Membrane Fractionation of Protein Hydrolysates from By-Products: Recovery of Valuable Compounds from Spent Yeasts
Abstract
1. Introduction
2. Processing Strategies of Protein By-Products Using Pressure-Driven Membrane Operations
3. Membrane Fractionation of Protein Hydrolysates: Challenges, Limitations, Advantages and Solutions
4. Peptide Separation and Purification by Charge
5. Membrane Fractionation and Purification of Yeast Protein Hydrolysates: Recovery of Bioactive Peptides
5.1. Challenges Involving Yeast-Product Separation by Membranes
5.2. Strategy of Fractionation of SBY and Yeast Protein Hydrolysates
| Yeast | Fractionation/Concentration | Peptide Analytical Techniques | Purpose | References |
|---|---|---|---|---|
| Sugarcane spent yeast (S. cerevisiae) | Fractionation: UF cartridges (5 kg mol−1 MWCO) and FPLC with IMAC-Fe(III) resin chromatography. Concentration by freeze-drying. | - | Iron-chelating peptides | de la Hoz et al. [71] |
| Cultivated yeast (S. cerevisiae) | Fractionation: UF cartridges (10 and 30 kg mol−1 MWCO). Concentration by freeze-drying. | - | Peptides with anti-obesogenic and anti-stress properties | Jung et al. [64], Park et al. [65], Jung et al. [66], Kim et al. [67], Lee et al. [68], Lee et al. [69], Kim et al. [70], Kim et al. [72] |
| Cultivated yeast (S. cerevisiae) | Fractionation: UF (10, 5 and 3 kg mol−1 MWCO) and RP-HPLC (C18 column). Concentration: freeze-drying. | MS (MALDI-TOF-TOF) for peptide sequencing | Peptides with antioxidant activity | Mirzaei et al. [73] |
| Cultivated yeast (S. cerevisiae) | Fractionation: Dialysis (6–8 kg mol−1 MWCO) and RP-SPE cartridges (C18). Concentration: vacuum evaporation. | RP-HPLC (C18 column) | Glyco-peptide with anti-inflammatory activity | Williams et al. [76] |
| Cultivated yeast (S. cerevisiae K-7) | Fractionation: labscale UF (5 kg mol−1 MWCO); SEC (Sephadex G-25) and RP-HPLC (C18 column). Concentration: freeze-drying | LC-MS (peptide sequencing) | Anti-angiogenic peptides | Jeong et al. [74] |
| Yeast | Fractionation/Concentration | Peptide Analytical Techniques | Purpose | References |
|---|---|---|---|---|
| SBY | Fractionation: adsorbing column (Amberlite XAD-2 resin), SEC (Sephadex G-25), RP-HPLC (C30 column). Purification: gel filtration phase HPLC (Diol column) | LC/MS/MS (amino acid sequencing) | Peptides with ACE-I activity | Kanauchi et al. [13] |
| SBY | Fractionation: UF (10 kg mol−1 MWCO). Concentration: acid precipitation, activated carbon adsorption. | HPLC (peptide profile) | Antioxidant and anti-diabetic peptides | Jung et al. [23] |
| SBY | Fractionation: UF module of effective permeation area of 7.4 m2 (10 and 3 kg mol−1 MWCO). Concentration: Reverse osmosis and freeze-drying. | RP-HPLC (C18 column); MS (MALDI-TOF-TOF for amino acid sequencing) | Nutritional ingredient rich in protein and polysaccharides 1; peptides with antioxidant and ACE-I properties 2 | Amorim et al. [16] 1; Amorim et al. [75] 2 |
