A Reevaluation of Chitosan-Decorated Nanoparticles to Cross the Blood-Brain Barrier
Abstract
1. Introduction
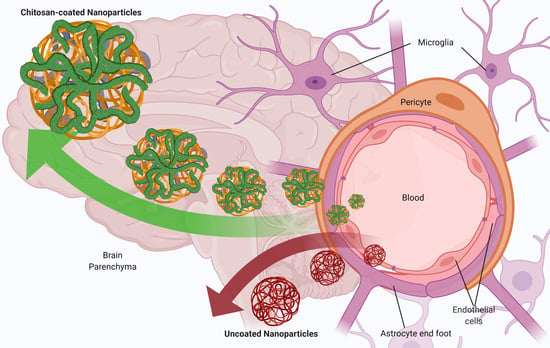
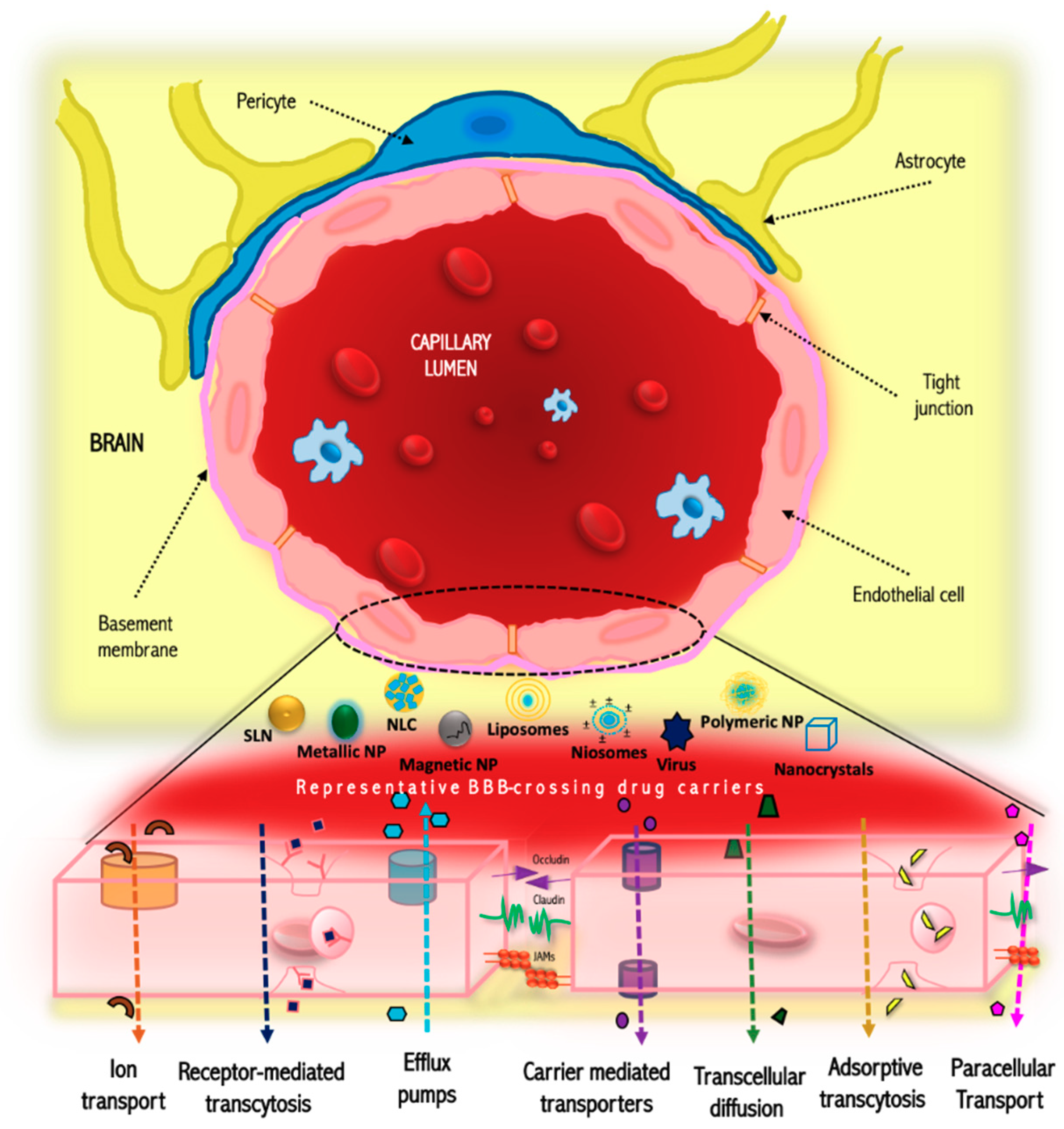
2. Structure and Function of the Blood-Brain Barrier
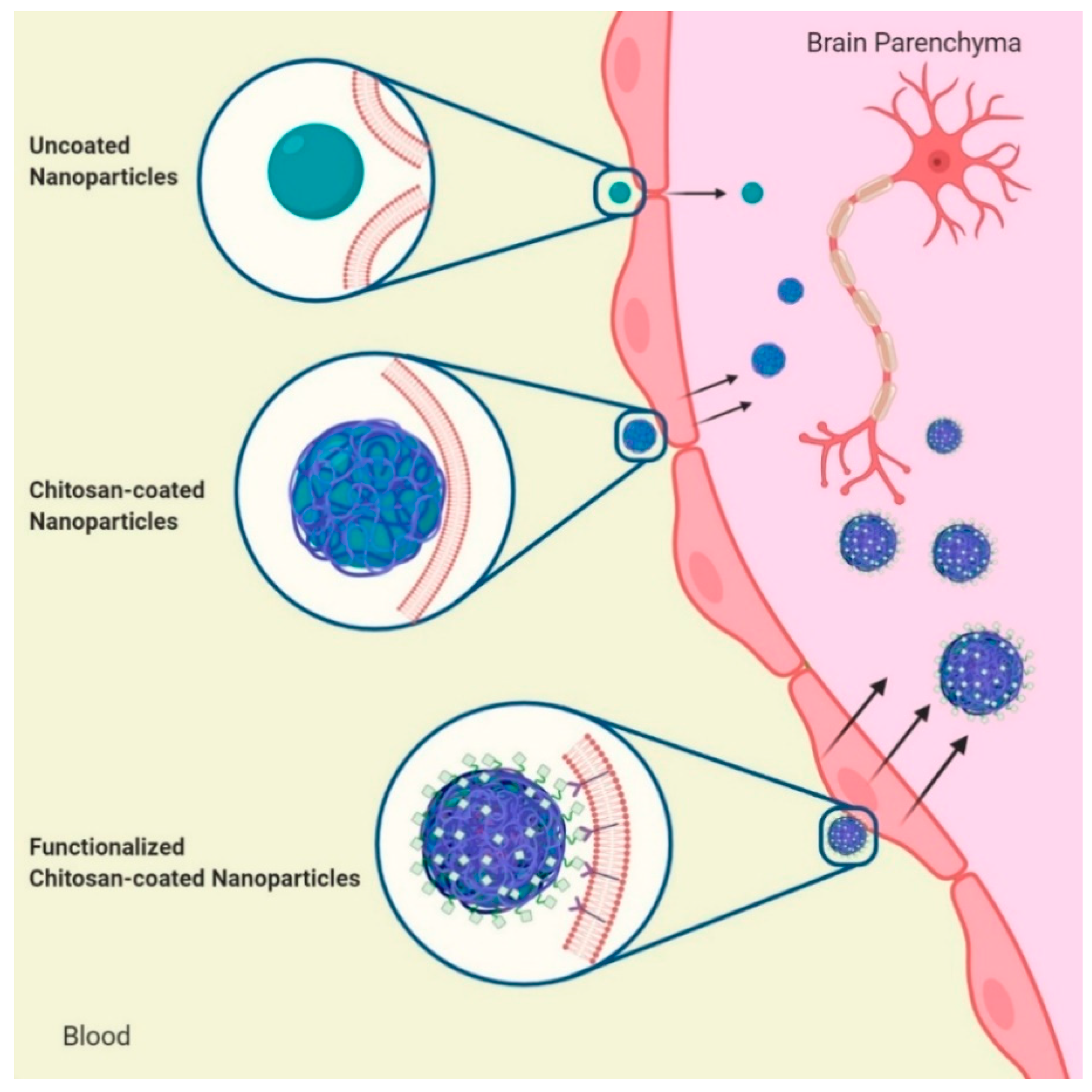
3. Chitosan Routes for Crossing the Blood-Brain Barrier
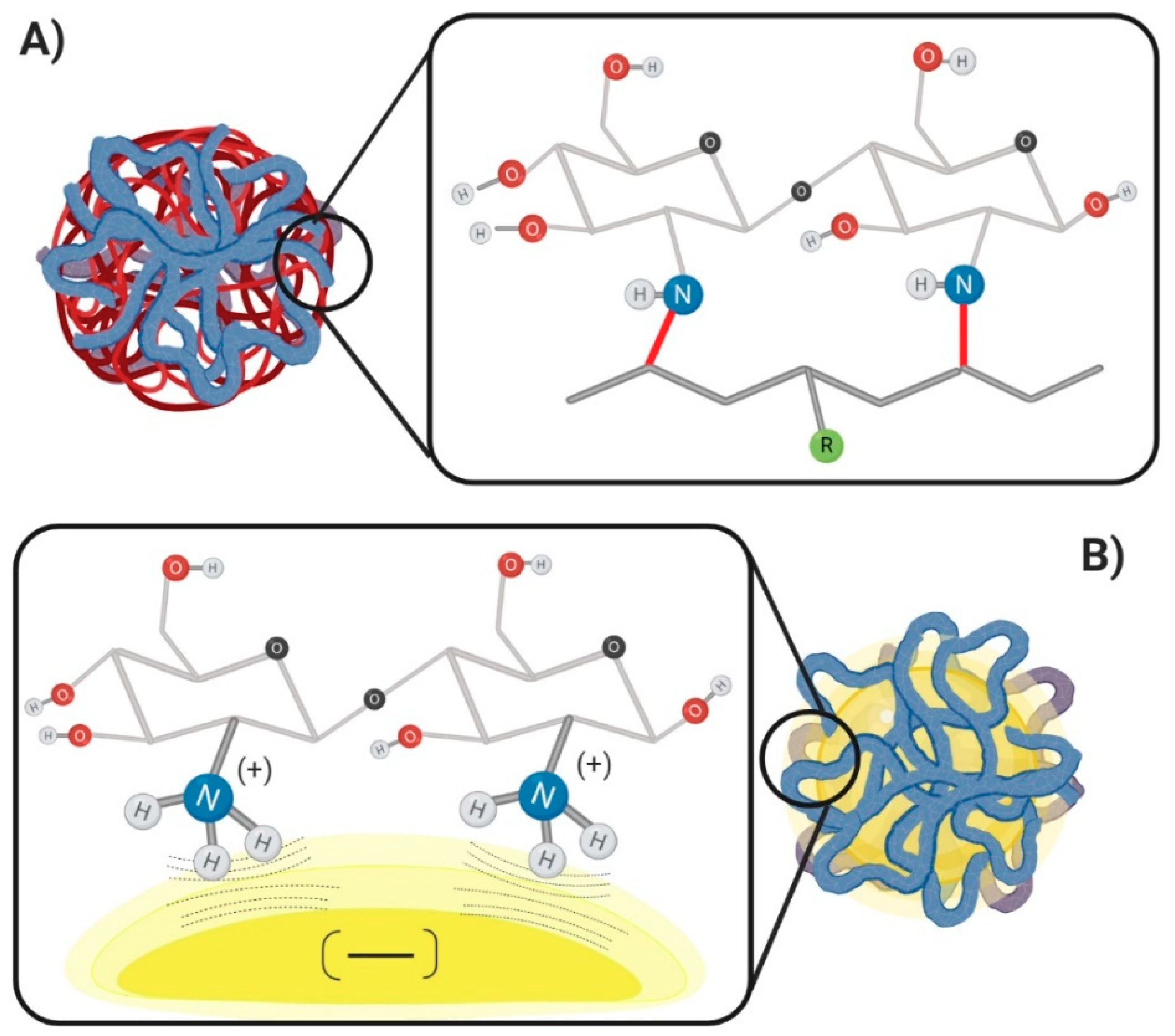
4. Decoration of Nanoparticles with Chitosan: Methods and Mechanisms
4.1. Non-Covalent Mechanism
4.2. Covalent Mechanism
5. Chitosan-decorated Nanoparticles for Brain Targeting
5.1. Polymeric Nanoparticles
5.2. Polymeric Micelles
5.3. Lipid Nanoparticles
5.4. Liposomes and Niosomes
5.5. Inorganic Nanoparticles
6. Conclusion and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hodson, R. The brain. Nature 2019, 571, S1. [Google Scholar] [CrossRef]
- Harilal, S.; Jose, J.; Parambi, D.J.G.; Kumar, R.; Unnikrishnan, M.K.; Uddin, M.S.; Mathew, G.E.; Pratap, R.; Marathakam, A.; Mathew, B. Revisiting the Blood-brain barrier: A hard nut to crack in the transportation of drug Molecules. Brain Res. Bull. 2020, 160, 121–140. [Google Scholar] [CrossRef]
- Tajes, M.; Ramos-Fernández, E.; Weng-Jiang, X.; Bosch-Morató, M.; Guivernau, B.; Eraso-Pichot, A.; Salvador, B.; Fernàndez-Busquets, X.; Roquer, J.; Muñoz, F.J. The blood-brain barrier: Structure, function and therapeutic approaches to cross it. Mol. Membr. Biol. 2014, 31, 152–167. [Google Scholar] [CrossRef]
- Feng, M.R. Assessment of Blood-Brain Barrier Penetration: In Silico, In Vitro and In Vivo. Curr. Drug Metab. 2002, 3, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Ciura, K.; Dziomba, S. Application of separation methods for in vitro prediction of blood-brain barrier permeability—The state of the art. J. Pharm. Biomed. Anal. 2020, 177, 112891. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Drug delivery systems, CNS protection, and the blood brain barrier. Biomed. Res. Int. 2014, 2014, 869269. [Google Scholar] [CrossRef] [PubMed]
- Mensch, J.; Oyarzabal, J.; Mackie, C.; Augustijns, P. In Vivo, In Vitro and In Silico Methods for Small Molecule Transfer Across the BBB. J. Pharm. Sci. 2009, 98, 4429–4468. [Google Scholar] [CrossRef]
- Cortes, H.; Alcalá-Alcalá, S.; Ávalos-Fuentes, A.; Mendoza-Muñoz, N.; Quintanar-Guerrero, D.; Leyva-Gomez, G.; Florán, B. Nanotechnology As Potential Tool for siRNA Delivery in Parkinson’s Disease. Curr. Drug Targets 2017, 18, 1866–1879. [Google Scholar] [CrossRef]
- Leyva-Gómez, G.; Cortés, H.; Magaña, J.J.; Leyva-García, N.; Quintanar-Guerrero, D.; Florán, B. Nanoparticle technology for treatment of Parkinson’s disease: The role of surface phenomena in reaching the brain. Drug Discov. Today 2015, 20, 824–837. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of curcumin nanoparticles for brain diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef]
- Mulvihill, J.J.; Cunnane, E.M.; Ross, A.M.; Duskey, J.T.; Tosi, G.; Grabrucker, A.M. Drug delivery across the blood-brain barrier: Recent advances in the use of nanocarriers. Nanomedicine 2020, 15, 205–214. [Google Scholar] [CrossRef]
- Sarkar, A.; Fatima, I.; Sajid Jamal, Q.M.; Sayeed, U.; Khan, M.K.A.; Akhtar, S.; Kamal, M.A.; Farooqui, A.; Siddiqui, M.H. Nanoparticles as a Carrier System for Drug Delivery Across Blood Brain Barrier. Curr. Drug Metab. 2017, 18, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Eslamifar, M.; Khezri, K.; Dizaj, S.M. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019, 111, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Sharifi-Rad, J.; Mendoza-Muñoz, N.; González-Torres, M.; Urbán-Morlán, Z.; Florán, B.; Cortes, H.; Leyva-Gómez, G. Chitosan-decorated nanoparticles for drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101896. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Wang, L.J.; Rajesh, R. Targeting human brain cancer stem cells by curcumin-loaded nanoparticles grafted with anti-aldehyde dehydrogenase and sialic acid: Colocalization of ALDH and CD44. Mater. Sci. Eng. C 2019, 102, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zheng, J.; Wang, K.; Tang, Y.; Zhang, X.; Zhang, H.; Huang, F.; Pei, Y.; Jiang, Y. Cationic core-shell nanoparticles with carmustine contained within O6-benzylguanine shell for glioma therapy. Biomaterials 2013, 34, 8968–8978. [Google Scholar] [CrossRef]
- Dhas, N.; Mehta, T. Cationic biopolymer functionalized nanoparticles encapsulating lutein to attenuate oxidative stress in effective treatment of Alzheimer’s disease: A non-invasive approach. Int. J. Pharm. 2020, 586, 119553. [Google Scholar] [CrossRef]
- Meng, Q.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for.pdf. Int. J. Nanomed. 2018, 13, 705–718. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Alrasheed, R.A.; Almatar, H.M.A.; Al-Ramadan, A.S.; Amir, M.; Sarafroz, M. Quantification and evaluations of catechin hydrate polymeric nanoparticles used in brain targeting for the treatment of epilepsy. Pharmaceutics 2020, 12, 203. [Google Scholar] [CrossRef]
- Kaur, S.; Manhas, P.; Swami, A.; Bhandari, R.; Sharma, K.K.; Jain, R.; Kumar, R.; Pandey, S.K.; Kuhad, A.; Sharma, R.K.; et al. Bioengineered PLGA-chitosan nanoparticles for brain targeted intranasal delivery of antiepileptic TRH analogues. Chem. Eng. J. 2018, 346, 630–639. [Google Scholar] [CrossRef]
