Forward Osmosis as Concentration Process: Review of Opportunities and Challenges
Abstract
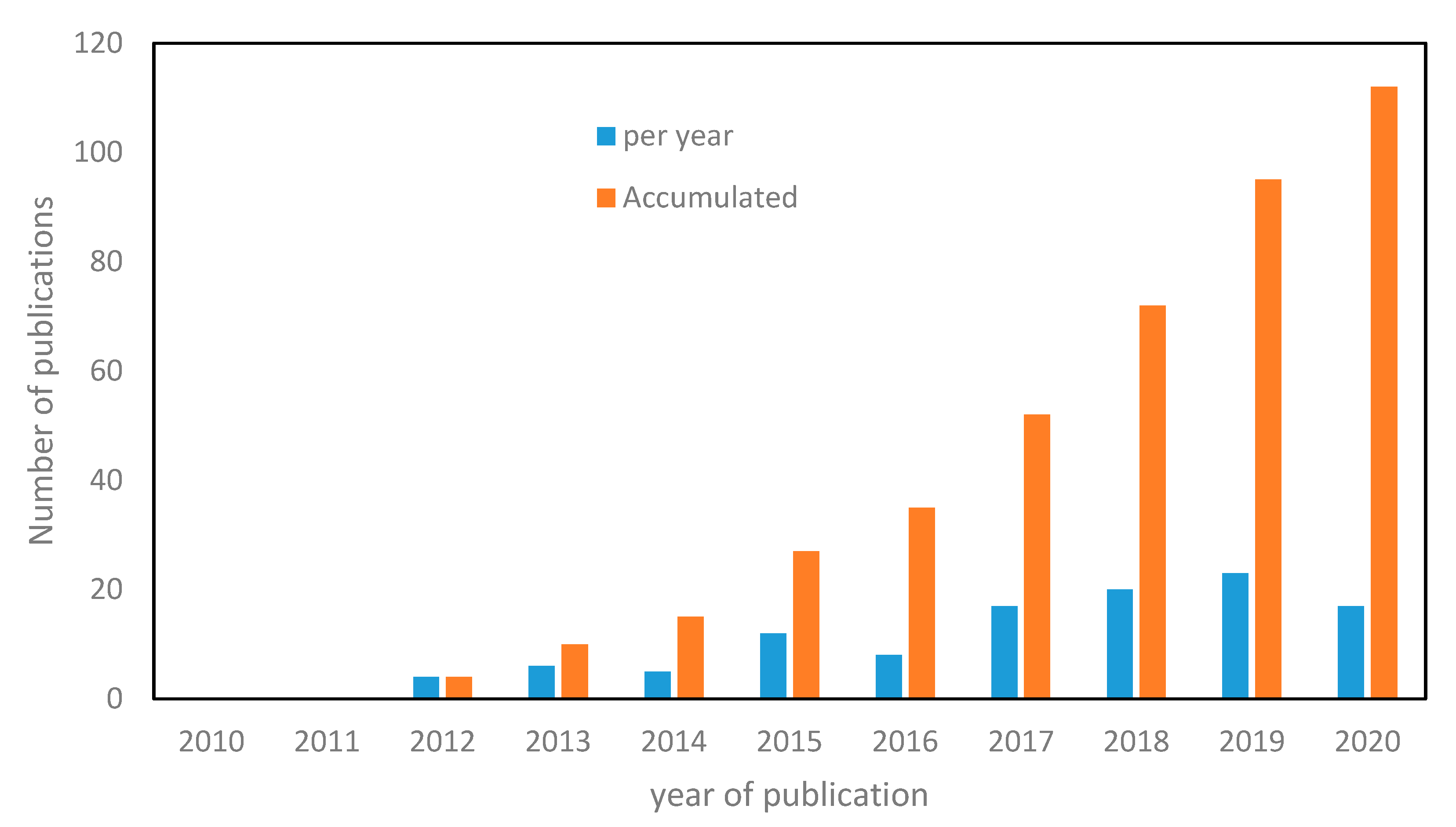
1. Introduction
1.1. State of the Art Concentration Processes
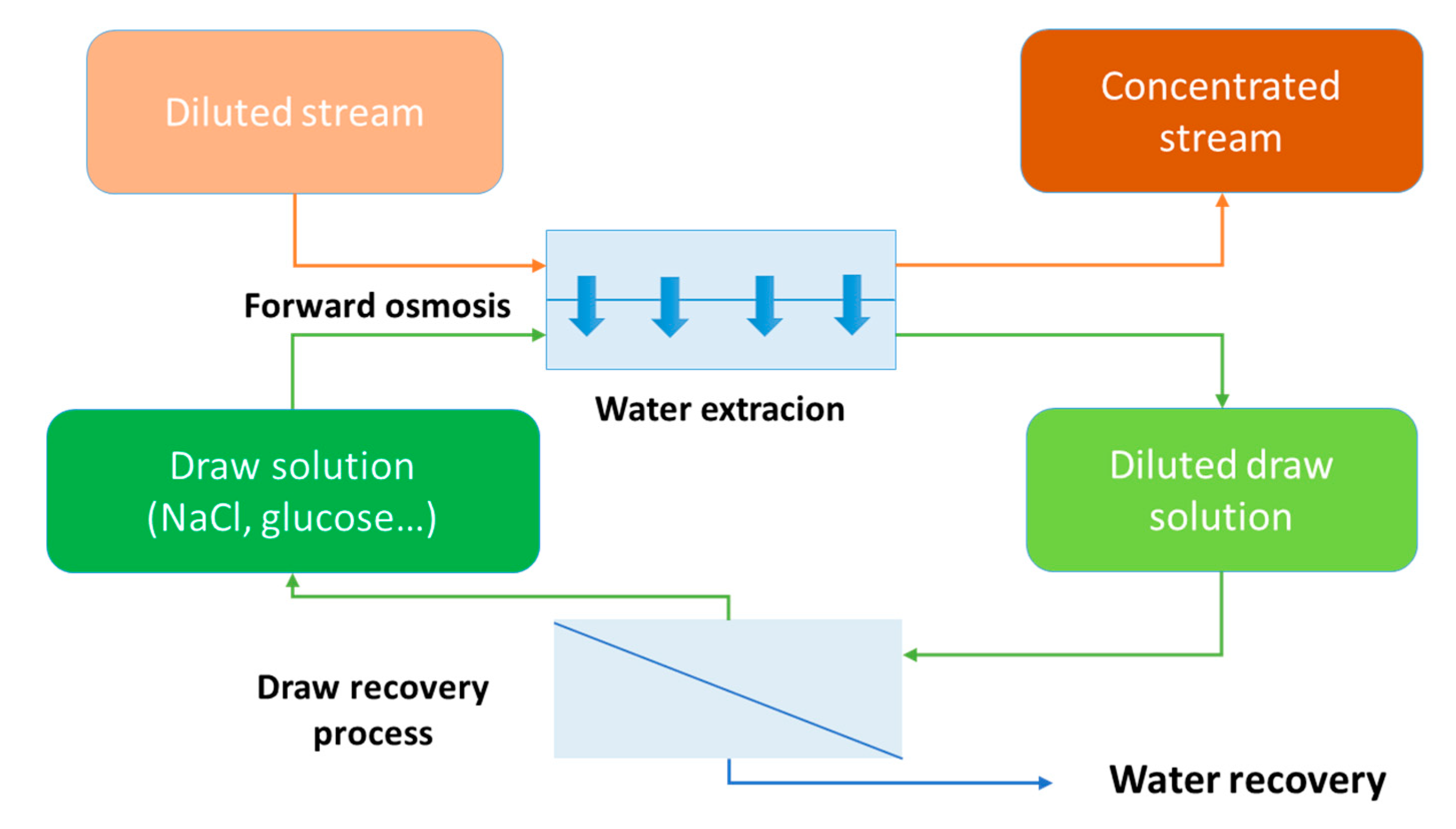
1.2. The Emergence of Forward Osmosis
2. Applications of FO as Concentrating Process
2.1. Food and Beverages
2.1.1. Liquid Food Concentration
2.1.2. Dairy
| Application | Membrane Type | Draw Solution | T (°C) | Initial Jw (L·m−2·h−1) | Initial Conc. | CF | Final Conc. | Ref. |
|---|---|---|---|---|---|---|---|---|
| Skim milk | CTA FO | 0.9 M NaCl | 16–19 | 4.5 | 8% | 2.8 | 22% | [62] |
| Whey | CTA FO | 0.9 M NaCl | 16–19 | 4 | 5.9% | 2.5 | 15% | [62] |
| Whey | TFC FO | 0.5 M NaCl | 22.5 | 12 | 6% | 3.6 | 22% | [66] |
| Whey | CTA FO | 2 M NaCl | 25 | 28 | 6.8% | 4 | 28% | [64] |
| Whey | CTA FO | 2 M NaCl | 25 | 12 | 2.5% | 8 | 21% | [65] |
| Whey | CTA FO | 3 M NaCl | 25 | 20 | 6.8% | 2.1 | 14% | [63] |
2.1.3. Alcohol
2.2. Organic Value-Added Compounds
2.2.1. Food Derived Products and by-Products
| Application | Membrane Type | Draw Solution | Initial Jw (L·m−2·h−1) | Initial Conc. | CF | Final Conc. | Ref. |
|---|---|---|---|---|---|---|---|
| Polyphenols from carob pulp | CTA FO | K-lactate 60° Brix | 13 | 1.13 g·L−1 TPC and 4.5° | 16.2 (TPC) | 18.4 g·L−1 TPC and 24.5° | [73] |
| Proteins/tuna cooking juice | CTA FO | 0.5–2 M NaCl | 5 | 5.5% | 1.6 | 9.0% | [74] |
| Biophenols/olive mill WW | CTA FO | 3 M MgCl2 | 6.5 | >2 concentration on analysed phenolic | [75] | ||
| Polyphenols and melanoidins/Molassess distillery WW | TFC FO | 4 M MgCl2 | 4 | 80 g·L−1 Mel, 10 g·L−1 Polyphenols | >2 concentration with 97% rejection of Melanoidins, 90% on antioxidant | [76] | |
| Polyphenols and melanoidins/Molassess distillery WW | CTA & TFC | 3 M MgCl2 | 4–6 | 80 g·L−1 Mel, 69 mM antioxydant | up to 4 | 141–267g·L−1 Mel, 102–213 mM antioxydant | [84] |
| Xylose | TFC FO | 1–3 M MgCl2/LaCl3 | 25 | 25 g·L−1 | 6 | 150 g·L−1 | [77] |
| Sugar cane juice/Sucrose | TFC FO | sea bittern (MgCl2) | 13 | 10.50% | 3.9 | 40.60% | [78] |
| Rice straw/Sugar | TFC FO | MgSO4 (saturated) | 20 g·L−1 | 3.6 | 72 g·L−1 | [79] | |
| Pretreated-rice straw/Sugars | TFC FO | 3.6 M TEA | 4 | 199 mM | 8.1 | 1612 mM | [80] |
| Fermentation broth/Succinic acid | TFC FO | 5 M NaCl | 16 | 40 g·L−1 | 4.5 | 180 g·L−1 | [81] |
| Fermentation broth/Lactic acid | TFC FO | 5 M NaCl | 13 | 15 g·L−1 | 3.8 | 57 g·L−1 | [81] |
| Fermentation broth/Ethanol | TFC FO | 5 M NaCl | 22 | 20 g·L−1 | 5.5 | 110 g·L−1 | [81] |
2.2.2. Microalgae Harvesting
2.2.3. Volatile Fatty Acid Production
2.3. Water Reuse and Nutrients Recovery
2.3.1. Primary Treated WW and Raw Sewage Concentration
2.3.2. Osmotic Membrane Bioreactor (OMBR)
2.3.3. Anaerobic OMBR (AnOMBR)
| Process | Membrane Type | Draw Solution | TOC Removal (%) or CF | COD Removal (%) or CF | NH4 Removal (%) or CF | TN Removal (%) or CF | TP Removal (%) or CF | Ref. |
|---|---|---|---|---|---|---|---|---|
| OMBR | CTA (cross-flow) | NaCl 1 M | 98% | - | 99% | - | - | [150] |
| TFC (cross-flow) | NaCl 1 M | 96% | - | 99% | - | - | [150] | |
