Dielectric Properties of Phosphatidylcholine Membranes and the Effect of Sugars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Giant Unilamellar Vesicles
2.2.2. Preparation of Large Unilamellar Vesicles
2.2.3. Preparation of Bilayer Lipid Membranes
2.2.4. Fluorescence Spectroscopy of Laurdan-, DPH- and Di-8-ANEPPS-Labeled LUVs
2.2.5. Electrodeformation of GUVs
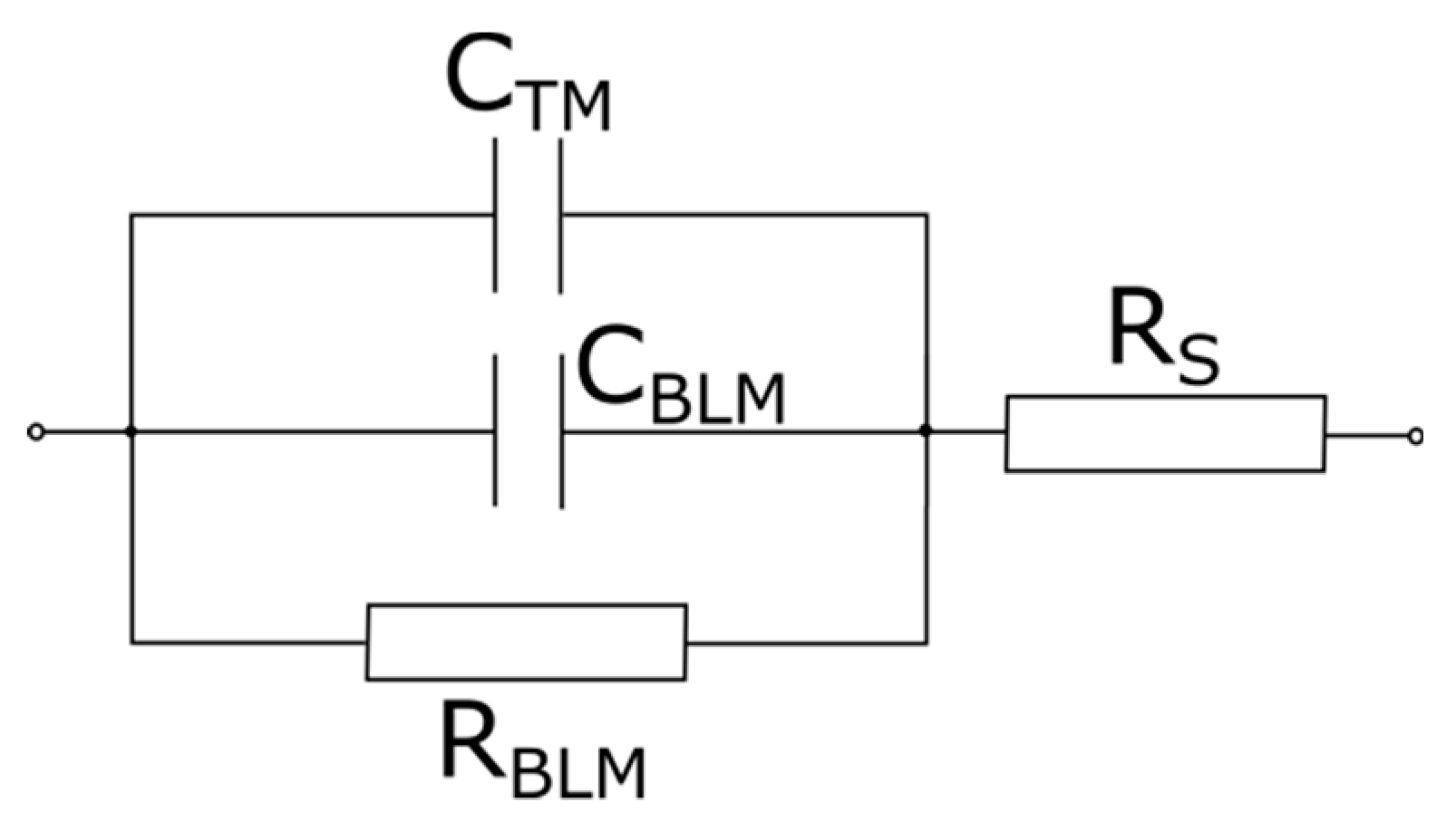
2.2.6. Fast Fourier Transform Impedance Spectroscopy of BLMs
3. Results
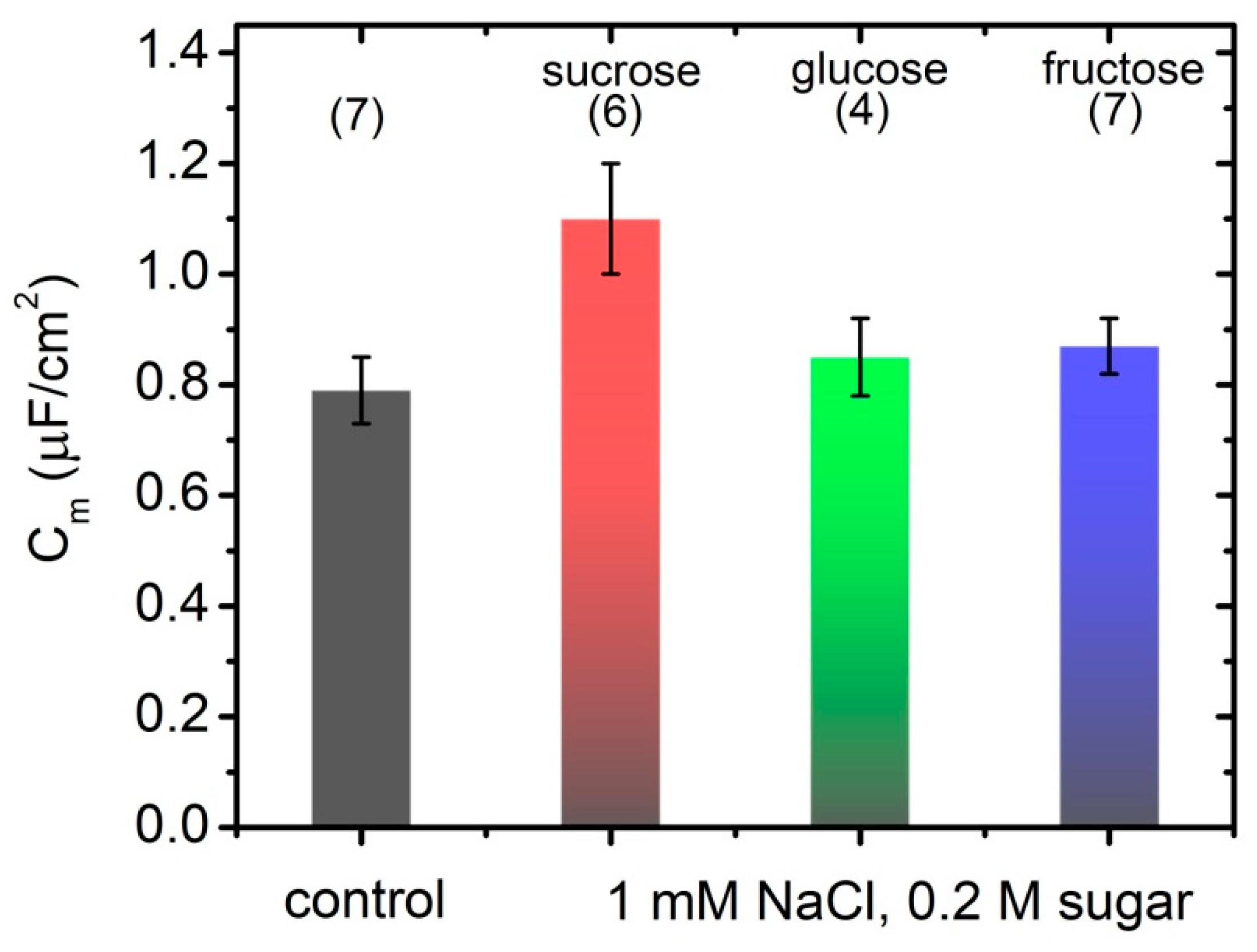
3.1. Specific Capacitance of Lipid Bilayers in the Presence of Simple Carbohydrates
3.1.1. Free-Standing Lipid Bilayers—Electrodeformation of GUVs
3.1.2. Suspended Planar Lipid Bilayers—FFT-EIS of BLMs
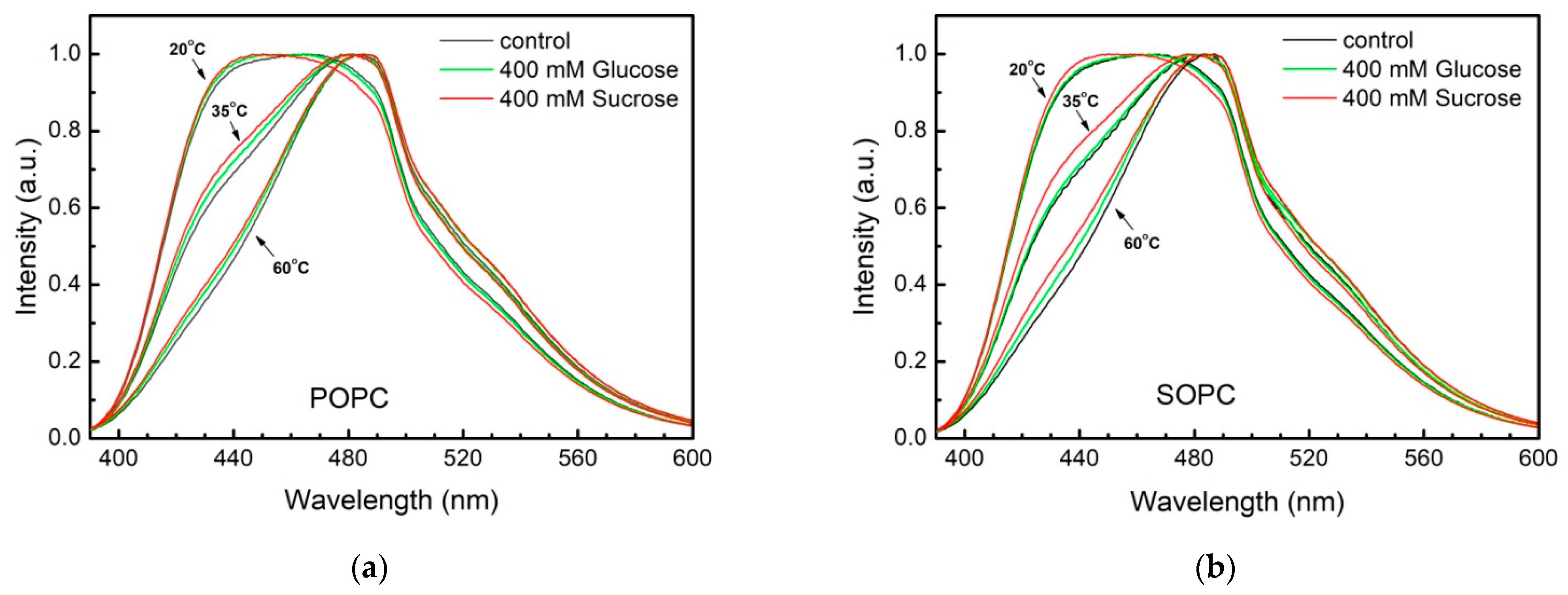
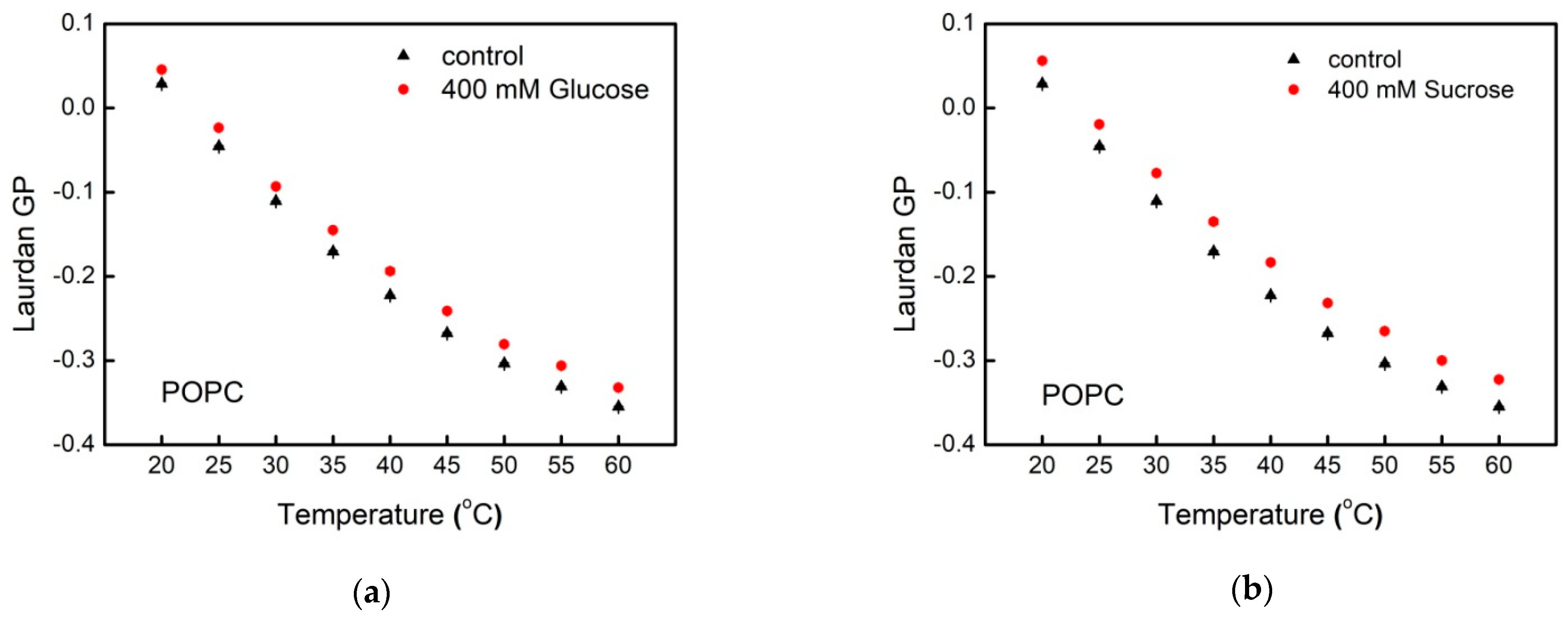
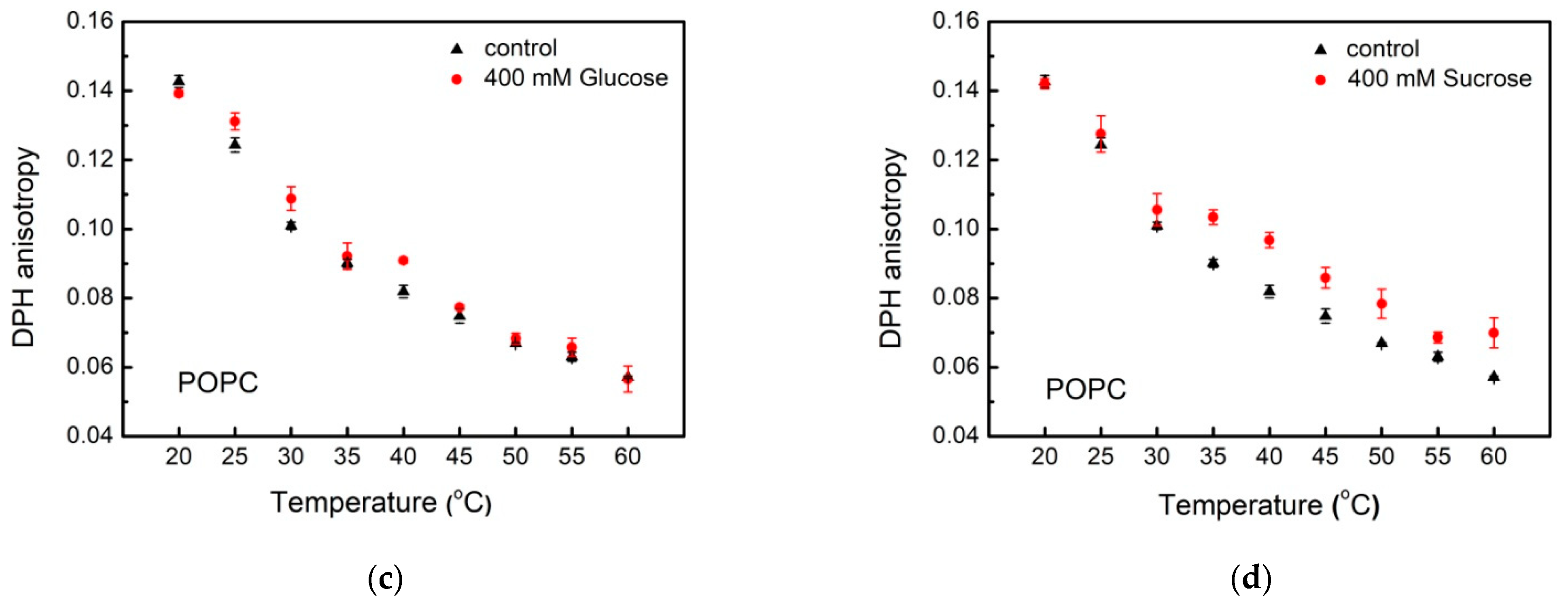
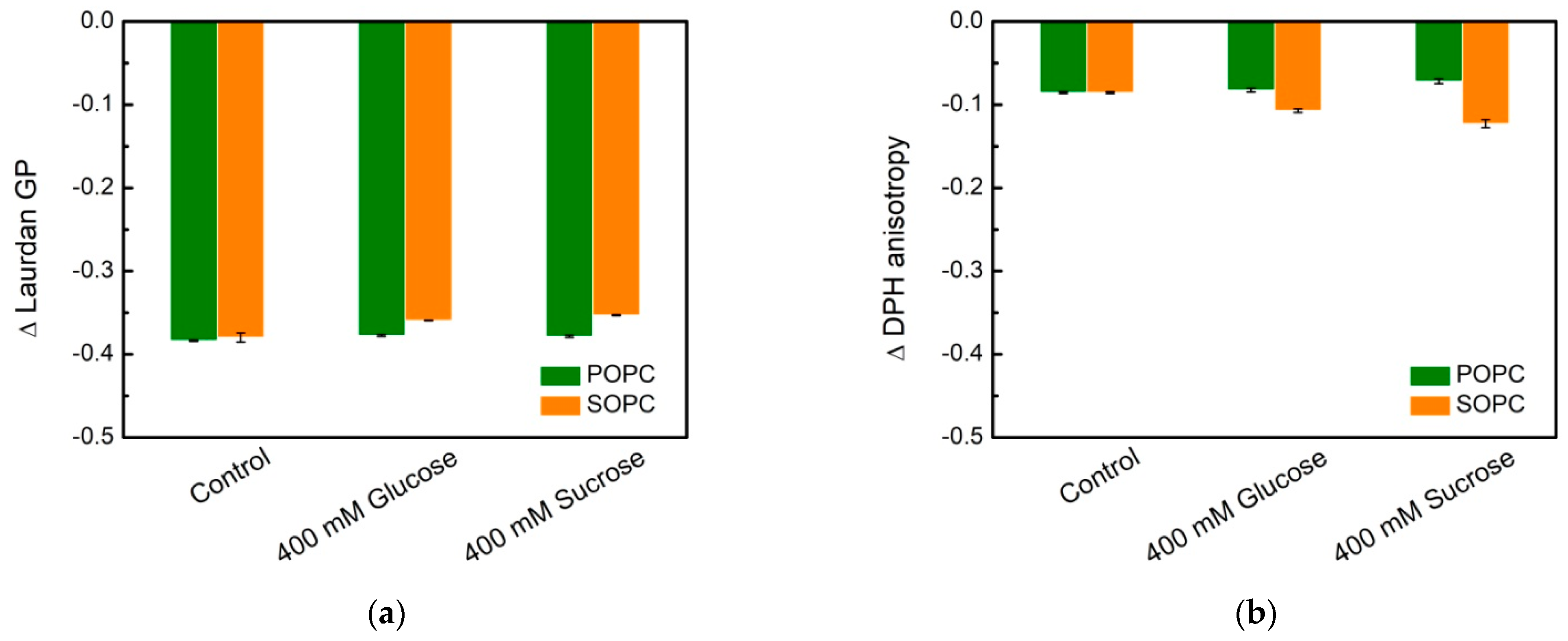
3.2. Lipid Packing in the Presence of Sugars
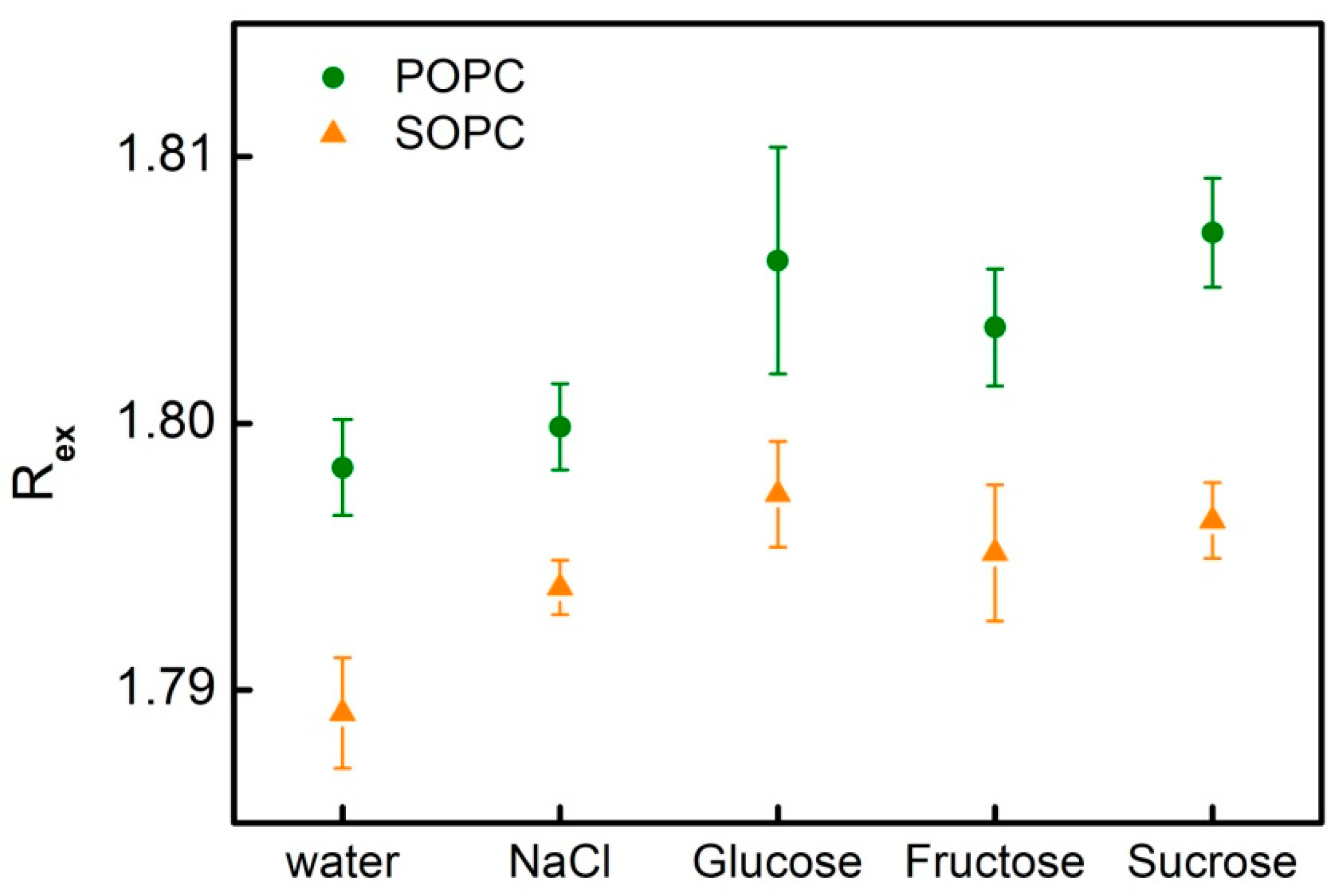
3.3. Dipole Potential in Lipid Bilayers and the Effect of Simple Carbohydrates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BLM | bilayer lipid membrane |
| Di-8-ANEPPS | 4-(2-[6-(Dioctylamino)-2-naphthalenyl]ethenyl)-1-(3-sulfopropyl) pyridinium inner salt |
| DPH | 1,6-diphenyl-1,3,5-hexatriene |
| FFT-EIS | fast Fourier transform electrochemical impedance spectroscopy |
| GP | generalized polarization |
| GUV | giant unilamellar vesicle |
| ITO | indium tin oxide |
| Laurdan | 6-dodecanoyl-N, N-dimethyl-2-naphthylamine |
| LUV | large unilamellar vesicle |
| PC | phosphatidylcholine |
| PDMS | polydimethylsiloxane |
| POPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| SOPC | 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine |
References
- Crowe, L.M. Lessons from nature: The role of sugars in anhydrobiosis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 505–513. [Google Scholar] [CrossRef]
- Panis, B.; Piette, B.; Swennen, R. Droplet vitrification of apical meristems: A cryopreservation protocol applicable to all Musaceae. Plant Sci. 2005, 168, 45–55. [Google Scholar] [CrossRef]
- O’Brien, C.; Hiti-Bandaralage, J.; Folgado, R.; Hayward, A.; Lahmeyer, S.; Folsom, J.; Mitter, N. Cryopreservation of Woody Crops: The Avocado Case. Plants 2021, 10, 934. [Google Scholar] [CrossRef]
- Rockinger, U.; Funk, M.; Winter, G. Current approaches of preservation of cells during (freeze-) drying. J. Pharm. Sci. 2021, 110, 2873–2893. [Google Scholar] [CrossRef]
- Vereyken, I.J.; Chupin, V.; Demel, R.A.; Smeekens, S.C.M.; Kruijff, B.D. Fructans insert between the headgroups of phospholipids. Biochim. Biophys. Acta Biomembr. 2001, 1510, 307–320. [Google Scholar] [CrossRef] [Green Version]
