1. Introduction
The increasing frequency and intensity of harmful cyanobacteria (algae) blooms in surface freshwater, due primarily to eutrophication and climate change [
1], poses challenges to drinking water treatment plants. The rapid changes in cell densities and their tendency to float can result in inefficient treatment by coagulation and cell breakthrough to settled water [
2]. Meanwhile, low-pressure ultrafiltration (UF) membranes can almost completely remove cyanobacteria cells, including the smallest ones (Microcystis aeruginosa, 3–6 μm), from water by size exclusion [
3] and with minimal cell breakage [
4]. However, membrane productivity rapidly decreases due to fouling by cyanobacteria cells and natural organic matter (NOM) present in surface water. Cyanobacteria cells form a compressible cake layer on the membrane [
4,
5] whereas NOM adsorbs on the membrane surface as well as within the membrane pores [
6,
7]. Consequently, fouling increases operation costs and remains the greatest obstacle to the application of membranes in drinking water treatment, especially the hydraulically irreversible fouling fraction (i.e., fouling not removed by hydraulic backwashes) [
8,
9].
To remove hydraulically irreversible fouling, chemical cleaning is most commonly executed as a clean-in-place (CIP) operation, which requires that the membrane be soaked in chemicals for extended periods of time [
9]. The drawbacks of a CIP are that it requires process adjustments, high chemical concentrations, and results in costly downtime [
9]. A chemically enhanced backwash (CEB) is an alternative strategy in which a chemical, at a relatively lower concentration compared to a CIP, is combined with a hydraulic backwash for an in-situ cleaning process that results in downtimes similar to those of hydraulic backwashes [
10].
Ozone is of particular interest for CEB applications, with ceramic membranes, as it reacts rapidly with organic material in water [
11] and only small exposures are required to damage cyanobacterial cells [
2]. Furthermore, the catalytic decomposition of ozone by ceramic materials leads to the formation of stronger and less selective oxidants, making the combination of ozone and ceramic membranes very attractive and yielding new opportunities for membrane fouling control [
11]. In comparison, polymeric membranes are damaged by repeated exposure with oxidants [
8], although polymeric membranes with improved ozone resistance are being developed.
The efficiency in which ozone can clean ceramic membranes has been previously demonstrated in the literature. For instance, when ozone was used in a CIP procedure to clean a ceramic ultrafiltration membrane fouled by humic acids and alginate fractions of NOM, over 98% of the unified membrane fouling index (UMFI) was recovered within an hour [
12]. In that application, a dissolved ozone solution was recirculated with a normalized dose of 0.5 mg O3 per mg of organic carbon on the membrane at the start of the CIP. The reported performance of the ozone CIP was comparable to a 4-h CIP with a mixed solution of sodium hydroxide and hypochlorite.
Sartor et al. [
13] used continuous in-situ ozonation, a technique in which ozone is continuously dosed in the membrane module during filtration, to control ceramic membrane fouling by surface water. In this study, the rate of fouling was reduced and hydraulic backwashes maintained the membrane’s specific flux at approximately 80% of its original value, which is up to 4 times higher than without in-situ ozonation. In the presence of the cyanobacterium M. aeruginosa (2 × 106 cells/mL, DOC: 1.67 mg/L), Wei et al. [
14] also reported that in-situ ozonation (0-5 mg/L) reduced the rate of UF membrane fouling. The authors of both studies hypothesized that the reactions between ozone, the extrapolymeric substances, and the natural organic matter in the feed water lead to the formation of a more porous cake layer on the membrane surface. This more porous cake resulted in a membrane foulant resistance that was smaller than foulant resistance caused by the cake formed without in-situ ozonation.
The application of in-situ ozonation reduces the rate of membrane fouling but this type of application is energy-intensive and requires the continuous addition of ozone to be effective [
15]. A greater ozone demand is expected as ozone can react with all the feed components, not only what fouls the membrane. Furthermore, in treatment applications involving cyanobacteria, ozone can induce the release of the cells’ internal organic metabolites [
2] which often exacerbates hydraulically irreversible fouling [
14]. Furthermore, these metabolites can be toxic [
1], and permeate through the membrane [
14] causing concerns for human health. In these cases, there is an increase in the permeate’s dissolved organic carbon concentration and an adsorptive media such as granular activated carbon is required downstream to remove these components and ensure regulatory compliance [
13].
Chemically enhanced backwashing (CEB) does not suffer from the disadvantages of CIPs and continuous in-situ ozonation. However, research addressing the mechanisms and kinetics involved in CEB fouling control applications is limited. The impact of an ozone CEB on membrane fouling was quantified, in which ozone could almost completely remove the fouling of a ceramic microfiltration treating municipal wastewater [
16]. The authors suggested that this performance was due to increased ozone reactions with foulants present within the membrane pores. Most of the other studies regarding CEB applications used a chlorine CEB for membrane fouling control in seawater desalination and feed water that has a low particulate organic matter concentration. The CEB was rarely the primary focus of these studies, which generally address the application of low-pressure membranes as a pretreatment step to reverse osmosis. Additionally, the frequency of the CEB and the oxidant dose varied greatly between studies (between every 1 to 24 h; 1 to 500 mg/L Cl2), and the chosen doses where not justified [
10]. Studies analyzing the impact of CEB parameters on performance have not been found for surface water applications nor cyanobacteria-laden water applications.
