Enhanced Performance of Carbon Nanotube Immobilized Membrane for the Treatment of High Salinity Produced Water via Direct Contact Membrane Distillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Water Sample Composition
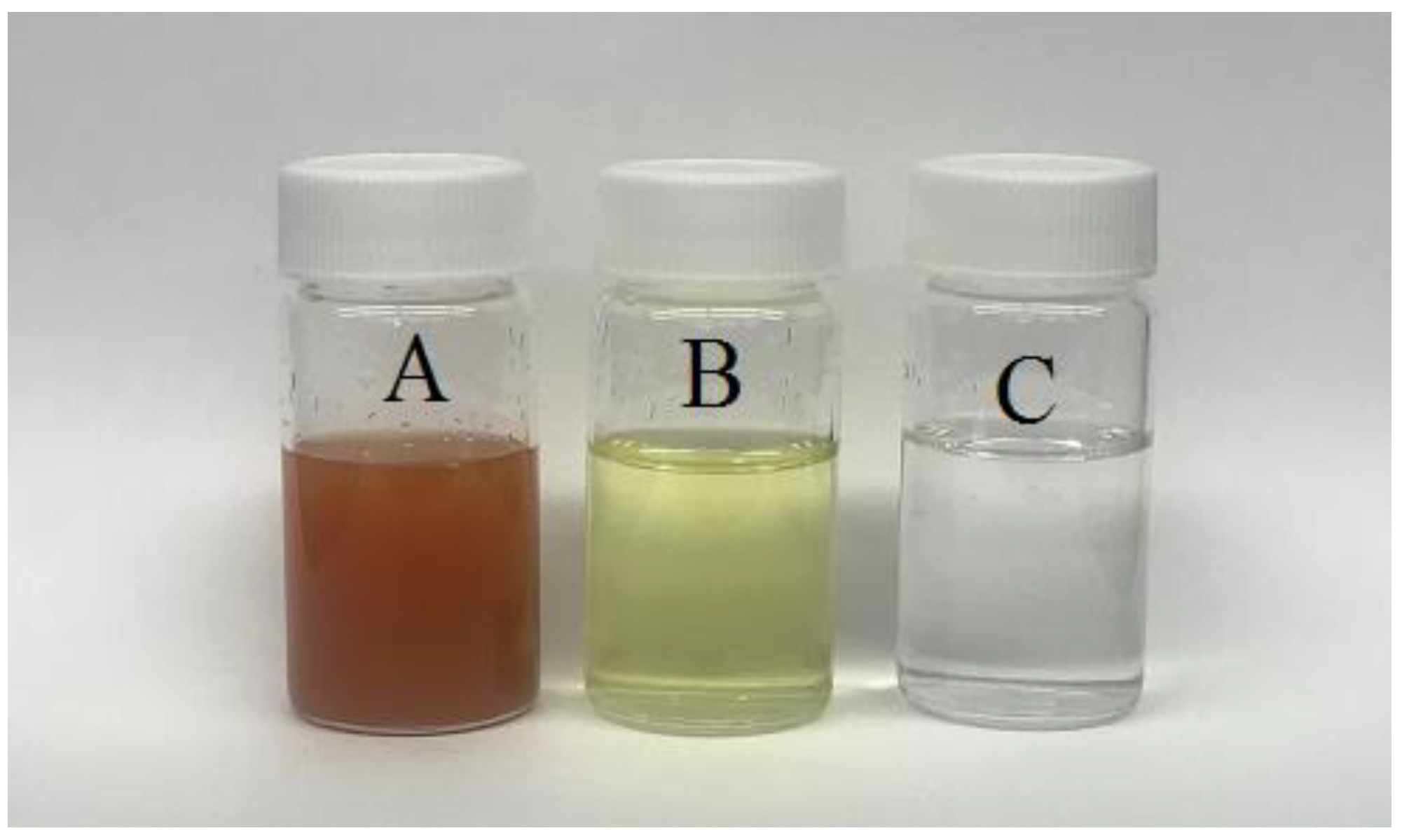
2.3. Water Sample and Pretreatment Methods
2.4. CNIM Fabrication
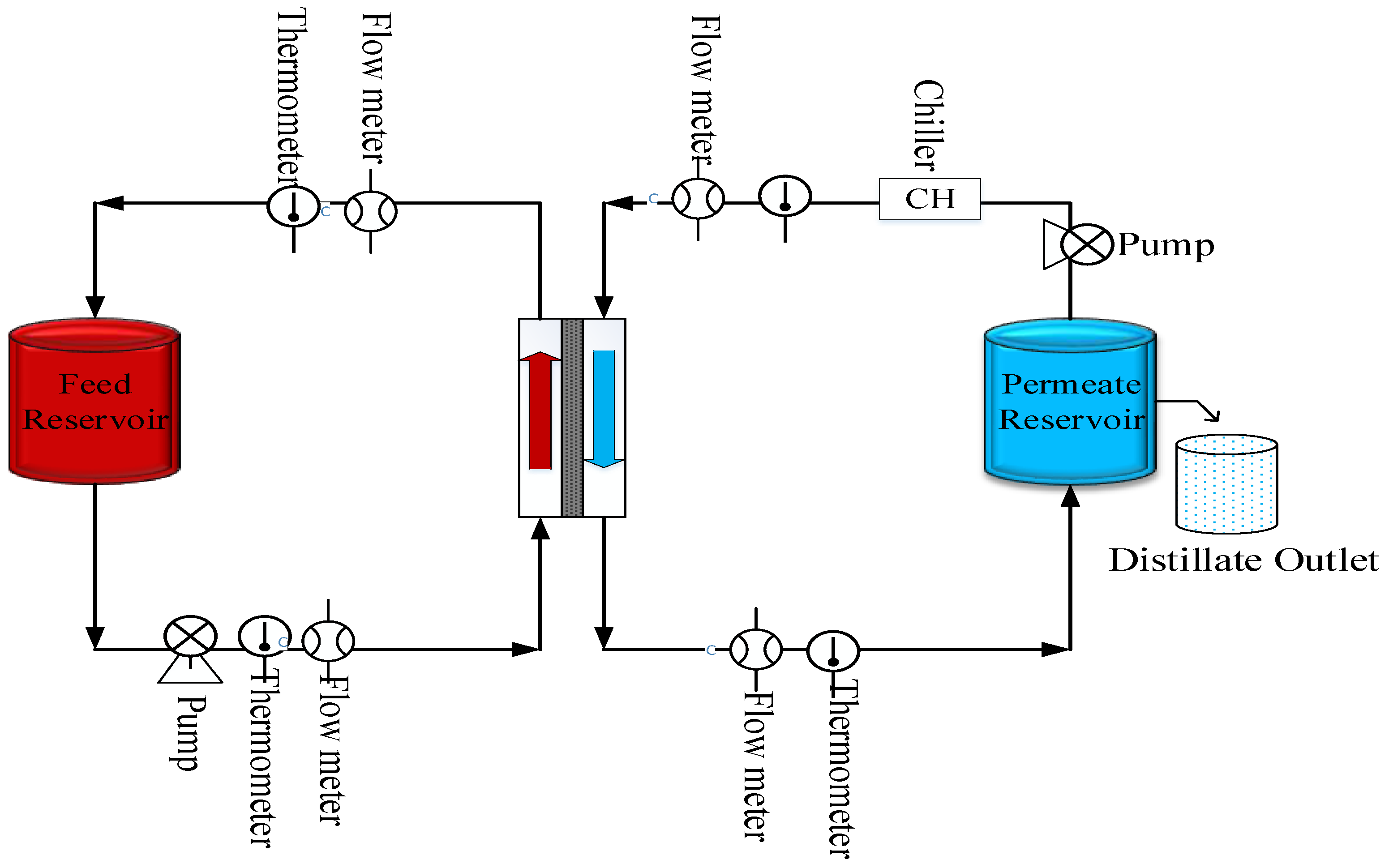
2.5. Experimental Procedure
2.6. DCMD Performance Using CNIM and PTFE Membrane
3. Results and Discussions
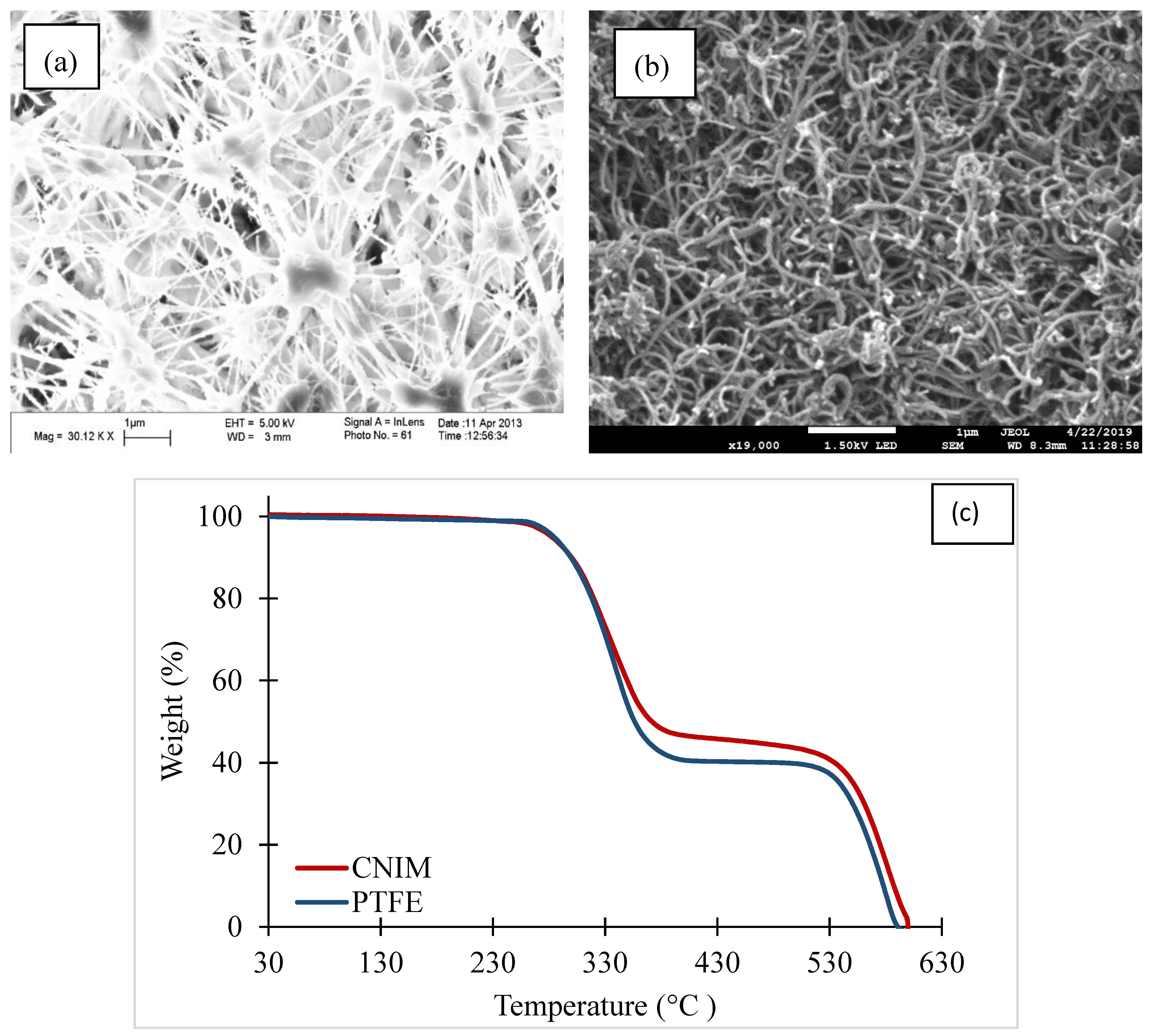
3.1. Membrane Characterization
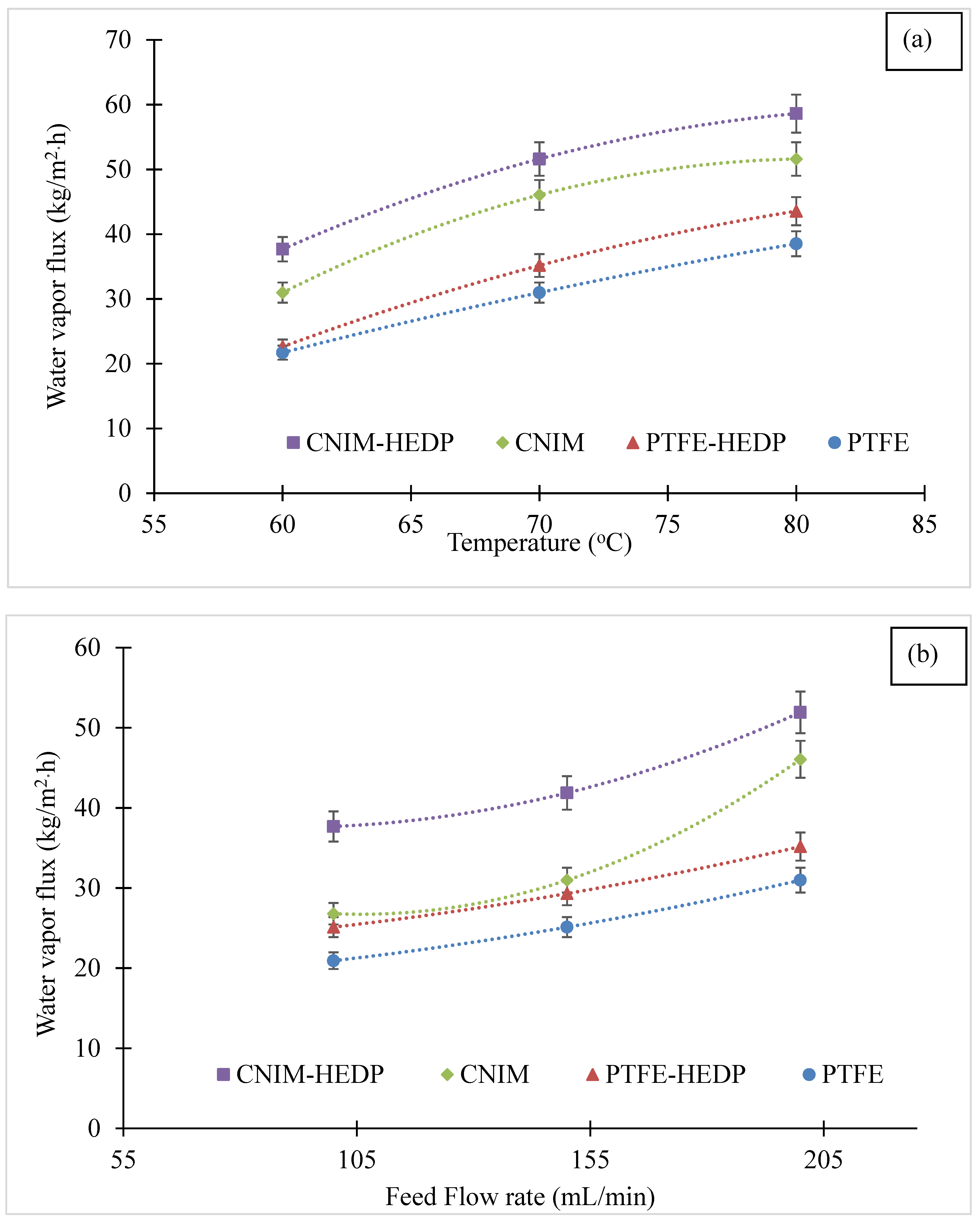
3.2. Effect of Temperature and Feed Flow Rate on Water Vapor Flux
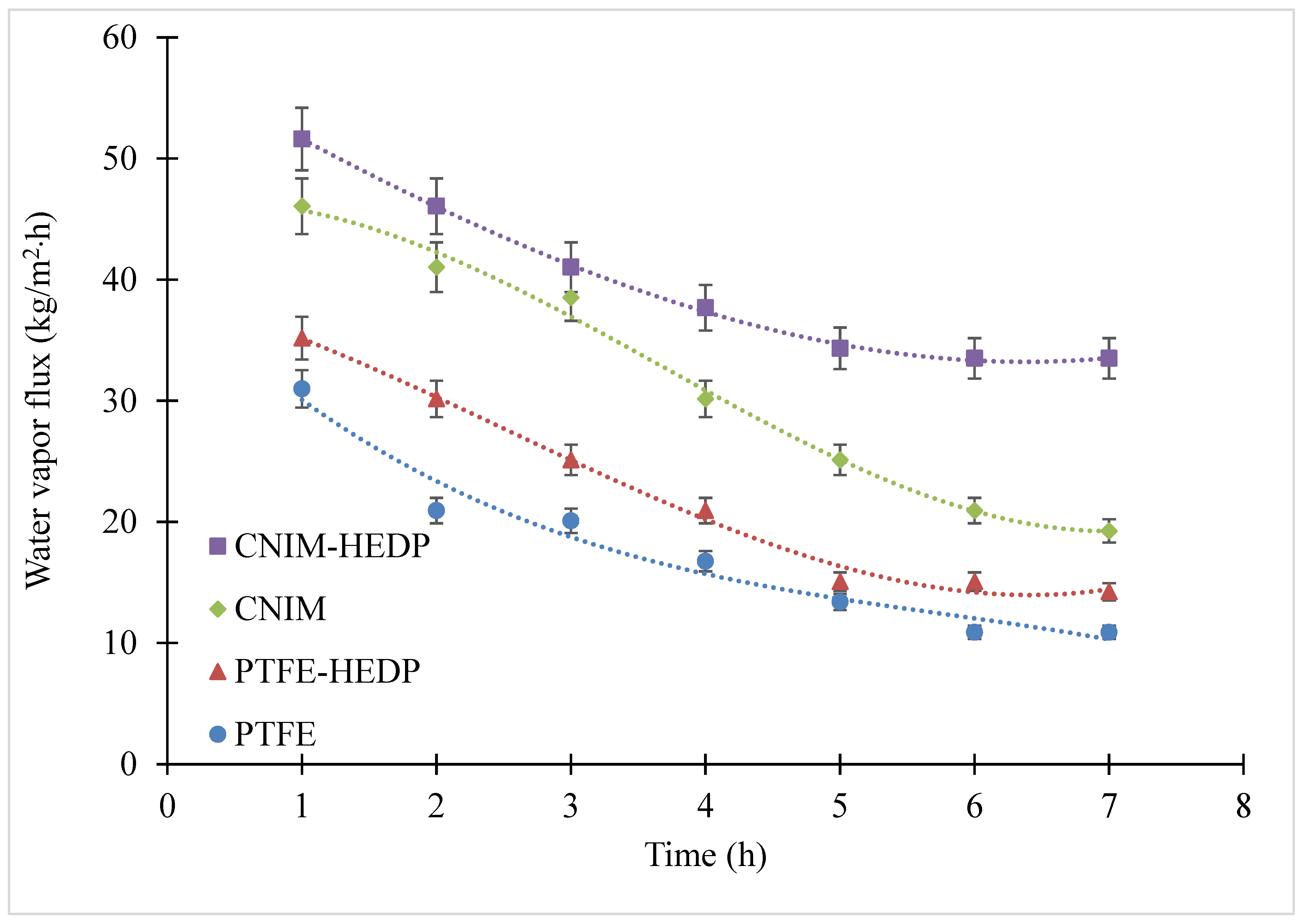
3.3. Fouling Behavior of Produced Water
3.4. Deposition of Foulants on the Membrane Surface
3.5. Membrane Regeneration
3.6. Mass Transfer Coefficient
4. Membrane Stability
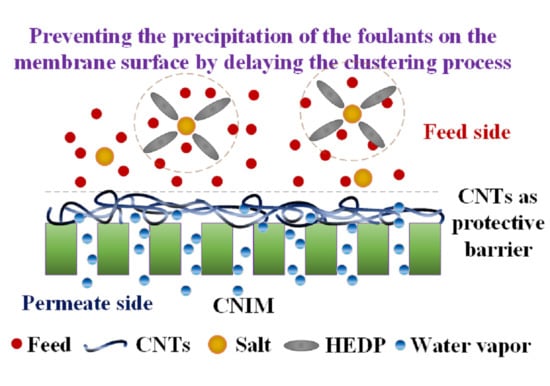
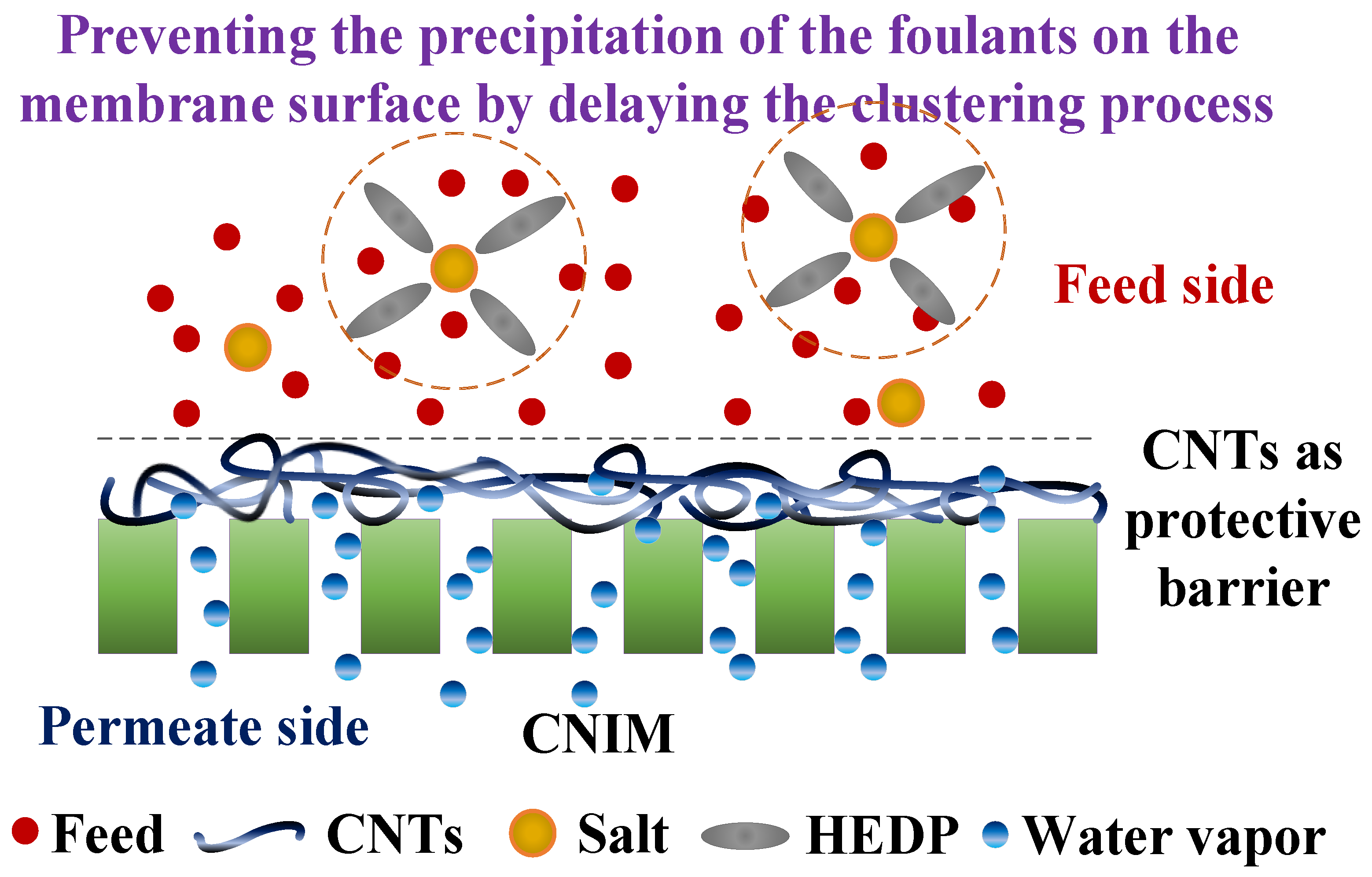
5. Proposed Mechanism
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Rourke, D.; Connolly, S. Just oil? The distribution of environmental and social impacts of oil production and consumption. Annu. Rev. Environ. Resour. 2003, 28, 587–617. [Google Scholar] [CrossRef]
- Gregory, K.B.; Vidic, R.D.; Dzombak, D.A. Water management challenges associated with the production of shale gas by hydraulic fracturing. Elements 2011, 7, 181–186. [Google Scholar] [CrossRef]
- Coday, B.D.; Xu, P.; Beaudry, E.G.; Herron, J.; Lampi, K.; Hancock, N.T.; Cath, T.Y. The sweet spot of forward osmosis: Treatment of produced water, drilling wastewater, and other complex and difficult liquid streams. Desalination 2014, 333, 23–35. [Google Scholar] [CrossRef]
- Choi, Y.C.; Kim, G.D.; Hendren, Z. Low-Energy Water Recovery from Subsurface Brines; RTI International, Research Triangle Park: Durham, NC, USA, 2018. [Google Scholar]