- Chalikwar, S.S.; Mene, B.S.; Pardeshi, C.V.; Belgamwar, V.S.; Surana, S.J. Self-Assembled, Chitosan Grafted PLGA Nanoparticles for Intranasal Delivery: Design, Development and Ex Vivo Characterization. Polym. Plast. Technol. Eng. 2013, 52, 368–380. [Google Scholar] [CrossRef]
- Ahmad, N.; Al-Subaie, A.M.; Ahmad, R.; Sharma, S.; Alam, M.A.; Ashafaq, M.; Abdur Rub, R.; Ahmad, F.J. Brain-targeted glycyrrhizic-acid-loaded surface decorated nanoparticles for treatment of cerebral ischaemia and its toxicity assessment. Artif. Cells Nanomed. Biotechnol. 2019, 47, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Alam, M.A.; Ahmad, F.J. Quantification and Brain Targeting of Eugenol-Loaded Surface Modified Nanoparticles Through Intranasal Route in the Treatment of Cerebral Ischemia. Drug Res. 2018, 68, 584–595. [Google Scholar] [CrossRef]
- Ferro, M.P.; Heilshorn, S.C.; Owens, R.M. Materials for blood brain barrier modeling in vitro. Mater. Sci. Eng. R Rep. 2020, 140, 100522. [Google Scholar] [CrossRef]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming blood–brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef]
- Arif, W.M.; Elsinga, P.H.; Gasca-Salas, C.; Versluis, M.; Martínez-Fernández, R.; Dierckx, R.A.J.O.; Borra, R.J.H.; Luurtsema, G. Focused ultrasound for opening blood-brain barrier and drug delivery monitored with positron emission tomography. J. Control. Release 2020, 324, 303–316. [Google Scholar] [CrossRef]
- Halder, S.K.; Kant, R.; Milner, R. Chronic mild hypoxia increases expression of laminins 111 and 411 and the laminin receptor α6β1 integrin at the blood-brain barrier. Brain Res. 2018, 1700, 78–85. [Google Scholar] [CrossRef]
- Tang, V.W.; Goodenough, D.A. Paracellular ion channel at the tight junction. Biophys. J. 2003, 84, 1660–1673. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2019. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Campbell, M. Tight junction modulation at the blood-brain barrier: Current and future perspectives. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183298. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.P.; Almeida, A.; Sarmento, B. The role of non-endothelial cells on the penetration of nanoparticles through the blood brain barrier. Prog. Neurobiol. 2017, 159, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, V.N.; Rowe, R.K.; Harrison, J.; Wu, C.; Anderson, T.R.; Lifshitz, J.; Adelson, P.D.; Kodibagkar, V.D.; Stabenfeldt, S.E. Blood–brainbarrier disruption dictates nanoparticle accumulation following experimental brain injury. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yao, J.; Zhang, Y.; Chen, Y.; Wang, K.; Lee, R.J.; Yu, B.; Zhang, X. Solid lipid nanoparticles as a drug delivery system to across the blood-brain barrier. Biochem. Biophys. Res. Commun. 2019, 519, 385–390. [Google Scholar] [CrossRef]
- Tamba, B.I.; Streinu, V.; Foltea, G.; Neagu, A.N.; Dodi, G.; Zlei, M.; Tijani, A.; Stefanescu, C. Tailored surface silica nanoparticles for blood-brain barrier penetration: Preparation and in vivo investigation. Arab. J. Chem. 2018, 11, 981–990. [Google Scholar] [CrossRef]
- Copeland, C.; Stabenfeldt, S.E. Leveraging the dynamic blood–brain barrier for central nervous system nanoparticle-based drug delivery applications. Curr. Opin. Biomed. Eng. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Gert, F.; Ott, M.; Mahringer, A. The Blood Brain Barrier (BBB). In Topics in Medicinal Chemistry; Fricker, G., Ott, M., Mahringer, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 10. [Google Scholar]
- De Jong, E.; Williams, D.S.; Abdelmohsen, L.K.E.A.; Van Hest, J.C.M.; Zuhorn, I.S. A filter-free blood-brain barrier model to quantitatively study transendothelial delivery of nanoparticles by fluorescence spectroscopy. J. Control. Release 2018, 289, 14–22. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Magaña, J.J.; Mejía-Contreras, B.A.; Borbolla-Jiménez, F.V.; Giraldo-Gomez, D.M.; Piña-Barba, M.C.; Quintanar-Guerrero, D.; Leyva-Gómez, G. In vitro cell uptake evaluation of curcumin-loaded PCL/F68 nanoparticles for potential application in neuronal diseases. J. Drug Deliv. Sci. Technol. 2019, 52, 905–914. [Google Scholar] [CrossRef]
- Modi, G.; Pillay, V.; Choonara, Y.E. Advances in the treatment of neurodegenerative disorders employing nanotechnology. Ann. N. Y. Acad. Sci. 2010, 1184, 154–172. [Google Scholar] [CrossRef]
