Effect of High Ammonium Salt Concentration and Temperature on the Structure, Morphology, and Ionic Conductivity of Proton-Conductor Solid Polymer Electrolytes Based PVA
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
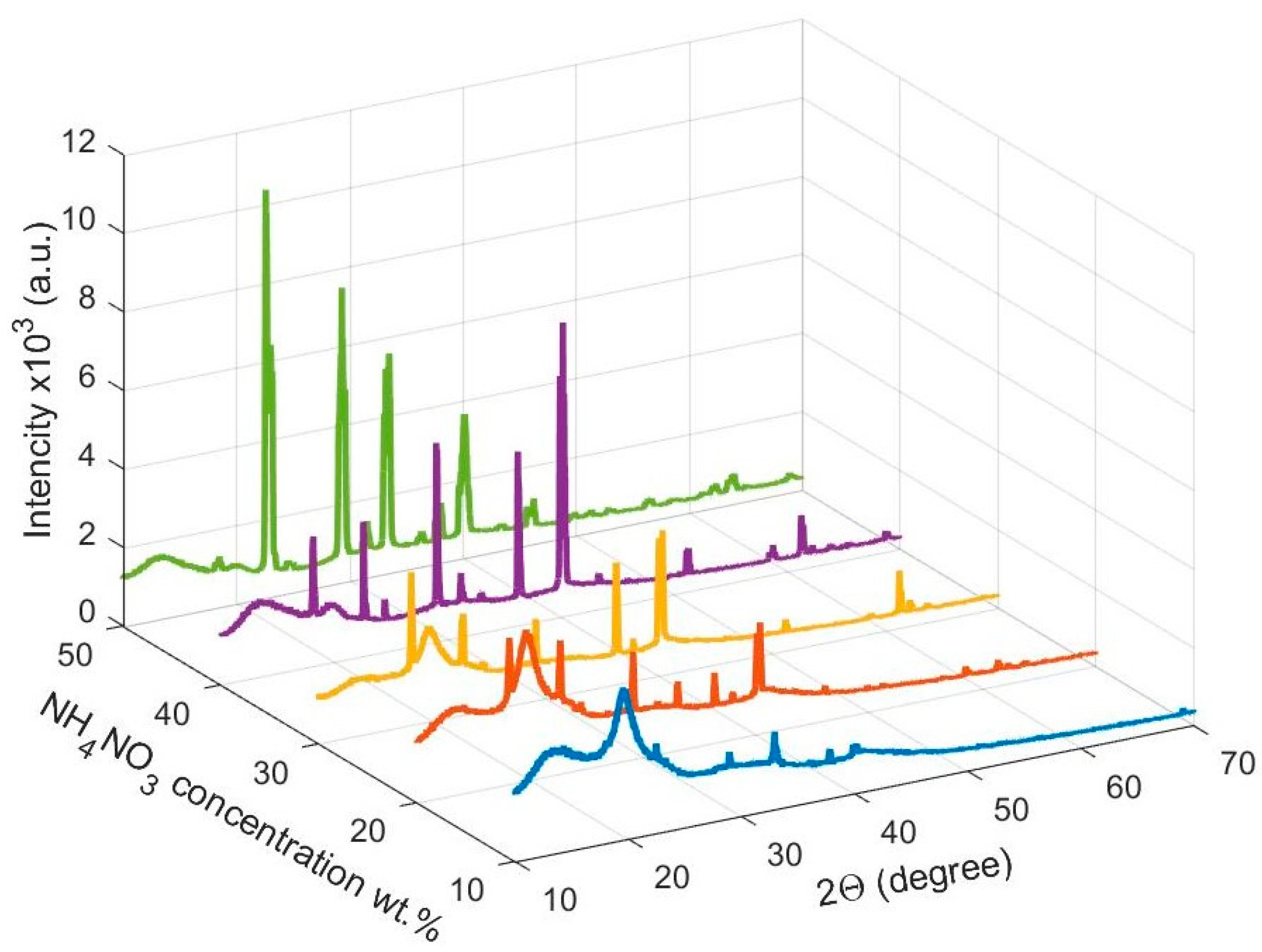
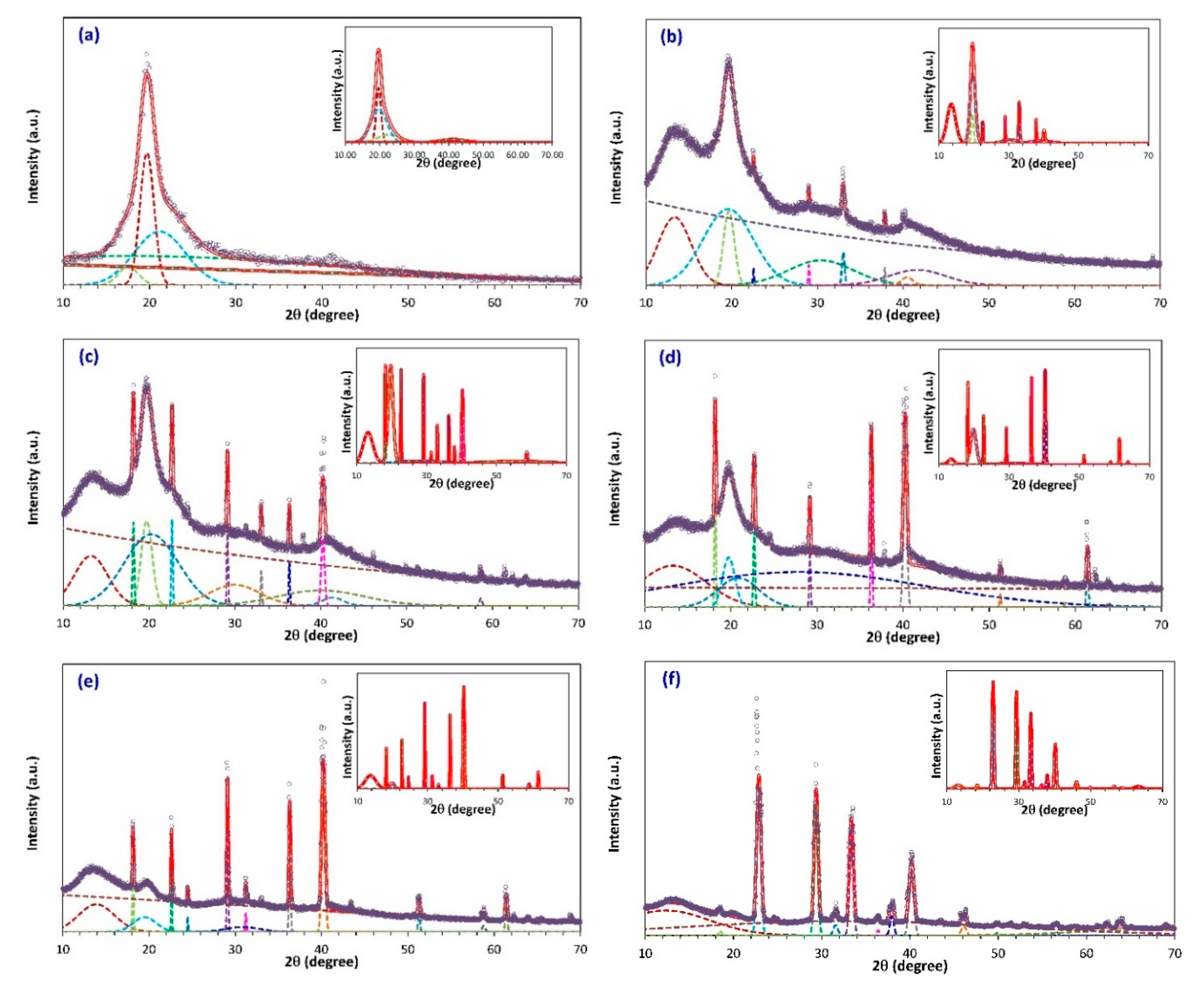
3.1. X-ray Diffraction Studies
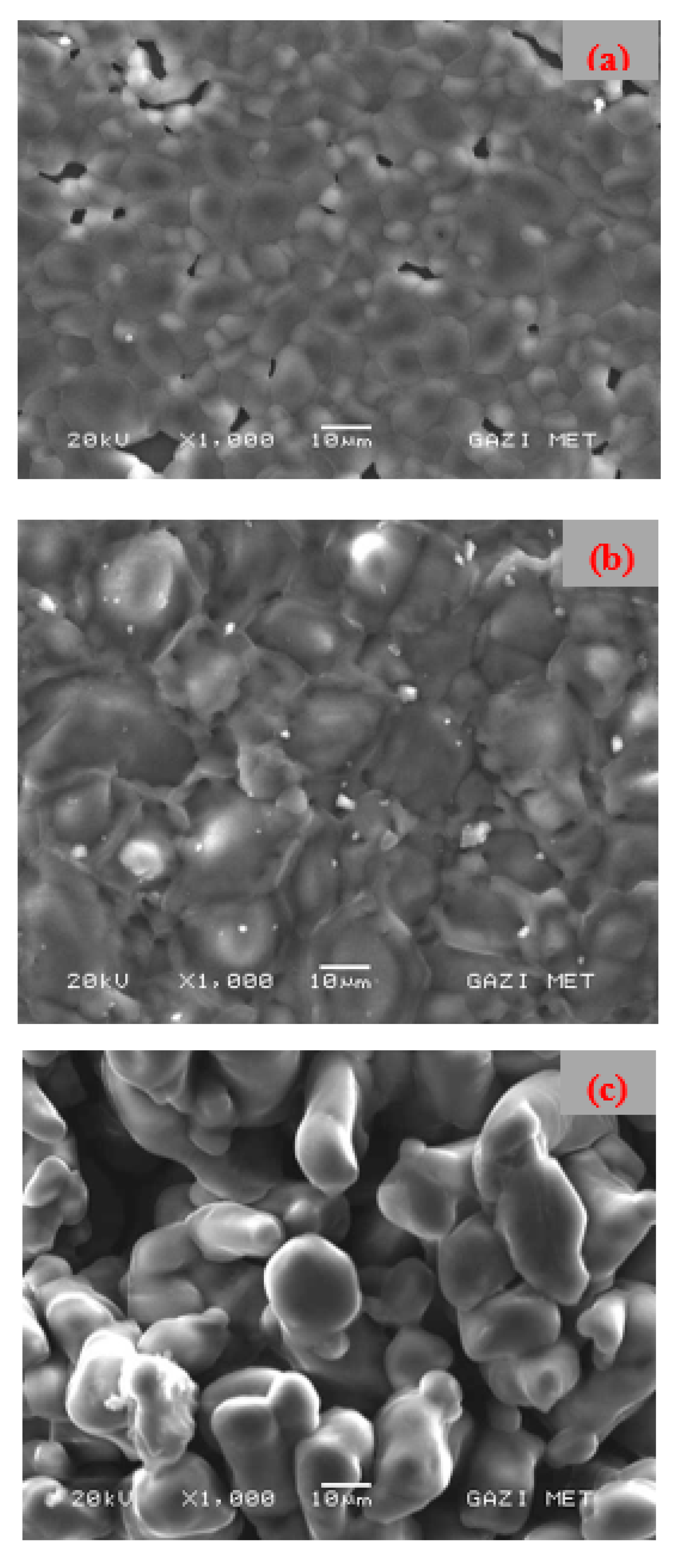
3.2. Morphological Study
3.3. Electrical Conductivity Studies
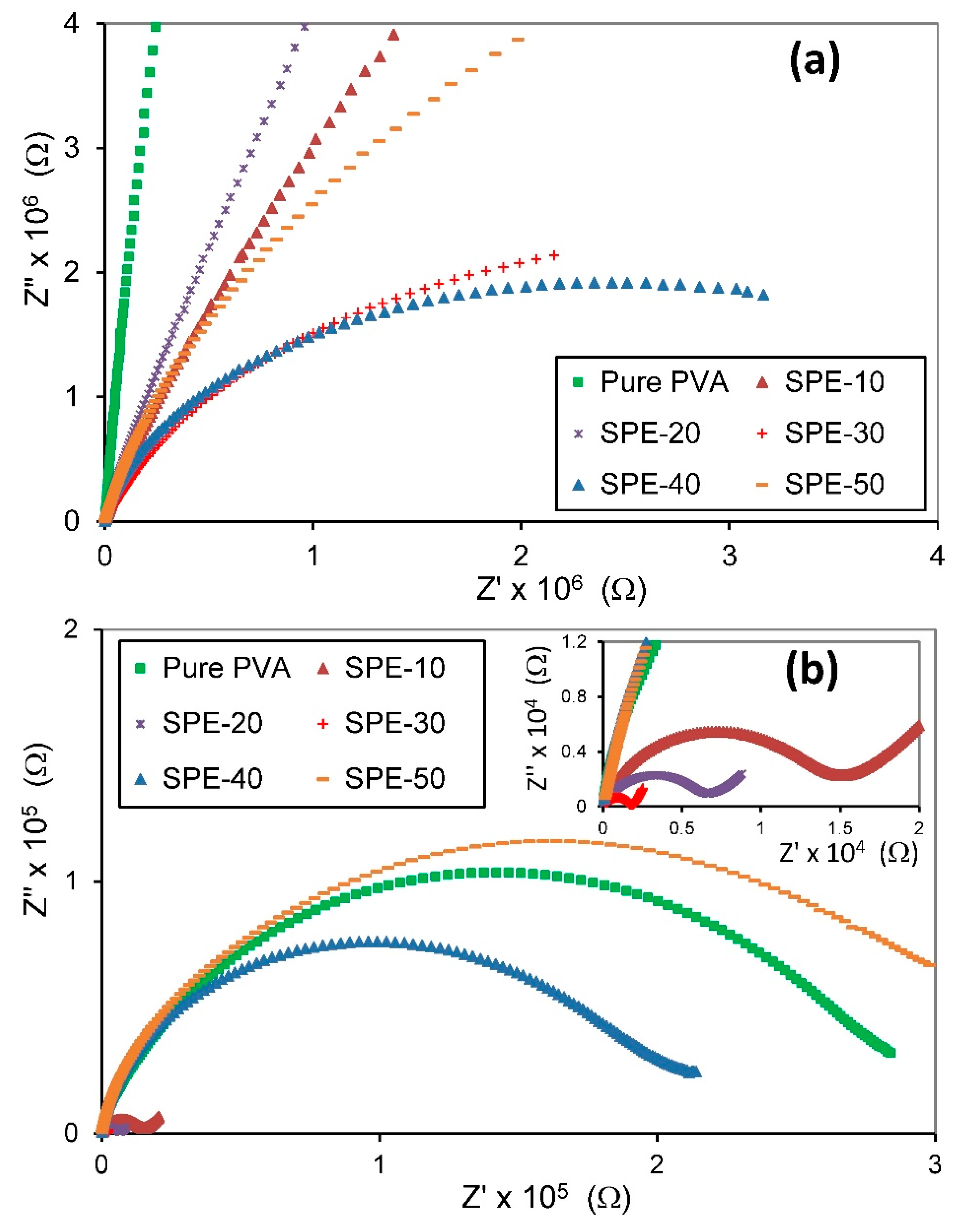
3.3.1. Cole-Cole Plot
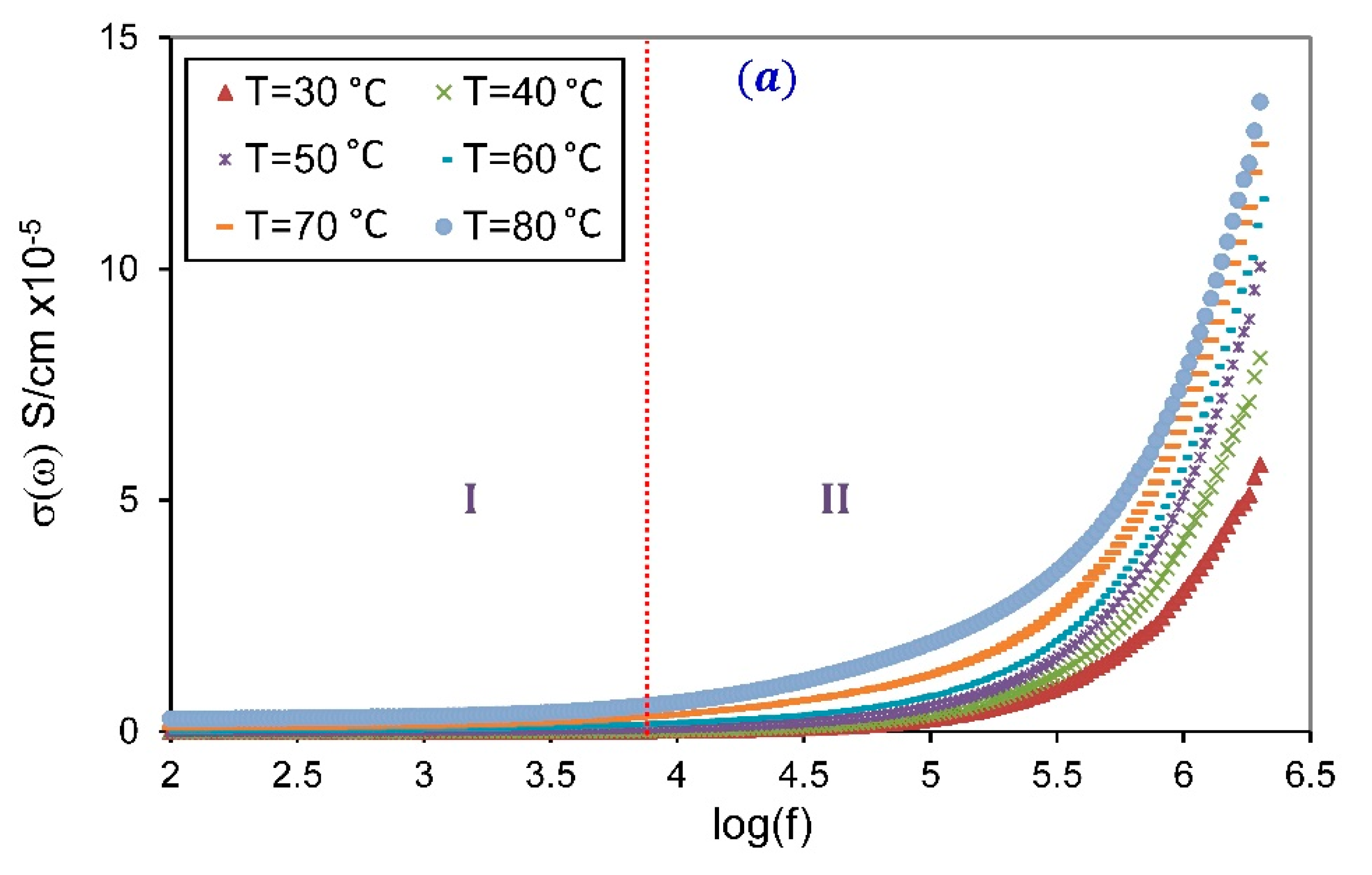
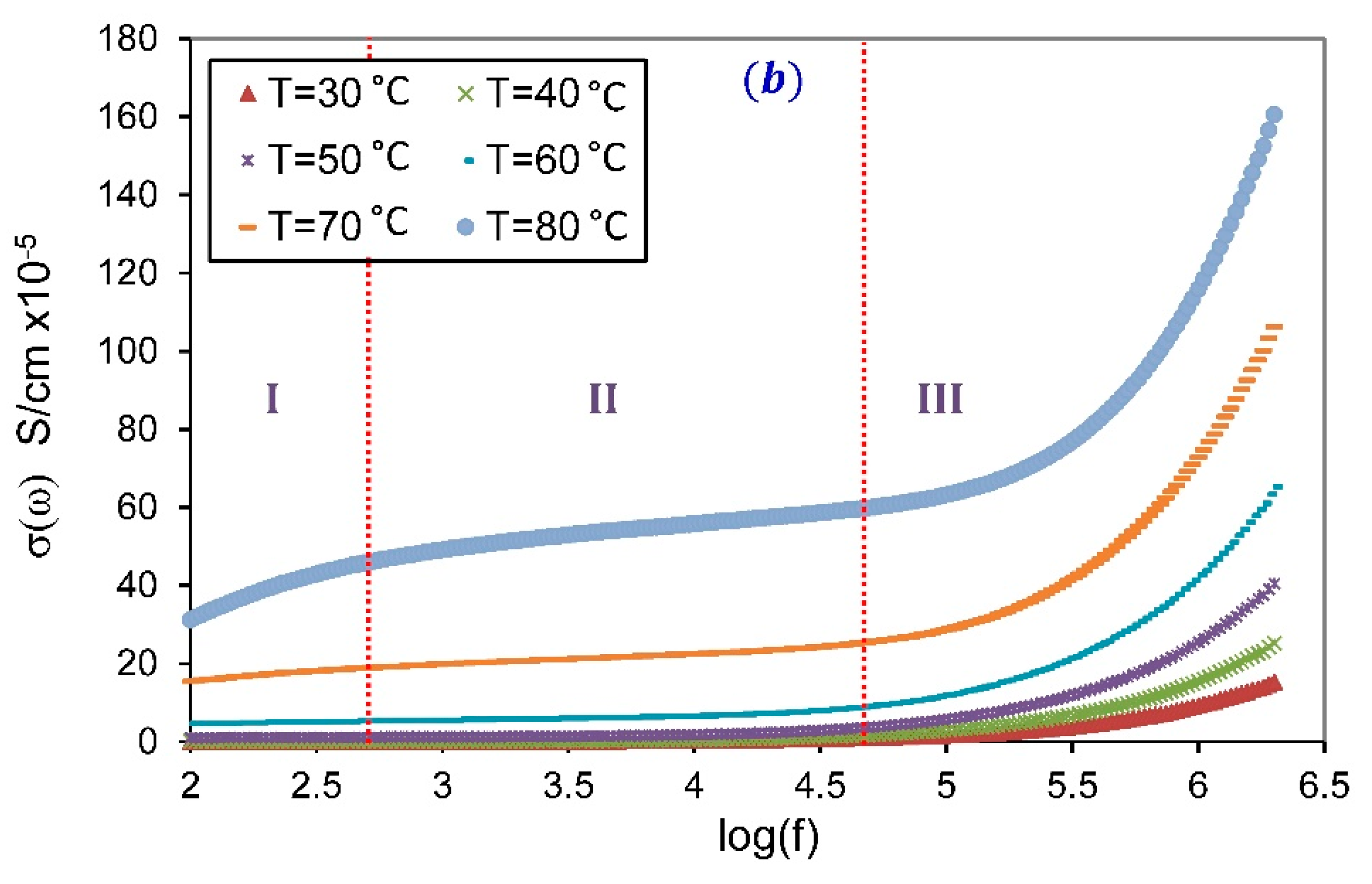
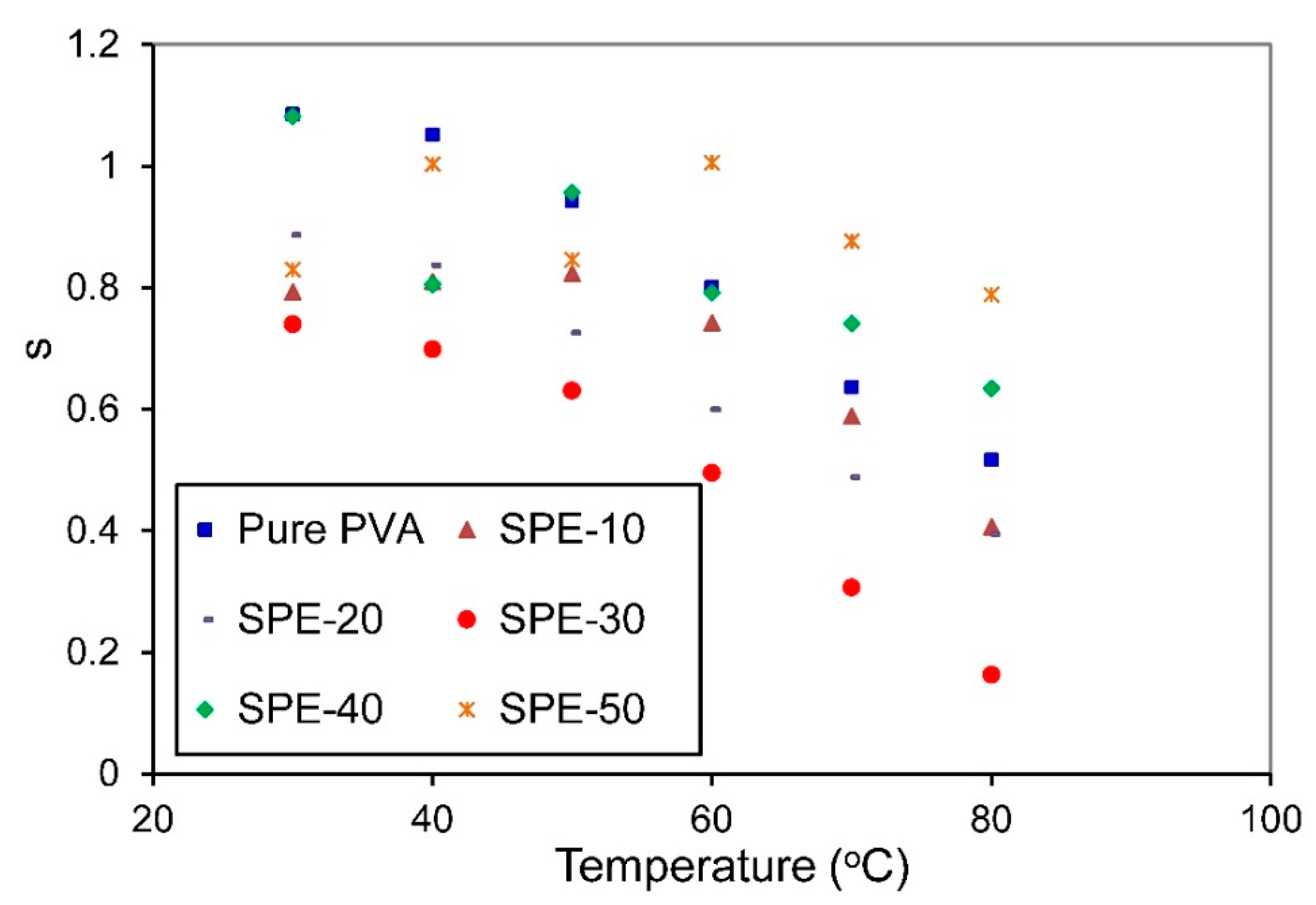
3.3.2. Frequency-Dependent Conductivity
3.3.3. Bulk Conductivity
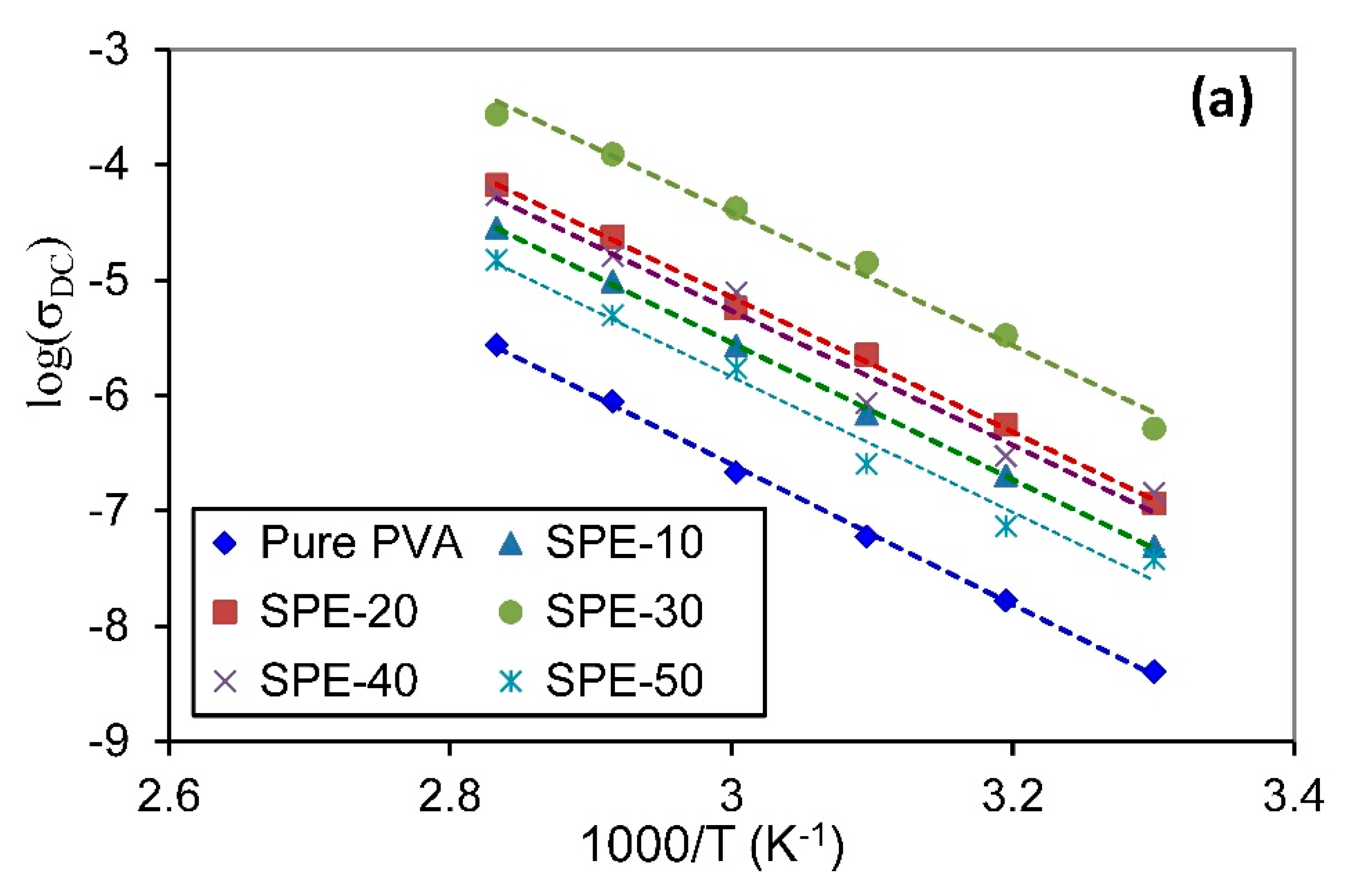
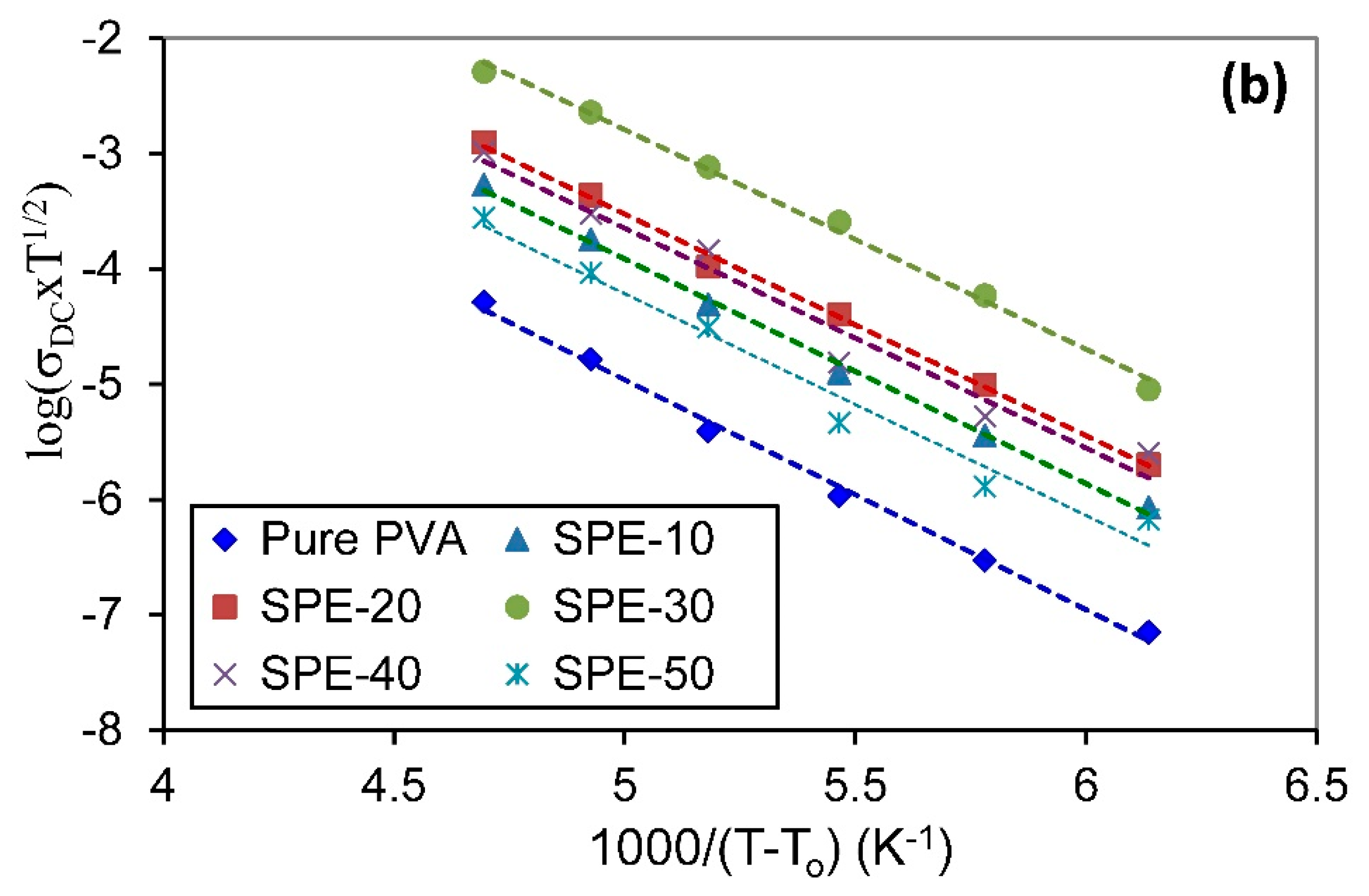
3.3.4. Temperature-Dependent Conductivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chandra, A.; Agrawal, R.C.; Mahipal, Y.K. Ion transport property studies on PEO-PVP blended solid polymer electrolyte membranes. J. Phys. D Appl. Phys. 2009, 42, 135107. [Google Scholar] [CrossRef] [Green Version]
- Gadjourova, Z.; Andreev, Y.G.; Tunstall, D.P.; Bruce, P.G. Ionic conductivity in crystalline polymer electrolytes. Nature 2001, 412, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Salman, Y.A.K.; Abdullah, O.G.; Hanna, R.R.; Aziz, S.B. Conductivity and electrical properties of chitosan—Methylcellulose blend biopolymer electrolyte incorporated with lithium tetrafluoroborate. Int. J. Electrochem. Sci. 2018, 13, 3185–3199. [Google Scholar] [CrossRef]
- Aziz, S.B.; Al-zangana, S.; Woo, H.J.; Kadir, M.F.Z.; Abdullah, O.G. The compatibility of chitosan with divalent salts over monovalent salts for the preparation of solid polymer electrolytes. Results Phys. 2018, 11, 826–836. [Google Scholar] [CrossRef]
- Ahmed, H.T.; Abdullah, O.G. Preparation and composition optimization of PEO:MC polymer blend films to enhance electrical conductivity. Polymers 2019, 11, 853. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.S.; Reddy, M.J.; Rao, U.V.S. Ion transport and battery studies of a new (PVP + KIO3) polymer electrolyte system. J. Mater. Sci. 2006, 41, 6171–6173. [Google Scholar] [CrossRef]
