The Impact of Various Natural Gas Contaminant Exposures on CO2/CH4 Separation by a Polyimide Membrane
Abstract
1. Introduction
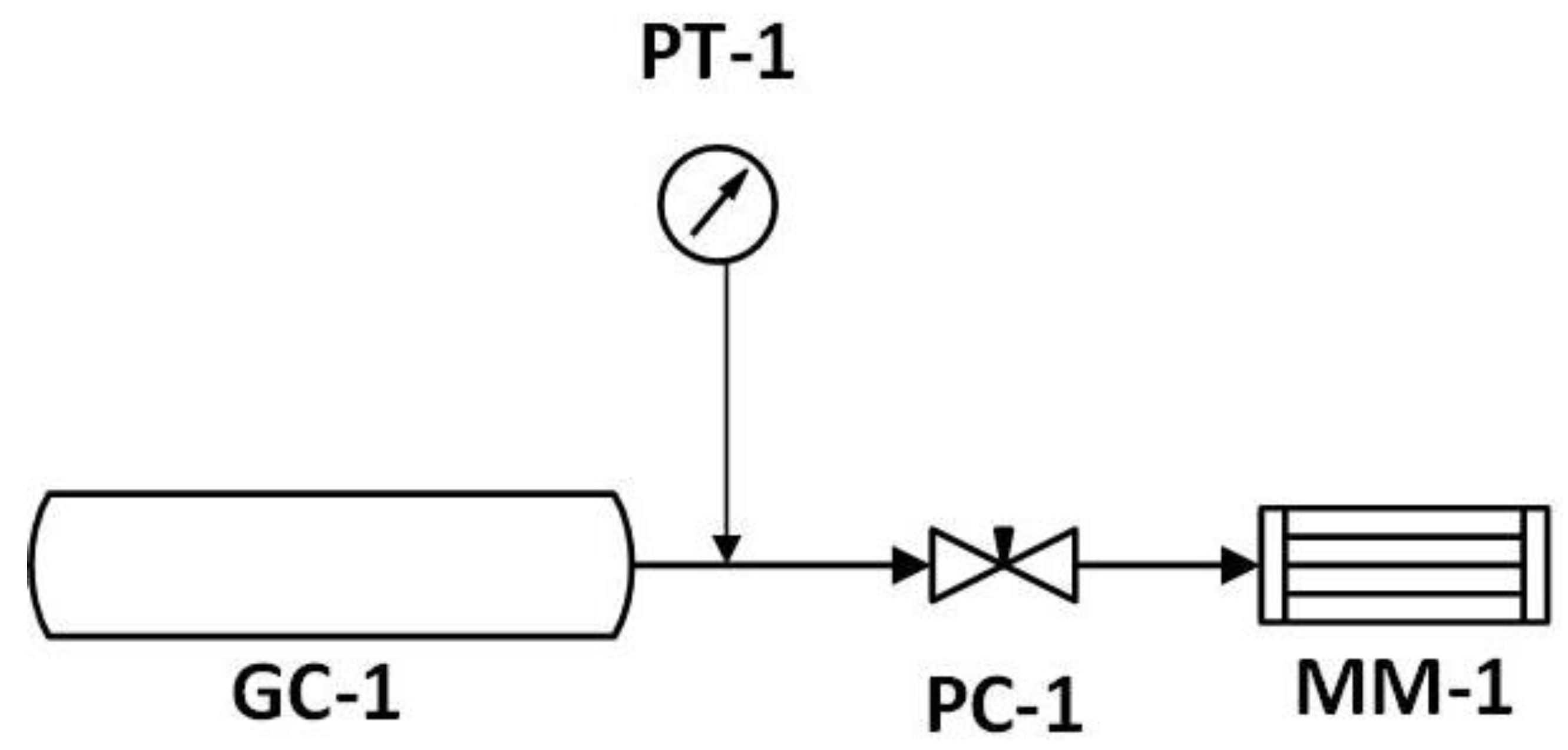
2. Materials and Methods
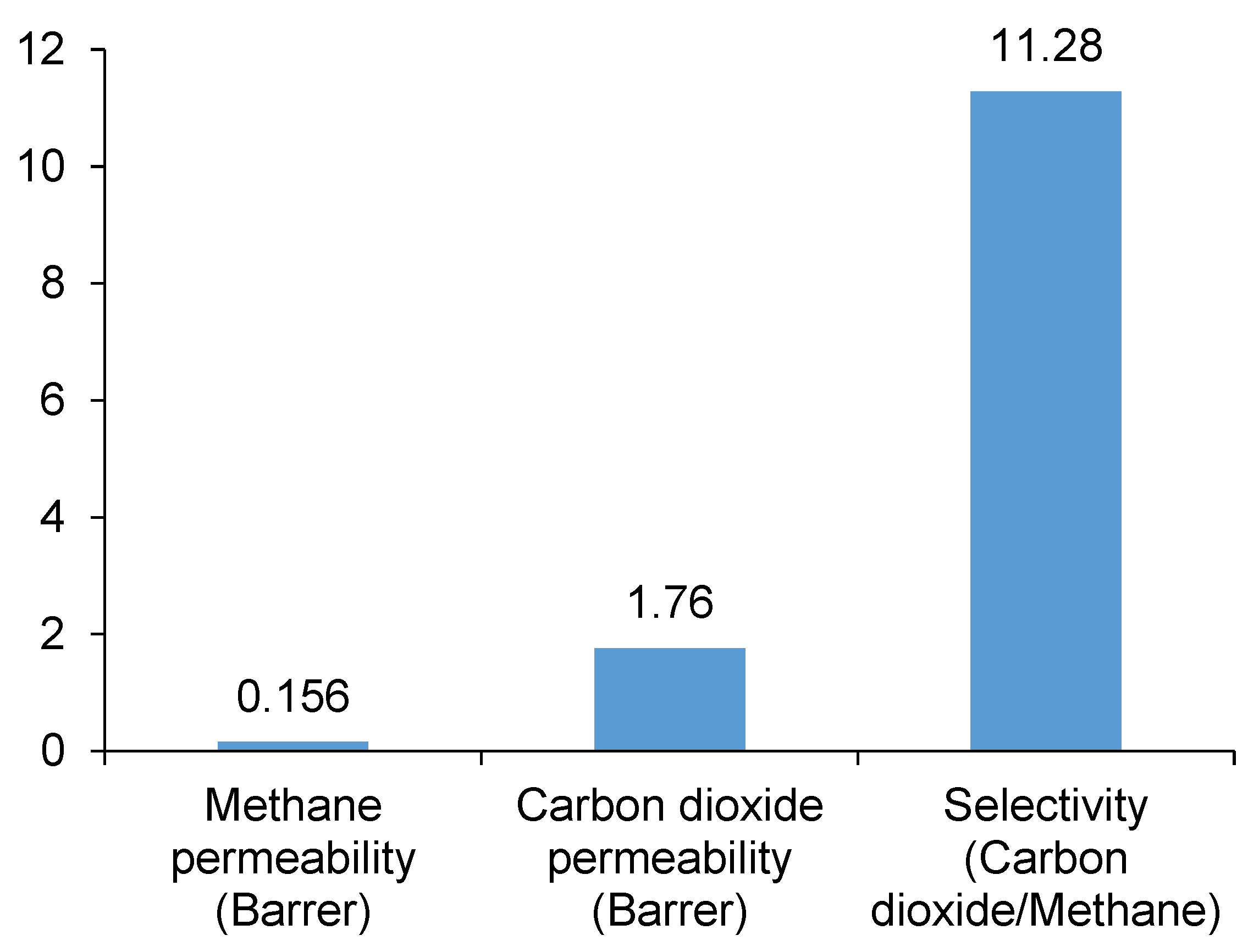
3. Results and Discussion
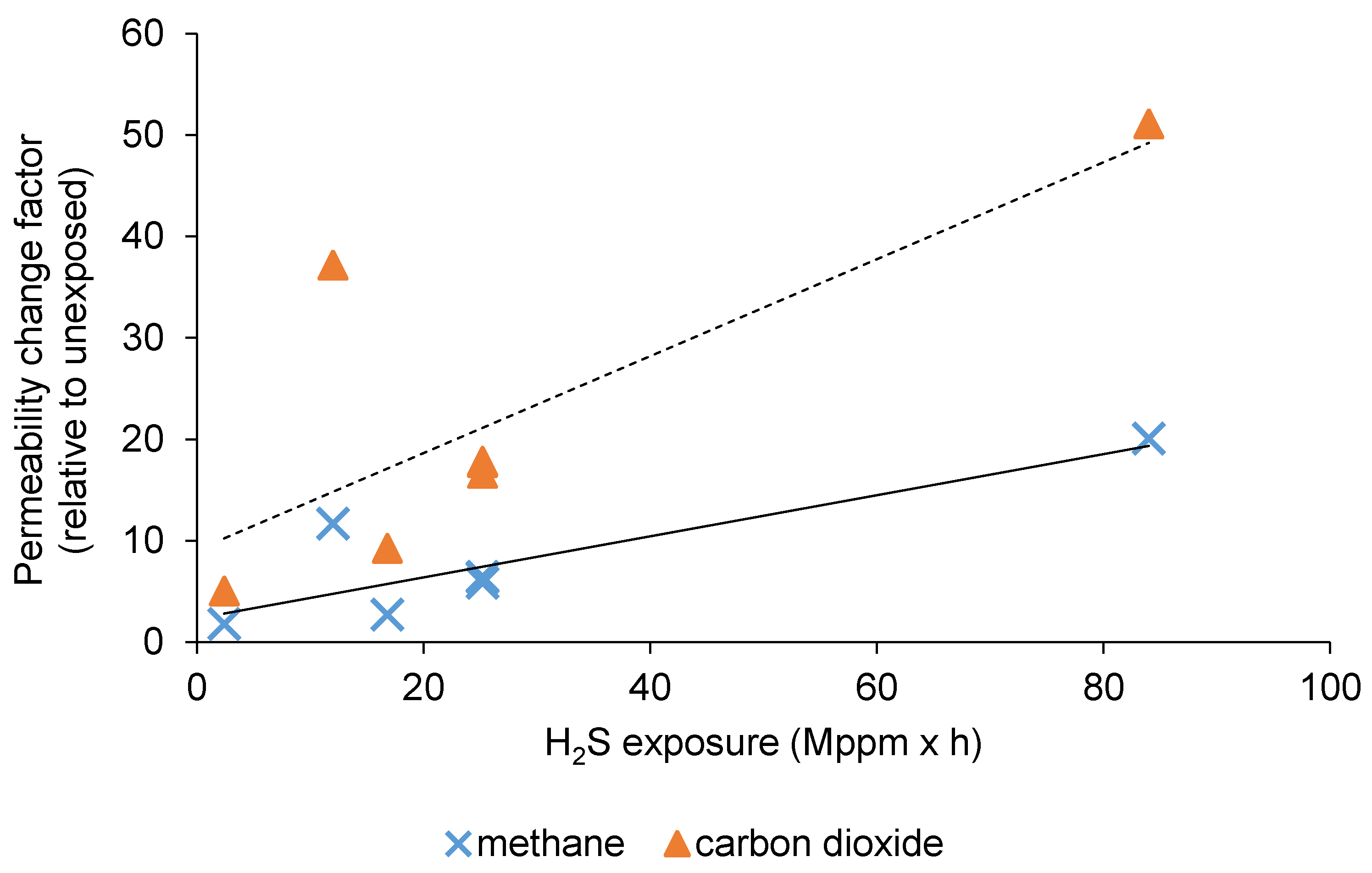
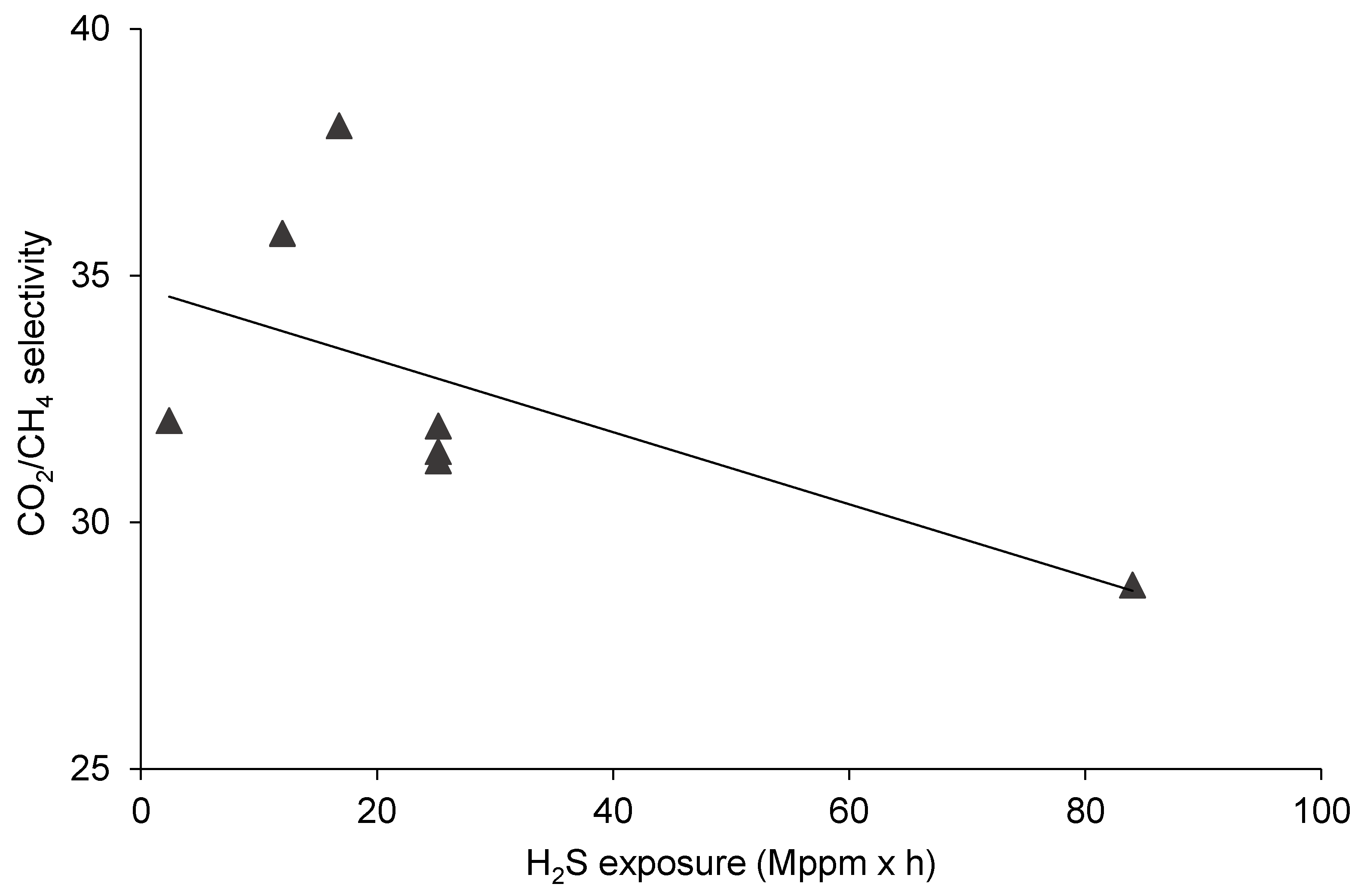
3.1. The Effect of H2S Exposure on CO2/CH4 Separation
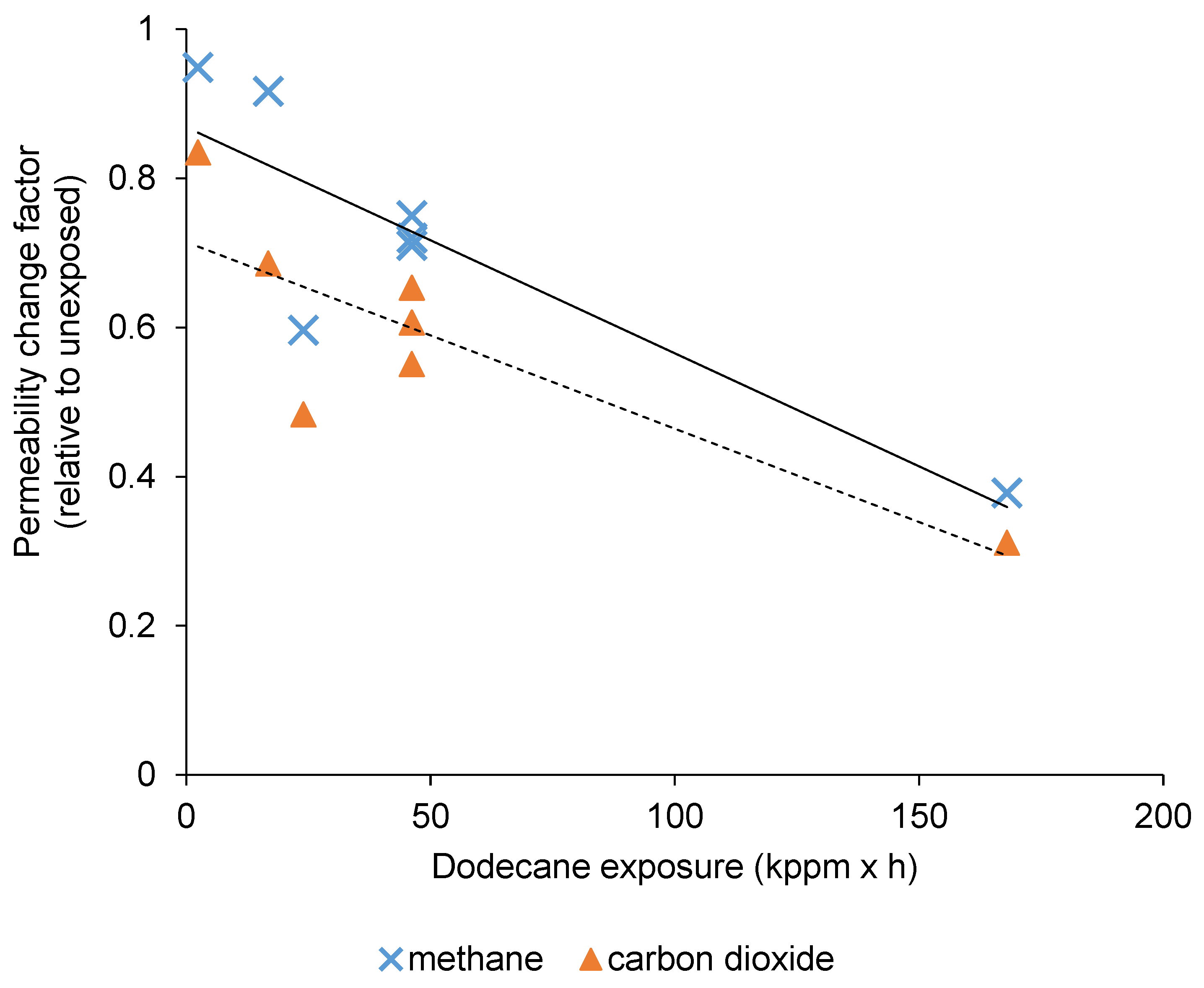
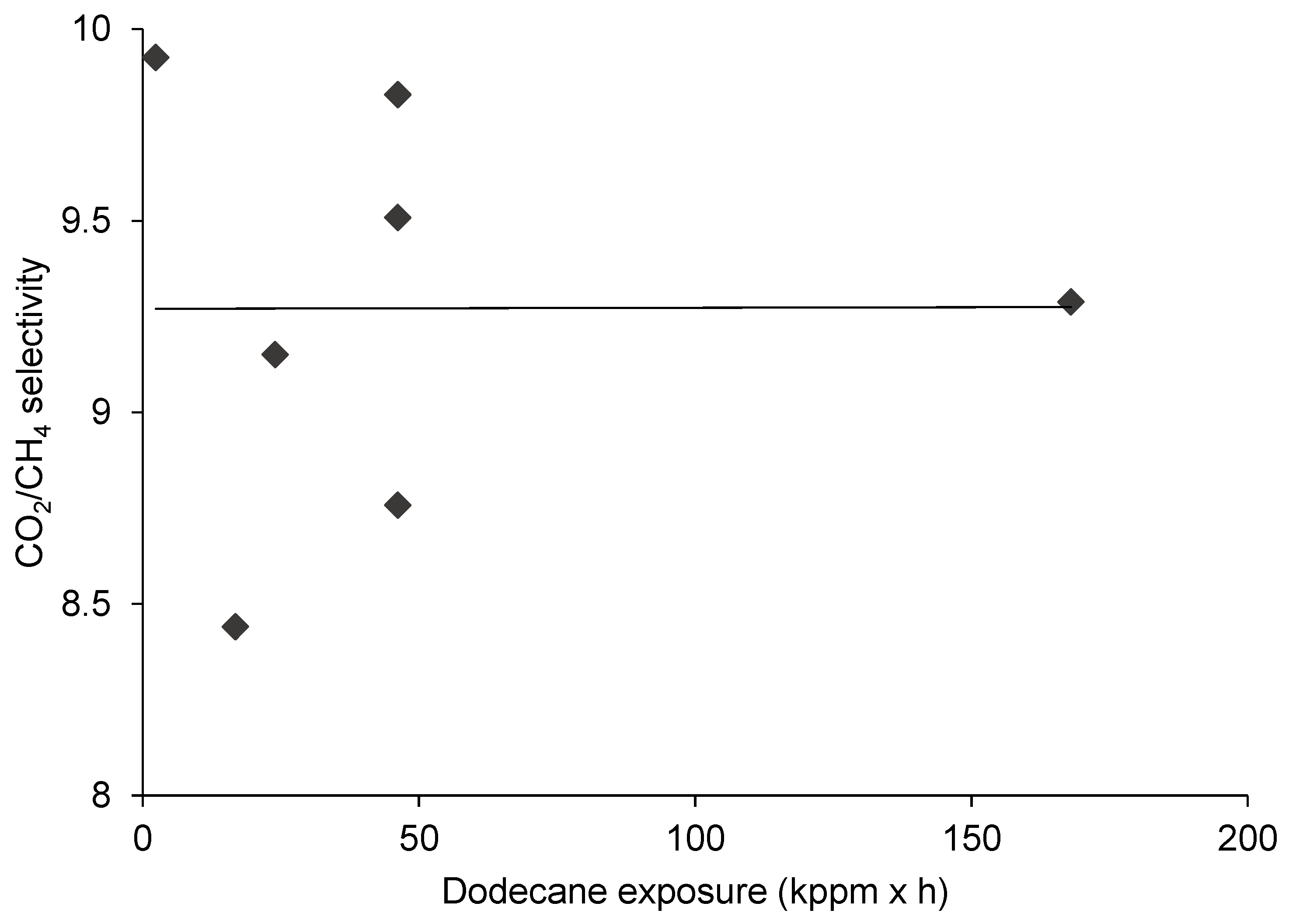
3.2. The Effect of Dodecane Exposure on CO2/CH4 Separation
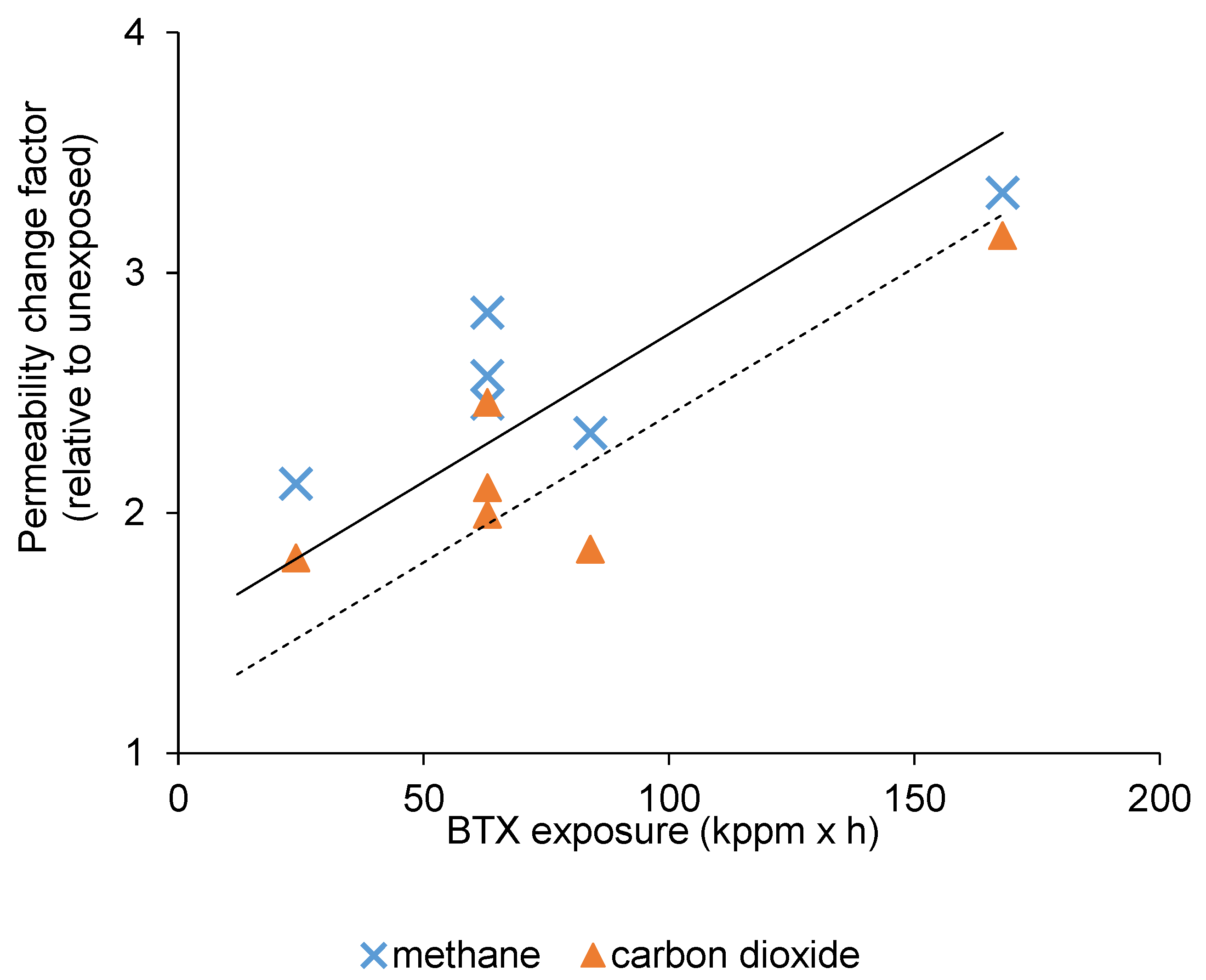
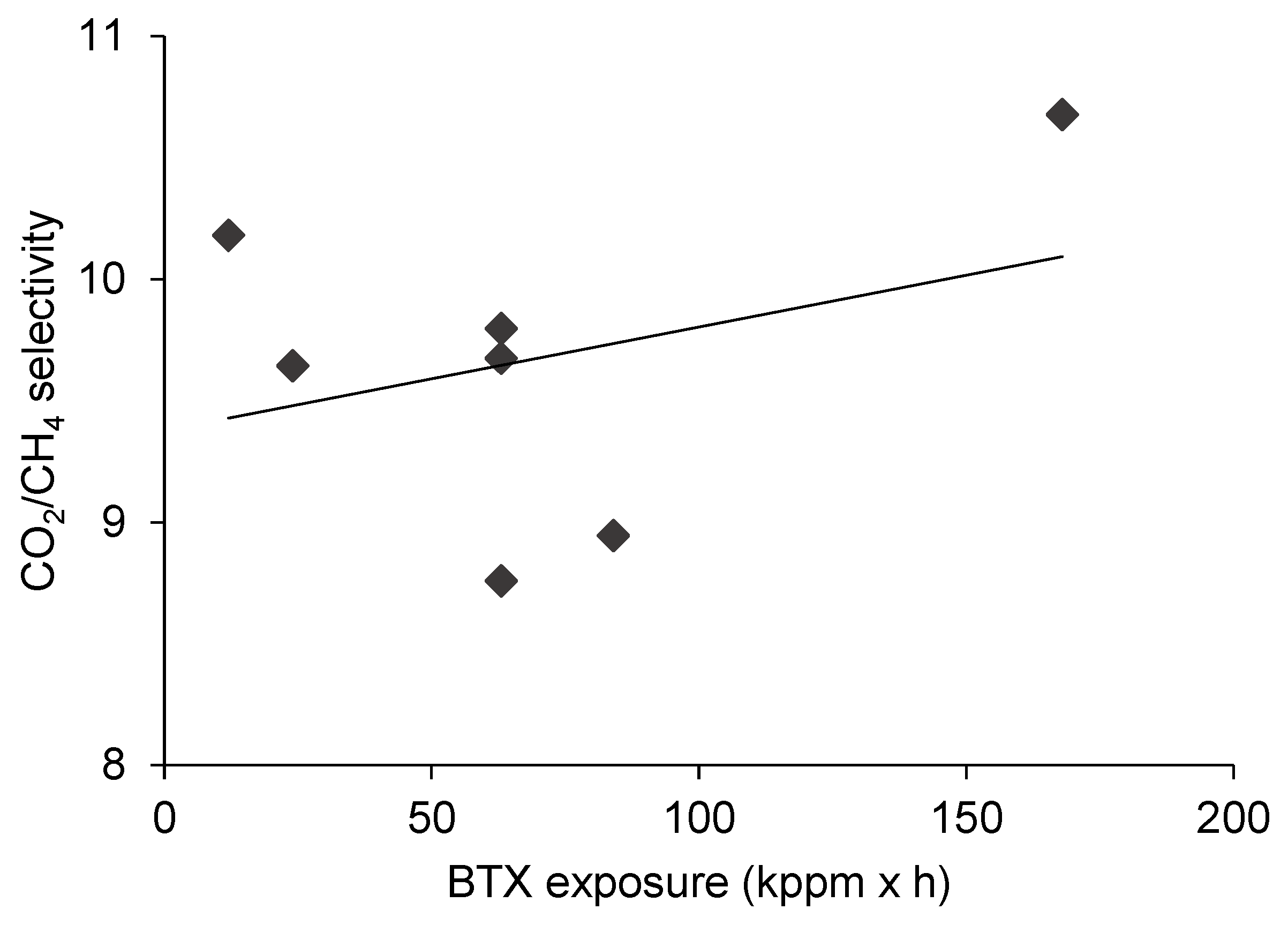
3.3. The Effect of BTX Exposure on CO2/CH4 Separation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane gas separation applications in natural gas processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