- Roy, S.; Ragunath, S. Emerging membrane technologies for water and energy sustainability: Future prospects, constraints and challenges. Energies 2018, 11, 2997. [Google Scholar] [CrossRef]
- Tavakkoli, S.; Lokare, O.R.; Vidic, R.D.; Khanna, V. A techno-economic assessment of membrane distillation for treatment of Marcellus shale produced water. Desalination 2017, 416, 24–34. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Goh, P.S.; Abdul Karim, Z.; Ismail, A.F. Thin film composite membrane for oily waste water treatment: Recent advances and challenges. Membranes 2018, 8, 86. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Hancock, N.T.; Nowosielski-Slepowron, M.S.; McGurgan, G.D. Pilot demonstration of the NH3/CO2 forward osmosis desalination process on high salinity brines. Desalination 2013, 312, 67–74. [Google Scholar] [CrossRef]
- Cath, T.Y.; Childress, A.E.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- El-Bourawi, M.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Camacho, L.M.; Dumée, L.; Zhang, J.; Li, J.-d.; Duke, M.; Gomez, J.; Gray, S. Advances in membrane distillation for water desalination and purification applications. Water 2013, 5, 94–196. [Google Scholar] [CrossRef]
- Ge, Q.; Ling, M.; Chung, T.-S. Draw solutions for forward osmosis processes: Developments, challenges, and prospects for the future. J. Membr. Sci. 2013, 442, 225–237. [Google Scholar] [CrossRef]
- Chung, T.-S.; Li, X.; Ong, R.C.; Ge, Q.; Wang, H.; Han, G. Emerging forward osmosis (FO) technologies and challenges ahead for clean water and clean energy applications. Curr. Opin. Chem. Eng. 2012, 1, 246–257. [Google Scholar] [CrossRef]
- Koschikowski, J.; Wieghaus, M.; Rommel, M. Solar thermal driven desalination plants based on membrane distillation. Water Sci. Technol. Water Supply 2003, 3, 49–55. [Google Scholar] [CrossRef]
