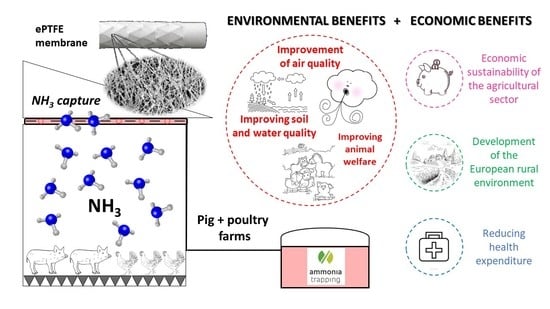
Pilot Plant for the Capture of Ammonia from the Atmosphere of Pig and Poultry Farms Using Gas-Permeable Membrane Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Location
2.2. Composition of Livestock Waste
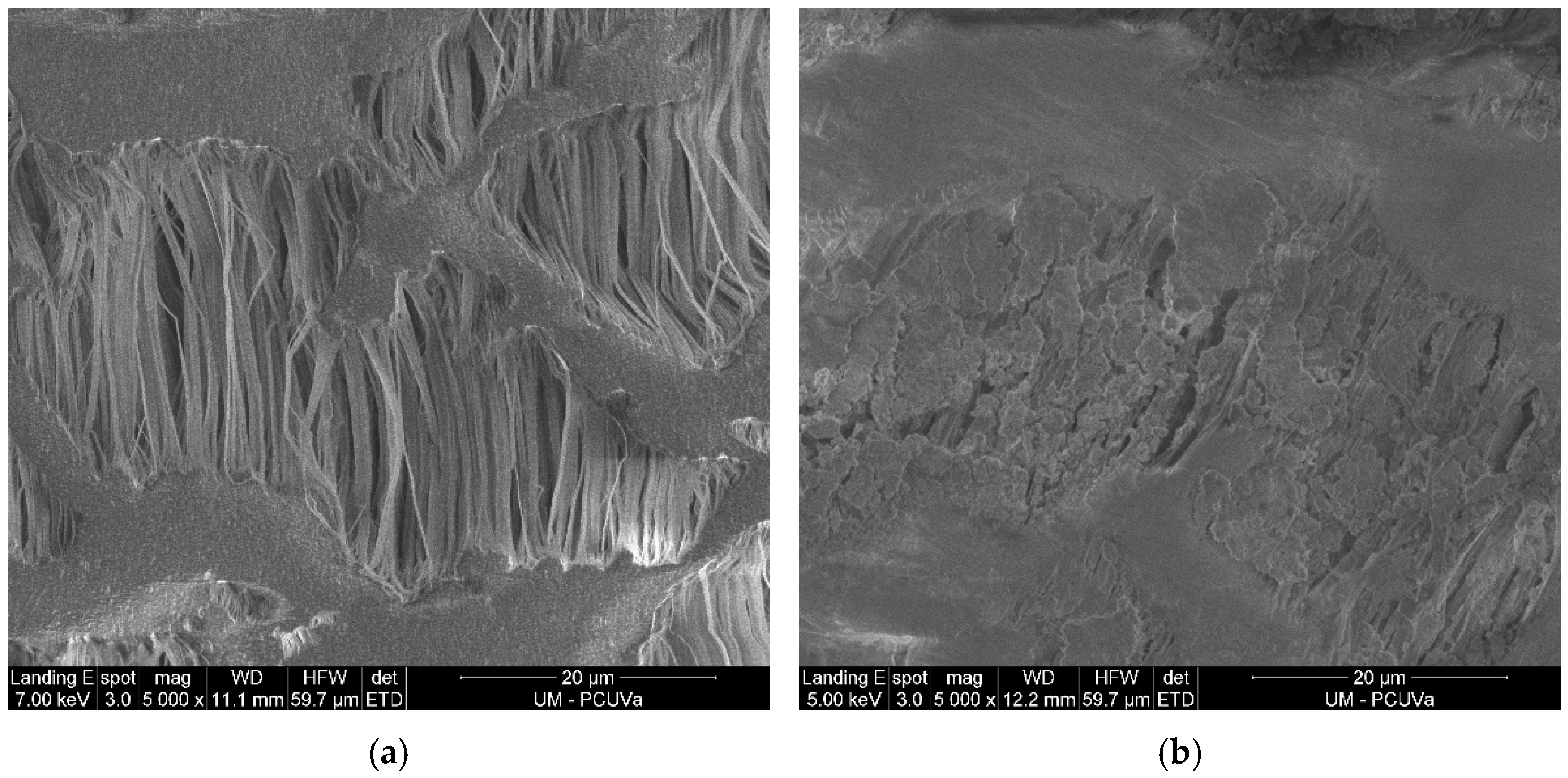
2.3. Characteristics of the e-PTFE Membrane
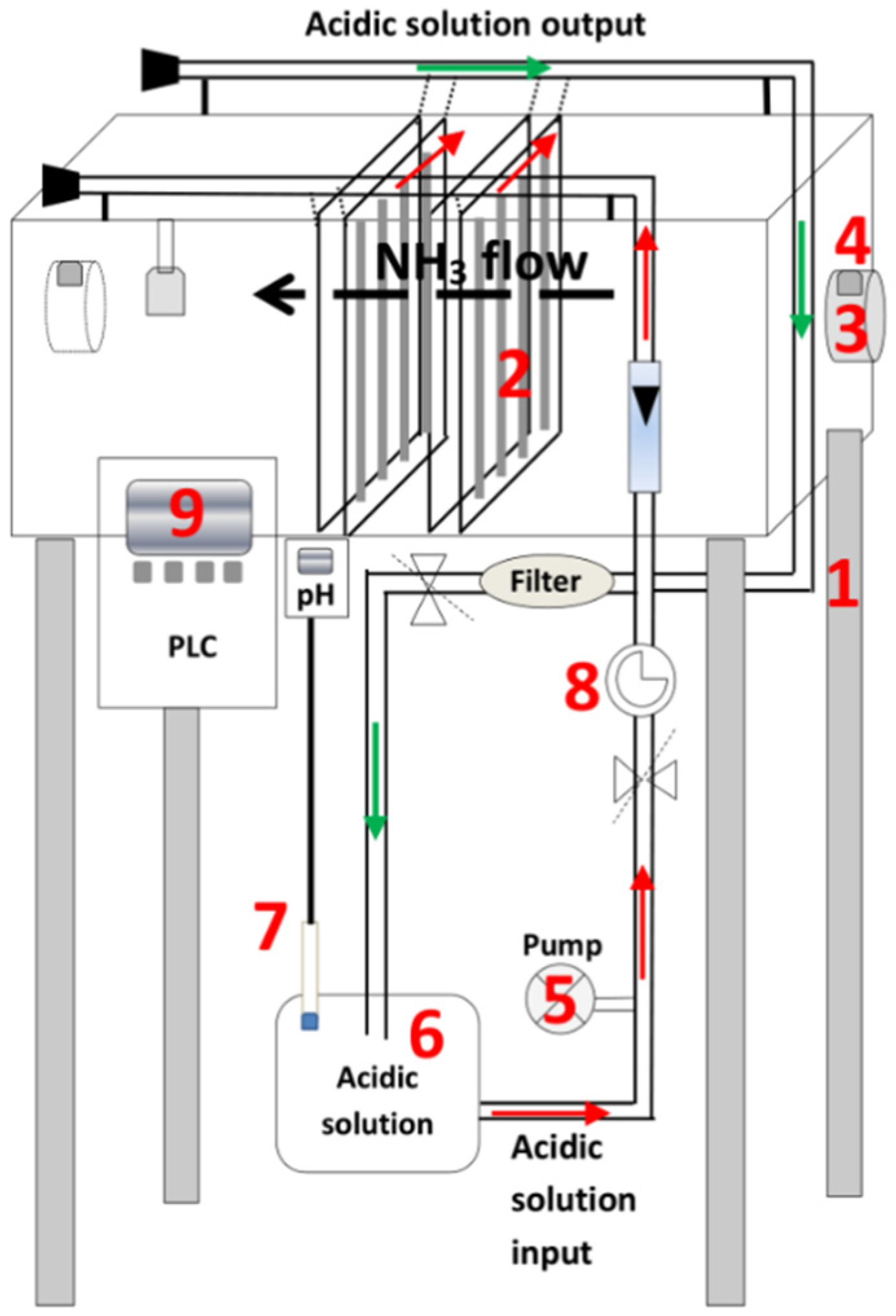
2.4. Components of the Pilot-Scale Gas-Recovery Prototype
- A 2.6 m3 steel structure.
- Thirty-two membrane panels arranged vertically within the steel structure. Each panel contains 14.8 m of e-PTFE tubular membrane (ZEUS, Orangeburg, SC, USA) attached by plastic connections to a plastic support net (1 cm mesh), attached to stainless steel frames (0.750 m × 0.495 m). The surface area of the membrane used is 7.7 m2.
- One 150 W single-phase wall-mounted fan.
- Two DURTOX IP65-v07 ammonia sensors (Duran® Electrónica, Madrid, Spain).
- A 0.56 kW acidic solution recirculation pump. A flow rate of 2.1 L·h−1 was used.
- A 0.25 m3 tank for the storage of the acidic solution. Specifically, the capture solution rises to a sealed distribution pipe connected to the 32 parallel membrane panels. Another pipe collects the acidic capture solution from the membranes of the 32 panels, returning it to the storage tank by gravity.
- pH and temperature probes.
- A pressure gauge to monitor the pressure of the acidic solution. This is an important control parameter, given that a low pressure can impede the circulation of the liquid through the membranes, slowing down the capture rate, whereas a high pressure can cause damage to the membranes. The pressure selected to lead the capture solution to the sealed distribution tube is 0.2 bar, with 0.5 bar being the highest admissible outlet pressure to avoid damaging the membranes used.
- A PLC system (Siemens, Munich, Germany) was used to control the equipment. It generates a continuous record of the temperature and pH of the acidic solution, keeping the latter below 2 to favor NH3 capture.