The objective of this research is to demonstrate the potential of ozone chemically enhanced backwashes to control the fouling of ceramic ultrafiltration membranes by cyanobacteria in a filtered surface water and to understand the cleaning mechanisms involved in the process. This will be achieved by: (1) comparing the performance of an ozone CEB to the performance of a hydraulic (water-only) backwash, (2) determining the rate of ozone demand and organic foulant removal from the membrane during an ozone CEB, and (3) evaluating the effect of ozone dose on foulant removal and membrane resistance. This research will help determine the importance of different CEB parameters in order to optimize the impact of an ozone chemically enhanced backwash on membrane fouling control.
2. Materials and Methods
2.1. Cyanobacterial Culture
Microcystis aeruginosa (CCPC 633, non-toxic strain) was purchased from the Canadian Phycological Culture Centre (Waterloo, Ontario, Canada). It was incubated in 3N-BBM growth media, at 21 ± 2 °C, with constant aeration, and under a 12-h light/dark cycle to simulate normal growth conditions (2000 lux, Phillips).
M. aeruginosa was chosen for this study as it is the predominant freshwater cyanobacterial species [
1] and has the smallest single cell diameter. Small single cells are problematic as they are the most likely to not be removed by coagulation and sedimentation [
3]. The culture’s growth was monitored by direct cell enumeration using an improved Neubauer hemocytometer (Marienfield). On average, the single cells had a diameter of 4.11 ± 0.75 μm once in the early stationary phase, between 30 to 36 days after inoculation.
2.2. Feed Water Characteristics
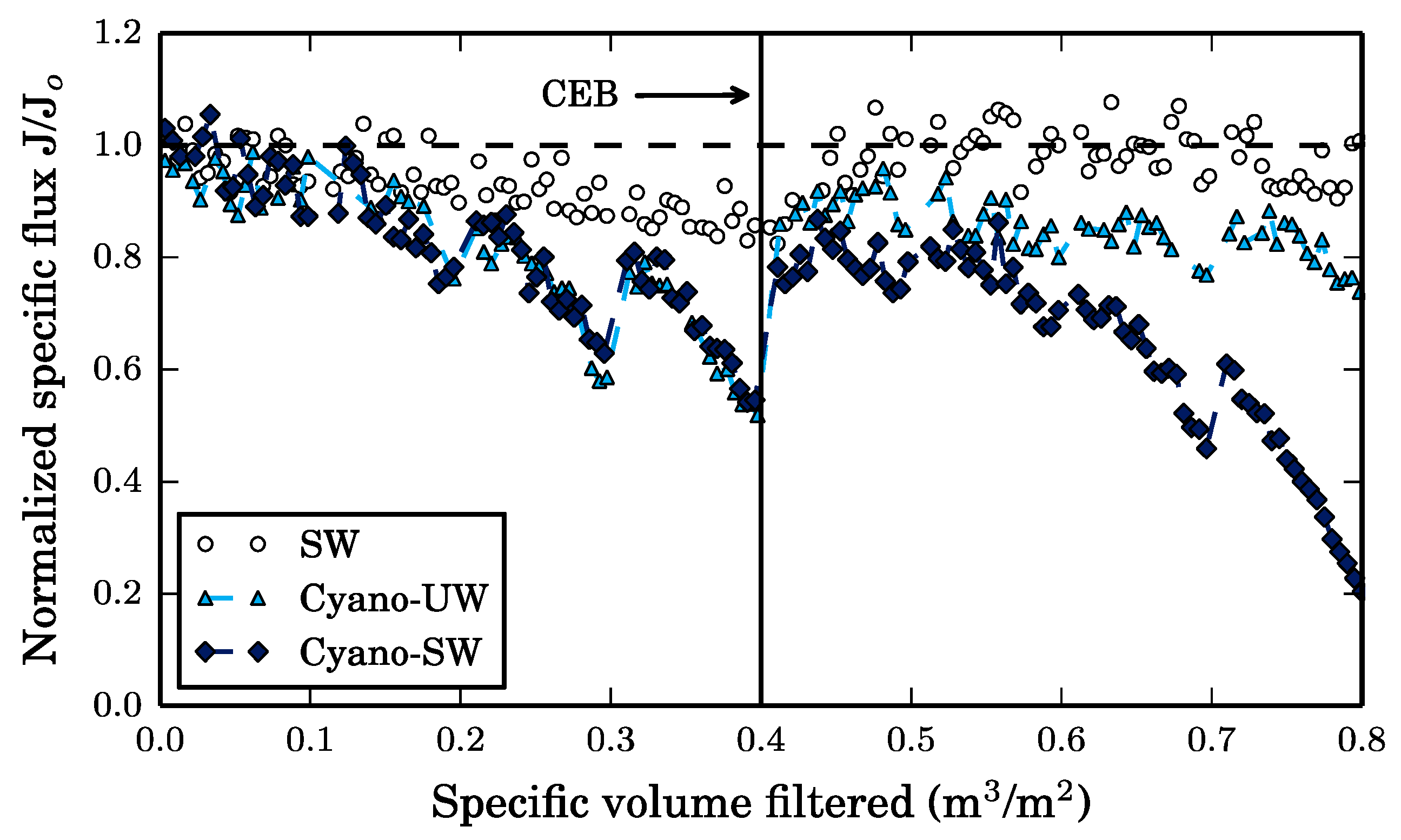
To fully understand the impact of different foulants present in surface water impacted by cyanobacteria on the performance of a chemically enhanced backwash, three feed solutions were used in this study: surface water (SW), cyanobacteria-spiked ultrapure water (Cyano-UW), and cyanobacteria-spiked surface water (Cyano-SW). The surface water was obtained from the Sainte-Rose Drinking Water Treatment Plant, of which the intake is located in the Rivière des Mille-Îles (Laval, Québec, Canada). The surface water used in the experiments was collected after alum coagulation and flocculation, sedimentation, and sand-anthracite filtration to minimize fluctuations in water quality throughout experiments.