| CTA (sub-FO P&F) | NaCl 0.7 M | - | >99% | - | >82% | >99% | [151] | |
| CTA (sub-FO P&F) | NaCl and MgCl2 | 98% | - | 80–90% | - | >99% (P-PO4) | [152] | |
| CTA (sub-FO P&F) | NaCl | >98% | - | >98% | - | - | [153] | |
| CTA (sub-FO P&F) | - | - | >95% | 70–80% | - | >99% | [154] | |
| An-OMBR | CTA (sub-FO P&F) | - | - | >95% | 70–80% | - | >99% | [146] |
| CTA (sub-FO P&F) | NaCl | - | 96.7% | 60% | - | 99% | [145] | |
| TFC (Aquaporin) | MgSO4 | - | >95% | >95% | - | >95% | [155] | |
| CTA (sub-FO P&F) | NaCl and Na2SO4 | 92.9% | - | - | - | [148] | ||
| WW pre conc. | CTA (sub-FO P&F) | - | - | 99% | - | 56–59% | 99% (P-PO4) | [113] |
| CTA (pilot-SW) | 0.5 M NaCl | - | 99.8% | 48.1% | 67.8% | 99.7% | [129] | |
| TFC | Synthetic seawater brine | - | CF 2.4 and 2.7 | CF 1.3 and 1.8 | CF 1.6 and 1.9 | CF 3.3 and 3.5 | [156] | |
| CTA | Synthetic seawater | 79.0 ± 6.7% | - | 85.4% | - | 93.3 ± 3.3% | [154] | |
| CTA-ES | 0.2–4 M NaCl | - | 96.5% | 89.4% | 93.3% | 95.4% | [157] | |
| TFC-Aquaporin | 1.5 M MgCl2 | - | 99% | CF 1.32 | - | 68–74% | [158] | |
| TFC | - | - | 93.0–97.5 | <59.06% | - | 93.7–98.5% (P-PO4) | [130] | |
| CTA | 1 M NaCl | - | 97% | - | 96% | >99% | [159] | |
| TFC Aquaporin | 1 M NaCl | - | 97% | - | 96% | >99% | [159] | |
| CTA | 0.6 M NaCl | 98% | - | 70% | - | 90% | [160] | |
| TFC (sub-FO P&F) | 0.2 M Seasalts | - | CF 2.4 | CF 0.75 | - | CF 2.2 | [128] | |
| CTA | Synthetic seawater | - | 80–95% | 89.2% | - | 99% | [161] |
2.4. Treatment of Waste Streams
2.4.1. Sludge Concentration
2.4.2. Urine Recovery and Concentration
2.4.3. Landfill Leachate
2.4.4. Digestate Treatment
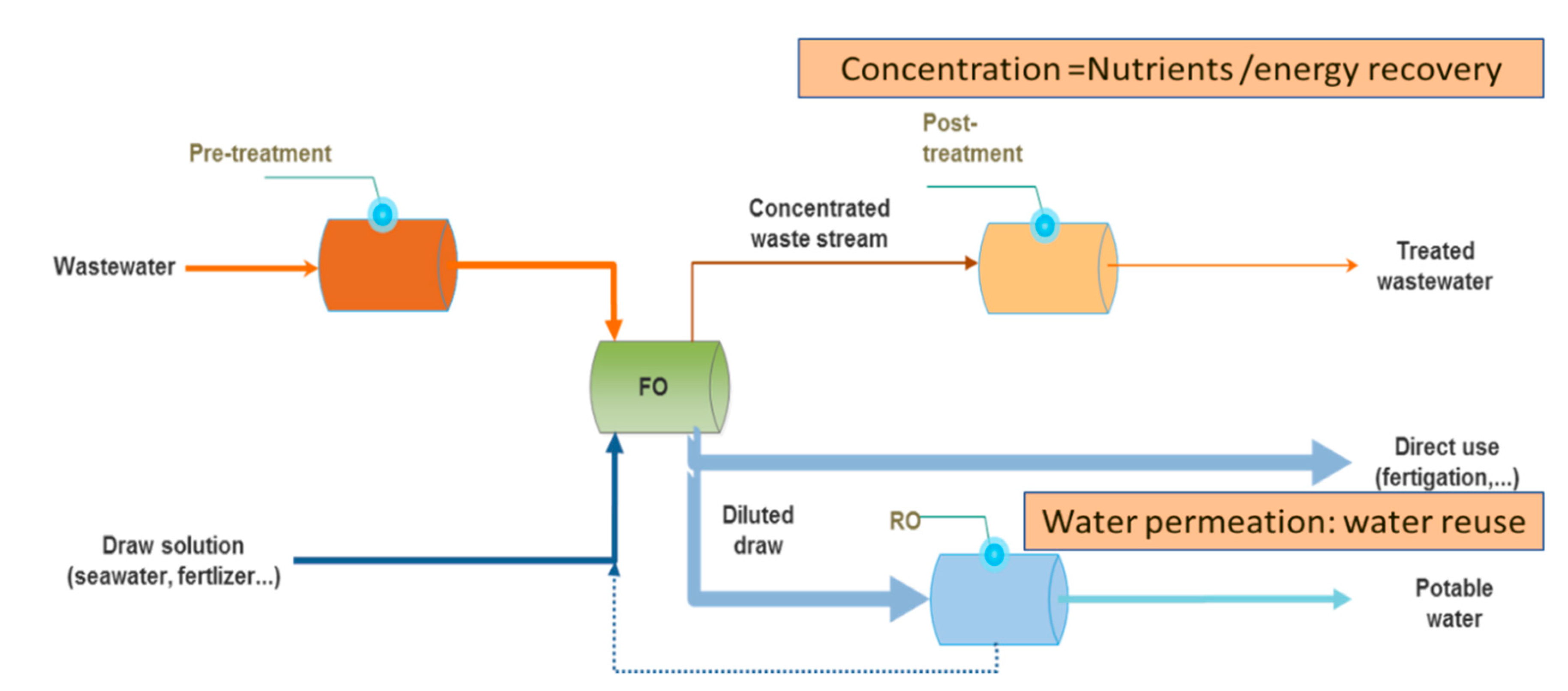
2.5. Brine Management: Concentration and Salts Mining
2.5.1. Brine Concentration Towards Zero Liquid Discharge
2.5.2. Seawater and Brine Mining/ Recovery of Valuable Minerals
3. Tuning FO Process to Tackle Current Limitations for Optimum Concentration
3.1. Better Understanding of FO Fundamentals in the Context of Concentration
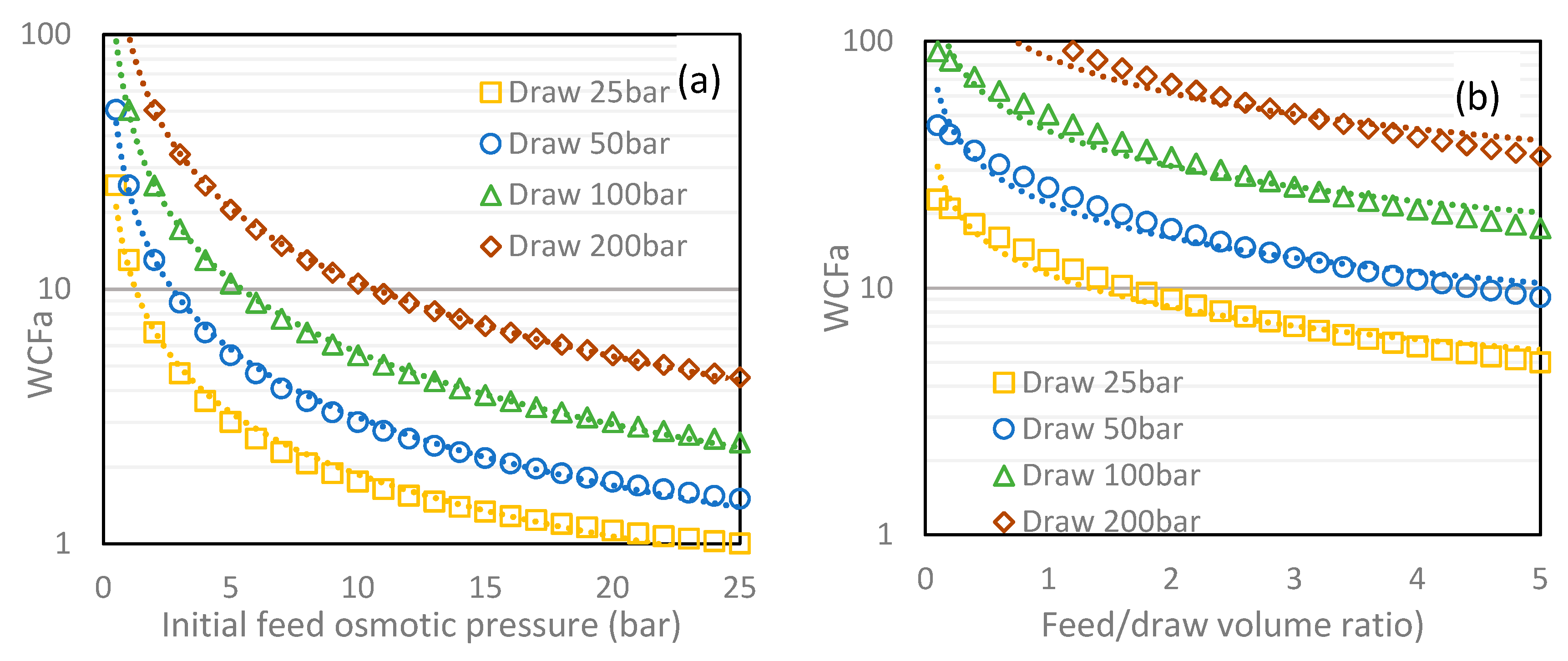
3.1.1. Water Concentration Factor (WCF)
3.1.2. Nutrients Rejections and Recovery
3.2. Membranes and Modules
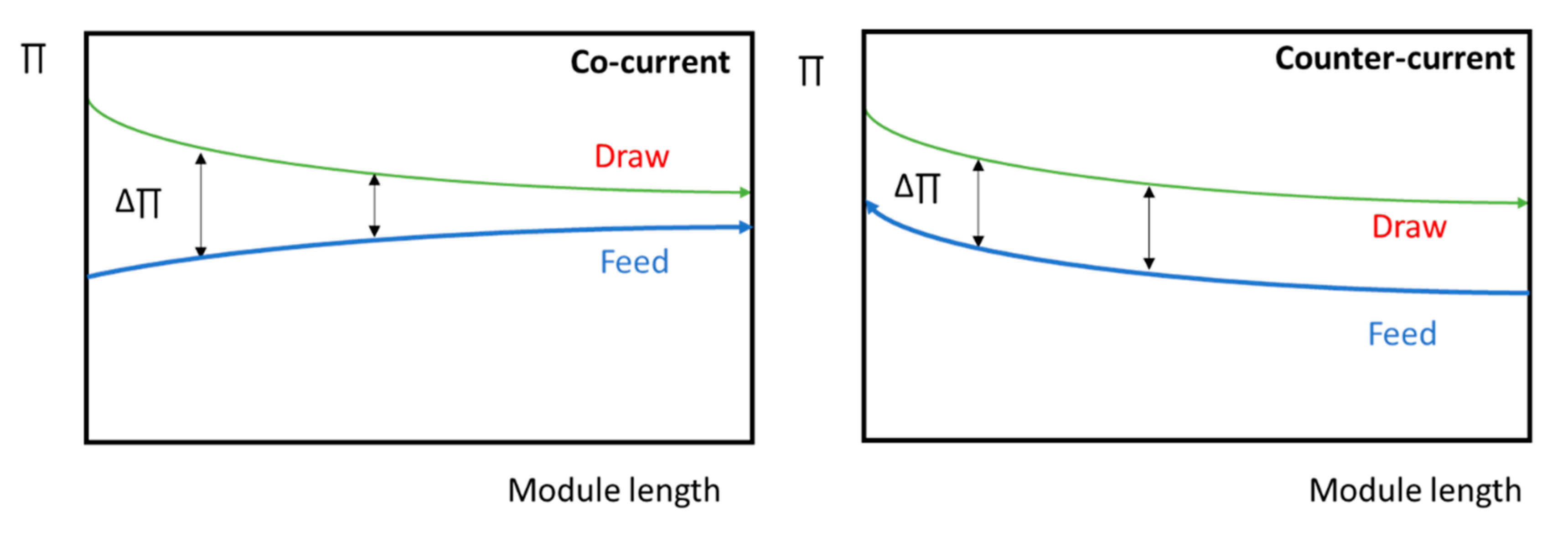
3.3. Process Design
3.4. Draw Selection
- Osmotic pressure should be in line with the required concentration level and the initial osmotic pressure of the feed solution to be treated. Required osmotic pressure will not only impact the draw selection but also the draw recovery method, since above 60 bar typical RO recovery is limited and thermal processes, such as MD, evaporation processes, or under development thermo-responsive draw solution processes are required [258]. According to Siew, the RO draw regeneration system can be used up to 50–60 bar draw osmotic pressure with a range of energy consumption between 1 and 4 Kwh·m−3. From 40 to 120 bar, the RO system can be operated in osmotically assisted RO mode but requires high energy input (5–15 Kwh·m−3). As alternatives, MD, evaporators or thermo-responsive systems allow for a much broader range of operation—i.e., even as high as 200 Bar of draw osmotic pressure but with very high energy requirements.