- Fath, H.E.; Elsherbiny, S.M.; Hassan, A.A.; Rommel, M.; Wieghaus, M.; Koschikowski, J.; Vatansever, M. PV and thermally driven small-scale, stand-alone solar desalination systems with very low maintenance needs. Desalination 2008, 225, 58–69. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, P.; Fu, X.; Chung, T.-S. Sustainable water recovery from oily wastewater via forward osmosis-membrane distillation (FO-MD). Water Res. 2014, 52, 112–121. [Google Scholar] [CrossRef]
- Cho, C.H.; Oh, K.Y.; Kim, S.K.; Yeo, J.G.; Sharma, P. Pervaporative seawater desalination using NaA zeolite membrane: Mechanisms of high water flux and high salt rejection. J. Membr. Sci. 2011, 371, 226–238. [Google Scholar] [CrossRef]
- Mozumder, M.S.I.; Picioreanu, C.; Van Loosdrecht, M.C.; Volcke, E.I. Effect of heterotrophic growth on autotrophic nitrogen removal in a granular sludge reactor. Environ. Technol. 2014, 35, 1027–1037. [Google Scholar] [CrossRef]
- Gude, V.G.; Nirmalakhandan, N.; Deng, S. Renewable and sustainable approaches for desalination. Renew. Sustain. Energy Rev. 2010, 14, 2641–2654. [Google Scholar] [CrossRef]
- Gude, V.G. Energy storage for desalination processes powered by renewable energy and waste heat sources. Appl. Energy 2015, 137, 877–898. [Google Scholar] [CrossRef]
- Lee, J. Carbon Nanotube-Based Membranes for Water Purification. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 309–331. [Google Scholar]
- Song, B.; Xu, P.; Zeng, G.; Gong, J.; Zhang, P.; Feng, H.; Liu, Y.; Ren, X. Carbon nanotube-based environmental technologies: The adopted properties, primary mechanisms, and challenges. Rev. Environ. Sci. Bio/Technol. 2018, 17, 571–590. [Google Scholar] [CrossRef]