- Ahmed, H.T.; Jalal, V.J.; Tahir, D.A.; Mohamad, A.H.; Abdullah, O.G. Effect of PEG as a plasticizer on the electrical and optical properties of polymer blend electrolyte MC-CH-LiBF4 based films. Results Phys. 2019, 15, 102735. [Google Scholar] [CrossRef]
- Hemalatha, R.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Moniha, V.; Boopath, G.; Selvin, P.C. Preparation and characterization of proton-conducting polymer electrolyte based on PVA, amino acid proline, and NH4Cl and its applications to electrochemical device. Ionics 2019, 25, 141–154. [Google Scholar] [CrossRef]
- Patra, S.; Puthirath, A.B.; Vineesh, T.V.; Narayanaru, S.; Soman, B.; Suriyakumar, S.; Stephan, A.M.; Narayanan, T.N. On the development of a proton conducting solid polymer electrolyte using poly (ethylene oxide). Sustain. Energy Fuels 2018, 2, 1870–1877. [Google Scholar] [CrossRef]
- Ahmed, H.T.; Abdullah, O.G. Structural and ionic conductivity characterization of PEO:MC-NH4I proton-conducting polymer blend electrolytes based films. Results Phys. 2020, 16, 102861. [Google Scholar] [CrossRef]
- Ramya, C.S.; Selvasekarapandian, S.; Hirankumar, G.; Savitha, T.; Angelo, P.C. Investigation on dielectric relaxations of PVP-NH4SCN polymer electrolyte. J. Non-Cryst. Solids 2008, 354, 1494–1502. [Google Scholar] [CrossRef]
- Agrawal, S.L.; Singh, M.; Tripathi, M.; Dwivedi, M.M.; Pandey, K. Dielectric relaxation studies on [PEO-SiO2]: NH4SCN nanocomposite polymer electrolyte films. J. Mater. Sci. 2009, 44, 6060–6068. [Google Scholar] [CrossRef]
- Borah, P.; Dutta, A. Conductivity study of some polymer electrolytes based on polyacrylonitrile. Ionics 2009, 15, 711–716. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Saleem, S.A. Effect of copper sulfide nanoparticles on the optical and electrical behavior of poly (vinyl alcohol) films. J. Electron. Mater. 2016, 45, 5910–5920. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Rasheed, M.A. Structural and electrical characteristics of PVA:NaTf based solid polymer electrolytes: Role of lattice energy of salts on electrical DC conductivity. J. Mater. Sci. Mater. Electron. 2017, 28, 12873–12884. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, S.B.; Rasheed, M.A. Incorporation of NH4NO3 into MC-PVA blend-based polymer to prepare proton-conducting polymer electrolyte films. Ionics 2018, 24, 777–785. [Google Scholar] [CrossRef]
