, S.; Flisar, K.; Petkovsek, M.; Nastran, J.; Miklavcic, D. Techniques of signal generation required for elec-tropermeabilization. Survey of electropermeabilization devices. Bioelectrochemistry 2004, 64, 113–124. [Google Scholar] [CrossRef]
- Morren, J.; Roodenburg, B.; de Haan, S.W.H. Electrochemical reactions and electrode corrosion in pulsed electric field (PEF) treatment chambers. Innov. Food Sci. Emerg. Technol. 2003, 4, 285–295. [Google Scholar] [CrossRef]
- Roodenburg, B.; Morren, J.; Berg, H.E.I.; de Haan, S.W.H. Metal release in a stainless steel pulsed electric field (PEF) system. Part II. The treatment of orange juice; related to legislation and treatment chamber lifetime. Innov. Food Sci. Emerg. Technol. 2005, 6, 337–345. [Google Scholar] [CrossRef]
- Saulis, G.; Satkauskas, S.; Praneviciute, R. Determination of cell electroporation from the release of intracellular potassium ions. Anal. Biochem. 2007, 360, 273–281. [Google Scholar] [CrossRef]
- Silkunas, M.; Bavirsa, M.; Saule, R.; Batiuskaite, D. To breathe or not to breathe? Hypoxia after pulsed-electric field treatment reduces the effectiveness of electrochemotherapy in vitro. Bioelectrochemistry 2021, 137, 107636. [Google Scholar] [CrossRef]
- Moonesan, M.S.; Jayaram, S.H. Effect of pulsewidth on medium temperature rise and microbial inactivation under pulsed electric field food treatment. IEEE Trans. Ind. Appl. 2013, 49, 1767–1772. [Google Scholar] [CrossRef]
- Van, L.A.; Verachtert, B.; Hendrickx, M. Effects of high electric field pulses on enzymes. Trends Food Sci. Technol. 2001, 12, 94–102. [Google Scholar] [CrossRef]
- Freshney, I.R. Culture of Animal Cells: A Manual of Basic Techniques; John Wiley & Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Cook, J.A.; Mitchell, J.B. Viability measurements in mammalian cell systems. Anal. Biochem. 1989, 179, 1–7. [Google Scholar] [CrossRef]
- Saulis, G.; Saule, R. Size of the pores created by an electric pulse: Microsecond vs millisecond pulses. Biochim. Biophys. Acta 2012, 1818, 3032–3039. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.K.; Cleary, S.F. Effects of pulsed electric fields on mouse spleen lymphocytes in vitro. Biochim. Biophys. Acta 1983, 763, 325–331. [Google Scholar] [CrossRef]
- Bowman, A.M.; Nesin, O.M.; Pakhomova, O.N.; Pakhomov, A.G. Analysis of plasma membrane integrity by fluorescent detection of Tl+ uptake. J. Membr. Biol. 2010, 236, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Schwister, K.; Deuticke, B. Formation and properties of aqueous leaks induced in human erythrocytes by electrical breakdown. Biochim. Biophys. Acta 1985, 816, 332–348. [Google Scholar] [CrossRef]
- Silve, A.; Leray, I.; Mir, L.M. Demonstration of cell membrane permeabilization to medium-sized molecules caused by a single 10 ns electric pulse. Bioelectrochemistry 2012, 87, 260–264. [Google Scholar] [CrossRef]
- Sozer, E.B.; Pocetti, C.F.; Vernier, P.T. Asymmetric patterns of small molecule transport after nanosecond and microsecond electropermeabilization. J. Membr. Biol. 2018, 251, 197–210. [Google Scholar] [CrossRef] [Green Version]
- Pakhomova, O.N.; Gregory, B.W.; Semenov, I.; Pakhomov, A.G. Two modes of cell death caused by exposure to nanosecond pulsed electric field. PLoS ONE 2013, 8, e70278. [Google Scholar] [CrossRef] [Green Version]
- Krassowska, N.W.; Neu, J.C. Mechanism of Irreversible Electroporation in Cells: Insight from the Models. In Irreversible Electroporationin; Rubinsky, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 85–122. [Google Scholar] [CrossRef]
- Hofmann, F.; Ohnimus, H.; Scheller, C.; Strupp, W.; Zimmermann, U.; Jassoy, C. Electric field pulses can induce apoptosis. J. Membr. Biol. 1999, 169, 103–109. [Google Scholar] [CrossRef]
- Rowan, N.J.; MacGregor, S.J.; Anderson, J.G.; Fouracre, R.A.; Farish, O. Pulsed electric field inactivation of diarrhoeagenic Bacillus cereus through irreversible electroporation. Lett. Appl. Microbiol. 2000, 31, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Calderon-Miranda, M.L.; Barbosa-Canovas, G.V.; Swanson, B.G. Transmission electron microscopy of Listeria innocua treated by pulsed electric fields and nisin in skimmed milk. Int. J. Food Microbiol. 1999, 51, 31–38. [Google Scholar] [CrossRef]
