Performance Evaluation of Tight Ultrafiltration Membrane Systems at Pilot Scale for Agave Fructans Fractionation and Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Agave Fructans
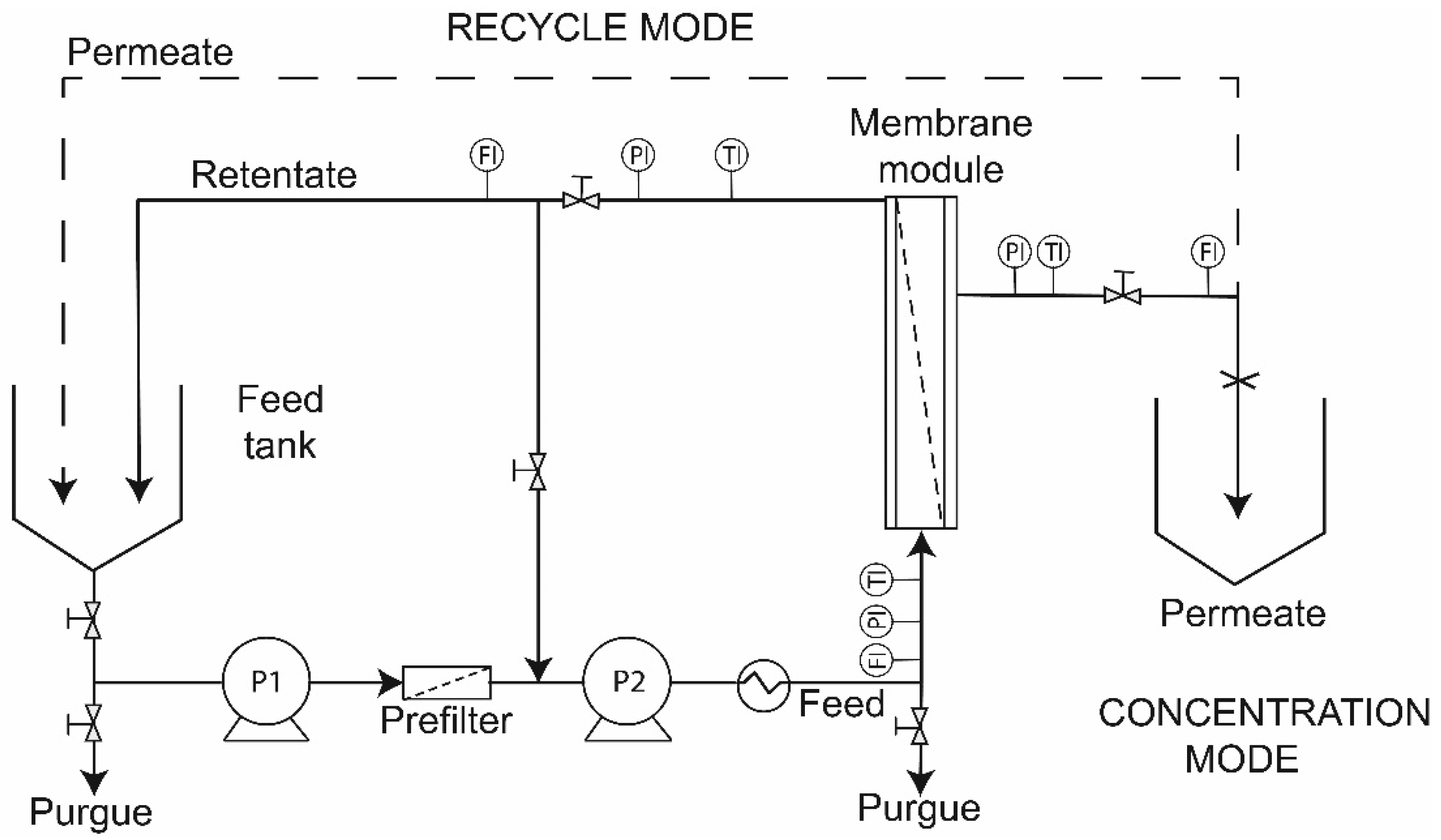
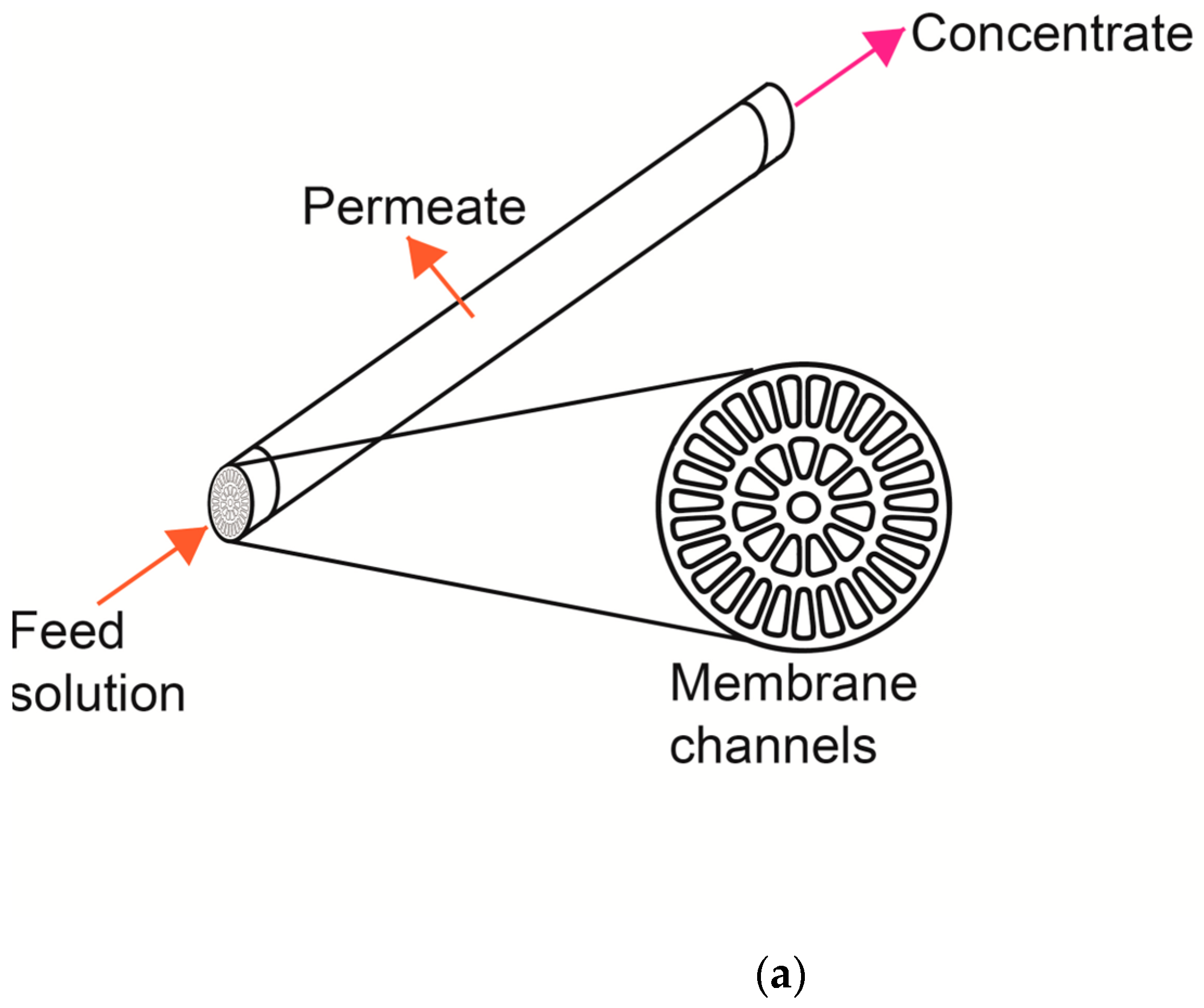
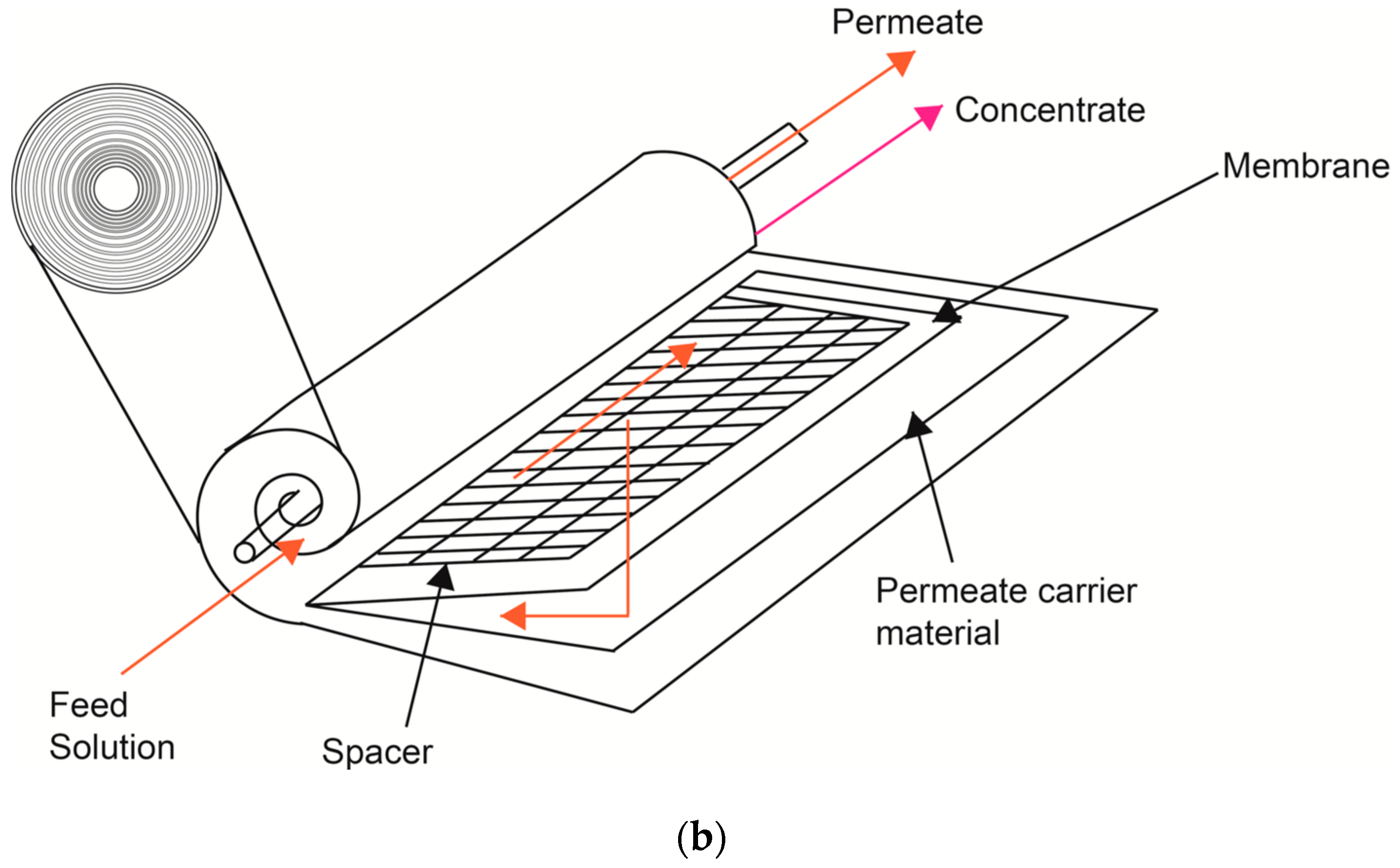
2.2. Pilot Scale Filtration System and Membranes
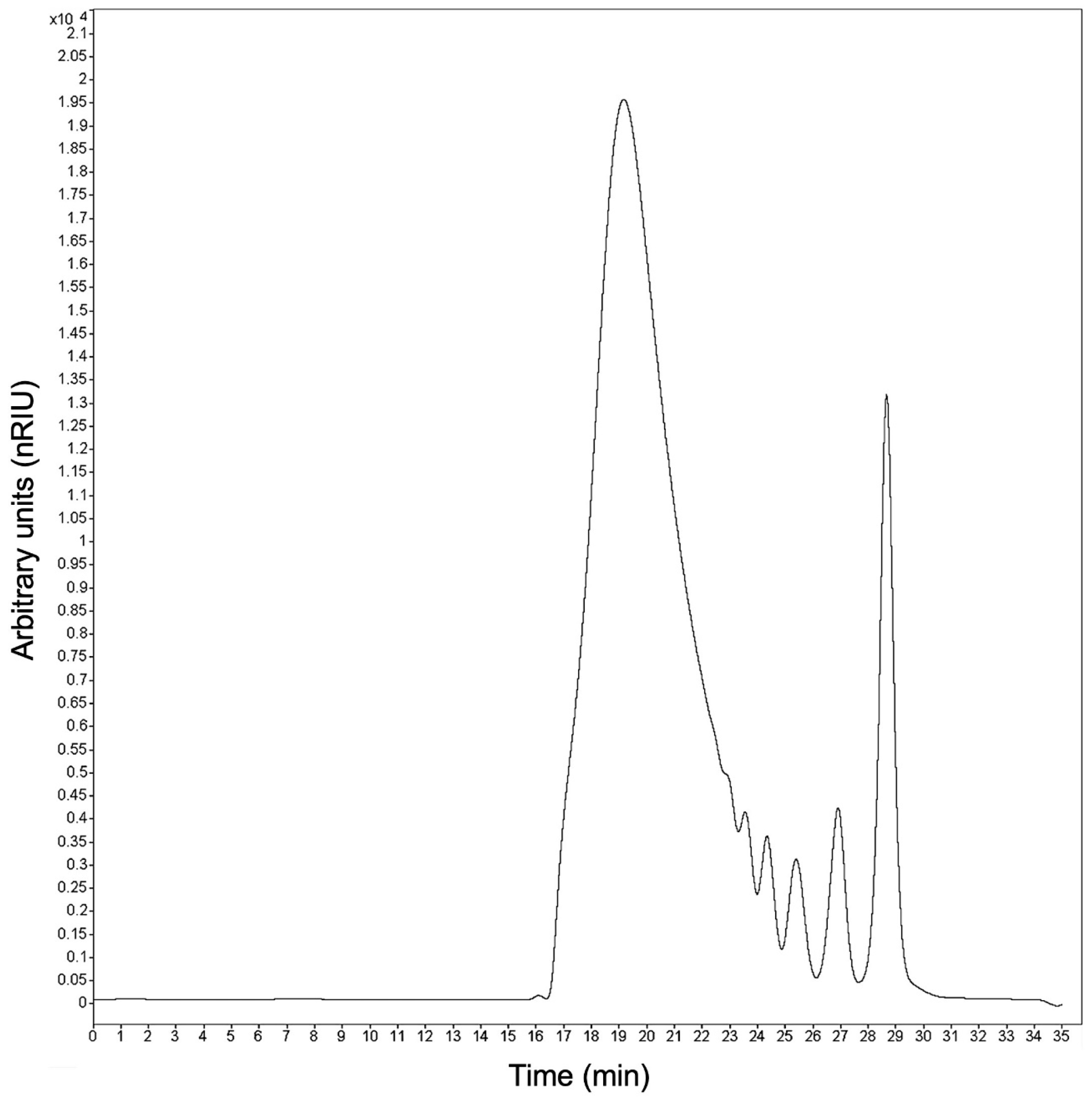
2.3. Measurement of Size Distribution of Fructans
2.4. Evaluation of Membrane Systems Performance
2.4.1. Water Permeability and Permeate Flux
2.4.2. Estimation of Rejection Coefficient of Agave Fractions
2.4.3. Analysis of the Fouling Resistance
2.4.4. Global Yield of the Process
2.4.5. Data Analysis
3. Results and Discussion
3.1. Size Distribution Profile of Agave Fructans
3.2. Full Recycle Mode Experiments
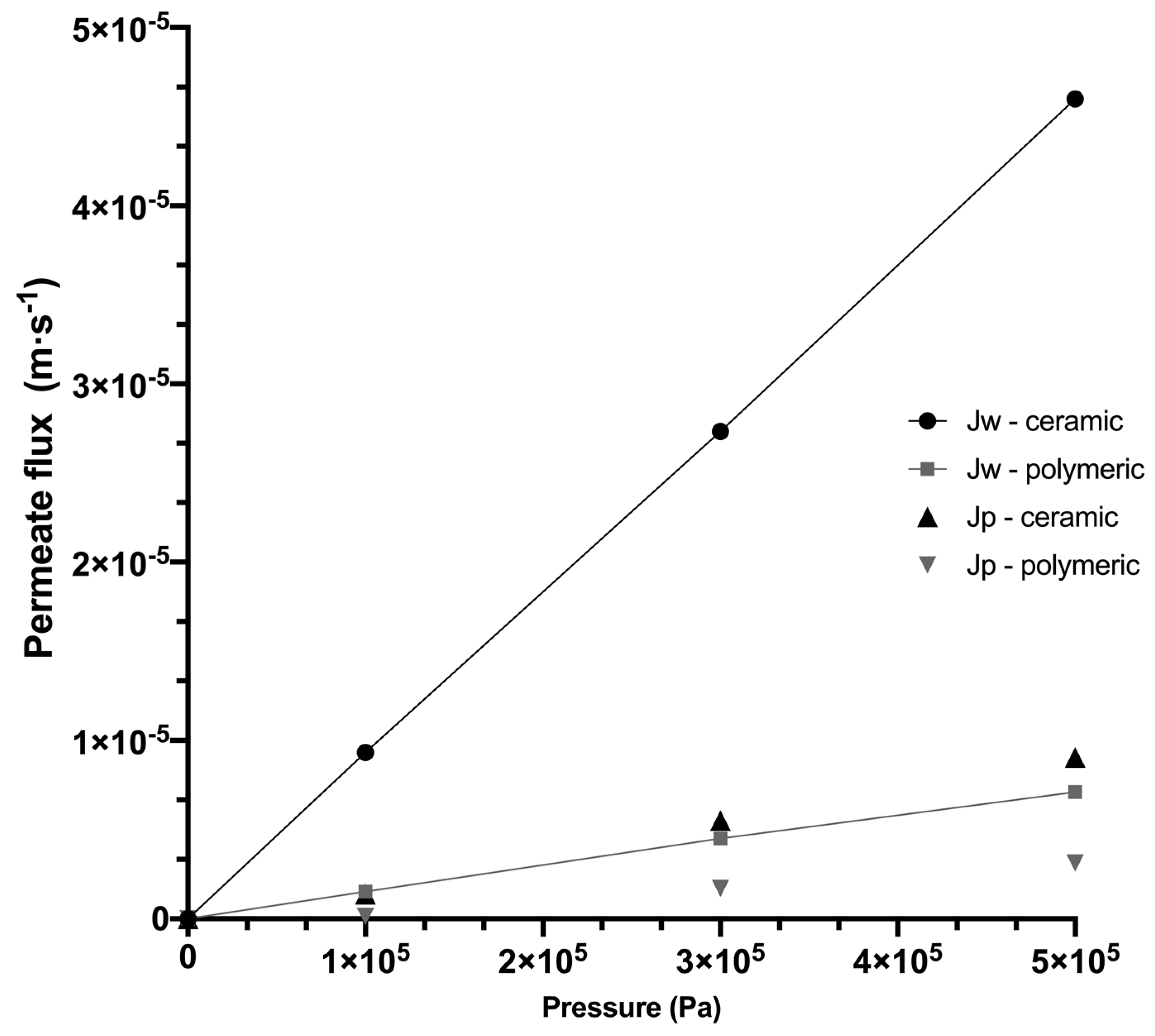
3.2.1. Water Permeability and Permeate Flux of Systems
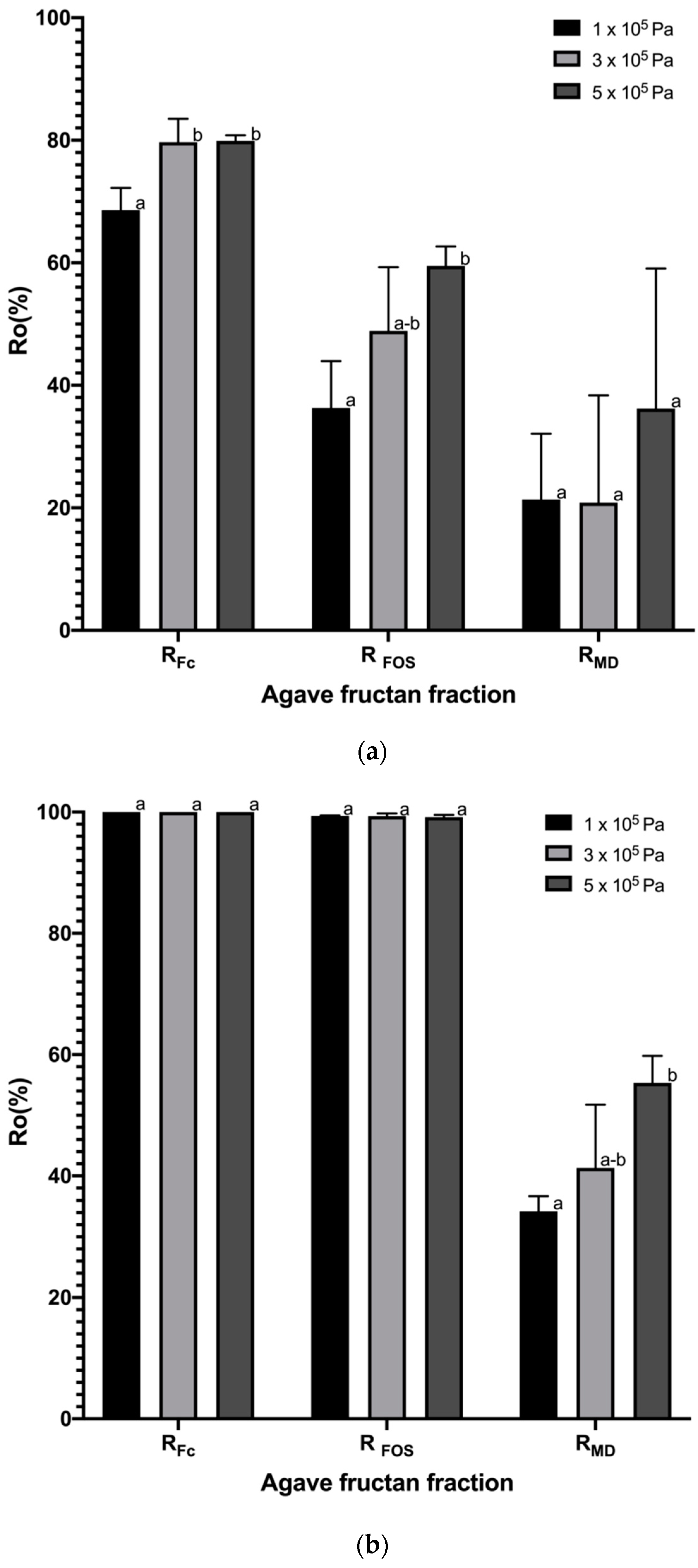
3.2.2. Rejection Coefficients
3.3. Experiments in Concentration Mode
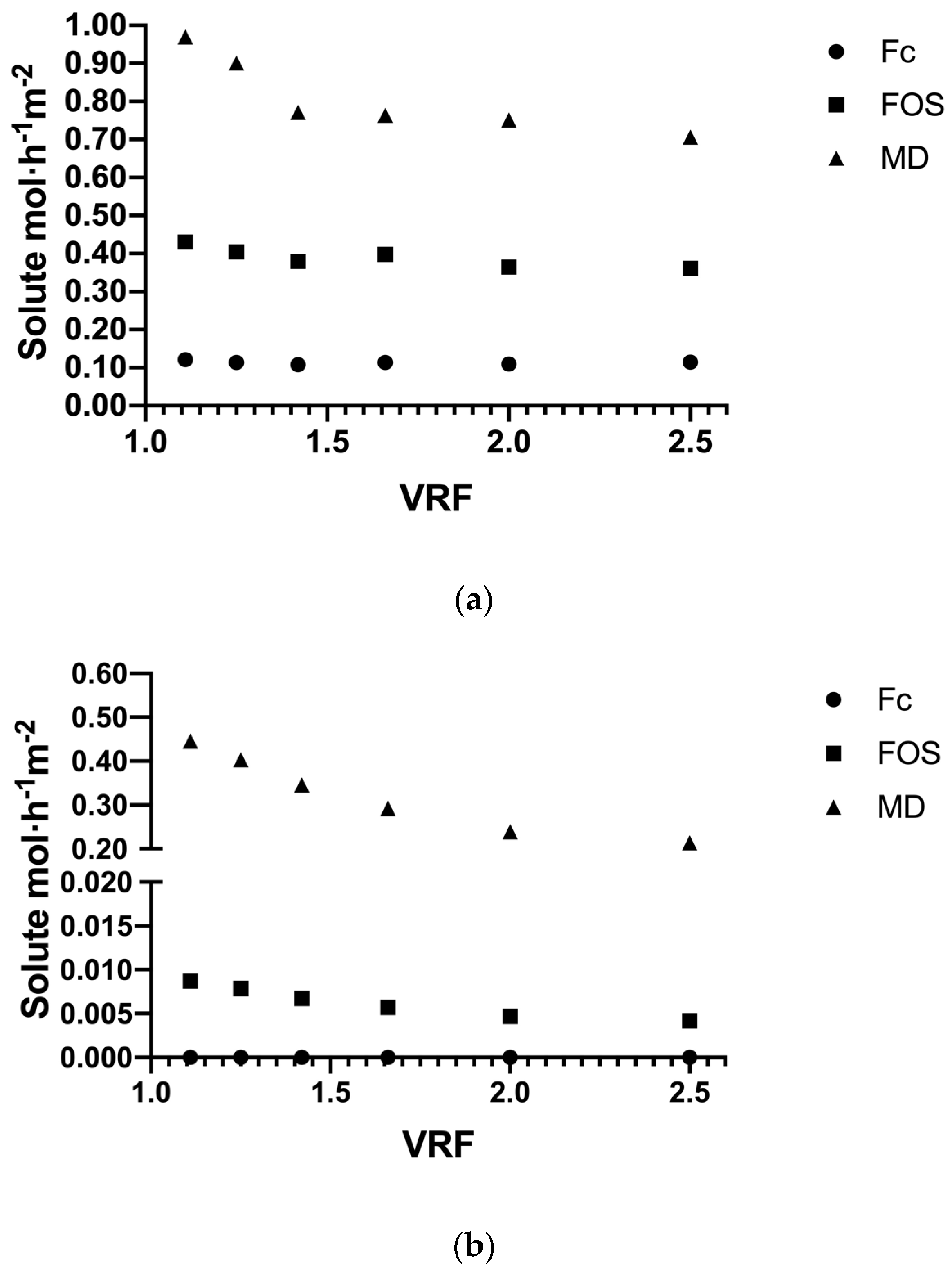
3.3.1. Solute Flux
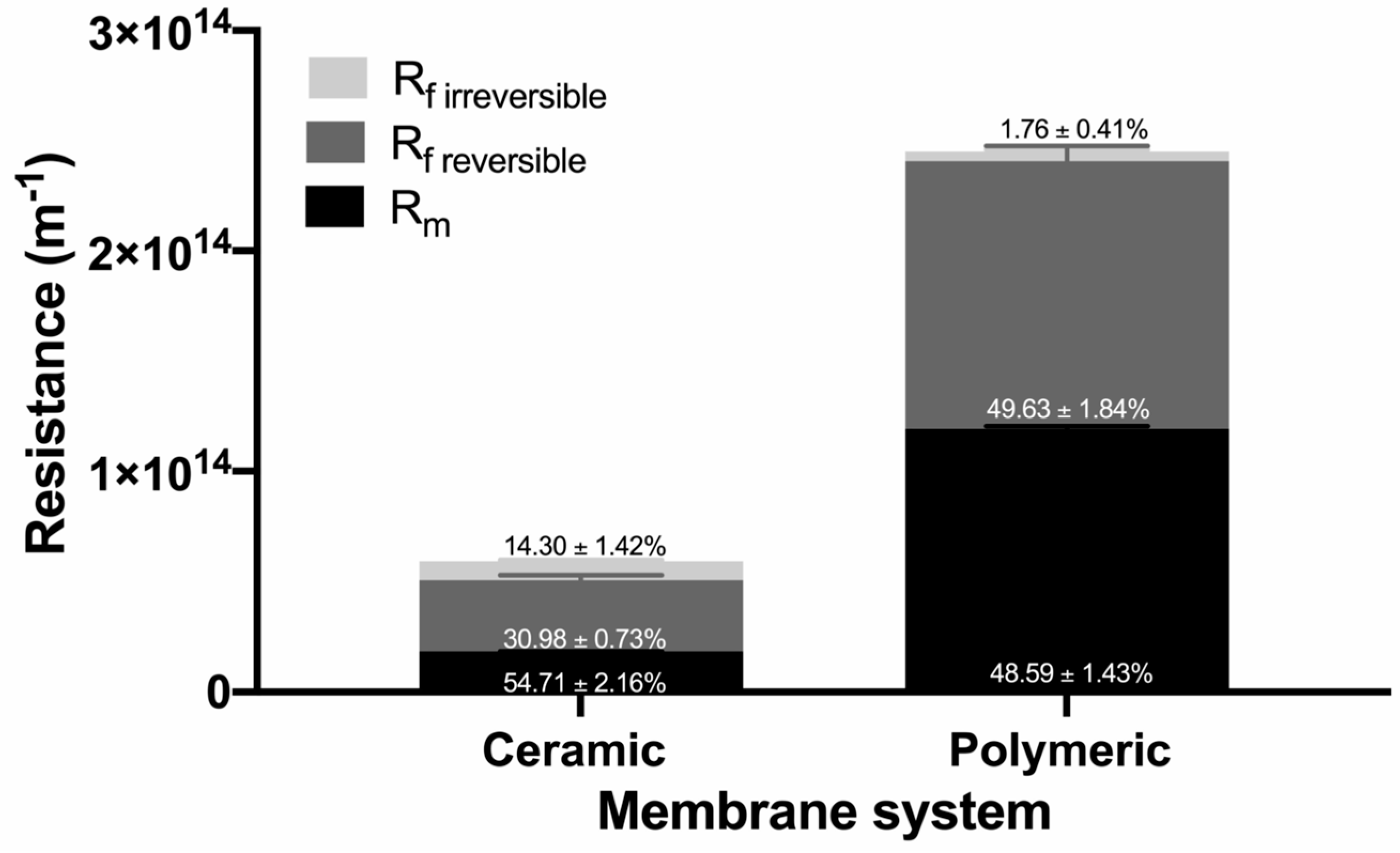
3.3.2. Analysis of the Fouling Resistance
3.3.3. Analysis of Global Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Symbols | |
| A | membrane area (m2) |
| concentration of solute in permeate stream (kg·m−3) | |
| concentration of the solute in feed (kg·m−3). | |
| viscosity of fructans in permeate stream (kg·m−1·s−1) | |
| water viscosity (kg·m−1·s−1) | |
| density of fructans solution (kg·m−3) | |
| molecular weight (kg·kmol−1) | |
| permeate flux with fructan solution (m·s−1) | |
| permeate flux with pure water (m·s−1) | |
| hydraulic permeability (m2·s·kg−1) | |
| Reynolds number (dimensionless) | |
| fouling resistance (m−1) | |
| total resistance of the membrane system (m−1) | |
| observed rejection (dimensionless) | |
| rejection coefficient of agave fructan with DP ˃ 10 (dimensionless) | |
| rejection coefficient of FOS (dimensionless) | |
| rejection coefficient of mono- and disaccharides (dimensionless) | |
| intrinsic membrane resistance (m−1) | |
| transmembrane pressure (Pa) | |
| hydraulic diameter of membrane (m) | |
| tangential velocity (m·s−1) | |
| retentate volume (m3) | |
| feed volume (m3) | |
References
- Lopez, M.G.; Mancilla-Margalli, N.A.; Mendoza-Díaz, G. Molecular Structures of Fructans from Agave tequilana Weber var.azul. J. Agric. Food Chem. 2003, 51, 7835–7840. [Google Scholar] [CrossRef]