- Adewole, J.; Ahmad, A.; Ismail, S.; Leo, C. Current challenges in membrane separation of CO2 from natural gas: A review. Int. J. Greenh. Gas Control. 2013, 17, 46–65. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural gas processing with membranes: An overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Lokhandwala, K.A.; Pinnau, I.; He, Z.; Amo, K.D.; Dacosta, A.R.; Wijmans, J.G.; Baker, R.W. Membrane separation of nitrogen from natural gas: A case study from membrane synthesis to commercial deployment. J. Membr. Sci. 2010, 346, 270–279. [Google Scholar] [CrossRef]
- Scholz, M.; Melin, T.; Wessling, M. Transforming biogas into biomethane using membrane technology. Renew. Sustain. Energy Rev. 2013, 17, 199–212. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymers 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Wind, J.D.; Paul, D.R.; Koros, W.J. Natural gas permeation in polyimide membranes. J. Membr. Sci. 2004, 228, 227–236. [Google Scholar] [CrossRef]
- Xiao, Y.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas-A review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
- Sridhar, S.; Smitha, B.; Aminabhavi, T. Separation of carbon dioxide from natural gas mixtures through polymeric membranes-A Review. Sep. Purif. Rev. 2007, 36, 113–174. [Google Scholar] [CrossRef]
- AlQaheem, Y.; Alomair, A.; Vinoba, M.; Pérez, A. Polymeric gas-separation membranes for petroleum refining. Int. J. Polym. Sci. 2017, 2017, 1–19. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Freeman, B.D. Basis of permeability/selectivity tradeoff relations in polymeric gas separation membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Yong, W.F.; Kwek, K.H.A.; Liao, K.-S.; Chung, T.-S. Suppression of aging and plasticization in highly permeable polymers. Polymers 2015, 77, 377–386. [Google Scholar] [CrossRef]
- Low, Z.-X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas permeation properties, physical aging, and its mitigation in high free volume glassy polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef] [PubMed]
