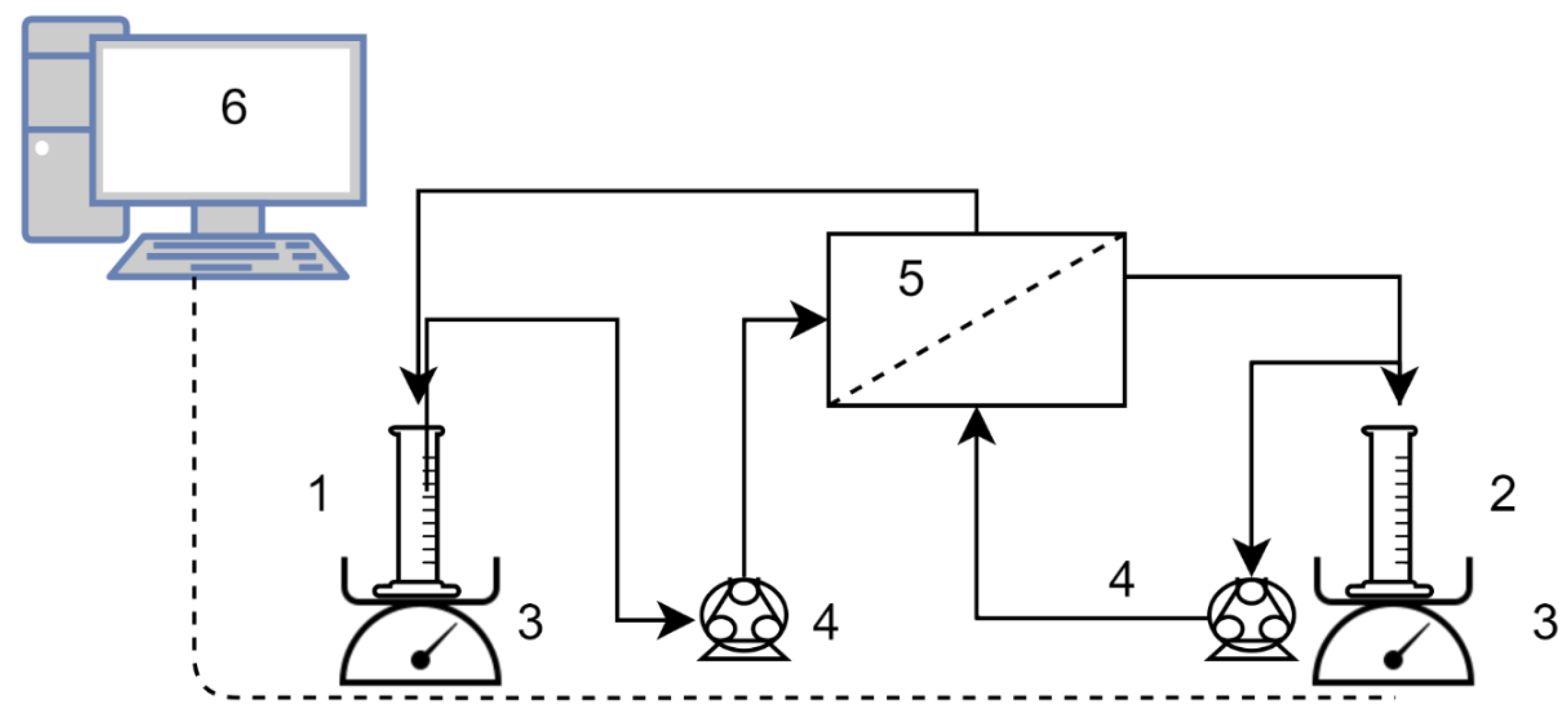
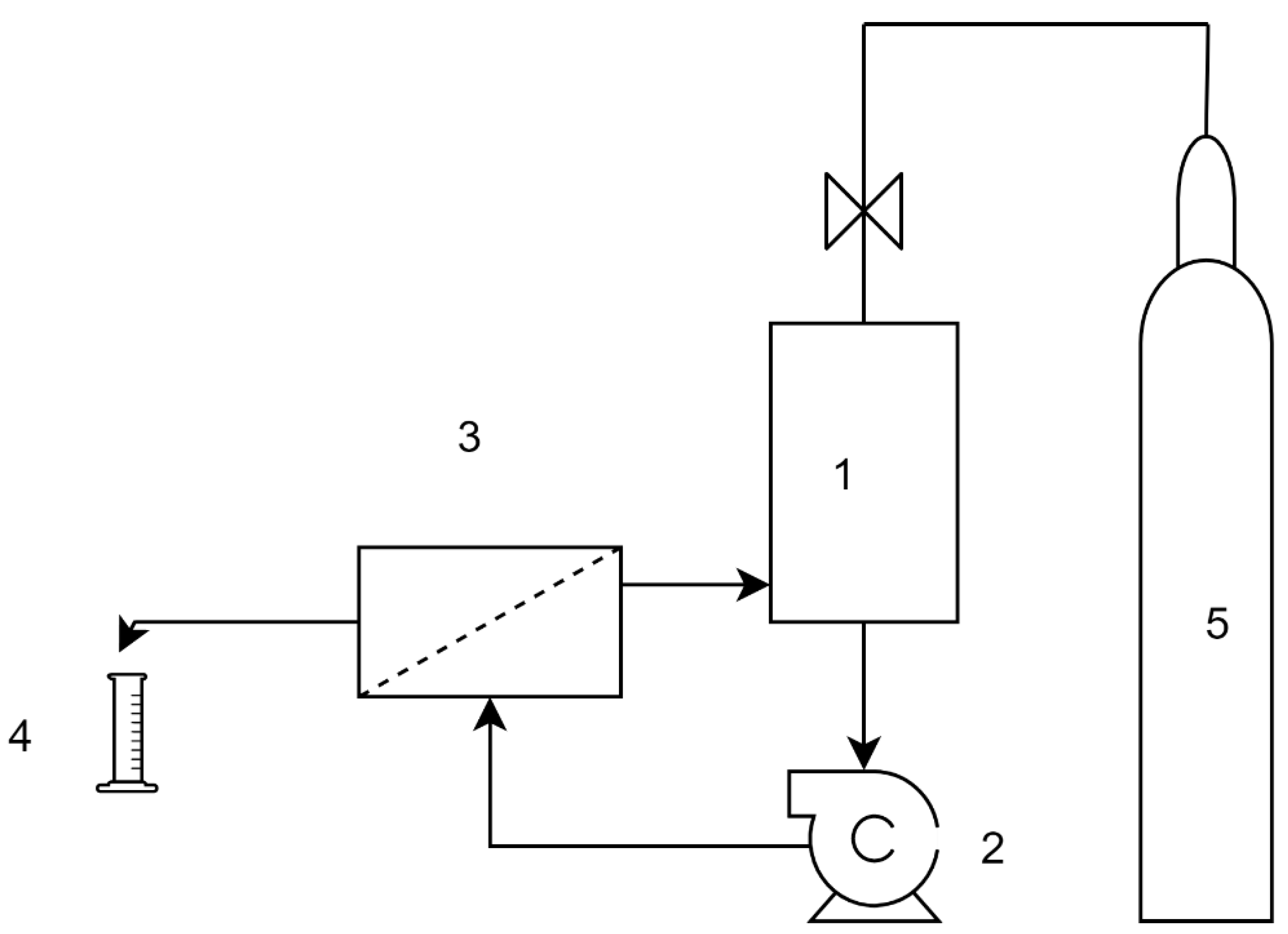
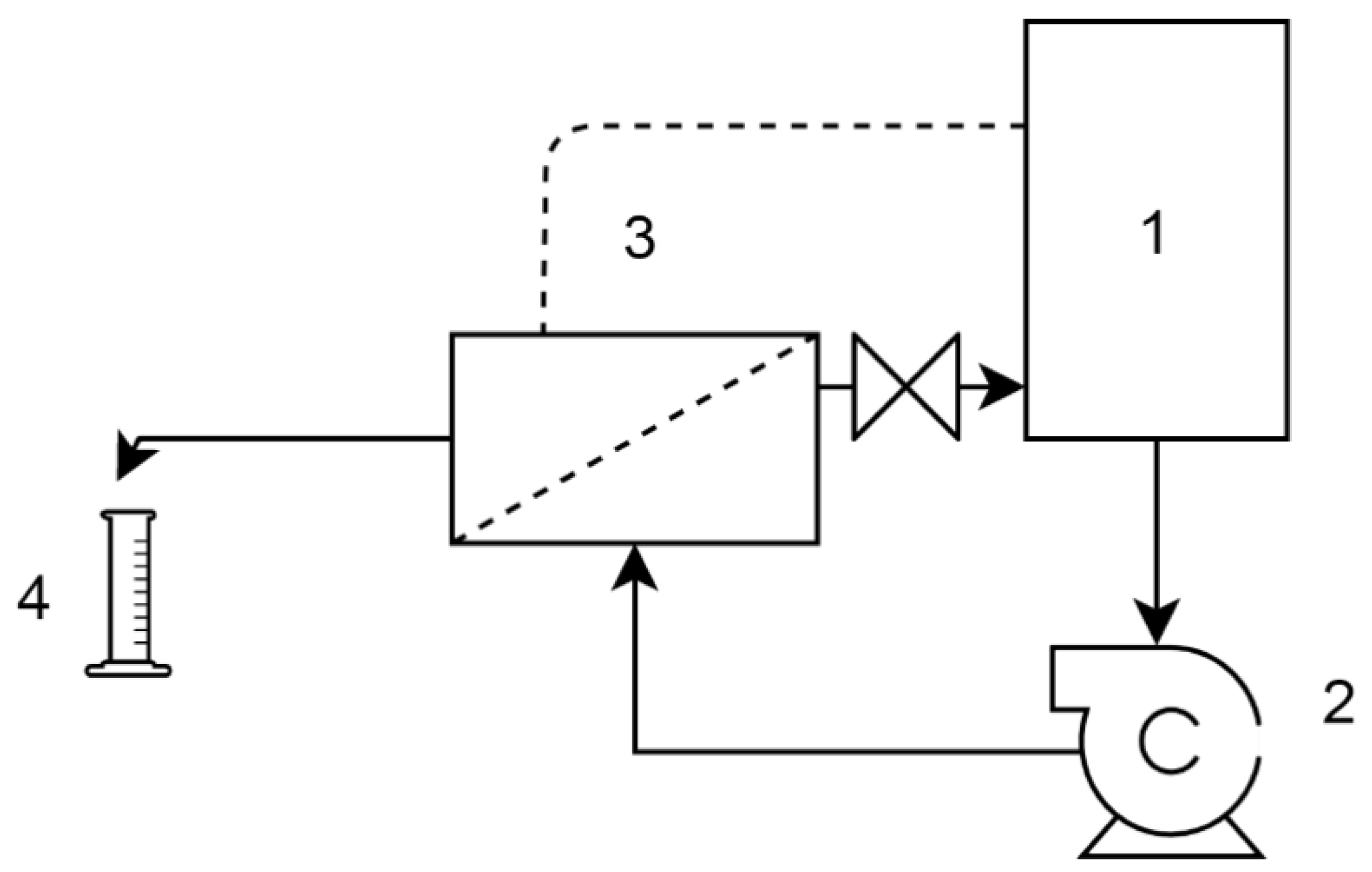
1. Introduction
Volatile fatty acids (VFAs) are carboxylic acids with six or fewer carbon atoms that can be saturated or unsaturated, the most common forms being acetic, formic, propionic and butyric acid [
1]. For industrial applications, VFAs are produced mostly via chemical routes [
2], nevertheless, microbial fermentations offer an alternative [
3]. VFAs are demanded by the chemical industry to synthetize alcohols, ketones, esters, olefins or aldehydes [
4]. Furthermore, VFAs can be used in textiles and cosmetics or in biopolymer plastic applications. They can also be seen as a feedstock in biofuel production or microbial electrochemical technologies [
5,
6]. VFA separation/rejection can be carried out by reverse osmosis (RO) and nanofiltration (NF) (as pressure-driven) membrane processes. Both RO and NF are well-established, not just for the chemical and water treatment industry, but also for biotechnological downstreaming [
7]. The low molecular mass and chemical properties of VFAs enable NF and RO to be plausible choices. RO membranes offer the tightest pores for pressure-driven processes and accordingly have the highest rejection for ionic species, along with a reasonable water flux and energy consumption thanks to the intensive R&D and optimization works of the past decades by the scientific and industrial community [
8,
9,
10]. In RO, solution pH levels where VFAs are mainly in ionized forms seem to increase the retention properties up to 99.7% [
11]. Still, Jänisch et al. found significant amounts of acetic acid in the permeate of various RO membranes fed by biogas plant hydrolysates [
12].