- Urías-Silvas, J.E.; Cani, P.D.; Delmée, E.; Neyrinck, A.; López, M.G.; Delzenne, N.M. Physiological effects of dietary fructans extracted from Agave tequilana Gto. and Dasylirion spp. Br. J. Nutr. 2008, 99, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Sáyago-Ayerdi, S.G.; Mateos, R.; Ortiz-Basurto, R.I.; Largo, C.; Serrano, J.; Granado-Serrano, A.B.; Sarriá, B.; Bravo, L.; Tabernero, M. Effects of consuming diets containing Agave tequilana dietary fibre and jamaica calyces on body weight gain and redox status in hypercholesterolemic rats. Food Chem. 2014, 148, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Ramnani, P.; Costabile, A.; Bustillo, A.G.R.; Gibson, G.R. A randomised, double- blind, cross-over study investigating the prebiotic effect of agave fructans in healthy human subjects. J. Nutr. Sci. 2015, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Padilla-Camberos, E.; Barragán-Álvarez, C.P.; Diaz-Martinez, N.E.; Rathod, V.; Flores-Fernández, J.M. Effects of Agave fructans (Agave tequilana Weber var. azul) on Body Fat and Serum Lipids in Obesity. Plant Foods Hum. Nutr. 2018, 73, 34–39. [Google Scholar] [CrossRef]
- Crispín-Isidro, G.; Lobato-Calleros, C.; Espinosa-Andrews, H.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of inulin and agave fructans addition on the rheological, microstructural and sensory properties of reduced-fat stirred yogurt. LWT Food Sci. Technol. 2015, 62, 438–444. [Google Scholar] [CrossRef]
- Palatnik, D.R.; Aldrete Herrera, P.; Rinaldoni, A.N.; Ortiz Basurto, R.I.; Campderrós, M.E. Development of reduced-fat cheeses with the addition of Agave fructans. Int. J. Dairy Technol. 2017, 70, 212–219. [Google Scholar] [CrossRef]
- Ortiz-Basurto, R.I.; Rubio-Ibarra, M.E.; Ragazzo-Sanchez, J.A.; Beristain, C.I.; Jiménez-Fernández, M. Microencapsulation of Eugenia uniflora L. juice by spray drying using fructans with different degrees of polymerisation. Carbohydr. Polym. 2017, 175, 603–609. [Google Scholar] [CrossRef]
- Santiago-García, P.A.; Mellado-Mojica, E.; León-Martínez, F.M.; López, M.G. Evaluation of Agave angustifolia fructans as fat replacer in the cookies manufacture. LWT Food Sci. Technol. 2017, 77, 100–109. [Google Scholar] [CrossRef]
- Jimenez-Sánchez, D.E.; Calderón-Santoyo, M.; Ortiz-Basurto, R.I.; Bautista-Rosales, P.U.; Ragazzo-Sánchez, J.A. Effect of maltodextrin reduction and native agave fructans addition on the physicochemical properties of spray-dried mango and pineapple juices. Food Sci. Technol. Int. 2018, 24, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Aguirre, A.L.; Camacho-Ruiz, R.M.; Arriaga-Alba, M.; Padilla-Camberos, E.; Kirchmayr, M.R.; Blasco, J.L.; González-Avila, M. Effects of Agave tequilana fructans with different degree of polymerization profiles on the body weight, blood lipids and count of fecal Lactobacilli/Bifidobacteria in obese mice. Food Funct. 2013, 4, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Aguirre, A.L.; Camacho-Ruíz, R.M.; Gutiérrez-Mercado, Y.K.; Padilla-Camberos, E.; González-Ávila, M.; Gálvez-Gastélum, F.J.; Díaz-Martínez, N.E.; Ortuño-Sahagún, D. Fructans from Agave tequilana with a Lower Degree of Polymerization Prevent Weight Gain, Hyperglycemia and Liver Steatosis in High-Fat Diet-Induced Obese Mice. Plant Foods Hum. Nutr. 2016, 71, 416–421. [Google Scholar] [CrossRef] [Green Version]