- Harasimowicz, M.; Orluk, P.; Zakrzewska-Trznadel, G.; Chmielewski, A. Application of polyimide membranes for biogas purification and enrichment. J. Hazard. Mater. 2007, 144, 698–702. [Google Scholar] [CrossRef]
- Uddin, M.W.; Hägg, M.-B. Natural gas sweetening-The effect on CO2-CH4 separation after exposing a facilitated transport membrane to hydrogen sulfide and higher hydrocarbons. J. Membr. Sci. 2012, 143–149. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Liu, G.; Qiu, W.; Bhuwania, N.; Chinn, D.; Koros, W.J. Surprising plasticization benefits in natural gas upgrading using polyimide membranes. J. Membr. Sci. 2020, 593, 117430. [Google Scholar] [CrossRef]
- Hao, J.; Rice, P.; Stern, S. Upgrading low-quality natural gas with H2S- and CO2-selective polymer membranes. J. Membr. Sci. 2008, 320, 108–122. [Google Scholar] [CrossRef]
- Scholes, C.A.; Kentish, S.E.; Stevens, G.W. Effects of minor components in carbon dioxide capture using polymeric gas separation membranes. Sep. Purif. Rev. 2009, 38, 1–44. [Google Scholar] [CrossRef]
- White, L.S.; Blinka, T.A.; Kloczewski, H.A.; Wang, I.-F. Properties of a polyimide gas separation membrane in natural gas streams. J. Membr. Sci. 1995, 103, 73–82. [Google Scholar] [CrossRef]
- Toth, G.; Nemestóthy, N.; Bélafi-Bakó, K.; Vozik, D.; Bakonyi, P. Degradation of hydrogen sulfide by immobilized Thiobacillus thioparus in continuous biotrickling reactor fed with synthetic gas mixture. Int. Biodeterior. Biodegrad. 2015, 105, 185–191. [Google Scholar] [CrossRef]
- Lashkari, S.; Tran, A.; Kruczek, B. Effect of back diffusion and back permeation of air on membrane characterization in constant pressure system. J. Membr. Sci. 2008, 324, 162–172. [Google Scholar] [CrossRef]
- Mohammadi, A.T.; Matsuura, T.; Sourirajan, S. Design and construction of gas permeation system for the measurement of low permeation rates and permeate compositions. J. Membr. Sci. 1995, 98, 281–286. [Google Scholar] [CrossRef]
