Fast Reduced Graphene-Based Membranes with High Desalination Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of GO Solutions
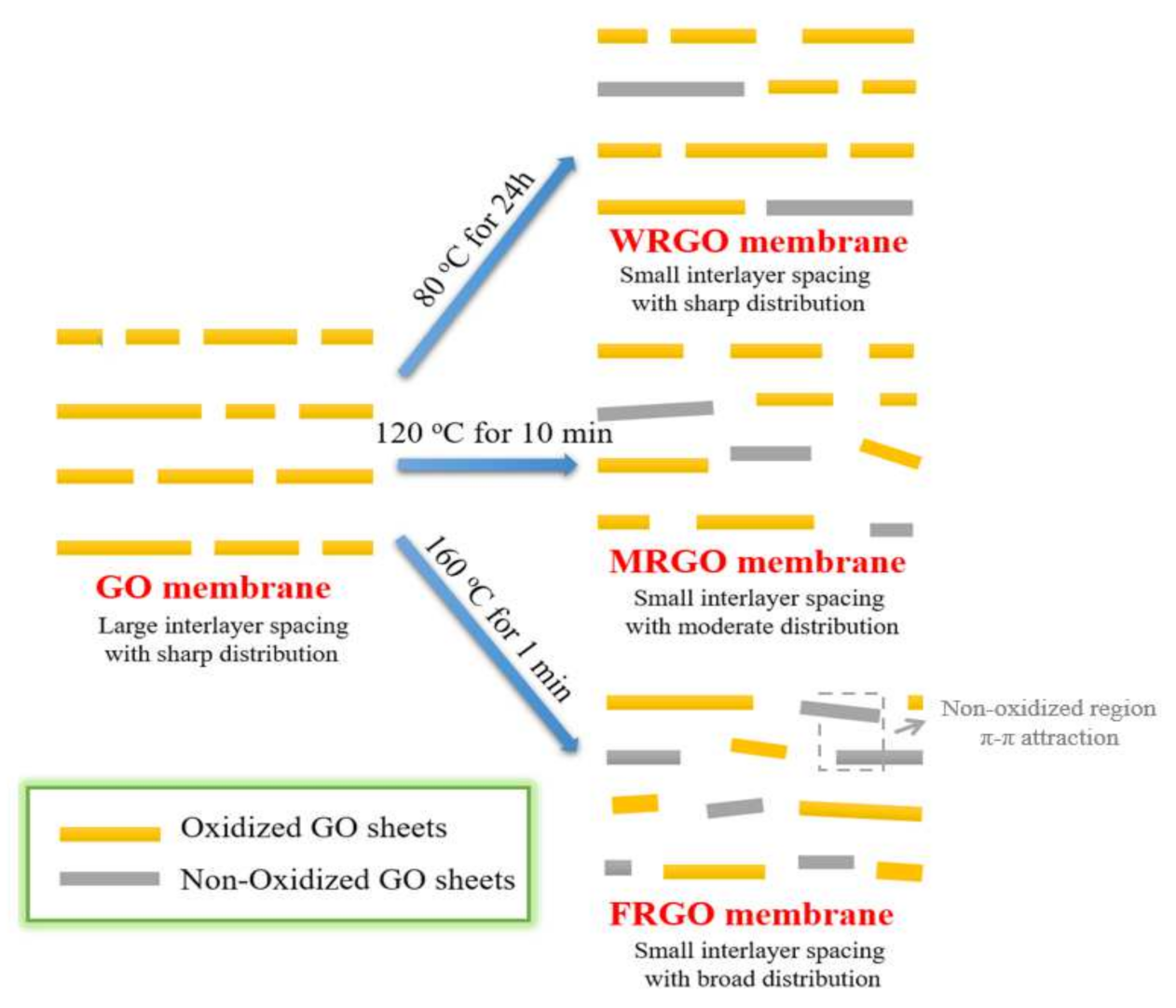
2.2. Fabrications of Different GO Membrane
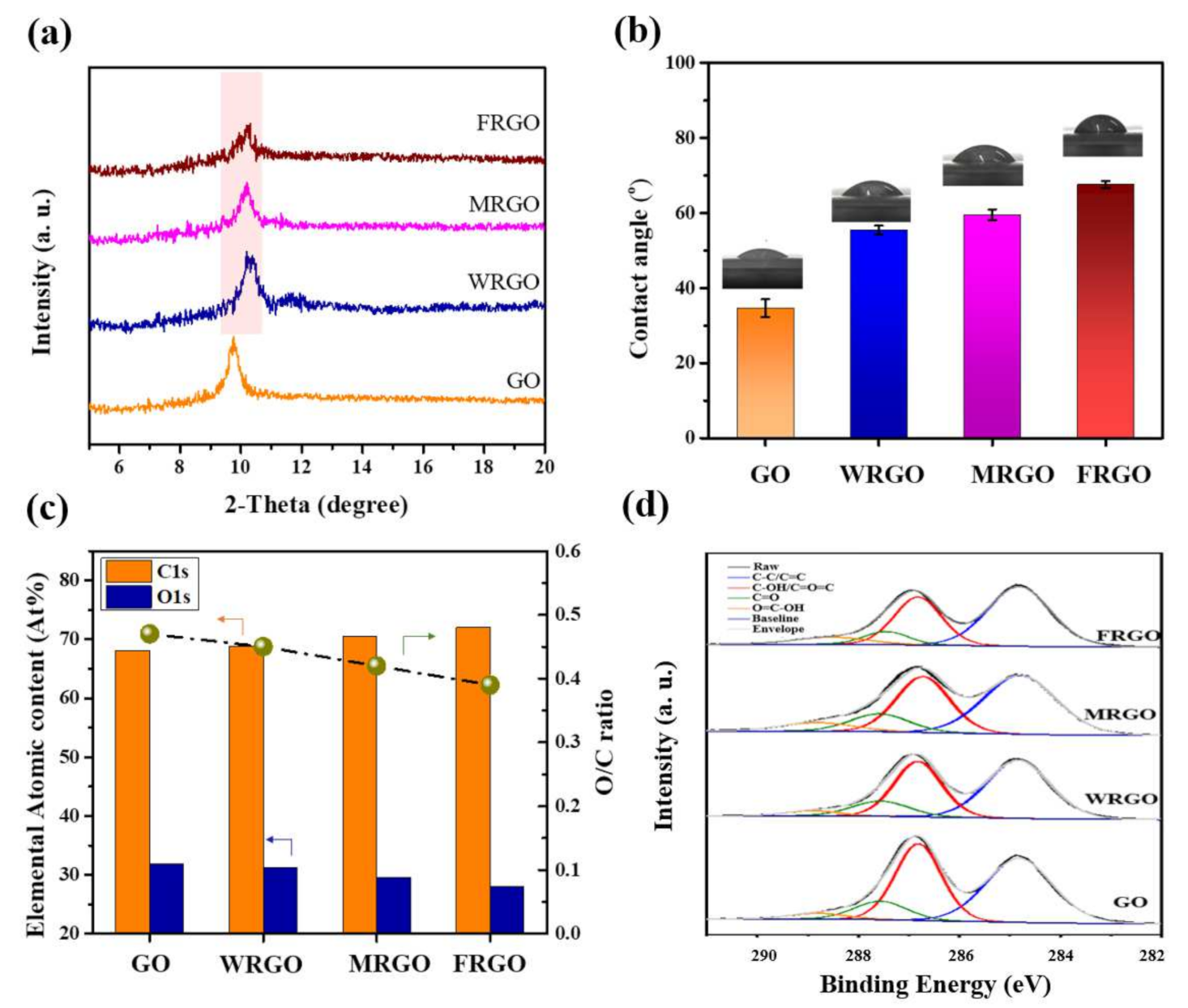
2.3. Characterization
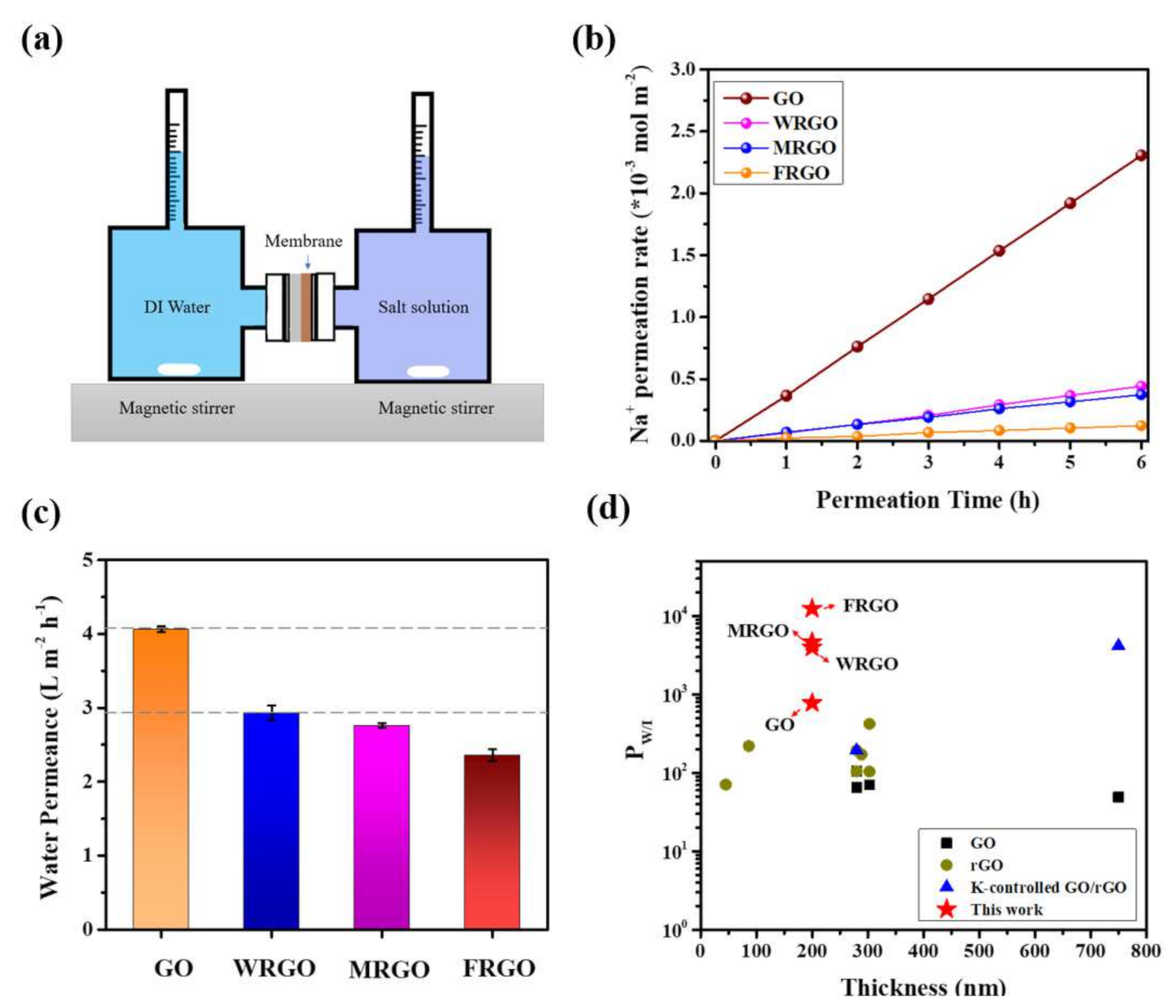
2.4. Ion Permeation Experiments
3. Result and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Werber, J.R.; Elimelech, M. Permselectivity limits of biomimetic desalination membranes. Sci. Adv. 2018, 4, eaar8266. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, B.; Liang, W.; VahidMohammadi, A.; Karnik, R.; Noy, A.; Wanunu, M. High permeability sub-nanometre sieve composite MoS2 membranes. Nat. Commun. 2020, 11, 2747. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Qian, L.; Wang, H.; Yang, J.; Chen, X.; Chang, X.; Nan, Y.; He, Z.; Hu, P.; Wu, W.; Liu, T. Amino Acid Cross-Linked Graphene Oxide Membranes for Metal Ions Permeation, Insertion and Antibacterial Properties. Membranes 2020, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded Permeation of Water Through Helium-Leak-Tight Graphene-Based Membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef]
- Mi, B. Graphene Oxide Membranes for Ionic and Molecular Sieving. Science 2014, 343, 740–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guan, K.; Ji, Y.; Liu, G.; Jin, W.; Xu, N. Controllable ion transport by surface-charged graphene oxide membrane. Nat. Commun. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mat. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, S.; Chen, L.; Fang, H. Controlling interlayer spacings of graphene oxide membranes with cationic for precise sieving of mono-/multi-valent ions. Sep. Purif. Technol. 2020, 241, 116738. [Google Scholar] [CrossRef]