2.5. Equipment Used at Laboratory Scale
2.6. Operating and Monitoring Procedure
2.7. Sampling and Analysis
2.8. Calculations
3. Results and Discussion
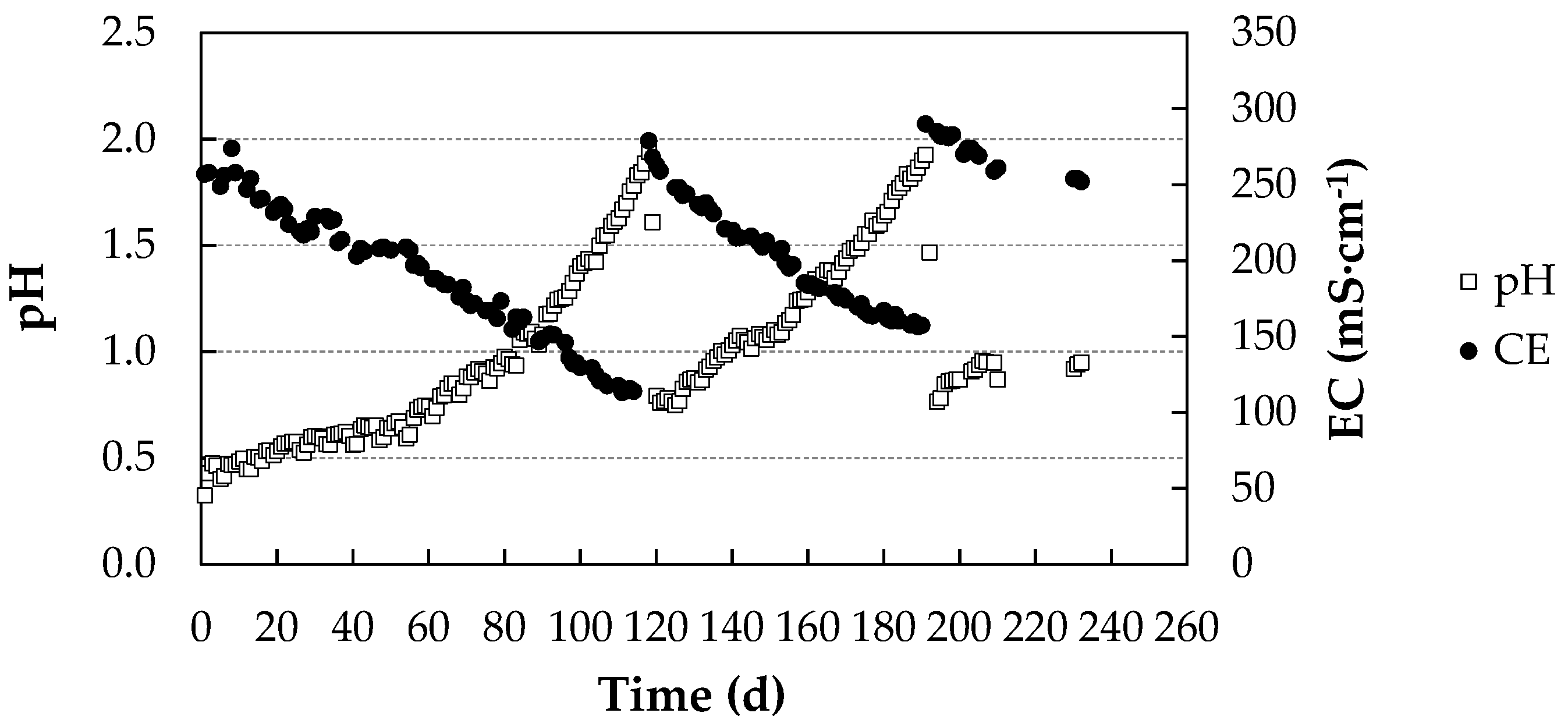
3.1. Process Parameters
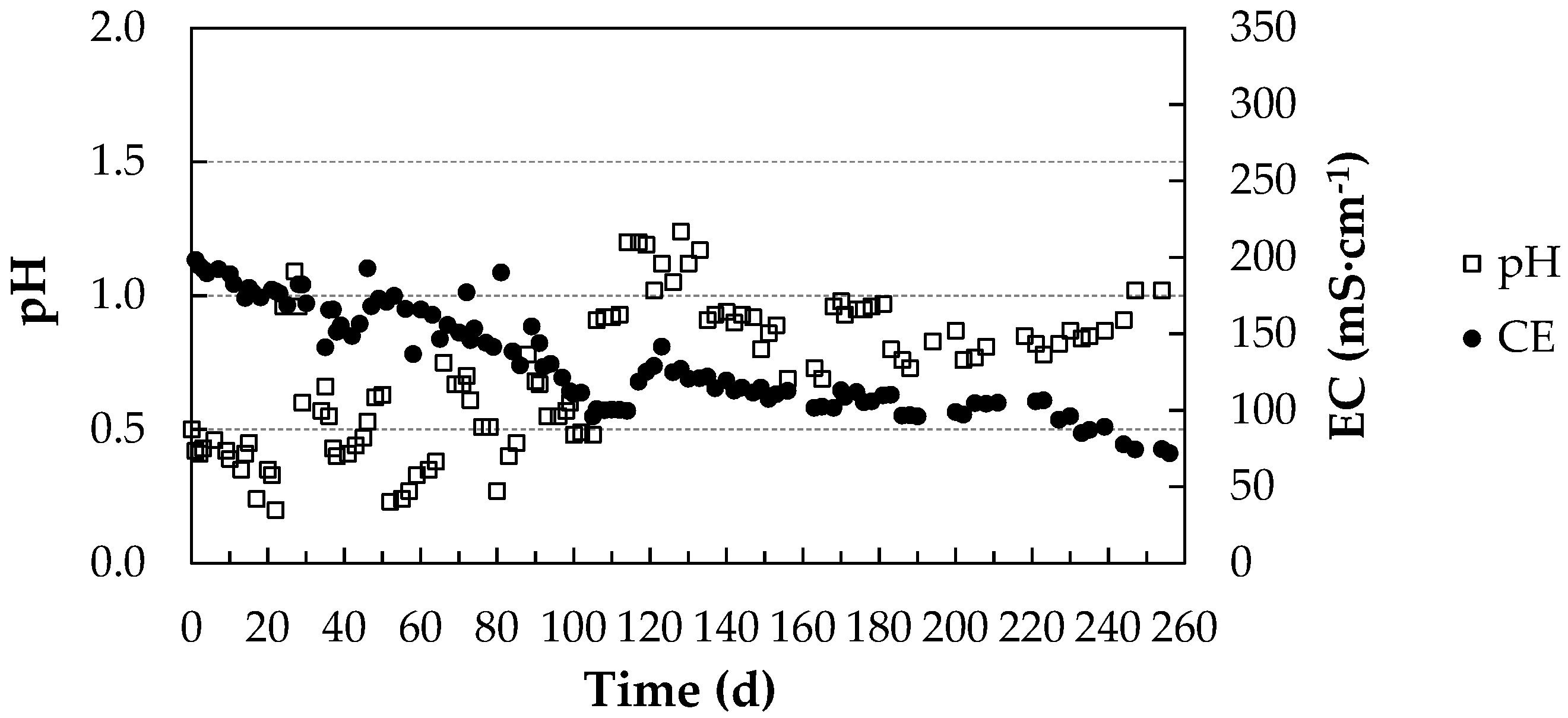
pH and Electrical Conductivity Values
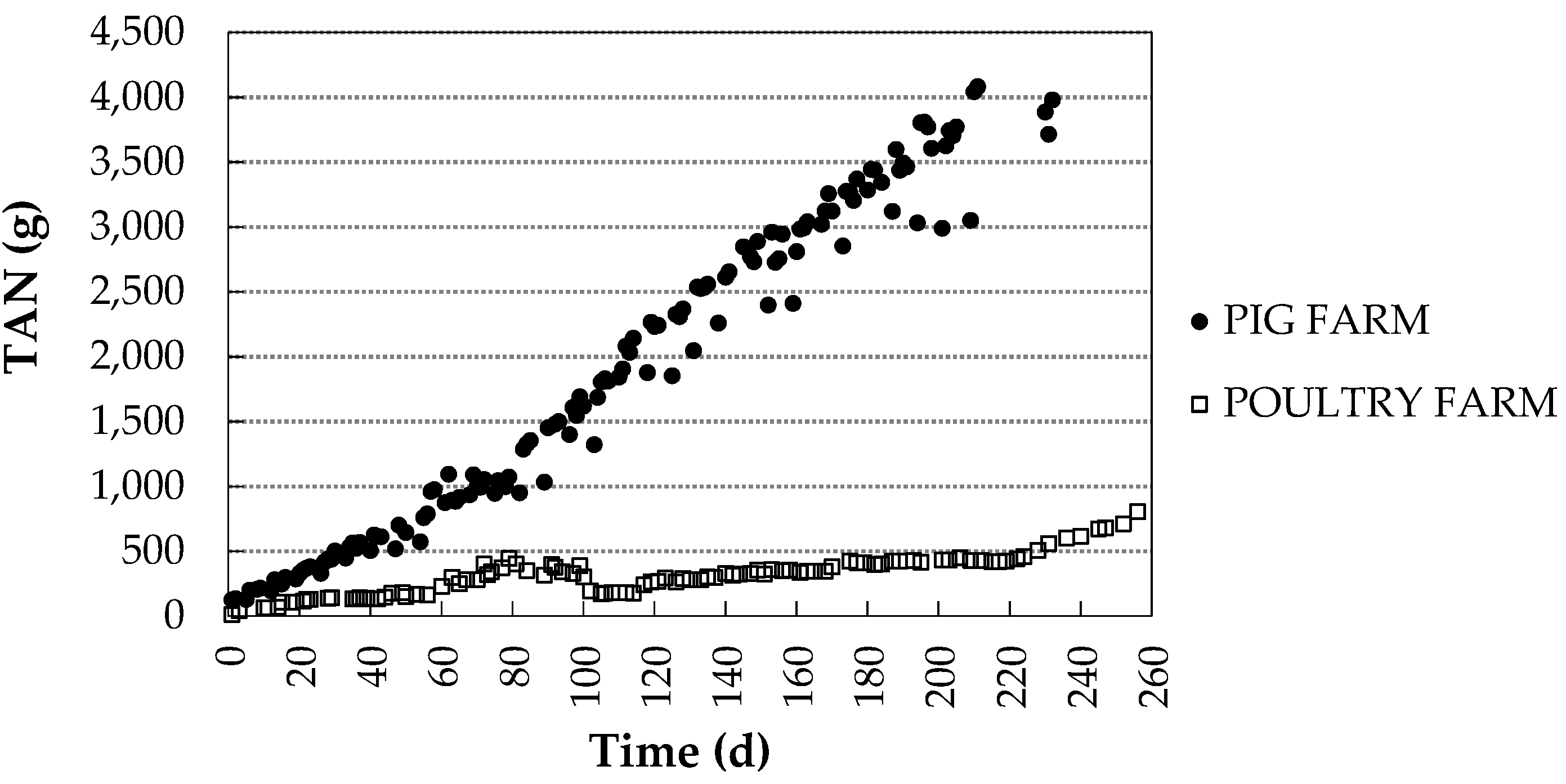
3.2. Differences in TAN Capture between Seasons
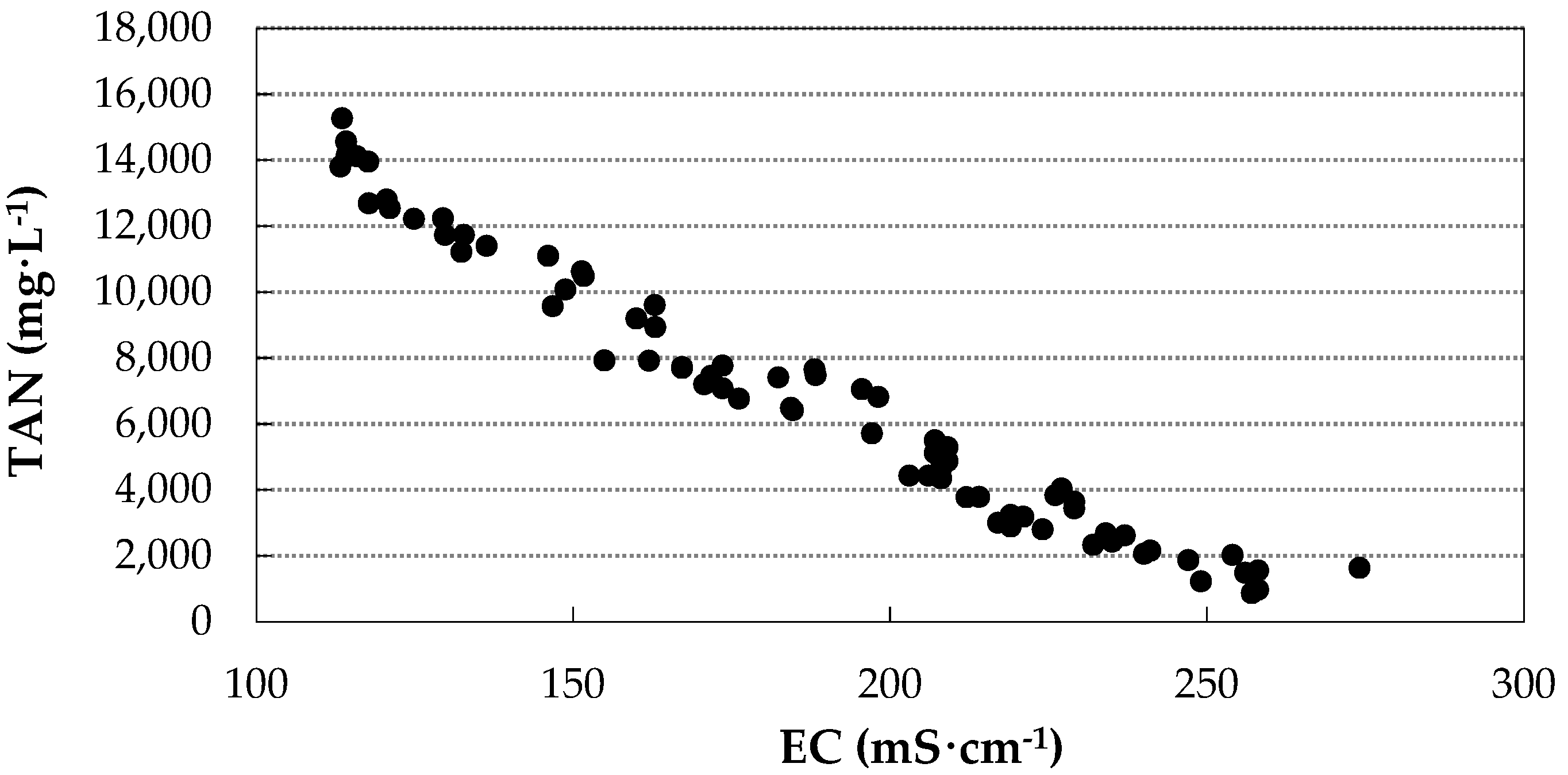
3.3. Correlation between TAN Concentration in the Acidic Solution and Electrical Conductivity
3.4. Estimation of Laboratory TAN Recovery Balances for Each Type of Waste
3.5. Analysis of TAN Recovery Results
3.5.1. Comparison with TAN Recovery Results Reported in the Literature
3.5.2. Comparison between Farm and Laboratory-Scale Results
3.6. Applicability of the Tested Ammonia Trapping System and Future Work
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giner Santonja, G.; Georgitzikis, K.; Scalet, B.M.; Montobbio, P.; Roudier, S.; Sancho, L.D. Best Avaible Techniques Reference Document for the Intensive Rearing of Poultry or Pigs; Publications Office of the European Union: Luxembourg, 2017; pp. 18–21. [Google Scholar]
- Ndegwa, P.M.; Hristov, A.N.; Arogo, J.; Sheffield, R.E. A review of ammonia emission mitigation techniques for concentrated animal feeding operations. Biosyst. Eng. 2008, 100, 453–469. [Google Scholar] [CrossRef]
- Erisman, J.W. The Nanjing declaration on management of reactive nitrogen. Bioscience 2004, 54, 286–287. [Google Scholar] [CrossRef][Green Version]
- Sutton, M.A.; Erisman, J.W.; Dentener, F.; Möller, D. Ammonia in the environment: From ancient times to the present. Environ. Pollut. 2008, 156, 583–604. [Google Scholar] [CrossRef]
- Aneja, V.P.; Roelle, P.A.; Murray, G.C.; Southerland, J.; Erisman, J.W.; Fowler, D.; Asman, W.A.H.; Patni, N. Atmospheric nitrogen compounds. II: Emissions, transport, transformation, deposition and assessment. Atmos. Environ. 2001, 35, 1903–1911. [Google Scholar] [CrossRef]
- Schiffman, S.S. Livestock Odors: Implications for Human Health and Well-Being. J. Anim. Sci. 1998, 76, 1343–1355. [Google Scholar] [CrossRef]
