Synthetic Poly(lactic-co-glycolic Acid) Microvesicles as a Feasible Carbon Monoxide-Releasing Platform for Cancer Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of IC-Loaded PLGA MVs
2.3. Characterization
2.4. Evaluation of CO Release from IC-Loaded PLGA MVs
2.5. Cell Culture
2.6. In Vitro Cytotoxicity Study
2.7. In Vitro Reactive Oxygen Species Evaluation
3. Results and Discussion
3.1. Synthetic Polymer Microvesicles with Cell Membrane-Mimic Shells for Drug Loading
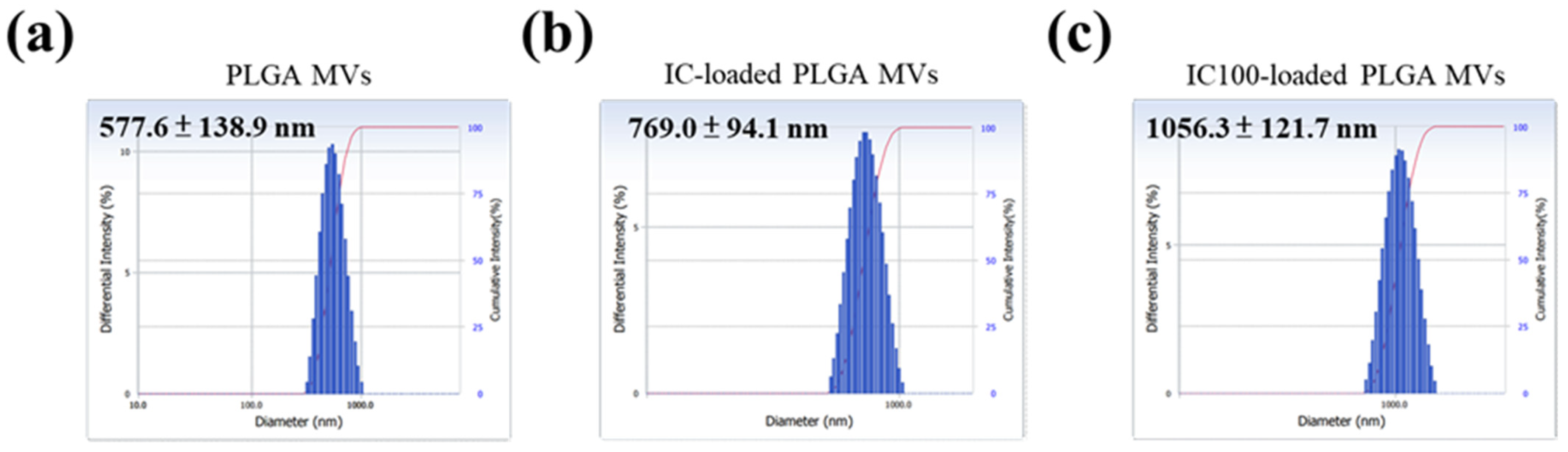
3.2. Morphology and Size Distribution of MVs with and without IC Loading
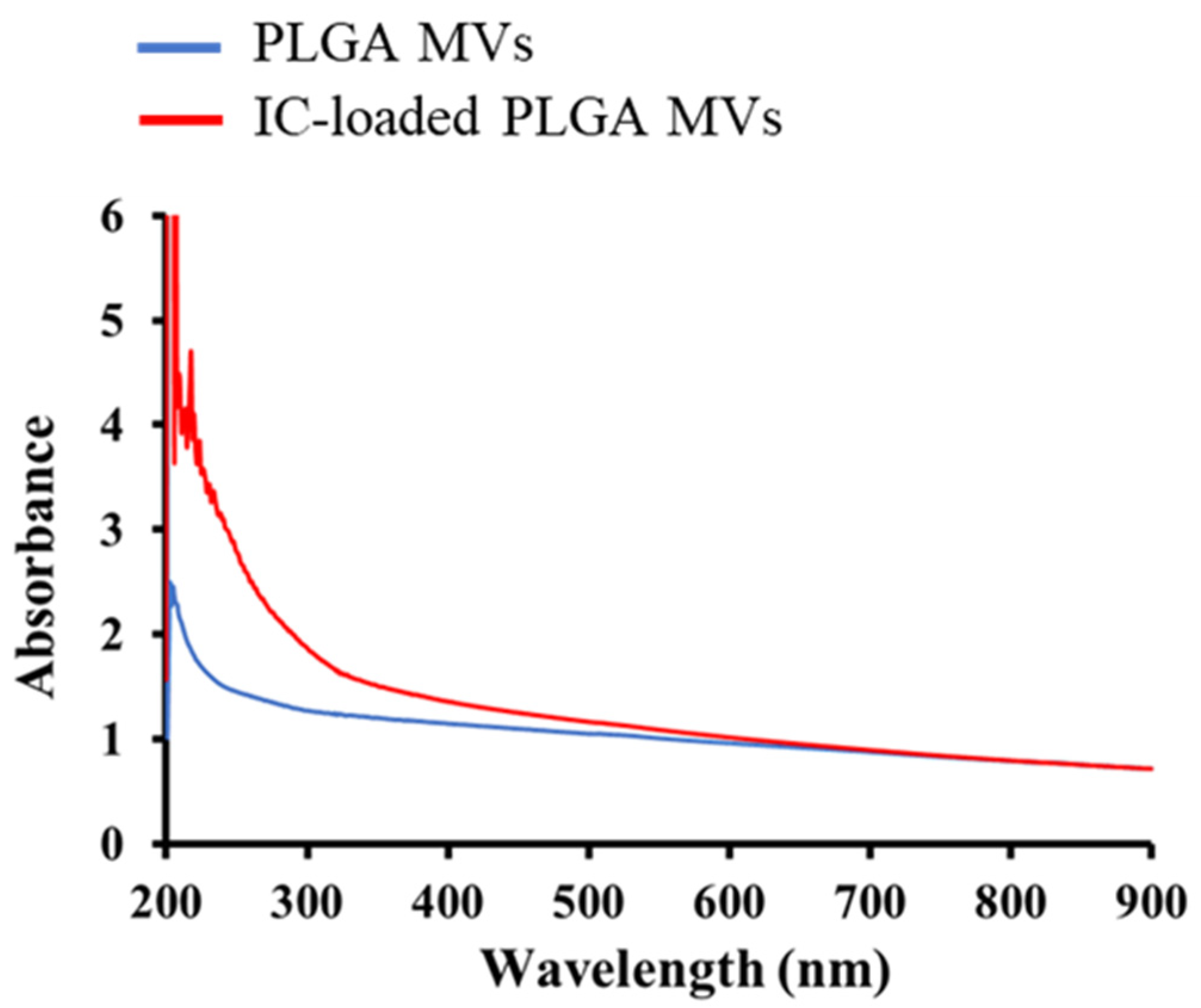
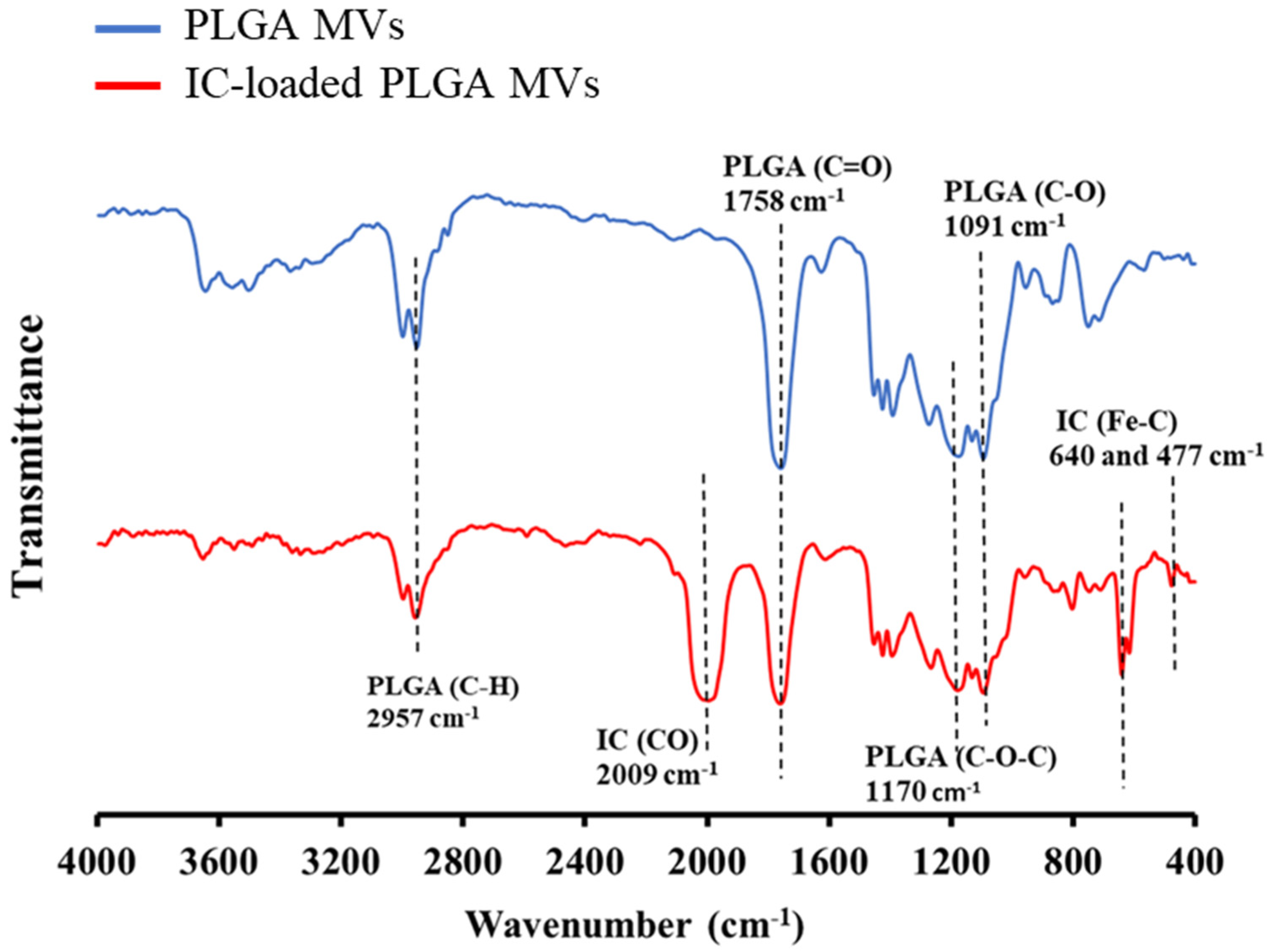
3.3. Characterization of IC Loading on the MVs
3.4. Evaluation of CO Release from IC-Loaded PLGA MVs
3.5. In Vitro Study for the Evaluation of CO-Induced Cytotoxicity by IC-Loaded PLGA MVs
3.6. The Future Development of Synthetic MVs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, E.H.; Solomon, P.S. Extracellular vesicles from the apoplastic fungal wheat pathogen Zymoseptoria tritici. Fungal Biol. Biotechnol. 2020, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Souza, W.; Barrias, E.S. Membrane-bound extracellular vesicles secreted by parasitic protozoa: Cellular structures involved in the communication between cells. Parasitol. Res. 2020, 119, 2005–2023. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Kuehn, M.J. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619. [Google Scholar] [CrossRef] [Green Version]
- Paolicelli, R.C.; Bergamini, G.; Rajendran, L. Cell-to-cell communication by extracellular vesicles: Focus on microglia. Neuroscience 2019, 405, 148–157. [Google Scholar] [CrossRef]