- Wu, T.; Wang, Z.; Lu, Y.; Liu, S.; Li, H.; Ye, G.; Chen, J. Graphene Oxide Membranes for Tunable Ion Sieving in Acidic Radioactive Waste. Adv. Sci. 2021, 8, 2002717. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-N.; Raidongia, K.; Shao, J.; Yang, Q.-H.; Huang, J. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 2015, 7, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Tu, Q.; Urban, J.J.; Li, S.; Mi, B. Swelling of Graphene Oxide Membranes in Aqueous Solution: Characterization of Interlayer Spacing and Insight into Water Transport Mechanisms. ACS Nano 2017, 11, 6440–6450. [Google Scholar] [CrossRef]
- Kang, Y.; Xia, Y.; Wang, H.; Zhang, X. 2D Laminar Membranes for Selective Water and Ion Transport. Adv. Funct. Mater. 2019, 29, 1902014. [Google Scholar] [CrossRef]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, W.; Li, Z. Controlling Interlayer Spacing of Graphene Oxide Membranes by External Pressure Regulation. ACS Nano 2018, 12, 9309–9317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mao, Y.; Liu, G.; Liu, G.; Fan, Y.; Jin, W. Molecular Bridges Stabilize Graphene Oxide Membranes in Water. Angew. Chem. Int. Ed. 2020, 59, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wang, M.; Wang, B.; Yang, F.; Quan, X.; Tang, C.Y.; Dong, Y. Cross-linked Graphene Oxide Framework Membranes with Robust Nano-Channels for Enhanced Sieving Ability. Environ. Sci. Technol. 2020, 54, 15442–15453. [Google Scholar] [CrossRef]
- Song, J.; Yu, H.-W.; Ham, M.-H.; Kim, I.S. Tunable Ion Sieving of Graphene Membranes through the Control of Nitrogen-Bonding Configuration. Nano Lett. 2018, 18, 5506–5513. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef]