- Kekez, M.M.; Savic, P.; Johnson, B.F. Contribution to the biophysics of the lethal effects of electric field on microorganisms. Biochim. Biophys. Acta 1996, 1278, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Wouters, P.C.; Bos, A.P.; Ueckert, J. Membrane permeabilization in relation to inactivation kinetics of Lactobacillus species due to pulsed electric fields. Appl. Environ. Microbiol. 2001, 67, 3092–3101. [Google Scholar] [CrossRef] [Green Version]
- Batista, N.T.; Polajzer, T.; Miklavcic, D. Cell death due to electroporation—A review. Bioelectrochemistry 2021, 141, 107871. [Google Scholar] [CrossRef]
- Saulis, G. Electroporation of cell membranes: The fundamental effects of pulsed electric fields in food processing. Food Eng. Rev. 2010, 2, 52–73. [Google Scholar] [CrossRef]
- Dutreux, N.; Notermans, S.; Wijtzes, T.; Gongora-Nieto, M.M.; Barbosa-Canovas, G.V.; Swanson, B.G. Pulsed electric fields inactivation of attached and free-living Escherichia coli and Listeria innocua under several conditions. Int. J. Food Microbiol. 2000, 54, 91–98. [Google Scholar] [CrossRef]
- Elez-Martinez, P.; Escola-Hernandez, J.; Soliva-Fortuny, R.C.; Martin-Belloso, O. Inactivation of Lactobacillus brevis in orange juice by high-intensity pulsed electric fields. Food Microbiol. 2005, 22, 311–319. [Google Scholar] [CrossRef]
- Tsong, T.Y. Electroporation of cell membranes. Biophys. J. 1991, 60, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Rowan, N.J.; MacGregor, S.J.; Anderson, J.G.; Cameron, D.; Farish, O. Inactivation of mycobacterium paratuberculosis by pulsed electric fields. Appl. Environ. Microbiol. 2001, 67, 2833–2836. [Google Scholar] [CrossRef] [Green Version]
- Kotnik, T.; Macek, L.; Miklavcic, D.; Mir, L.M. Evaluation of cell membrane electropermeabilization by means of a nonper-meant cytotoxic agent. Biotechniques 2000, 28, 921–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rols, M.P.; Teissie, J. Electropermeabilization of mammalian cells to macromolecules: Control by pulse duration. Biophys. J. 1998, 75, 1415–1423. [Google Scholar] [CrossRef] [Green Version]
- Cemazar, M.; Jarm, T.; Sersa, G. Cancer electrogene therapy with interleukin-12. Curr. Gene Ther. 2010, 10, 300–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gothelf, A.; Mir, L.M.; Gehl, J. Electrochemotherapy: Results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat. Rev. 2003, 29, 371–387. [Google Scholar] [CrossRef]
- Sun, J.; Bai, W.; Zhang, Y.; Liao, X.; Hu, X. Effects of electrode materials on the degradation, spectral characteristics, visual colour, and antioxidant capacity of cyanidin-3-glucoside and cyanidin-3-sophoroside during pulsed electric field (PEF) treat-ment. Food Chem. 2011, 128, 742–747. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990, 186, 1–85. [Google Scholar] [CrossRef]
- Conrad, M.E.; Umbreit, J.N. Iron absorption and transport—An update. Am. J. Hematol. 2000, 64, 287–298. [Google Scholar] [CrossRef]
- Young, S.P.; Garner, C. Delivery of iron to human cells by bovine transferrin: Implications for the growth of human cells in vitro. Biochem. J. 1990, 265, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Huang, N.; Xu, L.; Zhang, Y.; Liu, H.; Sun, H.; Leng, Y. Biocompatibility of pure iron: In vitro assessment of degra-dation kinetics and cytotoxicity on endothelial cells. Mater. Sci. Eng. C 2009, 29, 1589–1592. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Reddy, T.K.; Venkatesha, V.A.; Kedlaye, R. Influence of naringin on ferric iron induced oxidative damage in vitro. Clin. Chim. Acta 2004, 347, 189–197. [Google Scholar] [CrossRef]
- Papagianni, M.; Avramidis, N.; Filioussis, G. High efficiency electrotransformation of Lactococcus lactis spp. lactis cells pre-treated with lithium acetate and dithiothreitol. BMC Biotechnol. 2007, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Rhee, M.S.; Kim, J.W.; Qian, Y.; Ingram, L.O.; Shanmugam, K.T. Development of plasmid vector and electroporation condition for gene transfer in sporogenic lactic acid bacterium, Bacillus coagulans. Plasmid 2007, 58, 13–22. [Google Scholar] [CrossRef]