- Hu, A.; Zhang, X.; Oakes, K.D.; Peng, P.; Zhou, Y.N.; Servos, M.R. Hydrothermal growth of free standing TiO2 nanowire membranes for photocatalytic degradation of pharmaceuticals. J. Hazard. Mater. 2011, 189, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xue, S.; Bridges, D.; Yu, Y.; Zhang, L.; Pooran, J.; Hill, C.; Wu, J.; Hu, A. Fe-based ceramic nanocomposite membranes fabricated via e-spinning and vacuum filtration for Cd2+ ions removal. Chemosphere 2019, 230, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Noy, A.; Park, H.G.; Fornasiero, F.; Holt, J.K.; Grigoropoulos, C.P.; Bakajin, O. Nanofluidics in carbon nanotubes. Nano Today 2007, 2, 22–29. [Google Scholar] [CrossRef]
- Roy, S.; Humoud, M.S.; Intrchom, W.; Mitra, S. Microwave-induced desalination via direct contact membrane distillation. ACS Sustain. Chem. Eng. 2018, 6, 626–632. [Google Scholar] [CrossRef]
- Roy, S.; Ntim, S.A.; Mitra, S.; Sirkar, K.K. Facile fabrication of superior nanofiltration membranes from interfacially polymerized CNT-polymer composites. J. Membr. Sci. 2011, 375, 81–87. [Google Scholar] [CrossRef]
- Sae-Khow, O.; Mitra, S. Carbon nanotube immobilized composite hollow fiber membranes for pervaporative removal of volatile organics from water. J. Phys. Chem. C 2010, 114, 16351–16356. [Google Scholar] [CrossRef]
- Hylton, K.; Chen, Y.; Mitra, S. Carbon nanotube mediated microscale membrane extraction. J. Chromatogr. A 2008, 1211, 43–48. [Google Scholar] [CrossRef]
- Roy, S.; Bhadra, M.; Mitra, S. Enhanced desalination via functionalized carbon nanotube immobilized membrane in direct contact membrane distillation. Sep. Purif. Technol. 2014, 136, 58–65. [Google Scholar] [CrossRef]
- Bhadra, M.; Roy, S.; Mitra, S. Enhanced desalination using carboxylated carbon nanotube immobilized membranes. Sep. Purif. Technol. 2013, 120, 373–377. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Mitra, S. Enhanced membrane distillation of organic solvents from their aqueous mixtures using a carbon nanotube immobilized membrane. J. Member. Sci. 2018, 568, 134–140. [Google Scholar] [CrossRef]