- Ismail, A.F.; Khulbe, K.C.; Matsuura, T. Application of gas separation membranes. In Gas Separation Membranes; Springer: Berlin, Germany, 2015; pp. 978–983. [Google Scholar]
- Gaspar, D.J.; Phillips, S.D.; George, A.; Albrecht, K.O.; Jones, S.; Howe, D.T.; Landera, A.; Santosa, D.M.; Howe, D.T.; Baldwin, A.G.; et al. Measuring and predicting the vapor pressure of gasoline containing oxygenates. Fuel 2019, 243, 630–644. [Google Scholar] [CrossRef]
- Cecopierigomez, M.; Palaciosalquisira, J.; Dominguez, J. On the limits of gas separation in CO2/CH4, N2/CH4 and CO2/N2 binary mixtures using polyimide membranes. J. Membr. Sci. 2007, 293, 53–65. [Google Scholar] [CrossRef]
- Jeon, Y.-W.; Lee, D.-H. Gas Membranes for CO2/CH4 (Biogas) Separation: A Review. Environ. Eng. Sci. 2015, 32, 71–85. [Google Scholar] [CrossRef]
- Ohlrogge, K.; Brinkmann, T. Natural gas cleanup by means of membranes. Ann. New York Acad. Sci. 2003, 984, 306–317. [Google Scholar] [CrossRef]