NF membranes demonstrate several advantages compared to RO: the larger pore size and the typically higher surface charge are expected to enhance the selectivity (e.g., separation of divalent and monovalent salts), productivity and decrease the energy investment in the process. However, a trade-off is represented by lower rejections for most compounds [
10,
13,
14]. Actually, Zacharov et al. measured the acetate and butyrate rejection of commercial NF membranes to be between 20–75%, which could be somewhat increased by adding salts to the system [
15]. In fact, some process designs intentionally use the low VFA rejection of NF membranes to separate them from other organics, which can be sent back to the bioreactor and VFAs are recovered in the permeate [
16,
17]. Interestingly, Xiong et al. reported a superior, 100% rejection of butyric acid with NF membranes [
18].
The Layer-by-Layer (LbL) method represents a unique NF membrane fabrication technique where polycations and polyanions are alternatingly layered on a charged substrate, typically an ultrafiltration membrane (UF) to build a dense amorphous polyelectrolyte multilayer (PEM), which acts as the active separation layer. The high charge density of the PEM leads to a high monovalent salt passage, a relatively greater rejection of organics and, if the membrane has a high surface charge, a high mono/divalent salt selectivity [
19,
20,
21,
22]. PEM membranes have further advantages compared to polyamide membranes, which originate from the different chemistry: a much higher resistance to oxidants, such as chlorine, a higher pH tolerance [
23], high self-healing ability [
24,
25,
26] and, if the substrate allows, solvent resistance [
27,
28]. The LbL method lends itself to produce NF membranes with a hollow fiber geometry: by using UF membranes with such geometry as substrates. Hollow fiber membranes have distinct advantages: a higher crossflow tolerance, no spacer fouling and the option of backwashing [
29,
30]. On the other hand, in the case of longer fibers, concentration polarization can cause significant issues [
31], certain feed conditions can destabilize the top layer (very high salinity, surfactants) and the maximum recommended transmembrane pressure is only 6 bars (in the case of the products available on the market at the date of publication).
Forward osmosis (FO) is an osmotically driven separation process where the higher osmotic potential of the draw solution governs the water through a tight (comparable to RO) semipermeable membrane. To achieve an effective separation, not only does the feed side have to have a thin active layer, but the whole membrane has to be thin to mitigate dilutive internal concentration polarization [
32,
33]. In spite of some intrinsic advantages, the practical implementation of FO has not caught up with the vast number of scientific publications to date [
34]. According to McGovern et al., it seems unlikely that FO will outperform RO for seawater desalination [
35], however it might find uses for special separation applications, e.g., in the food industry [
36,
37] where fouling can pose serious limitations for pressure-driven membrane processes. Finding an economical and scalable draw solution is still the most important challenge for FO [
38]. At the moment, RO is still the most commonly used method to recover the draw solution, which inherently leads to a somewhat more energetically demanding process than simple RO; nonetheless, severe fouling might justify this setup in some cases [
39], as it was demonstrated by the treatment of various wastewater feeds [
40]. For feed solutions having lower osmotic potential which need dewatering (e.g., juice concentration), seawater, especially concentrated seawater from desalination plants, provides an economic and environmentally friendly, low-carbon footprint solution with obvious geographical limitations. VFA solutions, including real wastewater, can be effectively dewatered by FO [
41,
42], thanks to the tight nature of the membranes.
Liquid–liquid extraction also represents a potentially efficient method for VFA separation; however, it requires the acidification of the feed, and furthermore, the regeneration of the extractant adds burden to the process. Additionally, the toxic effect on the fermentation culture is a possible issue in the case of many solvents [
43]. Compared to traditional solvents, phosphonium-based ionic liquid (IL) extraction achieved a higher maximum VFA loading, leading to a higher efficiency [
44,
45,
46,
47], but 1-hexyl-3-methylimidazolium bromide was also successful in recovering lactic acid from wine [
48].