- García Gamboa, R.; Ortiz Basurto, R.I.; Calderón Santoyo, M.; Bravo Madrigal, J.; Ruiz Álvarez, B.E.; González Avila, M. In vitro evaluation of prebiotic activity, pathogen inhibition and enzymatic metabolism of intestinal bacteria in the presence of fructans extracted from agave: A comparison based on polymerization degree. LWT Food Sci. Technol. 2018, 92, 380–387. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Moscosa-Santillán, M.; Grajales-Lagunes, A.; González-Chávez, M.; Bonnin-Paris, J.; Bostyn, S.; Ruiz-Cabrera, M. Sugars and Fructans Separation by Nanofiltration from Model Sugar Solution and Comparative Study with Natural Agave Juice. Sep. Sci. Technol. 2013, 48, 1768–1776. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Bonnin-Paris, J.; Bostyn, S.; Ruiz-Cabrera, M.A.; Moscosa-Santillán, M. Assessment of sugars separation from a model carbohydrates solution by nanofiltration using a design of experiments (DoE) methodology. Sep. Purif. Technol. 2014, 131, 84–93. [Google Scholar] [CrossRef]
- Reynoso-Ponce, H.; Grajales-Lagunes, A.; Castillo-Andrade, A.; González-García, R.; Ruiz-Cabrera, M.A. Integration of nanofiltration and spray drying processes for enhancing the purity of powdered fructans from Agave salmiana juice. Powder Technol. 2017, 322, 96–105. [Google Scholar] [CrossRef]
- Pérez Martínez, F.J.; Gonzalez Avila, M.; Camacho Ruiz, R.M.; Márquez Aguirre, A.L.; Alonso Segura, D.; Gschaedler Mathis, A.C.; Prado Ramírez, R.; Flores Montaño, J.L.; Mateos Díaz, J.C.; Arrizón Gaviño, J.P. Fructanos Fraccionados de Agave, Proceso de Obtención y uso de los Mismos. MX Patent 367976 B, 30 April 2013. [Google Scholar]
- Flores Montaño, J.L.; Camacho Ruiz, R.M.; Prado Ramírez, R.; Morena Vilet, L.; Luiz Santos, N.; Mendoza Rivera, M.; de los, Á.; Ballón Villagrá, A. Fructanos Fraccionados de Agave y su Proceso de Obtención a Nivel Piloto e Industrial. Patent request MX/a/2015/014523, 15 October 2015. [Google Scholar]
- Fane, A.G.; Tang, C.Y.; Wang, R. Membrane Technology for Water: Microfiltration, Ultrafiltration, Nanofiltration, and Reverse Osmosis. Treatise Water Sci. 2010, 4, 301–335. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and tight ultrafiltration membranes for the recovery of polyphenols from agro-food by-products. Int. J. Mol. Sci. 2018, 19, 351. [Google Scholar] [CrossRef] [Green Version]
- Shang, R.; Verliefde, A.R.D.; Hu, J.; Zeng, Z.; Lu, J.; Kemperman, A.J.B.; Deng, H.; Nijmeijer, K.; Heijman, S.G.J.; Rietveld, L.C. Tight ceramic UF membrane as RO pre-treatment: The role of electrostatic interactions on phosphate rejection. Water Res. 2014, 48, 498–507. [Google Scholar] [CrossRef]