| SBY (S. pastorianus) | Fractionation: NF in amicon cell (3 kg mol−1 MWCO). Concentration: freeze-drying. | SEC (Superdex 200 and Superdex peptide 10/300GL) | Peptides with ACE-I activity | Amorim et al. [10] |
| SBY | Fractionation: MF and UF in hollow fibers with effective permeation area of 0.05 m2 (0.2 m and 10 kg mol−1 MWCO). | - | Polysaccharide and protein-rich fractions | Huang et al. [54] |
| SBY (S. pastorianus) | Fractionation: UF in flat sheet module of effective permeation area of 0.0016 m2 (30 and 10 kg mol−1 MWCO). | Electrophoresis (SDS-PAGE) | Antioxidant peptides | Marson et al. [9] |
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE-I | Inhibitory activity of the angiotensin-converting enzyme |
| EDUF | Electrodialysis using ultrafiltration membranes |
| EMF | Electrically enhanced membrane filtration |
| FPLC | Fast Protein Liquid Chromatography |
| HPLC | High-performance liquid chromatography |
| HPTFF | High-performance tangential flow filtration |
| IMAC | Immobilized metal affinity chromatography |
| LC | Liquid chromatography |
| MALDI | Matrix-assisted laser desorption/ionization |
| MF | Microfiltration |
| MS | Mass spectrometry |
| MW | Molecular weight |
| MWCO | Molecular weight cut-off |
| NF | Nanofiltration |
| pI | Isoelectric point |
| RNA | Ribonucleic acids |
| RO | Reverse osmosis |
| RP | Reversed-phase |
| SBY | Spent brewer’s yeast |
| SDS-PAGE | Sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| SEC | Size-exclusion chromatography |
| SPE | Solid phase extraction |
| TOF | Time-of-Flight |
| UF | Ultrafiltration |
References
- Nazir, A.; Khan, K.; Maan, A.; Zia, R.; Giorno, L.; Schroën, K. Membrane separation technology for the recovery of nutraceuticals from food industrial streams. Trends Food Sci. Technol. 2019, 86, 426–438. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Barragán-Huerta, B.; Fíla, V.; Denis, P.C.; Ruby-Figueroa, R. Current Role of Membrane Technology: From the Treatment of Agro-Industrial by-Products up to the Valorization of Valuable Compounds. Waste Biomass Valoriz. 2018, 9, 513–529. [Google Scholar] [CrossRef]
- Pouliot, Y.; Gauthier, S.F.; Groleau, P.E. Membrane-based fractionation and purification strategies for bioactive peptides. In Nutraceutical Proteins and Peptides in Health and Disease, 1st ed.; Mine, Y., Shahidi, F., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 109–143. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, B.P.; Kumar, M.; Shahi, V.K. Membrane-based techniques for the separation and purification of proteins: An overview. Adv. Colloid Interface Sci. 2009, 145, 1–22. [Google Scholar] [CrossRef]
- van Reis, R.; Zydney, A. Membrane separations in biotechnology. Curr. Opin. Biotechnol. 2001, 12, 208–211. [Google Scholar] [CrossRef]
- Belleville, M.P.; Vaillant, F. Membrane technology for production of nutraceuticals. In Functional Food Ingredients and Nutraceuticals: Processing Technologies, 2nd ed.; Shi, J., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 217–234. [Google Scholar]
