Bringing Preventive RSV Monoclonal Antibodies to Infants in Low- and Middle-Income Countries: Challenges and Opportunities
Abstract
:1. Introduction
2. RSV’s Burden of Disease
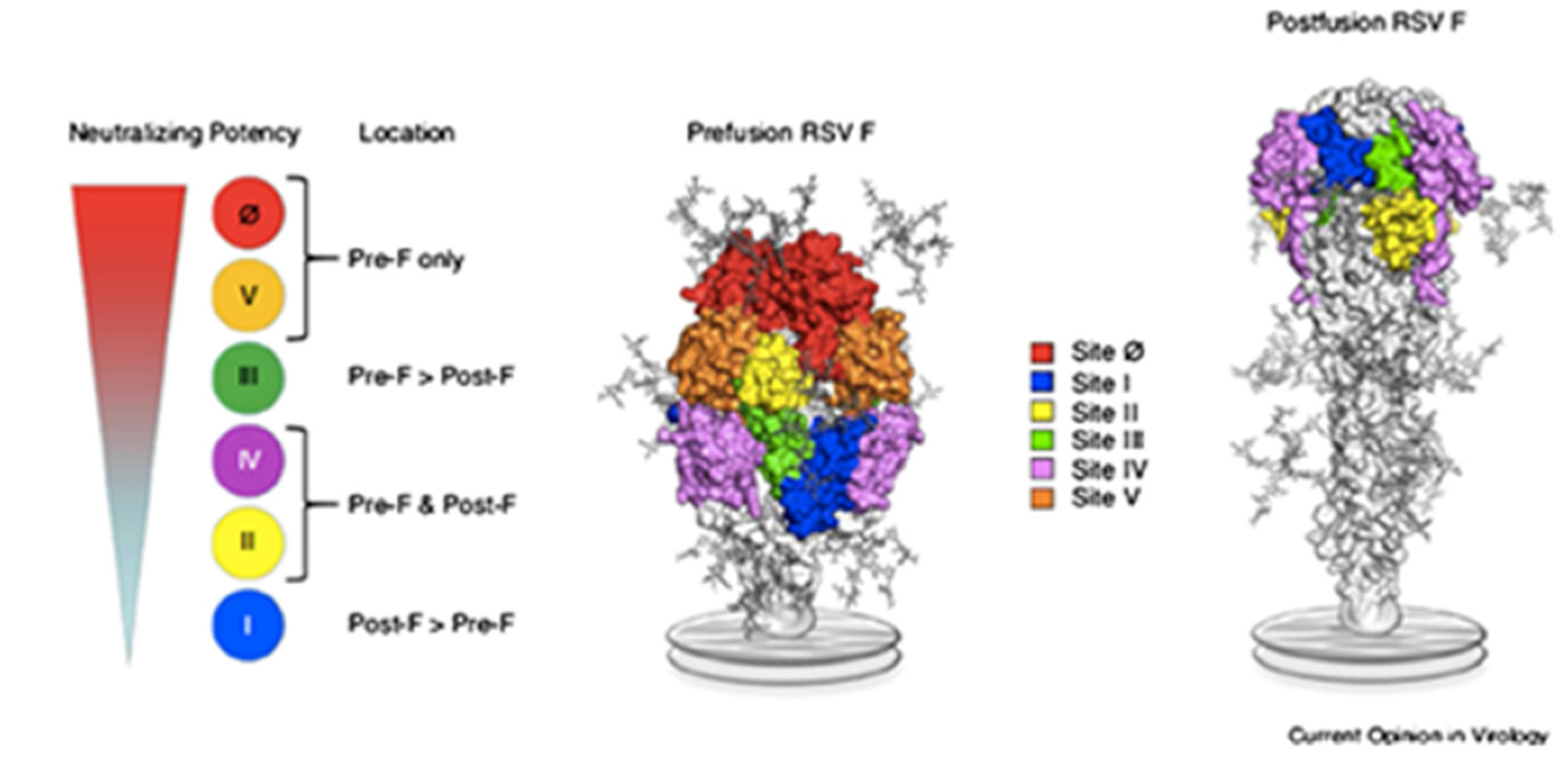
3. RSV’s Molecular Characteristics
4. Natural Immunity to RSV
5. RSV Prevention Strategies
6. RSV Monoclonal Antibody Studies
7. Challenges and Opportunities in Bringing RSV Monoclonal Antibody to LMICs
7.1. RSV Seasonality and Timing of RSV mAb Dosing
7.2. Escape Mutants
7.3. Cost of Goods (COGs) and Scalability for LMICs
7.4. Partnership with National and International Key Stakeholders
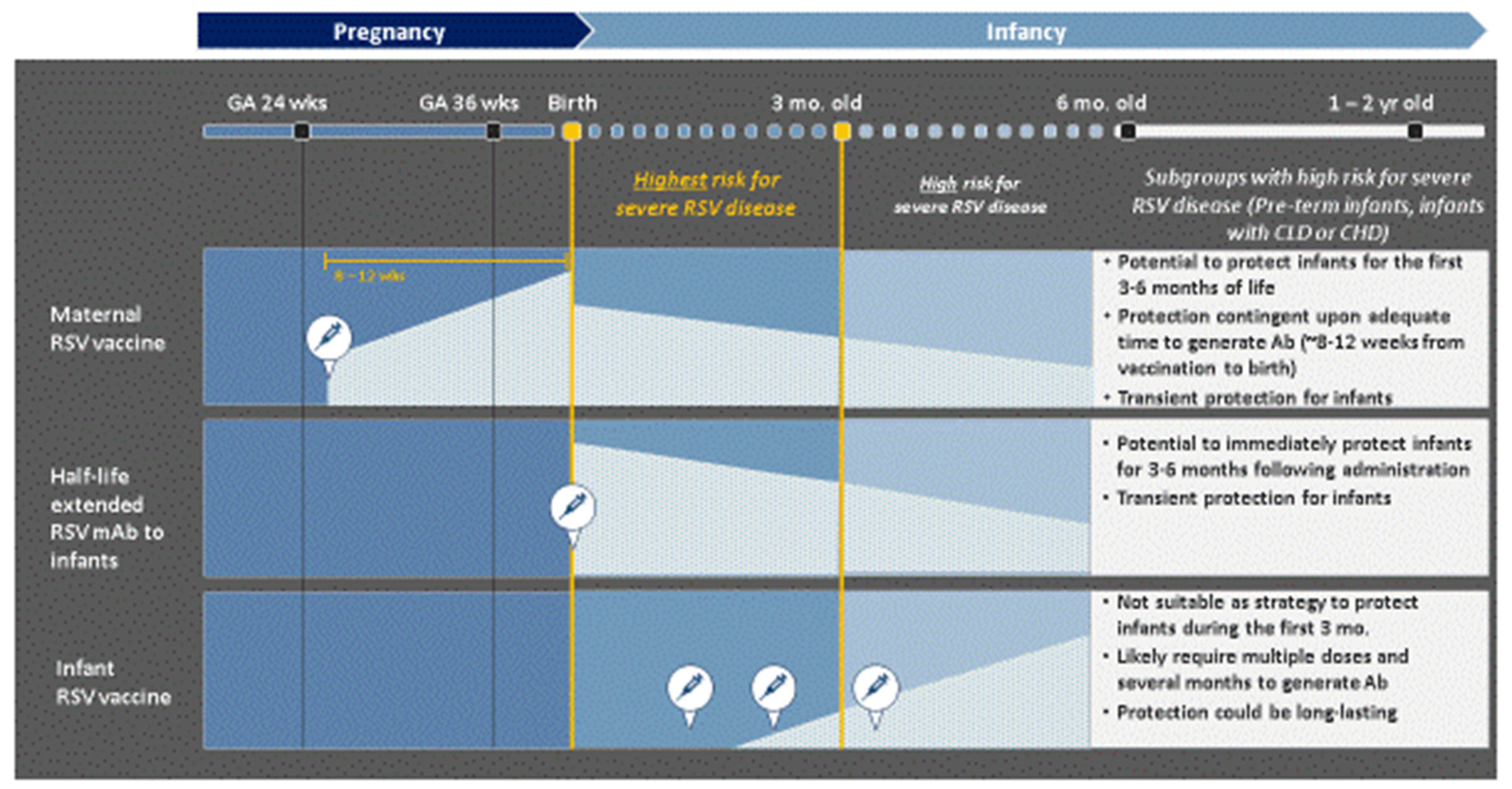
7.5. Complimentary Roles of RSV mAb and Maternal RSV Vaccines in Preventing Severe RSV LRTI in Infants
7.6. Lessons Learned from COVID-19
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simões, E.A.; Madhi, S.A.; Gessner, B.D.; Polack, F.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [Green Version]
- Ohuma, E.O.; Okiro, E.A.; Ochola, R.; Sande, C.J.; Cane, P.A.; Medley, G.F.; Bottomley, C.; Nokes, D.J. The natural history of respiratory syncytial virus in a birth cohort: The influence of age and previous infection on reinfection and disease. Am. J. Epidemiol. 2012, 176, 794–802. [Google Scholar] [CrossRef]