- Dunny, G.M.; Lee, L.N.; LeBlanc, D.J. Improved electroporation and cloning vector system for gram-positive bacteria. Appl. Environ. Microbiol. 1991, 57, 1194–1201. [Google Scholar] [CrossRef] [Green Version]
- Sabri, N.; Pelissier, B.; Teissie, J. Electropermeabilization of intact maize cells induces an oxidative stress. Eur. J. Biochem. 1996, 238, 737–743. [Google Scholar] [CrossRef]
- Gusbeth, C.; Frey, W.; Volkmann, H.; Schwartz, T.; Bluhm, H. Pulsed electric field treatment for bacteria reduction and its impact on hospital wastewater. Chemosphere 2009, 75, 228–233. [Google Scholar] [CrossRef]
- Pakhomova, O.N.; Gregory, B.W.; Khorokhorina, V.A.; Bowman, A.M.; Xiao, S.; Pakhomov, A.G. Electroporation-induced electrosensitization. PLoS ONE 2011, 6, e17100. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Yang, R.; Wang, M. Cold storage temperature following pulsed electric fields treatment to inactivate sublethally injured microorganisms and extend the shelf life of green tea infusions. Int. J. Food Microbiol. 2009, 129, 204–208. [Google Scholar] [CrossRef]
- Pastor, N.; Weinstein, H.; Jamison, E.; Brenowitz, M. A detailed interpretation of OH radical footprints in a TBP-DNA complex reveals the role of dynamics in the mechanism of sequence-specific binding. J. Mol. Biol. 2000, 304, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Baes, C.F.; Mesmer, R.E. The Hydrolysis of Cations; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Byrne, R.H.; Luo, Y.-R.; Young, R.W. Iron hydrolysis and solubility revisited: Observations and comments on iron hydrolysis characterizations. Mar. Chem. 2000, 70, 23–35. [Google Scholar] [CrossRef]
- Kobya, M.; Can, O.T.; Bayramoglu, M. Treatment of textile wastewaters by electrocoagulation using iron and aluminum electrodes. J. Hazard. Mater. 2003, B100, 163–178. [Google Scholar] [CrossRef]
- Walker, B.L.; Tiong, J.W.C.; Jefferies, W.A. Iron metabolism in mammalian cells. Int. Rev. Cytol. 2001, 211, 241–278. [Google Scholar] [CrossRef]
- Pakhomova, O.N.; Khorokhorina, V.A.; Bowman, A.M.; Rodaite-Riseviciene, R.; Saulis, G.; Xiao, S.; Pakhomov, A.G. Oxidative effects of nanosecond pulsed electric field exposure in cells and cell-free media. Arch. Biochem. Biophys. 2012, 527, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, D.; Stohs, S.J.; Downs, B.W.; Bagchi, M.; Preuss, H.G. Cytotoxicity and oxidative mechanisms of different forms of chromium. Toxicology 2002, 180, 5–22. [Google Scholar] [CrossRef]
- Alimba, C.G.; Dhillon, V.; Bakare, A.A.; Fenech, M. Genotoxicity and cytotoxicity of chromium, copper, manganese and lead, and their mixture in WIL2-NS human B lymphoblastoid cells is enhanced by folate depletion. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 798–799, 35–47. [Google Scholar] [CrossRef]
- Lu, X.; Bao, X.; Huang, Y.; Qu, Y.; Lu, H.; Lu, Z. Mechanisms of cytotoxicity of nickel ions based on gene expression profiles. Biomaterials 2009, 30, 141–148. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saulis, G.; Rodaitė-Riševičienė, R.; Saulė, R. Cytotoxicity of a Cell Culture Medium Treated with a High-Voltage Pulse Using Stainless Steel Electrodes and the Role of Iron Ions. Membranes 2022, 12, 184. https://doi.org/10.3390/membranes12020184
Saulis G, Rodaitė-Riševičienė R, Saulė R. Cytotoxicity of a Cell Culture Medium Treated with a High-Voltage Pulse Using Stainless Steel Electrodes and the Role of Iron Ions. Membranes. 2022; 12(2):184. https://doi.org/10.3390/membranes12020184
Chicago/Turabian StyleSaulis, Gintautas, Raminta Rodaitė-Riševičienė, and Rita Saulė. 2022. "Cytotoxicity of a Cell Culture Medium Treated with a High-Voltage Pulse Using Stainless Steel Electrodes and the Role of Iron Ions" Membranes 12, no. 2: 184. https://doi.org/10.3390/membranes12020184
APA StyleSaulis, G., Rodaitė-Riševičienė, R., & Saulė, R. (2022). Cytotoxicity of a Cell Culture Medium Treated with a High-Voltage Pulse Using Stainless Steel Electrodes and the Role of Iron Ions. Membranes, 12(2), 184. https://doi.org/10.3390/membranes12020184