- Radha, K.P.; Selvasekarapandian, S.; Karthikeyan, S.; Hema, M.; Sanjeeviraja, C. Synthesis and impedance analysis of proton-conducting polymer electrolyte PVA:NH4F. Ionics 2013, 19, 1437–1447. [Google Scholar] [CrossRef]
- Hema, M.; Selvasekarapandian, S.; Arunkumar, D.; Sakunthala, A.; Nithya, H. FTIR, XRD and AC impedance spectroscopic study on PVA based polymer electrolyte doped with NH4X (X = Cl, Br, I). J. Non-Cryst. Solids 2009, 355, 84–90. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Hussein, S.A.; Ahmed, H.M. Effect of PVA blending on structural and ion transport properties of CS:AgNt based polymer electrolyte membrane. Polymers 2017, 9, 622. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, O.G. Synthesis of single-phase zinc chromite nano-spinel embedded in polyvinyl alcohol films and its effects on energy band gap. J. Mater. Sci. Mater. Electron. 2016, 27, 12106–12111. [Google Scholar] [CrossRef]
- Bdewi, S.F.; Abdullah, O.G.; Aziz, B.K.; Mutar, A.A.R. Synthesis, structural and optical characterization of MgO nanocrystalline embedded in PVA matrix. J. Inorg. Organomet. Polym. Mater. 2016, 26, 326–334. [Google Scholar] [CrossRef]
- Zagorskaya, S.A.; Tretinnikov, O.N. Infrared spectra and structure of solid polymer electrolytes based on poly (vinyl alcohol) and lithium halides. Polym. Sci. Ser. A 2019, 61, 514–519. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Salman, Y.A.K.; Saleem, S.A. Electrical conductivity and dielectric characteristics of in-situ prepared PVA/HgS nanocomposite films. J. Mater. Sci. Mater. Electron. 2016, 27, 3591–3598. [Google Scholar] [CrossRef]
- Moniha, V.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Boopathi, G. Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J. Non-Cryst. Solids 2018, 481, 424–434. [Google Scholar] [CrossRef]
- Shuhaimi, N.E.A.; Teo, L.P.; Majid, S.R.; Arof, A.K. Transport studies of NH4NO3 doped methyl cellulose electrolyte. Synth. Met. 2010, 160, 1040–1044. [Google Scholar] [CrossRef]