- Leekumjorn, S.; Sum, A.K. Molecular dynamics study on the stabilization of dehydrated lipid bilayers with glucose and trehalose. J. Phys. Chem. B 2008, 112, 10732–10740. [Google Scholar] [CrossRef]
- van den Bogaart, G.; Hermans, N.; Krasnikov, V.; de Vries, A.H.; Poolman, B. On the decrease in lateral mobility of phospholipids by sugars. Biophys. J. 2007, 92, 1598–1605. [Google Scholar] [CrossRef] [Green Version]
- Demel, R.A.; Dorrepaal, E.; Ebskamp, M.J.M.; Smeekens, J.C.M.; de Kruijff, B. Fructans interact strongly with model membranes. Biochim. Biophys. Acta Biomembr. 1998, 1375, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Nagle, J.F.; Jablin, M.S.; Tristram-Nagle, S. Sugar does not affect the bending and tilt moduli of simple lipid bilayers. Chem. Phys. Lipids 2016, 196, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Nagle, J.F.; Jablin, M.S.; Tristram-Nagle, S.; Akabori, K. What are the true values of the bending modulus of simple lipid bilayers? Chem. Phys. Lipids 2015, 185, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitkova, V.; Genova, J.; Mitov, M.D.; Bivas, I. Sugars in the aqueous phase change the mechanical properties of lipid mono- and bilayers. Mol. Cryst. Liq. Cryst. 2006, 449, 95–106. [Google Scholar] [CrossRef]
- Shchelokovskyy, P.; Tristram-Nagle, S.; Dimova, R. Effect of the HIV-1 fusion peptide on the mechanical properties and leaflet coupling of lipid bilayers. New J. Phys. 2011, 13, 025004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitkova, D.; Vitkova, V. The aqueous surroundings alters the bending rigidity of lipid membranes. Russ. J. Electrochem. 2016, 52, 1172–1178. [Google Scholar] [CrossRef]
- Handbook of Electroporation; Miklavcic, D. (Ed.) Springer: Cham, Switzerland, 2017; Volume 3, p. 2998. [Google Scholar]
- Schwan, H.P. Dielectrophoresis and Rotation of Cells. In Electroporation and Electrofusion in Cell Biology; Neumann, E., Sowers, A.E., Jordan, C.A., Eds.; Springer: Boston, MA, USA, 1989. [Google Scholar] [CrossRef]
- Vitkova, V.; Mitkova, D.; Antonova, K.; Popkirov, G.; Dimova, R. Sucrose solutions alter the electric capacitance and dielectric permittivity of lipid bilayers. Colloids Surf. A Physicochem. Eng. Asp. 2018, 557, 51–57. [Google Scholar] [CrossRef]
- Heimburg, T. The capacitance and electromechanical coupling of lipid membranes close to transitions: The effect of electrostriction. Biophys. J. 2012, 103, 918–929. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Levitt, D.G. Theoretical calculation of the dielectric constant of a bilayer membrane. Biophys. J. 1977, 17, 111–128. [Google Scholar] [CrossRef] [Green Version]