- Shi, G.; Liu, J.; Wang, C.; Song, B.; Tu, Y.; Hu, J.; Fang, H. Ion Enrichment on the Hydrophobic Carbon-based Surface in Aqueous Salt Solutions due to Cation-π Interactions. Sci. Rep. 2013, 3, 3436. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, Y.; Sheng, S.; Wen, B.; Sheng, N.; Liu, X.; Wan, R.; Yan, L.; Hou, Z.; Lei, X.; et al. Controlling the coffee ring effect on graphene and polymer by cations. Chin. Phys. Lett. 2020, 37, 028103. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, W.; Weyland, M.; Yuan, S.; Xia, Y.; Liu, H.; Jian, M.; Yang, J.; Easton, C.D.; Selomulya, C.; et al. Thermally Reduced Nanoporous Graphene Oxide Membrane for Desalination. Environ. Sci. Technol. 2019, 53, 8314–8323. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Fan, Y.; Yu, R.; Dai, F.; Lan, J.; Wang, Z.; Chen, J.; Chen, L. Robust reduced graphene oxide membranes with high water permeance enhanced by K+ modification. J. Membr. Sci. 2021, 635, 119437. [Google Scholar] [CrossRef]
- Dai, F.; Yu, R.; Yi, R.; Lan, J.; Yang, R.; Wang, Z.; Chen, J.; Chen, L. Ultrahigh water permeance of a reduced graphene oxide nanofiltration membrane for multivalent metal ion rejection. Chem. Commun. 2020, 56, 15068–15071. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Zhang, X. Facile Fabrication of Freestanding Ultrathin Reduced Graphene Oxide Membranes for Water Purification. Adv. Mater. 2015, 27, 249–254. [Google Scholar] [CrossRef]
- Yi, R.; Xia, X.; Yang, R.; Yu, R.; Dai, F.; Chen, J.; Liu, W.; Wu, M.; Xu, J.; Chen, L. Selective reduction of epoxy groups in graphene oxide membrane for ultrahigh water permeation. Carbon 2021, 172, 228–235. [Google Scholar] [CrossRef]