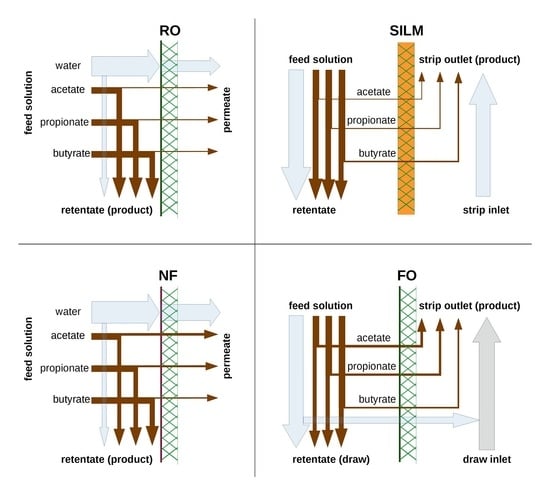
Supported ionic liquid membranes (SILMs) can be formed by immobilizing ionic liquids in porous matrices [
49,
50,
51,
52,
53]. Such membranes enable a continuous extraction and stripping process of VFAs via ionic liquids [
53,
54], which transport the extractants to the stripping solution on the other side of the membrane. The concentration-driven transport of the VFAs is facilitated through the ionic liquid membrane by circulating the feed on one side of the membrane, and a stripping solution (pure water in our case) on the other side. Contrary to the former three technologies above, where the VFAs are concentrated in the retentate, in SILM extraction the goal is to transport VFAs to the extractant side of the membrane from the feed side. The pure VFA solution obtained in this process can be concentrated by a second process step, e.g., by RO. Another major difference of SILMs—compared to pressure- and osmotically driven membranes—is that the passage on SILMs depends mainly on polarity (partition coefficients) and much less on molecular size [
55]. This difference in selectivity based on size vs. polarity is clearly shown in the work of Abejón et al., where the selectivity between monosaccharides and macromolecules (lignosulfonates, lignin) are compared for SILM vs. loose NF and tight UF, displaying a difference of almost two orders of magnitude [
56].
Membrane processes are able to recover VFAs by various methods [
57], but the original research studies compare only one or two methods in the same scope. In this study, four separation processes (RO, NF, FO, SILM) are investigated by a comparative methodology supported by statistical analysis according to the anaerobic effluents treating needs. Microbial processes can produce different VFA solutions, which can vary by composition, concentration and pH. To study the effect of the varying feed solution, we have set up an appropriate experimental design to investigate the efficiency of each separation process.
4. Discussion
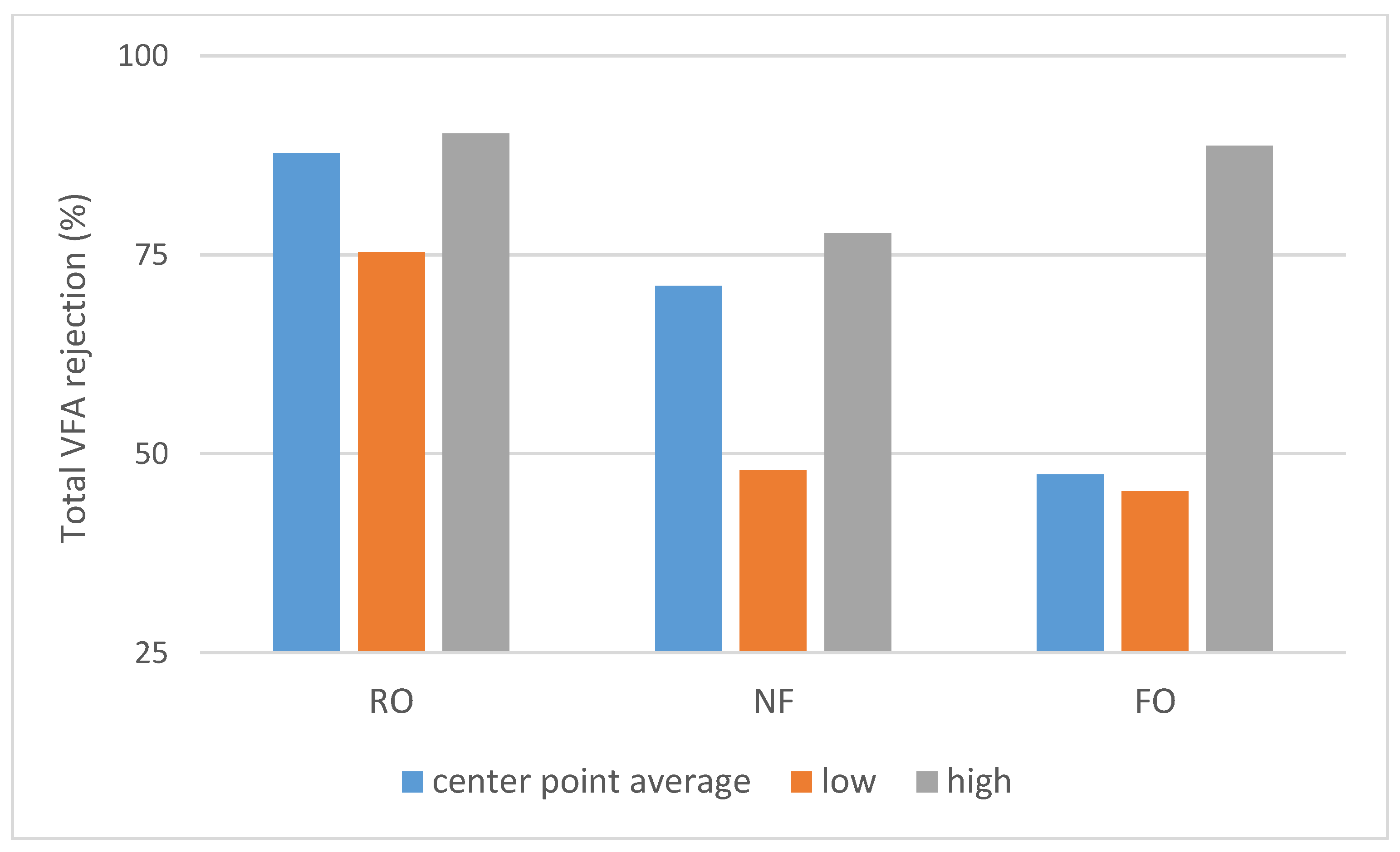
All selected separation methods are capable of purifying VFAs from the model solution. The highest VFA rejection can be achieved by RO filtration, but this method has the highest energetic demand and intensive pretreatment requirements.