- Stotz, H.U.; Brotherton, D.; Inal, J. Communication is key: Extracellular vesicles as mediators of infection and defence during host-microbe interactions in animals and plants. FEMS Microbiol. Rev. 2021, 45, 1–18. [Google Scholar]
- Chronopoulos, A.; Kalluri, R. Emerging role of bacterial extracellular vesicles in cancer. Oncogene 2020, 39, 6951–6960. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular vesicles in cancer: Cell-to-cell mediators of metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Kang, M.; Jordan, V.; Blenkiron, C.; Chamley, L.W. Biodistribution of extracellular vesicles following administration into animals: A systematic review. J. Extracell. Vesicles 2021, 10, e12085. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Svennerholm, K.; Crescitelli, R.; Lässer, C.; Gribonika, I.; Lötvall, J. Synthetic bacterial vesicles combined with tumour extracellular vesicles as cancer immunotherapy. J. Extracell. Vesicles 2021, 10, e12120. [Google Scholar] [CrossRef] [PubMed]
- Thonea, M.N.; Kwon, Y.J. Extracellular blebs: Artificially-induced extracellular vesicles for facile production and clinical translation. Methods 2020, 177, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Arab, T.; Mallick, E.R.; Huang, Y.; Dong, L.; Liao, Z.; Zhao, Z.; Gololobova, O.; Smith, B.; Haughey, N.J.; Pienta, K.J.; et al. Characterization of extracellular vesicles and synthetic nanoparticles with four orthogonal single-particle analysis platforms. J. Extracell. Vesicles 2021, 10, e12079. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, C.; Chen, J.; Xiao, Y.; Du, J. Multifunctional biocompatible and biodegradable folic acid conjugated poly(ε-caprolactone)−polypeptide copolymer vesicles with excellent antibacterial activities. Bioconjugate Chem. 2015, 26, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.G.; Chandrasekar, B.; Rooney, P.; Sivaraman, G.; Larrañaga, A.; Krishna, K.V.; Pandit, A.; Rochev, Y. Biomimetic lipid-based nanosystems for enhanced dermal delivery of drugs and bioactive agents. ACS Biomater. Sci. Eng. 2017, 3, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Koog, L.; Gandek, T.B.; Nagelkerke, A. Liposomes and extracellular vesicles as drug delivery systems: A comparison of composition, pharmacokinetics, and functionalization. Adv. Healthc. Mater. 2021, 2100639. [Google Scholar] [CrossRef]
- Motterlini, R.; Otterbein, L.E. The Therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef]
- Fujita, K.; Tanaka, Y.; Sho, T.; Ozeki, S.; Abe, S.; Hikage, T.; Kuchimaru, T.; Kizaka-Kondoh, S.; Ueno, T. Intracellular CO release from composite of ferritin and ruthenium carbonyl complexes. J. Am. Chem. Soc. 2014, 136, 16902–16908. [Google Scholar] [CrossRef]
- Wegiel, B.; Gallo, D.; Csizmadia, E.; Harris, C.; Belcher, J.; Vercellotti, G.M.; Penacho, N.; Seth, P.; Sukhatme, V.; Ahmed, A.; et al. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013, 73, 7009–7021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Yuan, F.; Chen, G.; Tu, K.; Wang, H.; Wang, L.Q. Dextran-based thermo-responsive hemoglobin–polymer conjugates with oxygen-carrying capacity. RSC Adv. 2014, 4, 52940–52948. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Wang, J.; Zhao, Y.; Ji, T.; Zhao, X.; Ding, Y.; Zhao, X.; Zhao, R.; Li, F.; et al. Nanoparticle-mediated local depletion of tumour-associated platelets disrupts vascular barriers and augments drug accumulation in tumours. Nat. Biomed. Eng. 2017, 1, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Garni, M.; Thamboo, S.; Schoenenberger, C.A.; Palivan, C.G. Biopores/membrane proteins in synthetic polymer membranes. Biochim. Biophys. Acta 2017, 1859, 619–638. [Google Scholar] [CrossRef] [PubMed]
- Li, W.P.; Su, C.H.; Chang, Y.C.; Lin, Y.J.; Yeh, C.S. Ultrasound-induced reactive oxygen species mediated therapy and imaging using a Fenton reaction activable polymersome. ACS Nano 2016, 10, 2017–2027. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Liu, S.; Wang, J.; Han, X.; Qin, H.; Lang, J.; Cheng, K.; Li, Y.; Qi, Y.; et al. Inhibition of platelet function using liposomal nanoparticles blocks tumor metastasis. Theranostics 2017, 7, 1062–1071. [Google Scholar] [CrossRef]
- Douliez, J.P.; Gaillard, C. Self-assembly of fatty acids: From foams to protocell vesicles. New J. Chem. 2014, 38, 5142–5148. [Google Scholar] [CrossRef]
- Dehaini, D.; Wei, X.; Fang, R.H.; Masson, S.; Angsantikul, P.; Luk, B.T.; Zhang, Y.; Ying, M.; Jiang, Y.; Kroll, A.V.; et al. Erythrocyte–platelet hybrid membrane coating for enhanced nanoparticle functionalization. Adv. Mater. 2017, 29, 1606209. [Google Scholar] [CrossRef] [Green Version]
- Egloff-Juras, C.; Bezdetnaya, L.; Dolivet, G.; Lassalle, H.P. NIR fluorescence-guided tumor surgery: New strategies for the use of indocyanine green. Int. J. Nanomed. 2019, 14, 7828–7838. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, K.; Li, T.; Maruf, A.; Qin, X.; Luo, L.; Zhong, Y.; Qiu, J.; McGinty, S.; Pontrelli, G.; et al. Macrophage membrane functionalized biomimetic nanoparticles for targeted anti-atherosclerosis applications. Theranostics 2021, 11, 164–180. [Google Scholar] [CrossRef]