- Mohamad, A.H.; Saeed, S.R.; Abdullah, O.G. Synthesis of very-fine PbS nanoparticles dispersed homogeneously in MC matrix: Effect of concentration on the structural and optical properties of the host polymer. Mater. Res. Express 2019, 6, 115332. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, S.B.; Rasheed, M.A. Structural and optical characterization of PVA:KMnO4 based solid polymer electrolyte. Results Phys. 2016, 6, 1103–1108. [Google Scholar] [CrossRef] [Green Version]
- Zainuddin, N.K.; Samsudin, A.S. Investigation on the effect of NH4Br at transport properties in K–Carrageenan based biopolymer electrolytes via structural and electrical analysis. Mater. Today Commun. 2018, 14, 199–209. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Rasheed, M.A.; Ahmed, H.M. Effect of high salt concentration (HSC) on structural, morphological and electrical characteristics of chitosan based solid polymer electrolytes. Polymers 2017, 9, 187. [Google Scholar] [CrossRef] [Green Version]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Rajendran, S.; Sivakumar, P. An investigation of PVdF/PVC-based blend electrolytes with EC/PC as plasticizers in lithium battery applications. Physica B 2008, 403, 509–516. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Hanna, R.R.; Salman, Y.A.K. Structural and electrical conductivity of CH:MC bio-poly-blend films: Optimize the perfect composition of the blend system. Bull. Mater. Sci. 2019, 42, 64. [Google Scholar] [CrossRef] [Green Version]
- Kadir, M.F.Z.; Majid, S.R.; Arof, A.K. Plasticized chitosan-PVA blend polymer electrolyte based proton battery. Electrochim. Acta 2010, 55, 1475–1482. [Google Scholar] [CrossRef]
- Patla, S.K.; Ray, R.; Asokan, K.; Karmakar, S. Investigation of ionic conduction in PEO-PVDF based blend polymer electrolytes. J. Appl. Phys. 2018, 123, 125102. [Google Scholar] [CrossRef]
- Kadir, M.F.Z.; Aspanut, Z.; Yahya, R.; Arof, A.K. Chitosan–PEO proton conducting polymer electrolyte membrane doped with NH4NO3. Mater. Res. Innov. 2011, 15, s164–s167. [Google Scholar] [CrossRef]