- Malhotra, M.; Tomaro-duchesneau, C.; Prakash, S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials 2013, 34, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Yemişci, M.; Gürsoy-Özdemir, Y.; Caban, S.; Bodur, E.; Çapan, Y.; Dalkara, T. Transport of a caspase inhibitor across the blood-brain barrier by chitosan nanoparticles. Methods Enzymol. 2012, 508, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Sahin, A.; Yoyen-Ermis, D.; Caban-Toktas, S.; Horzum, U.; Aktas, Y.; Couvreur, P.; Esendagli, G.; Capan, Y. Evaluation of brain-targeted chitosan nanoparticles through blood–brain barrier cerebral microvessel endothelial cells. J. Microencapsul. 2017, 34, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; De Giglio, E.; Cafagna, D.; Denora, N.; Agrimi, G.; Cassano, T.; Gaetani, S.; Cuomo, V.; Trapani, G. Characterization and evaluation of chitosan nanoparticles for dopamine brain delivery. Int. J. Pharm. 2011, 419, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, J.V.; Kalicharan, D.; Couraud, P.O.; Romero, I.A.; Weksler, B.; Hoekstra, D.; Zuhorn, I.S. Surface characteristics of nanoparticles determine their intracellular fate in and processing by human blood-brain barrier endothelial cells in vitro. Mol. Ther. 2011, 19, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Scherrmann, J.M. Drug delivery to brain via the blood-brain barrier. Vascul. Pharmacol. 2002, 38, 349–354. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, T.; Ma, M.; Hu, Y.; Zhang, J. Preparation and evaluation of anti-neuroexcitation peptide (ANEP) loaded N-trimethyl chitosan chloride nanoparticles for brain-targeting. Int. J. Pharm. 2010, 386, 249–255. [Google Scholar] [CrossRef]
- Kaiser, M.; Pereira, S.; Pohl, L.; Ketelhut, S.; Kemper, B.; Gorzelanny, C.; Galla, H.J.; Moerschbacher, B.M.; Goycoolea, F.M. Chitosan encapsulation modulates the effect of capsaicin on the tight junctions of MDCK cells. Sci. Rep. 2015, 5, 10048. [Google Scholar] [CrossRef]
- Lien, C.F.; Molnár, É.; Toman, P.; Tsibouklis, J.; Pilkington, G.J.; Górecki, D.C.; Barbu, E. In vitro assessment of alkylglyceryl-functionalized chitosan nanoparticles as permeating vectors for the blood-brain barrier. Biomacromolecules 2012, 13, 1067–1073. [Google Scholar] [CrossRef]
- Bruinsmann, F.A.; Pigana, S.; Aguirre, T.; Souto, G.D.; Pereira, G.G.; Bianchera, A.; Fasiolo, L.T.; Colombo, G.; Marques, M.; Pohlmann, A.R.; et al. Chitosan-coated nanoparticles: Effect of chitosan molecular weight on nasal transmucosal delivery. Pharmaceutics 2019, 11, 86. [Google Scholar] [CrossRef]
- Duskey, J.T.; Baraldi, C.; Gamberini, M.C.; Ottonelli, I.; Da Ros, F.; Tosi, G.; Forni, F.; Vandelli, M.A.; Ruozi, B. Investigating novel syntheses of a series of unique hybrid PLGA-chitosan polymers for potential therapeutic delivery applications. Polymers 2020, 12, 823. [Google Scholar] [CrossRef] [PubMed]
- Li, A.D.; Sun, Z.Z.; Zhou, M.; Xu, X.X.; Ma, J.Y.; Zheng, W.; Zhou, H.M.; Li, L.; Zheng, Y.F. Electrospun Chitosan-graft-PLGA nanofibres with significantly enhanced hydrophilicity and improved mechanical property. Colloids Surfaces B Biointerfaces 2013, 102, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Jiang, H.; Tu, K.; Wang, L.; Zhu, K. A facile route for regioselective conjugation of organo-soluble polymers onto chitosan. Macromol. Biosci. 2009, 9, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Gartziandia, O.; Herrán, E.; Ruiz-Ortega, J.A.; Miguelez, C.; Igartua, M.; Lafuente, J.V.; Pedraz, J.L.; Ugedo, L.; Hernández, R.M. Intranasal administration of chitosan-coated nanostructured lipid carriers loaded with GDNF improves behavioral and histological recovery in a partial lesion model of Parkinson’s disease. J. Biomed. Nanotechnol. 2016, 12, 2220–2230. [Google Scholar] [CrossRef]
- Hernando, S.; Herran, E.; Figueiro-Silva, J.; Pedraz, J.L.; Igartua, M.; Carro, E.; Hernandez, R.M. Intranasal administration of TAT-conjugated lipid nanocarriers loading GDNF for Parkinson’s disease. Mol. Neurobiol. 2018, 55, 145–155. [Google Scholar] [CrossRef]
- Chen, H.; Yang, W.; Chen, H.; Liu, L.; Gao, F.; Yang, X.; Jiang, Q.; Zhang, Q.; Wang, Y. Surface modification of Mitoxantrone-loaded PLGA nanospheres with chitosan. Colloids Surfaces B Biointerfaces 2009, 73, 212–218. [Google Scholar] [CrossRef]
- Pauluk, D.; Padilha, A.K.; Khalil, N.M.; Mainardes, R.M. Chitosan-coated zein nanoparticles for oral delivery of resveratrol: Formation, characterization, stability, mucoadhesive properties and antioxidant activity. Food Hydrocoll. 2019, 94, 411–417. [Google Scholar] [CrossRef]
- Piazzini, V.; Landucci, E.; D’Ambrosio, M.; Tiozzo Fasiolo, L.; Cinci, L.; Colombo, G.; Pellegrini-Giampietro, D.E.; Bilia, A.R.; Luceri, C.; Bergonzi, M.C. Chitosan coated human serum albumin nanoparticles: A promising strategy for nose-to-brain drug delivery. Int. J. Biol. Macromol. 2019, 129, 267–280. [Google Scholar] [CrossRef]
- Simon, L.C.; Stout, R.W.; Sabliov, C. Bioavailability of Orally Delivered Alpha-Tocopherol by Poly(Lactic-Co-Glycolic) Acid (PLGA) Nanoparticles and Chitosan Covered PLGA Nanoparticles in F344 Rats. Nanobiomedicine 2016, 3, 8. [Google Scholar] [CrossRef]
- Nafee, N.; Taetz, S.; Schneider, M.; Schaefer, U.F.; Lehr, C.-M. Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: Effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 173–183. [Google Scholar] [CrossRef]
- Piazzini, V.; Cinci, L.; D’Ambrosio, M.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Solid Lipid Nanoparticles and Chitosan-coated Solid Lipid Nanoparticles as Promising Tool for Silybin Delivery: Formulation, Characterization, and In vitro Evaluation. Curr. Drug Deliv. 2018, 16, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Mazzarino, L.; Travelet, C.; Ortega-Murillo, S.; Otsuka, I.; Pignot-Paintrand, I.; Lemos-Senna, E.; Borsali, R. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J. Colloid Interface Sci. 2012, 370, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kievit, F.M.; Jeon, M.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. Nanoparticle-Mediated Target Delivery of TRAIL as Gene Therapy for Glioblastoma. Adv. Healthc. Mater. 2015, 4, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Kievit, F.M.; Fang, C.; Mu, N.; Jana, S.; Leung, M.C.; Mok, H.; Ellenbogen, R.G.; Park, J.O.; Zhang, M. Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials 2010, 31, 8032–8042. [Google Scholar] [CrossRef] [PubMed]