- Wing, S.; Horton, R.A.; Marshall, S.W.; Thu, K.; Tajik, M.; Schinasi, L.; Schiffman, S.S. Air pollution and odor in communities near industrial swine operations. Environ. Health Perspect. 2008, 116, 1362–1368. [Google Scholar] [CrossRef]
- Donham, K.J.; Wing, S.; Osterberg, D.; Flora, J.L.; Hodne, C.; Thu, K.M.; Thorne, P.S. Community health and socioeconomic issues surrounding concentrated animal feeding operations. Environ. Health Perspect. 2007, 115, 317–320. [Google Scholar] [CrossRef]
- Leip, A.; Billen, G.; Garnier, J.; Grizzetti, B.; Lassaletta, L.; Reis, S.; Simpson, D.; Sutton, M.A.; De Vries, W.; Weiss, F.; et al. Impacts of European livestock production: Nitrogen, sulphur, phosphorus and greenhouse gas emissions, land-use, water eutrophication and biodiversity. Environ. Res. Lett. 2015, 10. [Google Scholar] [CrossRef]
- European Union. Decisión de Ejecución (UE) 2017/302 de la Comisión, de 15 de febrero de 2017, por la que se establecen las conclusiones sobre las mejores técnicas disponibles (MTD) en el marco de la Directiva 2010/75/UE del Parlamento Europeo y del Consejo respecto a la cría intensiva de aves de corral o de cerdos. Off. J. Eur. Union L 2017, 43, 231–279. [Google Scholar]
- Loyon, L.; Burton, C.H.; Misselbrook, T.; Webb, J.; Philippe, F.X.; Aguilar, M.; Doreau, M.; Hassouna, M.; Veldkamp, T.; Dourmad, J.Y.; et al. Best available technology for European livestock farms: Availability, effectiveness and uptake. J. Environ. Manag. 2016, 166, 1–11. [Google Scholar] [CrossRef] [PubMed]
- EC—European Commission. Directive (EU) 2016/2284 of the European Parlament and of the Council of 14 December 2016 on the reduction of national emissions of certain atmospheric polluTANts, amending Directive 2003/35/EC and repealing Directive 2001/81/EC. Off. J. Eur. Commun. 2016, 344, 1–31. [Google Scholar]
- Yang, S.; Yang, F. Nitrogen removal via short-cut simultaneous nitrification and denitrification in an intermittently aerated moving bed membrane bioreactor. J. Hazard. Mater. 2011, 195, 318–323. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, P.; Long, T.; Verstraete, W. The integration of methanogensis with simultaneous nitrification and denitrification in a membrane bioreactor. Process Biochem. 