- Ghosh, R. Chapter 18: Ultrafiltration-based Protein Bioseparation. In Handbook of Membrane Separations: Chemical, Pharmaceutical, Food and Biotechnological Applications, 1st ed.; Pabby, A.K., Rizvi, S.S.H., Sastre, A.M., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 497–512. [Google Scholar]
- Mathias, T.R.d.S.; Alexandre, V.M.F.; Cammarota, M.C.; Mello, P.P.M.; Sérvulo, E.F.C. Characterization and determination of brewer’s solid wastes composition. J. Inst. Brew. 2015, 121, 400–404. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; da Costa Machado, M.T.; da Silva Zandonadi, F.; de Freitas Queiroz Barros, H.D.; Júnior, M.R.M.; Sussulini, A.; Hubinger, M.D. Proteolytic enzymes positively modulated the physicochemical and antioxidant properties of spent yeast protein hydrolysates. Process Biochem. 2020, 91, 34–45. [Google Scholar] [CrossRef]
- Amorim, M.; Pinheiro, H.; Pintado, M. Valorization of spent brewer’s yeast: Optimization of hydrolysis process towards the generation of stable ACE-inhibitory peptides. LWT 2019, 111, 77–84. [Google Scholar] [CrossRef]
- Rakowska, R.; Sadowska, A.; Dybkowska, E.; Świderski, F. Spent yeast as natural source of functional food additives. Ann. Natl. Inst. Hyg. 2017, 68, 115–121. [Google Scholar]
- Chae, H.J.; Joo, H.; In, M.J. Utilization of brewer’s yeast cells for the production of food-grade yeast extract. Part 1: Effects of different enzymatic treatments on solid and protein recovery and flavor characteristics. Bioresour. Technol. 2001, 76, 253–258. [Google Scholar] [CrossRef]
- Kanauchi, O.; Igarashi, K.; Ogata, R.; Mitsuyama, K.; Andoh, A. A Yeast Extract High in Bioactive Peptides has a Blood-Pressure Lowering Effect in Hypertensive Model. Curr. Med. Chem. 2005, 12, 3085–3090. [Google Scholar] [CrossRef]
- Bączek, T. Fractionation of peptides and identification of proteins from Saccharomyces cerevisiae in proteomics with the use of reversed-phase capillary liquid chromatography and pI-based approach. J. Pharm. Biomed. Anal. 2004, 35, 895–904. [Google Scholar] [CrossRef]
- Ghosh, R.; Cui, Z.F. Protein purification by ultrafiltration with pre-treated membrane. J. Membr. Sci. 2000, 167, 47–53. [Google Scholar] [CrossRef]
- Amorim, M.; Pereira, J.O.; Gomes, D.; Pereira, C.D.; Pinheiro, H.; Pintado, M. Nutritional ingredients from spent brewer’s yeast obtained by hydrolysis and selective membrane filtration integrated in a pilot process. J. Food Eng. 2016, 185, 42–47. [Google Scholar] [CrossRef]
- Huang, S.; Gong, Y.; Li, Y.; Ruan, S.; Roknul Azam, S.M.; Duan, Y.; Ye, X.; Ma, H. Preparation of ACE-inhibitory peptides from milk protein in continuous enzyme membrane reactor with gradient dilution feeding substrate. Process Biochem. 2020, 92, 130–137. [Google Scholar] [CrossRef]