To prepare the Cyano-UW feed solution, cyanobacteria cells were spiked into ultrapure water (Milli-Q™). Cyanobacteria cells were harvested in the early stationary growth phase and were separated from the growth medium by centrifugation at 10,000
g and 4°C for 15 min [
4,
14]. To obtain a cyanobacteria cell concentration of 5 × 10
5 cells/mL in the feed solution, a concentration that could represent the fraction of a large bloom that breakthrough conventional treatment [
17], the centrifuged volume of growth medium was determined based on the growth medium’s cell concentration.
The preparation of the Cyano-SW feed was similar to the preparation of the Cyano-UW feed except that the cyanobacteria cells were spiked directly into the filtered surface water sampled at the Sainte-Rose treatment plant instead of ultrapure water.
The parameters of the three feed solutions are listed in
Table 1. Since divalent ions have been shown to impact membrane fouling [
18], the total hardness of all three feed solutions was adjusted to 60 mg CaCO
3/L using calcium chloride dihydrate (Fisher Scientific). During the experiments, the temperature of the feeds was maintained at room temperature (23 ± 1 °C).
2.3. Bench-Scale Membrane Filtration System
Experiments were conducted with a ceramic ultrafiltration membrane (Atech Innovations, Germany) installed on a semi-automated bench-scale filtration system. A schematic representation of the system is presented in
Figure 1. The membrane was tubular, with a surface area of 95 cm
2 and a molecular weight cut-off of 150 kDa. The membrane surface was composed of zirconium dioxide (ZrO
2) and supported by an α-aluminum oxide (α-Al
2O
3) layer.