- Stern, H.A.; Feller, S.E. Calculation of the dielectric permittivity profile for a nonuniform system: Application to a lipid bilayer simulation. J. Chem. Phys. 2003, 118, 3401–3412. [Google Scholar] [CrossRef] [Green Version]
- Nymeyer, H.; Zhou, H.-X. A Method to Determine Dielectric Constants in Nonhomogeneous Systems: Application to Biological Membranes. Biophys. J. 2008, 94, 1185–1193. [Google Scholar] [CrossRef] [Green Version]
- Structure and Dynamics of Membranes; Lipowsky, R.; Sackmann, E. (Eds.) Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Giant Vesicles; Luisi, P.L.; Walde, P. (Eds.) John Wiley & Sons, Ltd.: Chichester, UK, 2000. [Google Scholar]
- The Giant Vesicle Book, 1st ed.; Dimova, R.; Marques, C. (Eds.) CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [Green Version]
- Vitkova, V.; Antonova, K.; Popkirov, G.; Mitov, M.D.; Ermakov, Y.A.; Bivas, I. Electrical resistivity of the liquid phase of vesicular suspensions prepared by different methods. J. Phys. Conf. Ser. 2010, 253, 012059. [Google Scholar] [CrossRef] [Green Version]
- Khalifat, N.; Fournier, J.B.; Angelova, M.I.; Puff, N. Lipid packing variations induced by pH in cardiolipin-containing bilayers: The driving force for the cristae-like shape instability. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2724–2733. [Google Scholar] [CrossRef] [PubMed]
- Gutsmann, T.; Heimburg, T.; Keyser, U.; Mahendran, K.R.; Winterhalter, M. Protein reconstitution into freestanding planar lipid membranes for electrophysiological characterization. Nat. Protoc. 2015, 10, 188–198. [Google Scholar] [CrossRef]
- Montal, M.; Mueller, P. Formation of Bimolecular Membranes from Lipid Monolayers and a Study of Their Electrical Properties. Proc. Natl. Acad. Sci. USA 1972, 69, 3561–3566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blitterswijk, M.J.V.; Hoeven, R.P.V.; DerMeer, B.W.V. Lipid structural order parameters (reciprocal of fluidity) in biomembranes derived from steady-state fluorescence polarization measurements. Biochim. Biophys. Acta Biomembr. 1981, 644, 323–332. [Google Scholar] [CrossRef]
- Parasassi, T.; Gratton, E. Membrane lipid domains and dynamics as detected by Laurdan fluorescence. J. Fluoresc. 1995, 5, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Litman, B.J.; Barenholz, Y. Fluorescent probe: Diphenylhexatriene. Methods Enzymol. 1982, 81, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Vitkova, V.; Mitkova, D.; Yordanova, V.; Pohl, P.; Bakowsky, U.; Staneva, G.; Batishchev, O. Elasticity and phase behaviour of biomimetic membrane systems containing tetraether archaeal lipids. Colloids Surf. A Physicochem. Eng. Asp. 2020, 601, 124974. [Google Scholar] [CrossRef]