- Compatibility with the solution to be treated is critical, especially for food and beverages or potable water reuse applications. The non-food draw solution may simply not be approved. Additionally, RSD may alter taste or other organoleptic properties. RSD can also be a limiting factor with regard to achieving high recovery (by shifting the osmotic equilibrium) or may alter biological activity (by excess of saline compounds).
- Costs are very application-dependent. If in food and beverages or brine concentration, more flexibility in draw selection and recovery may be allowed thanks to the relative low cost of FO vs. other concentration process. In lower costs applications, such as in WW treatment, a low cost solution will be preferred [259,260].
3.5. Fouling, Mitigation and Cleaning
4. Concluding Remarks, Remaining Challenges and Future Research
4.1. Need for Dedicated Concentration Studies
4.2. Design of FO Concentration Units
4.3. To Demonstrate Competitiveness Versus Other Concentration Processes
Author Contributions
Funding
Conflicts of Interest
References
- Eykamp, W. Chapter 1 Microfiltration and ultrafiltration. In Membrane Science and Technology; Noble, R.D., Stern, S.A., Eds.; Membrane Separations Technology; Elsevier: Amsterdam, The Netherlands, 1995; Volume 2, pp. 1–43. [Google Scholar]
- Jiao, B.; Cassano, A.; Drioli, E. Recent advances on membrane processes for the concentration of fruit juices: A review. J. Food Eng. 2004, 63, 303–324. [Google Scholar] [CrossRef]
- Wenten, I.G. Khoiruddin Reverse osmosis applications: Prospect and challenges. Desalination 2016, 391, 112–125. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Kiss, A.A.; Readi, O.M.K. An industrial perspective on membrane distillation processes. J. Chem. Technol. Biotechnol. 2018, 93, 2047–2055. [Google Scholar] [CrossRef]
- Hongvaleerat, C.; Cabral, L.M.C.; Dornier, M.; Reynes, M.; Ningsanond, S. Concentration of pineapple juice by osmotic evaporation. J. Food Eng. 2008, 88, 548–552. [Google Scholar] [CrossRef]
- Aider, M.; de Halleux, D. Cryoconcentration technology in the bio-food industry: Principles and applications. LWT Food Sci. Technol. 2009, 42, 679–685. [Google Scholar] [CrossRef]
- Cassano, A.; Jiao, B.; Drioli, E. Production of concentrated kiwifruit juice by integrated membrane process. Food Res. Int. 2004, 37, 139–148. [Google Scholar] [CrossRef]
- Loeb, S.; Titelman, L.; Korngold, E.; Freiman, J. Effect of porous support fabric on osmosis through a Loeb-Sourirajan type asymmetric membrane. J. Membr. Sci. 1997, 129, 243–249. [Google Scholar] [CrossRef]
- Loeb, S. The Loeb-Sourirajan Membrane: How It Came About. In Synthetic Membranes; Turbak, A.F., Ed.; American Chemical Society: Washington, DC, USA, 1981; Volume 153, pp. 1–9. ISBN 978-0-8412-0622-9. [Google Scholar]
- Loeb, S. Production of energy from concentrated brines by pressure-retarded osmosis: I. Preliminary technical and economic correlations. J. Membr. Sci. 1976, 1, 49–63. [Google Scholar] [CrossRef]
- Beaudry, E.G.; Herron, J.R.; Peterson, S.W. Direct Osmosis Concentration of Waste Water: Final Report; Osmotek Inc.: Corvallis, OR, USA, 1999. [Google Scholar]
- Cath, T.Y.; Gormly, S.; Beaudry, E.G.; Flynn, M.T.; Adams, V.D.; Childress, A.E. Membrane contactor processes for wastewater reclamation in space: Part I. Direct osmotic concentration as pretreatment for reverse osmosis. J. Membr. Sci. 2005, 257, 85–98. [Google Scholar] [CrossRef]
- York, R.J.; Thiel, R.S.; Beaudry, E.G. Full-Scale Experience of Direct Osmosis Concentration Applied to Leachate Management; S. Margherita di Pala: Cagliari, Sardinia, Italy, 1999. [Google Scholar]
- Herron, J.R.; Beaudry, E.G.; Jochums, C.E.; Medina, L.E. Osmotic Concentration Apparatus and Method for Direct Osmotic Concentration of Fruit Juices. U.S. Patent 5,281,430, 25 January 1994. [Google Scholar]
- Cath, T.; Childress, A.; Elimelech, M. Forward osmosis: Principles, applications, and recent developments. J. Membr. Sci. 2006, 281, 70–87. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Elimelech, M. Global challenges in energy and water supply: The promise of engineered osmosis. Environ. Sci. Technol. 2008, 42, 8625–8629. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zou, L.; Tang, C.Y.; Mulcahy, D. Recent developments in forward osmosis: Opportunities and challenges. J. Membr. Sci. 2012, 396, 1–21. [Google Scholar] [CrossRef]
- Chung, T.-S.; Zhang, S.; Wang, K.Y.; Su, J.; Ling, M.M. Forward osmosis processes: Yesterday, today and tomorrow. Desalination 2012, 287, 78–81. [Google Scholar] [CrossRef]
- Klaysom, C.; Cath, T.Y.; Depuydt, T.; Vankelecom, I.F.J. Forward and pressure retarded osmosis: Potential solutions for global challenges in energy and water supply. Chem. Soc. Rev. 2013, 42, 6959. [Google Scholar] [CrossRef]
- She, Q.; Wang, R.; Fane, A.G.; Tang, C.Y. Membrane fouling in osmotically driven membrane processes: A review. J. Membr. Sci. 2016, 499, 201–233. [Google Scholar] [CrossRef]
- Coday, B.D.; Yaffe, B.G.M.; Xu, P.; Cath, T.Y. Rejection of Trace Organic Compounds by Forward Osmosis Membranes: A Literature Review. Environ. Sci. Technol. 2014, 48, 3612–3624. [Google Scholar] [CrossRef]
- Chekli, L.; Phuntsho, S.; Shon, H.K.; Vigneswaran, S.; Kandasamy, J.; Chanan, A. A review of draw solutes in forward osmosis process and their use in modern applications. Desalin. Water Treat. 2012, 43, 167–184. [Google Scholar] [CrossRef]
- Qin, J.-J.; Lay, W.C.L.; Kekre, K.A. Recent developments and future challenges of forward osmosis for desalination: A review. Desalin. Water Treat. 2012, 39, 123–136. [Google Scholar] [CrossRef]
- Shaffer, D.L.; Yip, N.Y.; Gilron, J.; Elimelech, M. Seawater desalination for agriculture by integrated forward and reverse osmosis: Improved product water quality for potentially less energy. J. Membr. Sci 2012, 415–416, 1–8. [Google Scholar] [CrossRef]
- Chekli, L.; Phuntsho, S.; Kim, J.E.; Kim, J.; Choi, J.Y.; Choi, J.-S.; Kim, S.; Kim, J.H.; Hong, S.; Sohn, J.; et al. A comprehensive review of hybrid forward osmosis systems: Performance, applications and future prospects. J. Membr. Sci. 2016, 497, 430–449. [Google Scholar] [CrossRef]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. Toward Resource Recovery from Wastewater: Extraction of Phosphorus from Digested Sludge Using a Hybrid Forward Osmosis–Membrane Distillation Process. Environ. Sci. Technol. Lett. 2014, 1, 191–195. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Verliefde, A.R.D.; Roest, K.; Rietveld, L.C.; Cornelissen, E.R. Forward osmosis for application in wastewater treatment: A review. Water Res. 2014, 58, 179–197. [Google Scholar] [CrossRef]
- Akther, N.; Sodiq, A.; Giwa, A.; Daer, S.; Arafat, H.A.; Hasan, S.W. Recent advancements in forward osmosis desalination: A review. Chem. Eng. J. 2015, 281, 502–522. [Google Scholar] [CrossRef]
- Rastogi, N.K. Opportunities and Challenges in Application of Forward Osmosis in Food Processing. Crit. Rev. Food Sci. Nutr. 2016, 56, 266–291. [Google Scholar] [CrossRef]
- Coday, B.D.; Xu, P.; Beaudry, E.G.; Herron, J.; Lampi, K.; Hancock, N.T.; Cath, T.Y. The sweet spot of forward osmosis: Treatment of produced water, drilling wastewater, and other complex and difficult liquid streams. Desalination 2014, 333, 23–35. [Google Scholar] [CrossRef]
- Petrotos, K.B.; Lazarides, H.N. Osmotic concentration of liquid foods. J. Food Eng. 2001, 49, 201–206. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Marczak, L.D.F.; Tessaro, I.C. Membrane concentration of liquid foods by forward osmosis: Process and quality view. J. Food Eng. 2012, 111, 483–489. [Google Scholar] [CrossRef]
- Popper, K.; Camirand, W.; Nury, F.; Stanley, W. Dialyzer concentrates beverages. Food Eng. 1966, 38, 102–104. [Google Scholar]
- Beaudry, E.; Lampi, K. Membrane technology for direct-osmosis concentration of fruit juices. Food Technol. 1990, 44, 121. [Google Scholar]
- Kim, D.I.; Gwak, G.; Zhan, M.; Hong, S. Sustainable dewatering of grapefruit juice through forward osmosis: Improving membrane performance, fouling control, and product quality. J. Membr. Sci. 2019, 578, 53–60. [Google Scholar] [CrossRef]
- Sant’Anna, V.; Gurak, P.D.; de Vargas, N.S.; da Silva, M.K.; Marczak, L.D.F.; Tessaro, I.C. Jaboticaba (Myrciaria jaboticaba) juice concentration by forward osmosis. Sep. Sci. Technol. 2016, 51, 1708–1715. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Mcdaniel, M.R.; Durst, R.W.; Micheals, N.; Lampi, K.A.; Beaudry, E.G. Composition and Sensory Characterization of Red Raspberry Juice Concentrated by Direct-Osmosis or Evaporation. J. Food Sci. 1993, 58, 633–637. [Google Scholar] [CrossRef]
- Dova, M.I.; Petrotos, K.B.; Lazarides, H.N. On the direct osmotic concentration of liquid foods. Part I: Impact of process parameters on process performance. J. Food Eng. 2007, 78, 422–430. [Google Scholar] [CrossRef]
- Petrotos, K.B.; Quantick, P.; Petropakis, H. A study of the direct osmotic concentration of tomato juice in tubular membrane—Module configuration. I. The effect of certain basic process parameters on the process performance. J. Membr. Sci. 1998, 150, 99–110. [Google Scholar] [CrossRef]
- Babu, B.R.; Rastogi, N.K.; Raghavarao, K.S.M.S. Effect of process parameters on transmembrane flux during direct osmosis. J. Membr. Sci. 2006, 280, 185–194. [Google Scholar] [CrossRef]
- Nayak, C.A.; Rastogi, N.K. Forward osmosis for the concentration of anthocyanin from Garcinia indica Choisy. Sep. Purif. Technol. 2010, 71, 144–151. [Google Scholar] [CrossRef]
- Nayak, C.A.; Valluri, S.S.; Rastogi, N.K. Effect of high or low molecular weight of components of feed on transmembrane flux during forward osmosis. J. Food Eng. 2011, 106, 48–52. [Google Scholar] [CrossRef]
- Valluri, S.S. Forward Osmosis for Concentration of Liquid Foods. Ph.D. Thesis, Central Food Technological Research Institute, Mysore, India, 2010. [Google Scholar]
- Coconut Milk Concentration. Aquaporin. 2019. Available online: http://aquaporin.com/coconut-concentration/ (accessed on 19 March 2020).
- Food & Beverage Solutions. Available online: http://www.porifera.com/food-solutions (accessed on 19 March 2020).
- Forward Osmosis for Natural Flavors/Colors—Ederna. Available online: http://www.ederna.com/beverages (accessed on 19 March 2020).