- Li, T.; Qin, X.; Li, Y.; Shen, X.; Li, S.; Yang, H.; Wu, C.; Zheng, C.; Zhu, J.; You, F.; et al. Cell membrane coated-biomimetic nanoplatforms toward cancer theranostics. Front. Bioeng. Biotechnol. 2020, 8, 371–380. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-J.; Kan, C.-D.; Chen, C.-Y.; Meng, Y.-Y.; Wang, J.-N.; Chen, W.-L.; Chen, C.-H.; Li, W.-P. Synthetic Poly(lactic-co-glycolic Acid) Microvesicles as a Feasible Carbon Monoxide-Releasing Platform for Cancer Treatment. Membranes 2021, 11, 818. https://doi.org/10.3390/membranes11110818
Wang W-J, Kan C-D, Chen C-Y, Meng Y-Y, Wang J-N, Chen W-L, Chen C-H, Li W-P. Synthetic Poly(lactic-co-glycolic Acid) Microvesicles as a Feasible Carbon Monoxide-Releasing Platform for Cancer Treatment. Membranes. 2021; 11(11):818. https://doi.org/10.3390/membranes11110818
Chicago/Turabian StyleWang, Wen-Jyun, Chung-Dann Kan, Chih-Yen Chen, Yi-Yao Meng, Jieh-Neng Wang, Wei-Ling Chen, Chia-Hsiang Chen, and Wei-Peng Li. 2021. "Synthetic Poly(lactic-co-glycolic Acid) Microvesicles as a Feasible Carbon Monoxide-Releasing Platform for Cancer Treatment" Membranes 11, no. 11: 818. https://doi.org/10.3390/membranes11110818
APA StyleWang, W.-J., Kan, C.-D., Chen, C.-Y., Meng, Y.-Y., Wang, J.-N., Chen, W.-L., Chen, C.-H., & Li, W.-P. (2021). Synthetic Poly(lactic-co-glycolic Acid) Microvesicles as a Feasible Carbon Monoxide-Releasing Platform for Cancer Treatment. Membranes, 11(11), 818. https://doi.org/10.3390/membranes11110818