- Clarke, R.J.; Kane, D.J. Optical detection of membrane dipole potential: Avoidance of fluidity and dye-induced effects. Biochim. Biophys. Acta Biomembr. 1997, 1323, 223–239. [Google Scholar] [CrossRef] [Green Version]
- Clarke, R.J. Effect of lipid structure on the dipole potential of phosphatidylcholine bilayers. Biochim. Biophys. Acta Biomembr. 1997, 1327, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Starke-Peterkovic, T.; Clarke, R.J. Effect of headgroup on the dipole potential of phospholipid vesicles. Eur. Biophys. J. 2009, 39, 103. [Google Scholar] [CrossRef]
- Parisio, G.; Marini, A.; Biancardi, A.; Ferrarini, A.; Mennucci, B. Polarity-Sensitive Fluorescent Probes in Lipid Bilayers: Bridging Spectroscopic Behavior and Microenvironment Properties. J. Phys. Chem B 2011, 115, 9980–9989. [Google Scholar] [CrossRef]
- Vlahovska, P.M.; Gracia, R.S.; Aranda-Espinoza, S.; Dimova, R. Electrohydrodynamic model of vesicle deformation in alternating electric fields. Biophys. J. 2009, 96, 4789–4803. [Google Scholar] [CrossRef] [Green Version]
- Salipante, P.F.; Knorr, R.L.; Dimova, R.; Vlahovska, P.M. Electrodeformation method for measuring the capacitance of bilayer membranes. Soft Matter 2012, 8, 3810–3816. [Google Scholar] [CrossRef]
- Dimova, R.; Bezlyepkina, N.; Jordö, M.D.; Knorr, R.L.; Riske, K.A.; Staykova, M.; Vlahovska, P.M.; Yamamoto, T.; Yang, P.; Lipowsky, R. Vesicles in electric fields: Some novel aspects of membrane behavior. Soft Matter 2009, 5, 3201–3212. [Google Scholar] [CrossRef]
- Bockris, J.O.M.; Khan, S.U.M. The Interphasial Structure. In Surface Electrochemistry: A Molecular Level Approach; Springer US, Plenum Press: New York, NY, USA, 1993; pp. 59–210. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: New York, NY, USA, 1985. [Google Scholar]
- Gongadze, E.; Velikonja, A.; Perutkova, Š.; Kramar, P.; Maček-Lebar, A.; Kralj-Iglič, V.; Iglič, A. Ions and water molecules in an electrolyte solution in contact with charged and dipolar surfaces. Electrochim. Acta 2014, 126, 42–60. [Google Scholar] [CrossRef]
- Iglič, A.; Gongadze, E.; Kralj-Iglič, V. Differential Capacitance of Electric Double Layer—Influence of Asymmetric Size of Ions, Thickness of Stern Layer and Orientational Ordering of Water Dipoles. Acta Chim. Slov. 2019, 66, 8. [Google Scholar] [CrossRef]