2005, 40, 541–547. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, P.; Xiao, J.; Xiao, D.; Gao, F. Repeatedly using the decomposition product of struvite by ultrasound stripping to remove ammonia nitrogen from landfill leachate. Ultrason. Sonochem. 2017, 38, 622–628. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Yang, M.; Du, W.; Harada, H. Repeated use of MAP decomposition residues for the removal of high ammonium concentration from landfill leachate. Chemosphere 2007, 66, 2233–2238. [Google Scholar] [CrossRef]
- Zangeneh, A.; Sabzalipour, S.; Takdatsan, A.; Yengejeh, R.J.; Khafaie, M.A. Ammonia removal form municipal wastewater by air stripping process: An experimental study. South African J. Chem. Eng. 2021, 36, 134–141. [Google Scholar] [CrossRef]
- Chen, T.L.; Chen, L.H.; Lin, Y.J.; Yu, C.P.; Ma, H.-w.; Chiang, P.C. Advanced ammonia nitrogen removal and recovery technology using electrokinetic and stripping process towards a sustainable nitrogen cycle: A review. J. Clean. Prod. 2021, 309, 127369. [Google Scholar] [CrossRef]
- Sürmeli, R.Ö.; Bayrakdar, A.; Çalli, B. Ammonia recovery from chicken manure digestate using polydimethylsiloxane membrane contactor. J. Clean. Prod. 2018, 191, 99–104. [Google Scholar] [CrossRef]
- Dube, P.; Vanotti, M.; Szogi, A.; González, M.C.G. Enhancing recovery of ammonia from swine manure anaerobic digester effluent using gas-permeable membrane technology. Waste Manag. 2016, 49, 372–377. [Google Scholar] [CrossRef]
- Daguerre-Martini, S.; Vanotti, M.B.; Rodriguez-Pastor, M.; Rosal, A.; Moral, R. Nitrogen recovery from wastewater using gas-permeable membranes: Impact of inorganic carbon content and natural organic matter. Water Res. 2018, 137, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Garcia-González, M.C.; Vanotti, M.B. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of waste strength and pH. Waste Manag. 2015, 38, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo-Salces, B.; Riaño, B.; Vanotti, M.B.; Hernández-González, D.; García-González, M.C. Pilot-scale demonstration of membrane-based nitrogen recovery from swine manure. Membranes 2020, 10, 270. [Google Scholar] [CrossRef]