- Thuanthong, M.; De Gobba, C.; Sirinupong, N.; Youravong, W.; Otte, J. Purification and characterization of angiotensin-converting enzyme-inhibitory peptides from Nile tilapia (Oreochromis niloticus) skin gelatine produced by an enzymatic membrane reactor. J. Funct. Foods 2017, 36, 243–254. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Olsen, H.S. Enzymic modification of food protein. In Enzymes in Food Technology; Whitehurst, R.J., Law, B.A., Eds.; Sheffield Academic Press: Sheffield, UK, 2002; pp. 109–143. [Google Scholar]
- Emin, C.; Kurnia, E.; Katalia, I.; Ulbricht, M. Polyarylsulfone-based blend ultrafiltration membranes with combined size and charge selectivity for protein separation. Sep. Purif. Technol. 2018, 193, 127–138. [Google Scholar] [CrossRef]
- Saidi, S.; Deratani, A.; Amar, R.B.; Belleville, M.P. Fractionation of a tuna dark muscle hydrolysate by a two-step membrane process. Sep. Purif. Technol. 2013, 108, 28–36. [Google Scholar] [CrossRef]
- Chabeaud, A.; Vandanjon, L.; Bourseau, P.; Jaouen, P.; Guérard, F. Fractionation by ultrafiltration of a saithe protein hydrolysate (Pollachius virens): Effect of material and molecular weight cut-off on the membrane performances. J. Food Eng. 2009, 91, 408–414. [Google Scholar] [CrossRef]
- Jung, E.Y.; Lee, H.S.; Choi, J.W.; Ra, K.S.; Kim, M.R.; Suh, H.J. Glucose Tolerance and Antioxidant Activity of Spent Brewer’s Yeast Hydrolysate with a High Content of Cyclo-His-Pro (CHP). J. Food Sci. 2011, 76, C272–C278. [Google Scholar] [CrossRef]
- Ortiz-Martinez, M.; Winkler, R.; García-Lara, S. Preventive and therapeutic potential of peptides from cereals against cancer. J. Proteom. 2014, 111, 165–183. [Google Scholar] [CrossRef]
- Ambrosi, A.; Cardozo, N.S.M.; Tessaro, I.C. Membrane Separation Processes for the Beer Industry: A Review and State of the Art. Food Bioprocess Technol. 2014, 7, 921–936. [Google Scholar] [CrossRef]
- Habert, A.C.; Borges, C.P.; Nobrega, R. Aspectos Gerais dos Processos com Membranas. In Processos de Separação por Membranas, 1st ed.; Habert, A.C., Borges, C.P., Nobrega, R., Eds.; E-papers: Rio de Janeiro, Brasil, 2006; pp. 9–24. [Google Scholar]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology, 1st ed.; Springer-Science + Business Media, B.V.: Dordrecht, The Netherlands, 1991. [Google Scholar] [CrossRef]
- Etzel, M.; Arunkumar, A. Chapter 6—Novel Membrane Technologies for Protein Concentration and Fractionation. In Innovative Food Processing Technologies; Woodhead Publishing Series in Food Science, Technology and Nutrition; Knoerzer, K., Juliano, P., Smithers, G., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 151–175. [Google Scholar] [CrossRef]
- Cui, Z.; Jiang, Y.; Field, R. Chapter 1—Fundamentals of Pressure-Driven Membrane Separation Processes. In Membrane Technology; Cui, Z., Muralidhara, H., Eds.; Butterworth-Heinemann: Oxford, UK, 2010; pp. 1–18. [Google Scholar] [CrossRef]
- Wang, Y.; Selomulya, C. Spray drying strategy for encapsulation of bioactive peptide powders for food applications. Adv. Powder Technol. 2020, 31, 409–415. [Google Scholar] [CrossRef]
- Kurozawa, L.E.; Park, K.J.; Hubinger, M.D. Effect of carrier agents on the physicochemical properties of a spray dried chicken meat protein hydrolysate. J. Food Eng. 2009, 94, 326–333. [Google Scholar] [CrossRef]