- Velikonja, A.; Perutkova, S.; Gongadze, E.; Kramar, P.; Polak, A.; Maček-Lebar, A.; Iglič, A. Monovalent ions and water dipoles in contact with dipolar zwitterionic lipid headgroups-theory and MD simulations. Int. J. Mol. Sci 2013, 14, 2846–2861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Needham, D.; Hochmuth, R.M. Electro-mechanical permeabilization of lipid vesicles. Role of membrane tension and compressibility. Biophys. J. 1989, 55, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Popkirov, G.S.; Schindler, R.N. Validation of experimental data in electrochemical impedance spectroscopy. Electrochim. Acta 1993, 38, 861–867. [Google Scholar] [CrossRef]
- Popkirov, G.S.; Schindler, R.N. A new approach to the problem of “good” and “bad” impedance data in electrochemical impedance spectroscopy. Electrochim. Acta 1994, 39, 2025–2030. [Google Scholar] [CrossRef]
- Velikonja, A.; Kramar, P.; Miklavčič, D.; Maček Lebar, A. Specific electrical capacitance and voltage breakdown as a function of temperature for different planar lipid bilayers. Bioelectrochemistry 2016, 112, 132–137. [Google Scholar] [CrossRef]
- Naumowicz, M.; Zając, M.; Kusaczuk, M.; Gál, M.; Kotyńska, J. Electrophoretic Light Scattering and Electrochemical Impedance Spectroscopy Studies of Lipid Bilayers Modified by Cinnamic Acid and Its Hydroxyl Derivatives. Membranes 2020, 10, 343. [Google Scholar] [CrossRef]
- Batishchev, O.V.; Indenbom, A.V. Alkylated glass partition allows formation of solvent-free lipid bilayer by Montal-Mueller technique. Bioelectrochemistry 2008, 74, 22–25. [Google Scholar] [CrossRef]
- Luzardo, M.d.C.; Amalfa, F.; Nunez, A.M.; Diaz, S.; Biondi de Lopez, A.C.; Disalvo, E.A. Effect of Trehalose and Sucrose on the Hydration and Dipole Potential of Lipid Bilayers. Biophys. J. 2000, 78, 2452–2458. [Google Scholar] [CrossRef] [Green Version]
- Starke-Peterkovic, T.; Turner, N.; Else, P.L.; Clarke, R.J. Electric field strength of membrane lipids from vertebrate species: Membrane lipid composition and Na+-K+-ATPase molecular activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R663–R670. [Google Scholar] [CrossRef] [Green Version]
- Wang, L. Measurements and Implications of the Membrane Dipole Potential. Annu. Rev. Biochem. 2012, 81, 615–635. [Google Scholar] [CrossRef]
- Benz, R.; Janko, K. Voltage-induced capacitance relaxation of lipid bilayer membranes Effects of membrane composition. Biochim. Biophys. Acta 1976, 455, 721–738. [Google Scholar] [CrossRef]
- Alvarez, O.; Latorre, R. Voltage-dependent capacitance in lipid bilayers made from monolayers. Biophys. J. 1978, 21, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Schuster, B.; Pum, D.; Braha, O.; Bayley, H.; Sleytr, U. Self-assembled α-hemolysin pores in an S-layer-supported lipid bilayer. Biochim. Biophys. Acta 1998, 1370, 280–288. [Google Scholar] [CrossRef] [Green Version]