- Birch, M.E.; Ruda-Eberenz, T.A.; Chai, M.; Andrews, R.; Hatfield, R.L. Properties that influence the specific surface areas of carbon nanotubes and nanofibers. Ann. Occup. Hyg. 2013, 57, 1148–1166. [Google Scholar]
- Gethard, K.; Sae-Khow, O.; Mitra, S. Water desalination using carbon-nanotube-enhanced membrane distillation. ACS Appl. Mater. Interfaces 2011, 3, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, M.; Mitra, S. Advances in nanostructured membranes for water desalination. In Nanotechnology Applications for Clean Water; Elsevier: Amsterdam, The Netherlands, 2014; pp. 109–122. [Google Scholar]
- Guo, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Kang, G.-d.; Cao, Y.-m. Development of antifouling reverse osmosis membranes for water treatment: A review. Water Res. 2012, 46, 584–600. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Peng, W.; Zhang, M.; Chen, J.; Hong, H.; Zhang, Y. A review on anaerobic membrane bioreactors: Applications, membrane fouling and future perspectives. Desalination 2013, 314, 169–188. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Wu, Z.; Wei, T.; Lu, F.; Tong, J.; Mai, S. Membrane fouling in an anaerobic dynamic membrane bioreactor (AnDMBR) for municipal wastewater treatment: Characteristics of membrane foulants and bulk sludge. Process Biochem. 2011, 46, 1538–1544. [Google Scholar] [CrossRef]
- Humoud, M.S.; Intrchom, W.; Roy, S.; Mitra, S. Reduction of scaling in microwave induced membrane distillation on a carbon nanotube immobilized membrane. Environ. Sci. Water Res. Technol. 2019, 5, 1012–1021. [Google Scholar] [CrossRef]