- Aguirre Montesdeoca, V.; Janssen, A.E.M.; Boom, R.M.; Van der Padt, A. Fine ultrafiltration of concentrated oligosaccharide solutions—Hydration and pore size distribution effects. J. Memb. Sci. 2019, 580, 161–176. [Google Scholar] [CrossRef]
- Singh, R. Introduction to Membrane Technology. In Membrane Technology and Engineering for Water Purification, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 1–80. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, W.; Wang, X.; Yang, H.; Xie, Y.F. Relating the rejections of oligomeric ethylene glycols and saccharides by nanofiltration: Implication for membrane pore size determination. Sep. Purif. Technol. 2018, 205, 151–158. [Google Scholar] [CrossRef]
- Mora, F.; Pérez, K.; Quezada, C.; Herrera, C.; Cassano, A.; Ruby-Figueroa, R. Impact of membrane pore size on the clarification performance of grape marc extract by microfiltration. Membranes 2019, 9, 146. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Dickson, J.M.; Kentish, S.E. Microfiltration for casein and serum protein separation. In Membrane Processing for Dairy Ingredient Separation, 1st ed.; Hu, K., Dickson, J.M., Eds.; Jonh Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–34. [Google Scholar] [CrossRef]
- Gitis, V.; Rothenberg, G. The basics. In Ceramic Membranes: New Opportunities and Practical Applications, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2016; pp. 1–395. [Google Scholar] [CrossRef]
- Urošević, T.; Povrenović, D.; Vukosavljević, P.; Urošević, I.; Stevanović, S. Recent developments in microfiltration and ultrafiltration of fruit juices. Food Bioprod. Process. 2017, 106, 147–161. [Google Scholar] [CrossRef]
- Teng, J.; Wu, M.; Chen, J.; Lin, H.; He, Y. Different fouling propensities of loosely and tightly bound extracellular polymeric substances (EPSs) and the related fouling mechanisms in a membrane bioreactor. Chemosphere 2020, 255, 126953. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Tang, J.; Hu, S.; Shen, L.; Xu, Y.; Li, R.; Lin, H. Inkjet printing assisted electroless Ni plating to fabricate nickel coated polypropylene membrane with improved performance. J. Colloid Interface Sci. 2020, 565, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, Y.; Lin, H.; Zhao, L.; Shen, L.; Li, R.; Xu, Y.; Hong, H.; He, Y. Membrane fouling caused by biological foams in a submerged membrane bioreactor: Mechanism insights. Water Res. 2020, 181, 115932. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, L.; Lin, H.; Yu, W.; Xu, Y.; Li, R.; Sun, T.; He, Y. A novel strategy based on magnetic field assisted preparation of magnetic and photocatalytic membranes with improved performance. J. Memb. Sci. 2020, 612, 118378. [Google Scholar] [CrossRef]
- Sun, T.; Liu, Y.; Shen, L.; Xu, Y.; Li, R.; Huang, L.; Lin, H. Magnetic field assisted arrangement of photocatalytic TiO 2 particles on membrane surface to enhance membrane antifouling performance for water treatment. Colloid Interface Sci. 2020, 570, 273–285. [Google Scholar] [CrossRef]