- Cui, Z. Protein separation using ultrafiltration—An example of multi-scale complex systems. China Particuol. 2005, 3, 343–348. [Google Scholar] [CrossRef]
- Corbatón-Báguena, M.J.; Álvarez-Blanco, S.; Vincent-Vela, M.C.; Ortega-Navarro, E.; Pérez-Herranz, V. Application of electric fields to clean ultrafiltration membranes fouled with whey model solutions. Sep. Purif. Technol. 2016, 159, 92–99. [Google Scholar] [CrossRef]
- Córdova, A.; Astudillo-Castro, C.; Ruby-Figueroa, R.; Valencia, P.; Soto, C. Recent advances and perspectives of ultrasound assisted membrane food processing. Food Res. Int. 2020, 133, 109163. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Hengl, N.; Baup, S.; Pignon, F.; Gondrexon, N.; Sztucki, M.; Gésan-Guiziou, G.; Magnin, A.; Abyan, M.; Karrouch, M.; et al. Effects of ultrasound on cross-flow ultrafiltration of skim milk: Characterization from macro-scale to nano-scale. J. Membr. Sci. 2014, 470, 205–218. [Google Scholar] [CrossRef]
- Li, X.; Li, J. Critical Flux. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–3. [Google Scholar] [CrossRef]
- Taddei, C.; Howell, J.A. On the effect of membrane conditioning in cell harvesting using microfiltration. Biotechnol. Tech. 1989, 3, 155–160. [Google Scholar] [CrossRef]
- Fane, A.; Fell, C.; Waters, A. Ultrafiltration of protein solutions through partially permeable membranes—The effect of adsorption and solution environment. J. Membr. Sci. 1983, 16, 211–224. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; John Wiley & Sons Ltd.: Sussex, UK, 2004. [Google Scholar] [CrossRef]
- Abejón, R.; Abejón, A.; Belleville, M.P.; Sánchez-Marcano, J.; Garea, A.; Irabien, A. Multiobjective Optimization of Membrane Networks for Fractionation of Protein Hydrolysate from Fish By-Products. In 26th European Symposium on Computer Aided Process Engineering; Computer Aided Chemical Engineering; Kravanja, Z., Bogataj, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 415–420. [Google Scholar] [CrossRef]
- Persico, M.; Daigle, G.; Kadel, S.; Perreault, V.; Pellerin, G.; Thibodeau, J.; Bazinet, L. Predictive models for determination of peptide fouling based on the physicochemical characteristics of filtration membranes. Sep. Purif. Technol. 2020, 240, 116602. [Google Scholar] [CrossRef]
- Kolakowski, E. Analysis of Proteins, Peptides and Amino Acids in Foods. In Methods of Analysis of Food Components and Additives, 2nd ed.; Ötleş, S., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 79–114. [Google Scholar]
- Przybylski, R.; Bazinet, L.; Firdaous, L.; Kouach, M.; Goossens, J.F.; Dhulster, P.; Nedjar, N. Harnessing slaughterhouse by-products: From wastes to high-added value natural food preservative. Food Chem. 2020, 304, 125448. [Google Scholar] [CrossRef] [PubMed]