- An, X.; Hu, Y.; Wang, N.; Zhou, Z.; Liu, Z. Continuous juice concentration by integrating forward osmosis with membrane distillation using potassium sorbate preservative as a draw solute. J. Membr. Sci. 2019, 573, 192–199. [Google Scholar] [CrossRef]
- Chanukya, B.S.; Rastogi, N.K. Ultrasound assisted forward osmosis concentration of fruit juice and natural colorant. Ultrason. Sonochem. 2017, 34, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Petrotos, K.B.; Tsiadi, A.V.; Poirazis, E.; Papadopoulos, D.; Petropakis, H.; Gkoutsidis, P. A description of a flat geometry direct osmotic concentrator to concentrate tomato juice at ambient temperature and low pressure. J. Food Eng. 2010, 97, 235–242. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; McCutcheon, J.R.; Elimelech, M. Performance evaluation of sucrose concentration using forward osmosis. J. Membr. Sci. 2009, 338, 61–66. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; McCutcheon, J.R. Dewatering press liquor derived from orange production by forward osmosis. J. Membr. Sci. 2011, 372, 97–101. [Google Scholar] [CrossRef]
- Hasanoğlu, A.; Gül, K. Concentration of Skim Milk and Dairy Products by forward Osmosis. J. Turk. Chem. Soc. Sect. B 2017, 1, 149–160. [Google Scholar]
- Schuck, P.; Jeantet, R.; Tanguy, G.; Méjean, S.; Gac, A.; Lefebvre, T.; Labussière, E.; Martineau, C. Energy Consumption in the Processing of Dairy and Feed Powders by Evaporation and Drying. Dry. Technol. 2015, 33, 176–184. [Google Scholar] [CrossRef]
- Burke, N.; Zacharski, K.A.; Southern, M.; Hogan, P.; Ryan, M.P.; Adley, C.C. The Dairy Industry: Process, Monitoring, Standards, and Quality. Descr. Food Sci. 2018. [CrossRef]
- Písecký, J. Handbook of Milk Powder Manufacture; GEA Process Engineering A/S: Copenhagen, Denmark, 2012; ISBN 87-87036-74-6. [Google Scholar]
- Ramírez, C.A.; Patel, M.; Blok, K. From fluid milk to milk powder: Energy use and energy efficiency in the European dairy industry. Energy 2006, 31, 1984–2004. [Google Scholar] [CrossRef]
- Available online: https://www.gea.com/en/binaries/gea-membrane-filtration-brochure-for-dairy-industry_tcm11-17109.pdf (accessed on 20 March 2020).
- Chen, G.Q.; Artemi, A.; Lee, J.; Gras, S.L.; Kentish, S.E. A pilot scale study on the concentration of milk and whey by forward osmosis. Sep. Purif. Technol. 2019, 215, 652–659. [Google Scholar] [CrossRef]
- Aydiner, C.; Topcu, S.; Tortop, C.; Kuvvet, F.; Ekinci, D.; Dizge, N.; Keskinler, B. A novel implementation of water recovery from whey: “forward–reverse osmosis” integrated membrane system. Desalin. Water Treat. 2013, 51, 786–799. [Google Scholar] [CrossRef]
- Aydiner, C.; Sen, U.; Topcu, S.; Sesli, D.; Ekinci, D.; Altınay, A.D.; Ozbey, B.; Koseoglu-Imer, D.Y.; Keskinler, B. Techno-economic investigation of water recovery and whey powder production from whey using UF/RO and FO/RO integrated membrane systems. Desalin. Water Treat. 2013, 1–11. [Google Scholar] [CrossRef]
- Aydiner, C.; Sen, U.; Topcu, S.; Ekinci, D.; Altinay, A.D.; Koseoglu-Imer, D.Y.; Keskinler, B. Techno-economic viability of innovative membrane systems in water and mass recovery from dairy wastewater. J. Membr. Sci. 2014, 458, 66–75. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Wang, R.; Li, W.; Tang, C.Y. Whey recovery using forward osmosis – Evaluating the factors limiting the flux performance. J. Membr. Sci. 2017, 533, 179–189. [Google Scholar] [CrossRef]
- Schrier, J. Ethanol concentration by forward osmosis with solar-regenerated draw solution. Sol. Energy 2012, 86, 1351–1358. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, Z.; Wang, D.K.; Diniz da Costa, J.C. A novel ethanol dehydration process by forward osmosis. Chem. Eng. J. 2013, 232, 397–404. [Google Scholar] [CrossRef]
- Ambrosi, A.; Corrêa, G.L.; de Vargas, N.S.; Gabe, L.M.; Cardozo, N.S.M.; Tessaro, I.C. Impact of osmotic agent on the transport of components using forward osmosis to separate ethanol from aqueous solutions. Aiche J. 2017, 63, 4499–4507. [Google Scholar] [CrossRef]
- Ambrosi, A.; Al-Furaiji, M.; McCutcheon, J.R.; Cardozo, N.S.M.; Tessaro, I.C. Transport of Components in the Separation of Ethanol from Aqueous Dilute Solutions by Forward Osmosis. Ind. Eng. Chem. Res. 2018, 57, 2967–2975. [Google Scholar] [CrossRef]
- Nazir, A.; Khan, K.; Maan, A.; Zia, R.; Giorno, L.; Schroën, K. Membrane separation technology for the recovery of nutraceuticals from food industrial streams. Trends Food Sci. Technol. 2019, 86, 426–438. [Google Scholar] [CrossRef]
- Wang, K.Y.; Teoh, M.M.; Nugroho, A.; Chung, T.-S. Integrated forward osmosis–membrane distillation (FO–MD) hybrid system for the concentration of protein solutions. Chem. Eng. Sci. 2011, 66, 2421–2430. [Google Scholar] [CrossRef]
- Wang, J.; Martínez-Hernández, A.; de Lamo-Castellví, S.; Romero, M.-P.; Kaade, W.; Ferrando, M.; Güell, C. Low-energy membrane-based processes to concentrate and encapsulate polyphenols from carob pulp. J. Food Eng. 2020, 109996. [Google Scholar] [CrossRef]
- Khongnakorn, W.; Youravong, W. Concentration and recovery of protein from tuna cooking juice by forward osmosis. J. Eng. Sci. Technol. 2016, 11, 962–973. [Google Scholar]
- Gebreyohannes, A.Y.; Curcio, E.; Poerio, T.; Mazzei, R.; Di Profio, G.; Drioli, E.; Giorno, L. Treatment of Olive Mill Wastewater by Forward Osmosis. Sep. Purif. Technol. 2015, 147, 292–302. [Google Scholar] [CrossRef]
- Singh, N.; Petrinic, I.; Hélix-Nielsen, C.; Basu, S.; Balakrishnan, M. Concentrating molasses distillery wastewater using biomimetic forward osmosis (FO) membranes. Water Res. 2018, 130, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Choi, J.; Hong, S. Evaluation on suitability of osmotic dewatering through forward osmosis (FO) for xylose concentration. Sep. Purif. Technol. 2018, 191, 225–232. [Google Scholar] [CrossRef]
- Mondal, D.; Nataraj, S.K.; Reddy, A.V.R.; Ghara, K.K.; Maiti, P.; Upadhyay, S.C.; Ghosh, P.K. Four-fold concentration of sucrose in sugarcane juice through energy efficient forward osmosis using sea bittern as draw solution. RSC Adv. 2015, 5, 17872–17878. [Google Scholar] [CrossRef]
- Zhang, Y.; Nakagawa, K.; Shibuya, M.; Sasaki, K.; Takahashi, T.; Shintani, T.; Yoshioka, T.; Kamio, E.; Kondo, A.; Matsuyama, H. Improved permselectivity of forward osmosis membranes for efficient concentration of pretreated rice straw and bioethanol production. J. Membr. Sci. 2018, 566, 15–24. [Google Scholar] [CrossRef]
- Shibuya, M.; Yasukawa, M.; Sasaki, K.; Tanaka, Y.; Takahashi, T.; Kondo, A.; Matsuyama, H. Up-concentration of sugars in pretreated-rice straw by an osmotic pressure-driven method. Biochem. Eng. J. 2017, 121, 13–16. [Google Scholar] [CrossRef]
- Garcia-Aguirre, J.; Alvarado-Morales, M.; Fotidis, I.A.; Angelidaki, I. Up-concentration of succinic acid, lactic acid, and ethanol fermentations broths by forward osmosis. Biochem. Eng. J. 2020, 155, 107482. [Google Scholar] [CrossRef]
- Blandin, G.; Rosselló, B.; Monsalvo, V.M.; Batlle-Vilanova, P.; Viñas, J.M.; Rogalla, F.; Comas, J. Volatile fatty acids concentration in real wastewater by forward osmosis. J. Membr. Sci. 2019, 575, 60–70. [Google Scholar] [CrossRef]
- Cui, Y.; Chung, T.-S. Pharmaceutical concentration using organic solvent forward osmosis for solvent recovery. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Singh, N.; Petrinic, I.; Hélix-Nielsen, C.; Basu, S.; Balakrishnan, M. Influence of Forward Osmosis (FO) membrane properties on dewatering of molasses distillery wastewater. J. Water Process. Eng. 2019, 32, 100921. [Google Scholar] [CrossRef]
- Draaisma, R.B.; Wijffels, R.H.; (Ellen) Slegers, P.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177. [Google Scholar] [CrossRef]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Trent, J.; Embaye, T.; Buckwalter, P.; Richardson, T.-M.; Kagawa, H.; Reinsch, S.; Martis, M. Offshore Membrane Enclosures for Growing Algae (OMEGA: A System for Biofuel Production, Wastewater Treatment, and CO2 Sequestration. 2010. Available online: https://ntrs.nasa.gov/citations/20100039342 (accessed on 4 April 2020).