The membrane was operated in dead-end and was fed by a gear pump (drive: Ismatec BVP-Z; head: MicroPump L3468) to obtain a constant permeate flux of 200 LMH. Given the high mechanical strength of ceramic membranes, the permeate flux was set higher than average to accelerate fouling. The permeate flowrate (flow sensor: McMillan Flow 101-3T) and trans-membrane pressure (transducers: Omega PX309-100G5V) were measured every 30 s. The trans-membrane pressure was calculated by subtracting the pressure measured at the membrane outlet (PT ahead of V-4) from the feed pressure (PT ahead of V-1, see
Figure 1). Hydraulic (water-only) backwashes were initiated every 30 min and had a duration of 27 s (equivalent to 3 membrane volume replacements). Ultrapure water at room temperature and pressurized to 0.78 atm (11.5 psig) by nitrogen gas was used to obtain a flowrate of approximately 600 LMH during the hydraulic backwash.
At the end of each experiment, the membrane was washed with a mixed solution of sodium hydroxide and sodium hypochlorite (pH 12, 500 mg/L as Cl
2, 35 °C) [
12]. To do so, the feed tank in
Figure 1 was replaced with the cleaning solution, which was recirculated at a cross-flow velocity of 0.1 m/s for 1 h and then left to soak for 3 h. This was followed by a hydrochloric acid wash to remove any residual organic and inorganic foulants. The HCl solution (pH 2) was recirculated at 0.1 m/s for 1 h, left to soak for 2 h, and recirculated again for 1 h (adapted from [
19]). Afterwards, the membrane system was flushed with ultrapure water until the pH of the permeate was stable.
2.4. Experimental Plan
Three sets of experiments were conducted to address the research objectives: batch ozonation tests, baseline membrane performance tests, and ozone CEB tests.
2.4.1. Batch Ozonation Tests
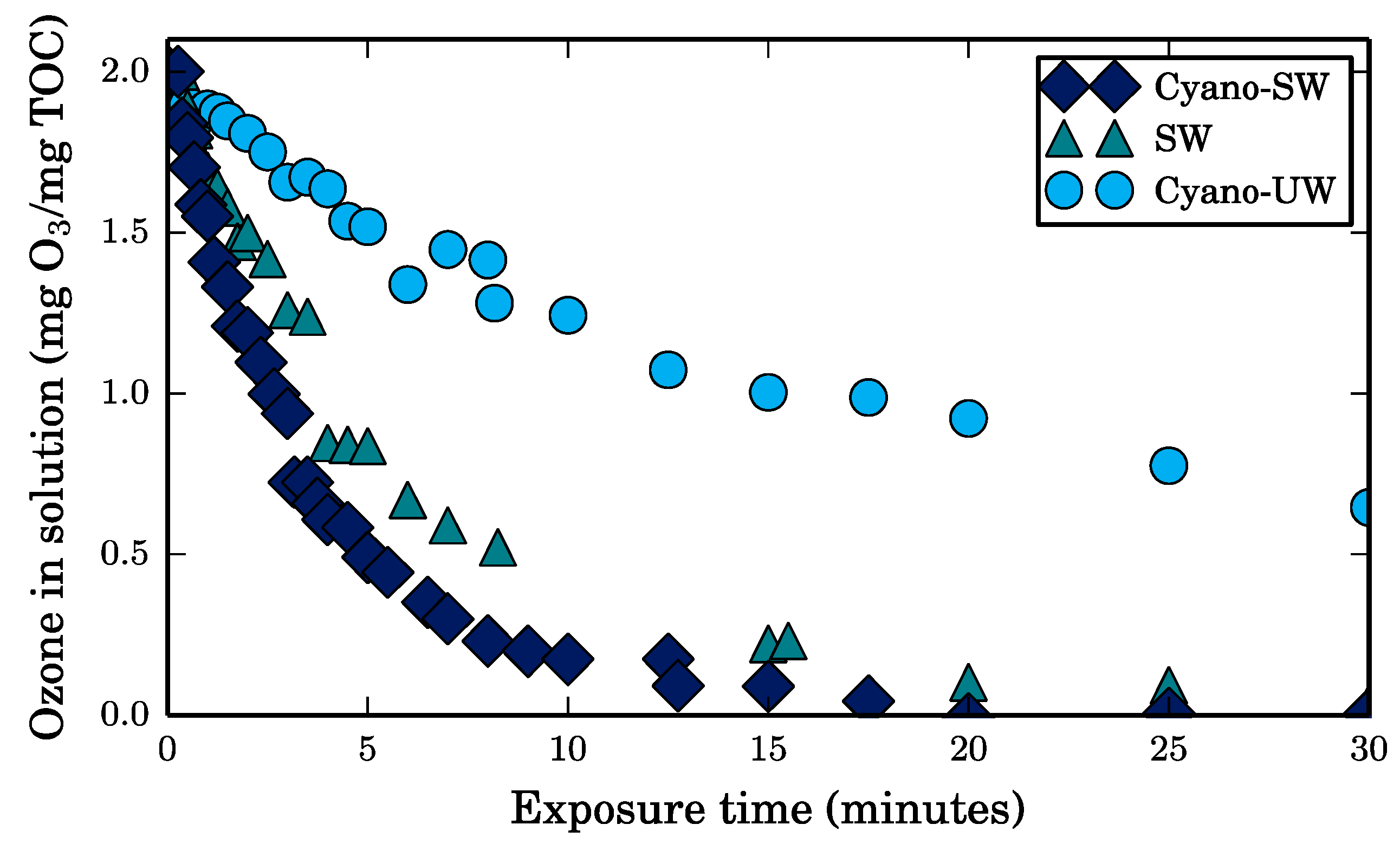