- Stephen, Z.R.; Kievit, F.M.; Veiseh, O.; Chiarelli, P.A.; Fang, C.; Wang, K.; Hatzinger, S.J.; Ellenbogen, R.G.; Silber, J.R.; Zhang, M. Redox-responsive magnetic nanoparticle for targeted convection-enhanced delivery of O6-benzylguanine to brain tumors. ACS Nano 2014, 8, 10383–10395. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-I.; Kim, J.C.; Kim, Y.H.; Tae, G.; Lee, S.Y.; Kim, K.; Kwon, I.C. The effect of surface functionalization of PLGA nanoparticles by heparin- or chitosan-conjugated Pluronic on tumor targeting. J. Control. Release 2010, 143, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.T.; Du, Y.Z.; Yuan, H.; Hu, F.Q. Brain-targeting study of stearic acid-grafted chitosan micelle drug-delivery system. Int. J. Nanomed. 2012, 7, 3235–3244. [Google Scholar] [CrossRef]
- Wang, Z.H.; Wang, Z.Y.; Sun, C.S.; Wang, C.Y.; Jiang, T.Y.; Wang, S.L. Trimethylated chitosan-conjugated PLGA nanoparticles for the delivery of drugs to the brain. Biomaterials 2010, 31, 908–915. [Google Scholar] [CrossRef]
- Chakravarthi, S.S.; Robinson, D.H. Enhanced cellular association of paclitaxel delivered in chitosan-PLGA particles. Int. J. Pharm. 2011, 409, 111–120. [Google Scholar] [CrossRef]
- Zhang, W.; Gilstrap, K.; Wu, L.; Remant Bahadur, K.C.; Moss, M.A.; Wang, Q.; Lu, X.; He, X. Synthesis and characterization of thermally responsive pluronic F127-chitosan nanocapsules for controlled release and intracellular delivery of small molecules. ACS Nano 2010, 4, 6747–6759. [Google Scholar] [CrossRef]
- Messaoudi, K.; Saulnier, P.; Boesen, K.; Benoit, J.P.; Lagarce, F. Anti-epidermal growth factor receptor siRNA carried by chitosan-transacylated lipid nanocapsules increases sensitivity of glioblastoma cells to temozolomide. Int. J. Nanomed. 2014, 9, 1479–1490. [Google Scholar] [CrossRef]
- Ping, Y.; Liu, C.; Zhang, Z.; Liu, K.L.; Chen, J.; Li, J. Chitosan-graft-(PEI-β-cyclodextrin) copolymers and their supramolecular PEGylation for DNA and siRNA delivery. Biomaterials 2011, 32, 8328–8341. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Choi, W.I.; Kim, Y.H.; Tae, G.; Lee, S.Y.; Kim, K.; Kwon, I.C. In-vivo tumor targeting of pluronic-based nano-carriers. J. Control. Release 2010, 147, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Peptu, C.A.; Ochiuz, L.; Alupei, L.; Peptu, C.; Popa, M. Carbohydrate based nanoparticles for drug delivery across biological barriers. J. Biomed. Nanotechnol. 2014, 10, 2107–2148. [Google Scholar] [CrossRef] [PubMed]
- Vllasaliu, D.; Exposito-Harris, R.; Heras, A.; Casettari, L.; Garnett, M.; Illum, L.; Stolnik, S. Tight junction modulation by chitosan nanoparticles: Comparison with chitosan solution. Int. J. Pharm. 2010, 400, 183–193. [Google Scholar] [CrossRef]
- Van der Lubben, I.M.; Verhoef, J.C.; Borchard, G.; Junginger, H.E. Chitosan and its derivatives in mucosal drug and vaccine delivery. Eur. J. Pharm. Sci. 2001, 14, 201–207. [Google Scholar] [CrossRef]
- Qiang, F.; Shin, H.J.; Lee, B.J.; Han, H.K. Enhanced systemic exposure of fexofenadine via the intranasal administration of chitosan-coated liposome. Int. J. Pharm. 2012, 430, 161–166. [Google Scholar] [CrossRef]
- Ong, W.-Y.; Shalini, S.-M.; Costantino, L. Nose-to-Brain Drug Delivery by Nanoparticles in the Treatment of Neurological Disorders. Curr. Med. Chem. 2014, 21, 4247–4256. [Google Scholar] [CrossRef]
- Bourganis, V.; Kammona, O.; Alexopoulos, A.; Kiparissides, C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur. J. Pharm. Biopharm. 2018, 128, 337–362. [Google Scholar] [CrossRef]
- Furtado, D.; Björnmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the Blood–Brain Barrier: The Role of Nanomaterials in Treating Neurological Diseases. Adv. Mater. 2018, 30, e1801362. [Google Scholar] [CrossRef]
- Xu, Y.; Wei, L.; Wang, H. Progress and perspectives on nanoplatforms for drug delivery to the brain. J. Drug Deliv. Sci. Technol. 2020, 57, 101636. [Google Scholar] [CrossRef]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Sonvico, F.; Clementino, A.; Buttini, F.; Colombo, G.; Pescina, S.; Guterres, S.S.; Pohlmann, A.R.; Nicoli, S. Surface-modified nanocarriers for nose-to-brain delivery: From bioadhesion to targeting. Pharmaceutics 2018, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Gartziandia, O.; Egusquiaguirre, S.P.; Bianco, J.; Pedraz, J.L.; Igartua, M.; Hernandez, R.M.; Préat, V.; Beloqui, A. Nanoparticle transport across in vitro olfactory cell monolayers. Int. J. Pharm. 2016, 499, 81–89. [Google Scholar] [CrossRef]
- de Moraes Profirio, D.; Pessine, F.B.T. Formulation of functionalized PLGA nanoparticles with folic acid-conjugated chitosan for carboplatin encapsulation. Eur. Polym. J. 2018, 108, 311–321. [Google Scholar] [CrossRef]