- Mobarak, N.N.; Ahmad, A.; Abdullah, M.P.; Ramli, N.; Rahman, M.Y.A. Conductivity enhancement via chemical modification of chitosan based green polymer electrolyte. Electrochim. Acta 2013, 92, 161–167. [Google Scholar] [CrossRef]
- Naiwi, T.S.R.T.; Aung, M.M.; Ahmad, A.; Rayung, M.; Suait, M.S.; Yusof, N.A.; Lae, K.Z.W. Enhancement of plasticizing effect on bio-based polyurethane acrylate solid polymer electrolyte and its properties. Polymers 2018, 10, 1142. [Google Scholar] [CrossRef] [Green Version]
- Chai, M.N.; Isa, M.I.N. Electrical characterization and ionic transport properties of carboxyl methylcellulose-oleic acid solid polymer electrolytes. Int. J. Polym. Anal. Charact. 2013, 18, 280–286. [Google Scholar] [CrossRef]
- Ramesh, S.; Arof, A.K. Ionic conductivity studies of plasticized poly (vinyl chloride) polymer electrolytes. Mater. Sci. Eng. B 2001, 85, 10–15. [Google Scholar] [CrossRef]
- Mohamad, A.H.; Abdullah, O.G.; Saeed, S.R. Effect of very fine nanoparticle and temperature on the electric and dielectric properties of MC-PbS polymer nanocomposite films. Results Phys. 2020, 16, 102898. [Google Scholar] [CrossRef]
- Hema, M.; Selvasekerapandian, S.; Sakunthala, A.; Arunkumar, D.; Nithya, H. Structural, vibrational and electrical characterization of PVA-NH4Br polymer electrolyte system. Physica B 2008, 403, 2740–2747. [Google Scholar] [CrossRef]
- Ravi, M.; Pavani, Y.; Kumar, K.K.; Bhavani, S.; Sharma, A.K.; Rao, V.V.R.N. Studies on electrical and dielectric properties of PVP:KBrO4 complexed polymer electrolyte films. Mater. Chem. Phys. 2011, 130, 442–448. [Google Scholar] [CrossRef]
- Karan, N.K.; Pradhan, D.K.; Thomas, R.; Natesan, B.; Katiyar, R.S. Solid polymer electrolytes based on polyethylene oxide and lithium trifluoro-methane sulfonate (PEO-LiCF3SO3): Ionic conductivity and dielectric relaxation. Solid State Ion. 2008, 179, 689–696. [Google Scholar] [CrossRef]
- Bandara, T.M.W.J.; DeSilva, L.A.; Gunasekara, L.B.E.; Dehipawala, S.; Mellander, B.E. Determination of charge carrier transport parameters in a polymer electrolyte intended for Li-ion batteries using electrochemical impedance analysis. J. Solid State Electr. 2020, 24, 1207–1216. [Google Scholar] [CrossRef]