- Andeweg, S.P.; Schepp, R.M.; van de Kassteele, J.; Mollema, L.; Berbers, G.A.M.; van Boven, M. Population-based serology reveals risk factors for RSV infection in children younger than 5 years. Sci. Rep. 2021, 11, 8953. [Google Scholar] [CrossRef]
- Buchwald, A.G.; Graham, B.S.; Traore, A.; Haidara, F.C.; Chen, M.; Morbito, K.; Lin, B.C.; Sow, S.O.; Levine, M.M.; Pasetti, M.F.; et al. Respiratory Syncytial Virus (RSV) Neutralizing Antibodies at Birth Predict Protection from RSV Illness in Infants in the First 3 Months of Life. Clin. Infect. Dis. 2020, ciaa648. [Google Scholar] [CrossRef]
- Carbonell-Estrany, X.; Fullarton, J.R.; Gooch, K.L.; Gouyon, J.B.; Lanari, M.; Rodgers-Gray, B.S.; Thwaites, R.J.; Vo, P.G.; Liese, J.G. The influence of birth weight amongst 33–35 weeks gestational age (wGA) infants on the risk of respiratory syncytial virus (RSV) hospitalization: A pooled analysis. J. Matern. Fetal. Neonatal. Med. 2017, 30, 134–140. [Google Scholar] [CrossRef]
- Domachowske, J.; Khan, A.A.; Esser, M.; Jensen, K.; Takas, T.; Villafana, T.; Dubovsky, F.; Griffin, M.P. Safety, tolerability and pharmacokinetics of MEDI8897, an extended half-life single