- Humoud, M.S.; Roy, S.; Mitra, S. Scaling Reduction in Carbon Nanotube-Immobilized Membrane during Membrane Distillation. Water 2019, 11, 2588. [Google Scholar] [CrossRef]
- Rahman, F. Calcium sulfate precipitation studies with scale inhibitors for reverse osmosis desalination. Desalination 2013, 319, 79–84. [Google Scholar] [CrossRef]
- Gray, S.R.; Zach-Maor, A.; Semiat, R.; Rahardianto, A.; Cohen, Y.; Wilson, S. Diagnostic analysis of RO desalting treated waste water. Desalination 2008, 230, 239–247. [Google Scholar]
- Hilal, N.; Ogunbiyi, O.O.; Miles, N.J.; Nigmatullin, R. Methods employed for control of fouling in MF and UF membranes: A comprehensive review. Sep. Sci. Technol. 2005, 40, 1957–2005. [Google Scholar] [CrossRef]
- Ho, B.P.; Wu, M.W.; Zeiher, E.K.; Chattoraj, M. Method of Monitoring Biofouling in Membrane Separation Systems. U.S. Patent 6,699,684, 2 March 2004. [Google Scholar]
- Dickinson, W.H. Use of Cerium Salts to Inhibit Manganese Deposition in Water Systems. U.S. Patent 7,252,769, 7 August 2007. [Google Scholar]
- Sherwood, N.S.; Yorke, M.A. Method For Controlling Calcium Carbonate Scaling in High pH Aqueous Systems. U.S. Patent 5,124,046, 23 June 1992. [Google Scholar]
- Kowalski, X. Methods of Scale Inhibition Using Substoichiometric Amounts of Amino Alcohol and Phosphonic Acids. U.S. Patent 3,671,448, 20 June 1972. [Google Scholar]
- Ritter, G. Method of Dissolving, and Solvents for, Difficult to Dissolve Carbonates. U.S. Patent 4,747,975, 31 May 1988. [Google Scholar]
- Intrchom, W.; Roy, S.; Mitra, S. Functionalized carbon nanotube immobilized membrane for low temperature ammonia removal via membrane distillation. Sep. Purif. Technol. 2020, 235, 116188. [Google Scholar] [CrossRef]
- Bhadra, M.; Roy, S.; Mitra, S. Flux enhancement in direct contact membrane distillation by implementing carbon nanotube immobilized PTFE membrane. Sep. Purif. Technol. 2016, 161, 136–143. [Google Scholar] [CrossRef]