The purpose of the batch ozonation tests is to determine and compare the reactivity of ozone with the different foulants in the feed solutions, without the interference of the CEB’s hydraulic force or the membrane material, which reacts with ozone. To do so, a liter of each feed solution was placed in 2-L borosilicate glass beakers, spiked with ozone, and continuously stirred. A small volume of highly concentrated ozone stock solution (50–60 mg O
3/L) was added to obtain a normalized ozone dose of 2 milligrams per milligram of TOC in the feed solution. The greatest sample dilution factor caused by the addition of ozone stock was 18% and was observed in the Cyano-SW feed, which had the largest TOC content as seen in
Table 1. The stock was prepared by bubbling gaseous ozone, which was produced by a bench-top ozone generator (Ozone Solutions TG-10), in ultrapure water at 4 °C. To minimize degassing, the aliquot of ozone stock solution was dosed with a syringe, feed solutions were covered with floating polytetrafluoroethylene lids, and water samples for analysis were taken with a syringe. Solutions were left to react with ozone for 30 min. Experiments were conducted at room temperature (23 °C). The ozone concentration in solution throughout the tests was determined using the indigo colorimetric method described elsewhere [
20].
2.4.2. Baseline Membrane Performance Tests
The purpose of the baseline tests is to determine the membrane fouling mechanisms involved when filtering the feed solutions and to evaluate their impact on fouling control by hydraulic backwashes. To do so, the feed solutions were filtered on the bench-scale membrane system discussed in
Section 2.3. The control condition consisted in hydraulic backwashes using a solution without ozone for the same duration/frequency as a 2 mg O
3/mg C CEB. The performance of these extended backwashes will be compared to the performance of CEBs in the ozone CEB tests.
2.4.3. Ozone CEB Tests
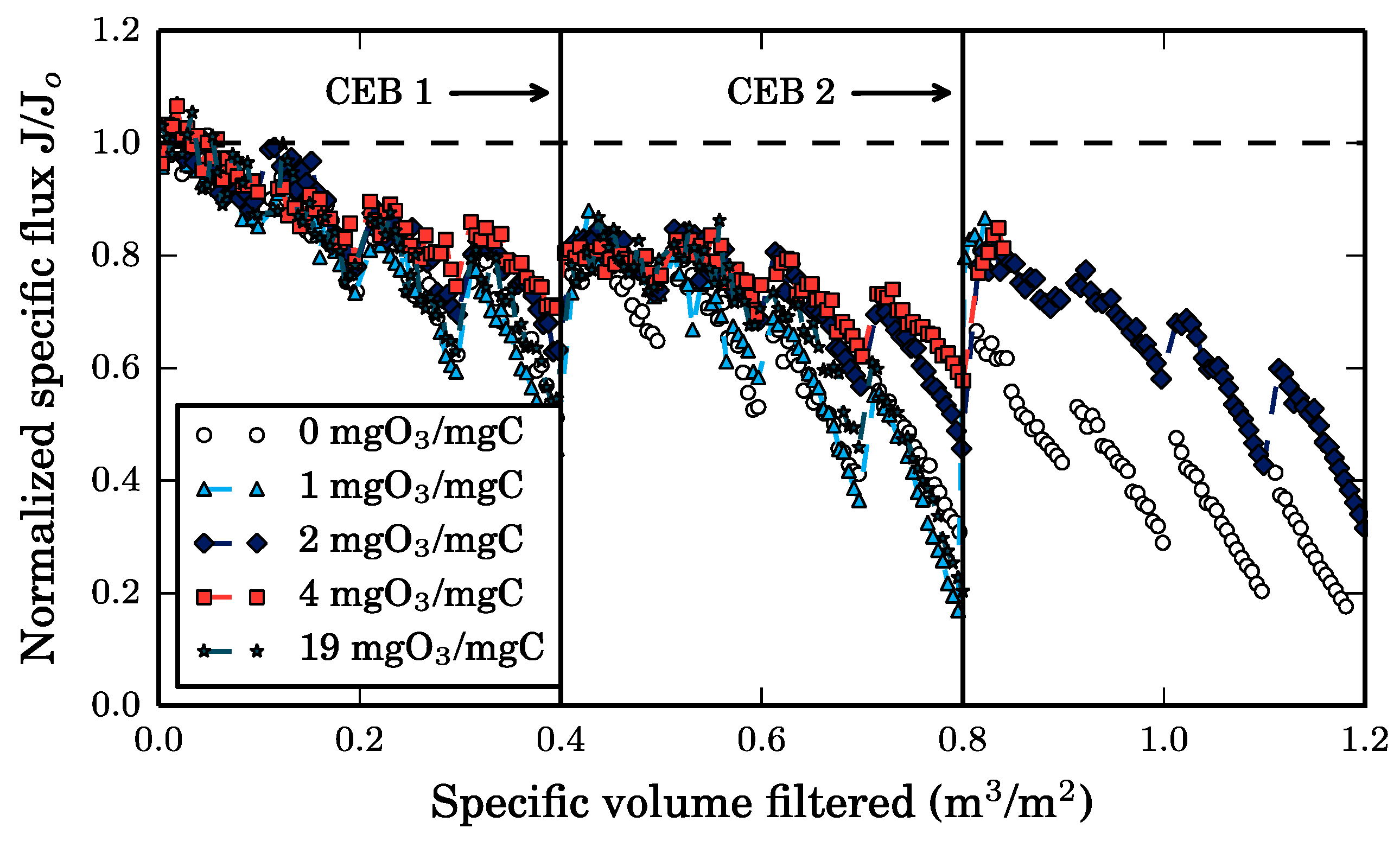