- Delrue, F.; Álvarez-Díaz, P.D.; Fon-Sing, S.; Fleury, G.; Sassi, J.-F. The Environmental Biorefinery: Using Microalgae to Remediate Wastewater, a Win-Win Paradigm. Energies 2016, 9, 132. [Google Scholar] [CrossRef]
- Bilad, M.R.; Arafat, H.A.; Vankelecom, I.F.J. Membrane technology in microalgae cultivation and harvesting: A review. Biotechnol. Adv. 2014, 32, 1283–1300. [Google Scholar] [CrossRef] [PubMed]
- Udom, I.; Zaribaf, B.H.; Halfhide, T.; Gillie, B.; Dalrymple, O.; Zhang, Q.; Ergas, S.J. Harvesting microalgae grown on wastewater. Bioresour. Technol. 2013, 139, 101–106. [Google Scholar] [CrossRef]
- Wang, J.-H.; Zhang, T.-Y.; Dao, G.-H.; Xu, X.-Q.; Wang, X.-X.; Hu, H.-Y. Microalgae-based advanced municipal wastewater treatment for reuse in water bodies. Appl. Microbiol. Biotechnol. 2017, 101, 2659–2675. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Wu, C.; Muylaert, K.; Vyverman, W.; Yu, H.-Q.; Muñoz, R.; Rittmann, B. Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: A review. Bioresour. Technol. 2017, 241, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Wibisono, Y.; Agung Nugroho, W.; Akbar Devianto, L.; Adi Sulianto, A.; Roil Bilad, M. Microalgae in Food-Energy-Water Nexus: A Review on Progress of Forward Osmosis Applications. Membranes 2019, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Onyshchenko, E.; Blandin, G.; Comas, J.; Dvoretsky, A. Influence of microalgae wastewater treatment culturing conditions on forward osmosis concentration process. Environ. Sci. Pollut. Res. 2018, 27, 1234–1245. [Google Scholar] [CrossRef]
- Praveen, P.; Heng, J.Y.P.; Loh, K.-C. Tertiary wastewater treatment in membrane photobioreactor using microalgae: Comparison of forward osmosis & microfiltration. Bioresour. Technol. 2016, 222, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Praveen, P.; Loh, K.-C. Nitrogen and phosphorus removal from tertiary wastewater in an osmotic membrane photobioreactor. Bioresour. Technol. 2016, 206, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, P.; Embaye, T.; Gormly, S.; Trent, J.D. Dewatering microalgae by forward osmosis. Desalination 2013, 312, 19–22. [Google Scholar] [CrossRef]
- Ye, J.; Zhou, Q.; Zhang, X.; Hu, Q. Microalgal dewatering using a polyamide thin film composite forward osmosis membrane and fouling mitigation. Algal Res. 2018, 31, 421–429. [Google Scholar] [CrossRef]
- Volpin, F.; Yu, H.; Cho, J.; Lee, C.; Phuntsho, S.; Ghaffour, N.; Vrouwenvelder, J.S.; Shon, H.K. Human urine as a forward osmosis draw solution for the application of microalgae dewatering. J. Hazard. Mater. 2019, 378, 120724. [Google Scholar] [CrossRef]
- Mazzuca Sobczuk, T.; Ibáñez González, M.J.; Molina Grima, E.; Chisti, Y. Forward osmosis with waste glycerol for concentrating microalgae slurries. Algal Res. 2015, 8, 168–173. [Google Scholar] [CrossRef]
- Ryu, H.; Kim, K.; Cho, H.; Park, E.; Chang, Y.K.; Han, J.-I. Nutrient-driven forward osmosis coupled with microalgae cultivation for energy efficient dewatering of microalgae. Algal Res. 2020, 48, 101880. [Google Scholar] [CrossRef]
- Onyshchenko, E.; Blandin, G.; Comas, J.; Dvoretsky, A. Design and Evaluation of a forward Osmosis Microalgae Bioreactor to Combine Microalgae Dewatering and Wastewater Reuse; EFCE Spain Group: Barcelona, Spain, 2017. [Google Scholar]
- Larronde-Larretche, M.; Jin, X. Microalgae (Scenedesmus obliquus) dewatering using forward osmosis membrane: Influence of draw solution chemistry. Algal Res. 2016, 15, 1–8. [Google Scholar] [CrossRef]
- Itliong, J.N.; Villagracia, A.R.C.; Moreno, J.L.V.; Rojas, K.I.M.; Bernardo, G.P.O.; David, M.Y.; Manrique, R.B.; Ubando, A.T.; Culaba, A.B.; Padama, A.A.B.; et al. Investigation of reverse ionic diffusion in forward-osmosis-aided dewatering of microalgae: A molecular dynamics study. Bioresour. Technol. 2019, 279, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Honda, R.; Rukapan, W.; Komura, H.; Teraoka, Y.; Noguchi, M.; Hoek, E.M.V. Effects of membrane orientation on fouling characteristics of forward osmosis membrane in concentration of microalgae culture. Bioresour. Technol. 2015, 197, 429–433. [Google Scholar] [CrossRef]
- Zou, S.; Gu, Y.; Xiao, D.; Tang, C.Y. The role of physical and chemical parameters on forward osmosis membrane fouling during algae separation. J. Membr. Sci. 2011, 366, 356–362. [Google Scholar] [CrossRef]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Cagnetta, C.; D’Haese, A.; Coma, M.; Props, R.; Buysschaert, B.; Verliefde, A.R.D.; Rabaey, K. Increased carboxylate production in high-rate activated A-sludge by forward osmosis thickening. Chem. Eng. J. 2017, 312, 68–78. [Google Scholar] [CrossRef]
- Jung, K.; Choi, J.; Lee, D.; Seo, C.; Lee, J.; Lee, S.Y.; Chang, H.N.; Kim, Y.-C. Permeation characteristics of volatile fatty acids solution by forward osmosis. Process. Biochem. 2015, 50, 669–677. [Google Scholar] [CrossRef]
- Lutchmiah, K.; Cornelissen, E.R.; Harmsen, D.J.H.; Post, J.W.; Lampi, K.; Ramaekers, H.; Rietveld, L.C.; Roest, K. Water recovery from sewage using forward osmosis. Water Sci. Technol. 2011, 64, 1443–1449. [Google Scholar] [CrossRef]
- Phuntsho, S.; Shon, H.K.; Hong, S.; Lee, S.; Vigneswaran, S. A novel low energy fertilizer driven forward osmosis desalination for direct fertigation: Evaluating the performance of fertilizer draw solutions. J. Membr. Sci. 2011, 375, 172–181. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Li, Z.; Abu-Ghdaib, M.; Wei, C.H.; Amy, G.; Vrouwenvelder, J.S. Water harvesting from municipal wastewater via osmotic gradient: An evaluation of process performance. J. Membr. Sci. 2013, 447, 50–56. [Google Scholar] [CrossRef]
- Lee, S.; Boo, C.; Elimelech, M.; Hong, S. Comparison of fouling behavior in forward osmosis (FO) and reverse osmosis (RO). J. Membr. Sci. 2010, 365, 34–39. [Google Scholar] [CrossRef]
- Cath, T.Y.; Hancock, N.T.; Lundin, C.D.; Hoppe-Jones, C.; Drewes, J.E. A multi-barrier osmotic dilution process for simultaneous desalination and purification of impaired water. J. Membr. Sci. 2010, 362, 417–426. [Google Scholar] [CrossRef]
- Hancock, N.T.; Black, N.D.; Cath, T.Y. A comparative life cycle assessment of hybrid osmotic dilution desalination and established seawater desalination and wastewater reclamation processes. Water Res. 2012, 46, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Blandin, G.; Verliefde, A.; Comas, J.; Rodriguez-Roda, I.; Le-Clech, P. Efficiently Combining Water Reuse and Desalination through Forward Osmosis—Reverse Osmosis (FO-RO) Hybrids: A Critical Review. Membranes 2016, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Blandin, G.; Verliefde, A.R.D.; Tang, C.Y.; Le-Clech, P. Opportunities to reach economic sustainability in forward osmosis–reverse osmosis hybrids for seawater desalination. Desalination 2015, 363, 26–36. [Google Scholar] [CrossRef]
- Teusner, A.; Blandin, G.; Le-Clech, P. Augmenting water supply by combined desalination/water recycling methods: An economic assessment. Environ. Technol. 2017, 38, 257–265. [Google Scholar] [CrossRef]
- Large Scale FO-Based Desalination|ForwardOsmosisTech. Available online: https://www.forwardosmosistech.com/0-26mgd-fo-swro-hybrid-for-seawater-desalination-achieves-25-energy-reduction-compared-to-mf-swro/ (accessed on 30 March 2020).
- Holloway, R.; Childress, A.; Dennett, K.; Cath, T. Forward osmosis for concentration of anaerobic digester centrate. Water Res. 2007, 41, 4005–4014. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Chen, S.-S.; Yang, H.-Y.; Hau, N.T. Application of forward osmosis on dewatering of high nutrient sludge. Bioresour. Technol. 2013, 132, 224–229. [Google Scholar] [CrossRef]
- Butler, R.; MacCormick, T. Opportunities for decentralized treatment, sewer mining and effluent re-use. Desalination 1996, 106, 273–283. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Ngo, H.H.; Guo, W.; Nghiem, L.D. Assessing the integration of forward osmosis and anaerobic digestion for simultaneous wastewater treatment and resource recovery. Bioresour. Technol. 2018, 260, 221–226. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Drewes, J.E.; Nghiem, L.D. Forward osmosis as a platform for resource recovery from municipal wastewater—A critical assessment of the literature. J. Membr. Sci. 2017, 529, 195–206. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, Z.; Wang, D.K.; Diniz da Costa, J.C. Processing municipal wastewaters by forward osmosis using CTA membrane. J. Membr. Sci. 2014, 468, 269–275. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Guo, W.; Ngo, H.H.; Price, W.E.; Nghiem, L.D. Factors governing the pre-concentration of wastewater using forward osmosis for subsequent resource recovery. Sci. Total Environ. 2016, 566–567, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Pijuan, M.; Rodriguez-Roda, I.; Blandin, G. Exploring Submerged Forward Osmosis for Water Recovery and Pre-Concentration of Wastewater before Anaerobic Digestion: A Pilot Scale Study. Membranes 2019, 9, 97. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, J.; Tang, J.; Wang, X.; Wu, Z. A pilot-scale forward osmosis membrane system for concentrating low-strength municipal wastewater: Performance and implications. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Bao, X.; Wu, Q.; Tian, J.; Shi, W.; Wang, W.; Zhang, Z.; Zhang, R.; Zhang, B.; Guo, Y.; Shu, S.; et al. Fouling mechanism of forward osmosis membrane in domestic wastewater concentration: Role of substrate structures. Chem. Eng. J. 2019, 370, 262–273. [Google Scholar] [CrossRef]
- Cornelissen, E.R.; Harmsen, D.; Beerendonk, E.F.; Qin, J.J.; Oo, H.; de Korte, K.F.; Kappelhof, J.W.M.N. The innovative Osmotic Membrane Bioreactor (OMBR) for reuse of wastewater. Water Sci. Technol. 2011, 63, 1557–1565. [Google Scholar] [CrossRef]
- Holloway, R.W.; Achilli, A.; Cath, T.Y. The osmotic membrane bioreactor: A critical review. Environ. Sci. Water Res. Technol. 2015, 1, 581–605. [Google Scholar] [CrossRef]
- Blandin, G.; Le-Clech, P.; Cornelissen, E.; Verliefde, A.R.D.; Comas, J.; Rodriguez-Roda, I. Can osmotic membrane bioreactor be a realistic solution for water reuse? NPJ Clean Water 2018, 1, 7. [Google Scholar] [CrossRef]
- Blandin, G.; Gautier, C.; Sauchelli Toran, M.; Monclús, H.; Rodriguez-Roda, I.; Comas, J. Retrofitting membrane bioreactor (MBR) into osmotic membrane bioreactor (OMBR): A pilot scale study. Chem. Eng. J. 2018, 339, 268–277. [Google Scholar] [CrossRef]
- Luo, W.; Phan, H.V.; Xie, M.; Hai, F.I.; Price, W.E.; Elimelech, M.; Nghiem, L.D. Osmotic versus conventional membrane bioreactors integrated with reverse osmosis for water reuse: Biological stability, membrane fouling, and contaminant removal. Water Res. 2017, 109, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, V.W.C.; Tang, C.Y. Osmotic membrane bioreactor (OMBR) technology for wastewater treatment and reclamation: Advances, challenges, and prospects for the future. J. Membr. Sci. 2016, 504, 113–132. [Google Scholar] [CrossRef]
- Holloway, R.W.; Wait, A.S.; Fernandes da Silva, A.; Herron, J.; Schutter, M.D.; Lampi, K.; Cath, T.Y. Long-term pilot scale investigation of novel hybrid ultrafiltration-osmotic membrane bioreactors. Desalination 2015, 363, 64–74. [Google Scholar] [CrossRef]
- Luo, W.; Hai, F.I.; Kang, J.; Price, W.E.; Nghiem, L.D.; Elimelech, M. The role of forward osmosis and microfiltration in an integrated osmotic-microfiltration membrane bioreactor system. Chemosphere 2015, 136, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Ting, Y.-P. Direct phosphorus recovery from municipal wastewater via osmotic membrane bioreactor (OMBR) for wastewater treatment. Bioresour. Technol. 2014, 170, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Zhang, S.; Srinivasa Raghavan, D.S.; Das, S.; Ting, Y.-P. The potential of hybrid forward osmosis membrane bioreactor (FOMBR) processes in achieving high throughput treatment of municipal wastewater with enhanced phosphorus recovery. Water Res. 2016, 105, 370–382. [Google Scholar] [CrossRef]
- Xue, W.; Tobino, T.; Nakajima, F.; Yamamoto, K. Seawater-driven forward osmosis for enriching nitrogen and phosphorous in treated municipal wastewater: Effect of membrane properties and feed solution chemistry. Water Res. 2015, 69, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Yamamoto, K.; Tobino, T. Membrane fouling and long-term performance of seawater-driven forward osmosis for enrichment of nutrients in treated municipal wastewater. J. Membr. Sci. 2016, 499, 555–562. [Google Scholar] [CrossRef]
- Giménez, J.B.; Robles, A.; Carretero, L.; Durán, F.; Ruano, M.V.; Gatti, M.N.; Ribes, J.; Ferrer, J.; Seco, A. Experimental study of the anaerobic urban wastewater treatment in a submerged hollow-fibre membrane bioreactor at pilot scale. Bioresour. Technol. 2011, 102, 8799–8806. [Google Scholar] [CrossRef]
- Pretel, R.; Robles, A.; Ruano, M.V.; Seco, A.; Ferrer, J. The operating cost of an anaerobic membrane bioreactor (AnMBR) treating sulphate-rich urban wastewater. Sep. Purif. Technol. 2014, 126, 30–38. [Google Scholar] [CrossRef]
- Chen, L.; Gu, Y.; Cao, C.; Zhang, J.; Ng, J.-W.; Tang, C. Performance of a submerged anaerobic membrane bioreactor with forward osmosis membrane for low-strength wastewater treatment. Water Res. 2014, 50, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Chen, L.; Ng, J.-W.; Lee, C.; Chang, V.W.-C.; Tang, C.Y. Development of anaerobic osmotic membrane bioreactor for low-strength wastewater treatment at mesophilic condition. J. Membr. Sci. 2015, 490, 197–208. [Google Scholar] [CrossRef]