- Durand, R.; Pellerin, G.; Thibodeau, J.; Fraboulet, E.; Marette, A.; Bazinet, L. Screening for metabolic syndrome application of a herring by-product hydrolysate after its separation by electrodialysis with ultrafiltration membrane and identification of novel anti-inflammatory peptides. Sep. Purif. Technol. 2020, 235, 116205. [Google Scholar] [CrossRef]
- Suwal, S.; Roblet, C.; Doyen, A.; Amiot, J.; Beaulieu, L.; Legault, J.; Bazinet, L. Electrodialytic separation of peptides from snow crab by-product hydrolysate: Effect of cell configuration on peptide selectivity and local electric field. Sep. Purif. Technol. 2014, 127, 29–38. [Google Scholar] [CrossRef]
- Marie, G.C.U.; Perreault, V.; Henaux, L.; Carnovale, V.; Aluko, R.E.; Marette, A.; Doyen, A.; Bazinet, L. Impact of a high hydrostatic pressure pretreatment on the separation of bioactive peptides from flaxseed protein hydrolysates by electrodialysis with ultrafiltration membranes. Sep. Purif. Technol. 2019, 211, 242–251. [Google Scholar] [CrossRef]
- He, R.; Girgih, A.T.; Rozoy, E.; Bazinet, L.; Ju, X.R.; Aluko, R.E. Selective separation and concentration of antihypertensive peptides from rapeseed protein hydrolysate by electrodialysis with ultrafiltration membranes. Food Chem. 2016, 197, 1008–1014. [Google Scholar] [CrossRef]
- Charcosset, C. 5—Membrane chromatography. In Membrane Processes in Biotechnology and Pharmaceutics; Charcosset, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 169–212. [Google Scholar] [CrossRef]
- Boi, C. Chapter 6—Membrane Chromatography for Biomolecule Purification. In Current Trends and Future Developments on (Bio-) Membranes; Basile, A., Charcosset, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 151–166. [Google Scholar] [CrossRef]
- Halász, A.; Lásztity, R. (Eds.) Chemical composition and biochemistry of yeast biomass. In Use of Yeast Biomass in Food Production; CRC Press: Boca Raton, FL, USA, 1991; pp. 23–41. [Google Scholar]
- Marson, G.V.; da Costa Machado, M.T.; de Castro, R.J.S.; Hubinger, M.D. Sequential hydrolysis of spent brewer’s yeast improved its physico-chemical characteristics and antioxidant properties: A strategy to transform waste into added-value biomolecules. Process Biochem. 2019, 84, 91–102. [Google Scholar] [CrossRef]
- Podpora, B.; Świderski, F.; Sadowska, A.; Rakowska, R.; Wasiak-zys, G. Spent brewer’s yeast extracts as a new component of functional food. Czech J. Food Sci. 2016, 34, 554–563. [Google Scholar] [CrossRef]
- Huang, K.; Gao, J.Y.; Ma, S.; Lu, J.J. Optimising separation process of protein and polysaccharide from spent brewer’s yeast by ultrafiltration. Int. J. Food Sci. Technol. 2012, 47, 1259–1264. [Google Scholar] [CrossRef]
- Patsioura, A.; Galanakis, C.M.; Gekas, V. Ultrafiltration optimization for the recovery of β-glucan from oat mill waste. J. Membr. Sci. 2011, 373, 53–63. [Google Scholar] [CrossRef]