Two series of CEB experiments were conducted. Firstly, 30-min long ozone chemically enhanced backwashes were initiated after 2 h of filtration on the bench-scale membrane system described in
Section 2.3, during which the residual ozone concentration in the effluent and the cumulative organic matter removed from the membrane were monitored. This experiment was repeated with each feed solution. A control experiment, which is a 30-min CEB of the clean membrane, was also conducted to estimate the ozone demand from the ceramic membrane itself given its reactivity with ozone. Overall, the purpose of the 30-min CEB is to determine the foulant removal kinetics by ozone and to compare them to the kinetics observed during the batch ozonation tests.
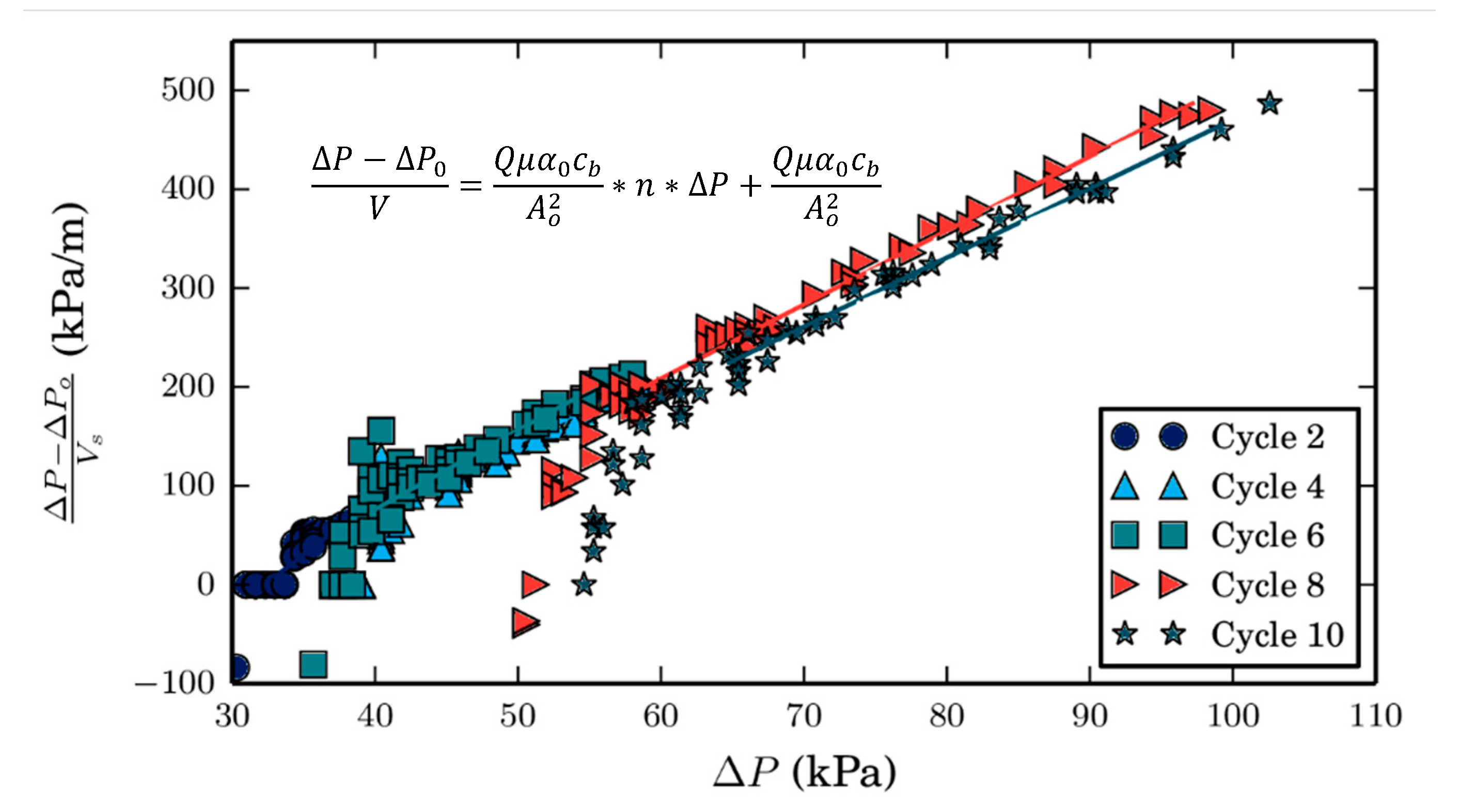
Secondly, the purpose of the second set of ozone CEB experiments is to evaluate the impact of the ozone dose on the post-CEB membrane resistance. To do so, the Cyano-SW feed was filtered for 6 h on the bench-scale membrane system described in
Section 2.3, during which an ozone CEB was initiated every 2 h. The experiment was repeated with different CEB ozone doses, which were varied by adjusting the CEB duration. To normalize the results, the doses were expressed as a function of the organic carbon mass remaining on the membrane surface prior to the CEB (m
CEB), as determined by mass balances. The mass balance calculation is expressed in Equations (1a) and (1b), where TOC
f, TOC
p, and TOC
BW are the TOC concentrations in the feed, permeate, and cumulative hydraulic backwash effluent, respectively; whereas V
f, V
p, V
BW, and V
m are the volumes of feed filtered, the volume of permeate (which is equal to the volume of feed filtered since the membrane is operated in dead-end filtration), the cumulative backwash effluent, and the internal membrane volume, respectively.