- Varan, C.; Bilensoy, E. Cationic PEGylated polycaprolactone nanoparticles carrying post-operation docetaxel for glioma treatment. Beilstein J. Nanotechnol. 2017, 8, 1446–1456. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Rajesh, R. Targeted delivery of rosmarinic acid across the blood–brain barrier for neuronal rescue using polyacrylamide-chitosan-poly(lactide-co-glycolide) nanoparticles with surface cross-reacting material 197 and apolipoprotein E. Int. J. Pharm. 2017, 528, 228–241. [Google Scholar] [CrossRef]
- Jaruszewski, K.M.; Ramakrishnan, S.; Poduslo, J.F.; Kandimalla, K.K. Chitosan enhances the stability and targeting of immuno-nanovehicles to cerebro-vascular deposits of Alzheimer’s disease amyloid protein. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 250–260. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Chai, G.; Zhang, X.; Li, F. Brain pharmacokinetics of neurotoxin-loaded PLA nanoparticles modified with chitosan after intranasal administration in awake rats. Drug Dev. Ind. Pharm. 2013, 39, 1618–1624. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.J.; Tang, X.J.; Du, L.; Li, F. In vitro and in vivo evaluation of functionalized chitosan-Pluronic micelles loaded with myricetin on glioblastoma cancer. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1263–1278. [Google Scholar] [CrossRef]
- Agrawal, P.; Sonali; Singh, R.P.; Sharma, G.; Mehata, A.K.; Singh, S.; Rajesh, C.V.; Pandey, B.L.; Koch, B.; Muthu, M.S. Bioadhesive micelles of D-α-tocopherol polyethylene glycol succinate 1000: Synergism of chitosan and transferrin in targeted drug delivery. Colloids Surfaces B Biointerfaces 2017, 152, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Meenu Vasudevan, S.; Ashwanikumar, N.; Vinod Kumar, G.S. Peptide decorated glycolipid nanomicelles for drug delivery across the blood-brain barrier (BBB). Biomater. Sci. 2019, 7, 4017–4021. [Google Scholar] [CrossRef] [PubMed]
- Chirio, D.; Gallarate, M.; Peira, E.; Battaglia, L.; Muntoni, E.; Riganti, C.; Biasibetti, E.; Capucchio, M.T.; Valazza, A.; Panciani, P.; et al. Positive-charged solid lipid nanoparticles as paclitaxel drug delivery system in glioblastoma treatment. Eur. J. Pharm. Biopharm. 2014, 88, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018, 116, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Soddu, E.; Posadino, A.M.; Pintus, G.; Sarmento, B.; Giunchedi, P.; Gavini, E. Nose-to-brain delivery of BACE1 siRNA loaded in solid lipid nanoparticles for Alzheimer’s therapy. Colloids Surfaces B Biointerfaces 2017, 152, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Belgamwar, V.S. Improved brain pharmacokinetics following intranasal administration of N,N,N-trimethyl chitosan tailored mucoadhesive NLCs. Mater. Technol. 2020, 35, 249–266. [Google Scholar] [CrossRef]
- Singh, S.K.; Hidau, M.K.; Gautam, S.; Gupta, K.; Singh, K.P.; Singh, S.K.; Singh, S. Glycol chitosan functionalized asenapine nanostructured lipid carriers for targeted brain delivery: Pharmacokinetic and teratogenic assessment. Int. J. Biol. Macromol. 2018, 108, 1092–1100. [Google Scholar] [CrossRef]
- Hansraj, G.P.; Singh, S.K.; Kumar, P. Sumatriptan succinate loaded chitosan solid lipid nanoparticles for enhanced anti-migraine potential. Int. J. Biol. Macromol. 2015, 81, 467–476. [Google Scholar] [CrossRef]
- Salem, L.H.; El-Feky, G.S.; Fahmy, R.H.; El Gazayerly, O.N.; Abdelbary, A. Coated Lipidic Nanoparticles as a New Strategy for Enhancing Nose-to-Brain Delivery of a Hydrophilic Drug Molecule. J. Pharm. Sci. 2020, 109, 2237–2251. [Google Scholar] [CrossRef]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surfaces B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- Ramalingam, P.; Ko, Y.T. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: Pharmacokinetic and brain distribution evaluations. Pharm. Res. 2015, 32, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Trotta, V.; Pavan, B.; Ferraro, L.; Beggiato, S.; Traini, D.; Des Reis, L.G.; Scalia, S.; Dalpiaz, A. Brain targeting of resveratrol by nasal administration of chitosan-coated lipid microparticles. Eur. J. Pharm. Biopharm. 2018, 127, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Popov, M.; Abu Hammad, I.; Bachar, T.; Grinberg, S.; Linder, C.; Stepensky, D.; Heldman, E. Delivery of analgesic peptides to the brain by nano-sized bolaamphiphilic vesicles made of monolayer membranes. Eur. J. Pharm. Biopharm. 2013, 85, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Hou, D.; Wang, L.; Li, S.; Sun, S.; Ping, Q.; Xu, Y. Effects and molecular mechanism of chitosan-coated levodopa nanoliposomes on behavior of dyskinesia rats. Biol. Res. 2016, 49, 1–9. [Google Scholar] [CrossRef]
- Salade, L.; Wauthoz, N.; Deleu, M.; Vermeersch, M.; De Vriese, C.; Amighi, K.; Goole, J. Development of coated liposomes loaded with ghrelin for nose-to-brain delivery for the treatment of cachexia. Int. J. Nanomed. 2017, 12, 8531–8543. [Google Scholar] [CrossRef]
- Rinaldi, F.; Hanieh, P.N.; Chan, L.K.N.; Angeloni, L.; Passeri, D.; Rossi, M.; Wang, J.T.W.; Imbriano, A.; Carafa, M.; Marianecci, C. Chitosan glutamate-coated niosomes: A proposal for nose-to-brain delivery. Pharmaceutics 2018, 10, 34. [Google Scholar] [CrossRef]