- Rothrock, M.J.; Szögi, A.A.; Vanotti, M.B. Recovery of ammonia from poultry litter using gas-permeable membranes. Trans. ASABE 2010, 53, 1267–1275. [Google Scholar] [CrossRef]
- Rothrock, M.J.; Szögi, A.A.; Vanotti, M.B. Recovery of ammonia from poultry litter using flat gas permeable membranes. Waste Manag. 2013, 33, 1531–1538. [Google Scholar] [CrossRef]
- Masse, L.; Massé, D.I.; Pellerin, Y.; Dubreuil, J. Osmotic pressure and substrate resistance during the concentration of manure nutrients by reverse osmosis membranes. J. Memb. Sci. 2010, 348, 28–33. [Google Scholar] [CrossRef]
- Milan, Z.; Sánchez, E.; Weiland, P.; De Las Pozas, C.; Borja, R.; Mayari, R.; Rovirosa, N. Ammonia removal from anaerobically treated piggery manure by ion exchange in columns packed with homoionic zeolite. Chem. Eng. J. 1997, 66, 65–71. [Google Scholar] [CrossRef]
- Bonmatí, A.; Flotats, X. Air stripping of ammonia from pig slurry: Characterisation and feasibility as a pre- or post-treatment to mesophilic anaerobic digestion. Waste Manag. 2003, 23, 261–272. [Google Scholar] [CrossRef]
- Zarebska, A.; Romero Nieto, D.; Christensen, K.V.; Fjerbæk Søtoft, L.; Norddahl, B. Ammonium fertilizers production from manure: A critical review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1469–1521. [Google Scholar] [CrossRef]
- Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Vanotti, M.B.; Martín-Ramos, P. Effect of acid flow rate, membrane surface area, and capture solution on the effectiveness of suspended gpm systems to recover ammonia. Membranes 2021, 11, 538. [Google Scholar] [CrossRef]
- Szogi, A.A.; Vanotti, M.B.; Rothrock, M.J. Gaseous Ammonia Removal System. U.S. Patent 8906,332 B2, 9 December 2014. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Blet, V.; Pons, M.-N.; Greffe, J. Separation of ammonia with a gas-permeable tubular membrane. Anal. Chim. Acta 1989, 219, 309–311. [Google Scholar] [CrossRef]
- Villalba, T. Código de Protección y Bienestar Animal. Agencia Estatal Boletín Oficial del Estado. 2021. Available online: https://boe.es/biblioteca_juridica/codigos/codigo.php?id=204&modo=2¬a=0 (accessed on 17 August 2021).