In both sets of experiments, each CEB was preceded by a hydraulic backwash to specifically evaluate the impact of ozone on the hydraulically irreversible fouling fraction. This strategy also mimics the usual CEB approach which is applied after a regular BW. The ozonated water used in the CEBs was prepared by diffusing gaseous ozone directly into the CEB reservoir filled with ultrapure water at 4 °C, similarly to the method presented in
Section 2.4.1. Once the ozone concentration reached 40 mg/L, the reservoir was pressurized to 0.78 atm (11.5 psig) and the CEB was started immediately. The dissolved ozone concentration in the stock CEB solution and in the CEB effluent was determined with the indigo method [
20]. To minimize ozone degassing when sampling, the extremity of the CEB effluent line was submerged in an overflowing 45 mL vial. Samples could then be taken from the center of the vial with a syringe.
2.5. Analytical Methods
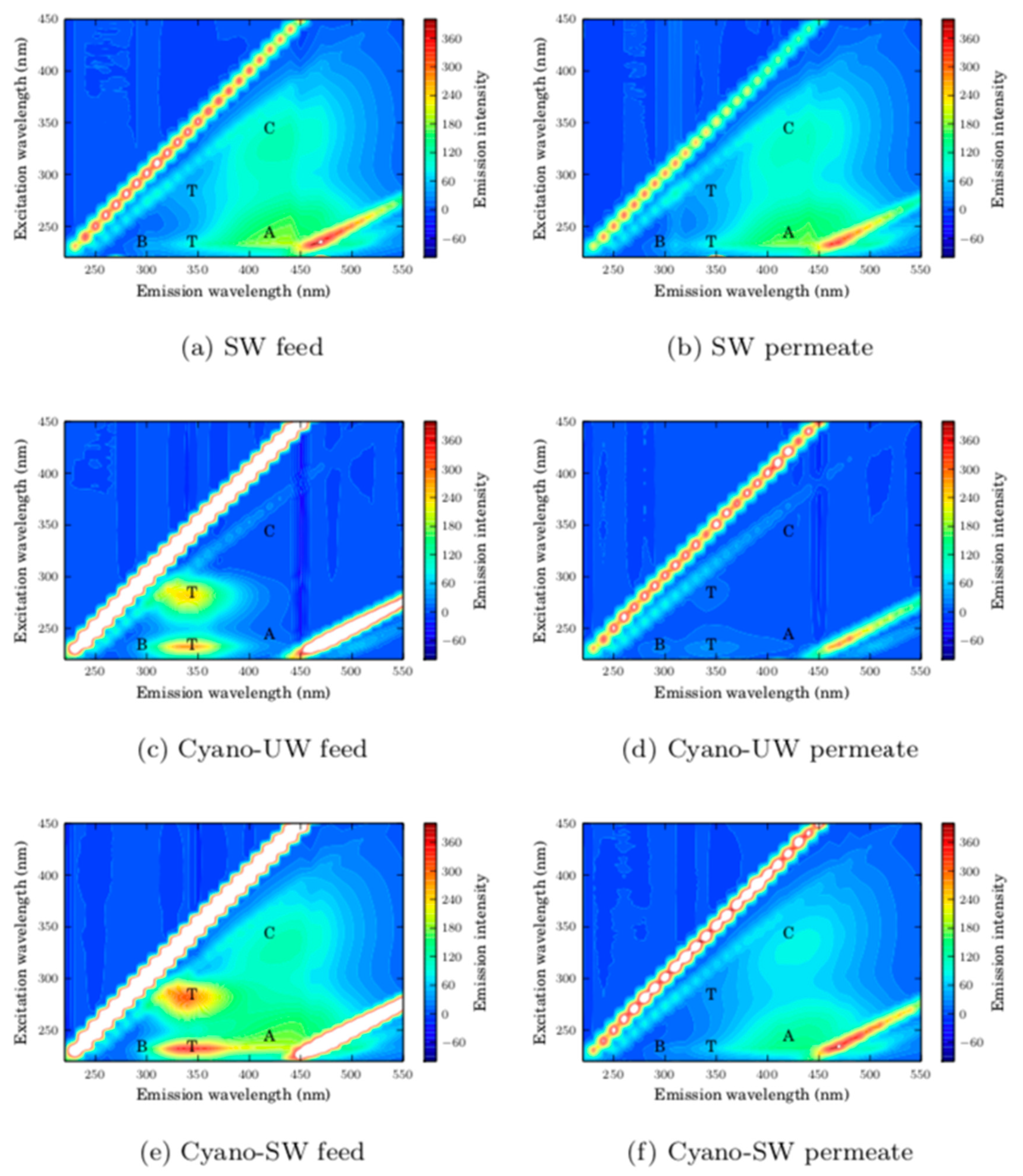
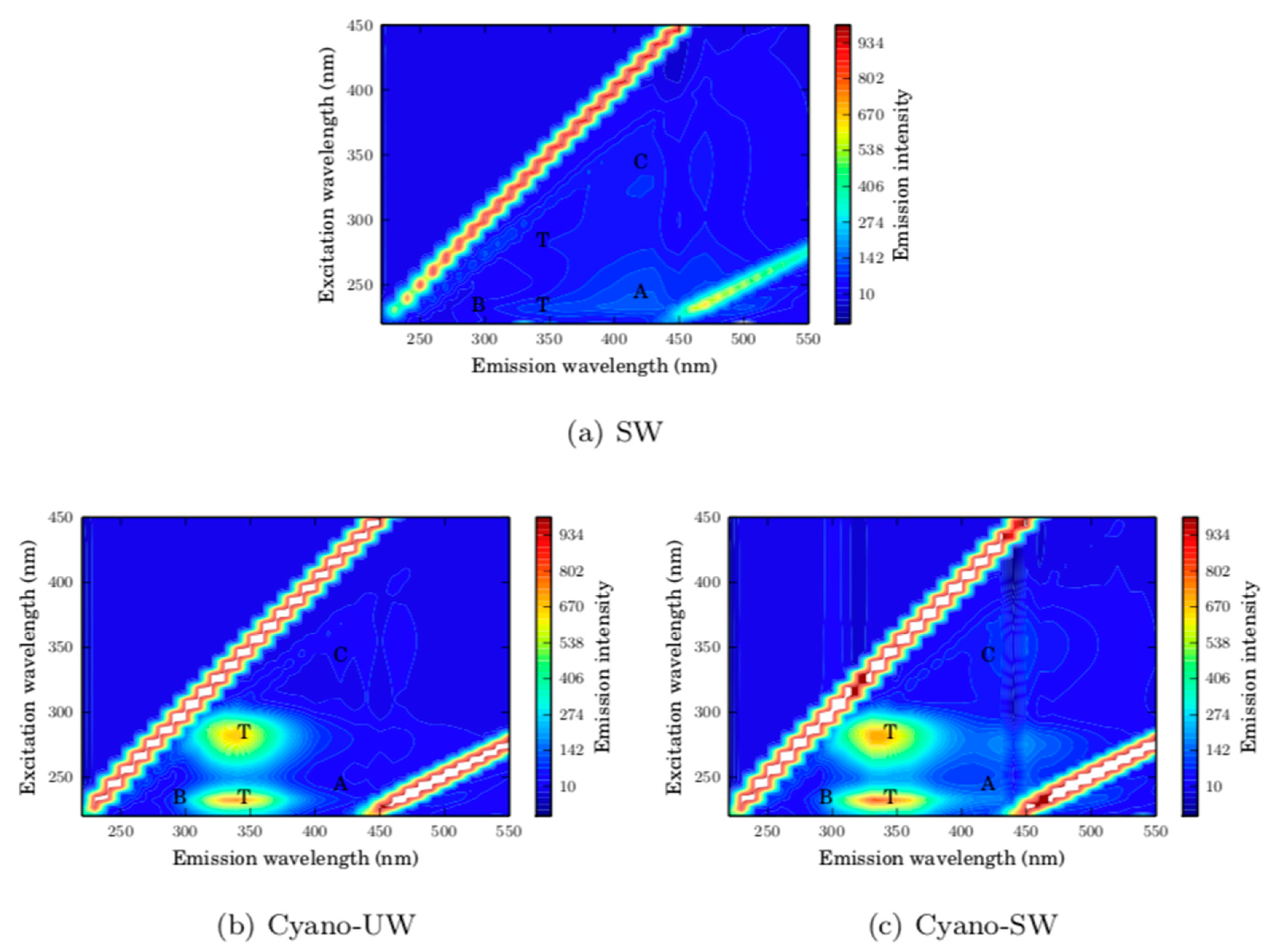
In addition to the specific monitored parameters discussed above, the pH, the turbidity (Hach 2100), the total and dissolved organic carbon concentrations (Sievers M5310C On-Line TOC Analyzer), the ultraviolet absorbance at 254 nm (Cary UV-vis, Varian) were measured. Fluorescence excitation-emission matrices (FEEM) were also obtained (Shimadzu 5301PC). Excitation and emission wavelengths were set to range between 220 and 600 nm, with an excitation increment of 10 nm, an excitation slit width of 10 nm, an emission slit width of 5 nm, and a sampling interval of 1.0 nm. In the batch tests, these measurements were taken for the feed before and after ozonation. In the baseline and CEB experiments, the measurements were taken for the feed, permeate, hydraulic backwash (cumulative for all hydraulic backwash between two CEBs), and CEB effluents.