- Rosli, N.H.A.; Chan, C.H.; Subban, R.H.Y.; Winie, T. Studies on the structural and electrical properties of hexanoyl chitosan/polystyrene-based polymer electrolytes. Phys. Procedia 2012, 25, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Funke, K.; Singh, P.; Banhatti, R.D. Conductivity dispersion in supercooled calcium potassium nitrate: Caged ionic motion viewed as part of standard behaviour. Phys. Chem. Chem. Phys. 2007, 9, 5582–5590. [Google Scholar] [CrossRef]
- Chaurasia, S.K.; Saroj, A.L.; Shalu; Singh, V.K.; Tripathi, A.K.; Gupta, A.K.; Verma, Y.L.; Singh, R.K. Studies on structural, thermal and AC conductivity scaling of PEO-LiPF6 polymer electrolyte with added ionic liquid [BMIMPF6]. AIP Adv. 2015, 5, 077178. [Google Scholar] [CrossRef]
- Pal, P.; Ghosh, A. Dielectric spectroscopy of PMMA-LiClO4 based polymer electrolyte plasticized with ethylene carbonate EC. J. Appl. Phys. 2016, 120, 045108. [Google Scholar] [CrossRef]
- Aziz, S.B.; Abdullah, O.G.; Saeed, S.R.; Ahmed, H.M. Electrical and dielectric properties of copper ion conducting solid polymer electrolytes based on chitosan: CBH model for ion transport mechanism. Int. J. Electrochem. Sci. 2018, 13, 3812–3826. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Aziz, S.B.; Saber, D.R. Characterizations of pure and Pb2+ ion doped methylcellulose based biopolymer electrolyte films: Optical and electrical properties. Int. J. Electrochem. Sci. 2018, 13, 11931–11952. [Google Scholar] [CrossRef]
- Abdullah, O.G.; Hanna, R.R.; Salman, Y.A.K. Structural, optical, and electrical characterization of chitosan: Methylcellulose polymer blends based film. J. Mater. Sci. Mater. Electron. 2017, 28, 10283–10294. [Google Scholar] [CrossRef]
- Parameswaran, V.; Nallamuthu, N.; Devendran, P.; Nagarajan, E.R.; Manikandan, A. Electrical conductivity studies on ammonium bromide incorporated with Zwitterionic polymer blend electrolyte for battery application. Physica B 2017, 515, 89–98. [Google Scholar] [CrossRef]
- Sengwa, R.J.; Choudhary, S. Investigation of correlation between dielectric parameters and nanostructures in aqueous solution grown poly (vinyl alcohol)-montmorillonite clay nanocomposites by dielectric relaxation spectroscopy. Express Polym. Lett. 2010, 4, 559–569. [Google Scholar] [CrossRef]