- Gross, L.; Heron, A.; Baca, S.; Wallace, M. Determining membrane capacitance by dynamic control of droplet interface bilayer area. Langmuir 2011, 27, 14335–14342. [Google Scholar] [CrossRef] [PubMed]
- Garten, M.; Mosgaard, L.D.; Bornschlögl, T.; Dieudonné, S.; Bassereau, P.; Toombes, G.E.S. Whole-GUV patch-clamping. Proc. Natl. Acad Sci. USA 2017, 114, 328–333. [Google Scholar] [CrossRef] [Green Version]
- Mitov, M.D.; Faucon, J.F.; Méléard, P.; Bothorel, P. Thermal fluctuations of membranes. In Advances in Supramolecular Chemistry; Gokel, G.W., Ed.; JAI Press Inc.: Stamford, CT, USA, 1992; Volume 2, pp. 93–139. [Google Scholar]
- Genova, J.; Vitkova, V.; Bivas, I. Registration and analysis of the shape fluctuations of nearly spherical lipid vesicles. Phys. Rev. E 2013, 88, 022707. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.J.; Venkatesan, G.A.; Collier, C.P.; Sarles, S.A. Direct in situ measurement of specific capacitance, monolayer tension, and bilayer tension in a droplet interface bilayer. Soft Matter 2015, 11, 7592–7605. [Google Scholar] [CrossRef]
- Beltramo, P.J.; Hooghten, R.V.; Vermant, J. Millimeter-area, free standing, phospholipid bilayers. Soft Matter 2016, 12, 4324–4331. [Google Scholar] [CrossRef]
- Gracia, R.S.; Bezlyepkina, N.; Knorr, R.L.; Lipowsky, R.; Dimova, R. Effect of cholesterol on the rigidity of saturated and unsaturated membranes: Fluctuation and electrodeformation analysis of giant vesicles. Soft Matter 2010, 6, 1472–1482. [Google Scholar] [CrossRef]
- Andersen, H.D.; Wang, C.; Arleth, L.; Peters, G.H.; Westh, P. Reconciliation of opposing views on membrane–sugar interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1874–1878. [Google Scholar] [CrossRef] [Green Version]
- Kučerka, N.; Nieh, M.-P.; Katsaras, J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2761–2771. [Google Scholar] [CrossRef]
- Matsuki, H.; Goto, M.; Tada, K.; Tamai, N. Thermotropic and barotropic phase behavior of phosphatidylcholine bilayers. Int. J. Mol. Sci 2013, 14, 2282–2302. [Google Scholar] [CrossRef] [Green Version]
- Jurkiewicz, P.; Cwiklik, L.; Jungwirth, P.; Hof, M. Lipid hydration and mobility: An interplay between fluorescence solvent relaxation experiments and molecular dynamics simulations. Biochimie 2012, 94, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Poojari, C.; Wilkosz, N.; Lira, R.B.; Dimova, R.; Jurkiewicz, P.; Petka, R.; Kepczynski, M.; Róg, T. Behavior of the DPH fluorescence probe in membranes perturbed by drugs. Chem. Phys. Lipids 2019, 223, 104784. [Google Scholar] [CrossRef]