- Wang, T.; Kievit, F.M.; Veiseh, O.; Arami, H.; Stephen, Z.R.; Fang, C.; Liu, Y.; Ellenbogen, R.G.; Zhang, M. Targeted cell uptake of a noninternalizing antibody through conjugation to iron oxide nanoparticles in primary central nervous system lymphoma. World Neurosurg. 2013, 80, 134–141. [Google Scholar] [CrossRef]
- Das, M.; Wang, C.; Bedi, R.; Mohapatra, S.S.; Mohapatra, S. Magnetic micelles for DNA delivery to rat brains after mild traumatic brain injury. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1539–1548. [Google Scholar] [CrossRef]
- Veiseh, O.; Sun, C.; Fang, C.; Bhattarai, N.; Gunn, J.; Kievit, F.; Du, K.; Pullar, B.; Lee, D.; Ellenbogen, R.G.; et al. Specific targeting of brain tumors with an optical/magnetic resonance imaging nanoprobe across the blood-brain barrier. Cancer Res. 2009, 69, 6200–6207. [Google Scholar] [CrossRef]
- Seyedebrahimi, R.; Razavi, S.; Varshosaz, J. Controlled Delivery of Brain Derived Neurotrophic Factor and Gold-Nanoparticles from Chitosan/TPP Nanoparticles for Tissue Engineering Applications. J. Clust. Sci. 2020, 31, 99–108. [Google Scholar] [CrossRef]
- Agyare, E.K.; Jaruszewski, K.M.; Curran, G.L.; Rosenberg, J.T.; Grant, S.C.; Lowe, V.J.; Ramakrishnan, S.; Paravastu, A.K.; Poduslo, J.F.; Kandimalla, K.K. Engineering theranostic nanovehicles capable of targeting cerebrovascular amyloid deposits. J. Control. Release 2014, 185, 121–129. [Google Scholar] [CrossRef] [PubMed]
| Brain Desease/CNS Disorder | Nanosytem | Size (nm) | PDI | Z-Potential (mV) | Model Drug | Drug Loading (%) | Chitosan Coating Conditions/Method | Additional Functionalization/Targeting Agents | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Nature of Nanocore | Non-Decorated | Decorated | Non-Decorated | Decorated | Non-Decorated | Decorated | ||||||
| Brain cancer (Glioblastoma, and tumor diagnosis) | Polymeric NPs | PLGA | 117.35 ± 2.37 | 140.88 ± 5.15 | −− | −− | −14.94 ± 4.05 | 10.33 ± 0.26 | Curcumin | LE: 39.48 to 82.67 | Adsorption by immersion in CS solution at 0.02, 0.04, 0.06 %w/v. Crosslinkers: NHS and EDC | Anti-ALDH to target BCSCs and Salic Acid as BBB permeator | [13] |
| PLGA | 121.0 | 178.0 | 0.120 | 0.200 | −34.0 | 46.0 | Carboplatin | EE: 35.5 and DL: 1.8 | Adsorption by immersion in solution of conjugated CS in a range concentration 0.0008-0.2 %w/v | Folic acid | [75] | ||
| PLGA | 168 | 177 | −− | −− | −6.2 ± 2.5 | 19.6 ± 4.8 | Carmustine/O6-Benzylguanine | LE: 8.97 | Adsorption by immersion in CS solution at 0.3 %w/v | None | [14] | ||
| PCL | 170 ± 0.1 | 196 to 218 | 0.07 ± 0.02 | 0.25 to 0.20 | −20 ± 0.6 | 39 to 54 | Docetaxel | EE: ≃76.0 ± 3.0 | Coating with CS amounts at 0, 0.01 and 0.025 %w/v | None | [79] | ||
| PCL | −− | 168 to 185 | −− | 0.12 to 0.16 | −− | 28.95 to 33.8 | Lipid-core with Simvastatin | EE: ≃100 | Interfacial deposition of pre-formed CS, aqueous phase contained 0.1 %w/v low-MW CS and high-MW CS | None | [47] | ||
| Polymeric Micelles | Pluronic P123/F68® | −− | 51.5 ± 12.3 | −− | 0.692 ± 0.09 | −− | 22.38 ± 4.15 | Myricetin | EE: 91.72 ± 12.68 and DL: 15.63 ± 1.82 | By adding an aqueous solution of CS (40 mg/20 mL) and incubation at 75 °C and dialysis against de-ionized water for 24 h | None | [80] | |
| D-α-tocopheryl glycol succinate 1000 (TPGS) | 13.89 ± 1.2 | 14.25 ± 2.9 | 0.22 ± 0.05 | 0.37 ± 0.03 | −4.91 ± 0.58 | −2.32 ± 0.05 | Docetaxel | EE: 98.8 ± 1.9 | TPGS-COOH activated was conjugated to the CS by EDC and NHS in phosphate buffer saline (pH 5.5) | Transferrin | [81] | ||
| Lipid NPs | SLN (Behenic Acid) | 299.2 to 386.4 | 374.5 to 624.3 | 0.053 to 0.167 | 0.196 to 0.227 | −3.04 to −3.41 | 15.46 to 23.79 | Paclitaxel (PTX) | EE: 24 to 83 and DL: 28 to 100 gPTX/mg | Glycol chitosan was added at the beginning of the preparation of SLN where micellar solution was formed | None | [82] | |
| SLN (Vitamin E and Gelucire 44/14®) | −− | 117 to 203 | −− | 0.131 to 0.216 | −− | −− | Temozolomide | EE: 71.33 to 88.45 and DL: 7.11 to 9.23 | NPs were converted to hydrogel using CS at 1.0 %w/v solution, adding slowly with constant stirring for half an hour | None | [86] | ||
| Inorganic NPs | Iron Oxide | 7.5 ± 1.3 | 76 ± 4 | −− | 0.16 | −− | 4 ± 7.4 | O6-Benzylguanine | DL: 150 ± 14 BG molecules/NP | Via a coprecipitation method. CS-grafted-PEG (150 mg) was mixed with iron chlorides (9 mg Fe2+, 15 mg Fe3+) in 2.18 mL of degassed DI water until complete nucleation of NPs | Tumor targeting peptide, Chlorotoxin | [57] | |
| 34.2 ± 5.4 | 57.1 ± 1.4 | −− | −− | 17.75 ± 0.64 | 18.63 ± 1.27 | DNA encoding Human tumor necrosis factor α-related apoptosis-inducing ligand (TRAIL) | Effcient DNA binding | By reaction with aminated poly(ethylene glycol) residues of CS–PEG and CS–PEG–PEI copolymers | Chlorotoxin | [55] | |||
| 7 | 33 | −− | −− | −− | 4.2 | −− | −− | NPs were synthesized in the presence of CS-graftedPEG via coprecipitation of ferrous and ferric chlorides with ammonium hydroxide. | Chlorotoxin and Cy5.5 (near-IR fluorophore) | [98] | |||
| 7.5 | 111.9 ± 52.4 | −− | −− | −− | 19.6 ± 5.7 | siRNA to knockdown green fluorescence protein (GFP) expression | DL: 3.8 siRNA molecules/NP | nanoparticles were coprecipitated in the presence of chitosan-grafted PEG polymer | Chlorotoxin | [56] | |||