- Yang, E.; Ham, M.-H.; Park, H.B.; Kim, C.-M.; Song, J.-H.; Kim, I.S. Tunable semi-permeability of graphene-based membranes by adjusting reduction degree of laminar graphene oxide layer. J. Membr. Sci. 2018, 547, 73–79. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Gao, T.; Zhang, M.; Li, Y.; Dai, L.; Qu, L.; Shi, G. Reduced Graphene Oxide Membranes for Ultrafast Organic Solvent Nanofiltration. Adv. Mater. 2016, 28, 8669–8674. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Sun, P.; Chen, Q.; Li, X.; Liu, H.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Ma, R.; Sasaki, T.; et al. Highly efficient quasi-static water desalination using monolayer graphene oxide/titania hybrid laminates. NPG Asia Mater. 2015, 7, e162. [Google Scholar] [CrossRef]
- Zhang, Q.; Qian, X.; Thebo, K.H.; Cheng, H.-M.; Ren, W. Controlling reduction degree of graphene oxide membranes for improved water permeance. Sci. Bull. 2018, 63, 788–794. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.E.; Seo, S.; Woo, J.Y.; Han, C.-S. Near-complete blocking of multivalent anions in graphene oxide membranes with tunable interlayer spacing from 3.7 to 8.0 angstrom. J. Membr. Sci. 2019, 592, 117394. [Google Scholar] [CrossRef]
- Zhao, Z.; Ni, S.; Su, X.; Gao, Y.; Sun, X. Thermally Reduced Graphene Oxide Membrane with Ultrahigh Rejection of Metal Ions’ Separation from Water. ACS Sustain. Chem. Eng. 2019, 7, 14874–14882. [Google Scholar] [CrossRef]
- Huang, H.-H.; Joshi, R.K.; De Silva, K.K.H.; Badam, R.; Yoshimura, M. Fabrication of reduced graphene oxide membranes for water desalination. J. Membr. Sci. 2019, 572, 12–19. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Y.; Xia, Y.; Kang, Y.; Yang, J.; Uddin, H.; Liu, H.; Selomulya, C.; Zhang, X. Minimizing Non-selective Nanowrinkles of Reduced Graphene Oxide Laminar Membranes for Enhanced NaCl Rejection. Environ. Sci. Technol. Lett. 2020, 7, 273–279. [Google Scholar] [CrossRef]