- Tabase, R.K.; Millet, S.; Brusselman, E.; Ampe, B.; De Cuyper, C.; Sonck, B.; Demeyer, P. Effect of ventilation control settings on ammonia and odour emissions from a pig rearing building. Biosyst. Eng. 2020, 192, 215–231. [Google Scholar] [CrossRef]
- Philippe, F.X.; Cabaraux, J.F.; Nicks, B. Ammonia emissions from pig houses: Influencing factors and mitigation techniques. Agric. Ecosyst. Environ. 2011, 141, 245–260. [Google Scholar] [CrossRef]
- Ni, J.Q.; Liu, S.; Diehl, C.A.; Lim, T.T.; Bogan, B.W.; Chen, L.; Chai, L.; Wang, K.; Heber, A.J. Emission factors and characteristics of ammonia, hydrogen sulfide, carbon dioxide, and particulate matter at two high-rise layer hen houses. Atmos. Environ. 2017, 154, 260–273. [Google Scholar] [CrossRef]
- Wang-Li, L.; Li, Q.F.; Wang, K.; Bogan, B.W.; Ni, J.Q.; Cortus, E.L.; Heber, A.J. The national air emissions monitoring study’s Southeast Layer Site: Part I. Site characteristics and monitoring methodology. Trans. ASABE 2013, 56, 1157–1161. [Google Scholar] [CrossRef]
- Fairchild, B.D.; Czarick, M.; Harper, L.A.; Worley, J.W.; Ritz, C.W.; Hale, B.D.; Naeher, L.P. Ammonia concentrations downstream of broiler operations. J. Appl. Poult. Res. 2009, 18, 630–639. [Google Scholar] [CrossRef]
- Guarino, M.; Claudio, F.; Navarotto, P.; Valli, L.; Mascatelli, G.; Rossetti, M.; Mazzotta, V. Ammonia, methane and nitrous oxide emissions and particulate matter concentrations in two different buildings for fattening pigs. Proc. Int. Symp. Gaseous Odour Emiss. from Anim. Prod. Facil. 2003, 140–149. [Google Scholar]
- Aarnink, A.J.A.; Wagemans, M.J.M. Volatilización de amoniaco y concentración de polvo afectado por los sistemas de ventilación de las naves de engorde de cerdos. Transacciones de la ASAE 1997, 40, 1161–1170. [Google Scholar]
- Hayes, M.D.; Xin, H.; Li, H.; Shepherd, T.A.; Zhao, Y.; Stinn, J.P. Ammonia, greenhouse gas, and particulate matter emissions of aviary layer houses in the Midwestern U.S. Trans. ASABE 2013, 56, 1921–1932. [Google Scholar] [CrossRef][Green Version]
- Moral, R.; Perez-Murcia, M.D.; Perez-Espinosa, A.; Moreno-Caselles, J.; Paredes, C. Estimation of nutrient values of pig slurries in Southeast Spain using easily determined properties. Waste Manag. 2005, 25, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Provolo, G.; Martínez-Suller, L. In situ determination of slurry nutrient content by electrical conductivity. Bioresour. Technol. 2007, 98, 3235–3242. [Google Scholar] [CrossRef]
- Buabeng, F.; Hashem, F.M.; Millner, P.; Matias, B.V.; Timmons, J.; Arthur, A. Controlling poultry house ammonia emmissions using gas permeable membrane systems. Br. J. Environ. Sci. 2018, 6, 1–11. [Google Scholar]
- Diario de la Unión Europea Diario de la Unión Europea. Diario Oficial de la Unión Europea L 2007, 182, 19–28.
- Guidance Document on Preventing and Abating Ammonia Emissions from Agricultural Sources; ECE/EB.AIR/120, United Nations, Economic and Social Council, Economis Commission for Europe, Executive Body for the Convention on Long-range Transoundary Air Polution, 100 s. 2014, pp. 1–100. Available online: https://www.unece.org/fileadmin/DAM/env/documents/2012/EB/ECE_EB.AIR_120_ENG.pdf (accessed on 17 August 2021).