- Jurkiewicz, P.; Olżyńska, A.; Langner, M.; Hof, M. Headgroup Hydration and Mobility of DOTAP/DOPC Bilayers: A Fluorescence Solvent Relaxation Study. Langmuir 2006, 22, 8741–8749. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.D.; Washabaugh, M.W. The Hofmeister effect and the behaviour of water at interfaces. Q. Rev. Biophys. 1985, 18, 323–422. [Google Scholar] [CrossRef]
- Brockman, H. Dipole potential of lipid membranes. Chem. Phys. Lipids 1994, 73, 57–79. [Google Scholar] [CrossRef]
- Cevc, G. Hydration force and the interfacial structure of the polar surface. J. Chem. Soc. Faraday Trans. 1991, 87, 2733–2739. [Google Scholar] [CrossRef]
- White, G.; Pencer, J.; Nickel, B.G.; Wood, J.M.; Hallett, F.R. Optical changes in unilamellar vesicles experiencing osmotic stress. Biophys. J. 1996, 71, 2701–2715. [Google Scholar] [CrossRef] [Green Version]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta Biomembr. 2000, 1469, 159–195. [Google Scholar] [CrossRef] [Green Version]
- Tristram-Nagle, S.; Petrache, H.I.; Nagle, J.F. Structure and Interactions of Fully Hydrated Dioleoylphosphatidylcholine Bilayers. Biophys. J. 1998, 75, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Nagle, J.F. Diffuse scattering provides material parameters and electron density profiles of biomembranes. Phys. Rev. E 2004, 69, 040901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucerka, N.; Tristram-Nagle, S.; Nagle, J.F. Structure of Fully Hydrated Fluid Phase Lipid Bilayers with Monounsaturated Chains. J. Membrane Biol. 2005, 208, 193–202. [Google Scholar] [CrossRef]
- Raudino, A.; Mauzerall, D. Dielectric properties of the polar head group region of zwitterionic lipid bilayers. Biophys. J. 1986, 50, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Vitkova, V.; Minetti, C.; Stoyanova-Ivanova, A. Bending rigidity of lipid bilayers in electrolyte solutions of sucrose. Bulg. Chem. Commun. 2020, 52, 35–40. [Google Scholar]
- Valley, C.C.; Perlmutter, J.D.; Braun, A.R.; Sachs, J.N. NaCl interactions with phosphatidylcholine bilayers do not alter membrane structure but induce long-range ordering of ions and water. J. Memb. Biol. 2011, 244, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Bakarić, D.; Petrov, D.; Mouvencherya, Y.K.; Heiβler, S.; Oostenbrink, C.; Schaumann, G.E. Ion-induced modification of the sucrose network and its impact on melting of freeze-dried liposomes. DSC and molecular dynamics study. Chem. Phys. Lipids 2018, 210, 38–46. [Google Scholar] [CrossRef] [PubMed]




| Sugar, mmol/L | λin, µS/cm | Λ | (Number of Vesicles) | GF | |
|---|---|---|---|---|---|
| Control | |||||