The rejection of VFAs is on the lower side in the case of NF compared to RO, especially considering the unusually low pressure applied for RO (with higher pressure, higher rejection can be expected). If the goal is also NaCl (or other monovalent salt) passage besides VFA retention, then further consideration of such LbL NF membranes might be worthwhile, especially if tighter LbL membranes become available commercially, such as the asymmetric membrane developed by te Brinke et al. [
62].
The selectivities between the different VFA components of all four methods are compared by presenting the separation factors (α
ij = passage
i/passage
j) in
Table 11. This represents only an approximate comparison between the membranes since the selectivity is also dependent on the feed concentrations. Passage on SILMs depends less the molecular size, and more on the polarity, so unsurprisingly the transport of butyrate is the most favored, which is not only the largest, but the most hydrophobic VFA in our study. The other three membranes exhibit an opposite selectivity, which is most likely governed by size.
The selectivity between the three VFAs was markedly larger in the case of LbL NF compared to RO, or to the acetate/butyrate selectivity measured by Zacharov et al. on TFC NF membranes [
15]. This feature might be useful if the separation of the different acids is also a goal during the process.
Non pressure-driven systems, such as FO and SILM, are less prone to fouling and can treat direct wastewater streams as well. In this study, FO gives a lower selectivity than RO; however, the used membranes were same. Selectivity can be improved by providing appropriate flow conditions and choosing a membrane which exhibits lower internal concentration polarization. In the case of SILM, the goal is to maximize transport of VFAs to the extractant side from the feed side of the membrane, which is the opposite of the other three methods studied. It is also different regarding the absence of water flux. VFAs are purified but diluted which means another concentration step is needed. In our study, the typical VFA flux was around 1 g/m2h, which means the process speed is approximately two orders of magnitude slower compared to a concentration step with RO or NF, which means further optimization would be needed to make this process industrially relevant.