| Parameters * | Pig Slurry | Poultry Manure |
|---|---|---|
| H (%) a | 84.7 ± 0.3 | 15.9 ± 0.2 |
| OM (%) b | 63.5 ± 0.7 | 75.2 ± 0.7 |
| C/N b | 8.70 | 9.70 |
| pH a | 8.17 ± 0.02 | 8.88 ± 0.00 |
| EC (mS·cm−1) a | 1154 ± 5.0 | 5.58 ± 0.16 |
| TN (mg·L−1) b | 6446 ± 202 | 10,723 ± 1710 |
| NH4-N (mg·L−1) a | 2524 ± 147 | 663 ± 69 |
| P2O5 (mg·L−1) b | 8674 ± 243 | 12,329 ± 670 |
| K (mg·kg−1) b | 51,984 ± 13,614 | 12,784 ± 463 |
| Na (mg·kg−1) b | 9567 ± 3853 | 1510 ± 57 |
| Ca (mg·kg−1) b | 9958 ± 162 | 40,115 ± 1665 |
| Mg (mg·kg−1) b | 7464 ± 165 | 4046 ± 120 |
| Cu (mg·kg−1) b | 124 ± 5 | 24 ± 1 |
| Fe (mg·kg−1) b | 962 ± 36 | 550 ± 17 |
| Mn (mg·kg−1) b | 213 ± 7 | 178 ± 8 |
| Zn (mg·kg−1) b | 369 ± 11 | 189 ± 11 |
| Cr (mg·kg−1) b | 4.42 ± 0.75 | 3.59 ± 0.21 |
| Ni (mg·kg−1) b | 8.10 ± 0.33 | 3.89 ± 0.30 |
| Pb (mg·kg−1) b | 1.60 ± 0.05 | 0.75 ± 0.32 |
| Cd (mg·kg−1) b | 0.16 ± 0.01 | 0.25 ± 0.02 |
| Hg (µg·kg−1) b | 0.24 ± 0.09 | 2.8 ± 0.05 |
| Parameters | Pig Farm | Poultry Farm | ||
|---|---|---|---|---|
| Summer | Winter | Summer | Winter | |
| Mass of TAN recovered (g) | 376 | 893 | 82.4 | 371.2 |
| Daily average TAN capture rate (g·d−1) | 38 | 185 | 8.6 | 12.7 |
| TAN capture efficiency (g TAN·m−2·d−1) | 1.62 | 3.85 | 0.33 | 1.20 |
| Type of Waste | TAN Initial Mass (g) | TAN Mass Removed (g) | TAN Mass Recovered (g) | Average Daily Capture (g·d−1) | Mass Flow (g·m−2·d−1) |
|---|---|---|---|---|---|
| Pig slurry | 1.91 | 1.78 | 1.31 | 0.022 | 1.4 |
| Poultry manure | 6.41 | 5.12 | 5.04 | 0.084 | 5.1 |
| Scale | Substrate | Type of Membrane Mounting | Ammonia Recovered (g) | Ammonia Recovery Rate (g·m−2·d−1) | Recovery Efficiency (%) | Reference |
|---|---|---|---|---|---|---|
| Pilot | Poultry litter | Suspended ePTFE | 794 | 0.41 | − | This study |
| 29.94–48.83 | 10.42–28.63 | 97.7–100 | [25] | |||
| 4.08 | − | − | [45] | |||
| Swine slurry | Suspended ePTFE | 4108 | 2.29 | − | This study | |
| Submerged ePTFE | 3111 | 19.72 | 62.03 | [23] | ||
| Laboratory | Poultry litter | Suspended ePTFE | 5.04 | 5.1 | 98.4 | This study |
| 0.240 | 1.37 | 89.9 | [24] | |||
| 0.107 | 1.25 | 88.4 | [25] | |||
| Synthetic solution (6000 mg·L−1 TAN) | 2.993 | 13.0 | 96.4 | [30] | ||
| Liquid fraction of digested chicken manure | Submerged PDMS | 0.088–0.110 | 1.22–1.48 | − | [19] | |
| Swine slurry | Suspended ePTFE | 1.31 | 1.4 | 73.3 | This study | |
| Synthetic solution (3000 mg·L−1 TAN) | 1.609 | 7.0 | 97.2 | [30] | ||
| Anaerobically digested liquid swine manure | Submerged ePTFE | 1.442–2.936 | 2.65–6.05 | 76–95 | [20] | |
| Swine slurry | 2.280 | 3.92 | 99 | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Herranz, M.; Sánchez-Báscones, M.; Antolín-Rodríguez, J.M.; Martín-Ramos, P. Pilot Plant for the Capture of Ammonia from the Atmosphere of Pig and Poultry Farms Using Gas-Permeable Membrane Technology. Membranes 2021, 11, 859. https://doi.org/10.3390/membranes11110859
Soto-Herranz M, Sánchez-Báscones M, Antolín-Rodríguez JM, Martín-Ramos P. Pilot Plant for the Capture of Ammonia from the Atmosphere of Pig and Poultry Farms Using Gas-Permeable Membrane Technology. Membranes. 2021; 11(11):859. https://doi.org/10.3390/membranes11110859
Chicago/Turabian StyleSoto-Herranz, María, Mercedes Sánchez-Báscones, Juan Manuel Antolín-Rodríguez, and Pablo Martín-Ramos. 2021. "Pilot Plant for the Capture of Ammonia from the Atmosphere of Pig and Poultry Farms Using Gas-Permeable Membrane Technology" Membranes 11, no. 11: 859. https://doi.org/10.3390/membranes11110859
APA StyleSoto-Herranz, M., Sánchez-Báscones, M., Antolín-Rodríguez, J. M., & Martín-Ramos, P. (2021). Pilot Plant for the Capture of Ammonia from the Atmosphere of Pig and Poultry Farms Using Gas-Permeable Membrane Technology. Membranes, 11(11), 859. https://doi.org/10.3390/membranes11110859