| 0 | 258 | 0.87 | 0.44 ± 0.03 (7) | 0.51 ± 0.04 | 0.75 |
| Sucrose | |||||
| 50 | 276 | 0.95 | 0.45 ± 0.02 (9) | 0.53 ± 0.03 | 0.73 |
| 100 | 238 | 0.93 | 0.49 ± 0.04 (11) | 0.59 ± 0.05 | 0.44 |
| 200 | 363 | 0.95 | 0.53 ± 0.03 (10) | 0.65 ± 0.04 | 0.82 |
| 300 | 347 | 0.94 | 0.54 ± 0.03 (8) | 0.66 ± 0.04 | 0.84 |
| Glucose | |||||
| 50 | 318 | 0.94 | 0.45 ± 0.04 (8) | 0.53 ± 0.05 | 0.93 |
| 100 | 257 | 0.88 | 0.45 ± 0.05 (6) | 0.53 ± 0.06 | 0.66 |
| 200 | 192 | 0.88 | 0.45 ± 0.05 (6) | 0.53 ± 0.07 | 0.31 |
| 300 | 223 | 0.90 | 0.44 ± 0.02 (8) | 0.52 ± 0.02 | 0.96 |
| Fructose | |||||
| 50 | 145 | 0.88 | 0.47 ± 0.04 (15) | 0.56 ± 0.04 | 0.53 |
| 100 | 312 | 0.95 | 0.43 ± 0.02 (7) | 0.50 ± 0.02 | 0.48 |
| 200 | 148 | 0.87 | 0.42 ± 0.02 (10) | 0.49 ± 0.02 | 0.80 |
| 300 | 145 | 0.87 | 0.45 ± 0.04 (10) | 0.55 ± 0.04 | 0.64 |
| Sugar | Number of Samples | GF | ||
|---|---|---|---|---|
| Control | 1.42 ± 0.05 | 0.79 ± 0.06 | 7 | 0.51 |
| Glucose | 1.62 ± 0.08 | 0.85 ± 0.07 | 4 | 0.16 |
| Fructose | 1.57 ± 0.06 | 0.87 ± 0.05 | 7 | 0.71 |
| Sucrose | 1.56 ± 0.02 | 1.10 ± 0.10 | 6 | 0.43 |
| Sample | POPC | SOPC | ||
|---|---|---|---|---|
| Rex | Rex | |||
| H2O, bidistilled | 1.798 ± 0.002 | 488 | 1.789 ± 0.002 | 486 |
| 1 mM NaCl | 1.800 ± 0.002 | 488 | 1.794 ± 0.001 | 487 |
| 200 mM Glucose, 1 mM NaCl | 1.806 ± 0.004 | 490 | 1.797 ± 0.002 | 488 |
| 200 mM Fructose, 1 mM NaCl | 1.804 ± 0.002 | 489 | 1.795 ± 0.003 | 487 |
| 200 mM Sucrose, 1 mM NaCl | 1.807 ± 0.002 | 490 | 1.796 ± 0.001 | 487 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitkova, V.; Yordanova, V.; Staneva, G.; Petkov, O.; Stoyanova-Ivanova, A.; Antonova, K.; Popkirov, G. Dielectric Properties of Phosphatidylcholine Membranes and the Effect of Sugars. Membranes 2021, 11, 847. https://doi.org/10.3390/membranes11110847
Vitkova V, Yordanova V, Staneva G, Petkov O, Stoyanova-Ivanova A, Antonova K, Popkirov G. Dielectric Properties of Phosphatidylcholine Membranes and the Effect of Sugars. Membranes. 2021; 11(11):847. https://doi.org/10.3390/membranes11110847
Chicago/Turabian StyleVitkova, Victoria, Vesela Yordanova, Galya Staneva, Ognyan Petkov, Angelina Stoyanova-Ivanova, Krassimira Antonova, and Georgi Popkirov. 2021. "Dielectric Properties of Phosphatidylcholine Membranes and the Effect of Sugars" Membranes 11, no. 11: 847. https://doi.org/10.3390/membranes11110847
APA StyleVitkova, V., Yordanova, V., Staneva, G., Petkov, O., Stoyanova-Ivanova, A., Antonova, K., & Popkirov, G. (2021). Dielectric Properties of Phosphatidylcholine Membranes and the Effect of Sugars. Membranes, 11(11), 847. https://doi.org/10.3390/membranes11110847