4. Conclusions
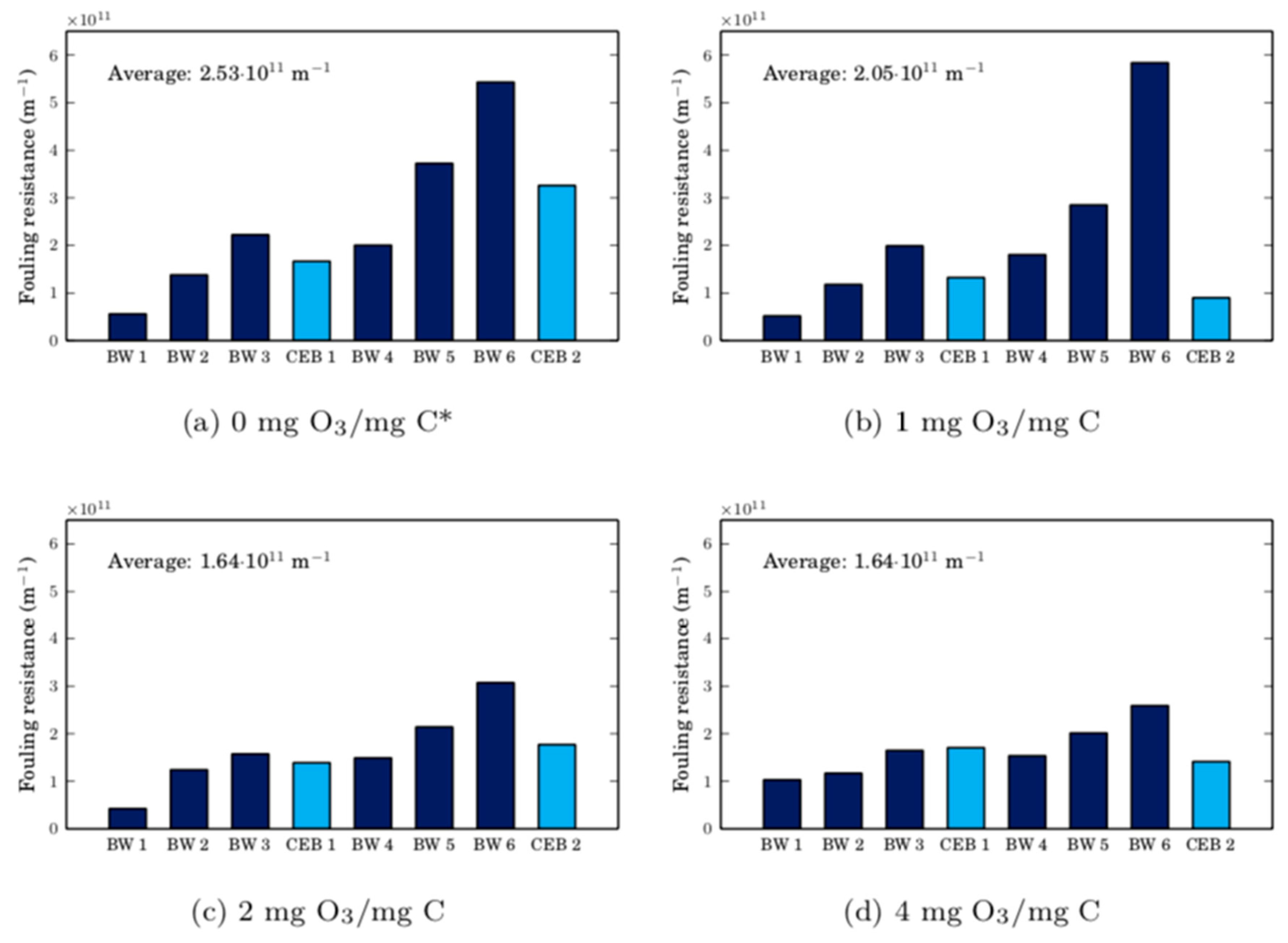
This research demonstrates that the removal of irreversible membrane fouling caused by cyanobacteria is not simply due to the cyanobacteria cells’ reactivity with ozone, but due to the combination of ozone and the CEB’s hydraulic force. Over two filtration cycles, the average fouling resistance was reduced by 35% when ozone chemically enhanced backwashes were initiated at doses of 2 mg O3/mg C or higher when compared to hydraulic backwashes only. In regard to the foulant removal mechanisms involved in the ozone CEB, the following insights were gained:
1. In batch test ozonation, the components in surface water react more rapidly with ozone than with cyanobacteria. During membrane filtration, this selectivity is not observed during the initial stages of a CEB. In this case, the foulant cake is likely weakened by ozone, increasing its porosity and allowing the cake to be more easily removed by the CEB’s hydraulic force.
2. All of the surface water foulants had been removed from the membrane module. Due to the incomplete removal of cyanobacteria foulants from the membrane surface, the membrane’s specific flux recovery was not maximized. Mass balances indicated that 74% of cyanobacterial foulants were still on the membrane surface after a 30-min CEB.