- Li, R.; Fan, H.; Shen, L.; Rao, L.; Tang, J.; Hu, S.; Lin, H. Inkjet printing assisted fabrication of polyphenol-based coating membranes for oil/water separation. Chemosphere 2020, 250, 126236. [Google Scholar] [CrossRef]
- Alresheedi, M.T.; Barbeau, B.; Basu, O.D. Comparisons of NOM fouling and cleaning of ceramic and polymeric membranes during water treatment. Sep. Purif. Technol. 2019, 209, 452–460. [Google Scholar] [CrossRef]
- Lee, S.J.; Dilaver, M.; Park, P.K.; Kim, J.H. Comparative analysis of fouling characteristics of ceramic and polymeric microfiltration membranes using filtration models. J. Memb. Sci. 2013, 432, 97–105. [Google Scholar] [CrossRef]
- Karasu, K.; Glennon, N.; Lawrence, N.D.; Stevens, G.W.; O’connor, A.J.; Barber, A.R.; Yoshikawa, S.; Kentish, S.E. A comparison between ceramic and polymeric membrane systems for casein concentrate manufacture. Int. J. Dairy Technol. 2010, 63, 284–289. [Google Scholar] [CrossRef]
- Dudziak, M.; Wyczarska-Kokot, J.; Łaskawiec, E.; Stolarczyk, A. Application of ultrafiltration in a swimming pool water treatment system. Membranes 2019, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-Razak, N.H.; Chew, Y.M.J.; Bird, M.R. Membrane fouling during the fractionation of phytosterols isolated from orange juice. Food Bioprod. Process. 2019, 113, 10–21. [Google Scholar] [CrossRef]
- Grangeon, A.; Lescoche, P. Flat ceramic membranes for the treatment of dairy products: Comparison with tubular ceramic membranes. Le Lait. 2000, 80, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, A.; Lehmann, S.; Haaksman, V.; Ogier, J.; Schellenberg, C.; van Loosdrecht, M.C.M.; Kruithof, J.C.; Vrouwenvelder, J.S. Porosity of spacer-filled channels in spiral-wound membrane systems: Quantification methods and impact on hydraulic characterization. Water Res. 2017, 119, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Chevereau, E.; Zouaoui, N.; Limousy, L.; Dutournié, P.; Déon, S.; Bourseau, P. Surface properties of ceramic ultrafiltration TiO2 membranes: Effects of surface equilibriums on salt retention. Desalination 2010, 255, 1–8. [Google Scholar] [CrossRef]
- Schmidt, C.M.; Sprunk, M.; Löffler, R.; Hinrichs, J. Relating nanofiltration membrane morphology to observed rejection of saccharides. Sep. Purif. Technol. 2020, 239, 116550. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Bostyn, S.; Flores-Montaño, J.L.; Camacho-Ruiz, R.M. Size-exclusion chromatography (HPLC-SEC) technique optimization by simplex method to estimate molecular weight distribution of agave fructans. Food Chem. 2017, 237, 833–840. [Google Scholar] [CrossRef]