dose respiratory syncytial virus prefusion F-targeting monoclonal antibody administered as a single dose to healthy preterm infants. Pediatr. Infect. Dis. J. 2018, 37, 886–892. [Google Scholar] [CrossRef]
- Obando-Pacheco, P.; Justicia-Grande, A.; Rivero-Calle, I.; Rodríguez-Tenreiro, C.; Sly, P.; Ramilo, O.; Mejías, A.; Baraldi, E.; Papadopoulos, N.G.; Nair, H.; et al. Respiratory syncytial virus seasonality: A global overview. Clin. Inf. Dis. 2018, 217, 1356–1364. [Google Scholar] [CrossRef]
- Scheltema, N.; Gentile, A.; Lucion, F.; Nokes, D.J.; Munywoki, P.K.; Madhi, S.A.; Groome, M.J.; Cohen, C.; Moyes, J.; Thorburn, K.; et al. Global respiratory syncytial virus-associated mortality in young children (RSV GOLD): A retrospective case series. Lancet Glob. Health 2017, 5, e984–e991. [Google Scholar] [CrossRef] [Green Version]
- WHO Department of Immunization, Vaccines and Biologicals’ Technical Advisory Group. Preferred Product Characteristics of Monoclonal Antibodies for Passive Immunization Against Respiratory Syncytial Virus (RSV). WHO/Draft-V0.1/Mar2020. Available online: https://www.who.int/immunization/research/ppc-tpp/PPC_RSV-MAbs_Draft_V-0.1-for-consultation.pdf?ua=1 (accessed on 4 June 2021).