- Morales, D.; Smiderle, F.R.; Piris, A.J.; Soler-Rivas, C.; Prodanov, M. Production of a β-d-glucan-rich extract from Shiitake mushrooms (Lentinula edodes) by an extraction/microfiltration/reverse osmosis (nanofiltration) process. Innov. Food Sci. Emerg. Technol. 2019, 51, 80–90. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- She, Q.; Tang, C.Y.; Wang, Y.N.; Zhang, Z. The role of hydrodynamic conditions and solution chemistry on protein fouling during ultrafiltration. Desalination 2009, 249, 1079–1087. [Google Scholar] [CrossRef]
- Fernández, A.; Suárez, A.; Zhu, Y.; FitzGerald, R.J.; Riera, F.A. Membrane fractionation of a β-lactoglobulin tryptic digest: Effect of the pH. J. Food Eng. 2013, 114, 83–89. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.-J. Yeast extract production using spent yeast from beer manufacture: Influence of industrially applicable disruption methods on selected substance groups with biotechnological relevance. Eur. Food Res. Technol. 2019, 245, 1169–1182. [Google Scholar] [CrossRef]
- Gao, F.; Wang, J.; Zhang, H.; Jia, H.; Cui, Z.; Yang, G. Role of ionic strength on protein fouling during ultrafiltration by synchronized UV–vis spectroscopy and electrochemical impedance spectroscopy. J. Membr. Sci. 2018, 563, 592–601. [Google Scholar] [CrossRef]
- Manzano, I.; Zydney, A.L. Quantitative study of RNA transmission through ultrafiltration membranes. J. Membr. Sci. 2017, 544, 272–277. [Google Scholar] [CrossRef]
- Manzano, I.; Vezeau, G.; Salis, H.; Zydney, A.L. RNA size and 3-dimensional structure determine ultrafiltration behavior of small RNA molecules. Sep. Purif. Technol. 2020, 237, 116372. [Google Scholar] [CrossRef]
- Jung, E.Y.; Cho, M.K.; Hong, Y.H.; Kim, J.H.; Park, Y.; Chang, U.J.; Suh, H.J. Yeast hydrolysate can reduce body weight and abdominal fat accumulation in obese adults. Nutrition 2014, 30, 25–32. [Google Scholar] [CrossRef]
- Park, Y.; Kim, J.H.; Lee, H.S.; Jung, E.Y.; Lee, H.; Noh, D.O.; Suh, H.J. Thermal stability of yeast hydrolysate as a novel anti-obesity material. Food Chem. 2013, 136, 316–321. [Google Scholar] [CrossRef]
- Jung, E.Y.; Hong, Y.H.; Kim, J.H.; Park, Y.; Bae, S.H.; Chang, U.J.; Suh, H.J. Effects of Yeast Hydrolysate on Hepatic Lipid Metabolism in High-Fat-Diet-Induced Obese Mice: Yeast Hydrolysate Suppresses Body Fat Accumulation by Attenuating Fatty Acid Synthesis. Ann. Nutr. Metab. 2012, 61, 89–94. [Google Scholar] [CrossRef]
- Kim, J.H.; Jung, E.Y.; Hong, Y.H.; Bae, S.H.; Kim, J.M.; Noh, D.O.; Nozaki, T.; Inoue, T.; Suh, H.J. Short Communication: Pet foods with yeast hydrolysate can reduce body weight and increase girth in beagle dogs. Can. J. Anim. Sci. 2012, 92, 207–210. [Google Scholar] [CrossRef][Green Version]
- Lee, H.; Jung, E.; Bae, S.H.; Kwon, K.H.; Kim, J.; Suh, H.J. Stimulation of osteoblastic differentiation and mineralization in MC3T3 E1 cells by yeast hydrolysate. Phytother. Res. 2011, 25, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jung, E.Y.; Suh, H.J. Chemical Composition and Anti-Stress Effects of Yeast Hydrolysate. J. Med. Food 2009, 12, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, S.; Jung, E.; Bae, S.H.; Suh, H.J. Yeast hydrolysate induces longitudinal bone growth and growth hormone release in rats. Phytother. Res. 2009, 23, 731–736. [Google Scholar] [CrossRef]