- Lee, J.S.; Koros, W.J.; Koros, W.J. Antiplasticization and plasticization of Matrimid® asymmetric hollow fiber membranes-Part A. Experimental. J. Membr. Sci. 2010, 350, 232–241. [Google Scholar] [CrossRef]
- Al-Juaied, M.; Koros, W.J. Performance of natural gas membranes in the presence of heavy hydrocarbons. J. Membr. Sci. 2006, 274, 227–243. [Google Scholar] [CrossRef]
- Omole, I.C.; Bhandari, D.A.; Miller, S.J.; Koros, W.J. Toluene impurity effects on CO2 separation using a hollow fiber membrane for natural gas. J. Membr. Sci. 2011, 369, 490–498. [Google Scholar] [CrossRef]


| Pollutant | Clow [ppm] | Ccent [ppm] | Chigh [ppm] | t1 [day] | t2 [day] | t3 [day] |
|---|---|---|---|---|---|---|
| H2S | 100,000 | 300,000 | 500,000 | 1 | 3.5 | 7 |
| BTX | 500 | 750 | 1000 | 1 | 3.5 | 7 |
| dodecane | 1000 | 5500 | 10,000 | 1 | 3.5 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemestóthy, N.; Bakonyi, P.; Lajtai-Szabó, P.; Bélafi-Bakó, K. The Impact of Various Natural Gas Contaminant Exposures on CO2/CH4 Separation by a Polyimide Membrane. Membranes 2020, 10, 324. https://doi.org/10.3390/membranes10110324
Nemestóthy N, Bakonyi P, Lajtai-Szabó P, Bélafi-Bakó K. The Impact of Various Natural Gas Contaminant Exposures on CO2/CH4 Separation by a Polyimide Membrane. Membranes. 2020; 10(11):324. https://doi.org/10.3390/membranes10110324
Chicago/Turabian StyleNemestóthy, Nándor, Péter Bakonyi, Piroska Lajtai-Szabó, and Katalin Bélafi-Bakó. 2020. "The Impact of Various Natural Gas Contaminant Exposures on CO2/CH4 Separation by a Polyimide Membrane" Membranes 10, no. 11: 324. https://doi.org/10.3390/membranes10110324
APA StyleNemestóthy, N., Bakonyi, P., Lajtai-Szabó, P., & Bélafi-Bakó, K. (2020). The Impact of Various Natural Gas Contaminant Exposures on CO2/CH4 Separation by a Polyimide Membrane. Membranes, 10(11), 324. https://doi.org/10.3390/membranes10110324