- Tang, M.K.Y.; Ng, H.Y. Impacts of different draw solutions on a novel anaerobic forward osmosis membrane bioreactor (AnFOMBR). Water Sci. Technol. 2014, 69, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C.; Tang, C.Y.; Hu, T.; Li, X.; Ren, Y. Development of a novel anaerobic membrane bioreactor simultaneously integrating microfiltration and forward osmosis membranes for low-strength wastewater treatment. J. Membr. Sci. 2017, 527, 1–7. [Google Scholar] [CrossRef]
- Vinardell, S.; Astals, S.; Mata-Alvarez, J.; Dosta, J. Techno-economic analysis of combining forward osmosis-reverse osmosis and anaerobic membrane bioreactor technologies for municipal wastewater treatment and water production. Bioresour. Technol. 2020, 297, 122395. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Y.; Yuan, B.; Wang, Z.; Li, X.; Ren, Y. Comparison of biofouling mechanisms between cellulose triacetate (CTA) and thin-film composite (TFC) polyamide forward osmosis membranes in osmotic membrane bioreactors. Bioresour. Technol. 2016, 202, 50–58. [Google Scholar] [CrossRef]
- Holloway, R.W.; Regnery, J.; Nghiem, L.D.; Cath, T.Y. Removal of trace organic chemicals and performance of a novel hybrid ultrafiltration-osmotic membrane bioreactor. Environ. Sci. Technol. 2014, 48, 10859–10868. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Law, Y.; Das, S.; Ting, Y. Direct and Complete Phosphorus Recovery from Municipal Wastewater Using a Hybrid Micro fi ltration-Forward Osmosis Membrane Bioreactor Process with Seawater Brine as Draw Solution. Environ. Sci. Technol. 2015, 49, 6156–6163. [Google Scholar] [CrossRef]
- Qiu, G.; Ting, Y.P. Osmotic membrane bioreactor for wastewater treatment and the effect of salt accumulation on system performance and microbial community dynamics. Bioresour. Technol. 2013, 150, 287–297. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, J.; Song, L.; Gao, S.; Shi, W.; Cui, F. Dynamic changes of the fouling layer in forward osmosis based membrane processes for municipal wastewater treatment. J. Membr. Sci. 2018, 549, 523–532. [Google Scholar] [CrossRef]
- Chang, H.M.; Sun, Y.C.; Chien, I.C.; Chang, W.S.; Ray, S.S.; Cao, D.T.N.; Cong Duong, C.; Chen, S.S. Innovative upflow anaerobic sludge osmotic membrane bioreactor for wastewater treatment. Bioresour. Technol. 2019, 287, 121466. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gao, B.; Jang, A.; kyong Shon, H.; Yue, Q. Municipal wastewater treatment by forward osmosis using seawater concentrate as draw solution. Chemosphere 2019, 237. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fang, Z.; Liang, P.; Huang, X. Direct concentration of municipal sewage by forward osmosis and membrane fouling behavior. Bioresour. Technol. 2017, 247, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Dhiman, S.; Basu, S.; Balakrishnan, M.; Petrinic, I.; Helix-Nielsen, C. Dewatering of sewage for nutrients and water recovery by Forward Osmosis (FO)using divalent draw solution. J. Water Process. Eng. 2019, 31, 100853. [Google Scholar] [CrossRef]
- Song, Y.-C.; Kim, M.; Shon, H.; Jegatheesan, V.; Kim, S. Modeling methane production in anaerobic forward osmosis bioreactor using a modified anaerobic digestion model No. 1. Bioresour. Technol. 2018, 264, 211–218. [Google Scholar] [CrossRef]
- Li, J.; Hou, D.; Li, K.; Zhang, Y.; Wang, J.; Zhang, X. Domestic wastewater treatment by forward osmosis-membrane distillation (FO-MD) integrated system. Water Sci. Technol. 2018, 77, 1514–1523. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, J.; Zhao, Z.; Shi, W.; Liu, D.; Cui, F. Membrane fouling of forward osmosis (FO) membrane for municipal wastewater treatment: A comparison between direct FO and OMBR. Water Res. 2016, 104, 330–339. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, L.; Wen, X.; Huang, X. Feasibility of applying forward osmosis to the simultaneous thickening, digestion, and direct dewatering of waste activated sludge. Bioresour. Technol. 2012, 113, 207–213. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Nguyen, H.T.; Ho, S.-T.; Chen, S.-S.; Ngo, H.H.; Guo, W.; Ray, S.S.; Hsu, H.-T. Exploring high charge of phosphate as new draw solute in a forward osmosis–membrane distillation hybrid system for concentrating high-nutrient sludge. Sci. Total Environ. 2016, 557–558, 44–50. [Google Scholar] [CrossRef]
- Hau, N.T.; Chen, S.-S.; Nguyen, N.C.; Huang, K.Z.; Ngo, H.H.; Guo, W. Exploration of EDTA sodium salt as novel draw solution in forward osmosis process for dewatering of high nutrient sludge. J. Membr. Sci. 2014, 455, 305–311. [Google Scholar] [CrossRef]
- Lee, S.; Shon, H.K.; Hong, S. Dewatering of activated sludge by forward osmosis (FO) with ultrasound for fouling control. Desalination 2017, 421, 79–88. [Google Scholar] [CrossRef]
- Nguyen, N.C.; Nguyen, H.T.; Chen, S.-S.; Nguyen, N.T.; Li, C.-W. Application of forward osmosis (FO) under ultrasonication on sludge thickening of waste activated sludge. Water Sci. Technol. 2015, 72, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Larsen*, T.A.; Alder, A.C.; Eggen, R.I.L.; Maurer, M.; Lienert, J. Source Separation: Will We See a Paradigm Shift in Wastewater Handling? Environ. Sci. Technol. 2009, 43, 6121–6125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; She, Q.; Chang, V.W.C.; Tang, C.Y.; Webster, R.D. Mining Nutrients (N, K, P) from Urban Source-Separated Urine by Forward Osmosis Dewatering. Environ. Sci. Technol. 2014, 48, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Volpin, F.; Chekli, L.; Phuntsho, S.; Cho, J.; Ghaffour, N.; Vrouwenvelder, J.S.; Kyong Shon, H. Simultaneous phosphorous and nitrogen recovery from source-separated urine: A novel application for fertiliser drawn forward osmosis. Chemosphere 2018, 203, 482–489. [Google Scholar] [CrossRef]
- Maurer, M.; Pronk, W.; Larsen, T.A. Treatment processes for source-separated urine. Water Res. 2006, 40, 3151–3166. [Google Scholar] [CrossRef]
- Nikiema, B.C.W.-Y.; Ito, R.; Guizani, M.; Funamizu, N. Estimation of Water Flux and Solute Movement during the Concentration Process of Hydrolysed Urine by Forward Osmosis. J. Water Environ. Technol. 2017, 15, 163–173. [Google Scholar] [CrossRef][Green Version]
- Xu, Y.; Zhou, L.; Jia, Q. Nutrients recovery of source-separated urine by forward osmosis and a pilot-scale resources-oriented sanitation system. Desalin. Water Treat. 2017, 91, 252–259. [Google Scholar] [CrossRef]
- Volpin, F.; Chekli, L.; Phuntsho, S.; Ghaffour, N.; Vrouwenvelder, J.S.; Shon, H.K. Optimisation of a forward osmosis and membrane distillation hybrid system for the treatment of source-separated urine. Sep. Purif. Technol. 2019, 212, 368–375. [Google Scholar] [CrossRef]
- Ray, H.; Perreault, F.; Boyer, T.H. Urea recovery from fresh human urine by forward osmosis and membrane distillation (FO–MD). Environ. Sci. Water Res. Technol. 2019, 5, 1993–2003. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, C.; Zhao, L.; Ma, W.; Liu, H.; Ma, J. Integrated forward osmosis-membrane distillation process for human urine treatment. Water Res. 2016, 91, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Volpin, F.; Heo, H.; Hasan Johir, M.A.; Cho, J.; Phuntsho, S.; Shon, H.K. Techno-economic feasibility of recovering phosphorus, nitrogen and water from dilute human urine via forward osmosis. Water Res. 2019, 150, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Renou, S.; Givaudan, J.G.; Poulain, S.; Dirassouyan, F.; Moulin, P. Landfill leachate treatment: Review and opportunity. J. Hazard. Mater. 2008, 150, 468–493. [Google Scholar] [CrossRef] [PubMed]
- Life Leachless. Available online: https://lifeleachless.eu/en/ (accessed on 3 June 2020).
- Lampi, K.; Shethji, J. Forward osmosis industial wastewater treatment: Landfill leachate and oil and gas porduced waters. Int. Forw. Osmosis Assoc. World Summit 2014. [CrossRef]
- Iskander, S.M.; Novak, J.T.; He, Z. Reduction of reagent requirements and sludge generation in Fenton’s oxidation of landfill leachate by synergistically incorporating forward osmosis and humic acid recovery. Water Res. 2019, 151, 310–317. [Google Scholar] [CrossRef]
- Wu, S.; Zou, S.; Liang, G.; Qian, G.; He, Z. Enhancing recovery of magnesium as struvite from landfill leachate by pretreatment of calcium with simultaneous reduction of liquid volume via forward osmosis. Sci. Total Environ. 2018, 610–611, 137–146. [Google Scholar] [CrossRef]
- Iskander, S.M.; Novak, J.T.; He, Z. Enhancing forward osmosis water recovery from landfill leachate by desalinating brine and recovering ammonia in a microbial desalination cell. Bioresour. Technol. 2018, 255, 76–82. [Google Scholar] [CrossRef]
- Qin, M.; Molitor, H.; Brazil, B.; Novak, J.T.; He, Z. Recovery of nitrogen and water from landfill leachate by a microbial electrolysis cell–forward osmosis system. Bioresour. Technol. 2016, 200, 485–492. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z.; Zhu, C.; Wang, Q.; Tang, J.; Wu, Z. A forward osmosis membrane system for the post-treatment of MBR-treated landfill leachate. J. Membr. Sci. 2014, 471, 192–200. [Google Scholar] [CrossRef]
- Li, J.; Niu, A.; Lu, C.-J.; Zhang, J.-H.; Junaid, M.; Strauss, P.R.; Xiao, P.; Wang, X.; Ren, Y.-W.; Pei, D.-S. A novel forward osmosis system in landfill leachate treatment for removing polycyclic aromatic hydrocarbons and for direct fertigation. Chemosphere 2017, 168, 112–121. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, M.; Deng, Q.; Cai, T. Combination and performance of forward osmosis and membrane distillation (FO-MD) for treatment of high salinity landfill leachate. Desalination 2017, 420, 99–105. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Logan, M.; Visvanathan, C. Management strategies for anaerobic digestate of organic fraction of municipal solid waste: Current status and future prospects. Waste Manag. Res. 2019, 37, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Rajmohan, R.S.; Zarebska, A.; Tsapekos, P.; Hélix-Nielsen, C. Treating anaerobic effluents using forward osmosis for combined water purification and biogas production. Sci. Total Environ. 2019, 647, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Camilleri-Rumbau, M.S.; Soler-Cabezas, J.L.; Christensen, K.V.; Norddahl, B.; Mendoza-Roca, J.A.; Vincent-Vela, M.C. Application of aquaporin-based forward osmosis membranes for processing of digestate liquid fractions. Chem. Eng. J. 2019, 371, 583–592. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Xie, M.; Zhang, B.; Li, G.; Luo, W. Resource recovery from digested manure centrate: Comparison between conventional and aquaporin thin-film composite forward osmosis membranes. J. Membr. Sci. 2020, 593, 117436. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Hai, F.I.; Ansari, A.J.; Roddick, F.A. Mining phosphorus from anaerobically treated dairy manure by forward osmosis membrane. J. Ind. Eng. Chem. 2019, 78, 425–432. [Google Scholar] [CrossRef]
- Wu, Z.; Zou, S.; Zhang, B.; Wang, L.; He, Z. Forward osmosis promoted in-situ formation of struvite with simultaneous water recovery from digested swine wastewater. Chem. Eng. J. 2018, 342, 274–280. [Google Scholar] [CrossRef]
- Soler-Cabezas, J.L.; Mendoza-Roca, J.A.; Vincent-Vela, M.C.; Luján-Facundo, M.J.; Pastor-Alcañiz, L. Simultaneous concentration of nutrients from anaerobically digested sludge centrate and pre-treatment of industrial effluents by forward osmosis. Sep. Purif. Technol. 2018, 193, 289–296. [Google Scholar] [CrossRef]
- Ansari, A.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. Phosphorus recovery from digested sludge centrate using seawater-driven forward osmosis. Sep. Purif. Technol. 2016, 163, 1–7. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Shu, L.; Jegatheesan, V. A review of the management and treatment of brine solutions. Environ. Sci. Water Res. Technol. 2017, 3, 625–658. [Google Scholar] [CrossRef]
- Morillo, J.; Usero, J.; Rosado, D.; El Bakouri, H.; Riaza, A.; Bernaola, F.-J. Comparative study of brine management technologies for desalination plants. Desalination 2014, 336, 32–49. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Haralambous, K.-J.; Loizidou, M. Desalination brine disposal methods and treatment technologies—A review. Sci. Total Environ. 2019, 693, 133545. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.; Dufour, V.; Al Marzooqi, F.; Al Kaabi, M.; Hasan, S.W. Brine management methods: Recent innovations and current status. Desalination 2017, 407, 1–23. [Google Scholar] [CrossRef]
- Tang, W.; Ng, H.Y. Concentration of brine by forward osmosis: Performance and influence of membrane structure. Desalination 2008, 224, 143–153. [Google Scholar] [CrossRef]
- Martinetti, C.R.; Childress, A.E.; Cath, T.Y. High recovery of concentrated RO brines using forward osmosis and membrane distillation. J. Membr. Sci. 2009, 331, 31–39. [Google Scholar] [CrossRef]
- McGinnis, R.L.; Hancock, N.T.; Nowosielski-Slepowron, M.S.; McGurgan, G.D. Pilot demonstration of the NH3/CO2 forward osmosis desalination process on high salinity brines. Desalination 2013, 312, 67–74. [Google Scholar] [CrossRef]
- Tow, E.W.; McGovern, R.K.; Lienhard, J.H.V. Raising forward osmosis brine concentration efficiency through flow rate optimization. Desalination 2015, 366, 71–79. [Google Scholar] [CrossRef]
- Desalination|Trevi Systems Inc.|United States. Available online: https://www.trevisystems.com (accessed on 26 March 2020).