- de la Hoz, L.; Ponezi, A.N.; Milani, R.F.; da Silva, V.S.N.; de Souza, A.S.; Bertoldo-Pacheco, M.T. Iron-binding properties of sugar cane yeast peptides. Food Chem. 2014, 142, 166–169. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, S.W.; Kim, K.M.; Chang, U.J.; Song, J.C.; Suh, H.J. Anti-stress effect and functionality of yeast hydrolysate SCP-20. Eur. Food Res. Technol. 2003, 217, 168–172. [Google Scholar] [CrossRef]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M.; Hosseini, E. Purification and identification of antioxidant and ACE-inhibitory peptide from Saccharomyces cerevisiae protein hydrolysate. J. Funct. Foods 2015, 19, 259–268. [Google Scholar] [CrossRef]
- Jeong, S.C.; Lee, D.H.; Lee, J.S. Production and characterization of an anti-angiogenic agent from Saccharomyces cerevisiae K-7. J. Microbiol. Biotechnol. 2006, 16, 1904–1911. [Google Scholar]
- Amorim, M.; Marques, C.; Pereira, J.; Guardão, L.; Martins, M.; Osório, H.; Moura, D.; Calhau, C.; Pinheiro, H.; Pintado, M. Antihypertensive effect of spent brewer yeast peptide. Process Biochem. 2019, 76, 213–218. [Google Scholar] [CrossRef]
- Williams, R.; Dias, D.A.; Jayasinghe, N.; Roessner, U.; Bennett, L.E. β-glucan-depleted, glycopeptide-rich extracts from Brewer’s and Baker’s yeast (Saccharomyces cerevisiae) lower interferon-γ production by stimulated human blood cells in vitro. Food Chem. 2016, 197, 761–768. [Google Scholar] [CrossRef]
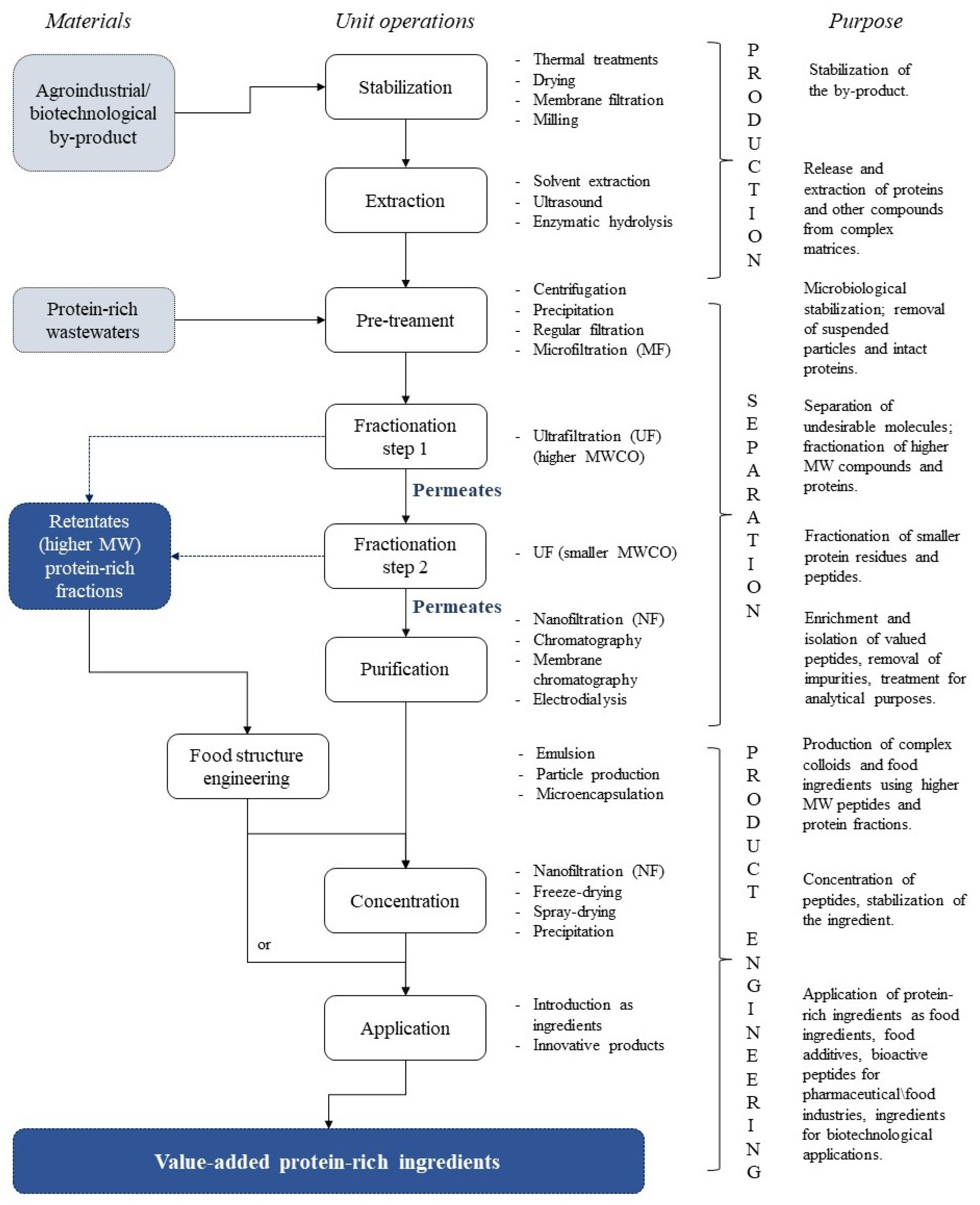
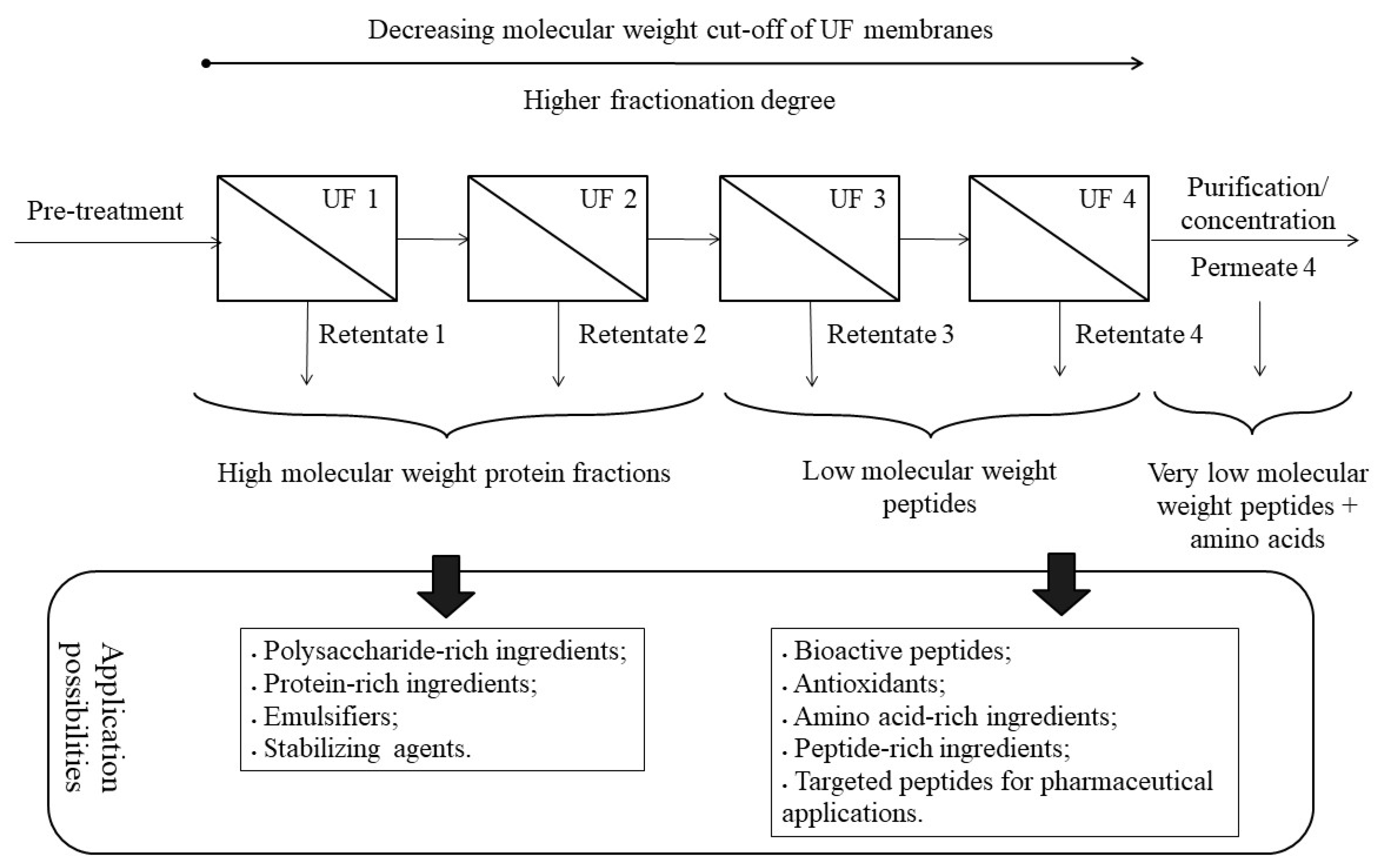
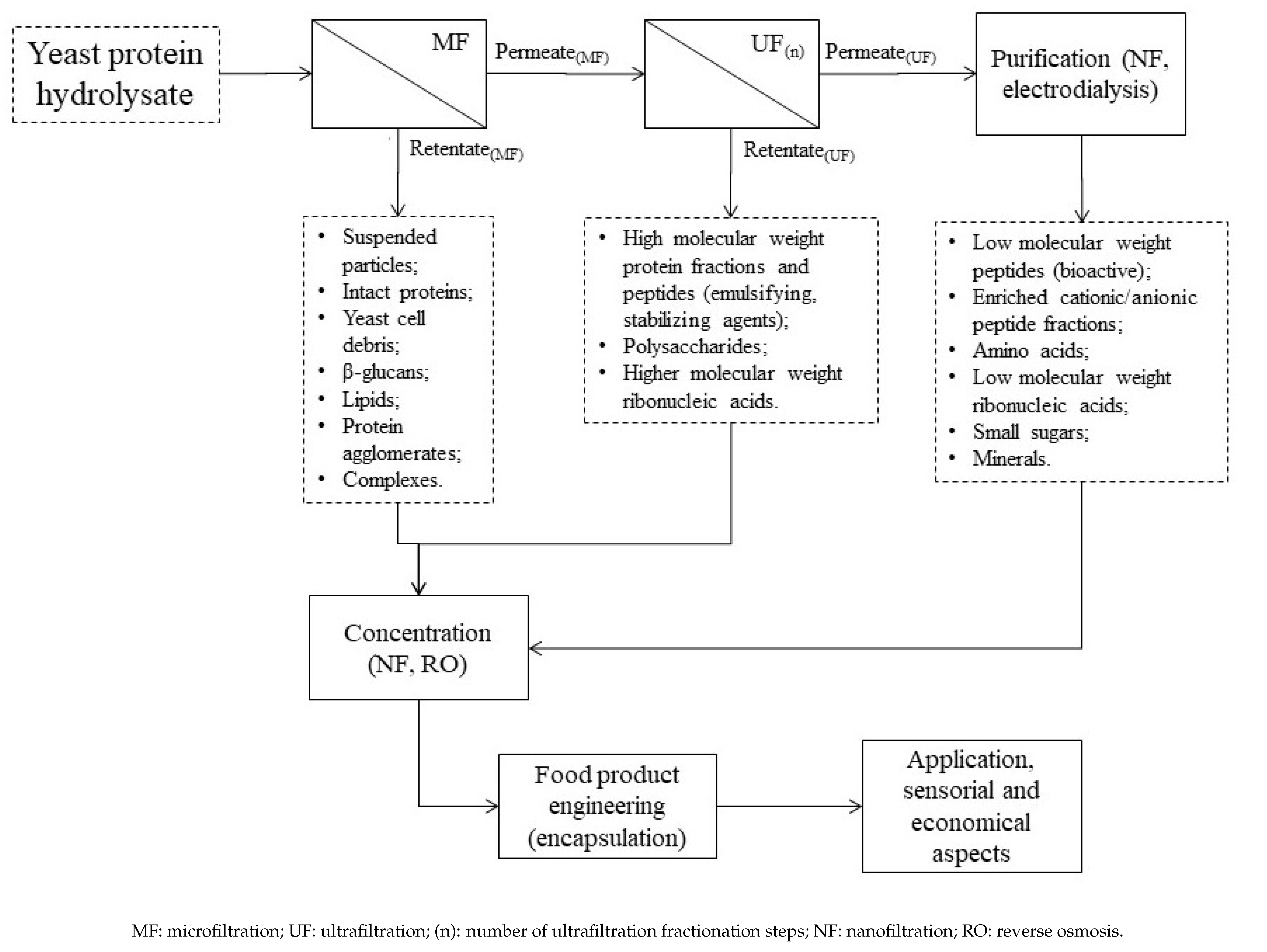
| Macronutrients (g 100 g−1, d.w.) | SBY Enzymatic Hydrolysate [9,12,16,52,53] |
|---|---|
| Total nitrogen | 1.5–12.0 |
| Protein nitrogen | 9.3–69.0 |
| Free amino nitrogen | 28–35 |
| Ribonucleic acids | 5.6 |
| Total sugars | 3.0–48 |
| Lipids | 0.2–1.0 |
| Ashes | 3.0–22.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vollet Marson, G.; Belleville, M.-P.; Lacour, S.; Dupas Hubinger, M. Membrane Fractionation of Protein Hydrolysates from By-Products: Recovery of Valuable Compounds from Spent Yeasts. Membranes 2021, 11, 23. https://doi.org/10.3390/membranes11010023
Vollet Marson G, Belleville M-P, Lacour S, Dupas Hubinger M. Membrane Fractionation of Protein Hydrolysates from By-Products: Recovery of Valuable Compounds from Spent Yeasts. Membranes. 2021; 11(1):23. https://doi.org/10.3390/membranes11010023
Chicago/Turabian StyleVollet Marson, Gabriela, Marie-Pierre Belleville, Stella Lacour, and Miriam Dupas Hubinger. 2021. "Membrane Fractionation of Protein Hydrolysates from By-Products: Recovery of Valuable Compounds from Spent Yeasts" Membranes 11, no. 1: 23. https://doi.org/10.3390/membranes11010023
APA StyleVollet Marson, G., Belleville, M.-P., Lacour, S., & Dupas Hubinger, M. (2021). Membrane Fractionation of Protein Hydrolysates from By-Products: Recovery of Valuable Compounds from Spent Yeasts. Membranes, 11(1), 23. https://doi.org/10.3390/membranes11010023