- Srikantiah, P.; Senior Program Officer, Global Health (Bill & Melinda Gates Foundation, Seattle, WA, USA). Personal communication, 2021.
- Taleb, S.; Al Thani, A.A.; Al Ansari, K.; Yassine, H.M. Human respiratory syncytial virus: Pathogenesis, immune responses, and current vaccine approaches. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1817–1827. [Google Scholar] [CrossRef]
- Ruckwardt, T.J.; Morabito, K.; Graham, B.S. Immunological lessons from respiratory syncytial virus vaccine development. Immunity 2019, 51, 429–442. [Google Scholar] [CrossRef]
- McLellan, J.S.; Chen, M.; Leung, S.; Graepel, K.W.; Du, X.; Yang, Y.; Zhou, T.; Baxa, U.; Yasuda, E.; Beaumont, T.; et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science 2013, 340, 1113–1117. [Google Scholar] [CrossRef] [Green Version]
- Stensballe, L.G.; Ravn, H.; Kristensen, K.; Meakins, T.; Aaby, P.; Simoes, E.A. Seasonal variation of maternally derived respiratory syncytial virus antibodies and association with infant hospitalizations for respiratory syncytial virus. J. Pediatrics 2009, 154, 296–298. [Google Scholar] [CrossRef]
- Mazur, N.; Horsley, N.; Englund, J.; Nederend, M.; Magaret, A.; Kumar, A.; Jacobino, S.R.; de Haan, C.A.M.; Khatry, S.K.; LeClerq, S.C.; et al. Breast milk prefusion F immunoglobulin G as a correlate of protection against respiratory syncytial virus acute respiratory illness. J. Infect. Dis. 2019, 219, 59–67. [Google Scholar] [CrossRef]
- Schmidt, M.; Varga, S. The CD8 T cell response to respiratory virus infections. Front. Immunol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Openshaw, P.; Chiu, C.; Culley, F.J.; Johansson, C. Protective and harmful immunity to RSV infection. Annu. Rev. Immunol. 2017, 35, 501–532. [Google Scholar] [CrossRef]
- Higgins, D.; Trujillo, C.; Keech, C. Advances in RSV vaccine research and development—A global agenda. Vaccine 2016, 34, 2870–2875. [Google Scholar] [CrossRef]
- PATH. RSV Vaccine and mAb Snapshot. Available online: https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/ (accessed on 4 June 2021).
- Clinicaltrials.gov No. NCT04605159. A Phase 3, Randomized, Double-Blind, Placebo-Controlled Multi-Country Study to Demonstrate Efficacy of a Single Dose of Unadjuvanted RSV Maternal Vaccine, Administered IM To Pregnant Women 18 to 49 Years of Age, for Prevention of RSV-Associated Lrtis in Their Infants up to 6 Months of Age. 7 May 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04605159 (accessed on 4 June 2021).
- Clinicaltrials.gov No. Nct04424316. A Phase 3, Randomized, Double-Blinded, Placebo-Controlled Trial to Evaluate the Efficacy and Safety of a Respiratory Syncytial Virus (Rsv) Prefusion F Subunit Vaccine in Infants Born to Women Vaccinated During Pregnancy. 27 May 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04424316 (accessed on 4 June 2021).