- Industrial Wastewater. Available online: https://www.blue-tec.nl/industrial-wastewater (accessed on 26 March 2020).
- Forward Water proves economic viability of forward osmosis Zero Liquid Discharge. Aquaporin. 2020. Available online: https://aquaporin.com/forward-osmosis-proves-economic-viability/ (accessed on 26 March 2020).
- Loganathan, P.; Naidu, G.; Vigneswaran, S. Mining valuable minerals from seawater: A critical review. Environ. Sci. Water Res. Technol. 2017, 3, 37–53. [Google Scholar] [CrossRef]
- Quist-Jensen, C.A.; Ali, A.; Drioli, E.; Macedonio, F. Perspectives on mining from sea and other alternative strategies for minerals and water recovery—The development of novel membrane operations. J. Taiwan Inst. Chem. Eng. 2019, 94, 129–134. [Google Scholar] [CrossRef]
- Diallo, M.S.; Kotte, M.R.; Cho, M. Mining Critical Metals and Elements from Seawater: Opportunities and Challenges. Environ. Sci. Technol. 2015, 49, 9390–9399. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, M.; Zhao, Y.; Yang, H.; Zhong, Y. Enrichment of lithium from salt lake brine by forward osmosis. R. Soc. Open Sci. 2018, 5, 180965. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H. Optimisation of crystallisation parameters for lithium carbonate microcrystals based on forward osmosis (FO) process. Mater. Res. Innov. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Van Wyk, L.A. Solute Transport in a Submerged Forward Osmosis Membrane System. 2019. Available online: https://scholar.sun.ac.za/bitstream/handle/10019.1/107235/vanwyk_solute_2019.pdf?sequence=1 (accessed on 18 December 2019).
- Farhat, A.; Ahmad, F.; Hilal, N.; Arafat, H.A. Boron removal in new generation reverse osmosis (RO) membranes using two-pass RO without pH adjustment. Desalination 2013, 310, 50–59. [Google Scholar] [CrossRef]
- Jin, X.; Tang, C.Y.; Gu, Y.; She, Q.; Qi, S. Boric Acid Permeation in Forward Osmosis Membrane Processes: Modeling, Experiments, and Implications. Environ. Sci. Technol. 2011, 45, 2323–2330. [Google Scholar] [CrossRef]
- Jin, X.; She, Q.; Ang, X.; Tang, C.Y. Removal of boron and arsenic by forward osmosis membrane: Influence of membrane orientation and organic fouling. J. Membr. Sci. 2012, 389, 182–187. [Google Scholar] [CrossRef]
- Luo, L.; Zhou, Z.; Chung, T.-S.; Weber, M.; Staudt, C.; Maletzko, C. Experiments and Modeling of Boric Acid Permeation through Double-Skinned Forward Osmosis Membranes. Environ. Sci. Technol. 2016, 50, 7696–7705. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Li, Z.Y.; Sarp, S.; Park, Y.G.; Amy, G.; Vrouwenvelder, J.S. Higher boron rejection with a new TFC forward osmosis membrane. Desalin. Water Treat. 2014, 55, 2734–2740. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Li, W.; Wang, R.; Tang, C.Y. Enhancing boron rejection in FO using alkaline draw solutions. Water Res. 2017, 118, 20–25. [Google Scholar] [CrossRef]
- Coday, B.D.; Heil, D.M.; Xu, P.; Cath, T.Y. Effects of Transmembrane Hydraulic Pressure on Performance of Forward Osmosis Membranes. Environ. Sci. Technol. 2013, 47, 2386–2393. [Google Scholar] [CrossRef]
- D’Haese, A.K.H.; De Leersnyder, I.; Vermeir, P.; Verliefde, A.R.D. On negative rejection of uncharged organic solutes in forward osmosis. J. Membr. Sci. 2018, 548, 22–31. [Google Scholar] [CrossRef]
- Qiu, G.; Wai Wong, G.K.; Ting, Y.-P. Electrostatic interaction governed solute transport in forward osmosis. Water Res. 2020, 115590. [Google Scholar] [CrossRef] [PubMed]
- Blandin, G.; Verliefde, A.R.D.; Le-Clech, P. Improving Trace Organic Contaminant Rejection Using Novel forward Osmosis Membranes; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- McCormick, P.; Pellegrino, J.; Mantovani, F.; Sarti, G. Water, salt, and ethanol diffusion through membranes for water recovery by forward (direct) osmosis processes. J. Membr. Sci. 2008, 325, 467–478. [Google Scholar] [CrossRef]
- Sanahuja-Embuena, V.; Khensir, G.; Yusuf, M.; Andersen, M.F.; Nguyen, X.T.; Trzaskus, K.; Pinelo, M.; Helix-Nielsen, C. Role of Operating Conditions in a Pilot Scale Investigation of Hollow Fiber Forward Osmosis Membrane Modules. Membranes 2019, 9, 66. [Google Scholar] [CrossRef]
- Irvine, G.J.; Rajesh, S.; Georgiadis, M.; Phillip, W.A. Ion Selective Permeation Through Cellulose Acetate Membranes in Forward Osmosis. Environ. Sci. Technol. 2013, 47, 13745–13753. [Google Scholar] [CrossRef]
- Lu, X.; Boo, C.; Ma, J.; Elimelech, M. Bidirectional Diffusion of Ammonium and Sodium Cations in Forward Osmosis: Role of Membrane Active Layer Surface Chemistry and Charge. Environ. Sci. Technol. 2014, 48, 14369–14376. [Google Scholar] [CrossRef]
- Sauchelli, M.; Pellegrino, G.; D’Haese, A.; Rodríguez-Roda, I.; Gernjak, W. Transport of trace organic compounds through novel forward osmosis membranes: Role of membrane properties and the draw solution. Water Res. 2018, 141, 65–73. [Google Scholar] [CrossRef]
- Hancock, N.T.; Phillip, W.A.; Elimelech, M.; Cath, T.Y. Bidirectional Permeation of Electrolytes in Osmotically Driven Membrane Processes. Environ. Sci. Technol. 2011, 45, 10642–10651. [Google Scholar] [CrossRef]
- Hancock, N.T.; Cath, T.Y. Solute coupled diffusion in osmotically driven membrane processes. Environ. Sci. Technol. 2009, 43, 6769–6775. [Google Scholar] [CrossRef]
- Yaroshchuk, A.; Bruening, M.L.; Licón Bernal, E.E. Solution-Diffusion–Electro-Migration model and its uses for analysis of nanofiltration, pressure-retarded osmosis and forward osmosis in multi-ionic solutions. J. Membr. Sci. 2013, 447, 463–476. [Google Scholar] [CrossRef]
- D’Haese, A.K.H. Interactions between feed solutes and inorganic electrolytic draw solutes in forward osmosis. J. Membr. Sci. 2020, 597, 117636. [Google Scholar] [CrossRef]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. Comparison of the removal of hydrophobic trace organic contaminants by forward osmosis and reverse osmosis. Water Res. 2012, 46, 2683–2692. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Shan, J.; Wang, C.; Wei, J.; Tang, C.Y. Rejection of pharmaceuticals by forward osmosis membranes. J. Hazard. Mater. 2012, 227–228, 55–61. [Google Scholar] [CrossRef]
- Verliefde, A.R.D.; Van der Meeren, P.; Van der Bruggen, B.; Hoek, E.M.V.; Tarabara, V.V. Solution-Diffusion Processes. In Encyclopedia of Membrane Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 978-1-118-52231-8. [Google Scholar]
- Jafarinejad, S.; Park, H.; Mayton, H.; Walker, S.L.; Jiang, S.C. Concentrating ammonium in wastewater by forward osmosis using a surface modified nanofiltration membrane. Environ. Sci. Water Res. Technol. 2019, 5, 246–255. [Google Scholar] [CrossRef]
- Fluid Technology Solutions, Inc.—Products. Available online: http://ftsh2o.com/products/ (accessed on 8 April 2020).
- Kim, J.; Blandin, G.; Phuntsho, S.; Verliefde, A.; Le-Clech, P.; Shon, H. Practical considerations for operability of an 8″ spiral wound forward osmosis module: Hydrodynamics, fouling behaviour and cleaning strategy. Desalination 2017, 404, 249–258. [Google Scholar] [CrossRef]
- Poriferanano Element Packing Density Comparison. Available online: http://www.porifera.com/pfo-advanteges/ (accessed on 2 August 2019).
- Saito, K.; Irie, M.; Zaitsu, S.; Sakai, H.; Hayashi, H.; Tanioka, A. Power generation with salinity gradient by pressure retarded osmosis using concentrated brine from SWRO system and treated sewage as pure water. Desalin. Water Treat. 2012, 41, 114–121. [Google Scholar] [CrossRef]
- Aquaporin Aquaporin InsideTM Forward Osmosis Membrane Products. Available online: https://aquaporin.com/fo (accessed on 2 August 2019).
- Berghof Membranes and Aquaporin Take Next Step in Tubular FO. berghofmembranes.com. 2018. Available online: https://www.berghofmembranes.com/berghof-membranes-and-aquaporin-announce-joint-development-agreement-2/ (accessed on 8 April 2020).
- Blandin, G.; Rodriguez-Roda, I.; Comas, J. Submerged Osmotic Processes: Design and Operation to Mitigate Mass Transfer Limitations. Membranes 2018, 8, 72. [Google Scholar] [CrossRef]
- Spier, M.R.; Vandenberghe, L.P.D.S.; Medeiros, A.B.P.; Soccol, C.R. Application of different types of bioreactors in bioprocesses. In Bioreactors: Design, Properties and Applications; Nova Science Publishers Inc.: New York, NY, USA, 2011; pp. 53–87. [Google Scholar]
- Lian, B.; Blandin, G.; Leslie, G.; Le-Clech, P. Impact of module design in forward osmosis and pressure assisted osmosis: An experimental and numerical study. Desalination 2018, 426, 108–117. [Google Scholar] [CrossRef]
- Gruber, M.F.; Aslak, U.; Hélix-Nielsen, C. Open-source CFD model for optimization of forward osmosis and reverse osmosis membrane modules. Sep. Purif. Technol. 2016, 158, 183–192. [Google Scholar] [CrossRef]
- Xiao, D.; Li, W.; Chou, S.; Wang, R.; Tang, C.Y. A modeling investigation on optimizing the design of forward osmosis hollow fiber modules. J. Membr. Sci. 2012, 392–393, 76–87. [Google Scholar] [CrossRef]
- Kim, Y.C.; Park, S.-J. Experimental Study of a 4040 Spiral-Wound Forward-Osmosis Membrane Module. Environ. Sci. Technol. 2011, 45, 7737–7745. [Google Scholar] [CrossRef] [PubMed]
- Bilad, M.R. Module-Scale Simulation of Forward Osmosis Module-Part A: Plate-and-Frame. IJOST 2016, 1, 249. [Google Scholar] [CrossRef]
- Bilad, M.R. Module-scale simulation of forward osmosis module-part B: Modified Spiral-Wound. IJOST 2017, 2, 211. [Google Scholar] [CrossRef][Green Version]
- Deshmukh, A.; Yip, N.Y.; Lin, S.; Elimelech, M. Desalination by forward osmosis: Identifying performance limiting parameters through module-scale modeling. J. Membr. Sci. 2015, 491, 159–167. [Google Scholar] [CrossRef]
- Gu, B.; Kim, D.Y.; Kim, J.H.; Yang, D.R. Mathematical model of flat sheet membrane modules for FO process: Plate-and-frame module and spiral-wound module. J. Membr. Sci. 2011, 379, 403–415. [Google Scholar] [CrossRef]
- Qing, L.; Bilad, M.R.; Sun, G.; Jaafar, J.; Fane, A.G. Flow uneven-distribution and its impact on performances of forward osmosis module. J. Water Process. Eng. 2020, 33, 101014. [Google Scholar] [CrossRef]
- Membrane Modules. Available online: http://www.porifera.com/modules (accessed on 20 April 2020).