- Khandale, A.P.; Bhoga, S.S.; Gedam, S.K. Study on ammonium acetate salt-added polyvinyl alcohol-based solid proton-conducting polymer electrolytes. Ionics 2013, 19, 1619–1626. [Google Scholar] [CrossRef]
- Gohel, K.; Kanchan, D.K. Effect of PC:DEC plasticizers on structural and electrical properties of PVDF-HFP:PMMA based gel polymer electrolyte system. J. Mater. Sci. Mater. Electron. 2019, 30, 12260–12268. [Google Scholar] [CrossRef]
- Ahmed, H.T.; Abdullah, O.G. Impedance and ionic transport properties of proton-conducting electrolytes based on polyethylene oxide/methylcellulose blend polymers. J. Sci. Adv. Mater. Devices 2020, 5, 125–133. [Google Scholar] [CrossRef]
- Yusof, Y.M.; Shukur, M.F.; Illias, H.A.; Kadir, M.F.Z. Conductivity and electrical properties of corn starch-chitosan blend biopolymer electrolyte incorporated with ammonium iodide. Phys. Scr. 2014, 89, 035701. [Google Scholar] [CrossRef]
- Sundaramahalingam, K.; Vanitha, D.; Nallamuthu, N.; Manikandan, A.; Muthuvinayagam, M. Electrical properties of lithium bromide poly ethylene oxide/poly vinyl pyrrolidone polymer blend electrolyte. Physica B 2019, 553, 120–126. [Google Scholar] [CrossRef]
- Sahu, G.; Rangasamy, E.; Li, J.; Chen, Y.; An, K.; Dudney, N.; Liang, C. A high-conduction Ge substituted Li3AsS4 solid electrolyte with exceptional low activation energy. J. Mater. Chem. A 2014, 2, 10396–10403. [Google Scholar] [CrossRef]
- Idris, N.H.; Majid, S.R.; Khiar, A.S.A.; Hassan, M.F.; Arof, A.K. Conductivity studies on chitosan/PEO blends with LiTFSI salt. Ionics 2005, 11, 375–377. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Shukur, M.F.; Kadir, M.F.Z. NH4NO3 as charge carrier contributor in glycerolized potato starch-methyl cellulose blend-based polymer electrolyte and the application in electrochemical double-layer capacitor. Ionics 2017, 23, 3429–3453. [Google Scholar] [CrossRef]
- Vijaya, N.; Selvasekarapandian, S.; Hirankumar, G.; Karthikeyan, S.; Nithya, H.; Ramya, C.S.; Prabu, M. Structural, vibrational, thermal, and conductivity studies on proton-conducting polymer electrolyte based on poly (N-vinylpyrrolidone). Ionics 2012, 18, 91–99. [Google Scholar] [CrossRef]