- Initiative for Vaccine Research (IVR) of the Department of Immunization, Vaccines and Biologicals. World Health Organization (WHO). WHO Preferred Product Characteristics for Respiratory Syncytial Virus (RSV) Vaccines. Geneva: WHO, 2021. License: CC BY-NC-SA 3.0 IGO. Available online: https://www.who.int/publications/i/item/9789240021853 (accessed on 16 August 2021).
- Modjarrad, K.; Giersing, B.; Kaslow, D.C.; Smith, P.G.; Moorthy, V.S. WHO RSV Vaccine Consultation Expert Group. WHO consultation on respiratory syncytial virus vaccine development report from a World Health Organization meeting held on 23–24 March 2015. Vaccine 2016, 34, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Madhi, S.A.; Polack, F.P.; Piedra, P.A.; Munoz, F.M.; Trenholme, A.A.; Simões, E.A.; Swamy, G.K.; Agrawal, S.; Ahmed, K.; August, A.; et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N. Engl. J. Med. 2020, 383, 426–439. [Google Scholar] [CrossRef]
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998, 102, 531–537. [Google Scholar] [CrossRef]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Kim, H.; Leikin, S.; Arrobio, J.; Brandt, C.; Chanock, R.M.; Parrott, R.H. Cell-mediated immunity to respiratory syncytial virus induced by inactivated vaccine or by infection. Pediatr. Res. 1976, 10, 75–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, M.F.; Polack, F.P. Involvement of antibody, complement and cellular immunity in the pathogenesis of enhanced respiratory syncytial virus disease. Expert Rev. Vaccines 2004, 3, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.; Pletneva, L.; Otoa, R.; Patel, M.C.; Vogel, S.N.; Boukhvalova, M.S. Preclinical assessment of safety of maternal vaccination against respiratory syncytial virus (RSV) in cotton rats. Vaccine 2017, 35, 3951–3958. [Google Scholar] [CrossRef]
- Feltes, T.; Sondheimer, H.; Tulloh, R.; Harris, B.; Jensen, K.M.; Losonsky, G.A.; Griffin, M.P. A randomized controlled trial of motavizumab versus palivizumab for the prophylaxis of serious respiratory syncytial virus disease in children with hemodynamically significant congenital heart disease. Pediatric Res. 2011, 70, 186–191. [Google Scholar] [CrossRef]
- O’Brien, K.L.; Chandran, A.; Weatherholtz, R.; Jafri, H.S.; Griffin, M.P.; Bellamy, T.; Millar, E.V.; Jensen, K.M.; Harris, B.S.; Reid, R.; et al. Respiratory syncytial virus (RSV) Prevention study group. Efficacy of motavizumab for the prevention of respiratory syncytial virus disease in healthy Native American infants: A phase 3 randomized double-blind placebo-controlled trial. Lancet Infect. Dis. 2015, 15, 1398–1408. [Google Scholar] [CrossRef]
- Simões, E.; Forleo-Neto, E.; Geba, G.; Kamal, M.; Yang, F.; Cicirello, H.; Houghton, M.R.; Rideman, R.; Zhao, Q.; Benvin, S.L.; et al. Suptavumab for the prevention of medically-attended respiratory syncytial virus infection in preterm infants. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov No. NCT02325791. Phase 3, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Efficacy and Safety of a Human Monoclonal Antibody, Suptavumab (REGN2222), for the Prevention of Medically Attended RSV Infection in Preterm Infants. 6 November 2018. Available online: https://clinicaltrials.gov/ct2/show/study/NCT02325791 (accessed on 4 June 2021).
- Griffin, P.; Yuan, Y.; Takas, T.; Domachowske, J.; Madhi, S.A.; Manzoni, P.; Simões, E.A.; Esser, M.T.; Khan, A.A.; Dubovsky, F.; et al. Single-dose nirsevimab for prevention of RSV in preterm infants. NEJM 2020, 383, 415–425. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov No. NCT02878330. Phase 2b Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of MEDI8897, a Monoclonal Antibody with an Extended Half-Life Against Respiratory Syncytial Virus, in Healthy Preterm Infants. Available online: https://clinicaltrials.gov/ct2/show/NCT02878330 (accessed on 4 June 2021).