- Phuntsho, S.; Hong, S.; Elimelech, M.; Shon, H.K. Osmotic equilibrium in the forward osmosis process: Modelling, experiments and implications for process performance. J. Membr. Sci. 2014, 453, 240–252. [Google Scholar] [CrossRef]
- Sahebi, S.; Phuntsho, S.; Eun Kim, J.; Hong, S.; Kyong Shon, H. Pressure assisted fertiliser drawn osmosis process to enhance final dilution of the fertiliser draw solution beyond osmotic equilibrium. J. Membr. Sci. 2015, 481, 63–72. [Google Scholar] [CrossRef]
- Ge, Q.; Ling, M.; Chung, T.-S. Draw solutions for forward osmosis processes: Developments, challenges, and prospects for the future. J. Membr. Sci. 2013, 442, 225–237. [Google Scholar] [CrossRef]
- Long, Q.; Jia, Y.; Li, J.; Yang, J.; Liu, F.; Zheng, J.; Yu, B. Recent Advance on Draw Solutes Development in Forward Osmosis. Processes 2018, 6, 165. [Google Scholar] [CrossRef]
- Guide to Commercial Draw Solutions|ForwardOsmosisTech. Available online: https://www.forwardosmosistech.com/guide-to-forward-osmosis-draw-solutions-and-recovery-methods/ (accessed on 8 April 2020).
- Corzo, B.; de la Torre, T.; Sans, C.; Ferrero, E.; Malfeito, J.J. Evaluation of draw solutions and commercially available forward osmosis membrane modules for wastewater reclamation at pilot scale. Chem. Eng. J. 2017, 326, 1–8. [Google Scholar] [CrossRef]
- De la torre, T.; Arnaldos, M.; Corzo, B.; Navea, S.; Ferrero, E.; Bacarit, J.; Malfeito, J. Evaluation of FO for Wastewater Reclamation with a Focus on the Selection of Optimal Draw Solutions; IFOA: Lisbon, Portugal, 2014. [Google Scholar]
- Siddiqui, F.A.; She, Q.; Fane, A.G.; Field, R.W. Exploring the differences between forward osmosis and reverse osmosis fouling. J. Membr. Sci. 2018, 565, 241–253. [Google Scholar] [CrossRef]
- Xie, M.; Lee, J.; Nghiem, L.D.; Elimelech, M. Role of pressure in organic fouling in forward osmosis and reverse osmosis. J. Membr. Sci. 2015, 493, 748–754. [Google Scholar] [CrossRef]
- Blandin, G.; Verliefde, A.R.D.; Le-Clech, P. Pressure enhanced fouling and adapted anti-fouling strategy in pressure assisted osmosis (PAO). J. Membr. Sci. 2015, 493, 557–567. [Google Scholar] [CrossRef]
- Sauchelli Toran, M.; D’Haese, A.; Rodríguez-Roda, I.; Gernjak, W. Fouling propensity of novel TFC membranes with different osmotic and hydraulic pressure driving forces. Water Res. 2020, 175, 115657. [Google Scholar] [CrossRef]
- Blandin, G.; Vervoort, H.; Le-Clech, P.; Verliefde, A.R.D. Fouling and cleaning of high permeability forward osmosis membranes. J. Water Process. Eng. 2016, 9, 161–169. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Kook, S.; Lee, C.; Field, R.W.; Kim, I.S. Critical flux-based membrane fouling control of forward osmosis: Behavior, sustainability, and reversibility. J. Membr. Sci. 2019, 570–571, 380–393. [Google Scholar] [CrossRef]
- Mi, B.; Elimelech, M. Organic fouling of forward osmosis membranes: Fouling reversibility and cleaning without chemical reagents. J. Membr. Sci. 2010, 348, 337–345. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, J.; Zhu, C.; Dong, Y.; Wang, Q.; Wu, Z. Chemical cleaning protocols for thin film composite (TFC) polyamide forward osmosis membranes used for municipal wastewater treatment. J. Membr. Sci. 2015, 475, 184–192. [Google Scholar] [CrossRef]
- Linares, R.V.; Li, Z.; Yangali-Quintanilla, V.; Li, Q.; Amy, G. Cleaning protocol for a FO membrane fouled in wastewater reuse. Desalin. Water Treat. 2013, 51, 4821–4824. [Google Scholar] [CrossRef]
- Low, K.S. Vibration-Assisted Forward Osmosis Process for Mitigation of Concentration Polarization in Submerged Hollow Fiber Membrane System. Master’s Thesis, Nanyang Technological University, Singapore, 2019. [Google Scholar]
- Aslan, M.; Saatçi, Y.; Hanay, Ö.; Hasar, H. Effect of biogas sparging with different membrane modules on membrane fouling in anaerobic submerged membrane bioreactor (AnSMBR). Environ. Sci. Pollut. Res. 2014, 21, 3285–3293. [Google Scholar] [CrossRef] [PubMed]
- Motsa, M.M.; Mamba, B.B.; Thwala, J.M.; Verliefde, A.R.D. Osmotic backwash of fouled FO membranes: Cleaning mechanisms and membrane surface properties after cleaning. Desalination 2017, 402, 62–71. [Google Scholar] [CrossRef]
- Antony, A.; Low, J.H.; Gray, S.; Childress, A.E.; Le-Clech, P.; Leslie, G. Scale formation and control in high pressure membrane water treatment systems: A review. J. Membr. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Phuntsho, S.; Lotfi, F.; Hong, S.; Shaffer, D.L.; Elimelech, M.; Shon, H.K. Membrane scaling and flux decline during fertiliser-drawn forward osmosis desalination of brackish groundwater. Water Res. 2014, 57, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Mi, B.; Elimelech, M. Silica scaling and scaling reversibility in forward osmosis. Desalination 2013, 312, 75–81. [Google Scholar] [CrossRef]
- Zhang, M.; Hou, D.; She, Q.; Tang, C.Y. Gypsum scaling in pressure retarded osmosis: Experiments, mechanisms and implications. Water Res. 2014, 48, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M. Influence of Feed and Draw Solutions Chemistry on Scaling in Osmotically Driven Membrane Process; Nanyang Technological University: Singapore, 2015. [Google Scholar]
- Oren, S.; Birnhack, L.; Lehmann, O.; Lahav, O. A different approach for brackish-water desalination, comprising acidification of the feed-water and CO2(aq) reuse for alkalinity, Ca2+ and Mg2+ supply in the post treatment stage. Sep. Purif. Technol. 2012, 89, 252–260. [Google Scholar] [CrossRef]
- Liu, Y.; Mi, B. Combined fouling of forward osmosis membranes: Synergistic foulant interaction and direct observation of fouling layer formation. J. Membr. Sci. 2012, 407–408, 136–144. [Google Scholar] [CrossRef]
- Liu, Y.; Mi, B. Effects of organic macromolecular conditioning on gypsum scaling of forward osmosis membranes. J. Membr. Sci. 2014, 450, 153–161. [Google Scholar] [CrossRef]
- Zhang, J.; Loong, W.L.C.; Chou, S.; Tang, C.; Wang, R.; Fane, A.G. Membrane biofouling and scaling in forward osmosis membrane bioreactor. J. Membr. Sci. 2012, 403–404, 8–14. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
| UF/MF | NF/RO | MD/OD | Evaporation | Cryo-Concentration | Forward Osmosis | |
|---|---|---|---|---|---|---|
| Temperature | Ambient | Ambient | Moderate | High | Low | Ambient |
| Pressure | Moderate | High | No | No | No | No |
| Concentration capability | Limited | High | High | Medium | High | |
| Organoleptic preservation | Good | Good | Good–moderate | Poor | Good | Good |
| Applications | WW, food, waste streams, desalination | WW, food, waste streams. desalination | Desalination (MD)/food (OD) | Food, Desalination | Food | WW, food, waste streams, desalination |
| Energy | Moderate | High | Low–high | Very high | High | Low–high |
| Technology readiness level | Established technology | Established technology | Pilot scale | Established technology | Established technology | Reaching market |
| Application | Membrane Type | Draw Solution | Initial Jw (L·m−2·h−1) | Initial Conc. | CF | Final Conc. | Ref. |
|---|---|---|---|---|---|---|---|
| Pineapple | CTA-FO | Sucrose/NaCl | <1 | 12° | 5 | 60° | [44] |
| Kokum | CTA-FO | 6 M NaCl | 12.3 | 2° | 26 | 52° | [45] |
| Tomato | TFC-RO | 4 M NaCl | 6 | 5.5° | 2.9 | 16° | [53] |
| Grapefruit | TFC-FO | 2 M NaCl | 13 | Pure juice | 4 | 22° | [39] |
| Grapefruit | TFC-FO | 2 M Glucose | 13 | Pure juice | 4 | 22° | [39] |
| Grape | CTA FO | 6 M NaCl | 8.5 | 4.4° | 12.3 | 54° | [46] |
| Beetroot | CTA FO | 6 M NaCl | 12.4 | 2.3° | 22.6 | 52° | [46] |
| Pineapple | CTA FO | 6 M NaCl | 10.5 | 8° | 6.8 | 54.6° | [46] |
| Raspberries | CTA FO | 10.5 | 10° | 4.5 | 45° | [41] | |
| Sweetlime | CTA FO | 6 M NaCl | 9 | 11° | 4.5 | 50° | [52] |
| Sucrose | CTA | 4 M NaCl | 5.8 | 10° | 5.6 | 56.8° | [54] |
| Orange press liquor | CTA | 4 M NaCl | 16.8 | 8° | 1.3 | 10.5° | [55] |
| Rose extract anthocyanin | CTA FO | 6 M NaCl | 11 | 4° | 12.5 | 50° | [52] |
| Draw Solute Type | Examples | Draw Recovery | Advantages | Limitations |
|---|---|---|---|---|
| Inorganic salts | NaCl (or seawater), MgCl2, Na2SO4, (NH4)2SO4, KNO3 | NF/RO, MD, etc. Direct use for fertilizers | High osmotic pressure Low cost (replenishment) | High RSD Divalent ions: scaling/fouling precursor NH4+, SO42−, salinity: Biological process inhibitor |
| Organic salts | Zwitterions (amino acids) Organic ionic salts Hydroacid complex | NF, MD | Low RSD Recovery through NF Biodegradation in feed (OMBR, AnOMBR) | High cost (replenishment) Toxicity (?) |
| Organic coumponds | Sucrose, fructose, etc. | NF/RO | Suitable for food and beverages applications Recovery through NF | Low osmotic pressure |
| Stimuli responsive solutes (temperature, electricity, CO2, magnetic) | NH4HCO3 | Distillation | High osmotic pressure | High RSD, Scaling precursor |
| Ionic liquid, glycol ether, nanoparticles, polymer, switchable hydrogels | MF, magnetic field, phase separation, etc., | Possibly lower energy for draw recovery | Cost, lab scale studies |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blandin, G.; Ferrari, F.; Lesage, G.; Le-Clech, P.; Héran, M.; Martinez-Lladó, X. Forward Osmosis as Concentration Process: Review of Opportunities and Challenges. Membranes 2020, 10, 284. https://doi.org/10.3390/membranes10100284
Blandin G, Ferrari F, Lesage G, Le-Clech P, Héran M, Martinez-Lladó X. Forward Osmosis as Concentration Process: Review of Opportunities and Challenges. Membranes. 2020; 10(10):284. https://doi.org/10.3390/membranes10100284
Chicago/Turabian StyleBlandin, Gaetan, Federico Ferrari, Geoffroy Lesage, Pierre Le-Clech, Marc Héran, and Xavier Martinez-Lladó. 2020. "Forward Osmosis as Concentration Process: Review of Opportunities and Challenges" Membranes 10, no. 10: 284. https://doi.org/10.3390/membranes10100284
APA StyleBlandin, G., Ferrari, F., Lesage, G., Le-Clech, P., Héran, M., & Martinez-Lladó, X. (2020). Forward Osmosis as Concentration Process: Review of Opportunities and Challenges. Membranes, 10(10), 284. https://doi.org/10.3390/membranes10100284