- Ávila-Fernández, Á.; Rendón-Poujol, X.; Olvera, C.; González, F.; Capella, S.; Peña-Alvarez, A.; López-Munguía, A. Enzymatic hydrolysis of fructans in the tequila production process. J. Agric. Food Chem. 2009, 57, 5578–5585. [Google Scholar] [CrossRef]
- Ávila-Fernández, Á.; Galicia-Lagunas, N.; Rodríguez-Alegría, M.E.; Olvera, C.; López-Munguía, A. Production of functional oligosaccharides through limited acid hydrolysis of agave fructans. Food Chem. 2011, 129, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.J.; Bird, M.R. Solute-membrane fouling interactions during the ultrafiltration of black tea liquor. Food Bioprod. Process. 2006, 84, 292–301. [Google Scholar] [CrossRef] [Green Version]
- Macedo, A.; Duarte, E.; Fragoso, R. Assessment of the performance of three ultrafiltration membranes for fractionation of ovine second cheese whey. Int. Dairy J. 2015, 48, 31–37. [Google Scholar] [CrossRef]
- Nair, R.R.; Protasova, E.; Strand, S.; Bilstad, T. Implementation of spiegler–Kedem and steric hindrance pore models for analyzing nanofiltration membrane performance for smart water production. Membranes 2018, 8, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acero, J.L.; Benitez, F.J.; Real, F.J.; García, C. Removal of phenyl-urea herbicides in natural waters by UF membranes: Permeate flux, analysis of resistances and rejection coefficients. Sep. Purif. Technol. 2009, 65, 322–330. [Google Scholar] [CrossRef]
- Kuhn, R.C.; Maugeri Filho, F.; Silva, V.; Palacio, L.; Hernández, A.; Prádanos, P. Mass transfer and transport during purification of fructooligosaccharides by nanofiltration. J. Memb. Sci. 2010, 365, 356–365. [Google Scholar] [CrossRef]
- González-Muñoz, M.J.; Parajó, J.C. Diafiltration of Eucalyptus wood autohydrolysis liquors: Mathematical modeling. J. Memb. Sci. 2010, 346, 98–104. [Google Scholar] [CrossRef]
- Muthukumaran, S.; Nguyen, D.A.; Baskaran, K. Performance evaluation of different ultrafiltration membranes for the reclamation and reuse of secondary effluent. Desalination 2011, 279, 383–389. [Google Scholar] [CrossRef]
- Arrizon, J.; Morel, S.; Gschaedler, A.; Monsan, P. Comparison of the water-soluble carbohydrate composition and fructan structures of Agave tequilana plants of different ages. Food Chem. 2010, 122, 123–130. [Google Scholar] [CrossRef]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook; Technomic Publishing Company, Inc.: Lancaster, PA, USA, 1998. [Google Scholar]
- Vegas, R.; Moure, A.; Domínguez, H.; Parajó, J.C.; Alvarez, J.R.; Luque, S. Evaluation of ultra- and nanofiltration for refining soluble products from rice husk xylan. Bioresour. Technol. 2008, 99, 5341–5351. [Google Scholar] [CrossRef]
- Cassano, A.; Marchio, M.; Drioli, E. Clarification of blood orange juice by ultrafiltration: Analyses of operating parameters, membrane fouling and juice quality. Desalination 2007, 212, 15–27. [Google Scholar] [CrossRef]

| Specification | Ceramic Membrane | Polymeric Membrane |