- Majka, T.M.; Leszczyńska, A.; Pielichowski, K. Thermal stability and degradation of polymer nanocomposites. In Polymer Nanocomposites; Springer: Berlin, Germany, 2016; pp. 167–190. [Google Scholar]
- Gullinkala, T.; Digman, B.; Gorey, C.; Hausman, R.; Escobar, I.C. Desalination: Reverse osmosis and membrane distillation. Sustain. Sci. Eng. 2010, 2, 65–93. [Google Scholar]
- Antony, A.; Low, J.H.; Gray, S.; Childress, A.E.; Le-Clech, P.; Leslie, G. Scale formation and control in high pressure membrane water treatment systems: A review. J. Member. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Member. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Wang, J.; Wu, X.; Wang, R.; Zhang, M. Detection of carbon nanotubes using tip-enhanced Raman spectroscopy. In Electronic Properties Carbon Nanotubes; InTech Rijeka: Rijeka, Croatia, 2011. [Google Scholar]
- Bhadra, M.; Roy, S.; Mitra, S. A bilayered structure comprised of functionalized carbon nanotubes for desalination by membrane distillation. ACS Appl. Mater. Interfaces 2016, 8, 19507–19513. [Google Scholar] [CrossRef]
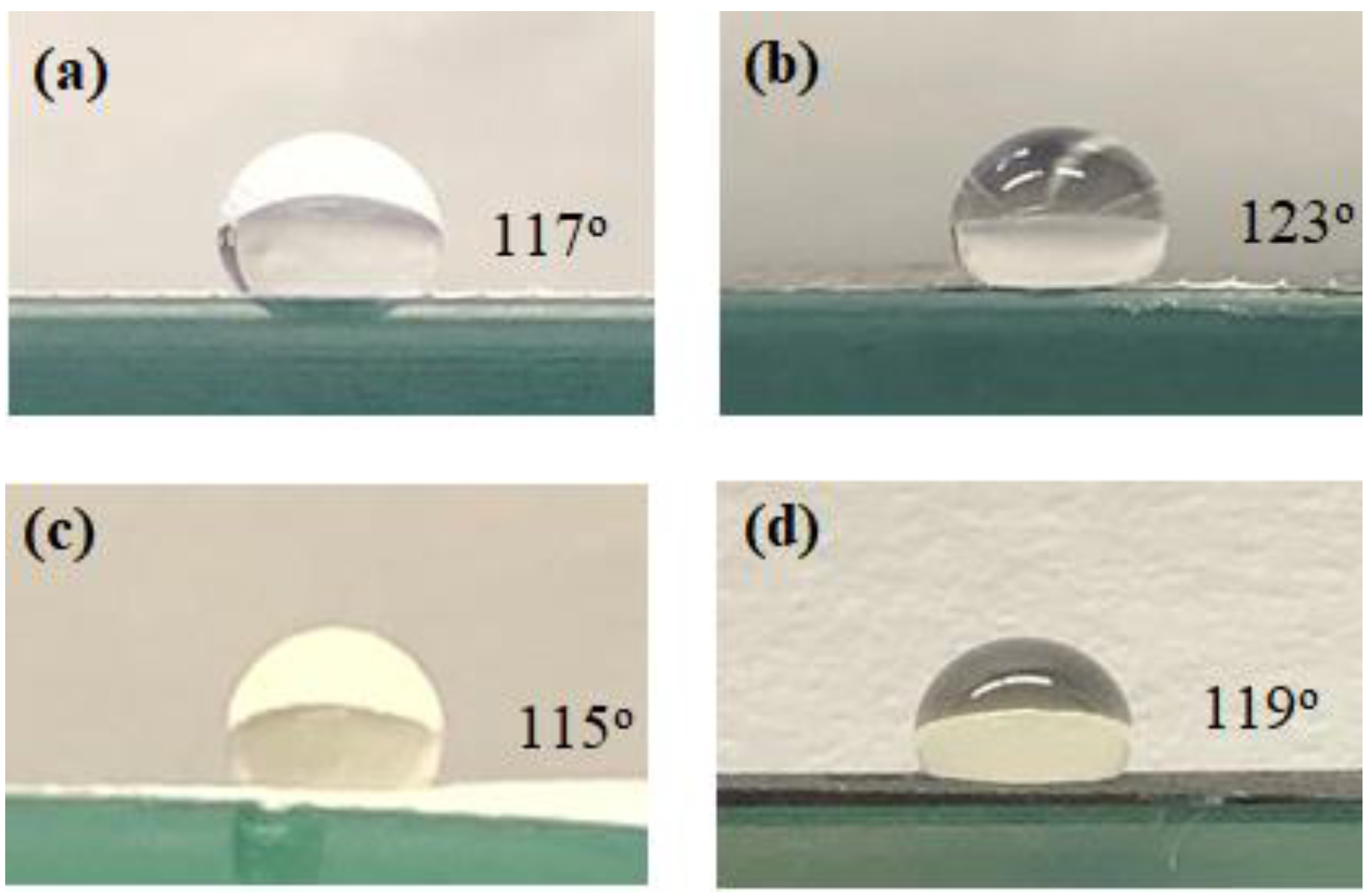

| Parameters/Dissolved Solids (mg/L) | Produced Water | After Filtration |
|---|---|---|
| Calcium | 119,500 | 1455 |
| Magnesium | 12,590 | 52 |
| Barium | 856 | 289 |
| Iron | 90 | 1.4 |
| Copper | <1.0 | 0.014 |
| Zinc | 2.4 | 0.025 |
| Sodium | 71,820 | 154 |
| Potassium | 1780 | 13 |
| Chloride | 118 | - |
| Sulfate | 130 | - |
| Nitrate | <100 | - |
| Ortho−Phosphate | <500 | - |
| Silica | 41 | - |
| Solution | FDn (%) of Produced Water Solution | |||
|---|---|---|---|---|
| PTFE | CNIM | PTFE-HEDP | CNIM-HEDP | |
| Produced Water | 64.8 | 58.2 | 59.5 | 35.1 |
| Solution | Amount of Salt Deposited on the Membrane Surface (mg) | % Weight Decrease | |