| 8 | 67.2 to 71.2 | 0.203 | 0.204 | −0.028 | 0.182 | Anti-CD20 single chain variable fragment-streptavidin fusion protein | DL: 0.7 FP molecules per NP | Via a coprecipitation method. CS-grafted-PEG (150 mg) was mixed with iron chlorides (9 mg Fe2+, 15 mg Fe3+) in 2.18 mL of degassed DI water until complete nucleation of NPs | Oregon Green 488 | [96] | |||
| Alzheimer’s Disease | Polymeric NPs | PLGA | 136.2 ± 1.09 | 142.3 ± 2.57 | 0.093 ± 0.005 | 0.091 ± 0.09 | −27.29 ± 0.97 | 46.6 ± 1.87 | Lutein | EE: 83.97 ± 1.03 and DL: 3.95 ± 0.03 | By electrostatic interaction of CS at 0.01, 0.02, 0.03 and 0.04 %w/v | None | [15] |
| PLGA | 78.1 ± 3.7 | 125.4 ± 9.1 | 0.182 ± 0.027 | 0.197 ± 0.025 | −21.2 ± 0.8 | 36.3 ± 4.0 | Huperzine A | EE: 77.0 ± 3.9 | By reaction with Mal-TMC (3 mg/7.5 mL) | Lactoferrin | [16] | ||
| PLGA | −− | ≈191.0 ± 5.0 | −− | −− | −− | ≈23.0 ± 3.0 | Rosmarinic Acid | EE: 50.0 ± 2.0 (max) | Crosslink of PAAM-CS at 0.05 %w/v with EDC and NHS | Material 197 and ApoE | [76] | ||
| PLGA | 217.33 ± 6.82 | 267.67 ± 2.52 | −− | −− | −7.64 ± 0.74 | 32.02 ± 2.65 | 6-Coumarin Probe | EE: 84.24 ± 4.22 | Redispersion of NP in CS solution at 0.3, 0.5, and 0.7 %w/v by sonication and mix | Anti-Aβ antibody | [77] | ||
| Lipid NPs | SLN (Witepsol E 85®) | 335.76 ± 34.81 | 358.44 ± 25.89 | 0.013 ± 0.00 | 0.028 ± 0.02 | −17.31 ± 0.68 | 10.54 ± 0.75 | BACE1 siRNA | −− | A CS solution at 1 %w/v is added at 1:1 w/w NP suspension, magnetically stirred overnight | Rabies virus glycoprotein known as RVG-9R | [83] | |
| Nanovesicles | Niosomes (Tween-20®) | 165.2 ± 3.1 | 180.2 ± 1.5 | 0.211 ± 0.020 | 0.248 ± 0.016 | −41.6 ± 1.4 | 29.5 ± 1.6 | Pentamidine | EE: 10 | By adding CS (0.05 mg/mL in acetate buffer 0.2 M, pH 4.4) solution to the different niosome samples (1:1 ratio), stirring for 1 h at room temperature | None | [94] | |
| Parkinson’s Disease | Lipid NPs | NLC (Precirol ATO5® and Mygliol®) | 201.5 ± 5.6 | 205.9 ± 6.3 | 0.315 ± 0.03 | 0.275 ± 0.02 | 19.9 ± 3.1 | 21.9 ± 1.8 | Glial cell-derived neurotrophic factor (GDNF) | EE: 87.62 to 87.66 and DL: 1.31 μgGDNF/mgNP | TAT was covalently linked to CS, then NLC dispersion was added dropwise to the TAT-CS solution under continuous agitation for 20 min at room temperature | Cell-penetrating peptides; transactivator of transcription (TAT) | [49] |
| NLC (Flaxseed oil and Tristearin) | 38.41 ± 2.23 | 44.45 ± 1.5 | 0.309 ± 0.02 | 0.281 ± 0.05 | −11.4 ± 0.98 | 16.15 ± 0.9 | Ropinirole-dextran sulphate nanoplex | EE: 92.75 ± 2.30 and DL: 17.26 ± 1.10 | 0.5% w/v of aqueous TMC-CS solution was added to the aqueous dispersion of NLC, stirring for 2 h | None | [87] | ||
| Liposomes | Phosphatidylcholine | −− | −− | −− | −− | −− | −− | Levodopa | −− | Electrostatic adsorption using a CS solution | None | [92] | |
| Emulsions | Microemulsion (Capmul MCM L8®, Tween-80®, PEG400 or Transcutol®) | 24.9 ± 4.60 | 37.1 ± 8.80 | −− | −− | −6.82 ± 2.80 | 13.7 ± 2.90 | Cabergoline | Load of 0.167 %w/w | By adding CS solution (1 %w/w in acetate buffer pH 5) with stirring to the continuous phase such that the final content of chitosan in the formulations is 0.5 %w/w | None | [102] | |
| Epilepsy | Polymeric NPs | PLGA | 93.46 ± 3.94 | 106.31 to 142.43 | 0.106 ± 0.01 | 0.239 to 0.364 | −12.63 ± 0.08 | 21.64 to 24.34 | Catechin Hydrate | EE: 80.36 to 81.66 and DL: 5.98 to 6.87 | Immersion in acidic (0.50% of acetic acid) CS solution (2.0 or 4.0 mg/mL) with 2.0 h of interaction | None | [17] |
| PLGA | 85.12 to 89.3 | 91.9 to 96.5 | 0.314 to 0.332 | 0.111 to 0.255 | −2.53 to −3.47 | 17.47 to 20.29 | Analogues of thyrotropin releasing hormone (NP-355 and NP-647) | EE: 47.94 to 52.56 and DL: 51.5 to 188.01 μg/mg of NPs | Electrostatic adsorption in CS solution at 1 mg/mL followed by stirring for 2 h at 400 rpm at room temperature | None | [18] | ||
| Cerebral Ischaemia | Polymeric NPs | PCL | 163.4 to 234.6 | 201.3 to 283.6 | 0.146 to 0.364 | 0.253 to 0.409 | −21.22 to −6.22 | 17.8 to 25.9 | Glycyrrhizic Acid | EE: 77.94 to 74.43 and DL: 4.17 to 4.84 | By incubation (2 h) with drug of an equal volume of CS solution (2 mg/mL in 65% acetic acid) | None | [20] |
| PCL | 181.3 to 254.0 | 224.5 to 284.0 | 0.143 to 0.297 | 0.216 to 0.419 | − 22.31 to − 28.17 | 18.64 to 26.04 | Eugenol | EE: 68.13 to 71.04 and DL: 4.29 to 5.14 | [21] | ||||
| Emulsions | Nanoemulsion (Capmul MCM®, Tween-80® and PEG-400) | 91.39 ± 1.89 | 98.31 ± 1.17 | 0.372 ± 0.014 | 0.386 ± 0.021 | −19.24 | 13.91 | Naringenin | Load of 2.0 %w/v | At the end of emulsion formation, CS Solution is added (2.3 mL at 0.50 %w/v) | None | [100] | |
| Schizophrenia and Bipolar Disorders | Polymeric NPs | PLGA | −− | 306.1 to 700.0 | −− | 0.173 to 0.462 | −− | 5.67 to 24.7 | Chlorpromazine Hydrochloride | EE: 18.61 to 36.72 and DL: 2.32 to 4.59 | Via amide bond formation mediated by carbodiimide, with 12 h immersion at room temperature | None | [19] |
| Lipid NPs | NLC (Glyceryl monostearate and Oleic Acid) | 167.30 ± 7.52 | 181.58 to 186.97 | −− | −− | −4.34 ± 1.37 | 5.51 to 18.88 | Asenapine Maleate | EE: 82.46 to 84.24 | Glycol CS solutions at 0.01, 0.05, 0.1, 0.2 and 0.4 %w/v were added to NCL suspension, stirring for 24 h | None | [88] | |