- Jang, J.-H.; Woo, J.Y.; Lee, J.; Han, C.-S. Ambivalent Effect of Thermal Reduction in Mass Rejection through Graphene Oxide Membrane. Environ. Sci. Technol. 2016, 50, 10024–10030. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, S.; Xia, Y.; Zhao, W.; Easton, C.D.; Selomulya, C.; Zhang, X. Mild annealing reduced graphene oxide membrane for nanofiltration. J. Membr. Sci. 2020, 601, 117900. [Google Scholar] [CrossRef]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 73. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhao, G.; He, Y.; Zhu, H. The application feasibility of graphene oxide membranes for pressure-driven desalination in a dead-end flow system. Desalination 2020, 477, 114271. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Wang, K.; Zhong, M.; Wu, D.; Zhu, H. Ultrafast liquid water transport through graphene-based nanochannels measured by isotope labelling. Chem. Commun. 2015, 51, 3251–3254. [Google Scholar] [CrossRef] [PubMed][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Zhu, L.; Wang, S.; Chen, L.; Fang, H. Fast Reduced Graphene-Based Membranes with High Desalination Performance. Membranes 2021, 11, 846. https://doi.org/10.3390/membranes11110846
Liang S, Zhu L, Wang S, Chen L, Fang H. Fast Reduced Graphene-Based Membranes with High Desalination Performance. Membranes. 2021; 11(11):846. https://doi.org/10.3390/membranes11110846
Chicago/Turabian StyleLiang, Shanshan, Liuyuan Zhu, Shuai Wang, Liang Chen, and Haiping Fang. 2021. "Fast Reduced Graphene-Based Membranes with High Desalination Performance" Membranes 11, no. 11: 846. https://doi.org/10.3390/membranes11110846
APA StyleLiang, S., Zhu, L., Wang, S., Chen, L., & Fang, H. (2021). Fast Reduced Graphene-Based Membranes with High Desalination Performance. Membranes, 11(11), 846. https://doi.org/10.3390/membranes11110846