- Buraidah, M.H.; Teo, L.P.; Majid, S.R.; Arof, A.K. Ionic conductivity by correlated barrier hopping in NH4I doped chitosan solid electrolyte. Physica B 2009, 404, 1373–1379. [Google Scholar] [CrossRef]



| Samples | |||
|---|---|---|---|
| Pure PVA | 9734.15 | 24,827.16 | 39.20 |
| SPE-10 | 4864.19 | 27,295.78 | 17.82 |
| SPE-20 | 7320.78 | 65,735.29 | 11.13 |
| SPE-30 | 5410.93 | 54,434.50 | 9.94 |
| SPE-40 | 7433.04 | 53,423.04 | 13.91 |
| SPE-50 | 13,342.05 | 61,947.55 | 21.53 |
| Samples | Arrhenius Model | VTF Model | |||
|---|---|---|---|---|---|
| Pure PVA | 1.207 | 0.9988 | 140 | 0.396 | 0.9959 |
| SPE-10 | 1.179 | 0.9994 | 140 | 0.387 | 0.9973 |
| SPE-20 | 1.161 | 0.9977 | 140 | 0.382 | 0.9974 |
| SPE-30 | 1.147 | 0.9896 | 140 | 0.378 | 0.9954 |
| SPE-40 | 1.156 | 0.9811 | 140 | 0.379 | 0.9684 |
| SPE-50 | 1.166 | 0.9808 | 140 | 0.382 | 0.9745 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, M.A.M.; Abdullah, O.G. Effect of High Ammonium Salt Concentration and Temperature on the Structure, Morphology, and Ionic Conductivity of Proton-Conductor Solid Polymer Electrolytes Based PVA. Membranes 2020, 10, 262. https://doi.org/10.3390/membranes10100262
Saeed MAM, Abdullah OG. Effect of High Ammonium Salt Concentration and Temperature on the Structure, Morphology, and Ionic Conductivity of Proton-Conductor Solid Polymer Electrolytes Based PVA. Membranes. 2020; 10(10):262. https://doi.org/10.3390/membranes10100262
Chicago/Turabian StyleSaeed, Maryam A. M., and Omed Gh. Abdullah. 2020. "Effect of High Ammonium Salt Concentration and Temperature on the Structure, Morphology, and Ionic Conductivity of Proton-Conductor Solid Polymer Electrolytes Based PVA" Membranes 10, no. 10: 262. https://doi.org/10.3390/membranes10100262
APA StyleSaeed, M. A. M., & Abdullah, O. G. (2020). Effect of High Ammonium Salt Concentration and Temperature on the Structure, Morphology, and Ionic Conductivity of Proton-Conductor Solid Polymer Electrolytes Based PVA. Membranes, 10(10), 262. https://doi.org/10.3390/membranes10100262