| Emulsions | Nanoemulsion: Capmul MCM®, Tween-80® and polyethylene glycol 400) | 20.1 ± 1.65 | 23.6 ± 2.11 | 0.264 ± 0.08 | 0.292 ± 0.06 | −28.41 ± 2.14 | −25.5 ± 1.32 | Olanzapine | Load of 8.5 mg/mL (Drug Content of 97.96 0.24%) | By addition of CS (0.50 %w/w) to aqueous phase in emulsion preparation. The dispersion was stirred for 1 h | None | [101] | |
| Migraine and pain | Lipid NPs | SLN (Glycerol Tripalmitate) | −− | 192.0 to 301.4 | −− | −− | −− | 30.2 to 51.4 | Sumatriptan Succinate | EE: 76.3 to 91.1 | CS solution (1% glacial acetic acid) as aqueous phase (with 150 a 250 mg of CS) is incorporated in the solvent injection method | None | [84] |
| NLC (Compritol® and Labrafil®) | −− | 255 | −− | 0.27 | −− | 34.1 | Almotriptan Maleate | EE: 80 | Adsorption by immersion in CS solution | None | [89] | ||
| Nanovesicles | Bolaamphiphilic vesicles (bolalipids GLH-19 and GLH-20) | 67.8 to 117.2 | −− | 0.155 to 0.277 | −− | 39.5 to 53.0 | −− | Kyotorphin and Leu-Enkephalin (analgesic peptides) | EE: 5.8 to 11.2 | CS-Vernolic acid conjugate was added at a molar concentration 5-fold lower than GLH-19 or GLH-20, at a molar ratio of 2:1, during film formation | None | [95] | |
| Polymeric NPs | PLA | 121.2 ± 5.2 | 140.5 ± 5.4 | −− | −− | −29.28 ± 2.39 | 33.71 ± 3.24 | Neurotoxin from venom of Naja naja atra | EE: 75.17 to 83.51 | Addition of CS solution (0.2 %w/v) in second aqueous phase in the resulting w/o/w emulsion, sonicated for 53 s | None | [78] | |
| Neuroprotection | Polymeric NPs | PLGA | 99.6 ± 6.3 | 146.7 ± 5.1 | −− | −− | −18.3 ± 1.2 | 21.0 ± 2.9 | Coenzyme Q10 | DL: 8.8 | By covalently coupled of TMC via a carbodiimide-mediated link | None | [59] |
| Lipid NPs | SLN (Palmitic Acid) | 138.8 ± 7.6 | 311.9 to 412.0 | 0.15 ± 0.04 | 0.24 to 0.26 | −29.67 ± 1.20 | 27.08 to 35.70 | Curcumin | EE: 93.12 ± 0.06 and LC: 4.04 ± 0.01 | Surface modified with TMC by charge interaction (50:1-CS:SLN w/w) by dispersion in distilled water and stirring for 10 h | None | [85] | |
| NLC (Precirol ATO5® or Dynasan 114® and Miglyol®) | 107.12 and 159.35 | 114.48 and 191.89 | 0.342 and 0.361 | 0.287 and 0.386 | −30.30 and − 19.12 | 28.40 and 41.50 | Neurotrophic factor human insulin-like growthfactor-I | EE: 90.28 ± 0.4 | NCL dispersion is added dropwise to an equal volume of a CS solution (0.5 %w/v) kept under continuous agitation at room temperature for 20 min | None | [90] | ||
| Lipid microparticles | Stearic Acid | 68.5 ± 3.1 μm | 76.3 and 84.5 μm | −− | −− | −12.7 ± 2.1 | 24.0 and 44.6 | Resveratrol | EE: 76.5 and 81.0 | Microparticles were added to CS solution (1.75 and 8.75 %w/v), during the cooling phase of emulsion method | None | [91] | |
| Cerebrovascular Inflammation | Inorganic NPs | Gadolinium-Magnevist® (MRI contrast agent) | 164 ± 1.2 | 239 ± 4.1 | −− | −− | 11.9 ± 0.5 | 21.6 ± 1.7 | Cyclophosphamide | DL: 21.7 ± 1.3 | By emulsion-droplet coalescence technique developed at CS polymer concentration of 2.5 %w/v | Anti-amyloid antibody, IgG4.1 | [99] |
| Others (Cachexia, Traumatic Brain Injury) | Liposomes | Lipoid S100® | 147.3 ± 4.3 | 194 ± 6.1 | 0.119 | 0.198 | −0.6 ± 0.3 | 6 ± 0.4 | Ghrelin | EE: 9.8 ± 3.7 | N-([2-hydroxy-3-trimethylammonium]propyl) CS chloride at 1 mg/mL is added dropwise to LP suspension under magnetic stirring at 3,000 rpm | None | [93] |
| Inorganic NPs | Iron Oxide | 7.0 ± 0.6 (22.7) | 50 to 70 | −− | −− | −− | −− | Reporter DNA (pCMV-td Tomato plasmid) | Efficient plasmid complex | CS-PEI magnetic-micelles (CPMMs) were prepared in a weight 1:1 ratio of CS-PEI (polyethyleneimine) (2 mg/mL) | MRI: Gadolinium chelates | [97] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés, H.; Alcalá-Alcalá, S.; Caballero-Florán, I.H.; Bernal-Chávez, S.A.; Ávalos-Fuentes, A.; González-Torres, M.; González-Del Carmen, M.; Figueroa-González, G.; Reyes-Hernández, O.D.; Floran, B.; et al. A Reevaluation of Chitosan-Decorated Nanoparticles to Cross the Blood-Brain Barrier. Membranes 2020, 10, 212. https://doi.org/10.3390/membranes10090212
Cortés H, Alcalá-Alcalá S, Caballero-Florán IH, Bernal-Chávez SA, Ávalos-Fuentes A, González-Torres M, González-Del Carmen M, Figueroa-González G, Reyes-Hernández OD, Floran B, et al. A Reevaluation of Chitosan-Decorated Nanoparticles to Cross the Blood-Brain Barrier. Membranes. 2020; 10(9):212. https://doi.org/10.3390/membranes10090212
Chicago/Turabian StyleCortés, Hernán, Sergio Alcalá-Alcalá, Isaac H. Caballero-Florán, Sergio A. Bernal-Chávez, Arturo Ávalos-Fuentes, Maykel González-Torres, Manuel González-Del Carmen, Gabriela Figueroa-González, Octavio D. Reyes-Hernández, Benjamín Floran, and et al. 2020. "A Reevaluation of Chitosan-Decorated Nanoparticles to Cross the Blood-Brain Barrier" Membranes 10, no. 9: 212. https://doi.org/10.3390/membranes10090212
APA StyleCortés, H., Alcalá-Alcalá, S., Caballero-Florán, I. H., Bernal-Chávez, S. A., Ávalos-Fuentes, A., González-Torres, M., González-Del Carmen, M., Figueroa-González, G., Reyes-Hernández, O. D., Floran, B., Del Prado-Audelo, M. L., & Leyva-Gómez, G. (2020). A Reevaluation of Chitosan-Decorated Nanoparticles to Cross the Blood-Brain Barrier. Membranes, 10(9), 212. https://doi.org/10.3390/membranes10090212