- Clinicaltrials.gov No. NCT03959488. Phase 2/3 Randomized, Double-Blind, Palivizumab-Controlled Study to Evaluate the Safety of MEDI8897, a Monoclonal Antibody with an Extended Half-Life Against Respiratory Syncytial Virus, in High-Risk Children (MEDLEY). 12 May 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03959488 (accessed on 13 August 2021).
- Clinicaltrials.gov No. NCT03979313. Phase 3 Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of MEDI8897, A Monoclonal Antibody with an Extended Half-Life Against Respiratory Syncytial Virus, in Healthy Late Preterm and Term Infants (MELODY). 20 May 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03979313. (accessed on 4 June 2021).
- AstraZeneca. First Potential Passive Immunization to Show Protection Against RSV in the General Infant Population. Press Release 26 April 2021 0:700 BST. Available online: https://www.astrazeneca.com/media-centre/press-releases/2021/nirsevimab-phase-iii-trial-met-primary-endpoint.html (accessed on 5 June 2021).
- Clinicaltrials.gov No. NCT03524118. A Double-Blind, Randomized, Placebo-Controlled, Single Ascending Dose Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of MK-1654 in Preterm and Tull-term Infants. 25 March 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03524118 (accessed on 4 June 2021).
- Clinicaltrials.gov No. NCT04086472. Phase 2a Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Efficacy and Safety of MK-1654 in Healthy Participants Inoculated with Experimental Respiratory Syncytial Virus.11 May 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04086472 (accessed on 4 June 2021).
- Clinicaltrials.gov No. NCT04767373. Phase 2b/3 Double-Blind, Randomized, Placebo-Controlled Study to Evaluate The Efficacy and Safety of MK-1654 in Healthy Pre-Term And Full-Term Infants. 2 June 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04767373 (accessed on 4 June 2021).
- American Academy of Pediatrics Committee on Infectious Diseases; American Academy of Pediatrics Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014, 134, 415–420. [Google Scholar] [CrossRef] [Green Version]
- Fergie, J.; Goldstein, M.; Krilov, L.R.; Wade, S.W.; Kong, A.M.; Brannman, L. Update on respiratory syncytial virus hospitalizations among U.S. preterm and term infants before and after the 2014 American Academy of Pediatrics policy on immunoprophylaxis: 2011–2017. Hum. Vaccin Immunother. 2021, 17, 1536–1545. [Google Scholar] [CrossRef]
- Papenburg, J.; Defoy, I.; Massé, E.; Caouette, G.; Lebel, M.H. Impact of the withdrawal of palivizumab immunoprophylaxis on the incidence of respiratory syncytial virus (RSV) hospitalizations among infants born at 33 to 35 weeks’ gestational age in the province of Quebec, Canada: The RSV-Quebec study. J. Pediatric Infect. Dis. Soc. 2021, 10, 237–244. [Google Scholar] [CrossRef]
- American Academy of Pediatrics (AAP). Interim Guidance for Use of Palivizumab Prophylaxis to Prevent Hospitalization From Severe Respiratory Syncytial Virus Infection During the Current Atypical Interseasonal RSV Spread. American Academy of Pediatrics. 10 August 2021. Available online: https://www.aap.org/en/pages/2019-novel-coronavirus-COVID-19-infections/clinical-guidance/interim-guidance-for-use-of-palivizumab-prophylaxis-to-prevent-hospitalization/ (accessed on 16 August 2021).