|---|---|---|
| Manufacturer | Tami industries | Hydranautics Nitto Group Company |
| Material | Zirconium/titanium dioxide | Sulfonated polyethersulfone |
| Configuration | Tubular | Spiral |
| Molecular weight cut-off | 1 kDa | 1 kDa |
| Membrane area | 0.5 m2 | 7.4 m2 |
| Operating pressure | <100 × 105 Pa | <41 × 105 Pa |
| Operating pH | 0–14 | 2–11 |
| Operating temperature | <350 °C | <50 °C |
| Spacer height * | NA | 8.63 × 10−4 m * |
| Spacer porosity * | NA | 0.89 * |
| Mean pore radio (nm) | 3 ** | 2.12 *** |
| DP | Name | Formula | MW kg·kmol−1 | % |
|---|---|---|---|---|
| 1 | Glucose and Fructose | C6H12O6 | 180 | 8.60 |
| 2 | Sucrose | C12H22O11 | 342 | 2.73 |
| 3 | Fructose (Fructose)1 Glucose | C18H32O16 | 504 | 2.30 |
| 4 | Fructose (Fructose)2 Glucose | C24H42O21 | 666 | 2.43 |
| 5 | Fructose (Fructose)3 Glucose | C30H52O26 | 828 | 2.66 |
| 6 | Fructose (Fructose)4 Glucose | C36H62O31 | 990 | 2.65 |
| 7 | Fructose (Fructose)5 Glucose | C42H72O36 | 1152 | 4.31 |
| 8 | Fructose (Fructose)6 Glucose | C48H82O41 | 1314 | 3.03 |
| 9 | Fructose (Fructose)7 Glucose | C54H92O46 | 1476 | 3.09 |
| 10 | Fructose (Fructose)8 Glucose | C60H102O51 | 1638 | 3.29 |
| 11 | Fructose (Fructose)9 Glucose | C66H112O56 | 1800 | 3.30 |
| 12 | Fructose (Fructose)10 Glucose | C72H122O61 | 1962 | 3.54 |
| 13 | Fructose (Fructose)11 Glucose | C78H132O66 | 2124 | 3.58 |
| 14 | Fructose (Fructose)12 Glucose | C84H142O71 | 2286 | 3.75 |
| 15 | Fructose (Fructose)13 Glucose | C90H152O76 | 2448 | 3.88 |
| 16 | Fructose (Fructose)14 Glucose | C96H162O81 | 2610 | 3.95 |
| 17 | Fructose (Fructose)15 Glucose | C102H172O86 | 2772 | 3.96 |
| 18 | Fructose (Fructose)16 Glucose | C108H182O91 | 2934 | 3.88 |
| 19 | Fructose (Fructose)17 Glucose | C114H192O96 | 3096 | 3.71 |
| 20 | Fructose (Fructose)18 Glucose | C120H202O101 | 3258 | 3.74 |
| 21 | Fructose (Fructose)19 Glucose | C126H212O106 | 3420 | 3.41 |
| 22 | Fructose (Fructose)20 Glucose | C132H222O111 | 3582 | 3.29 |
| 23 | Fructose (Fructose)21 Glucose | C138H232O116 | 3744 | 2.85 |
| 24 | Fructose (Fructose)22 Glucose | C144H242O121 | 3906 | 2.42 |
| 25 | Fructose (Fructose)23 Glucose | C150H252O126 | 4068 | 2.43 |
| 26 | Fructose (Fructose)24 Glucose | C156H262O131 | 4230 | 1.80 |
| 27 | Fructose (Fructose)25 Glucose | C162H272O136 | 4392 | 1.80 |
| 28 | Fructose (Fructose)26 Glucose | C168H282O141 | 4554 | 1.44 |
| 29 | Fructose (Fructose)27 Glucose | C174H292O146 | 4716 | 1.28 |
| 30 | Fructose (Fructose)28 Glucose | C180H302O151 | 4878 | 1.01 |
| 31 | Fructose (Fructose)29 Glucose | C186H312O156 | 5040 | 1.01 |
| 32 | Fructose (Fructose)30 Glucose | C192H322O161 | 5202 | 0.79 |
| 33 | Fructose (Fructose)31 Glucose | C198H332O166 | 5364 | 0.71 |
| 34 | Fructose (Fructose)32 Glucose | C204H342O171 | 5526 | 0.56 |
| 35 | Fructose (Fructose)33 Glucose | C210H352O176 | 5688 | 0.58 |