|---|---|---|---|
| PTFE | PTFE-HEDP | ||
| Produced Water | 15.76 | 4.68 | 70.3 |
| CNIM | CNIM-HEDP | ||
| 1.02 | 0.79 | 22.5 | |
| Membrane | Initial Flux (kg/m2·h) | Flux after 24 h (kg/m2·h) | Flux Regenerated (%) |
|---|---|---|---|
| PTFE | 30.9 | 25.1 | 81.1 |
| PTFE-HEDP | 35.2 | 33.5 | 95.2 |
| CNIM | 46.1 | 41.9 | 90.9 |
| CNIM-HEDP | 51.9 | 49.4 | 95.2 |
| (a) | ||
| Mass Transfer Coefficient (kg/m2 sec−1 Pa) × 10−7 | ||
| Feed Flow Rate (mL/min) | PTFE | CNIM |
| 100 | 1.9 | 2.6 |
| 150 | 2.4 | 3.0 |
| 200 | 3.7 | 4.4 |
| (b) | ||
| Mass Transfer Coefficient (kg/m2 sec−1 Pa) × 10−7 | ||
| Temperature (°C) | PTFE | CNIM |
| 60 | 3.4 | 4.9 |
| 70 | 3.0 | 4.4 |
| 80 | 2.4 | 3.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humoud, M.S.; Roy, S.; Mitra, S. Enhanced Performance of Carbon Nanotube Immobilized Membrane for the Treatment of High Salinity Produced Water via Direct Contact Membrane Distillation. Membranes 2020, 10, 325. https://doi.org/10.3390/membranes10110325
Humoud MS, Roy S, Mitra S. Enhanced Performance of Carbon Nanotube Immobilized Membrane for the Treatment of High Salinity Produced Water via Direct Contact Membrane Distillation. Membranes. 2020; 10(11):325. https://doi.org/10.3390/membranes10110325
Chicago/Turabian StyleHumoud, Madihah Saud, Sagar Roy, and Somenath Mitra. 2020. "Enhanced Performance of Carbon Nanotube Immobilized Membrane for the Treatment of High Salinity Produced Water via Direct Contact Membrane Distillation" Membranes 10, no. 11: 325. https://doi.org/10.3390/membranes10110325
APA StyleHumoud, M. S., Roy, S., & Mitra, S. (2020). Enhanced Performance of Carbon Nanotube Immobilized Membrane for the Treatment of High Salinity Produced Water via Direct Contact Membrane Distillation. Membranes, 10(11), 325. https://doi.org/10.3390/membranes10110325