- Carbonell-Estrany, X.; Simões, E.A.; Dagan, R.; Hall, C.B.; Harris, B.; Hultquist, M.; Connor, E.M.; Losonsky, G.A.; Motavizumab Study Group. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: A noninferiority trial. Pediatrics 2010, 125, e35–e51. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; McLellan, J.S.; Kallewaard, N.L.; Ulbrandt, N.D.; Palaszynski, S.; Zhang, J.; Moldt, B.; Khan, A.; Svabek, C.; McAuliffe, J.M.; et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci. Transl. Med. 2017, 9, eaaj1928. [Google Scholar] [CrossRef]
- Tang, A.; Chen, Z.; Cox, K.S.; Su, H.P.; Callahan, C.; Fridman, A.; Zhang, L.; Patel, S.B.; Cejas, P.J.; Swoye, R. A potent broadly neutralizing human RSV antibody targets conserved site IV of the fusion glycoprotein. Nat. Commun. 2019, 10, 4153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.H.; Kang, T.H.; Godon, O.; Watanabe, M.; Delidakis, G.; Gillis, C.M.; Sterlin, D.; Hardy, D.; Cogné, M.; Macdonald, L.E.; et al. An engineered human Fc domain that behaves like a pH-toggle switch for ultra-long circulation persistence. Nat. Commun. 2019, 10, 5031. [Google Scholar] [CrossRef] [PubMed]
- Pierre, V.; Griffin, P.M.; Esser, M.T.; Yuan, Y.; Takas, T.; Esser, M.T.; Villafana, T.; Roskos, L.; Narwal, R.; Khan, A.A.; et al. Population pharmacokinetics and exposure-response analysis of Nirsevimab in healthy preterm infants. In Proceedings of the American Conference on Pharmacometrics, CoP11 Virtual Conference, 9–13 November 2020. Poster 1085-P. [Google Scholar]
- Griffin, M.P.; Khan, A.A.; Esser, M.; Jensen, K.; Takas, T.; Kankam, M.K.; Villafana, T.; Dubovsky, F. Safety, tolerability, and pharmacokinetics of MEDI8897, the respiratory syncytial virus prefusion F-targeting monoclonal antibody with an extended half-life, in healthy adults. Antimicrob. Agents Chemother. 2017, 61, e017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliprantis, A.O.; Wolford, D.; Caro, L.; Maas, B.M.; Ma, H.; Montgomery, D.L.; Sterling, L.M.; Hunt, A.; Cox, K.S.; Vora, K.A.; et al. A phase 1 randomized, double-blind, placebo-controlled trial to assess the safety, tolerability, and pharmacokinetics of a respiratory syncytial virus neutralizing monoclonal antibody MK-1654 in healthy adults. Clin. Pharm. Drug Dev. 2021, 10, 556–566. [Google Scholar] [CrossRef]
- Li, Y.; Hodgson, D.; Wang, X.; Atkins, K.; Feikin, D.R.; Nair, H. Respiratory syncytial virus seasonality and prevention strategy planning for passive immunization of infants in low-income and middle-income countries: A modelling study. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Zhu, Q.; Lu, B.; McTamney, P.; Palaszynski, S.; Diallo, S.; Ren, K.; Ulbrandt, N.D.; Kallewaard, N.; Wang, W.; Fernandes, F.; et al. Prevalence and Significance of Substitutions in the Fusion Protein of Respiratory Syncytial Virus Resulting in Neutralization Escape from Antibody MEDI8897. J. Infect. Dis. 2018, 218, 572–580. [Google Scholar] [CrossRef]
- Tabor, D.E.; Fernandes, F.; Langedijk, A.C.; Wilkins, D.; Lebbink, R.J.; Tovchigrechko, A.; Ruzin, A.; Kragten-Tabatabaie, L.; Jin, H.; Esser, M.T.; et al. Global molecular epidemiology of respiratory syncytial virus from the 2017−2018 INFORM-RSV Study. J. Clin. Microbiol. 2021, 59. [Google Scholar] [CrossRef]