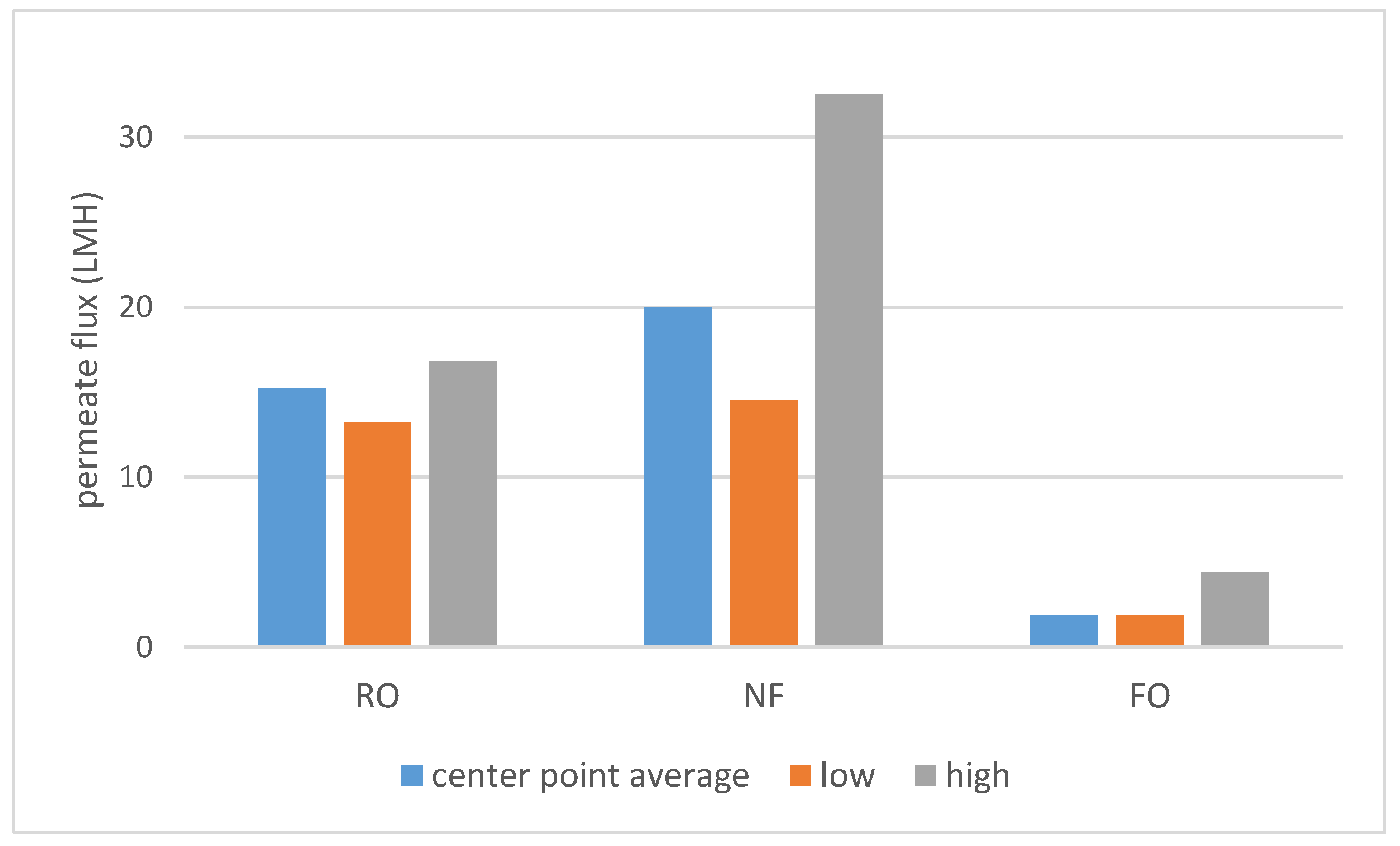
As shown on
Scheme 1, a large difference in permeate flux between FO and pressure-driven processes was observed. SILM was omitted from both
Scheme 1 and
Scheme 2 due to the different process properties. The dNF membrane exhibited a lower average rejection compared to RO (
Scheme 2), which can be expected due to the looser membrane structure. On the other hand, the nominally 400 Da MWCO dNF40 membrane provided VFA rejection values, which are on the higher side for NF, the typical being 20–75% [
15].
Summarizing the effect sizes of each system (see
Table 12), RO and NF were mostly affected by pH, while FO and SILM were most affected by acid concentrations. This behaviour provides directions for future aplications: RO consistenly provides good rejection, with a small dependencene on pH. The rejection properties of NF membranes are more sensitive to feed conditions, particularly to pH, and furthermore a larger difference can be seen between the retention of different VFAs compared to RO, which opens the possibility to separate them via membrane technology.
Switching the RO membrane to an FO mode provided no significant advantage over the pressure-driven mode; however, this might be different in the case of real anaerobic wastewater because of different fouling mechanisms. SILM might prove to be a feasible solution if the feed pH is acidic and severe fouling takes place, and if the extraction speed can be significantly improved in the future.
Our study demonstrates that the dependence of membrane transport properties from the feed conditions indicates that pilot testing is essential for appropriate process design.