| 36 | Fructose (Fructose)34 Glucose | C216H362O181 | 5850 | 0.46 |
| 37 | Fructose (Fructose)35 Glucose | C222H372O186 | 6012 | 0.41 |
| 38 | Fructose (Fructose)36 Glucose | C228H382O191 | 6174 | 0.32 |
| 39 | Fructose (Fructose)37 Glucose | C234H392O196 | 6336 | 0.32 |
| 40 | Fructose (Fructose)38 Glucose | C240H402O201 | 6498 | 0.27 |
| 41 | Fructose (Fructose)39 Glucose | C246H412O206 | 6660 | 0.18 |
| 42 | Fructose (Fructose)40 Glucose | C252H422O211 | 6822 | 0.29 |
| Total | 100 | |||
| Specification | Ceramic Membrane | Polymeric Membrane |
|---|---|---|
| Operating pressure (Pa) | 3 × 105 | 3 × 105 |
| Tangential velocity (m·s−1) | 3 | 0.16 |
| Reynolds number * | 12,460 | 398.74 |
| Operating temperature (K) | 318 | 318 |
| Feed Concentration (kg·m−3) | 100 | 100 |
| Hydraulic diameter (m) | 0.0025 | 0.0015 |
| Ceramic Membrane System | Polymeric Membrane System | |||||
|---|---|---|---|---|---|---|
| Fc | FOS | MD | Fc | FOS | MD | |
| Feed solution (kg) | 6.490 | 2.377 | 1.133 | 6.490 | 2.377 | 1.133 |
| Final yield of the permeate (%) | 11.05 ± 3.69a | 40.06 ± 0.70a | 52.08 ± 1.76a | 0.00b | 0.98 ± 0.88b | 33.22 ± 0.79b |
| Purity of the permeate (%) | 37.05 ± 2.73a | 42.16 ± 2.09a | 20.78 ± 0.63a | 0.00b | 5.87 ± 2.23b | 94.12 ± 2.23b |
| Final yield of the retentate (%) | 70.91 ± 3.11a | 48.66 ± 9.45a | 43.72 ± 9.43a | 89.07 ± 8.00b | 45.30 ± 2.97a | 13.90 ± 0.09b |
| Purity of the retentate (%) | 78.23 ± 0.73a | 16.24 ± 0.62a | 5.52 ± 0.10a | 82.38 ± 0.48b | 15.36 ± 0.28a | 2.25 ± 0.20b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luiz-Santos, N.; Prado-Ramírez, R.; Arriola-Guevara, E.; Camacho-Ruiz, R.-M.; Moreno-Vilet, L. Performance Evaluation of Tight Ultrafiltration Membrane Systems at Pilot Scale for Agave Fructans Fractionation and Purification. Membranes 2020, 10, 261. https://doi.org/10.3390/membranes10100261
Luiz-Santos N, Prado-Ramírez R, Arriola-Guevara E, Camacho-Ruiz R-M, Moreno-Vilet L. Performance Evaluation of Tight Ultrafiltration Membrane Systems at Pilot Scale for Agave Fructans Fractionation and Purification. Membranes. 2020; 10(10):261. https://doi.org/10.3390/membranes10100261
Chicago/Turabian StyleLuiz-Santos, Noe, Rogelio Prado-Ramírez, Enrique Arriola-Guevara, Rosa-María Camacho-Ruiz, and Lorena Moreno-Vilet. 2020. "Performance Evaluation of Tight Ultrafiltration Membrane Systems at Pilot Scale for Agave Fructans Fractionation and Purification" Membranes 10, no. 10: 261. https://doi.org/10.3390/membranes10100261
APA StyleLuiz-Santos, N., Prado-Ramírez, R., Arriola-Guevara, E., Camacho-Ruiz, R.-M., & Moreno-Vilet, L. (2020). Performance Evaluation of Tight Ultrafiltration Membrane Systems at Pilot Scale for Agave Fructans Fractionation and Purification. Membranes, 10(10), 261. https://doi.org/10.3390/membranes10100261