- Pollack, J.; Coffman, J.; Ho, S.V.; Farid, S. Integrated continuous bioprocessing: Economic, operational, and environmental feasibility for clinical and commercial antibody manufacture. Biotechnol. Prog. 2017, 33, 854–866. [Google Scholar] [CrossRef]
- Li, Y.; Johnson, E.K.; Shi, T.; Campbell, H.; Chaves, S.S.; Commaille-Chapus, C.; Dighero, I.; James, S.L.; Mahé, C.; Ooi, Y.; et al. National burden estimates of hospitalisations for acute lower respiratory infections due to respiratory syncytial virus in young children in 2019 among 58 countries: A modelling study. Lancet Respir. Med. 2021, 2, 175–185. [Google Scholar] [CrossRef]
- Rid, A.; Saxena, A.; Baqui, A.H.; Bhan, A.; Bines, J.; Bouesseau, M.-C.; Caplan, A.; Colgrove, J.; Dhai, A.; Gomez-Diaz, R.; et al. Placebo use in vaccine trials: Recommendations of a WHO expert panel. Vaccine 2014, 32, 4708–4712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baral, R.; Higgins, D.; Regan, K.; Pecenka, C. Impact and cost-effectiveness of potential interventions against infant respiratory syncytial virus (RSV) in 131 low-income and middle-income countries using a static cohort model. BMJ 2021, 11, e046563. [Google Scholar] [CrossRef]
- Clinicaltrials.gov No. NCT04504032. A Randomized, Controlled, Phase 2b Study to Evaluate Safety and Efficacy of Rivaroxaban (Xarelto®) for High Risk People with Mild COVID-19. 19 April 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04504032 (accessed on 4 June 2021).
| mAb | Stage | Populations and Outcomes |
|---|---|---|
| Palivizumab Commercial product | FDA- and EMA-approved in 1998 (n = 1502) [25] |
|
| Motavizumab Manufacturer voluntarily withdrew product from FDA submission (discontinued in 2010) | Cardiac study (n = 1248; 2005 to 2008) [30] |
|
| Phase 3 (n = 2127; 2004 to 2007) [31] |
| |
| Suptavumab (REGN2222) | Phase 3 (n = 1149; 2015 to 2017) [32,33] |
|
| Nirsevimab (MEDI8897) | Phase 2b (n = 1453; 2016 to 2018) [34,35] |
FDA granted breakthrough status; EMA granted Prime status
|
| Phase 2b/3 (n = 925; 2019 to 2022) [36] |
| |
| Phase 3 (n = 3000; 2019 to 2023) [37,38] |
| |
| MK-1654 | Phase 2a (n = 180; 2018 to 2022) [39] |
|
| Phase 2a (n = 80; 2019 to 2020) [40] |
| |
| Phase 2b/3 (n = 3300; 2021 to 2025) [41] |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ananworanich, J.; Heaton, P.M. Bringing Preventive RSV Monoclonal Antibodies to Infants in Low- and Middle-Income Countries: Challenges and Opportunities. Vaccines 2021, 9, 961. https://doi.org/10.3390/vaccines9090961
Ananworanich J, Heaton PM. Bringing Preventive RSV Monoclonal Antibodies to Infants in Low- and Middle-Income Countries: Challenges and Opportunities. Vaccines. 2021; 9(9):961. https://doi.org/10.3390/vaccines9090961
Chicago/Turabian StyleAnanworanich, Jintanat, and Penny M. Heaton. 2021. "Bringing Preventive RSV Monoclonal Antibodies to Infants in Low- and Middle-Income Countries: Challenges and Opportunities" Vaccines 9, no. 9: 961. https://doi.org/10.3390/vaccines9090961
APA StyleAnanworanich, J., & Heaton, P. M. (2021). Bringing Preventive RSV Monoclonal Antibodies to Infants in Low- and Middle-Income Countries: Challenges and Opportunities. Vaccines, 9(9), 961. https://doi.org/10.3390/vaccines9090961





